Abstract
Plant-derived products such as date syrup (DS) have demonstrated antibacterial activity and can inhibit bacteria through numerous different mechanisms, which may be attributed to bioactive compounds including plant-derived phenolic molecules. DS is rich in polyphenols and this study hypothesized that DS polyphenols demonstrate inherent antimicrobial activity, which cause oxidative damage. This investigation revealed that DS has a high content of total polyphenols (605 mg/100 g), and is rich in tannins (357 mg/100 g), flavonoids (40.5 mg/100 g), and flavanols (31.7 mg/100 g) that are known potent antioxidants. Furthermore, DS, and polyphenols extracted from DS, the most abundant bioactive constituent of DS are bacteriostatic to both Gram positive and Gram negative Escherichia coli and Staphylococcus aureus, respectively. It has further been shown that the extracted polyphenols independently suppress the growth of bacteria at minimum inhibitory concentration (MIC) of 30 and 20 mg/mL for E. coli and S. aureus, and have observed that DS behaves as a prooxidant by generating hydrogen peroxide that mediates bacterial growth inhibition as a result of oxidative stress. At sub-lethal MIC concentrations DS demonstrated antioxidative activity by reducing hydrogen peroxide, and at lethal concentrations DS demonstrated prooxidant activity that inhibited the growth of E. coli and S. aureus. The high sugar content naturally present in DS did not significantly contribute to this effect. These findings highlight that DS’s antimicrobial activity is mediated through hydrogen peroxide generation in inducing oxidative stress in bacteria.
Keywords: Phoenix dactylifera L., date syrup, polyphenol, S. aureus
Introduction
Staphylococcus aureus is affiliated to chronic wounds that have a strong association with chronic inflammation leading to high morbidity (Orsi et al., 2002). Furthermore, the increase in antibiotic-resistant bacteria poses a threat to health care worldwide resulting in a revived interest in plant products as adjunct antimicrobial agents to control pathogenic microorganisms (Cowan, 1999). Naturally derived compounds such as aloe vera, honey and curcumin (De et al., 2009) are gaining popularity as alternative antimicrobial compounds. A major plant group used for traditional medicinal applications is Phoenix Dactylifera L., more commonly known as the date palm. Fruit of the date palm have been used customarily in the treatment of intestinal disturbances (Vyawahare et al., 2009). In Egypt, date palm pollen grains have historically been used to enhance fertility (Al-Qarawi et al., 2003). Bauza (2002) has demonstrated that date palm kernels are included in medicinal skin treatment and nomadic tribes in the Middle East have been known to use traditional date syrup (DS) as an antimicrobial agent for wound healing (Tahraoui et al., 2007).
Date products such as DS are used in the food industry as a sweetening alternative and in the production of beverages and alcohol (Aboubacar et al., 2010). More than often, the perceived health benefits for the consumption and utilization in date-derived medicinal concoctions are attributed to the bioactive and nutritious compounds found in DS and date fruit. DS is a rich source of phenolic compounds which are known potent radical scavengers (Vayalil, 2002), various studies addressing the composition of DS have identified significant antioxidant potential (Guo et al., 2003) which may allude to the scientific basis of date fruit and DS’s traditional medicinal application. Numerous phenolic compounds such as polyphenols and flavonoids are antibacterial as a result of their oxidizing potential (Daglia, 2012), which may offer a rationale for date fruit and DS’s medicinal application as an antimicrobial agent.
Whilst it remains unclear as to precisely how the antioxidant scavenging potential contribute to the bacteriostatic and bactericidal activity of DS. Prooxidants are known to cause physiochemical and structural changes to microorganisms that results in growth retardation (Halliwell, 2008).
The challenge to this notion is the ability to determine by which mode of action DS inhibits microorganisms, and which bioactive compounds contribute to this effect. The topic of antioxidants as powerful scavengers of reactive oxygen species (ROS) has recently gained considerable attention in applied food microbiology, food science and technology and cell immunology. The antioxidant/prooxidant activity of secondary metabolites such as polyphenols can depend on factors such as pH, metal-reducing potential, chelating activity, and solubility (Sakihama et al., 2002). Polyphenols have antioxidant activity (radical scavenging, and metal chelating activity) or prooxidant activity depending on environmental conditions, interaction, structural changes and exposure to microorganisms (Yordi et al., 2012). Polyphenols are able to act as prooxidants in systems that utilize redox active metals such as iron and copper. Binding of the polyphenol complex ligand to Fe3+, the complex is able to reduce the iron to Fe2+ and is oxidized to a semiquinone, which is capable of reducing further Fe3+ oxidizing the semiquinone to a quinone. The reduction of Fe3+ generates Fe2+ that consequently participates in the Fenton reaction and results in ROS generation.
Bacterial aerobic respiration produces oxygen (O2) required in cellular energy production (Macvanin and Hughes, 2010). The incomplete reduction of O2 by microorganisms during respiration generates ROS including hydrogen peroxide (H2O2) and the hydroxyl radical (OH-). Bacteria that undergo aerobic respiration defend themselves against the oxidative stress associated with the accumulation of ROS such as exposure to polyphenols through several mechanisms one of which is the production of enzymes catalase and superoxide dismutase which combat ROS. Superoxide dismutase reduces OH- to H2O2, and catalase consequently converts H2O2 to water and O2. The detoxification process of ROS is efficient and with intracellular H2O2 concentration being controlled at a steady state value of 0.2 μM in Escherichia coli (Brudzynski et al., 2011). Antioxidants such as polyphenols and flavonoids induce bacterial lysis through increased ROS and H2O2 production.
Given that DS is known to have various bioactive polyphenols, reported as potential antimicrobial agents, this study aimed to identify the bacteriostatic and bactericidal activity of DS against Gram positive and Gram negative bacteria and to establish whether this activity is influenced by (a) DS phytochemical compounds, namely polyphenols, (b) the susceptibility of bacteria to oxidative stress resulting from hydrogen peroxide generated and mediated by the presence of polyphenols, and (c) osmolarity in regards to sugar content is the not the principal factor contributing to the antibacterial activity.
Materials and Methods
Standards, Solvents, and Reagents
The following reagents were obtained from Sigma (Sigma–Aldrich, UK): XAD-2 Resin, Folin-Ciocalteu reagent, Butylated hydroxytuolene (BHT), polyvinylpolypyrrolidone (PVPP), gallic acid, and catechin. Acetone and methanol (HPLC grade), Xylenol orange, aluminum chloride, glucose, fructose, and sucrose (analytical grade) and hydrogen peroxide were obtained from Fisher Scientific (UK) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) was purchased from Merck (Darmstadt, Germany).
