Abstract
AIM
To investigate whether umbilical cord human mesenchymal stem cell (UC-MSC) was able to differentiate into neural stem cell and neuron in vitro.
METHODS
The umbilical cords were obtained from pregnant women with their written consent and the approval of the Clinic Ethnics Committee. UC-MSC were isolated by adherent culture in the medium contains 20% fetal bovine serum (FBS), then they were maintained in the medium contain 10% FBS and induced to neural cells in neural differentiation medium. We investigated whether UC-MSC was able to differentiate into neural stem cell and neuron in vitro by using flow cytometry, reverse transcriptase-polymerase chain reaction (RT-PCR) and immunofluorescence (IF) analyzes.
RESULTS
A substantial number of UC-MSC was harvested using the tissue explants adherent method at about 2wk. Flow cytometric study revealed that these cells expressed common markers of MSCs, such as CD105 (SH2), CD73 (SH3) and CD90. After induction of differentiation of neural stem cells, the cells began to form clusters; RT-PCR and IF showed that the neuron specific enolase (NSE) and neurogenic differentiation 1-positive cells reached 87.3%±14.7% and 72.6%±11.8%, respectively. Cells showed neuronal cell differentiation after induced, including neuron-like protrusions, plump cell body, obviously and stronger refraction. RT-PCR and IF analysis showed that microtubule-associated protein 2 (MAP2) and nuclear factor-M-positive cells reached 43.1%±10.3% and 69.4%±19.5%, respectively.
CONCLUSION
Human umbilical cord derived MSCs can be cultured and proliferated in vitro and differentiate into neural stem cells, which may be a valuable source for cell therapy of neurodegenerative eye diseases.
Keywords: human umbilical cord, mesenchymal stem cells, neural stem cells, neuron, neurodegenerative eye diseases
INTRODUCTION
Neurodegenerative eye diseases, including glaucoma, optic neuritis, ischemic optic neuropathy and diabetic retinopathy (DR) are caused by chronic degeneration of retinal nerve tissue, including neuronal apoptosis and reactive glial cell hyperplasia. However, mechanisms underlying neurodegenerative eye diseases initiation and progression are not fully understood[1]. Ongoing basic and clinical researches are currently underway with the aim of curing neurodegenerative eye diseases[2]. Mesenchymal stem cells (MSCs) are derived from the embryonic mesoderm during fetal development. MSC distribute in many somatic tissues and cells, then later MSC confine to bone marrow (BM) and a lot of connective tissues in adults[3]. They have the ability of adherent, fibroblastic, cloning and expression of the unique properties of the cell surface phenotype. Because the cells are highly expandable ex vivo and capable of differentiating along a variety of different cell lineages, they are considered as a potential resources for stem cell based therapy and transplantation[4].
Because of the unique morphologic properties of human umbilical cord, it represents an important alternate source for MSCs, especially as compared with umbilical cord blood [5]. MSCs derived from human umbilical cord and human BM is similar in the proliferation and differentiation capability. Furthermore, human umbilical cord appears to be more advantageous than other stem cell sources with respect to cell procurement, storage and transplantation [6]. Therefore, human umbilical cord MSCs (UC-MSCs) are suitable for the development of cell-based therapeutics against various peripheral or central nerve injuries, and UC-MSCs may be a potential MSC source for neural stem cell therapies[7]. In the present study, we investigated the capacity of UC-MSCs to differentiate into neural stem cells in vitro and verified a positive expression of neurons in response to induction by using reverse transcription-polymerase chain reaction (RT-PCR) and immunofluorescence (IF) studies. We demonstrate that these UC-MSCs can also be a potential source of functional neurons for cell therapy of neurodegenerative eye diseases.
