Abstract
AIM
To investigate the changes in the expression of microRNA-181a (miR-181a) and Bim in a rat model of retinal ischemia-reperfusion (RIR), to explore their target relationship in RIR and their involvement in regulating apoptosis of retinal ganglion cells (RGCs).
METHODS
Target gene prediction for miR-181a was performed with the aid of bioinformatics and Bim was identified as a potential target gene of miR-181a. A rat model of RIR was created by increasing the intraocular pressure. RGCs in the flatmounted retinas were labeled with Brn3, a marker for alive RGCs, by immunofluorescent staining. The changes in the number of RGCs after RIR were recorded. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was used to determine the expression level of miR-181a in the retina. Bim/Brn3 double immunofluorescence was used to detect the localization of Bim. The expression of Bim in the retina was determined with the aids of Western blot and qRT-PCR.
RESULTS
Compared with the negative control group, the density of RGCs was significantly lower in the ischemia/reperfusion (I/R)-24h and I/R-72h groups (P<0.001). The expression level of miR-181a started to decrease at 0h after RIR, and further decreased at 24h and 72h compared with the negative control group (P<0.001). Bim was significantly upregulated at 12h after RIR (P<0.05) and reached peak at 24, 72h compared with the negative control group (P<0.01). Pearson correlation analysis showed that the expression level of Bim was negatively correlated with the expression level of miR-181a and the density of RGCs.
CONCLUSION
Bim may be a potential target gene of miR-181a. Both miR-181a and Bim are involved in RGCs death in RIR. RIR may promote RGCs apoptosis in the retina via downregulation of miR-181a and its inhibition on Bim expression.
Keywords: microRNA-181a, Bim, retinal ischemia reperfusion, target gene, retinal ganglion cells, apoptosis
INTRODUCTON
Retinal ischemia-reperfusion (RIR) injury is a common pathological process that occurs in many retinal diseases such as retinal vascular occlusion disease[1], diabetic retinopathy[2], anterior ischemic optic neuropathy[3], and acute glaucoma[4]–[5] or surgical procedures leading to fluctuation of intraocular pressure (IOP). RIR can aggravate reversible ischemia-induced damage, especially on retinal ganglion cells (RGCs) and optic nerves, and promote neuronal death via increasing apoptosis and necrosis[6], eventually leading to irreversible damage of visual function. Therefore, exploring the mechanisms of retinal damage caused by RIR and identifying potential therapeutic targets are important to reduce or prevent RIR-induced injury to RGCs and to protect retinal function, and thus can play an important role in the prevention and treatment of retinal ischemic diseases and glaucoma.
Apoptosis is the main pathogenesis of RIR to cause injury to RGCs[7]–[9]. Apoptosis is associated with many regulatory factors such as Bcl-2 family proteins. Bim, a BH3-only Bcl-2 family member, is required for apoptosis initiation[10]. MicroRNA-181a (miR-181a) is one of the most abundant microRNAs in retina[11]–[12], and mainly expressed in the ganglion cell layer (GCL) and inner layer of the inner nuclear layer (INL)[11]–[14]. Using microarray technique, we have previously found that miR-181a is significantly downregulated in a rat model of RIR. The microRNA target gene database predicts that Bim is one of the target genes of miR-181a. The target relationship between Bim and miR-181a is confirmed in the studies of non-Hodgkin's lymphoma[15] and tibial plateau fracture[16]. However, the target relationship between miR-181a and Bim and their involvment in RIR need to be elucidated.
In this study, we created a rat model of RIR and examined the changes in the expression of miR-181a and Bim in the retina of RIR rats. We further analyzed whether Bim was the target gene of miR-181a in RIR rats, and investigated their correlation with apoptosis in RGCs. This study, as per our knowledge, explored for the first time the possible anti-apoptotic role of miR-181a in inhibiting RGCs death in RIR rats, and thus provided with a novel research direction and therapeutic target for the treatment of RIR.
MATERIALS AND METHODS
Animals
Healthy male Sprague-Dawley (SD) rats weighing 200-220 g were purchased from the Dashuo Laboratory Animal Center (Chengdu, Sichuan Province, China), and totally 94 rats were used in this study. The animals used in this study were treated in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Target Gene Prediction of MicroRNA-181a
To explore the relations between miR-181a and apoptosis, we performed target gene prediction for miR-181a by using online bioinformatics tools TargetScan (Version 6.2) and miRBase (Version 20.0). The pro-apoptotic factor BCL2L11 (Bim) was identified as a potential target gene of miR-181a.
Induction of Retinal Ischemia-reperfusion Model in Rats
The mean IOP of normal SD rats under the condition of anesthesia was 18.09±3.87 mm Hg as reported by Ma et al[17]. We induced RIR injury in the rats by elevating the IOP according to the methods described by Tong et al[18]. Briefly, the rats were anesthetized by an intraperitoneal injection of 1% pentobarbital sodium (10 mg/kg). Corneal analgesia was achieved using 0.4% oxybuprocaine hydrochloride, pupillary dilatation was maintained with 0.5% tropicamide and 0.5% phenylephrine. After dilation of the pupil, the anterior chamber of the left eye was cannulated with a 30-gauge needle connected to a physiological saline reservoir. The IOP was raised up to 110 mmHg by keeping the reservoir at 150 cm above the eye (1 mm Hg=13.6 mm H2O). The pressure was monitored with the aid of a rebound tonometer (Icare, Finland), which showed that 90-100 mm Hg IOP was virtually acquired in most cases. Retinal ischemia was confirmed by corneal edema and examination of the fundus. After 60min, the retinal blood supply was recovered by slowly lowering the infusion bottle to animal eye level and removing the infusion needle from the anterior chamber. Erythromycin eye ointment 0.5% was applied to the eye for preventing infection after the procedure. Only the left eye was used in all experiments. The anterior chamber of the left eye in the sham control groups was similarly cannulated for 60min without raising the IOP.