Date Syrup Preparation for Antibacterial Testing
Date syrup was produced from the date fruit cultivar Khadrawi, belonging to the family Arecaceae, genus Phoenix and species dactylifera during the wet seasons of 2012–2013. The DS was raw and unprocessed; it was stored at 4°C on receipt. The DS was unsterile and not immediately suitable for antibacterial susceptibility testing. Therefore, different sterilization methods were undertaken to determine which method was the most ideal for DS with minimum effect on DS’s constituents. Sterilization of DS using the solvent acetone was determined to be most suitable. A 200 g of DS was mixed thoroughly and soaked in 200 mL acetone (Analytical grade) for 48 h at room temperature. After 48 h the homogenous mixture was filtered through Whatman No. 1 filter paper and the solvent was evaporated under rotary evaporation (Bibby RE-100, Bibby Scientific) at 40°C to ensure all acetone was removed. Crude DS extract was rehydrated in nutrient broth (NB) medium and passed through a 0.22 μm filter (Millex-GV, Millipore, UK) and stored at –80°C for analysis, the final concentration resulted in 50 mg/mL DS.
Preparation of Artificial DS
High Performance Liquid Chromatography (HPLC) analysis was conducted on the sugars present in DS to determine the percentage of individual sugar constituents. Artificial DS per 100 g was prepared by mixing 4.79 g sucrose (7.6% of total), 29.05 g fructose (46.13% of total), and 29.13 g glucose (46.3% of total) in sterile deionised water and warmed in a water bath at 50°C for 10 min to ensure complete dissolving of sugars.
Extraction of Flavonoid and Phenolic Fraction of DS on XAD-2 Resin
Date syrup (50 g) was mixed with 250 mL of pH2 HCl water for 24 h; the mixture was filtered through cotton wool to remove un-dissolved solid particles. XAD-2 resin (approximately 47 g) was initially conditioned in 2 M HCl for 1 h, conditioned by soaking in 1:1 methanol and water for pre-swelling overnight. The slurry with the resin was packed into a glass column (50 cm3) and the solution removed for an approximate bed volume of 1 × 50 cm3 and rinsed with 1 L of deionised water.
The filtered DS solution was passed slowly through the packed resin column, followed by 250 mL of acidified water (pH2), deionised water (300 mL), and phenolic fractions were finally eluted with 300 mL pure methanol. A 50 mL of collected methanol extract was concentrated to dryness under vacuum at 40°C, re-dissolved in water and stored at –80°C for analysis, and the remaining methanol extract was stored at –80°C for and dissolved accordingly for antibacterial analysis.
Determination of Antioxidant Activity
Quantification of Total Phenol Content
The total phenolic content of DS was determined by the Folin-Ciocalteu colorimetric assay based on the procedure previously identified by Al-Farsi et al. (2005). Gallic acid was used as a spectrophotometric standard (0–100 mg/mL) and results were expressed and means ± SD mg of gallic acid equivalents (GAE) per 100 g of DS. Measurements were taken in triplicate.
Total Flavonoid Content
Total flavonoid content was measured by the aluminium chloride colorimetric assay described by Zhishen et al. (1999). Absorbance was measured at 510 nm against a blank control. Total flavonoid content was expressed as mg GAE per 100 g DS.
Total Flavanol Content
Total flavonol content was adapted from the method described by Jimoh et al. (2010); 200 μl of DS (25 mg/mL) was mixed with 250 μl of 2% AlCl3 and 250 μl of 5% sodium acetate solution. Mixtures were sealed and incubated for 2.5 h at room temperature. The absorbance was measured at 440 nm and results were expressed as mg of catechin equivalents per 100 g of DS (mg catechin/100 g DS).
Total Tannin Content
The total tannin content was determined by the Folin-Ciocalteu method after the removal of tannins by their adsorption to the insoluble matrix PVPP. This method was based on Hagerman et al. (2000) and Kchaou et al. (2013); 1 mL of DS extract (25 mg/mL) was added to 100 mg of PVPP and incubated for 15 min at 4°C. The mixture was vigorously shaken and centrifuged for another 15 min at 13,000 g, where the supernatant was collected and non-adsorbed phenolics were subjected to the Folin-Ciocalteu assay for total phenolic content. Results were subtracted from total phenolic content and total tannins was expressed as mg GAE / 100 g fresh weight.
Total Carotenoid Content
Total carotenoids were extracted according to the method of Talcott and Howard (1999), working under red light and in dark conditions total carotenoids were calculated using the following equation and expressed as mg per 100 g of DS:
Where OD = absorbance at 470 nm, V = volume of sample extract, A1% = the average extinction coefficient for a 1% mixture of carotenoids at 2500, and W = sample weight in g.
Total Anthocyanin Content
Total anthocyanin was determined and calculated according to the pH-differential method as described by Giusti and Wrolstad (2001). Total anthocyanin content was expressed as mg/100 g of DS and calculated according to the following two equations:
(1) The difference in absorbance between the two anthocyanin extracts were calculated by:
(2) The monomeric anthocyanin pigment concentration in the original sample is expressed as cyaniding3-glucoside equivalents and calculated on the basis of the following formula:
Where MW = molecular weight of cyaniding3-glucoside (449.2 g/mol), DF = dilution factor, V = final volume in mL, 𝜀 = molar extinction coefficient for cyaniding3-glucoside (26,900), L = cell path length of 1 cm and W = sample weight in g.
Evaluation of Antioxidant Activity
DPPH Radical Scavenging Activity
DS’s anti radical scavenging capacity was assessed based on the scavenging activity of the stable free radical DPPH. Briefly, 100 μl of different DS concentrations (5–50 mg/mL) dissolved in deionized water were aliquoted into a 96-well plate (Costar), 50 μl of ultrapure (ELGA) water was added followed by 50 μl of 400 μm of DPPH (in absolute ethanol). The plate was sealed and shaken for 5 minutes and subsequently incubated in the dark for 25 min at room temperature. Absorbance was measured spectrophotometrically at 490 nm against a blank solution. The commercially available antioxidant BHT was used as a positive control (10 mg/mL in ethanol) and the percentage inhibition activity was calculated based on the following equation and expressed as % antioxidant activity:
where OD1 is absorbance of blank control and OD2 is absorbance of sample extract.
Antibacterial Susceptibility Testing
Bacterial Strains
Escherichia coli (reference strain NCTC 10418) and S. aureus (reference strain NCTC 13142) were used throughout the study. Cultures were grown aerobically in NB (Fluka) for 24 h at 37°C to promote planktonic growth.
Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
Minimum inhibitory concentration (MIC) for DS and extracted DS polyphenol against E. coli and S. aureus was determined using a broth-micro dilution method and spectrophotometric assay. MICs were determined in sterile 96 well round bottomed polystyrene microtitre plates (Corning Costar Ltd, New York, NY, USA) in accordance to methods of the Clinical and Laboratory Standards Institute [CLSI] (2012) MIC was determined by serial dilution (5–50 mg/mL in increments of 5 mg/mL). Bacterial inoculum corresponding to 0.5 McFarland standard of pre-culture [16 h at 37° C and equivalent to 106 colony forming units (CFU)] was added to test samples at each concentration. Samples were measured in triplicate. Plates were incubated at 37°C for 24 h and turbidity was measured spectrophotometrically at 650 nm in a plate reader (SPECTROstar Nano, BMG Labtech). The MBC was assessed in accordance to Clinical and Laboratory Standards Institute [CLSI] (2012) standards whereby those wells described for the MIC above, showing no apparent growth were streaked onto nutrient agar (NA) (Fluka). Plates were incubated overnight at 37°C, the plates with the lowest concentration of DS and DS polyphenol sample showing no growth following incubation overnight was recorded as the MBC. Tetracycline was used as an antibiotic control with a stock concentration of 33 μg/ml.
Measurement of H2O2 Concentration
The generation of hydrogen peroxide in NB medium without bacterial cells (cell free medium) after the addition of DS, DS polyphenols, or artificial DS for 1 h at 37°C was measured by the ferrous ion oxidation-xylenol orange (FOX) assay as described by Packer and Sies (2001), and Maeta et al. (2007). DS was prepared fresh in NB medium corresponding to concentrations sub-lethal (15 mg/mL) and lethal (30 mg/mL) to bacteria as identified in MIC studies. DS polyphenols and artificial DS were prepared at concentrations of 30 mg/mL to investigate their independent effect on H2O2 production. A working FOX reagent was prepared from two separate reagents; reagent 1 consisting of 4.4 mM BHT in methanol and reagent 2 compromised of 1 mM xylenol orange and 2.56 mM ammonium ferrous sulfate in 250 mM H2SO4, reagents were prepared fresh daily for each assay.
Samples of DS, extracted DS polyphenols or artificial DS (90 μl) were mixed with 10 μl of methanol, vortexed, and left to incubate at room temperature for 30 min, 900 μl of working FOX reagent was added to each sample assayed in triplicate and incubated for another 30 min followed by centrifugation at 15,000 g for 10 min. Absorbance was read at 560 nm against a methanol blank containing the necessary amount of sample to correct for background associated with sample. The FOX assay was calibrated using standard H2O2, diluted from stock (500 μM) and its concentration assessed using molar extinction coefficient of 43 M-1 cm-1 at 240 nm.
Analysis of Bacterial Survival
Escherichia coli and S. aureus were cultured in NB medium at 37°C for 16 h in accordance to Clinical and Laboratory Standards Institute [CLSI] (2012) standards, DS, DS polyphenols, or artificial DS with or without 100 U/mL catalase or H2O2 (1 mmol/L) were added to NB medium and allowed to equilibrate for 4 h. This was followed by inoculation with bacteria corresponding to 106 CFU/mL (0.5 McFarland). After incubation at 37°C for 4 h with shaking, cells were diluted (10-1–10-8) and enumerated using the surface drop count method to determine CFU.
Statistical Analysis
All data were expressed as mean ± SD of independent triplicates unless otherwise stated. One way ANOVA with Tukey’s post hoc analysis was used for multiple comparisons within groups of normally distributed data Statistical analysis was performed using GraphPad Prism® Version 6 software and results were significant at p < 0.05 and p < 0.01.
Results
The antimicrobial activity of DS might be associated with the presence of antioxidative compounds in DS that possess bioactive behavior. It was hypothesized that the phytochemical compounds present in DS may be involved in redox reactions mediated by the production of H2O2 that results in bacterial inhibition providing justification for DS’s traditional medicinal application.
Determination of DS Antioxidant Behavior
The determination of secondary metabolites as antioxidants is outlined in Table 1. In comparison to previous literature investigating date fruit and DS (Al-Farsi et al., 2007; Dhaouadi et al., 2010; Abbès et al., 2013) the results are in agreement that DS has sufficient secondary metabolites that are typically associated with bioactive behavior and radical scavenging (Vayalil, 2012).
Table 1.
Antioxidant determination of date syrup (DS).
| DS (mg/100 g) | |
|---|---|
| Total phenol content | 605.1 ± 31.6 |
| Tannins | 357.4 ± 18.7 |
| Flavanoids | 40.5 ± 28.9 |
| Flavanols | 31.7 ± 8.6 |
| Anthocyanins | 6.63 ± 1.9 |
| Carotenoids | 1.59 ± 0.1 |
Results are expressed as means ± SD mg/100g of fresh DS weight.
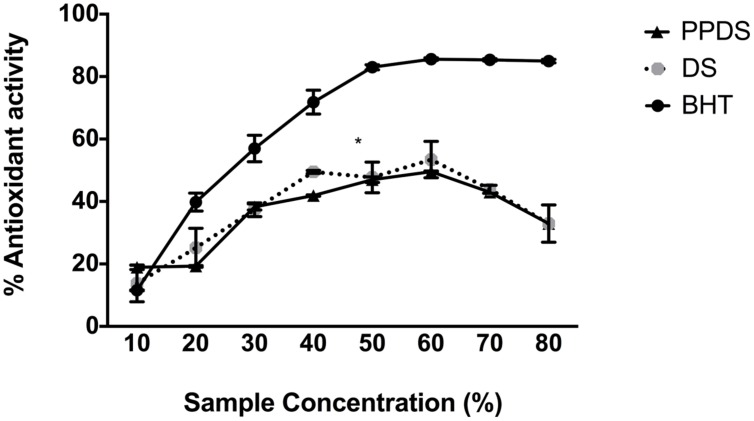
As the concentration of DS and DS polyphenols (PPDS) increases, so does the percentage antioxidant behavior, which is a demonstration of the free radical scavenging activity (Figure 1). However, this antioxidant behavior was only evident up until a concentration of 60-70% DS, therefore at a greater concentration of DS, the antioxidant power began to decline.
FIGURE 1.
Comparison of the antioxidant potential of date syrup (DS) (round gray scale dot) and extracted DS polyphenol (PPDS) (black triangle) against the commercially available antioxidant butylated hydroxytuolene (BHT) (black dot). Results are expressed as mean ± SD of three independent experiments and significant difference between DS treatments and BHT is indicated as ∗p < 0.05.