MATERIALS AND METHODS
Isolation and Culture of Umbilical Cord Human Mesenchymal Stem Cell
The study was approved by Tianjin Medical University Medical Ethics Committee and complied with the Declaration of Helsinki, including current revisions, and with the Good Clinical Practice guidelines. The procedures followed were in accordance with institutional guidelines, the subject provided written informed consent for human umbilical cord sampling according to the Declaration of Helsinki. The sample of human umbilical cord was obtained from healthy mother in Tianjin Medical University. The gestational age was 40wk. The sample was processed within 4h and stored at 4°C in sterile saline until they were used. The cords were rinsed several times in sterile phosphate-buffered saline (PBS) to remove blood components and cut into small pieces (2-3 cm). Cord vessels (2 arteries and 1 vein) were removed to avoid endothelial cell contamination. The cord were cut into pieces 0.5-1 cm3 and placed directly into 75-cm2 flasks for culture expansion in low-glucose Dulbecco's modified Eagle's medium (LG-DMEM) containing 20% fetal bovine serum (FBS), 100 U/mL penicillin/streptomycin, 2ng/mL epidermal growth factor (EGF) at 37°C, and 5% (v/v) CO2. After 5d, non-adherent cells were discarded and adherent cells were continued to culture with two medium changes/week. Prior to neural differentiation, the fifth-passage (P5) cells were plated at a density of 1×106 cells/cm2 in culture medium and grew greater than 60% confluence.
Characterization of Cultured Mesenchymal Stem Cells
The cultured MSCs were examined by flow cytometry. For detection of CD73, CD105, CD11b, CD45, CD90 and HLA-DR, the cells were harvested by treatment with 0.1% trypsin-EDTA, and detached cells were washed with cold PBS (pH 7.3) and incubated at 48°C for 20min with the respective monoclonal antibody (Becton Dickinson, San Jose, CA, USA), which were all conjugated with either fluorescein isothiocyanate (FITC) or P-phycoerythrin (PE). Two isotype controls of PE- and FITC-conjugated mouse immunoglobulin Gs (IgGs) were used for background non-specific binding. After washing with cold PBS, the cells were resuspended in 500 mL 2% FBS/PBS. PE- or FITC-labeled cells were then fixed with 2% formaldehyde/PBS and analyzed by flow cytometry (BD FACS ArisTM, Beckman-Dickinson, San Jose, CA, USA). Data were analyzed with CellQuest software (Becton Dickinson).
To confirm the multipotential differentiation capacity of cultured MSCs, MSCs at P5 were treated with the different induction media for 14d. Osteogenic induction medium contained 10% FBS, 10 mmol/L β-glycerophosphate, 100 nmol/L dexamethasone and 100 µg/mL ascorbate in high-glucose Dulbecco's Modified Eagle's Medium (HG-DMEM). Adipogenic induction medium comprised 10% FBS, 10 nmol/L dexamethasone, 5 µg/mL insulin, 0.5 µmol/L 3-isobutyl-1-methylxantine and 200 µg/mL indomethacin in HG-DMEM. After the differentiation process was completed, cover slips were fixed with 4% paraformaldehyde and stained for osteoblasts and adipocytes with Alizarin red and Oil-red O respectively.
Neural Differentiation
UC-MSC were incubated for 3wk in neural differentiation medium containing 100 ng/mL neurobasal media (Santa Cruz Biotechnology, Santa Cruz, CA, USA), 50 ng/mL EGF(Sigma-Aldrich, St Louis, MO, USA) and 2% N2/B27 (N2 and B27 Supplement, Abcam, Cambridge, MA, USA), 5% FBS and 1% penicillin/streptomycin. Neural stem cell and neuron differentiation were evaluated by RT-PCR and IF respectively.
Reverse Transcription-polymerase Chain Reaction Analysis
To analyze the relative expression of different mRNAs in differentiated neural stem cell and neuron, the amount of complementary DNA (cDNA) was normalized to the signal from expressed β-actin. Total RNA was isolated using a tissue total RNA extraction kit (GeneP-harma, Hi-Tech Park, Shanghai, China) according to the manufacture's direction. The first-strand cDNA was synthesized using RNA PCR kit (GeneP-harma, Hi-Tech Park, Shanghai, China). PCR reactions were performed at an initial denaturation at 95°C for 5min, followed by 35 cycles at 94°C for 2min, 94°C for 0.5min, 55°C for 0.5min and finally extension at 72°C for 7min using a DNA engine dyad peltier thermal cycler (MJ Research Inc., Waltham, MA, USA). The primers used were: 5′-CATGGGTCAGAAGGATTCCT-3′ and 5′-TGATCTGGGTCATCTTCTCG-3′ for β-actin; 5′-CGCTGGAGCCCTTCTTTGAA-3′ and 5′-GCGGACGGTTCGTGTTTGAA-3′ for neurogenic differentiation 1 (NeuroD1); 5′-AGCTGGCGCACCTCAAGATG-3′ and 5′-AGGGAAGTTGGGCTCAGGAC-3′ for Nestin; 5′-AATCAGCTCTGGCTCCCAGT-3′ and 5′-AGTGGGTGTTGAGGTACCAC-3′ for microtubule-associated protein (MAP2); 5′-GAGGCGGCCAGTTATCAGGA-3′ and 5′-GTTCTCCTCGCCCTCTAGCA-3′ for nuclear factor-M (NF-M). The amplified products were separated on 1.5% agarose gels, stained with ethidium bromide and photographed with Chemi Doc XRS (TaKaRa Biotechnology, Dalian, Liaoning Province, China).