Immunohistofluorescence
Flatmounted retinas
Brn3 was an ideal antigenic marker for active RGCs and was used to label alive RGCs in this study. The preparation of flatmounted retinas was refered to the method described by Yang et al[19]. The eyes were immediately enucleated and fixed with 4% paraformaldehyde at 4°C for 1h, and the retinas were dissected from the ora serrata. Retinal flatmounts were prepared by making four radial incisions, post-fixed for 1h in 4% paraformaldehyde, rinsed in 0.1 mol/L phosphate-buffered saline (PBS). The retinas were permeabilized in PBS with 0.5% Triton X-100 for 15min at -80°C, rinsed in new PBS with 0.5% Triton X-100, and incubated overnight at 4°C with goat anti-Brn3 antibody (Santa Cruz Biotechnology, Heidelberg, Germany) diluted 1:100 in blocking buffer (PBS, 2% Bovine serum albumin, 2% Triton X-100). The retinas were washed in PBS for three times and incubated for 2h at room temperature with Alexa Fluor-488 donkey anti-goat IgG antibody (Invitrogen-Molecular Probes, Eugene, Oregon, USA) which was diluted 1:500 in blocking buffer. Finally, they were thoroughly washed in PBS and mounted vitreous side up on slides and covered with glycerin solution.
Retinal sections
The eyes were enucleated, embedded in opti-mum cutting temperature compound (Tissue-Tek, Sakura, Tokyo, Japan) and quickly frozen in -80°C. Eight micrometer thick sections, which included a full length of retina approximately along the horizontal meridian, passing through the optic nerve, were cut and mounted on slides. The sections were blocked with 5% donkey serum in PBS and stained with a combination of goat anti-Brn3 antibody and rabbit anti-Bim antibody (Abcam, Cambridge, Cambridge, MA, USA), which were diluted 1:100 in 0.3% Saponin (Sigma-Aldrich, Alcobendas, Madrid, Spain). The mixtures were incubated overnight at 4°C. The goat anti-Brn3 antibody was detected with Alexa Fluor-488 donkey anti-goat IgG antibody (Invitrogen-Molecular Probes, Eugene, Oregon, USA). The anti-Bim antibody was detected with Alexa Fluor-568 donkey anti-rabbit IgG antibody (Invitrogen-Molecular Probes, Eugene, Oregon, USA). The mixtures of secondary antibodies were diluted 1:300 in PBS with 0.1% Triton-100 and incubated for 60min at room temperature avoiding light. The sections were counterstained with 4′, 6′-diamino-2-phenylindole (DAPI) and a fluorescence microscope (Carl Zeiss, Jena, Germany) was used to capture the images.
Manual quantification of Brn3-positive retinal ganglion cells in wholemounted retinas
We photographed the retinas under an fluorescence microscope (Carl Zeiss, Jena, Germany) and counted the Brn3-positive (Brn3+) RGCs manually according to a previously described method by Biermann et al[20]. Brn3+ RGCs were counted by three investigators blinded to procedure in photographs of 12 rectangular areas. We photographed 3 standard square areas (0.2×0.2 mm=0.04 mm2) at 1, 2 and 3 mm respectively from the optic disc of each retinal quadrant. To determine the density of Brn3+ RGCs per square millimeter, we multiplied the number of cells per 0.04 mm2 by 25. All averaged data are presented as mean RGCs density (RGCs/mm2) ±standard deviation (SD).
Quantitative Reverse Transcription-polymerase Chain Reaction for MicroRNA and mRNA Expression Analysis
Total RNA, including miRNA, was extracted from the retinas using a Trizol® Plus RNA Purification Kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. cDNA synthesis was conducted using 800 ng of extracted total RNA (including miRNAs) and specific primers (Invitrogen, Carlsbad, CA, USA) for miR-181a (GSP: 5′GGGAACATTCAACGCTGTCG3′; R: 5′CAGTGCGTGTCGTGGAGT3′) or small nuclear RNA U6 (an endogenous small nuclear RNA, snRNA). The qRT-PCR was conducted using a ViiA 7 Real-time PCR System (Applied Biosystems, Foster City, CA, USA) with 40 cycles of 95°C for 10s (denature) and 60°C for 60s (annealing and extension). miRNA expression was normalized to that of U6 and expressed as fold of control group. Data were analyzed according to the comparative Ct method.
For quantification of mRNAs in retinal tissues, the synthesized cDNA, iQ SYBR Green Supermix (BioRad, CA, USA) and primers for Bim (F: 5′CAATGAGACTTACACGAGGAGG3′; R: 5′CCAGACCAGACGGAAGATGAA3′) were used in qRT-PCR, with 40 cycles of 95°C for 10s and 60°C for 30s followed by melt-curve analysis. The Bim mRNA level was normalized to that of endogenous control, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and expressed as fold of control. The primers for GAPDH were: F: 5′GGAAAGCTGTGGCGTGAT3′; R: 5′AAGGTGGAAGAATGGGAGTT3′. Data were analyzed according to the comparative Ct method. The primers were designed using the Primer 5.0 Plus application.
