Abstract
Leishmaniasis is a neglected tropical disease transmitted by Phlebotomus spp. sand flies. Cutaneous leishmaniasis (CL) in Sri Lanka is caused by Leishmania donovani. Transmission patterns are different in Southern and Northern Sri Lanka. Current study examined the prevalence, risk factors and distribution of CL in Matara District, Southern Sri Lanka. Total of 2260 individuals from four District Secretariat divisions (DSDs) were screened by house to house surveys using an interviewer administered questionnaire. The study population had an age range of 1–90 years (median = 43 ± 17.31), low monthly income ( < 20 000 LKR, 52.8%) and a male to female ratio of 1 : 2. Thirty eight patients were diagnosed by light microscopy, culture and/or PCR with a disease prevalence of 1.68%. Spatial mapping provided evidence for significant case clustering, which tended to be more prominent with proximity to forest areas. The risk factors identified were un-plastered brick walls, absence or low usage of protective measures against insect bites, low income and excessive time (>4 hours/day) spent outdoors. However, exposure of limbs while outdoors, unawareness about the disease, type of occupation, common water source as the mode of water supply and presence of animal shelters within 200 m were not associated with the risk of acquiring the disease. Peri-domestic transmission is likely to contribute to the observed case clustering with all age groups at risk of acquiring the infection. Human behavioural habits coinciding with that of the vector, sand fly are likely to enable host-vector contact promoting its spread. Appropriate vector control measures, improvement of housing conditions, public education regarding preventive measures are required to contain the spread of disease.
Keywords: Peri-domestic transmission, Neglected tropical disease, Parasitic disease, Spatial mapping, Risk factors, Skin lesions, Vector-borne infections, L. donovani
Introduction
Leishmaniases are a group of parasitic diseases associated with high morbidity and mortality.1 The infection is caused by a protist species of the genus Leishmania and the clinical manifestations take three forms, viz., cutaneous leishmaniasis (CL), muco-CL (MCL) and visceral leishmaniasis (VL), depending on the causative species.1,2
Leishmaniasis burden spans across 88 countries, with 350 million people living at risk of infection.1–4 An approximate 500 000 new cases of VL and 1–1.5 million cases of CL occur annually with the disease mostly concentrated in few countries, hence the limited investments in its diagnosis, treatment and control.1,2 The progressive increase of risk factors that enable emergence and re-emergence of the disease have increased the level of public health concern. Migration, urbanisation, deforestation, new irrigation schemes, HIV, malnutrition and genetic factors are few examples.1–4 Such multiple and diverse risk factors have resulted the disease to emerge in new foci.1–7 The transmission of the disease may occur zoonotically in some endemic settings,8,9 while peri-domestic transmission is known in some others.5,9 Disease control or prevention strategies are primarily defined according to the transmission characteristics of the endemic setting. Therefore, novel insights gained with regard to the risk factors of the endemic setting and true disease burden can provide a comprehensive scientific platform that can assist in defining effective disease prevention and control strategies, for leishmaniasis.
Sri Lanka was recognised as a new focus of the disease in 1990s,10 where its main clinical form remains as CL.10–12 L. donovani, the usually visceralizing species, is known to cause CL in Sri Lanka.13,14 More virulent clinical forms such as VL and MCL have also emerged,15–18 calling for urgent preventive and control measures. Hence, disease control activities and scientific research leading towards such activities have been identified as priorities in the recently updated national action plan for leishmaniasis control in Sri Lanka.19
A few population-based case-control studies have been carried out in the past for risk factor analysis in the endemic settings in Sri Lanka. However, these studies were limited due to their restricted geographical coverage and the small size of the population covered. With reference to the aforementioned studies, zoonotic transmission was suspected in the Northern parts of the country, during the early stages of the outbreak,20–25 with gender (male), age (between 21 and 40 years) and outdoor occupation as risk factors. The traditional view of anthroponotic L. donovani transmission has been challenged with some evidence pointing towards the presence of animal reservoirs.25–27 However, a study conducted in the Southern part of the country 10 years later provided clues as to the higher prevalence of disease in ages between 11 and 40 years, with house-hold clustering and poor housing conditions as risk factors.11,25,28 The vector habits in the two areas are believed to be different,29–32 which might explain the different epidemiological patterns observed in the two locations within Sri Lanka. Another limiting factor apparent in the past studies was the primary dependence on passive methods for case detection, where only the patients who were referred to a local hospital were studied,11,28,33 which may understate disease prevalence, fail to represent geographical boundaries and may not illustrate an unbiased picture of the risk factors involved.24 Therefore, the current study was aimed at defining disease prevalence and risk factors using more precise methods (active-case detection),20,24,34,35 in a defined geographical area that is representative of a recognised administrative unit (a district), combined with spatial mapping of study subjects.
