Abstract
AIM
To investigate the effects of triptolide on proinflammatory cytokine and chemokine expression induced by the fungal component zymosan in cultured human corneal fibroblasts (HCFs).
METHODS
HCFs were cultured in the absence or presence of zymosan or triptolide. The release of interleukin (IL)-6, IL-8, and monocyte chemoattractant protein-1 (MCP-1) into culture supernatants was measured with enzyme-linked immunosorbent assays. The cellular abundance of the mRNAs for these proteins was determined by reverse transcription and real-time polymerase chain reaction analysis. The phosphorylation of mitogen-activated protein kinases (MAPKs) and the endogenous nuclear factor-κB (NF-κB) inhibitor IκB-α was examined by immunoblot analysis. The release of lactate dehydrogenase (LDH) activity from HCFs was measured with a colorimetric assay.
RESULTS
Triptolide inhibited the zymosan-induced release of IL-6, IL-8, and MCP-1 from HCFs in a concentration- and time-dependent manner. It also inhibited the zymosan-induced up-regulation of IL-6, IL-8, and MCP-1 mRNA abundance in these cells. Furthermore, triptolide attenuated zymosan-induced phosphorylation of the MAPKs extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK), and p38 as well as the phosphorylation and degradation of IκB-α. Triptolide did not exhibit cytotoxicity for HCFs.
CONCLUSION
Triptolide inhibited proinflammatory cytokine and chemokine production by HCFs exposed to zymosan, with this action likely being mediated by suppression of MAPK and NF-κB signaling pathways. This compound might thus be expected to limit the infiltration of inflammatory cells into the cornea associated with fungal infection.
Keywords: fungal keratitis, zymosan, triptolide, inflammation, corneal fibroblast
INTRODUCTION
Fungal keratitis, which refers to inflammation of the cornea as a result of ocular infection with a fungus, is a serious health problem in developing countries[1]. Without prompt and effective treatment, fungal keratitis can result in perforation of the cornea, permanent loss of vision, and even loss of the eye. Inflammation associated with this condition results from the infiltration and activation of inflammatory cells triggered by various chemokines and other cytokines[2]. Keratocytes are the resident cells of the corneal stroma, but these cells transform into corneal fibroblasts in response to corneal damage[3]. Corneal fibroblasts play an important role in modulation of local immune and inflammatory responses to pathogen infection in the corneal stroma through pathogen recognition and consequent expression and secretion of proinflammatory cytokines and chemokines[4].
Triptolide, the major component of the Chinese herb Tripterygium wilfordii Hook f, possesses multiple pharmacological activities including anti-inflammatory, immunosuppressive, antitumor, and proapoptotic effects[5]. It thus inhibits the production of proinflammatory cytokines and chemokines induced by various inflammatory stimuli such as lipopolysaccharide (LPS), interleukin (IL)-1β, and tumor necrosis factor-α (TNF-α) in corneal fibroblasts[6]–[7]. Triptolide was also shown to suppress the IL-1β-induced production of matrix metalloproteinases 1, 2, 3, and 9 in corneal fibroblasts and to attenuate collagen degradation mediated by these cells[8]. The pharmacological potential of this agent for the treatment of fungal keratitis has not previously been examined, however.
Zymosan is derived from the cell wall of yeast, consists of protein-carbohydrate complexes, binds to toll-like receptor 2 (TLR2) expressed on the surface of immune cells, and has been widely studied as a phagocytic stimulus[9]. Zymosan triggers production of the proinflammatory cytokine TNF-α in RAW264.7 cells[10] as well as that of the chemokine IL-8 in human monocytic cells[11]. Exposure of corneal fibroblasts to zymosan results in the up-regulation of IL-6 and IL-8 production[12]. To explore the potential of triptolide for suppression of the inflammation associated with corneal fungal infection, we have now examined the effects of this agent on zymosan-induced production of the proinflammatory cytokine IL-6 and the chemokines IL-8 and monocyte chemoattractant protein-1 (MCP-1) by cultured human corneal fibroblasts (HCFs). The intracellular signaling pathways responsible for such effects were also examined.