Immunocytochemistry
Differentiated neural stem cell and neuron were placed on glass slides. The slides were briefly washed with PBS, fixed in 4% paraformaldehyde for 15min at room temperature (RT) and then washed three times, 5min each time, with PBS. The fixed cells were permeabilized by incubation with 0.5% Triton X-100 for 15min at RT and then rinsed three times with PBS. The slides were treated with chondroitinase ABC (0.4 U/mL) and keratanase (0.4 U/mL) for 1h at 37°C and then rinsed briefly with PBS. To prevent non-specific binding, the slides were incubated with a blocking agent (2% bovine serum albumin in PBS) for 30min at RT. The slides were then incubated with anti-MAP2 (diluted to 1:50 in blocking agent; Sigma, MO, USA), anti-NF-M (diluted to 1:50 in blocking agent; Sigma, MO, USA), anti-SNCA (diluted to 1:50 in blocking agent; Sigma, MO, USA), anti-Nestin (diluted to 1:50 in blocking agent; Sigma, MO, USA) and anti-NeuroD1 (diluted to 1:50 in blocking agent; Sigma, MO, USA). After three rinses in PBS for 5min each time, the slides were incubated with secondary antibodies conjugated with FITC (1:100, Bethyl Laboratories, TX, USA) for 1h in the dark. After washing with PBS (5min each time, three times for each slide), cover slips were mounted using Vectashield (Vector Laboratories, CA, USA) containing 40, 6-diamidino-2-phenylindole (DAPI) to visualize nuclei and combat fading of the immunolabeling. For a background control, in some slides the primary antibody was replaced with normal serum of the species from which the primary antibody was derived.
Statistical Analysis
The data were presented as mean±SEM. All experiments were repeated at least three times. Comparison of data involved one-way ANOVA. A P<0.05 was considered statistically significant. All analyses were performed with a statistical software package (SPSS 13.0; SPSS Inc., Chicago, IL, USA)
RESULTS
Identification of Human Umbilical Cord Human Mesenchymal Stem Cell
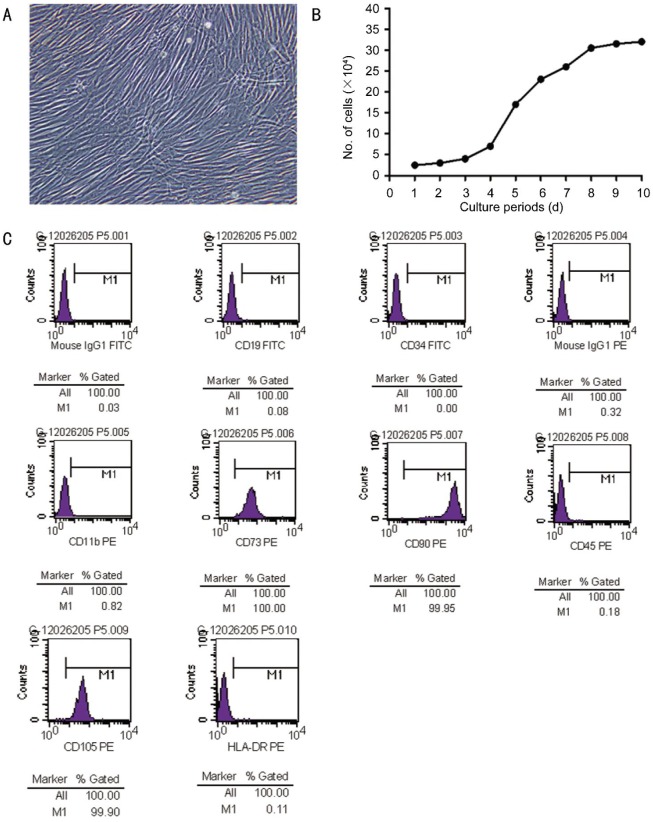
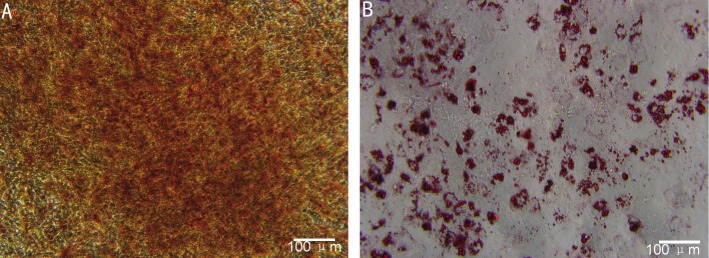