Western Blot Analysis for Protein Expression of Bim
Western blot analysis was performed as previously described[21]–[22]. Protein was extracted from freshly dissected retinas of the rats. Bim signal was detected with rabbit-anti Bim antibody (Abcam, Cambridge, Cambridge, MA, USA) diluted 1:500 in PBS-Tween 0.1% and 5% non-fat milk powder. Secondary detection was performed with horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (Santa Cruz Biotechnology, Dallas, Texas, USA), visualized by chemiluminescence (Merck Millipore, Darmstadt, Germany). As loading control, β-actin detection was performed with mouse anti-actin (Zhongshanjinqiao, Beijing, China) detected with goat anti-mouse-HRP secondary antibody (Zhongshanjinqiao, Beijing, China).
Statistical Analysis
Statistical analyses were undertaken using SPSS (version 19.0). The one-way ANOVA followed by LSD test was used to compare data of multi-groups. All data were expressed as means±SEM. Differences were regarded as significant when P<0.05. The correlation between the expression level of miR-181a, Bim and the densities of RGCs were examined by Pearson's correlation analysis. The correlation coefficient |ρ|>0.8 indicated a linear relationship between two data.
RESULTS
The Density of Brn3+ Retinal Ganglion Cells in Retinal Flatmounts
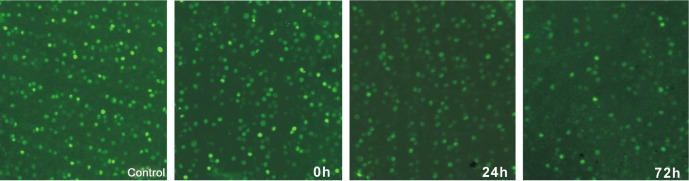
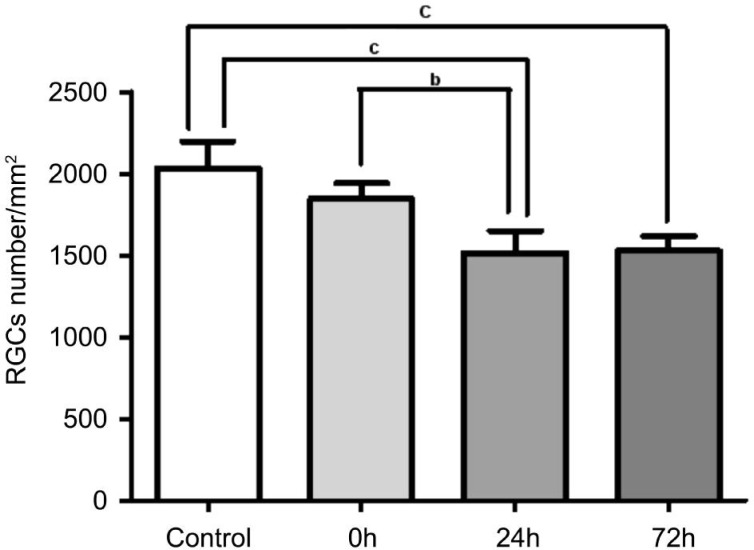
Figure 1 showed the Brn3+ RGCs in the normal and RIR rats. The densities of RGCs (RGCs/mm2) in the sham control group, ischemia/reperfusion (I/R)-0h, I/R-24h, and I/R-72h groups were respectively: 2031.25±165.41/mm2, 1845.75±92.45/mm2, 1514.5±135.2/mm2 and 1527.0±88.31/mm2. The densities of RGCs in the I/R-0h group were not significantly different from those in the sham controls (n=5, P>0.05). The densities of RGCs in the I/R-24h and I/R-72h groups were significantly lower than those in the sham control and I/R-0h groups (n=5, P<0.05). There were no significant differences in the densities of RGCs between the I/R-24h and I/R-72h groups (n=5, P>0.05) (Figure 2).
Figure 1. Immunofluorescence labeling of retinal ganglion cells with Brn3, a marker for alive retinal ganglion cells, in the flatmounted retinas from the control and retinal ischemia-reperfusion rats (×400).
Figure 2. The densities of retinal ganglion cells in the control and retinal ischemia-reperfusion rats.
The densities of RGCs was assessed by counting the cells with Brn3 immunoreactivity in wholemounted retinas. The number of RGCs exhibited a significantly decrease in the I/R-24h and I/R-72h groups. Data are expressed as means±SEM (bP<0.01, cP<0.001).
The Expression of MicroRNA-181a in the Retina from Retinal Ischemia-reperfusion Rats
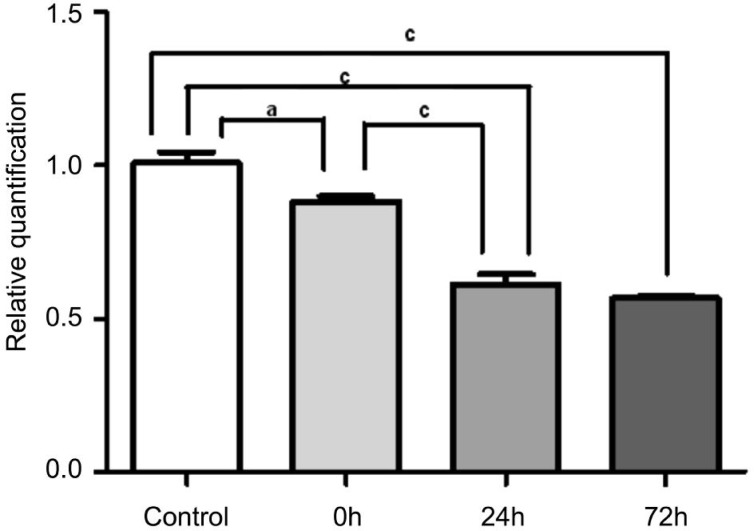
qRT-PCR results showed that the expression of miR-181a was significantly lower in the I/R-0h, I/R-24h, and I/R-72h groups compared with the sham control group (n=5, P<0.05). The expression of miR-181a was significantly lower in the I/R-24h and I/R-72h groups than in the I/R-0h group (n=5, P<0.001). There was no significant difference in the expression of miR-181a between the I/R-24h and I/R-72h groups (n=5, P>0.05) (Figure 3).