MATERIALS AND METHODS
Materials
Eagle's minimum essential medium (MEM) and fetal bovine serum were obtained from Gibco (Carlsbad, CA, USA), and 24-well culture plates and 60-mm culture dishes were from Corning-Costar (Corning, NY, USA). Zymosan A was from Sigma (St. Louis, MO, USA), and triptolide was from Alexis Biochemicals (Carlsbad, CA, USA). A CytoTox 96 Non-Radioactive Cytotoxicity Assay was obtained from Promega (Madison, WI, USA). Enzyme-linked immunosorbent assay (ELISA) kits for IL-6, IL-8, and MCP-1 were from Boster (Wuhan, Hubei Province, China), and a Quantscript reverse transcription (RT) kit was obtained from Tiangen Biotechnology (Beijing, China). Trizol was from Invitrogen (Carlsbad, CA, USA), and both RNA isolation and EvaGreen quantitative polymerase chain reaction (qPCR) kits were from CapitalBio (Beijing, China). Rabbit polyclonal antibodies to total or phosphorylated forms of extracellular signal-regulated kinase (ERK), p38, c-Jun NH2-terminal kinase (JNK), or IκB-α were obtained from Cell Signaling (Beverly, MA, USA), and horseradish peroxidase-conjugated secondary antibodies, nitrocellulose membranes, and an enhanced chemiluminescence (ECL) kit were from GE Healthcare (Uppsala, Sweden). All media and reagents used for cell culture were endotoxin minimized.
Isolation and Culture of Human Corneal Fibroblasts
HCFs were isolated and cultured as described previously[13]. The rim of tissue remaining after the center of donor corneas had been punched out for corneal transplantation surgery was used as the source of corneal fibroblasts, and the human tissue was used in strict accordance with the tenets of the Declaration of Helsinki. In brief, the endothelial layer of the corneal rim was removed mechanically, and the remaining tissue was incubated with dispase (2 mg/mL, in MEM) for 1h at 37°C. After mechanical removal of the epithelial sheet, the tissue was treated with collagenase (2 mg/mL, in MEM) at 37°C until a single-cell suspension was obtained. Isolated corneal fibroblasts were cultured under 5% CO2 at 37°C in MEM supplemented with 10% fetal bovine serum. The cells were used for experiments after three to seven passages and were harvested at subconfluence, in the actively proliferating state.
Measurement of Interleukin-6, Interleukin-8, and Monocyte Chemoattractant Protein-1 Release
HCFs were cultured in 24-well plates until they achieved confluence, after which the culture medium was replaced with serum-free MEM for 1d. The cells were incubated first for 1h with various concentrations of triptolide (1 to 30 nmol/L) and then for the indicated times in the same medium supplemented with zymosan (600 µg/mL). The medium was then collected and centrifuged at 120×g for 5min, and the resulting supernatant was stored at -80°C until subsequent measurement of IL-6, IL-8, and MCP-1 concentrations with ELISA kits.
Real-time Quantitative Polymerase Chain Reaction Analysis
The abundance of IL-6, IL-8, and MCP-1 mRNAs in HCFs was determined by RT and real-time qPCR analysis, as described previously[14]. Total RNA was isolated from cells cultured in 60-mm dishes with the use of the Trizol reagent and was subjected to RT with the use of a kit. The resulting cDNA was subjected to qPCR analysis with the use of an EvaGreen qPCR kit and a real-time fluorescence quantitative instrument (RT-Cycler, CapitalBio) and with primers specific for IL-6, IL-8, MCP-1, or glyceraldehyde-3-phosphate dehydrogenase (GAPDH, internal control). The sequences of the PCR primers (forward and reverse, respectively) were as follows: IL-6, 5′-TACCCCCAGGAGAAGATTCC-3′ and 5′-TTTTCTGCCAGTGCCTCTTT-3′; IL-8, 5′-TACTCCAAACCTTTCCACCC-3′ and 5′-AACTTCTCCACAACCCTCTG-3′; MCP-1, 5′-GCAATCAATGCCCCAGTCA-3′ and 5′-TGCTGCTGGTGATTCTTCTATAGCT-3′; and GAPDH, 5′-ATTCCACCCATGGCAAATTC-3′ and 5′-CGCTCCTGGAAGATGGTGAT-3′. The PCR protocol comprised an initial denaturation at 95°C for 10min; 40 cycles of denaturation at 95°C for 30s, annealing at 50°C or 60°C for 30s, and elongation at 72°C for 30s; and a final elongation at 72°C for 7min. The specificity of amplification was verified by melting curve analysis. The fold change in target gene expression was normalized by that in GAPDH mRNA abundance by the 2−ΔΔCt method.
