Abstract
AIM
To investigate the role of Aquaporin-1 (AQP-1) in lens epithelial cells (LECs) and its potential target genes. AQP-1 is specifically expressed in LECs of eyes and is significant for lens homeostasis and transparency maintenance. Herein, AQP-1 expression in LECs was investigated to evaluate its influence on cell survival in association with its potential role in cataract formation.
METHODS
LECs were transfected with lentivirus carrying AQP-1 small interfering RNA (siRNA). Real-time polymerase chain reaction (PCR) and Western blotting were conducted to detect AQP-1 expression in LECs from different groups. Meanwhile, cell counting kit-8 (CCK-8) assay and flow cytometry were performed to measure LEC proliferation and apoptosis, respectively.
RESULTS
AQP-1 expression was significantly reduced in LECs, both at mRNA and protein levels (P<0.05), after siRNA treatment. Decreased cell viability was detected by CCK-8 assay in LECs with siRNA interference, compared to control cells (P<0.05). The apoptosis rate significantly increased in cells after siRNA interference (P<0.05).
CONCLUSION
The decreased cell viability following AQP-1 down regulation is largely due to its induction of apoptosis of LECs. AQP-1 reduction might lead to changes of physiological functions in LECs, which might be associated with the occurrence and development of cataracts.
Keywords: Aquaporin-1, small interfering RNA, lens epithelial cells proliferation, apoptosis, cell counting kit-8, flow cytometry
INTRODUCTION
Cataract is a leading cause of blindness. Age-related cataract (ARC) is the most common type of cataract[1]–[2]. About 46% of 45 million cases of blindness worldwide are due to cataracts[3]. With increase of life expectancy and aged population, the prevalence of cataracts and the number of cataract cases are increasing. The only effective treatment for the disease is surgery, which is rather expensive[4]–[6]. Cataracts significantly decrease the eyesight and quality of life, and impose extra burden on economic well-being of patients. Numerous studies have investigated the pathogenesis of cataracts in order to find effective measures to prevent them and treat the disease.
The Aquaporin family (AQPs) is a group of membrane proteins that not only allow the rapid transportation of the water molecule into the cell but also block the transportation of charged molecules, such as protons (H+) [7]. To date, 13 membrane proteins have been identified in the AQP family of mammals, AQP 0-12. These AQPs have similar functions, but their expression is differently distributed. Thus, different AQPs have distinct physiological functions. Aquaporin-1 (AQP-1) is widely expressed in the eyes; in the lens, it is expressed only in lens epithelial cells (LECs) [8] and is a major membrane protein of those cells. Recently, increasing attention has been paid to the role of AQP-1 in the pathogenesis of cataracts. Some experiments have confirmed that altered water permeability of the basement membrane (lens capsule) has an important influence on lens transparency. AQP-1 in LECs may regulate the transmembrane transport of water and maintain the water/electrolyte balance in LECs, a balance crucial for maintaining lens homeostasis, transparency and resistance to cataracts [9]–[11]. In addition, some investigators have reported that AQP-1 expression in ARC LECs was significantly reduced, compared with the epithelial cells of transparent lens, suggesting that AQP-1 is related to the development and progression of cataract. However, the specific mechanism underlying the effect of AQP-1 on lens function is still poorly understood.
The pathogenesis of cataract formation is very complex. The disease is usually considered as a result of numerous factors. LEC apoptosis is a common cytological basis for the pathogenesis of cataracts, except for congenital cataracts. In studies on ARC, LEC apoptosis increases significantly, suggesting that LEC apoptosis is important in the development and progression of cataracts[12]–[14].
Thus, we speculate that LEC apoptosis is related to ARC since AQP-1 plays an important role in maintaining lens transparency and may counteract cataract development. The relationship between AQP-1 and LECs remains unknown, as does the role of AQP-1 in ARC. In this study, AQP-1 expression was down-regulation using RNA interference (RNAi), and other confounding factors were also removed, to allow us to focus on the relationship between AQP-1 and LECs. After down-regulation of AQP-1 expression, we detected the proliferation and apoptosis of LECs, to investigate the role of AQP-1 in the pathophysiology of LECs and the influence of AQP-1 on lens function. Our findings may help elucidate the etiology and pathogenesis of ARC and provide a theoretical basis for early prevention and treatment of cataracts.