Methodology
Study setting
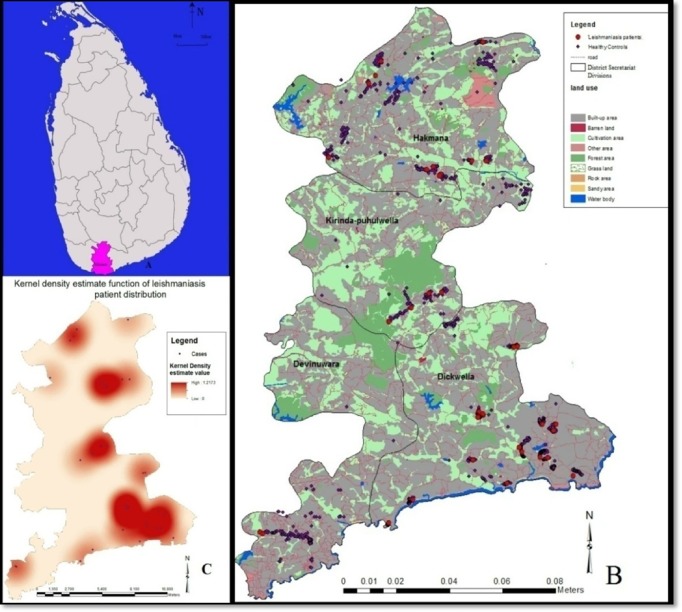
The study was carried out in the district of Matara in Southern Sri Lanka, an area recognised to have high prevalence of leishmaniasis (Fig. 1A).35
Figure 1.
(A) Map of Sri Lanka with the district of Matara shaded. (B) Geographical distribution of leishmaniasis patients in Matara district. (C) Kernel density estimate function of leishmaniasis patient distribution. A patient cluster was defined as the occurrence of a patient count of three or more with a maximum distance of 500 m between two adjacent points. The dark colour areas indicate the distribution of risk of acquiring the disease in relation to the epicentres.
Study population
The study population was the inhabitants of the district of Matara, who were residents of the area for more than 12 months.
Study design
A population-based cross sectional survey was carried out followed by a case-control study. Patients with suspicious skin lesions were subjected to laboratory diagnosis of CL. Age-sex matched, healthy controls were selected from the same clusters where the cases were found. A cluster was defined as a geographical area consisting of 45 households in a single Grama Niladhari Division (GND).
Sample selection
District of Matara consists of 16 Divisional Secretariat Divisions (DSDs) of which four DSDs viz., Dickwella, Devinuwara, Hakmana and Kirind-puhulwella, were randomly selected, for the study (Fig. 1B). Each DSD is divided into Grama Niladhari Divisions (GNDs), the smallest administrative units. Fifty two GNDs in the selected four DSDs, which covered 20% of district population (which was 810 627 according to latest census data (http://www.statistics.gov.lk/page.asp?page=population%20and%20housing)), were included in the study as described below.36 The sample size, representative of the district population was calculated using a standard formula with the following parameters {n = Z2 P (1–P)/d2 where Z = 1.96, P = 0.02, d = 0.01, n is the sample size and P is the expected prevalence with 5% alpha error}. A calculated sample size of 2260 was arrived upon, based on the aforementioned formula.
Four stage random cluster sample selection was used to select the study population. In the first stage, four DSDs were randomly selected from 16 DSDs and then 52 GNDs were randomly selected from four DSDs. In the third stage, 45 households were selected and in the last stage, individuals from each households were selected. The centre of each GND was identified as the starting point and the household selection was performed using right hand side selection, until the cluster was completed.
Data collection
Data collection on possible risk factors was done using an interviewer-administered questionnaire that has been previously used and validated for similar purposes.22 The extent of usage of protective measures (impregnated mosquito nets, insect repellents, long sleeved cloths with long trousers or dressers and other protective methods) against insect bites as stated by the study subjects were categorised as absent (none of the protective measures used); low (only one method used); moderate (two methods of protection used) or high (three or more methods of protection used). Data collections were done during the months of February, March, July, August and December, which cover the periods during which patients are reported, including the peak periods of patient reporting in southern Sri Lanka as per previous observations.28 Biological specimens were collected from clinically suspected patients in the form of lesion aspirate and slit skin scrapings for laboratory confirmation of diagnosis.