With a success rate of 80%, human UC-MSC appeared as a monolayer of large, flat cells. The morphology of cells was uniform spindle with spiral-shaped or racially arranged between the four and six passage of culture (Figure 1A). The average number of these cells was estimated to be 30×104 originally seeded mononuclear cells (MNC) (Figure 1B). Flow cytometric studies indicated that MSC exhibited a characteristic immunophenotype, CD73, CD90 and CD105 highly expressed but CD34, CD45, CD11b, CD19 and HLA-D weakly expressed (Figure 1C), which indicated the isolated MSCs were not contaminated with cells of hematopoietic or endothelial origin. Osteogenic differentiation was determined by the production of calcium deposits detected by Alizarin red staining (Figure 2A), and adipogenic differentiation was confirmed by Oil-red O staining (Figure 2B). As these properties were highly similar to those of BM-derived MSC, we concluded that the cell population prepared in this study represented human UC-MSC.
Figure 1. Morphologic, proliferative and immunophenotypic characterization of human UC-MSC.
A: Morphology at a 40×magnification; B: Growth curve measured by a trypan blue exclusion assay; C: Immunophenotype of MSC population that was derived from human umbilical cord. An open profile represents an isotype control for background fluorescence and a shaded one shows a positive signal.
Figure 2. Multilineage potential of MSC.
Cultured MSC differentiated into osteocytes (A: Alizarin red staining) and adipocytes (B: Oil-red O staining). Scale bar: 100 µm.
Characterization of Neural Potential of Mesenchymal Stem Cells
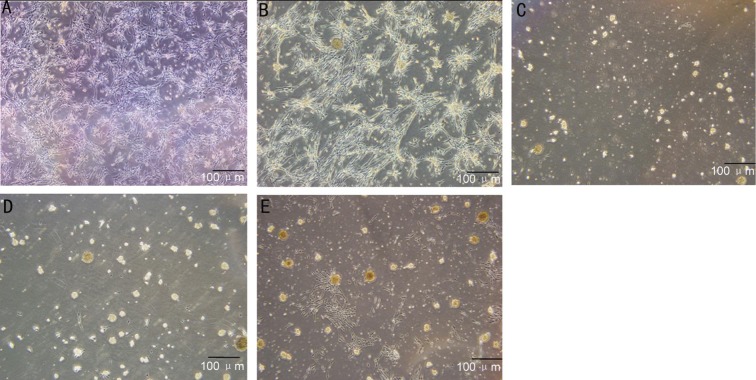
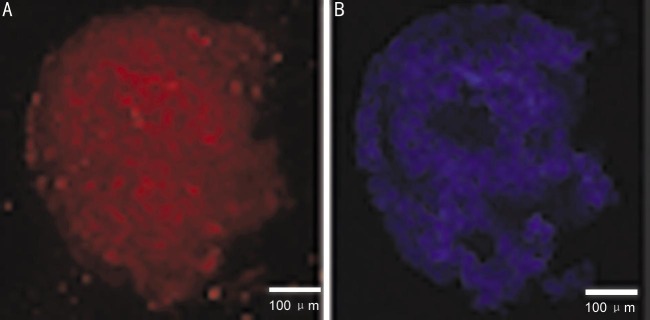
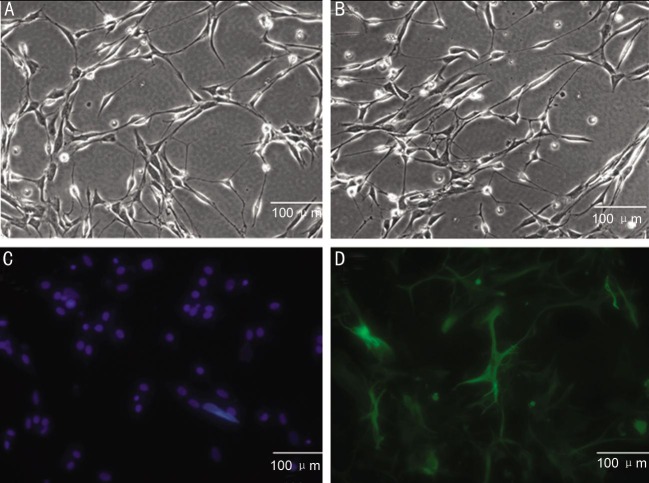
To determine the neural potential of MSCs, MSCs from P4-6 were treated with neural-specific differentiation medium containing N2/B27, EGF and neurobasal media. Most of the cells were bi-polar and spindle-like shaped at the beginning of induction (Figure 3A, 3B), and the cells spindle-like cells could proliferate to a much higher density after 3-4d induction (Figure 3C, 3D). They exhibited neurosphere-like morphologic change at the end of the third week (Figure 3E). Immunocytochemical staining indicated that the cells were reactive to differentiation medium, such as they began to express a number of neural stem cell surface markers, including Nestin and NeuroD1 at week 3. Among these markers, Nestin, one of the specific markers of neural stem cells, showed a most marked elevation in protein expression level (Figure 4A). Similarly, protein expressions of NeuroD1 were gradually increased during this course of differentiation (Figure 4B). This immunophenotypic change suggested that the cells underwent a fate change, from a mesenchymal to neural lineage, under this differentiation condition.