Antibacterial Susceptibility Testing
Date syrup and extracted DS polyphenols were investigated for their antibacterial activity. The bacteriostatic activity of DS and DS polyphenols was tested against E. coli and S. aureus and represented as the MIC. The MIC of DS and DS polyphenols is outlined in Table 2. DS’s MIC for the tested bacteria was determined at 30 mg/mL. For extracted DS polyphenols, the MIC was 30 mg/mL for E. coli and 20 mg/mL for S. aureus, these results are not significantly different (p < 0.05) from DS’s MIC suggesting that both DS and DS polyphenols exert the same effect in retarding bacterial growth.
Table 2.
Minimum inhibitory concentration (MIC) and Minimum bactericidal concentration (MBC) of DS, and extracted DS polyphenols necessary to inhibit microbial growth in vitro expressed in mg/mL.
| Microorganism | DS mg/mL (SD) |
DS polyphenols mg/mL (SD) |
||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| Escherichia coli | 30 (±0.83) | 40 (±0.97)∗ | 30 (±0.11) | 32 (±0.73)∗ |
| Staphylococcus aureus | 30 (±0.76) | 35 (±0.54)∗ | 20 (±0.82) | 23 (±0.94)∗ |
Mean and SD of results are expressed as three independent experiments in triplicates. Significant differences between each treatment and microorganism indicated as ∗p < 0.05.
Furthermore, it was found that treatment of both E. coli and S. aureus with the different DS treatments using concentrations of DS corresponding to sub-MIC (15 mg/mL), above MIC (30 mg/mL), extracted DS polyphenol (PPDS) and a concentration of artificial DS sugar (consisting of 7.6% w/v sucrose, 46.13% w/v fructose, and 46.3% w/v glucose) (Sugar) significantly decreased the survival rates as represented in Figure 2.
FIGURE 2.
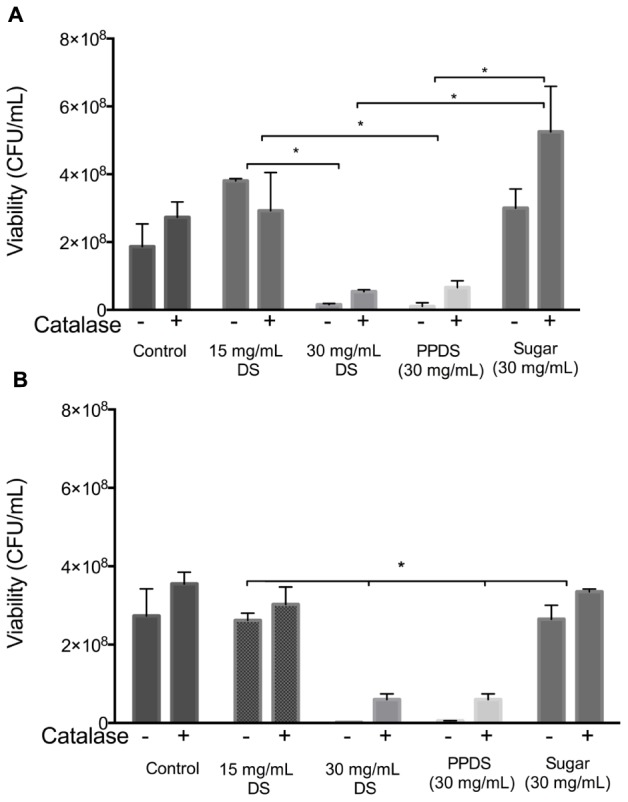
Inhibitory effects of catalase on the antibacterial action of DS. DS treatments; DS, extracted DS polyphenols (PPDS) and artificial DS sugar (Sugar) were added to (A) Escherichia coli and (B) Staphylococcus aureus cell suspensions with or without 100 U/mL catalase. After being incubated at 37°C for 4 h with shaking, cell viability was determined using surface drop count methods and expressed as viability in colony forming units (CFU). Data is mean ± SD of three independent experiments. Significant differences between treatment groups are indicated as ∗p < 0.05.
To assess whether extracted DS polyphenol (PPDS) derived hydrogen peroxide was responsible for the suppression of E. coli and S. aureus growth, the effect of catalase on the antibacterial activity of the different DS treatments including extracted DS polyphenol and artificial DS sugar was examined. The addition of 100U/mL catalase restored the growth of E. coli significantly (p < 0.05) and S. aureus medium containing different DS treatments as outlined in Figure 2. This suggests that H2O2 mediates the antibacterial activity of DS.
Hydrogen Peroxide Mediates the Antimicrobial Action of DS
To obtain evidence that H2O2 is generated by DS, the hydrogen peroxide production was determined in NB medium at concentrations of DS corresponding to 15 mg/mL DS, 30 mg/mL DS, extracted DS polyphenol (PPDS) and a concentration of artificial DS sugar (Sugar) corresponding to the MIC, this was achieved by the FOX method an assay sensitive to hydrogen peroxide production by measuring the formation of a complex between xylenol orange and ferric ion as identified in Figure 3. The addition of 100 U/mL catalase on the hydrogen peroxide activity of DS was also further investigated, the enzyme catalase quenches the generation of H2O2 and the addition of catalase significantly (p < 0.05) decreased the hydrogen peroxide generated.
FIGURE 3.
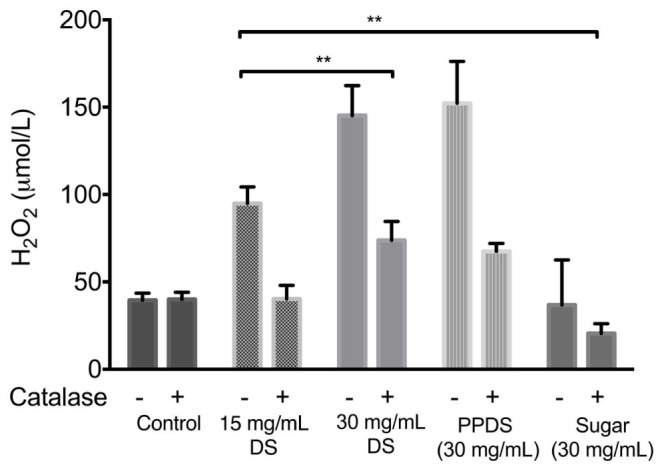
H2O2 generation by different sample treatments of DS, extracted DS polyphenols (PPDS), and artificial DS sugar (Sugar) and the effect of 100 U/mL catalase on the production of hydrogen peroxide by DS. The concentration of H2O2 in the medium was immediately determined by the FOX method 1 h after the addition of different DS treatments to NB medium (pH7.5). Data is mean ± SD of three independent experiments. Significant differences between treatment groups are indicated as ∗∗p < 0.01.