Diagnosis
Aspirates and slit skin smears obtained from skin lesions were microscopically examined under oil immersion ( × 1000 magnificent), both before and after in vitro culture.38 PCR was done for subset of samples using standard protocol.32 The target gene for PCR amplification was the gene coding for 18S r RNA, a 20–40 repeated sequence specific for the genus Leishmania.38,39
Laboratory-confirmed patients were referred to the local dermatologist for treatment.
Statistical analysis
A database was constructed from the field data with SPSS version 19.0. Analyses were done using SPSS version 19.0. The prevalence was estimated as the proportion of individuals who were positive, with 95% CI. Prevalence was disaggregated through cross-tabulation against possible risk factors.
Pearson chi-squared test was used to assess the difference between groups for categorical variables. Logistic regression analysis was used to calculate unadjusted and adjusted odds ratios (OR) with 95% confidence intervals (95% CI). Spatial distribution of CL cases was mapped and spatial cluster analysis methods such as nearest neighbour analysis, Ripley's K function analysis and kernel density estimation was performed using the ArcGIS 10-Desktop and the CrimeStat, with logistic regression analysis based on maximum likelihood estimation.40–42 Kernel density estimate provides an estimate of the prevalence that can occur in any given map location. Nearest neighbour analysis and the Ripley's K function were used to screen the study area for existence of case clustering followed by nearest neighbour hierarchical analysis routine to detect the actual places of case clustering.41
Ethical considerations
Written informed consent was obtained from individuals or from parents/guardians for those aged under 12 years. Ethical approval for the study was granted by the Ethics review committee, Faculty of Medicine, University of Colombo.
Results
A total of 2260 inhabitants, equivalent to 0.28% of the total population of Matara district, were studied. Age of the study population ranged between 1 and 90 years (median = 43 ± 17.31) where the majority were between 21 and 50 years (50%, n = 1130) and had a relatively low monthly income ( < 20, 000 LKR, 52.8%, n = 1193). Male to female ratio in the study sample was 1 : 2 (males 31.7%, n = 716, females 68.3%, n = 1544).
Prevalence and distribution of CL
Out of 2260 individuals studied, 80 participants (3.54%) were clinically suspected to have CL but only 38/2260 (1.68%) were confirmed as CL by light microscopy (LM), culture and/or PCR. Out of the three tests, PCR had the highest sensitivity (100%), while culture had the lowest (44.7%). A combination of culture with PCR and light microscopy with PCR had the sensitivity of 81.5 and 63.2%, respectively.
Over 1/2 of the laboratory-confirmed CL patients were from the DS of Dickwella (n = 21; 55.3%; P < 0.05) (Fig. 1B). Other patients were from the DSDs of Hakmana (n = 11), Devinuwara (n = 1) and Kirinda-Puhulwella (n = 5) (Fig. 1B). Disease prevalence in the study population was 1.68%. The regional prevalence values of leishmaniasis in four selected study sites (DSs) were higher, when compared to the disease prevalence and ranged from 1.73% in Devinuwara to 7.26% in Dickwella. Majority of the patients had nodular type lesions (n = 13), mostly localised in the face (n = 10) (excluding nose and ear) and the hand (n = 15). Only single lesions were evident in most of the patients (32/38).
Risk factors for CL
Cutaneous leishmaniasis affected a wide age range (13–84 years, median = 39 ± 18.99) with majority of patients belonging to 21–50 years age group (median = 38.5 ± 18.36; P > 0.05). More females (n = 23, 60.5%; P > 0.05) were affected than males. A significant level of risk to acquire CL in the study population was associated with the nature of walls in the houses (un-plastered brick walls), absence or low usage of protective measures against insect bites, excessive time (>4 hours/day) spent outdoors and a monthly income of less than 20 000 LKR (Table 1).
Table 1.