Figure 3. Characterization of neural stem cell induced from human MSCs.
MSCs were treated with neural-specific differentiation medium for 3wk; differentiated MSC were bi-polar and spindle-like shaped (A, B); spindle-like cells could proliferate to a much higher density (C, D); they exhibited neurosphere-like morphologic change at the end of the third week (E). Scale bar: 100 µm.
Figure 4. Immunofluorescence analyzes of neural-specific cell-surface markers.
Human umbilical cord derived MSC were cultured with neural differentiation medium for 3wk and stained with mouse monoclonal Ab raised against Nestin and NeuroD1 (A). After nuclei were stained with DAPI (B), the cells were observed by fluorescence microscopy. Scale bar: 100 µm.
To determine the potency of MSCs, cells were treated with appropriated neuron-specific induction medium. Most of the cells exhibited neuron-protrusion morphologic change at the end of the first week (Figure 5A, 5B). Neuron differentiation was determined by expressions of MAP2 and NF-M immunocytochemical staining (Figure 5C, 5D).
Figure 5. Cells incubated for 3wk in neuron differentiation medium.
A, B: Most of cells exhibited neuron-protrusion morphologic change. C, D: IF analyzes of neuron specific cell-surface markers. Neuron differentiation was determined by expressions of MAP2 and NF-M IF staining. Scale bar: 100 µm.
Reverse Transcription-polymerase Chain Reaction Analysis of Neural Potential of Mesenchymal Stem Cells
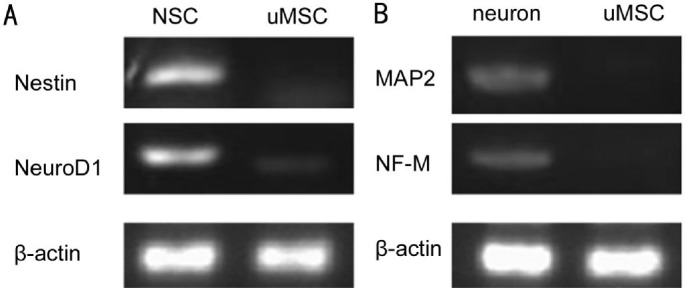
In order to confirm further neural differentiation of UC-MSCs, we analyzed the mRNA expression pattern for neural stem cell-specific genes, Nestin and NeuroD1. It was found that differentiated cells expressed an appreciable amount of mRNA for Nestin and NeuroD1. The expressions of Nestin and NeuroD1 were most high in the differentiated neural stem cell and negative in undifferentiated MSC, implying that molecular machineries for the neural function were activated in differentiated MSCs (Figure 6A).