The levels of H2O2 increased significantly as the concentration of DS increased, this was also evident with extracted DS polyphenols. This demonstrated that the addition of catalase had an effect on hydrogen peroxide activity regardless of DS treatment and concentration, and this effect was further corroborated (Figures 2A,B) with the addition of catalase in the presence of bacteria. DS sugar appears to generate the least hydrogen peroxide and is influenced least by catalase activity suggesting no direct effect in DS’s antimicrobial activity in inhibiting E. coli and S. aureus.
When E. coli cells were treated with hydrogen peroxide, the addition of 15 mg/mL concentration of DS appeared to behave as an antioxidant, as outlined in Figure 4, indicating that this concentration of DS in conjunction with hydrogen peroxide reduced any excessive accumulation of hydrogen peroxide that would otherwise be lethal (Figure 4A). Interestingly, a 15 mg/mL concentration of DS appeared to enhance the growth of bacterial cells implying antioxidative behavior, this result was supported by previous MIC’s whereby this concentration is not inhibitory to bacteria and it is possible that the % antioxidant activity as outlined in Figure 1 is not strong enough to inhibit bacterial growth.
FIGURE 4.
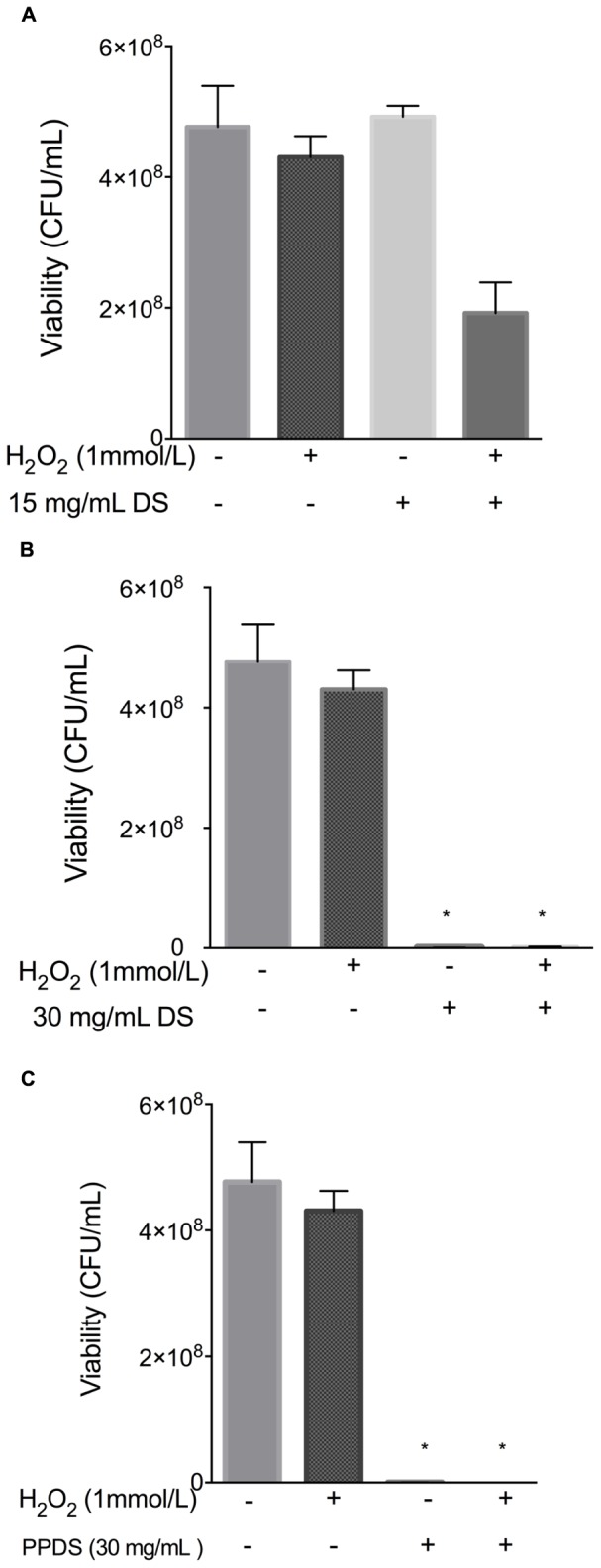
Evaluation of antioxidant activity of DS treatments. (A) 15 mg/mL DS, (B) 30 mg/mL DS, and (C) polyphenol DS (PPDS)(30 mg/mL). DS treatments were added to NB medium and incubated at 37°C with E. coli for 4 h with shaking, cell viability was determined using the surface drop count method and expressed as viability in CFU. Significant differences between treatment groups are indicated as ∗p < 0.05.
Date syrup and DS polyphenol where then further evaluated for synergistic activity with hydrogen peroxide as identified in Figure 5.
FIGURE 5.
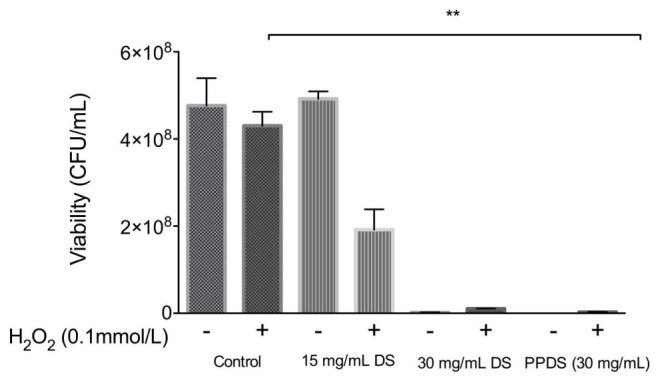
Evaluation of the synergistic effect of DS and H2O2 on cellular viability. Significant differences between treatment groups are indicated as ∗∗p < 0.01.
Date syrup and extracted DS polyphenol at the highest antioxidant activity potential, function as a prooxidants in inhibiting E. coli, whereas at a lower concentration it behaves as an antioxidant in allowing bacteria to survive, which corresponds to the MIC values.