MATERIALS AND METHODS
Cell Lines and Cell Culture
Our study was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University. A human LEC line, SRA 01/04, which was established by transfection with a large T-antigen of SV40, was cultured in antibiotic-free Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (all from Gibco-Invitrogen, Beijing, China). Incubation was carried out at 37°C in a humidified atmosphere with 5% CO2. Four groups of cells were used: interfering group 1, interfering group 2, non-specific interfering group and normal control group.
Construction of Vectors Carrying Small Interfering RNA and Packaging of Lentivirus
Using the human AQP-1 sequence, we designed small interfering RNA (siRNA) targeting this sequence. Two siRNAs were designed: AQP-1 siRNA-1 (5′-CCACGACCCTCTTTGTCTT-3′) and AQP-1 siRNA-2 (5′-GGAGGAGTATGACCTGGAT-3′). Meanwhile, a non-specific sequence without homology to any human genes was designed as a negative control: NC siRNA (5′-TTCTCCGAACGTGTCACGT-3′). Standard molecular techniques were employed to clone these oligonucleotide sequences into pLKD.CMV.GFP.U6.shRNA plasmids, which, together with psPAX2 packaging plasmids and pMD2.G adventitia plasmid, were transfected into 293T cells in the presence of DNA-Lipofectamine™ 2000. At 2-3d after transfection, lentiviruses were produced in 293T cells and were present in the medium. Lentiviruses were concentrated by centrifugation at 26 000 rpm. The lentivirus was used to infect human LECs.
Transfection of Lens Epithelial Cells with Lentivirus to Down-regulate Aquaporin-1
LECs were seeded into 6-well plates at a density of 2×105/well followed by incubation overnight. On the second day, cells were at about 70% confluence. The medium was removed, and fresh medium without antibiotics was added, followed by addition of diluted lentivirus solution. Then, incubation was done for 24h. The medium was refreshed. At 48h after transfection, fluorescence was observed. It reached a maximal level at 96h.
RNA Extraction and Quantitative Reverse Transcription-Polymerase Chain Reaction
Total RNA was extracted from LECs by using Trizol (Invitrogen, Beijing, China) and reverse transcribed into cDNA with a M-MLV reverse transcription kit (Promega, Shanghai, China). The housekeeping gene GAPDH served as a control. The primers for GAPDH were as follows: 5′-AGAAGGCTGGGGCTCATTTG-3′ (forward), 5′-AGGGGCCATCCACAGTCTTC-3′ (reverse); primers for AQP-1 were as follows: 5′-AGGCTACAAAGCAGAGATCGAC-3′ (forward), 5′-CACCCTCTAAATGGCTTCATTC-3′ (reverse). Polymerase chain reaction (PCR) was done according to the manufacturer's instructions (Takara Bio, Inc., Shanghai, China). The reaction mixture included 10 µL of SYBR-Premix Ex TaqTM, 0.4 µL of PCR forward primer, 0.4 µL of PCR reverse primer, 0.4 µL of ROX reference dye, 0.2 µL of cDNA template and 6.8 µL of dH2O (total volume 20 µL). The PCR conditions were as follows: pre-denaturation at 95°C for 30s and PCR at 95°C for 5s and 60°C for 30-34s. The 2−ΔΔCt method was used to calculate the AQP-1 expression. All groups were performed in triplicate.