Figure 3. The expression of microRNA-181a in retina.
The expression level of miR-181a was significantly downregulated at 0h after RIR compared with the sham control group. Further decrease was found in the I/R-24h and I/R-72h groups when compared with the I/R-0h group. The error bars represent SEM (aP<0.05, cP<0.001).
The Expression of Bim in the Retina from Retinal Ischemia-reperfusion Rats
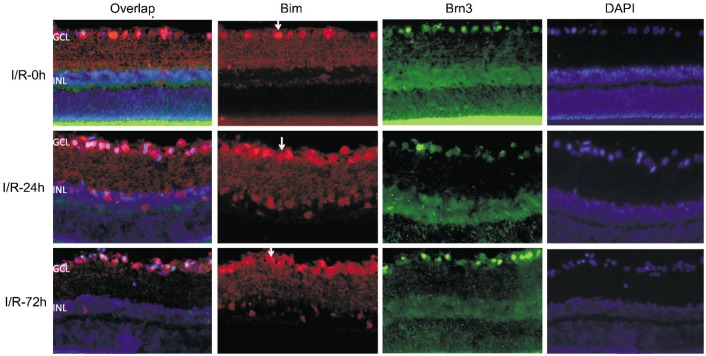
Bim was expressed in a few cells of the GCL in sham control rats (Figure 4). However, Bim was strongly expressed in the GCL and INL in the I/R-24h and the I/R-72h group. The expression of Bim was colocalized with the expression of Brn3 (Figure 5).
Figure 4. Immunofluorescence staining of Bim in the retina from sham control rats (×400).
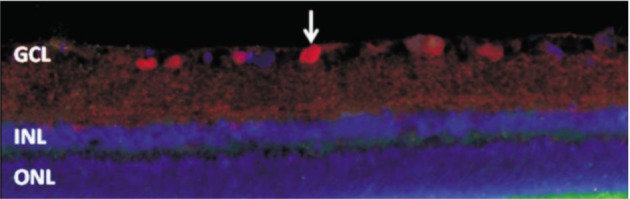
Bim (red) was expressed in a few cells in the GCL (arrows). Bim was not expressed in other layers of the retina.
Figure 5. The double labeled staining of Bim (red) and Brn3 (green) in retinal sections (×400).
The first colum: overlap of Bim, Brn3 and DAPI; the second column: Bim signal (red); the third column: Brn3 signal (green); the last column: DAPI (blue). Arrows represented the positive staining of Bim.
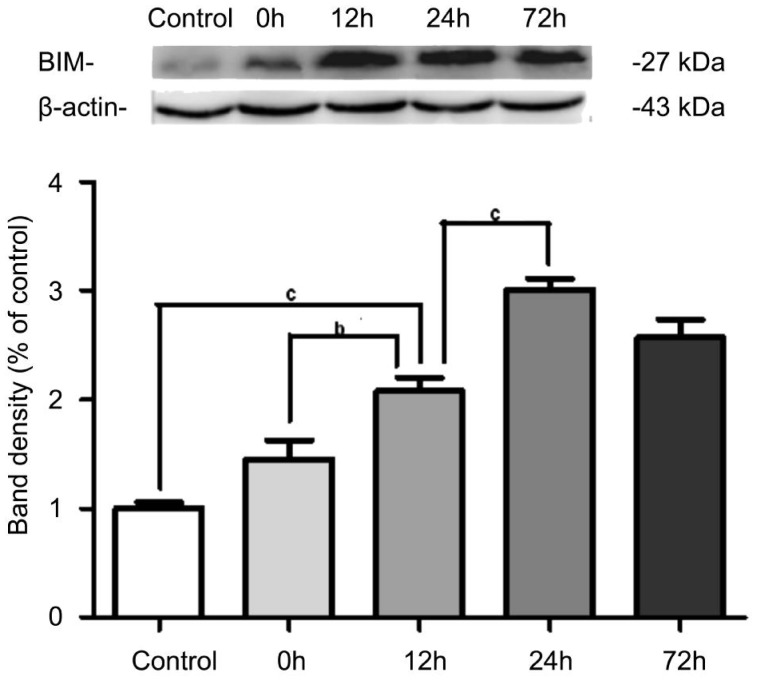
The Protein Expression of Bim in the Retina
A significant increase in Bim protein expression was observed at the I/R-12h when compared to the sham control group (n=5, P<0.001). The expression of Bim further increased and reached a peak at 24h after RIR compared with the I/R-12h group (n=5, P<0.001). But there was no significant difference in the expression of Bim between the I/R-24h and I/R-72h groups (n=5, P>0.05) (Figure 6).
Figure 6. The protein expression of Bim in retina.
The expression of Bim was significantly higher in the I/R-12h, I/R-24h, and I/R-72h groups compared with the sham control group and reached a peak at 24h after RIR. The error bars represent SEM (bP<0.01, cP<0.001).