Association between leishmaniasis and socio-demographic variables (OR = odds ratio, CI = confident interval)
| Variables | Lab confirmed n (%) | Unadjusted OR(CI 95%) P-Value | Adjusted OR (CI 95%) P-Value | |
|---|---|---|---|---|
| Nature of walls: | Bricks with plaster | 4 (10.5) | 41.47 (13.8–124.8) < 0.05 | 18.34 (4.4–76.8) < 0.05 |
| Bricks without plaster | 34 (89.5) | |||
| Protective measures against insect bites | None | 15 (39.5) | 10.04 (3.8–26.2) < 0.05 | 6.13 (1.3–30.3) < 0.05 |
| Nets | 10 (26.3) | |||
| Other methods | 13 (34.2) | |||
| Time spent outdoors | >4 hours outdoor activities | 33 (86.8) | 22.50 (8.3–61.1) < 0.05 | 24.06 (5.4–106.3) < 0.05 |
| < 4 hours outdoor activities | 5 (13.2) | |||
| Monthly income | < 20 000 LKR per month | 37 (97.4) | 33.37 (4.5–248.1) < 0.05 | 28.66 (2.7–307.7) < 0.05 |
| >20 000 LKR per month | 1 (2.6) | |||
| Disease awareness | Yes | 7 (18.4) | 10.73 (2.9–38.8) < 0.05 | 33.83 (0.5–2345) >0.05 |
| No | 31 (81.6) | |||
| Covering of arms and legs with clothing while outdoors | Yes | 9 (23.7) | 19.76 (5.3–77.3) < 0.05 | 4.87 (0.5–44.7) >0.05 |
| No | 29 (76.3) | |||
| Mode of water supply | Common water source | 26 (68.4) | 1.17 (1.1–2.4) >0.05 | – |
| Tube wells | 1 (2.6) | |||
| Own water source | 11 (28.9) | |||
| Presence of animal shelters | Within 200 m | 11 (28.9) | 0.9 (0.8–1.1) >0.05 | – |
| No animal shelters | 3 (7.9) | |||
| No animals | 24 (63.2) | |||
| Occupation | Indoor occupation (>80% of the time) | 24 (63.2) | 0.5 (0.3–1.0) >0.05 | – |
| outdoor occupation (>80% of the time) | 14 (36.8) |
Adjusted for out-door activities, mosquito repellent methods, monthly income, clothing type and awareness
However, lack of awareness of the disease, lack of coverage of arms and legs with clothing while outdoors, type of occupation, common water source as the mode of water supply and presence of animal shelters in their gardens within 200 m were not independently associated with the risk of acquiring the disease (Table 1).
Spatial distribution of CL
The observed distribution pattern of the leishmaniasis patients in this study was analysed using the aforementioned spatial analytical methods. Significant case clustering was observed in nearest neighbour analysis, with the calculated nearest neighbour ratio of 0.46 (P < 0.05). This result was further augmented by the findings of Ripley's K function where observed values were higher than the expected values, which also favours clustering. The distribution of the risk of acquiring the disease, in relation to the epicentres was determined using the kernel density estimation for the four DSDs, indicated as dark red areas in the kernel density estimation map (Fig. 1C). Two areas of localised clustering were identified, with the hierarchical nearest neighbour analysis (Fig. 1B and C). Furthermore, for each subject in the sample, the nearest environmental feature (out of four features) was identified within a 500 m buffer zone and the data were tabulated (Table 2). Logistic regression analysis was carried out to determine the effect of environmental factor(s) on the occurrence of disease. There were more patients who lived in close proximity to forests, though the association between the two was only marginally significant (P = 0.058).
Table 2.
Association between environmental factors and leishmaniasis patient distribution correlation between environmental factors identified within a radius of 500 m.
| Environmental feature | Patients | Healthy controls | Total number | % of the patients (n = 1919) | OR (CI 95%) | P- value |
|---|---|---|---|---|---|---|
| Forest area | 4 | 66 | 70 | 0.2 | 5.27 (0.94–29.47) | 0.058 |
| Paddy cultivation | 21 | 1031 | 1052 | 1.1 | 1.77 (0.41–7.62) | 0.442 |
| Coconut plantation | 11 | 610 | 621 | 0.6 | 1.57 (0.34–7.14) | 0.560 |
| Other features | 2 | 174 | 176 | 0.1 | 1.92 (0.51–8.56) | 0.89 |
Discussion and Conclusions
Tropical diseases, such as leishmaniasis, and the environment are linked together by human behavioural factors, vector habits and their interaction with the environment. This study was aimed at determining the disease prevalence and risk factors of leishmaniasis, within a defined geographical region in Southern Sri Lanka. A disease prevalence of 1.68% was observed in the study population. The prevalence in different sub-district level units (DSs) was not equal, indicating a non-homogenic case distribution of leishmaniasis within the Matara district. The Highest prevalence was recorded in the DS of Dickwella (7.26%), indicating an endemic hot spot affecting the district of Matara with Dickwella as its epicentre (Fig. 1B and C). These areas may be serving as reservoirs for leishmaniasis transmission from which the disease can spread to adjoining areas as well.