Western Blotting Analysis
Total proteins were extracted after cell lysis, the protein concentration was determined with the bicinchoninic acid (BCA) method. Subsequently, 30 µg of proteins were denatured by heating and subjected to 12% sodium dodecyl sulfate - polyacrylamide gel electrophoresis (SDS-PAGE). Then, proteins were transferred onto a polyvinylidene fluoride membrane blocked with 5% non-fat milk in Tris buffer for 2h. The membrane was incubated with primary AQP-1 antibody (1:1000; Santa Cruz Technology, Santa Cruz, CA, USA) at 4°C overnight and then with horseradish peroxidase conjugated secondary antibody (1:2000; Santa Cruz Technology, Santa Cruz, CA, USA) at room temperature for 1h followed by visualization. β-actin served as an internal reference. Protein bands were input into a computer and their optical density was determined with software. All groups were performed in triplicate.
Cell Counting Kit-8 Assay
At 96h after transfection, cells were detached and harvested. Then, the cells were seeded into 96-well plates at a density of 10 000 cells/µL. A total of 6 wells were included in each group, and a blank control group was also set up. Cells were incubated at 37°C for 24h in an environment with 5% CO2. Then, 10 µL of cell counting kit solution was added to each well and incubation was done for 2h. Absorbance was measured with a microplate reader (Model 123, Biosource Company, USA) at 450 nm. All groups were performed in triplicate.
Flow Cytometry
LECs were detached with EDTA-free trypsin and washed with phosphate buffer saline (PBS) twice. The cells were harvested (1-5×105). The binding buffer (50 µL) was mixed with 7-amino-actinomycin D (7-ADD) solution, and the cells were added to the mixture, followed by incubation at room temperature in the dark for 5-15min. Flow cytometry was done by using annexin V-PE apoptosis detection kit (Invitrogen, Beijing, China) at the FL2 channel. At the excitation wavelength of 488 nm and the emission wavelength of 578 nm, orange fluorescence was observed. The 7-ADD was detected via the FL3 channel. Red fluorescence was observed at the excitation wavelength of 546 nm and emission wavelength of 647 nm. All groups were performed in triplicate.
Statistical Analysis
Data were present as mean±SD. Each experiment was triplicated with one-way analysis of variance for comparison among groups. P-value<0.05 was considered as statistical difference. Statistical analysis was performed using the SPSS® software package, version 19.0 (SPSS Inc., Chicago, IL, USA) for Windows®.
RESULTS
Infection of Human Lens Epithelial Cells by Lentiviruses
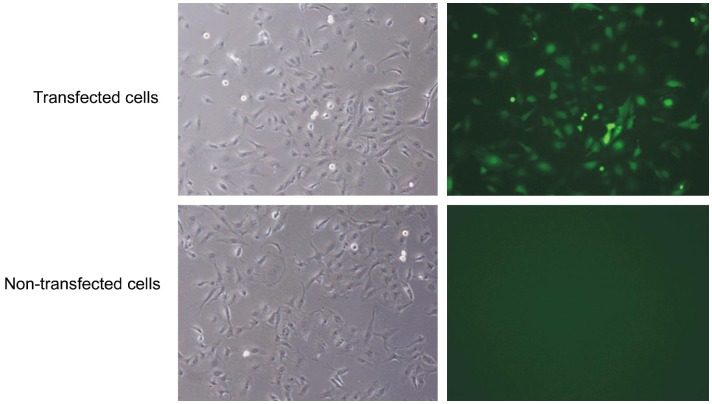
To infect human LECs with lentivirus, pLKD.CMV.GFP.U6.shRNA was constructed with green fluorescence to observe transfection efficiency. Under a fluorescence microscope, lentiviruses-infected LECs exhibited green fluorescence, revealing the transfection efficiency greater than 70%. No fluorescence was observed in non-transfected cells (Figure 1).
Figure 1. Human LECs after infection with lentiviruses.
Under a fluorescence microscope, LECs undergoing lentivirus transfection showed green fluorescence and the transfection efficiency was 70%. No fluorescence was observed in non-transfected cells.