Discussion
This study demonstrated that DS, and DS polyphenols, the most abundant bioactive constituent in DS, have antibacterial activity against the disease causing pathogens E. coli and S. aureus. The study has also shown that the extracted polyphenols retard bacterial growth and has observed that DS behaves as a prooxidant by generating hydrogen peroxide that mediates bacterial growth inhibition as a result of oxidative stress. Furthermore, low concentrations of DS demonstrated antioxidative activity by reducing hydrogen peroxide, whereas at optimal bacterial growth and weakly alkaline conditions DS demonstrated prooxidant activity that inhibited the growth of E. coli and S. aureus. The osmolarity as a result of the high sugar content naturally present in DS did not significantly contribute to this effect. These findings highlight that DS and DS polyphenols interaction with bacteria are involved in prooxidant mediated bacterial inhibition.
The determination of secondary metabolites as antioxidants is outlined in Table 1. In comparison to previous literature investigating date fruit and DS (Al-Farsi et al., 2007; Dhaouadi et al., 2010; Abbès et al., 2013), the results indicate that DS contains secondary metabolites that are associated with bioactive behavior (Vayalil, 2012).
The extent to which the bacterial growth was inhibited by DS and DS polyphenols was related to the content of redox active phenolic compounds and H2O2. These results support the assertion that the structural interaction between these bioactive compounds is responsible for growth inhibition beyond an osmotic effect of sugars alone. This offers a new possibility that redox active phenolic compound present in DS; date fruit and other antioxidant rich fruits are active intermediates contributing to microbial impairment.
Although polyphenols and individual phenolic compounds have long demonstrated antioxidant behavior, the present study has demonstrated that DS and DS polyphenols inhibit the growth of E. coli and S. aureus by generating H2O2. Furthermore, it has also been demonstrated that DS polyphenols, one of the most abundant constituents in DS, function similarly to whole DS suggesting that DS polyphenols are the major constituents contributing to DS’s antibacterial activity (Figures 2 and 3). The capacity for DS and DS polyphenols to generate H2O2 in culture medium is consistent with current literature (Nakagawa et al., 2004; Yamamoto et al., 2004; Liu et al., 2013) describing H2O2 generation in various mediums, implying that organic components of medium (such as vitamins, proteins, and inorganic salts) do not directly affect DS and DS polyphenol mediated H2O2 generation.
Critically, it was also observed that the contribution of osmolarity of DS had no significant influence on its antibacterial activity with MIC for artificial DS being higher than DS or extracted DS polyphenols (Figures 2A,B).
Polyphenols are able to inhibit microorganisms and the antimicrobial activity of polyphenols is dependent on their chemical structure and environmental conditions (Almajano et al., 2007). This study investigated whether DS and extracted DS polyphenols function as an antioxidant or as an antimicrobial. The naturally weak acidic DS (pH 5.1) at low concentrations behaved as an antioxidant and protected both E. coli and S. aureus from H2O2 induced oxidative damage, whereas at MIC concentrations DS and extracted DS polyphenols demonstrate prooxidant activity (Figures 4 and 5) thus behaving as an antimicrobial. The exact mechanism contributing to this effect remains unclear but polyphenols exist as esters of organic acids and can be readily bound to protein (Kroll et al., 2003); the interaction of polyphenols with proteins present in the bacteria result in ionic bonding and hydrogen bonding interactions (Canillac and Mourey, 2004) this will alter protein activity in the microorganism and make it more susceptible to treatment, but will also influence the antioxidant activity of polyphenols (Rawel et al., 2001, 2002).
These observations could be the result of changes to the proteins on and within the bacteria as a result of the interaction with DS and DS polyphenols, making it more susceptible to attack, and oxidative stress. Oxidants such as polyphenols cause oxidative stress and as aerobic bacteria, both E. coli and S. aureus have evolved intricate molecular mechanisms in response to oxidative stress by the activation of several stress genes (Macvanin and Hughes, 2010; Brudzynski et al., 2012).
Oxidative stress and damage is often associated with DNA damage due to the breakdown of fragments in DNA and further transcriptional changes in antioxidant associated genes such as superoxide dismutase and catalase (Brudzynski et al., 2012), which are induced and influenced by H2O2. The oxyR and perR genes control the expression of inducible forms of katG, and ahpCF genes, which function to homeostatically control the concentration of H2O2 once it becomes too high. Therefore it can be suggested that the antibacterial activity of DS mediated by hydrogen peroxide will most likely demonstrate transcriptional changes associated with antioxidant genes and oxidative stress genes.
In agreement with previous literature (Brudzynski et al., 2012; Chen et al., 2012; Liu et al., 2013) pre-treatment of DS and extracted DS polyphenols with catalase to remove H2O2 reduced the bacteriostatic activity of DS to a conservative level (Figures 2A,B), this was particularly significant between 15 and 30 mg/mL DS and was independent of the initial H2O2 concentration (Figure 3) thus suggesting that H2O2 generated as a result of DS induces antibacterial activity.
It has been recently documented that DS is an antioxidant fruit with specific compounds possessing antioxidant activity (Cadenas and Packer, 2005; Dhaouadi et al., 2010), of DS constituents the polyphenol compounds are renowned for their antioxidant behavior, Figure 1 illustrates this behavior. As shown in Figure 1 the antioxidant behavior of both DS and extracted DS polyphenols increase linearly as the concentration increases (p < 0.05). This assay revealed two particular insights; firstly, there was no significant difference between DS antioxidant activity and DS polyphenol antioxidant activity which suggests that the polyphenols in DS compromise predominantly the bioactive constituents and these bioactive compounds influence H2O2 in mediating it as an antimicrobial agent. Secondly, the increase in antioxidant behavior (activity) was observed repeatedly up until 60% (corresponding to 30 mg/mL), further supporting the role of DS and DS polyphenols as both antioxidants and prooxidants in antibacterial activity. At a concentration corresponding to 15 mg/mL, which is sub-lethal MIC, DS demonstrates antioxidative behavior signifying that it scavenges any free radicals and reduces H2O2 generated thus allowing bacterial cells to proliferate and grow. This is evident in the antibacterial results in both the presence and absence of catalase, signifying that this concentration is not lethal to bacteria implying minimal stress responses are activated by bacteria at this concentration. Previous research conducted on DS and date fruit support this finding (Procházková et al., 2011; Abbès et al., 2013; Kchaou et al., 2013; Martín-Sánchez et al., 2014).