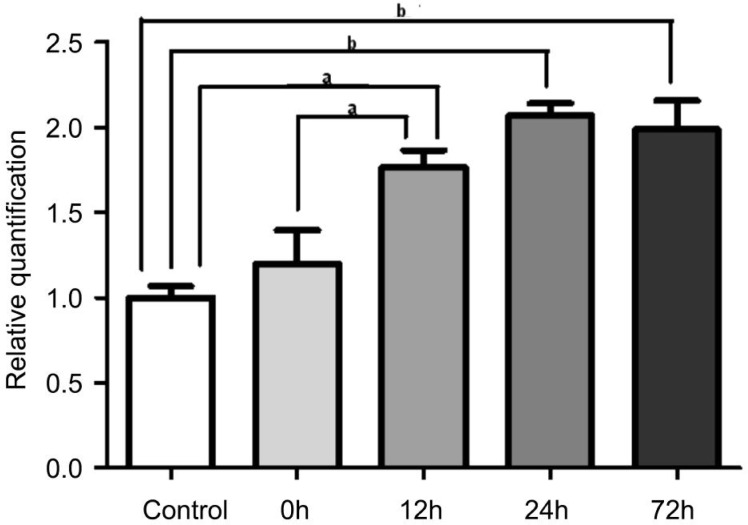
The mRNA Expression of Bim in the Retina
Corresponding to the expression pattern of Bim protein, there was no significant change in the expression level of Bim gene in the I/R-0h group compared to the sham control group (n=5, P>0.05). The expression of Bim was found to be significantly upregulated in the I/R-12h group (n=5, P<0.05), the I/R-24h group (n=5, P<0.01) and I/R-72h groups (n=5, P<0.01) compared with the sham control group (Figure 7). The expression level of Bim reached a peak at I/R-24h.
Figure 7. The mRNA expression of Bim in retina.
The qRT-PCR results showed that the expression of Bim was significantly upregulated in the I/R-12h group, the I/R-24h group and the I/R-72h group when compared to the sham control. The error bars represent SEM (aP<0.05, bP<0.01).
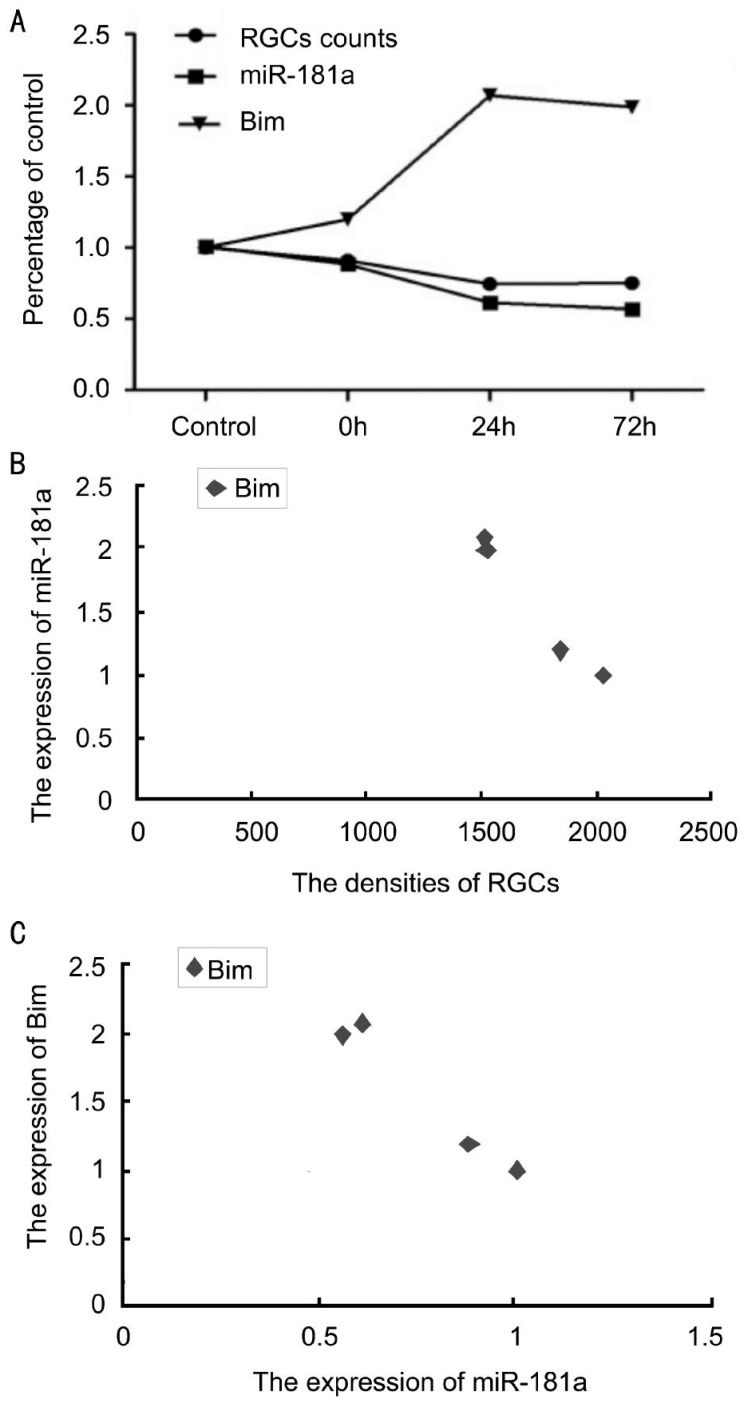
Correlations among the Expression of MicroRNA-181a, Bim, and the Densities of Retinal Ganglion Cells
Pearson correlation analysis showed that the expression level of Bim was negatively correlated with the expression of miR-181a (ρ=-0.984, P<0.05) and the densities of RGCs (ρ=-0.987, P<0.05). The expression level of miR-181a was positively correlated with the densities of RGCs (ρ=0.995, P<0.05) (Figure 8).
Figure 8. Correlations among the expression of miR-181a, Bim, and the densities of retinal ganglion cells.
A: Change trends of RGC numbers, miR-181a and Bim expression levels with RIR time; B, C: Correlation analysis.