Down-regulation of Aquaporin-1 Expression after RNA Interference
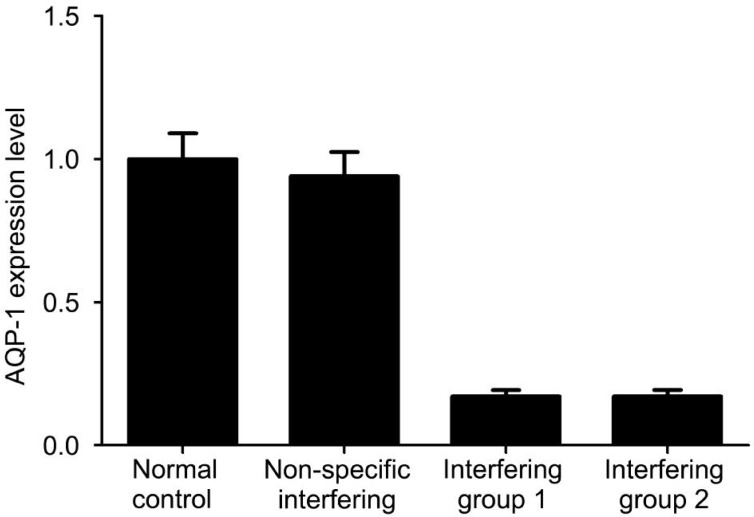
Real time fluorescence PCR was done to detect AQP-1 expression after RNAi. Results showed that the mRNA expression of AQP-1 was comparable between the normal control group and the non-specific interfering group. After RNAi with siRNAs targeting AQP-1, the AQP-1 mRNA level was reduced significantly (P<0.05; Figure 2).
Figure 2. Down-regulation of AQP-1 expression after RNA interference.
AQP-1 mRNA expression is reduced significantly after RNA interference with siRNA. GAPDH serves as an internal reference and ΔCt is calculated in each group.
Detection of Aquaporin-1 Protein Expression
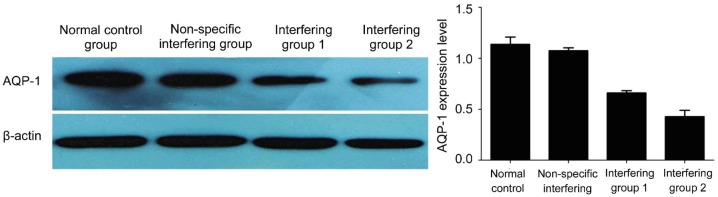
Western blotting assay was performed to detect AQP-1 expression after interference with siRNA. In both interference groups, AQP-1 expression was reduced markedly, compared to the non-specific interfering group and the control group (P<0.05). However, AQP-1 expression was comparable between the control group and the non-specific interfering group (P>0.05). This result suggests that RNAi of AQP-1 expression was successful, and AQP-1 expression was significantly down-regulated in human LECs (Figure 3).
Figure 3. Detection of AQP-1 protein expression after RNA interference with siRNA.
AQP-1 protein expression is reduced significantly
Detection of Lens Epithelial Cells Proliferation by Cell Counting Kit-8 Assay
Cell counting kit-8 (CCK-8) assay was done on the basis of WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium], an MTT-like compound with high sensitivity widely used in the rapid detection of cell proliferation. In the presence of an electron-coupling reagent, water-soluble tetrazolium (WST) may be reduced by dehydrogenases in the mitochondria into orange formazan. The formazan content is proportional to the cell proliferation activity. Optical density (OD) is measured with a microplate reader at 450 nm and may indirectly reflect the number of viable cells. In our study, the OD after interference with siRNA was significantly lower than that in the control group and the non-specific interfering group (P<0.05), suggesting compromised cell proliferation (Figure 4).
Figure 4. OD value detected of LEC proliferation by CCK-8 assay.
Detection was done at 450 nm; one way analysis of variance was used for statistical analysis. P<0.05: interfering group 1 or 2 vs the normal control group; P>0.05: the non-specific interfering group vs the normal control group. Cell viability was reduced significantly after RNAi. OD: Optical density.