Figure 6. RT-PCR analyzes of neural stem cells and neuron specific cell-surface markers.
A: RT-PCR analysis showed mRNA levels of factors involved in neural stem cell differentiation of NSC (neural stem cells) and uMSC. B: RT-PCR analysis showed mRNA levels of factors involved in neuron differentiation of neuron and uMSC. β-actin is a positive loading control.
Under the neuron differentiation condition, we analyzed the mRNA expression pattern for neuron-specific genes, MAP2 and NF-M. The expressions of these genes were increased in differentiated neural stem cell-neuron (Figure 6B). Expressions of MAP2 and NF-M appeared to be negative in undifferentiated MSC (uMSC). Together, these data suggested that differentiated neuron expressed mature neural surface molecules.
DISCUSSION
DR nerve damage was first reported by Barber et al[8], the study showed retinal ganglion cells are the earliest and highest rate damaged, and photoreceptor apoptosis was significantly increased with the progression of DR. But there is no effective measures to promote nerve repair after optic nerve damage and exogenous cell replacement therapy could be a potential clinical management for optic nerve damage[9]. Nowadays, MSC has become a promising source of stem cell-based regenerative medicine. They are regarded as superior to other types of stem cells for the reasons, such as convenient isolation ex vivo expansion and long-term cryopreservation, extraordinary plasticity and easy genetic manipulation, few ethical and legal concerns[10]. Although the cells have been traditionally obtained from BM, umbilical cord and associated tissues, such as placenta, umbilical cord vein and artery and Wharton's jelly, were proven recently to be an alternative source of MSC[11]. Therefore, UC-MSCs are expected to be the choice of cells in development of stem cell-based therapy and transplantation.
Human BM-MSCs and human umbilical cord blood-derived MSCs were recently found to differentiate to a neural phenotype[12]. Given the clinical advantages of human umbilical cord as an alternative source of stem cells, in the present study, we investigated whether neural stem cells could be derived from UC-MSCs and whether differentiated neural stem cells functions were able to differentiate into neuron in vitro. We found MSCs treated with a mixture of neurobasal growth factors expressed cell markers which were typical for neural stem cell including neuron. As we know MSCs secrete high levels of neurotrophic factors that known to stimulate peripheral nerve regeneration. MSCs could be differentiated and displayed trophic influences similar to those of neuron, which suggested that MSCs were a viable alternative to native neuron and may be used in cell-based therapy for central or peripheral nerve injuries[13].
In this study, we isolated MSCs without enzymatic digestion treatment, which favors cell adhesion and viability. We first characterized MSCs from umbilical cord by assessing the fibroblastic-like morphologic features, expression of a classical set of surface proteins, and neuropotential differentiation capability, which was consistent with previous research[14]–[15]. Recent reports demonstrated the function of differentiated human UC- derived MSCs by co-culturing with neurons, differentiated MSCs could significantly promote neuron outgrowth and elongation as compared with untreated MSCs. In our study, we found greater neuron-sprouting neurites and longer neurites on culture of MSCs. The data also confirmed that differentiated MSCs had a neurite outgrowth. Numerous studies had shown that neurons failed to extend neurites during the first day of culture and neurite extension was influenced by growth factors[16]. Therefore, this effect of MSCs may be due to soluble factors released by the cells. A recent study involving ELISA and blocking antibodies showed that the factors causing the neurite outgrowth phenomenon were Brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) secreted by differentiated rat BM-MSCs[17].