Immunoblot Analysis
Immunoblot analysis of ERK, p38, JNK, and IκB-α was performed as described previously. Cell lysates (10 µg of protein) prepared from cells cultured in 60-mm dishes were subjected to SDS-polyacrylamide gel electrophoresis on a 10% gel, the separated proteins were transferred electrophoretically to a nitrocellulose membrane, and nonspecific sites on the membrane were blocked. The membrane was then incubated with antibodies to total or phosphorylated forms of ERK, p38, JNK, or IκB-α, after which immune complexes were detected with horseradish peroxidase-conjugated secondary antibodies and ECL reagents.
Cytotoxicity Assay
HCFs (2×104 per well) seeded in a 96-well plate were incubated in MEM with or without triptolide for 24h, after which the amount of lactate dehydrogenase (LDH) released into the medium was determined with the use of an assay kit. Absorbance at 490 nm was measured with a microplate reader.
Statistical Analysis
Quantitative data are presented as means±SD and analyzed with Dunnett's multiple-comparison test, Student's t-test, or the Tukey-Kramer test. A P value of <0.05 was considered statistically significant.
RESULTS
Effects of Triptolide on Zymosan-induced Expression of Interleukin-6, Interleukin-8, and Monocyte Chemoattractant Protein-1 in Human Corneal Fibroblasts
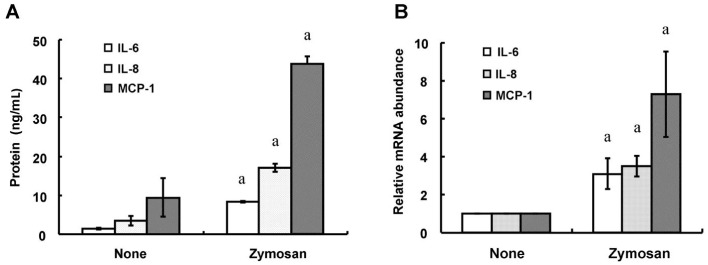
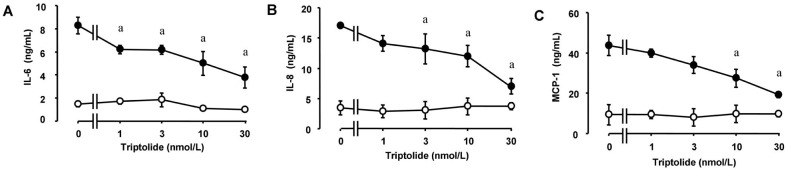
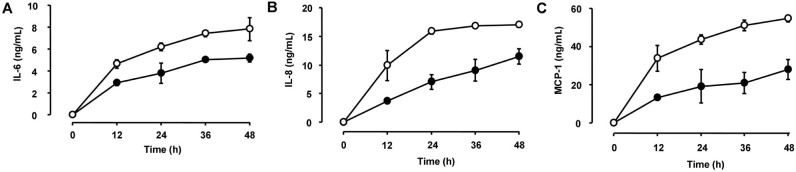
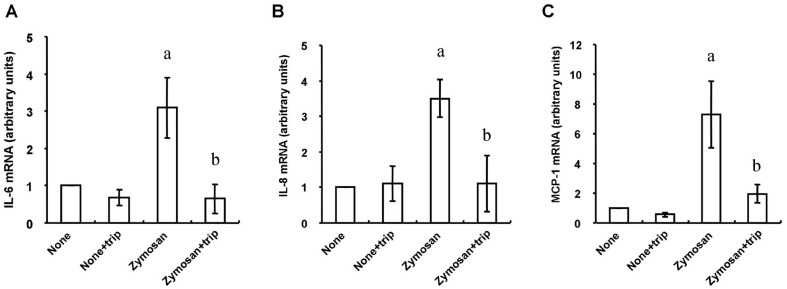
We first examined the effects of zymosan on the release of the proinflammatory cytokine IL-6 and the chemokines IL-8 and MCP-1 from HCFs. Consistent with previous observations[12], ELISAs and RT-qPCR analysis revealed that incubation of the cells with zymosan (600 µg/mL) for 12 or 24h induced the release of IL-6, IL-8, and MCP-1 (Figure 1A) as well as increased the cellular abundance of the corresponding mRNAs (Figure 1B), respectively. Whereas triptolide alone had no effect on cytokine or chemokine release, the release of IL-6, IL-8, and MCP-1 from HCFs induced by zymosan (600 µg/mL) was inhibited by triptolide in a concentration-dependent (Figure 2) and time-dependent (Figure 3) manner. In addition, triptolide (30 nmol/L) inhibited the zymosan-induced up-regulation of IL-6, IL-8, and MCP-1 mRNA abundance by 79%, 68%, and 71%, respectively (Figure 4).