Detection of Lens Epithelial Cells Apoptosis by Flow Cytometry
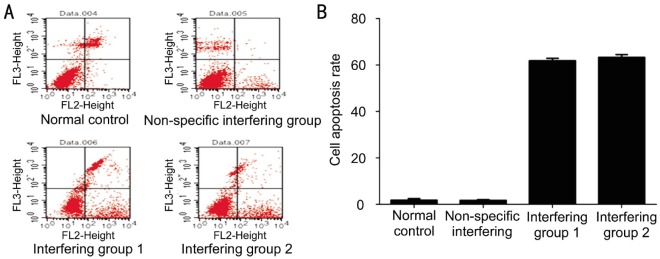
Flow cytometry was done to detect cell apoptosis with an Annexin V-PE/7-AAD apoptosis detection kit (KeyGEN BioTECH, Beijing, China). The scatter plots after flow cytometry showed that normal cells were negative for Annexin V-PE and 7-AAD (lower left quadrant). Cells positive for 7-AAD but negative for Annexin V-PE (upper left quadrant) were necrotic cells. Cells positive for Annexin V-PE but negative for 7-AAD (lower right quadrant) were cells in the early phase of apoptosis. Cells positive for Annexin V-PE and 7-AAD (upper right quadrant) were cells in the mid or late phase of apoptosis (Figure 5).
Figure 5. The apoptosis rate after interfering with siRNA.
A: Detection of LEC apoptosis by flow cytometry. The scatter plots showed that normal cells were negative for Annexin V-PE and 7-AAD (lower left quadrant). Cells positive for 7-AAD but negative for Annexin V-PE (upper left quadrant) were necrotic cells. Cells positive for Annexin V-PE but negative for 7-AAD (lower right quadrant) were cells in the early apoptosis phase. Cells positive for Annexin V-PE and 7-AAD (upper right quadrant) were cells in the mid or late apoptosis phase. B: Apoptosis rate after interference with siRNA. The apoptosis was significantly higher in the interference group than in the control group or the non-specific interfering group. The apoptosis rate of cells after RNA interference with siRNA was significantly higher than that in the normal control group and the non-specific interfering group. The experiment was done thrice with one way analysis of variance used for statistical analysis.
The results showed that the apoptosis rate after interfering with siRNA was significantly higher than that in the control group and the non-specific interfering group (P<0.05)
DISCUSSION
Real-time PCR and Western blotting assay showed that the mRNA and protein expression of AQP-1 after RNA interference with siRNA was significantly reduced when compared with the control group. This result suggests that the siRNA is correctly designed to halt AQP-1 expression. At the same time, a non-specific short hairpin RNA (shRNA) was also designed in our study as a control, to exclude the influence of other experimental factors (such as reagents) on LECs during transfection. The results showed that the AQP-1 expression was comparable between the non-specific interfering group and the control group, indicating that the procedure of RNAi has no influence on the AQP-1 expression of cells. Thus, this method is a feasible procedure by which to knock out the AQP-1 gene.
CCK-8 assay and flow cytometry showed that cell viability and proliferation were markedly compromised and the percentage of apoptotic cells increased significantly after the addition of siRNA targeting AQP-1. This result also indicates that AQP-1 helps maintain the normal physiological function of LECs. AQP-1 exists in the form of a tetramer in the cell membrane and each monomer has an independent functional unit of 6 transmembrane α helixes as the skeleton and 2 short non-transmembrane α helixes[15]–[16]. The hollow portion of 6 monomers forms a channel with high selectivity, which allows for the bidirectional transport of water. A single AQP-1 protein allows for the transport of 3 billion water molecules per second, a permeability 10-100 fold that of the lipid bilayer[17]. Thus, AQP-1 mainly mediates the transmembrane transport of free water and acts to regulate the water balance in cells, a balance crucial for maintaining the stability of the cell microenvironment.