DISCUSSION
RIR mainly leads to injury to cells in the GCL and INL in the retina, especially the RGCs. Cell apoptosis is the major mechanism underlying RIR-induced cell injury[7]–[9]. The rat model of RIR created by elevation of IOP is a well known animal model of RIR, which shows that increase of the IOP above the systolic pressure levels (up to 110 mm Hg) for periods of 60min or more results in RGCs loss in the rat retina[23]. Apoptosis of retinal cells starts to increase at 6h after RIR, reaches the peak at 24h, and declines thereafter [24]–[26]. In the present study, we labeled alive RGCs with Brn3 using the immunofluorescence method, and found that the average densities of RGCs was significantly decreased at 24h and 72h after RIR. These findings suggested that we successfully created the RIR model in which RGCs injury was obvious at 24h after RIR. Our findings are consistent with previous reports in the literature[24]–[26].
miRNAs are a class of small non-coding single-stranded RNA molecules with 17-24 nucleotides in length and are expressed in cells from plants to animals[27]–[28]. miRNAs post-transcriptionally regulate gene expression by imperfectly complementary binding to the 3′ untranslated region (3′-UTR) of target mRNAs to regulate cell apoptosis, proliferation, differentiation, development and metabolism[29]. miRNAs that are richly expressed in the central nervous system play an important role in ischemia-induced neuronal apoptosis by negatively regulating apoptosis-related proteins[30]–[32], as well as regulating neuronal regeneration and repair after ischemia[33]–[34]. To date, more than 250 miRNAs have been reportedly expressed in the retina[35], and 21 of them are specifically expressed in the retina[13],[36]–[37]. miR-181a, one of the most abundant miRNAs in the retina, is mainly expressed in the RGCs and amacrine cells in the INL[11]–[14], and thus may be involved in the pathophysiology of these cells. Using microarray technique, we have previously found that many miRNAs including miR-181a are downregulated in a rat model of RIR. In the present study, we used qRT-PCR to further examine the expression level of miR-181a in the retina of RIR rats. The results indicated that miR-181a was significantly downregulated immediately (I/R-0h) after RIR, and further decreased at 24h and 72h after RIR. These findings suggested that miR-181a may be involved in the pathogenesis of RIR, and may act as an upstream regulatory gene to inhibit mRNAs that contribute to the pathogenesis of RIR. Ulitsky et al[38] indicated that miRNAs may participate in a certain biological process or pathway if their target genes are involved. With the aid of bioinformatics analysis, we firstly identified Bim as a possible and potential downstream target gene of miR-181a, and then further investigated the changes in expression of Bim in the early phase of RIR.
Bim, belonging to the Bcl-2 family proteins, is a 129-amino-acid protein that is encoded by the gene located at chromosome 7p15.2[39]. Bim is one of the important members of BH3-only pro-apoptotic proteins. It selectively binds to five anti-apoptotic proteins (Bcl-2, Bcl-XL, Bcl-W, Mcl-1, and A1) and two pro-apoptotic proteins (Bax and Bak)[10]. Similar to other BH3-only members, Bim is upregulated in response to apoptotic stimuli, and inhibits anti-apoptotic protein and activates pro-apoptotic proteins, leading to increased permeability of the outer mitochondrial membrane, release of cytochrome C, activation of caspases, and eventually apoptosis[10]. It has been reported that BH3-only family proteins including Bim are upregulated after optic nerve transection, and Bim-knockout mice exhibit resistance to RGCs death induced by optic nerve transection[40]–[41]. Also Bim is an important activator of Bax, which plays a key role in RGCs death. Knockout of Bim has been found to reduce optic nerve injury in a chronic mouse model of glaucoma[42]. In the present study, we found that Bim was mainly expressed in the GCL of the retina from RIR rats, and colocalized with the expression of Brn3, a marker for RGCs. In addition, Bim was also expressed in a few cells in the INL. The localization of Bim is consistent with the injury site of RIR. Western blot and qRT-PCR results showed that Bim started to increase significantly at 12h after RIR, and reached peak at 24h after RIR. The time course of Bim expression is consistent with that of RGCs apoptosis, suggesting that Bim is likely to be involved in the pathological process of RGCs apoptosis.
In order to detect the possible target relationship of miR-181a and Bim, we investigated the changes in expression level of miR-181a and Bim in the rat model of RIR. We found that in the early phase of RIR (0-72h after RIR), miR-181a was downregulated in the retina. Correspondingly, expression of Bim increased with time of RIR. Correlation analysis showed that the expression level of miR-181a was negatively correlated with the expression of Bim. The expression of miR-181a was significantly lower in the I/R-0h group, and the change in the expression of miR-181a was earlier than that of Bim. These findings suggest that miR-181a may be an upstream regulator of Bim, and Bim is likely the downstream target gene of miR-181a in RIR. Thus, we inferred that miR-181a may be the upstream sensor of Bim-mediated apoptosis pathway. Bim may promote RGCs apoptosis via regulation of anti-apoptosis or pro-apoptotic proteins. Future functional studies are required to confirm the target relationship between miR-181a and Bim, and to investigate the effect of miR-181a on RGCs apoptosis using miR-181a mimics or inhibitors, etc.
In summary, our findings suggest that miR-181a may be involved in inhibiting RGCs apoptosis via negative regulation of pro-apoptotic gene such as Bim, and thus providing with the function of neuroprotection in RIR. miR-181a may be a potential therapeutic target for the neuroprotective treatment of RIR.
Acknowledgments
Fundations: Supported by the National Natural Science Foundation of China (No. 81070742/H1205); the International Collaboration Foundation from The Department of Science and Technology of Sichuan Province, China (No. 2010HH0030).