Figure 1. Effects of zymosan on the expression of IL-6, IL-8, and MCP-1 in HCFs.
A: Serum-deprived cells were incubated for 24h in the absence or presence of zymosan (600 µg/mL), after which the release of IL-6, IL-8, and MCP-1 into the culture medium was determined with ELISAs. B: Serum-deprived cells were incubated for 12h in the absence or presence of zymosan (600 µg/mL), after which total RNA was isolated from the cells and subjected to RT-qPCR analysis of IL-6, IL-8, and MCP-1 mRNA abundance. All data are means±SD of triplicates from experiments that were repeated a total of three times with similar results. aP<0.05 (Student's t-test) versus the corresponding value for cells cultured without zymosan.
Figure 2. Concentration-dependent inhibition by triptolide of zymosan-induced IL-6, IL-8, and MCP-1 release from HCFs.
Serum-deprived cells were incubated first for 1h in the absence or presence (1 to 30 nmol/L) of triptolide and then for 24h in the additional absence (open circles) or presence (closed circles) of zymosan (600 µg/mL), after which the release of IL-6 (A), IL-8 (B), and MCP-1 (C) into the culture medium was determined with ELISAs. Data are means±SD of triplicates from experiments that were repeated a total of three times with similar results. aP<0.05 versus the corresponding value for cells cultured without triptolide (Tukey-Kramer test).
Figure 3. Time course of the inhibitory effects of triptolide on IL-6, IL-8, and MCP-1 release from HCFs.
Serum-deprived cells were incubated first for 1h in the absence (open circles) or presence (closed circles) of 30 nmol/L triptolide and then for the indicated times in the additional presence of zymosan (600 µg/mL). A: The release of IL-6 into the culture medium was determined with ELISAs; B: The release of IL-8 into the culture medium was determined with ELISAs; C: The release of MCP-1 into the culture medium was determined with ELISAs. Data are means±SD of triplicates from experiments that were repeated a total of three times with similar results.
Figure 4. Effects of triptolide on the zymosan-induced up-regulation of IL-6, IL-8, and MCP-1 mRNA abundance in HCFs.
Serum-deprived cells were incubated first with or without 30 nmol/L triptolide (trip) for 1h and then in the additional absence or presence of zymosan (600 µg/mL) for 12h, after which total RNA was isolated from the cells and subjected to RT-qPCR analysis of IL-6 (A), IL-8 (B), and MCP-1 (C) mRNA abundance. Data are means±SD from three separate experiments. aP<0.001 (Tukey-Kramer test) versus the corresponding value for cells cultured without zymosan; bP<0.01 (Tukey-Kramer test) versus the corresponding value for cells cultured without triptolide.
Effects of Triptolide on Zymosan-induced MAPK Phosphorylation and IκB-α Phosphorylation and Degradation in Human Coreanl Fibroblasts
We next examined the possible effects of triptolide on zymosan-induced signaling by mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) pathways in HCFs. Consistent with previous observations[12], immunoblot analysis with antibodies to total or phosphorylated forms of MAPKs and the endogenous NF-κB inhibitor IκB-α revealed that zymosan induced phosphorylation (activation) of the MAPKs ERK, p38, and JNK as well as the phosphorylation and degradation of IκB-α in a time-dependent manner (Figure 5). Triptolide (30 nmol/L) inhibited the zymosan-induced phosphorylation of ERK, p38, JNK, and IκB-α by 52%, 30%, 30%, and 48%, respectively (Figure 6).