Since noticeable level of case clustering was observed near forest areas, it can be inferred that the forest area might be acting as anchors, assisting the spread of the sand fly and may even promote transmission through zoonotic reservoirs. However, the logistic regression failed to provide convincing level of statistical support for this association, which may be due to the sample inadequacy, an assumption that is supported by the wide confidence interval. It is fascinating to observe that, though the current evidence suggests that forests can have an effect on the spread of leishmaniasis, more prominent disease clustering was observed near paddy fields. This may be due to the fact that, the study recorded the geographic location of the subjects’ residence (most of the Sri Lankan community establishments are centred around the paddy fields rather than forests), which can differ from the location of their outdoor activities and the place of infection acquisition, thus a reflection of a selection bias. However, the findings confirm that the spatial pattern of leishmaniasis transmission could be used to identify the risk areas for leishmaniasis, which could be utilised in the design of infection control strategies.
The observed prevalence in Matara district, during this study could be deemed as high, in comparison to prevalence figures recorded in previous local studies, during the last decade.28 Possible reasons behind this may be the methodology used that included active case detection through house to house surveys, that was done covering a large area to represent a larger geographical administrative entity within the country. However, other factors such as progressive changes in the infra-structure developments that are likely to have provided convenient access to the district of Matara, facilitating free movements of disease carriers from other high risk areas (North-Central province) and the awareness-raising activities, which may have led to increased self-referrals and improved case diagnosis also may have contributed. Although, the first local case of CL was detected from Ambalantota, in Southern Sri Lanka in 1992,10 cases were clearly sporadic and were rare until early 2000.25 Recent outbreak of CL was first reported from Northern Sri Lanka, in 2001 and has continued during the decade that followed.11,25 This may indicate the possibility of Southern Sri Lanka silently harbouring a disease focus, which may have facilitated gradual spatial expansion of case distribution in this region.
Leishmania infection appears to affect a wide age range of both genders in Southern Sri Lanka. However, among the laboratory-confirmed cases, majority were middle aged, though the numbers were comparable to other age groups. Higher proportion of females being affected was true when total study subjects were considered and also within different age groups except in the very young ( < 10 years) and those between 60 and 80 years (data not shown). Furthermore, an increasing number of females were found to be harbouring the disease when compared to the previous studies,25,28 possibly indicating peri-domestication of the transmission cycle, since most of these females were unemployed and were house-bound, further supporting this assumption. However, since the study population consisted of a higher proportion of females, the results might be female-biassed; hence caution must be exercised in its interpretation. Low monthly income, poor housing conditions, absence or low usage of protective measures against insect bites and spending over 4 hours on outdoor activities were significantly associated with occurrence of disease among individuals, hence were identified as risk factors for disease acquisition and therefore should be taken into consideration when planning disease preventive measures in this region.
Poverty that prevail in these regions may play a major role in facilitating sub-standard housing with un-plastered brick walls and poor domestic sanitary conditions that increase peri-domestic sand fly propagation and resting sites, as well as their contact with humans.25,29 Absence or low usage of protective measures against insect bites such as bed nets, with or without impregnation with insecticides was identified as a risk factor for disease acquisition. However, the regular bed nets (without insecticide impregnation) are known to be ineffective in preventing sand fly bites, due to the fact that sand flies can easily penetrate these nets due to their large pore sizes.