Conflicts of Interest: He Y, None; Liu JN, None; Zhang JJ, None; Fan W, None.
REFERENCES
- 1.Archer DB. Tributary vein obstruction: pathogenesis and treatment of sequelae. Doc Ophthalmol. 1976;40(2):339–360. doi: 10.1007/BF00155046. [DOI] [PubMed] [Google Scholar]
- 2.Verma D. Pathogenesis of diabetic retinopathy-the missing link? Med Hypotheses. 1993;41(3):205–210. doi: 10.1016/0306-9877(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 3.Hayreh SS. Ischemic optic neuropathy. Int Ophthalmol. 1978;1(1):9–18. doi: 10.1007/BF00133273. [DOI] [PubMed] [Google Scholar]
- 4.Osborne NN, Ugarte M, Chao M, Chidlow G, Bae JH, Wood JP, Nash MS. Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Surv Ophthalmol. 1999;43(Suppl. 1):102–128. doi: 10.1016/s0039-6257(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 5.Buckingham BP, Inman DM, Lambert W, Oglesby E, Calkins DJ, Steele MR, Vetter ML, Marsh-Armstrong N, Horner PJ. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J Neurosci. 2008;28(11):2735–2744. doi: 10.1523/JNEUROSCI.4443-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joo CK, Choi JS, Ko HW, Park KY, Sohn S, Chun MH, Oh YJ, Gwag BJ. Necrosis and apoptosis after retinal ischemia: involvement of NMDA-mediated excitotoxicity and p53. Invest Ophthalmol Vis Sci. 1999;40(3):713–720. [PubMed] [Google Scholar]
- 7.Hu SX, Wang X, Lin SC, Zheng HL. The retinal cell apoptosis induced by ischemia reperfusion injury in rat. Chin J Ocul Fundus Dis. 2002;18(4):296–298. [Google Scholar]
- 8.Buchi ER. Cell death in the rat retina after a pressure-induced ischaemia-reperfusion insult: an electron microscopic study. I. Ganglion cell layer and inner nuclear layer. Exp Eye Res. 1992;55(4):605–613. doi: 10.1016/s0014-4835(05)80173-3. [DOI] [PubMed] [Google Scholar]
- 9.Lam TT, Abler AS, Tso MO. Apoptosis and caspases after ischemia-reperfusion injury in rat retina. Invest Ophthalmol Vis Sci. 1999;40(5):967–975. [PubMed] [Google Scholar]
- 10.Vaux DL. Apoptogenic factors released from mitochondria. Biochim Biophys Acta. 2011;1813(4):546–550. doi: 10.1016/j.bbamcr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Ryan DG, Oliveira-Fernandes M, Lavker RM. MicroRNAs of the mammalian eye display distinct and overlapping tissue specificity. Mol Vis. 2006;12:1175–1184. [PubMed] [Google Scholar]
- 12.Loscher CJ, Hokamp K, Kenna PF, Ivens AC, Humphries P, Palfi A, Farrar GJ. Altered retinal microRNA expression profile in a mouse model of retinitis pigmentosa. Genome Biol. 2007;8(11):R248. doi: 10.1186/gb-2007-8-11-r248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karali M, Peluso I, Marigo V, Banfi S. Identification and characterization of microRNAs expressed in the mouse eye. Invest Ophthalmol Vis Sci. 2007;48(2):509–515. doi: 10.1167/iovs.06-0866. [DOI] [PubMed] [Google Scholar]
- 14.Kapsimali M, Kloosterman WP, de Bruijn E, Rosa F, Plasterk RH, Wilson SW. MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome Biol. 2007;8(8):R173. doi: 10.1186/gb-2007-8-8-r173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lwin T, Lin J, Choi YS, Zhang X, Moscinski LC, Wright KL, Sotomayor EM, Dalton WS, Tao J. Follicular dendritic cell–dependent drug resistance of non-Hodgkin lymphoma involves cell adhesion–mediated Bim down-regulation through induction of microRNA-181a. Blood. 2010;116(24):5228–5236. doi: 10.1182/blood-2010-03-275925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, Tang C, Zhang Q, Chen W. Reduced miR-9 and miR-181a expression down-regulates Bim concentration and promote osteoclasts survival. Int J Clin Exp Pathol. 2014;7(5):2209–2218. [PMC free article] [PubMed] [Google Scholar]
- 17.Ma JZ, Xie L, He XG, Sun YL. Distribution regularity of intraocular pressure of normal rats under pentobarbital anesthesia. Zhongguo Linchuang Kangfu. 2005;9(38):115–117. [Google Scholar]
- 18.Tong N, Zhang Z, Gong Y, Yin L, Wu X. Diosmin protects rat retina from ischemia/reperfusion injury. J Ocul Pharmacol Ther. 2012;28(5):459–466. doi: 10.1089/jop.2011.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Mao D, Chen X, Zhao L, Tian Q, Liu C, Zhou BL. Decrease in retinal neuronal cells in streptozotocin-induced diabetic mice. Mol Vis. 2012;18:1411–1420. [PMC free article] [PubMed] [Google Scholar]
- 20.Biermann J, Lagrèze WA, Dimitriu C, Stoykow C, Goebel U. Preconditioning with inhalative carbon monoxide protects rat retinal ganglion cells from ischemia/reperfusion injury. Invest Ophthalmol Vis Sci. 2010;51(7):3784–3791. doi: 10.1167/iovs.09-4894. [DOI] [PubMed] [Google Scholar]
- 21.Agudo M, Pérez-Marín MC, Lönngren U, Sobrado P, Conesa A, Cánovas I, Salinas-Navarro M, Miralles-Imperial J, Hallböök F, Vidal-Sanz M. Time course profiling of the retinal transcriptome after optic nerve transection and optic nerve crush. Mol Vis. 2008;14:1050–1063. [PMC free article] [PubMed] [Google Scholar]