Despite apparent antioxidative activity, this was diminished at concentrations of 60%, above 60% it acts as a prooxidant suggesting high concentration of DS and DS polyphenols are required to achieve prooxidant mediated bacterial inhibition. It is possible that the prooxidant activity and subsequent H2O2 generation are affiliated with the presence of metal ions. The co-incubation of bacteria with DS polyphenols may disrupt bacterial respiration by sequestering metal ions leading to generation of H2O2. As a traditional medicinal application, this provides a preliminary scientific basis for DS’s medicinal use as an antimicrobial agent and it’s potential for future bacterial infection treatment. This observation is supported by previous literature highlighting the closely related relationship in polyphenols behaving as prooxidants and antioxidants, suggesting that dietary polyphenols exhibit both antioxidative and prooxidative properties under certain conditions such as pH, metal reducing potential, solubility and a natural defense in response to attack (Sakihama et al., 2002; Perron and Brumaghim, 2009; Procházková et al., 2011). This implies that prooxidant environment is beneficial, since, by imposing a mild degree of oxidative stress, the levels of antioxidant defenses and xenobiotic-metabolizing enzymes might be raised, leading to protection through cytotoxicity in inhibiting microorganisms (Halliwell, 2008).
Conclusion
It has been demonstrated for the first time that DS and DS polyphenols are able to inhibit Gram negative E. coli and Gram positive S. aureus by generating H2O2, and that DS polyphenols are active intermediates directly involved in inducing oxidative stress in bacteria as a result of hydrogen peroxide generation. These results confirm the critical relationship between antioxidants and prooxidants of DS polyphenols in bacterial growth and bacterial inhibition. It has also been shown that the high content of naturally occurring sugars in DS do not significantly contribute to its antibacterial activity. These results confirm the critical role of the relationship of antioxidants and prooxidants of DS polyphenols in bacterial inhibition and as an antimicrobial agent.
Author Contributions
HT, SM, RM, and AK conceived of the experimental ideas and wrote the paper; HT carried out the experiments.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Dr. Sam Hooper for his technical support and assistance throughout this investigation.
References
- Abbès F., Kchaou W., Blecker C., Ongena M., Lognay G., Attia H., et al. (2013). Effect of processing conditions on phenolic compounds and antioxidant properties of date syrup. Ind. Crops Prod. 44 634–642. 10.1016/j.indcrop.2012.09.008 [DOI] [Google Scholar]
- Aboubacar A., Hashim I. B., Afifi H. S. (2010). “Quality characteristics of muffin containing date syrup as sweetener,” in Poster Presented in Fourth International Date Palm Conference (FIDPC), Abu Dhabi, 15–17. [Google Scholar]
- Al-Farsi M., Alasalvar C., Al-Abid M., Al-Shoaily K., Al-Amry M., Al-Rawahy F. (2007). Compositional and functional characteristics of dates, syrups, and their by-products. Food Chem. 104 943–947. 10.1016/j.foodchem.2006.12.051 [DOI] [Google Scholar]
- Al-Farsi M., Alasalvar C., Morris A., Baron M., Shahidi F. (2005). Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J. Agric. Food Chem. 53 7592–7599. 10.1021/jf050578y [DOI] [PubMed] [Google Scholar]
- Almajano M. P., Carbo R., Delgado M. E., Gordon M. H. (2007). Effect of pH on the antimicrobial activity and oxidative stability of oil-in-water emulsions containing caffeic acid. J. Food Sci. 72 C258–C263. 10.1111/j.1750-3841.2007.00387.x [DOI] [PubMed] [Google Scholar]
- Al-Qarawi A. A., Ali B. H., Al-Mougy S. A., Mousa H. M. (2003). Gastrointestinal transit in mice treated with various extracts of date (Phoenix dactylifera L.). Food Chem. Toxicol. 41 37–39. 10.1016/S0278-6915(02)00203-X [DOI] [PubMed] [Google Scholar]
- Bauza E. (2002). Date palm kernel extract exhibits antiaging properties and significantly reduces skin wrinkles. Int. J. Tissue React. 24 131–136. [PubMed] [Google Scholar]
- Brudzynski K., Abubaker K., Miotto D. (2012). Unraveling a mechanism of honey antibacterial action: polyphenol/H2O2-induced oxidative effect on bacterial cell growth and on DNA degradation. Food Chem. 133 329–336. 10.1016/j.foodchem.2012.01.035 [DOI] [PubMed] [Google Scholar]
- Brudzynski K., Abubaker K., St-Martin L., Castle A. (2011). Re-examining the role of hydrogen peroxide in bacteriostatic and bactericidal activities of honey. Front. Microbiol. 2:213 10.3389/fmicb.2011.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E., Packer L. (2005). Handbook of Antioxidants. Los Angeles, CA: Marcel Dekker Inc. [Google Scholar]
- Canillac N., Mourey A. (2004). Effects of several environmental factors on the anti-Listeria monocytogenes activity of an essential oil of Picea excelsa. Int. J. Food Microbiol. 92 95–103. 10.1016/j.ijfoodmicro.2003.09.001 [DOI] [PubMed] [Google Scholar]
- Chen C., Campbell L. T., Blair S. E., Carter D. A. (2012). The effect of standard heat and filtration processing procedures on antimicrobial activity and hydrogen peroxide levels in honey. Front. Microbiol. 3:265 10.3389/fmicb.2012.00265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute [CLSI] (2012). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard: CLSI document M07-A 9, 9th Edn. Wayne, PA: Clinical Laboratory Standards Institute [CLSI]. [Google Scholar]
- Cowan M. M. (1999). Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12 564–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daglia M. (2012). Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 23 174–181. 10.1016/j.copbio.2011.08.007 [DOI] [PubMed] [Google Scholar]
- De R., Kundu P., Swarnakar S., Ramamurthy T., Chowdhury A., Nair G. B., et al. (2009). Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob. Agents Chemother. 53 1592–1597. 10.1128/AAC.01242-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaouadi K., Raboudi F., Estevan C., Barrajón E., Vilanova E., Hamdaoui M., et al. (2010). Cell viability effects and antioxidant and antimicrobial activities of Tunisian date syrup (Rub El Tamer) polyphenolic extracts. J. Agric. Food Chem. 59 402–406. 10.1021/jf103388m [DOI] [PubMed] [Google Scholar]