Interestingly, neural differentiation of umbilical cord-derived MSC appeared to proceed with manifesting noticeable morphologic change, which was in accordance with a previous report with BM-derived MSC[18]. Including morphologic change, the cell fate appeared to change progressively from a mesenchymal to neural lineage, because our RT-PCR analysis indicated that expression of several neural lineage surface markers increased. The temporal expression pattern for these markers was confirmed further by immunocytochemical staining. Moreover, differentiated neural stem cells were showed to gain steadily the capabilities to differentiate into neuron as neural differentiation progressed. This transdifferentiation potential is extraordinary because MSC are regarded as distant from neural lineage cells with respect to developmental stages and biologic properties[19].
In this study, we did not investigate the expression pattern of intracellular molecules because no neural specific intracellular markers had been known to date. In assessing neural differentiation, therefore, we relied on the cell-surface markers that played important role in developmental and physiologic processes for neural lineage cells. Among these surface markers, Nestin and NeuroD1 have been best characterized[20]–[21]. The upstream molecular mechanisms of these receptors are currently not completely understood, but a number of studies have indicated that Nestin and NeuroD1 interact with MAP3K10 and Cyclin D1 respectively[22]. Other surface determinants for mature neron include two closely related receptor, MAP2 and NF-M, as well as cell membrane-associated proteins such as tubulin beta 4 (TUBB4). Although all these molecules are essential for full neural function, they appeared to be expressed in different stages of neural differentiation, TUBB4 was expressed at an early stage, while expression of MAP2, growth associated protein 43 (GAP43) and NF-M appeared only in the later stage. This observation is in part consistent with the results from previous studies indicating that TUBB4 is an early marker[23] whereas MAP2, GAP43 and NF-M is restricted to later stages of vascular development[24].
In conclusion, we have demonstrated that human UC-MSCs could be induced into neural stem cells in terms of morphologic features, phenotype, and functional capacities. As an alternative source for MSCs, our results suggested that neural stem cells induced from MSCs maybe useful for neurodegenerative eye diseases. As these cells possess numerous clinical and practical advantages over other stem cells, they may be a valuable source for neurodegenerative eye diseases. This study included a small sample; and our study was the preliminary exploration of MSC differentiation into neural stem cell and neuron. The exact mechanism and outcome remain to be elucidated in next studies. Future work will assess the translational potential of local MSC transplantation for the treatment neurodegenerative eye diseases such as glaucoma, optic neuritis, ischemic optic neuropathy and disorders like DR, through drug development with targeting signaling pathways by stem cell-independent methods.
Acknowledgments
The authors do not discuss the use of off-label products, which includes unlabeled, unapproved, or investigative products or devices.
Foundation: Supported by Tianjin Science and Technology Project of China (13ZCZDSY01500).
Conflicts of Interest: Chen S, None; Zhang W, None; Wang JM, None; Duan HT, None; Kong JH, None; Wang YX, None; Dong M, None; Bi X, None; Song J, None.
REFERENCES
- 1.Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(2):283–290. doi: 10.1016/S0278-5846(03)00023-X. [DOI] [PubMed] [Google Scholar]
- 2.Arden GB, Sivaprasad S. The pathogenesis of early retinal changes of diabetic retinopathy. Doc Ophthalmol. 2012;124(1):15–26. doi: 10.1007/s10633-011-9305-y. [DOI] [PubMed] [Google Scholar]
- 3.Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Bio Med (Maywood) 2001;226(6):507–520. doi: 10.1177/153537020122600603. [DOI] [PubMed] [Google Scholar]
- 4.Si YL, Zhao YL, Hao HJ, Fu XB, Han WD. MSCs: biological characteristics, clinical applications and their outstanding concerns. Ageing Res Rev. 2011;10(1):93–103. doi: 10.1016/j.arr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Iftimia-Mander A, Hourd P, Dainty R, Thomas RJ. Mesenchymal stem cell isolation from human umbilical cord tissue: understanding and minimizing variability in cell yield for process optimization. Biopreserv Biobank. 2013;11(5):291–298. doi: 10.1089/bio.2013.0027. [DOI] [PubMed] [Google Scholar]