Our results showed that altered AQP-1 expression could disturb water transport, resulting in suppression of cell proliferation and, eventually, apoptosis. Morphologically, loss of water and shrinkage of cells are characteristics of apoptosis, also known as apoptotic volume decrease (AVD). King et al[18] and Jablonski et al[19] proposed that AQPs are important for the transmembrane transport of water during AVD. When AQP-1 expression is down-regulated, the permeability to water is significantly reduced, cell volume is also reduced and apoptosis, finally, occurs, a process consistent with our findings. Since AQP-1 in the cell membrane mediates water transport and regulates the balance of water and electrolytes in cells, reduced AQP-1 expression may decrease water transportation, leading to increases in intracellular potassium and calcium levels. In addition, loss of water in cells may cause pyknosis which may initiate programmed cell death. Apoptosis may involve the mitochondria or other pathways in which caspase and endonucleases are activated to induce apoptosis. This process requires further investigation in future studies.
The lens is transparent with no vascular structure. The lens acquires nutrition via the lens capsule (especially the LECs under the lens capsule). The lens fibroblasts must be maintained in a relatively dehydrated status to maintain lens transparency. The maintenance of this dehydrated status is dependent on LECs, which contact with the aqueous humor. It is obvious that LECs require sufficient water transportation to provide nutrition and excrete metabolites, to help maintain the self-balance and functional stability of the lens. Our results showed that reduced AQP-1 expression leads to reduced LEC proliferation and apoptosis of LECs (reduction in LECs), which may be one of the mechanisms underlying the role of AQP-1 in the occurrence and development of cataracts. Alterations in AQP-1 expression may directly influence LEC function because AQP-1 is expressed only in LECs in the eye. Once LECs become pathological, lesions will develop in the lens[20]–[21]. Ruiz-Ederra and Verkman[9] found that reduction in AQP-1 expression could increase the permeability of the lens capsule to water, which promotes the occurrence of cataracts. This finding also suggests that AQP-1 is crucial to maintain lens transparency and to counteract the formation of cataracts, an assertion further confirmed by our findings. We speculate that changes in AQP-1 may alter the physiological functioning of LECs to promote apoptosis, which influences the functioning of the lens capsule, inducing swelling and opacification of the lens fibers, finally resulting in cataracts.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81070715); Innovative Platform Foundation of Fujian Province, China (No.2010Y2003).
Conflicts of Interest: Zheng HH, None; Xu GX, None; Guo J, None; Fu LC, None; Yao Y, None.
REFERENCES
- 1.Resnikoff S, Keys TU. Future trends in global blindness. Indian J Ophthalmol. 2012;60(5):387–395. doi: 10.4103/0301-4738.100532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asbell PA, Dualan I, Mindel J, Brocks D, Ahmad M, Epstein S. Age-related cataract. Lancet. 2005;365(9459):599–609. doi: 10.1016/S0140-6736(05)17911-2. [DOI] [PubMed] [Google Scholar]
- 3.WHO . Blindness: Vision 2020-the global initiative for the elimination of avoidable blindness. Fact sheet number 213. Geneva: WHO; 2000. Available at http://www.who.int/mediacentre/factsheets/fs213/en/ Accessed Dec.10, 2004. [Google Scholar]
- 4.Riaz Y, Mehta JS, Wormald R, Evans JR, Foster A, Ravilla T, Snellingen T. Surgical interventions for age-related cataract. Cochrane Database Syst Rev. 2006;18(4):CD001323. doi: 10.1002/14651858.CD001323.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberg EP, Javitt JC, Sharkey PD, Zuckerman A, Legro MW, Anderson GF, Bass EB, O'Day D. The content and cost of cataract surgery. Arch Ophthalmol. 1993;111(8):1041–1049. doi: 10.1001/archopht.1993.01090080037016. [DOI] [PubMed] [Google Scholar]