- 22.Agudo M, Perez-Marin MC, Sobrado-Calvo P, Lönngren U, Salinas-Navarro M, Cánovas I, Nadal-Nicolás FM, Miralles-Imperial J, Hallböök F, Vidal-Sanz M. Immediate upregulation of proteins belonging to different branches of the apoptotic cascade in the retina after optic nerve transection and optic nerve crush. Invest Ophthalmol Vis Sci. 2009;50(1):424–431. doi: 10.1167/iovs.08-2404. [DOI] [PubMed] [Google Scholar]
- 23.Selles-Navarro I, Villegas-Perez MP, Salvador-Silva M, Ruiz-Gómez JM, Vidal-Sanz M. Retinal ganglion cell death after different transient periods of pressure-induced ischemia and survival intervals. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996;37(10):2002–2014. [PubMed] [Google Scholar]
- 24.Kaneda K, Kashii S, Kurosawa T, Kaneko S, Akaike A, Honda Y, Minami M, Satoh M. Apoptotic DNA fragmentation and upregulation of Bax induced by transient ischemia of the rat retina. Brain Res. 1999;815(1):11–20. doi: 10.1016/s0006-8993(98)01074-9. [DOI] [PubMed] [Google Scholar]
- 25.Niu YJ, Zhao YS, Gao YX, Zhou ZY, Wang HY. An experimental study of the therapeutical effect of basic fibroblast growth factor in retinal ischemia/reperfusion injury. Chin J Ophthalmol. 2003;39(11):664–668. [PubMed] [Google Scholar]
- 26.Zhao Y, Niu YJ, Zhou ZY, Gao YX, Wang HY. Expression of fas/fasl and the apoptosis in rat ischemia/reperfusion-induced retinal injury and effects of bfgf. Int J Ophthalmol. 2008;1(3):226–229. [Google Scholar]
- 27.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107(7):823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 28.Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet. 2007;8(2):93–103. doi: 10.1038/nrg1990. [DOI] [PubMed] [Google Scholar]
- 29.Cheng AM, Byrom MW, Shelton J, Ford LP. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. 2005;33(4):1290–1297. doi: 10.1093/nar/gki200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin KJ, Deng Z, Huang H, Hamblin M, Xie C, Zhang J, Chen YE. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis. 2010;38(1):17–26. doi: 10.1016/j.nbd.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buller B, Liu X, Wang X, Zhang RL, Zhang L, Hozeska-Solgot A, Chopp M, Zhang ZG. MicroRNA-21 protects neurons from ischemic death. FEBS J. 2010;277(20):4299–4307. doi: 10.1111/j.1742-4658.2010.07818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi G, Liu Y, Liu T, Yan W, Liu X, Wang Y, Shi J, Jia L. Upregulated miR-29b promotes neuronal cell death by inhibiting Bcl2L2 after ischemic brain injury. Exp Brain Res. 2012;216(2):225–230. doi: 10.1007/s00221-011-2925-3. [DOI] [PubMed] [Google Scholar]
- 33.Benoit ME, Tenner AJ. Complement protein C1q-mediated neuroprotection is correlated with regulation of neuronal gene and microRNA expression. J Neurosci. 2011;31(9):3459–3469. doi: 10.1523/JNEUROSCI.3932-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silber J, Lim DA, Petritsch C, Persson AI, Maunakea AK, Yu M, Vandenberg SR, Ginzinger DG, James CD, Costello JF, Bergers G, Weiss WA, Alvarez-Buylla A, Hodgson JG. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;6:14. doi: 10.1186/1741-7015-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundermeier TR, Palczewski K. The physiological impact of microRNA gene regulation in the retina. Cell Mol Life Sci. 2012;69(16):2739–2750. doi: 10.1007/s00018-012-0976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arora A, McKay GJ, Simpson DA. Prediction and verification of miRNA expression in human and rat retinas. Invest Ophthalmol Vis Sci. 2007;48(9):3962–3967. doi: 10.1167/iovs.06-1221. [DOI] [PubMed] [Google Scholar]
- 37.Xu S, Witmer PD, Lumayag S, Kovacs B, Valle D. MicroRNA (miRNA) transcriptome of mouse retina and identification of a sensory organ-specific miRNA cluster. J Biol Chem. 2007;282(34):25053–25066. doi: 10.1074/jbc.M700501200. [DOI] [PubMed] [Google Scholar]
- 38.Ulitsky I, Laurent LC, Shamir R. Towards computational prediction of microRNA function and activity. Nucleic Acids Res. 2010;38(15):e160. doi: 10.1093/nar/gkq570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S, Huang DC. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17(2):384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKernan DP, Cotter TG. A Critical role for Bim in retinal ganglion cell death. J Neurochem. 2007;102(3):922–930. doi: 10.1111/j.1471-4159.2007.04573.x. [DOI] [PubMed] [Google Scholar]
- 41.Näpänkangas U, Lindqvist N, Lindholm D, Hallböök F. Rat retinal ganglion cells upregulate the pro-apoptotic BH3-only protein Bim after optic nerve transection. Brain Res Mol Brain Res. 2003;120(1):30–37. doi: 10.1016/j.molbrainres.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 42.Harder JM, Fernandes KA, Libby RT. The Bcl-2 family member Bim has multiple glaucoma-relevant functions in DBA/2J mice. Sci Rep. 2012;2:530. doi: 10.1038/srep00530. [DOI] [PMC free article] [PubMed] [Google Scholar]