- 6.Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J Stem Cells. 2014;6(2):195–202. doi: 10.4252/wjsc.v6.i2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsieh JY, Wang HW, Chang SJ, Liao KH, Lee IH, Lin WS, Wu CH, Lin WY, Cheng SM. Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS One. 2013;8(8):e72604. doi: 10.1371/journal.pone.0072604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102(4):783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding DC, Chang YH, Shyu WC, Lin SZ. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24(3):339–347. doi: 10.3727/096368915X686841. [DOI] [PubMed] [Google Scholar]
- 10.Jin HJ, Bae YK, Kim M, Kwon SJ, Jeon HB, Choi SJ, Kim SW, Yang YS, Oh W, Chang JW. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14(9):17986–18001. doi: 10.3390/ijms140917986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahrlund-Richter L, De Luca M, Marshak DR, Munsie M, Veiga A, Rao M. Isolation and production of cells suitable for human therapy: challenges ahead. Cell Stem Cell. 2009;4(1):20–26. doi: 10.1016/j.stem.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25(6):1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 13.Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010;5(6):933–946. doi: 10.2217/rme.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H, Xie Z, Wei L, Yang H, Yang S, Zhu Z, Wang P, Zhao C, Bi J. Human umbilical cord mesenchymal stem cell-derived neuron-like cells rescue memory deficits and reduce amyloid-beta deposition in an AβPP/PS1 transgenic mouse model. Stem Cell Res Ther. 2013;4(4):76. doi: 10.1186/scrt227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang YK, Chen CS. Cell adhesion and mechanical stimulation in the regulation of mesenchymal stem cell differentiation. J Cell Mol Med. 2013;17(7):823–832. doi: 10.1111/jcmm.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leong WS, Wu SC, Pal M, Tay CY, Yu H, Li H, Tan LP. Cyclic tensile loading regulates human mesenchymal stem cell differentiation into neuron-like phenotype. J Tissue Eng Regen Med. 2012;6(Suppl. 3):s68–79. doi: 10.1002/term.1548. [DOI] [PubMed] [Google Scholar]
- 17.Foudah D, Redondo J, Caldara C, Carini F, Tredici G, Miloso M. Human mesenchymal stem cells express neuronal markers after osteogenic and adipogenic differentiation. Cell Mol Biol Lett. 2013;18(2):163–186. doi: 10.2478/s11658-013-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaculik C, Schuster C, Bauer W, Iram N, Pfisterer K, Kramer G, Reinisch A, Strunk D, Elbe-Bürger A. Human dermis harbors distinct mesenchymal stromal cell subsets. J Invest Dermatol. 2012;132(3 Pt 1):563–574. doi: 10.1038/jid.2011.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu YS, Cheng YC, Lin MY, Cheng H, Chu PM, Chou SC, Shih YH, Ko MH, Sung MS. Conversion of human umbilical cord mesenchymal stem cells in Wharton's jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. 2006;24(1):115–124. doi: 10.1634/stemcells.2005-0053. [DOI] [PubMed] [Google Scholar]
- 20.Harris DT. Umbilical cord tissue mesenchymal stem cells: characterization and clinical applications. Curr Stem Cell Res Ther. 2013;8(5):394–399. doi: 10.2174/1574888x11308050006. [DOI] [PubMed] [Google Scholar]
- 21.Xiao L, Tsutsui T. Human dental mesenchymal stem cells and neural regeneration. Hum Cell. 2013;26(3):91–96. doi: 10.1007/s13577-013-0069-4. [DOI] [PubMed] [Google Scholar]
- 22.Lederer CW, Santama N. Neural stem cells: mechanisms of fate specification and nuclear reprogramming in regenerative medicine. Biotechnol J. 2008;3(12):1521–1538. doi: 10.1002/biot.200800193. [DOI] [PubMed] [Google Scholar]
- 23.Munoz JL, Greco SJ, Patel SA, Sherman LS, Bhatt S, Bhatt RS, Shrensel JA, Guan YZ, Xie G, Ye JH, Rameshwar P, Siegel A. Feline bone marrow-derived mesenchymal stromal cells (MSCs) show similar phenotype and functions with regards to neuronal differentiation as human MSCs. Differentiation. 2012;84(2):214–222. doi: 10.1016/j.diff.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foudah D, Monfrini M, Donzelli E, Niada S, Brini AT, Orciani M, Tredici G, Miloso M. Expression of neural markers by undifferentiated mesenchymal-like stem cells from different sources. J Immunol Res. 2014;2014:987678. doi: 10.1155/2014/987678. [DOI] [PMC free article] [PubMed] [Google Scholar]