Abstract
It is generally accepted that the current guidelines for the primary prevention of sudden arrhythmic death, which are based on ejection fraction, do not allow the optimal selection of patients with low left ventricular ejection fraction of ischemic and nonischemic etiology for implantation of a cardioverter-defibrillator. Ejection fraction alone is limited in both sensitivity and specificity. An analysis of the risk of sudden arrhythmic death with a combination of multiple tests (ejection fraction associated with one or more arrhythmic risk markers) could partially compensate for these limitations. We propose a polyparametric approach for defining the risk of sudden arrhythmic death using ejection fraction in combination with other clinical and arrhythmic risk markers (i.e. late gadolinium enhancement cardiac magnetic resonance, T-wave alternans, programmed ventricular stimulation, autonomic tone, and genetic testing) that have been validated in nonrandomized trials. In this article, we examine these approaches to identify three subsets of patients who cannot be comprehensively assessed by the current guidelines: patients with ejection fraction of 35% or less and a relatively low risk of sudden arrhythmic death despite the ejection fraction value; patients with ejection fraction of 35% or less and high competitive risk of death due to evolution of heart failure or noncardiac causes; and patients with ejection fraction between 35 and 45% with relatively high risk of sudden arrhythmic death despite the ejection fraction value.
Keywords: heart failure, implantable cardioverter-defibrillator, late gadolinium enhancement, sudden death, T-wave alternans
Introduction
The present article is partially based on the Position Paper of ‘Associazione Nazionale Medici Cardiologi Ospedalieri (ANMCO)’ by Disertori et al.,1 issued in Giornale Italiano di Cardiologia, and developed on behalf of ANMCO.
The implantable cardioverter-defibrillator (ICD) is widely utilized in clinical practice, and its efficacy in reducing sudden arrhythmic death has been proven by a number of studies.2–6 In the current international guidelines,7–10 left ventricular ejection fraction of 35% or less is the major determinant for ICD implantation for the primary prevention of sudden arrhythmic death in patients with left ventricular dysfunction of ischemic or nonischemic etiology. In these patients, even the recent European Society of Cardiology (ESC) guidelines10 do not suggest the use of markers of arrhythmic risk other than ejection fraction and New York Heart Association (NYHA) functional class. However, as a risk marker for sudden arrhythmic death, low ejection fraction has limited sensitivity and specificity.11 Most patients implanted with an ICD according to the current guidelines do not actually benefit from it3,12,13 and may suffer from side effects.6,14,15 The randomized Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT),3 published in 2005 and still implemented in the ICD guidelines, had a low rate of appropriate ICD therapy (5-year event rate of 21%). Moreover, in recent years improvements in drug treatment for heart failure and myocardial revascularization have reduced the incidence of sudden arrhythmic death in patients with low left ventricular ejection fraction.16 Consequently, currently the rate of appropriate ICD therapy is even lower. In the recently published analysis of an Israeli ICD Registry including 2349 consecutive cases,13 the rate of appropriate ICD shocks among primary prevention patients was 2.6% at 30 months of follow-up. Although the rates of appropriate ICD therapy vary widely depending mainly on patient selection and device programming,4,5,17 many patients are unlikely to benefit from ICD implantation. By contrast, many patients who are at risk of sudden arrhythmic death are not identified, because the largest population of sudden arrhythmic death patients exhibit only mildly depressed ejection fraction.18–20
Thus, identifying patients who are at risk of sudden arrhythmic death solely based on ejection fraction appears to be an oversimplified method that does not maximize the benefit of ICD therapy. However, to improve the selection of patients for ICD therapy, two important obstacles that have barred the modification of guidelines in the last 10 years have to be overcome: the wait for new randomized trials and the search for a single marker to replace ejection fraction in sudden arrhythmic death risk stratification.
Overcoming the obstacles
The wait for new randomized trials
Current indications for ICD implantation for primary prevention of sudden arrhythmic death are based on randomized studies performed in the 2000s that showed a reduction in mortality among patients undergoing ICD implantation based mainly on ejection fraction.21 Specifically, the inclusion criteria of the MADIT-II2 and SCD-HeFT3 trials have been implemented in the guidelines. No randomized studies using markers other than ejection fraction for risk stratification of sudden arrhythmic death have been published subsequently. In patients with ejection fraction of 35% or less, who are indicated for ICD therapy based on the guidelines,7,10 the lack of subsequent randomized trials is due to both ethical and economic considerations. It is infeasible for both patients and doctors to randomize subjects who are indicated for ICD therapy according to the established guidelines to receive non-ICD implantation. Moreover, in the last few years, improvements in therapy have reduced the incidence of sudden arrhythmic death in patients with low left ventricular ejection fraction.16 To reach statistical significance, compared with the studies performed in the 2000s, the randomization of many more patients would be necessary, and this approach would be economically infeasible.22 Thus, in these patients, randomized trials using markers of sudden arrhythmic death other than ejection fraction are not available, not ongoing, and unlikely to be performed in the future.