The risk factors for leishmaniasis in Southern Sri Lanka appear to be associated with the life style of the people as well, whose habit of increased outdoor activities during the evenings, coincides with that of the vector, sand fly. The positively diagnosed patients spent more time in outdoor environment, for activities like farming/gardening in areas adjacent to their residence, in much contrast to military or deep jungle activities that are considered to be facilitators of zoonotic transmission. Therefore, this may not be a direct suggestion of zoonotic transmission, rather a peri-domestic nature of transmission, and is in contrast to the findings of the previously conducted studies in Northern Sri Lanka.24–26
Since peri-domestic environment appears to be associated with L. donovani transmission, further spread of infection within Sri Lanka might be unavoidable under present circumstances. Early detection and treatment of patients would therefore, considerably help to contain the situation. Due to the subjective nature of clinical diagnosis and the absence of pathognomonic features of CL,33,43 improvement of laboratory facilities across the country would facilitate more effective patient management. The disease established within the country, specifically in the Southern and North Central provinces, is bound to have many drawbacks including negative impacts on the tourism industry, thus becoming a hindrance to the country's economic development. Necessity of urgent preventive activities have been highlighted in the past,33 and a national action plan has been drawn up19 to facilitate the process. However, such plans need to be translated into action without further delay in order to limit transmission and the progressive spread of infection, as evidenced by this study. Furthermore, detailed entomological survey and studies on potential reservoir hosts, such as dogs, are needed to better understand the local disease transmission patterns, which would enable development of effective strategies for prevention and control of leishmaniasis in Sri Lanka.
Acknowledgements
We acknowledge Mr Sudath weerasinghe, Ms Dilhani Samarakoon and Mr Charith Bandara for technical assistance, Dr N. Ekneligoda for field assistance, Dr D.Somaratne, Regional Epidemiologist and Medical officers of health in Dickwella, Devinuwara, Kirinda and Hakmana for administrative assistance. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclaimer Statements
Contributors Peri-domestic transmission, neglected tropical disease, parasitic disease, spatial mapping, risk factors, skin lesions, vector-borne infections, L. donovani
Funding Research reported in this article was supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health, USA under Award Number R01AI099602. Funding to YS through WHO/SEARO(55760) and University of Colombo grant AP/3/2011/CG/01.
Conflicts of interest None of the authors of this paper has a financial or personnel relationship with other people or organisation that could inappropriately influence or bias the content of the paper.
Ethics approval Written informed consent was obtained from individuals or from parents / guardians for those aged under 12 years. Ethical approval for the study was granted by the Ethics review committee, Faculty of Medicine, University of Colombo.
References
- 1.Who Expert Committee Control of the leishmaniases. Technical. Geneva: Who Expert Committee; 2010; p. 1–86. [Google Scholar]
- 2.WHO , Leishmaniasis [Internet]. World Health Organization; 2014 [cited 2014 Sep 1]. Available from: http://www.who.int/mediacentre/factsheets/fs375/en/. [Google Scholar]
- 3.Desjeux P. Leishmaniasis current situation 2004. Comp Immunol Microbiol Infect Dis. 2004;27(5):305–18. [DOI] [PubMed] [Google Scholar]
- 4.Alvar J., Velez I. D., Bern C., Herrero M., Desjeux P., Cano J., et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma U., Singh S.. Insect vectors of Leishmania: distribution, physiology and their control. J Vector Borne Dis. 2008;45(4):255–72. [PubMed] [Google Scholar]
- 6.Moreno E. C., Melo M. N., Genaro O., Lambertucci J. R., Serufo J. C., Silva A., et al. Risk factors for Leishmania chagasi infection in an urban area of Minas Gerais State. Rev Soc Bras Med Trop. 2005;38(6):456–63. [DOI] [PubMed] [Google Scholar]