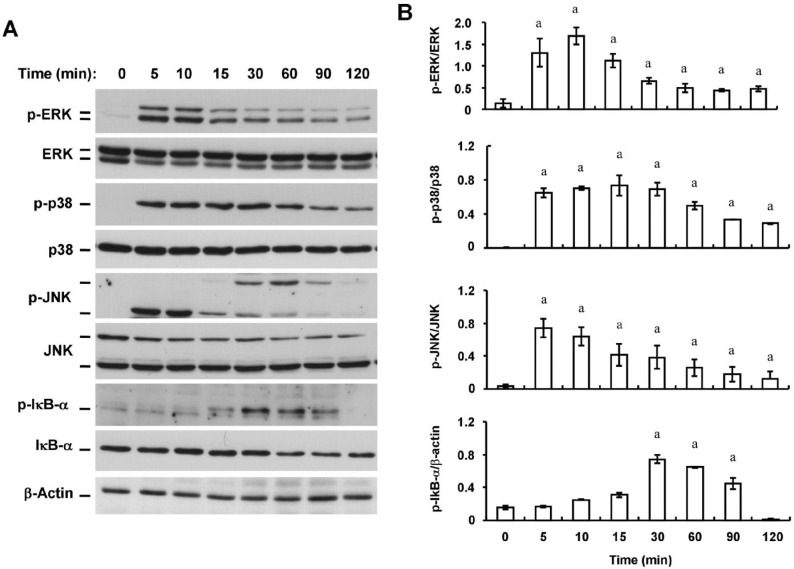
Figure 5. Time-dependent effects of zymosan on MAPK phosphorylation and IκB-α phosphorylation and degradation in HCFs.
A: Serum-deprived cells were incubated with zymosan (600 µg/mL) for the indicated times, after which cell lysates were prepared and subjected to immunoblot analysis with antibodies to phosphorylated (p-) or total forms of ERK1/2, p38, JNK, or IκB-α. β-Actin was examined as a loading control; B: Immunoblots similar to those in (A) were subjected to densitometric analysis to determine the intensity of the bands for phosphorylated forms of ERK1/2, p38, JNK, or IκB-α relative to that of those for the corresponding total forms or β-actin. Data are means±SD from three independent experiments. aP<0.05 (Dunnett's test) versus the corresponding value for time zero.
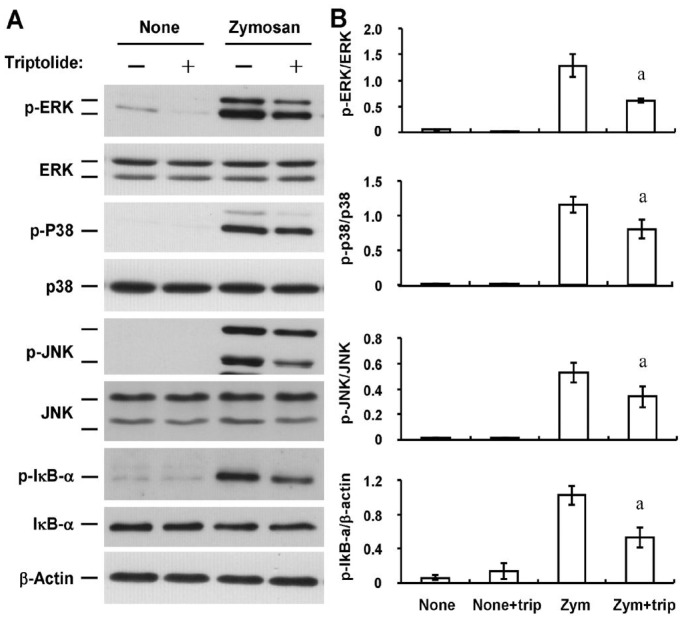
Figure 6. Effects of triptolide on the zymosan-induced activation of MAPK and NF-κB signaling pathways in HCFs.