- Giusti M. M., Wrolstad R. E. (2001). “UNIT F1.2 Characterization and measurement of anthocyanins by UV-visible spectroscopy,” in Current Protocols in Food Analytical Chemistry, ed. Wrolstad R. E. (New York, NY: Wiley; ). [Google Scholar]
- Guo C., Yang J., Wei J., Li Y., Xu J., Jiang Y. (2003). Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 23 1719–1726. 10.1155/2010/871379 [DOI] [Google Scholar]
- Hagerman A., Harvey-Mueller I., Makkar H. P. S. (2000). Quantification of Tannins in Tree Foliage–A Laboratory Manual. Vienna: Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, 4–7. [Google Scholar]
- Halliwell B. (2008). Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 476 107–112. 10.1016/j.abb.2008.01.028 [DOI] [PubMed] [Google Scholar]
- Jimoh F. O., Adedapo A. A., Afolayan A. J. (2010). Comparison of the nutritional value and biological activities of the acetone, methanol and water extracts of the leaves of Solanum nigrum and Leonotis leonorus. Food Chem. Toxicol. 48 964–971. 10.1016/j.fct.2010.01.007 [DOI] [PubMed] [Google Scholar]
- Kchaou W., Abbès F., Blecker C., Attia H., Besbes S. (2013). Effects of extraction solvents on phenolic contents and antioxidant activities of Tunisian date varieties (Phoenix dactylifera L.). Ind. Crops Prod. 45 262–269. 10.1016/j.indcrop.2012.12.028 [DOI] [Google Scholar]
- Kroll N. G., Rawel H. M., Rohn S. (2003). Reactions of plant phenolics with food proteins and enzymes under special consideration of covalent bonds. Food Sci. Technol. Res. 9 205–218. 10.3136/fstr.9.205 [DOI] [Google Scholar]
- Liu X., Li J., Wang Y., Li T., Zhao J., Zhang C. (2013). Green tea polyphenols function as prooxidants to inhibit Pseudomonas aeruginosa and induce the expression of oxidative stress-related genes. Folia Microbiol. 58 211–217. 10.1007/s12223-012-0198-2 [DOI] [PubMed] [Google Scholar]
- Macvanin M., Hughes D. (2010). “Assays of sensitivity of antibiotic-resistant bacteria to hydrogen peroxide and measurement of catalase activity,” in Antibiotic Resistance Protocols, 103 Edn Vol. 642 eds Gillespie S. H., McHugh T. D. (New York, NY: Humana Press; ), 95–103. [DOI] [PubMed] [Google Scholar]
- Maeta K., Nomura W., Takatsume Y., Izawa S., Inoue Y. (2007). Green tea polyphenols function as prooxidants to activate oxidative-stress-responsive transcription factors in yeasts. Appl. Environ. Microbiol. 73 572–580. 10.1128/AEM.01963-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Sánchez A. M., Cherif S., Ben-Abda J., Barber-Vallés X., Pérez-Álvarez J. Á., Sayas-Barberá E. (2014). Phytochemicals in date co-products and their antioxidant activity. Food Chem. 158 513–520. 10.1016/j.foodchem.2014.02.172 [DOI] [PubMed] [Google Scholar]
- Nakagawa H., Hasumi K., Woo J. T., Nagai K., Wachi M. (2004). Generation of hydrogen peroxide primarily contributes to the induction of Fe (II)-dependent apoptosis in Jurkat cells by (-)-epigallocatechin gallate. Carcinogenesis 25 1567–1574. 10.1093/carcin/bgh168 [DOI] [PubMed] [Google Scholar]
- Orsi G. B., Stefano L. D., Noah N. (2002). Hospital-acquired, laboratory-confirmed bloodstream infection: increased hospital stay and direct costs. Infect. Control 23 190–197. [DOI] [PubMed] [Google Scholar]
- Packer L., Sies H. (2001). Flavonoids and Other Polyphenols: Methods in Enzymology, Vol. 335 Cambridge, MA: Academic Press [Google Scholar]
- Perron N. R., Brumaghim J. L. (2009). A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 53 75–100. 10.1007/s12013-009-9043-x [DOI] [PubMed] [Google Scholar]
- Procházková D., Boušová I., Wilhelmová N. (2011). Antioxidant and prooxidant properties of flavonoids. Fitoterapia 82 513–523. 10.1016/j.fitote.2011.01.018 [DOI] [PubMed] [Google Scholar]
- Rawel H. M., Kroll J., Hohl U. C. (2001). Model studies on reactions of plant phenols with whey proteins. Nahrung 45 72–81. [DOI] [PubMed] [Google Scholar]
- Rawel H. M., Rohn S., Kruse H. P., Kroll J. (2002). Structural changes induced in bovine serum albumin by covalent attachment of chlorogenic acid. Food Chem. 78 443–455. 10.1016/S0308-8146(02)00155-3 [DOI] [Google Scholar]
- Sakihama Y., Cohen M. F., Grace S. C., Yamasaki H. (2002). Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicology 177 67–80. 10.1016/S0300-483X(02)00196-8 [DOI] [PubMed] [Google Scholar]
- Tahraoui A., El-Hilaly J., Israili Z. H., Lyoussi B. (2007). Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in south-eastern Morocco (Errachidia province). J. Ethnopharmacol. 110 105–117. 10.1016/j.jep.2006.09.011 [DOI] [PubMed] [Google Scholar]
- Talcott S. T., Howard L. R. (1999). Phenolic autoxidation is responsible for color degradation in processed carrot puree. J. Agric. Food Chem. 47 2109–2115. 10.1021/jf981134n [DOI] [PubMed] [Google Scholar]
- Vayalil P. K. (2002). Antioxidant and antimutagenic properties of aqueous extract of date fruit (Phoenix dactylifera L. Arecaceae). J. Agric. Food Chem. 50 610–617. 10.1021/jf010716t [DOI] [PubMed] [Google Scholar]
- Vayalil P. K. (2012). Date fruits (Phoenix dactylifera Linn): an emerging medicinal food. Crit. Rev. Food Sci. Nutr. 52 249–271. 10.1080/10408398.2010.499824 [DOI] [PubMed] [Google Scholar]
- Vyawahare N. S., Pujari R. R., Rajendran R., Khsirsagar A. D., Ingawale D. K., Patil M. N. (2009). Neurobehavioral effects of Phoenix dactylifera in mice. J. Young Pharm. 1 225–232. 10.4103/0975-1483.57069 [DOI] [Google Scholar]
- Yamamoto T., Lewis J., Wataha J., Dickinson D., Singh B., Bollag W. B., et al. (2004). Roles of catalase and hydrogen peroxide in green tea polyphenol-induced chemopreventive effects. J. Pharmacol. Exp. Ther. 308 317–323. 10.1124/jpet.103.058891 [DOI] [PubMed] [Google Scholar]
- Yordi E. G., Pérez E. M., Villares E. U., Matos M. J. (2012). Antioxidant and Pro-Oxidant Effects of Polyphenolic Compounds and Structure-Activity Relationship Evidence. Nutrition: INTECH Open Access Publisher. [Google Scholar]
- Zhishen J., Mengcheng T., Jianming W. (1999). The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64 555–559. 10.1016/S0308-8146(98)00102-2 [DOI] [Google Scholar]