- 7.Picado A., Ostyn B., Singh S. P., Uranw S., Hasker E., Rijal S., et al. Risk factors for visceral leishmaniasis and asymptomatic Leishmania donovani infection in India and Nepal. PLoS One. 2014;9(1):e87641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bates P. A. Transmission of Leishmania metacyclic promastigotes by Phlebotomine sand flies. Int J Parasitol. 2007;37(10):1097–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ready P. D. Epidemiology of visceral leishmaniasis. Clin Epidemiol. 2014;6:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Athukorale D. N., Seneviratne J. K., Ihalamulla R., Premaratne U. N.. Locally acquired cutaneous leishmaniasis in Sri Lanka. J Trop Med Hyg. 1992;95(6):423–3. [PubMed] [Google Scholar]
- 11.Siriwardena H., Udagedara C., Karunaweera N. D.. Clinical features, risk factors and efficacy of cryotherapy in cutaneous leishmaniasis in Sri Lanka. Ceylon Med J. 2003;48(1):10–12. [DOI] [PubMed] [Google Scholar]
- 12.Karunaweera N. D. Leishmania donovani causing cutaneous leishmaniasis in Sri Lanka: a wolf in sheep's clothing? Trends Parasitol. 2009;25(10):458–63. [DOI] [PubMed] [Google Scholar]
- 13.Siriwardena H. V. Y. D., Noyes H. A., Beeching N. J., Chance M. L., Karunaweera N. D., Bates P. A.. Leishmania donovani and cutaneous leishmaniasis, Sri Lanka. Emerg Infect Dis. 2007;13(3):3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karunaweera N. D., Pradong F., Siriwardane H. Y. D., Ihalamulla R. L., Dedet J. P.. Sri Lankan cutaneous leishmaniasis is caused by Leishmania donovani zymodeme MON-37. Trans R Soc Trop Med Hyg. 2003;97:380–1. [DOI] [PubMed] [Google Scholar]
- 15.Abeygunasekara P., Costa Y., Seneviratne N., Ranatunga N., Wijesundera M. D. S.. Locally acquired visceral leishmaniasis in Sri Lanka. Ceylon Med J. 2007;52(1):30–1. [DOI] [PubMed] [Google Scholar]
- 16.Rajapaksa U. S., Ihalamulla R. L., Karunaweera N. D.. First report of mucosal tissue localisation of leishmaniasis in Sri Lanka. Ceylon Med J. 2005;50(2):90–1. [PubMed] [Google Scholar]
- 17.Ranasinghe S., Zhang W-W., Wickremasinghe R., Abeygunasekera P., Chandrasekharan V., Athauda S., et al. Leishmania donovani zymodeme MON-37 isolated from an autochthonous visceral leishmaniasis patient in Sri Lanka. Pathog Glob Health. 2012;106(7):421–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rathnayake D., Ranawake R. R., Sirimanna G., Siriwardhane Y., Karunaweera N., De Silva R.. Co-infection of mucosal leishmaniasis and extra pulmonary tuberculosis in a patient with inherent immune deficiency. Int J Dermatol. 2010;49(5):549–51. [DOI] [PubMed] [Google Scholar]
- 19.Update on Clinical, Diagnostic, Chemotherapeutic and Entamological aspects of Leishmaniasis Proceedings and Action plan for prevention and control. 2013; p. 42–6. [Google Scholar]
- 20.Ranasinghe S., Wickremasinghe R., Munasinghe A., Hulangamuwa S., Sivanantharajah S., Seneviratne K., et al. Cross-sectional study to assess risk factors for leishmaniasis in an endemic region in Sri Lanka. Am J Trop Med Hyg. 2013;89(4):742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandanayaka R., Kahawita I., Gamage A., Siribaddana S., Agampodi S.. Emergence of cutaneous leishmaniasis in Polonnaruwa, Sri Lanka 2008-2011. Trop Med Int Health. 2014;19(2):140–5. [DOI] [PubMed] [Google Scholar]
- 22.Siriwardana H., Thalagala N., Karunaweera N.. Clinical and epidemiological studies on the cutaneous leishmaniasis caused by Leishmania (Leishmania) donovani in Sri Lanka. Ann Trop Med Parasitol. 2010;104(3):213–23. [DOI] [PubMed] [Google Scholar]
- 23.Semage S. N., Pathirana K. P. N., Agampodi S. B.. Cutaneous leishmaniasis in Mullaitivu, Sri Lanka: a missing endemic district in the leishmaniasis surveillance system. Int J Infect Dis. 2014;25:53–5. [DOI] [PubMed] [Google Scholar]
- 24.Nawaratna S. S. K., Weilgama D. J.. Cutaneous leishmaniasis, Sri Lanka. Emerg Infect Dis. 2007;13(7):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siriwardana H., Chandrawansa P. H., Sirimanna G., Karunaweera N. D.. Leishmaniasis in Sri Lanka: a decade old story. J Infect Dis. 2012;2(2):2–12. [Google Scholar]