A: Serum-deprived cells were incubated first for 1h in the absence or presence of 30 nmol/L triptolide (trip) and then for 10min (for MAPKs) or 30min (for IκB-α) in the additional absence or presence of zymosan (Zym, 600 µg/mL). Cell lysates were then prepared and subjected to immunoblot analysis with antibodies to total or phosphorylated (p-) forms of ERK1/2, p38, JNK, or IκB-α. B: Immunoblots similar to those in (A) were subjected to densitometric analysis to determine the intensity of the bands for phosphorylated forms of ERK1/2, p38, JNK, or IκB-α relative to that of those for the corresponding total forms or β-actin. Data are means±SD from three independent experiments. aP<0.05 (Tukey-Kramer test) versus the corresponding value for cells incubated with zymosan alone.
Effect of Triptolide on the Viability of Human Corneal Fibroblasts
Finally, we examined whether triptolide might affect the viability of HCFs. Exposure of the cells to various concentrations (1 to 30 nmol/L) of triptolide for 24h had no effect on the release of LDH (Figure 7), indicative of a lack of cytotoxicity.
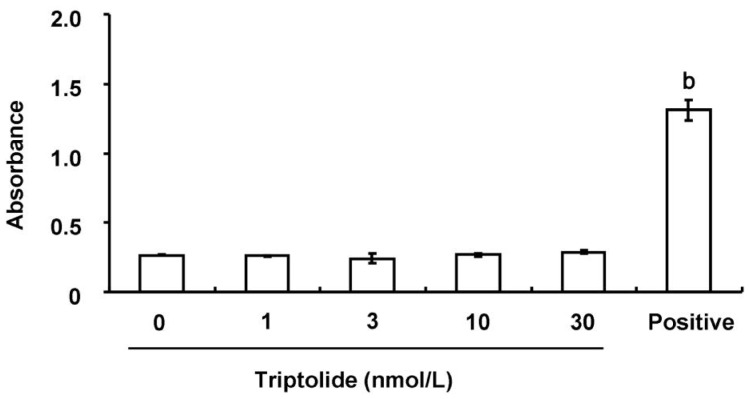
Figure 7. Lack of a cytotoxic effect of triptolide on HCFs.
Cells were incubated in the absence or presence of the indicated concentrations of triptolide for 24h, after which the activity of LDH in culture supernatants was measured. The amount of LDH released from cells by a cell lysis solution was determined as a positive control. Data are means±SD of triplicates from an experiment that was repeated a total of three times with similar results. bP<0.01 (Dunnett's test) versus the value for cells cultured without triptolide.
DISCUSSION
We have shown here that triptolide inhibited the zymosan-induced expression of the proinflammatory cytokine IL-6 and the chemokines IL-8 and MCP-1 in HCFs. Furthermore, the zymosan-induced activation of MAPK and NF-κB signaling in these cells was inhibited by triptolide.
Infiltration of various inflammatory cells into the cornea contributes to the pathophysiology of fungal keratitis. Histopathologic analysis has revealed that most inflammatory cells present in corneal tissue of individuals with fungal keratitis are polymorphonuclear neutrophils[2]. Leukocytes are attracted to the cornea by the local production of chemokines during the initial stages of fungal keratitis, and they promote fungal clearance[14]. However, excessive inflammation can lead to stromal damage and corneal opacification.
Cytokines and chemokines play a central role in ocular inflammatory responses through regulation of the activation, infiltration, and proliferation of immunocompetent cells in the cornea. IL-6 promotes the differentiation of B cells and mediates the acute-phase response[15]. Effective recruitment of neutrophils into the cornea is dependent on the production of IL-6[16]. IL-8 and MCP-1 are potent and selective chemoattractants for neutrophils and monocytes-macrophages, respectively. MCP-1 also promotes neovascularization associated with corneal inflammation[17]. The concentrations of these three molecules have been found to be increased in tear fluid of individuals with fungal keratitis or other corneal infections[18]–[19]. We have now shown that triptolide inhibited the zymosan-induced production of IL-6, IL-8, and MCP-1 by HCFs in a time- and concentration-dependent manner, suggesting that triptolide might attenuate corneal inflammation associated with fungal infection by blocking the expression of inflammatory cytokines and chemokines in these cells.