Patients with ejection fraction higher than 35% are not indicated for ICD therapy based on the current guidelines,7,10 and thus, these patients do not have ethical contraindications for inclusion in randomized trials. However, randomized trials are lacking even in this subset of patients. The DETERMINE trial23 randomized postinfarction patients with left ventricular ejection fraction higher than 35% and a left ventricular infarct mass higher than 10% to ICD or optimal medical therapy and assessed the included subjects using late gadolinium enhancement cardiac magnetic resonance (LGE-CMR) imaging with the primary endpoint of total mortality. However, this trial was stopped due to the low level of enrolment. There is only one ongoing study (REFINE-ICD; ClinicalTrial.gov Identifier NCT00673842) that is expected to randomize 1400 survivors of myocardial infarction with an ejection fraction between 36 and 50%, a positive microvolt T-wave alternans (TWA) test, and impaired heart rate turbulence (HRT) to receive ICD or optimal medical therapy with the primary outcomes of cardiac death and resuscitated cardiac arrest. However, the conclusion of the REFINE-ICD trial is not expected within the next few years.
Although randomized trials are the best analysis tools and are often advocated10,20,24 it is unlikely that they will be performed to address this issue in the near future. Thus, other research options to improve the selection of patients for ICD therapy should be adopted. For example, in the 2014 ESC guidelines on the diagnosis and management of hypertrophic cardiomyopathy,25 the majority of recommendations are based on observational cohort studies.
The polyparametric approach
Because of the complexity of the substrates that underlie sudden arrhythmic death, it is very unlikely for a single test to achieve significantly better predictive accuracy than ejection fraction. To overcome this limitation, a combination of markers could be used, combining ejection fraction evaluation with tests that investigate different arrhythmic mechanisms. Encouraging observational data on this topic obtained using multiple techniques already exists.26–28 For instance, Buxton et al.27 conducted a study of 674 patients with ischemic heart disease to correlate 25 prognostic variables with total and arrhythmic mortality. Patients with ejection fraction greater than 30% in the presence of other arrhythmic risk factors had a higher risk of sudden arrhythmic death than patients with ejection fraction of 30% or less and no other arrhythmic risk factors. Similar results were reported by Klem et al.,29 who combined ejection fraction with myocardial scar assessment by LGE-CMR. Recently, Merchant et al.30 conducted a multisite study of 3335 patients and showed significant improvement in sudden arrhythmic death risk prediction with a multimarker strategy employing ejection fraction, presence of ischemic heart disease, and TWA test result. The use of ejection fraction and TWA variables alone resulted in C-index values of 0.637 and 0.716, respectively, both significantly lower than the C-index of the multivariate model (0.817).
This finding suggests that a polyparametric approach is likely to predict the risk of sudden arrhythmic death more accurately than any individual risk marker. Therefore, it seems that a more promising option to improve sudden arrhythmic death risk stratification may be to evaluate the available data from nonrandomized studies that used a polyparametric approach to determine sudden arrhythmic death risk.
Sudden arrhythmic death risk stratification by polyparametric approaches in patients with low left ventricular ejection fraction
We propose the hypothesis that a polyparametric approach, applied if possible to the majority of patients before ICD implantation, would better define the risk of sudden arrhythmic death than any individual risk marker. In particular, the polyparametric approach could be useful to identify three subsets of patients with low left ventricular ejection fraction who cannot be thoroughly analyzed by the current guidelines.7,10 We examined polyparametric approaches using ejection fraction in combination with other clinical and arrhythmic risk markers that have been validated in nonrandomized trials.
Patients with ejection fraction 35% or less and a relatively low risk of sudden arrhythmic death
Patients with an ejection fraction of 35% or less are indicated for ICD implantation for primary prevention of sudden arrhythmic death according to the current guidelines.7,10 However, this patient subset is a heterogeneous group, with widely varying levels of sudden arrhythmic death risk. To date, the most useful techniques for identifying those at relatively low risk of sudden arrhythmic death seem to be LGE-CMR and the TWA test.