- 26.Nawaratna S. S. K., Weilgama D. J., Rajapaksha K.. Cutaneous leishmaniasis in Sri Lanka: a study of possible animal reservoirs. Int J Infect Dis. 2009;13(4):513–7. [DOI] [PubMed] [Google Scholar]
- 27.Rosypal A. C., Tripp S., Kinlaw C., Hailemariam S., Tidwell R. R., Lindsay D. S., et al. Surveillance for antibodies to Leishmania spp. in dogs from Sri Lanka. J Parasitol. 2010;96(1):230–1. [DOI] [PubMed] [Google Scholar]
- 28.Rajapaksa U. S., Ihalamulla R. L., Udagedera C., Karunaweera N. D.. Cutaneous leishmaniasis in southern Sri Lanka. Trans R Soc Trop Med Hyg. 2007;101(8):799–803. [DOI] [PubMed] [Google Scholar]
- 29.Premachandra W. T. S., Senarath D. P. C., de Silva M. P. K. S. K., Lalanthika Peiris B. S.. A study on phlebotomine sandflies (Diptera: Phlebotomidae) in Dickwella, southern Sri Lanka, an endemic focus for cutaneous leishmaniasis. Int J Trop Insect Sci. 2012;32(01):32–8. [Google Scholar]
- 30.Gajapathy K., Peiris L. B. S., Goodacre S. L., Silva A., Jude P. J., Surendran S. N.. Molecular identification of potential leishmaniasis vector species within the Phlebotomus (Euphlebotomus) argentipes species complex in Sri Lanka. Parasit Vectors. 2013;6(1):302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozbel Y., Sanjoba C., Alten B., Asada M., Depaquit J., Matsumoto Y., et al. Distribution and ecological aspects of sand fly (Diptera: Psychodidae) species in Sri Lanka. J Vector Ecol. 2011;36(Suppl 1):S77–S86. [DOI] [PubMed] [Google Scholar]
- 32.Gajapathy K., Surendran S. N.. Report of the presence of Phlebotomus (Phlebotomus) salehi mesghali in Sri Lanka: a potential cutaneous leishmaniasis vector. J Natl Sci Found. 2012;40(2):168–72. [Google Scholar]
- 33.Karunaweera N. D., Rajapaksa U. S.. Is leishmaniasis in Sri Lanka benign and be ignored? J Vector Borne Dis. 2009;46(1):13–17. [PubMed] [Google Scholar]
- 34.Bern C., Maguire J. H., Alvar J.. Complexities of assessing the disease burden attributable to leishmaniasis. PLoS Negl Trop Dis. 2008;2(10):e313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Epidemiology unit, Ministry of Health-Sri Lanka [Internet]. Weekly Epidemiological report, Jan 07-13, 2012 [cited 2013 Feb 14.Available from: http://epid.gov.lk/web. [Google Scholar]
- 36.Department of Census and Statistics-Sri Lanka [Internet]. Population of Sri Lanka by District. Feb-Mar, 2012 [cited 2012 Apr 20.Available from: http://www.statistics.gov.lk/PopHouSat/CPH2011/Pages/Activities/Reports/CPH_2012_5Per_Rpt.pdf.
- 37.Ihalamulla R., Siriwardana H., Gamage S., Perera A., Karunaweera N.. First successful in vitro culture of autochthonous Leishmania sp. in Sri Lanka. Ceylon Med J. 2002;47(2):58. [PubMed] [Google Scholar]
- 38.Alam MS, Ghosh D, Khan MGM, Islam MF, Mondal D, Itoh M, et al. Survey of domestic cattle for anti-Leishmania antibodies and Leishmania DNA in a visceral leishmaniasis endemic area of Bangladesh. BMC Vet Res. 2011;7(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lachaud L, Chabbert E, Dubessay P, Reynes J, Lamothe J, Bastien P. Comparison of various sample preparation methods for PCR diagnosis of visceral leishmaniasis using peripheral blood comparison of various sample preparation methods for PCR diagnosis of visceral leishmaniasis using peripheral blood. J Clin Microbiol. 2001;39(2):613–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez-Barroso D., Herrador Z., San Martín JV, Gherasim A, Aguado M, Romero-Maté A, et al. Spatial distribution and cluster analysis of a leishmaniasis outbreak in the south-western Madrid region, Spain, September 2009 to April 2013. Euro Surveill. 2015;20(7):1–10. [DOI] [PubMed] [Google Scholar]
- 41.Smith SC, Bruce CW. CrimeStat III: User workbook [Internet]. 2008; p. 1–113. Available from: http://www.icpsr.umich.edu/CrimeStat/workbook/CrimeStat_Workbook.pdf. [Google Scholar]
- 42.Marcelle P, Kimberlee G. Principles of biostatictics. CA, USA: Duxbury Thomsons learning; 2000;. [Google Scholar]
- 43.Siriwardana H. V. Y. D., Senarath U., Chandrawansa P. H., Karunaweera N. D.. Use of a clinical tool for screening and diagnosis of cutaneous leishmaniasis in Sri Lanka. Pathog Glob Health. 2015;109(4):174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]