Zymosan is derived from the cell wall of yeast, binds to TLR2 on the surface of immune cells, and induces the expression of proinflammatory cytokines in such cells[20]. Activation of TLRs triggers MAPK and NF-κB signaling and thereby promotes inflammation. Consistent with previous observations[12], we have now shown that zymosan induced the activation of MAPK (ERK, p38 and JNK) and NF-κB signaling pathways in a time-dependent manner in corneal fibroblasts. Furthermore, triptolide inhibited the zymosan-induced phosphorylation of ERK, p38 and JNK as well as that of IκB-α, suggesting that the inhibitory effects of triptolide on the zymosan-induced expression of IL-6, IL-8, and MCP-1 in HCFs might be mediated by inhibition of these signaling pathways.
Corneal fibroblasts play an important role in inflammation of the corneal stroma associated with bacterial, viral, or fungal infection by up-regulating the expression of chemokines, cytokines, and adhesion molecules in response to various stimuli including LPS, viral nucleic acid, and zymosan, respectively. LPS derived from Gram-negative bacteria and polyinosinic-polycytidylic acid [poly (I:C)] as a synthetic analog of viral double-stranded RNA are ligands for TLR4 and TLR3, respectively[21]. Triptolide inhibits the expression of chemokines, cytokines, and adhesion molecules induced by LPS in corneal fibroblasts[7]. It also attenuates the poly (I:C)-induced expression of matrix metalloproteinases in these cells[22], with these enzymes playing a key role in the degradation of extracellular matrix proteins. Together with our present results, these observations indicate that triptolide inhibits signaling pathways activated by various TLRs and might therefore be of therapeutic benefit in infectious diseases characterized by corneal stromal inflammation.
In conclusion, we have shown that triptolide inhibited the production of the proinflammatory cytokine IL-6 and the chemokines IL-8 and MCP-1 in HCFs exposed to zymosan and that these inhibitory effects were accompanied by inhibition of MAPK and NF-κB signaling pathways. Further studies are thus warranted to determine whether triptolide might prove effective for limiting the infiltration of inflammatory cells into the cornea associated with fungal infection.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No. 81100635); the Norman Bethune Program of Jilin University (No. 2012213).
Conflicts of Interest: Liu Y, None; Li J, None; Liu Y, None; Wang P, None; Jia H, None.
REFERENCES
- 1.Whitcher JP, Srinivasan M. Corneal ulceration in the developing world-a silent epidemic. Br J Ophthalmol. 1997;81(8):622–623. doi: 10.1136/bjo.81.8.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vemuganti GK, Garg P, Gopinathan U, Naduvilath TJ, John RK, Buddi R, Rao GN. Evaluation of agent and host factors in progression of mycotic keratitis: a histologic and microbiologic study of 167 corneal buttons. Ophthalmology. 2002;109(8):1538–1546. doi: 10.1016/s0161-6420(02)01088-6. [DOI] [PubMed] [Google Scholar]
- 3.Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999;18(3):311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- 4.Xiaoyan Zhang, Xinyi Wu, Li Gao. Pretreatment with lipopolysaccharide modulates innate immunity in corneal fibroblasts challenged with Aspergillus fumigatus. Innate Immun. 2011;17(3):237–244. doi: 10.1177/1753425910365364. [DOI] [PubMed] [Google Scholar]
- 5.Liu Q. Triptolide and its expanding multiple pharmacological functions. Int Immunopharmacol. 2011;11(3):377–383. doi: 10.1016/j.intimp.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Lu Y, Fukuda K, Nakamura Y, Kimura K, Kumagai N, Nishida T. Inhibitory effect of triptolide on chemokine expression induced by proinflammatory cytokines in human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2005;46(7):2346–2352. doi: 10.1167/iovs.05-0010. [DOI] [PubMed] [Google Scholar]