- 6.Hosler MR, Scott IU, Kunselman AR, Wolford KR, Oltra EZ, Murray WB. Impact of resident participation in cataract surgery on operative time and cost. Ophthalmology. 2012;119(1):95–98. doi: 10.1016/j.ophtha.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 7.Agre P, Kozono D. Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett. 2003;555(1):72–78. doi: 10.1016/s0014-5793(03)01083-4. [DOI] [PubMed] [Google Scholar]
- 8.Verkman AS. Role of aquaporin water channels in eye function. Exp Eye Res. 2003;76(2):137–143. doi: 10.1016/s0014-4835(02)00303-2. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Ederra J, Verkman AS. Accelerated cataract formation and reduced lens epithelial water permeability in aquaporin-1-deficient mice. Invest Ophthalmol Vis Sci. 2006;47(9):3960–3967. doi: 10.1167/iovs.06-0229. [DOI] [PubMed] [Google Scholar]
- 10.Varadaraj K, Kumari S, Shiels A, Mathias RT. Regulation of aquaporin water permeability in the lens. Invest Ophthalmol Vis Sci. 2005;46(4):1393–1402. doi: 10.1167/iovs.04-1217. [DOI] [PubMed] [Google Scholar]
- 11.Varadaraj K, Kumari SS, Mathias RT. Functional expression of aquaporins in embryonic, postnatal, and adult mouse lenses. Dev Dyn. 2007;236(5):1319–1328. doi: 10.1002/dvdy.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan Q, Liu JP, Li DW. Apoptosis in lens development and pathology. Differentiation. 2006;74(5):195–211. doi: 10.1111/j.1432-0436.2006.00068.x. [DOI] [PubMed] [Google Scholar]
- 13.Martinez G, de Iongh RU. The lens epithelium in ocular health and disease. Int J Biochem Cell Biol. 2010;42(12):1945–1963. doi: 10.1016/j.biocel.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Wielgus AR, Zhao B, Chignell CF, Hu DN, Roberts JE. Phototoxicity and cytotoxicity of fullerol in human retinal pigment epithelial cells. Toxicol Appl Pharmacol. 2010;242(1):79–90. doi: 10.1016/j.taap.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Ren G, Cheng A, Reddy V, Melnyk P, Mitra AK. Three-dimensional fold of the human AQP1 water channel determined at 4 A resolution by electron crystallography of two-dimensional crystals embedded in ice. J Mol Bio. 2000;301(2):369–387. doi: 10.1006/jmbi.2000.3949. [DOI] [PubMed] [Google Scholar]
- 16.Matsuzald T, Tajika Y, Ablimit A, Aoki T, Hagiwara H, Takata K. Aquaporins in the digestive system. Med Electron Microsc. 2004;37(2):71–80. doi: 10.1007/s00795-004-0246-3. [DOI] [PubMed] [Google Scholar]
- 17.Park JH, Saier MH. Phylogenetic characterization of the MIP family of transmembrane channel proteins. J Membr Biol. 1996;153(3):171–180. doi: 10.1007/s002329900120. [DOI] [PubMed] [Google Scholar]
- 18.King LS, Yasui M, Agre P. Aquaporins in health and disease. Mol Med Today. 2000;6(2):60–65. doi: 10.1016/s1357-4310(99)01636-6. [DOI] [PubMed] [Google Scholar]
- 19.Jablonski EM, Webb AN, McConnell NA, Riley MC, Hughes FM. Plasma membrane aquaporin activity can affect the rate of apoptosis but is inhibited after apoptotic volume decrease. Am J Physiol Cell Physiol. 2004;286(4):C975–C985. doi: 10.1152/ajpcell.00180.2003. [DOI] [PubMed] [Google Scholar]
- 20.Hightower KR, Reddan JR, McCready JP, Dziedzic DC. Lens epithelium: a primary target of UVB irradiation. Exp Eye Res. 1994;59(5):557–564. doi: 10.1006/exer.1994.1141. [DOI] [PubMed] [Google Scholar]
- 21.Kim J, Kim CS, Sohn E, Jeong IH, Kim JS. Lens epithelial cell apoptosis initiates diabetic cataractogenesis in the Zucker diabetic fatty rat. Graefes Arch Clin Exp Ophthalmol. 2010;248(6):811–818. doi: 10.1007/s00417-010-1313-1. [DOI] [PubMed] [Google Scholar]