- 7.Lu Y, Liu Y, Fukuda K, Nakamura Y, Kumagai N, Nishida T. Inhibition by triptolide of chemokine, proinflammatory cytokine, and adhesion molecule expression induced by lipopolysaccharide in corneal fibroblasts. Invest Ophthalmol Vis Sci. 2006;47(9):3796–3800. doi: 10.1167/iovs.06-0319. [DOI] [PubMed] [Google Scholar]
- 8.Lu Y, Fukuda K, Seki K, Nakamura Y, Kumagai N, Nishida T. Inhibition by triptolide of IL-1-induced collagen degradation by corneal fibroblasts. Invest Ophthalmol Vis Sci. 2003;44(12):5082–5088. doi: 10.1167/iovs.03-0476. [DOI] [PubMed] [Google Scholar]
- 9.Li DQ, Zhou N, Zhang L, Ma P, Pflugfelder SC. Suppressive effects of azithromycin on zymosan-induced production of proinflammatory mediators by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2010;51(11):5623–5629. doi: 10.1167/iovs.09-4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato M, Sano H, Iwaki D, Kudo K, Konishi M, Takahashi H, Takahashi T, Imaizumi H, Asai Y, Kuroki Y. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-kappa B activation and TNF-alpha secretion are down-regulated by lung collectin surfactant protein A. J Immunol. 2003;171(1):417–425. doi: 10.4049/jimmunol.171.1.417. [DOI] [PubMed] [Google Scholar]
- 11.Friedland JS, Constantin D, Shaw TC, Stylianou E. Regulation of interleukin-8 gene expression after phagocytosis of zymosan by human monocytic cells. J Leukoc Biol. 2001;70(3):447–454. [PubMed] [Google Scholar]
- 12.Nomi N, Kimura K, Nishida T. Release of interleukins 6 and 8 induced by zymosan and mediated by MAP kinase and NF-kappaB signaling pathways in human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2010;51(6):2955–2959. doi: 10.1167/iovs.09-4823. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Kimura K, Yanai R, Chikama T, Nishida T. Cytokine, chemokine, and adhesion molecule expression mediated by MAPKs in human corneal fibroblasts exposed to poly (I:C) Invest Ophthalmol Vis Sci. 2008;49(8):3336–3344. doi: 10.1167/iovs.07-0972. [DOI] [PubMed] [Google Scholar]
- 14.Zhong W, Yin H, Xie L. Expression and potential role of major inflammatory cytokines in experimental keratomycosis. Mol Vis. 2009;15:1303–1311. [PMC free article] [PubMed] [Google Scholar]
- 15.Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol. 2006;2(11):619–626. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 16.Cole N, Bao S, Stapleton F, Thakur A, Husband AJ, Beagley KW, Willcox MD. Pseudomonas aeruginosa keratitis in IL-6-deficient mice. Int Arch Allergy Immunol. 2003;130(2):165–172. doi: 10.1159/000069006. [DOI] [PubMed] [Google Scholar]
- 17.Ogawa S, Yoshida S, Ono M, Onoue H, Ito Y, Ishibashi T, Inomata H, Kuwano M. Induction of macrophage inflammatory protein-1alpha and vascular endothelial growth factor during inflammatory neovascularization in the mouse cornea. Angiogenesis. 1999;3(4):327–334. doi: 10.1023/a:1026554404941. [DOI] [PubMed] [Google Scholar]
- 18.Vasanthi M, Prajna NV, Lalitha P, Mahadevan K, Muthukkaruppan V. A pilot study on the infiltrating cells and cytokine levels in the tear of fungal keratitis patients. Indian J Ophthalmol. 2007;55(1):27–31. doi: 10.4103/0301-4738.29491. [DOI] [PubMed] [Google Scholar]
- 19.Yuan X, Hua X, Wilhelmus KR. Proinflammatory chemokines during Candida albicans keratitis. Exp Eye Res. 2010;90(3):413–419. doi: 10.1016/j.exer.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401(6755):811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 21.Manavalan B, Basith S, Choi S. Similar structures but different roles - an updated perspective on TLR structures. Front Physiol. 2011;2:41. doi: 10.3389/fphys.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura K, Nomi N, Yan ZH, Orita T, Nishida T. Inhibition of poly(I:C)-induced matrix metalloproteinase expression in human corneal fibroblasts by triptolide. Mol Vis. 2011;17:526–532. [PMC free article] [PubMed] [Google Scholar]