Late gadolinium enhancement cardiac magnetic resonance
Ventricular fibrosis plays an important role in the genesis of ventricular arrhythmias in patients with low left ventricular ejection fraction.31 Fibrotic tissue may constitute a substrate for ventricular arrhythmias, where the slow and heterogeneous conduction associated with fibrosis may favor the instauration of re-entrant circuits, increasing the vulnerability to ventricular tachycardia or ventricular fibrillation.32,33 Thus, the assessment of ventricular fibrosis by LGE-CMR imaging has recently been suggested as a candidate marker for sudden arrhythmic death risk stratification. Numerous studies have shown that LGE is a powerful predictor of ventricular tachyarrhythmic events both in ischemic and nonischemic cardiomyopathy patients and in patients with moderately to severely depressed ejection fraction.29,34–53
Table 1 outlines 19 studies29,34–51 (2692 patients) in which it was possible to identify an arrhythmic endpoint. All studies reported a statistically significant correlation between the presence or extent of ventricular fibrosis assessed with LGE-CMR and arrhythmic events. Moreover, in many of these studies, the negative predictive value (NPV) for sudden arrhythmic death was very high (>95%) when the single evaluation of ejection fraction was added to the evaluation of fibrosis by LGE-CMR. The largest study was published by Gulati et al.,42 who prospectively followed 472 patients with nonischemic cardiomyopathy for a median of 5.3 years. They found that the presence of midwall fibrosis was correlated with the occurrence of ventricular tachyarrhythmic events [adjusted hazard ratio (HR) 4.61; 95% confidence interval (CI): 2.75–7.74]. The combination of ventricular fibrosis with ejection fraction significantly improved risk reclassification for the arrhythmic end point (net reclassification improvement 0.29; 95% CI: 0.11–0.48). These data were also confirmed in two meta-analyses. In the meta-analysis of 1063 patients (572 with coronary artery disease and 491 with nonischemic cardiomyopathy) by Scott et al.,52 a greater extent of fibrosis as assessed by LGE-CMR was strongly associated with the occurrence of ventricular arrhythmias, with a relative risk of 4.33 (95% CI: 2.98–6.29). In the group of patients with implanted ICD, the relative risk increased to 6.22 (95% CI: 2.41–16.05). The meta-analysis of Kuruvilla et al.,53 which included 1488 patients with nonischemic cardiomyopathy, found that patients with fibrosis had an annualized risk of arrhythmic events of 6% compared with 1.2% in patients without fibrosis (P < 0.001), with an odds ratio of 5.32 (95% CI: 3.45–8.20).
Table 1.
Studies on late gadolinium enhancement cardiac magnetic resonance testing for arrhythmic risk stratification in patients with with low left ventricular ejection fraction of ischemic and nonischemic etiology
| Studies | Patients, n | AE, n | Arrhythmic end point | F-U (months) | Ejection fraction (%) | LGE-CMR patterns | Univariate analysis: HR (95% CI) | Multivariate analysis: HR (95% CI) | P |
| Studies with only ischemic cardiomyopathy patients | |||||||||
| Roes et al. (2009)34 | 91 | 18 | ICD therapya | 9 | 28 | Gray zone extent per 10-g increase | 1.56 (1.19–2.06) | 1.49 (1.01–2.20) | 0.04 |
| Scott et al. (2011)35 | 64 | 19 | ICD therapya | 19 | 30 | Transmural LGE segments, n | 1.40 (1.15–1.70) | 1.48 (1.18–1.84) | 0.001 |
| Alexandre et al. (2013)36 | 66 | 14 | ICD therapya | 42 | 23 | LGE extent per 1-g increase | 1.08 (104–1.12) | 3.15 (1.35–7.33) | <0.001 |
| Demirel et al. (2014)37 | 94 | 34 | ICD therapya, ventricular tachycardia | 65 | 32 | Peri- to core-infarct mass ratio % increase | 2.03 (1.18–3.48) | 2.01 (1.17–3.44) | 0.01 |
| Zeidan-Shwiri et al. (2015)38 | 43 | 28 | ICD therapya | 30 | 27 | Gray zone extent per 1 g increase | 1.25 (1.08–1.44) | 2.09 (1.14–3.85) | 0.0018 |
| Studies with only nonischemic cardiomyopathy patients | |||||||||
| Assomull et al. (2006)39 | 101 | 7 | Sudden death, ventricular tachycardiab | 22 | 36 | Midwall LGE presence | 5.2 (1.0–26.9) | 5.9 (1.1–32.2) | 0.04 |
| Iles et al. (2011)40 | 61 | 9 | ICD therapya | 19 | 25 | LGE presence | 25.8 (1.4–466.0)c | NR | <0.01 |
| Leyva et al. (2012)41 | 97 | 3 | Sudden death | 35 | 22 | Midwall LGE presence | 31.0 (1.5–627.8)c | NR | 0.0029 |
| Gulati et al. (2013)42 | 472 | 65 | ICD therapy, sudden death, aSDb | 64 | 37 | Midwall LGE presence | 5.24 (3.15–8.72) | 4.61 (2.75–7.74) | <0.001 |
| Neilan et al. (2013)43 | 162 | 37 | ICD therapya, sudden deathb | 29 | 26 | LGE presence | 14 (4.39–45.65) | NR | <0.0001 |
| Perazzolo et al. (2014)44 | 137 | 22 | ICD therapya, ventricular tachycardia / fibrillation, sudden death | 36 | 32 | LGE presence | 4.17 (1.56–11.2) | 3.8 (1.3–10.4) | 0.01 |
| Masci et al. (2014)45 | 228 | 8 | ICD therapy, aSD | 23 | 43 | LGE presence | 8.31 (1.66–41.55) | NR | 0.01 |
| Chimura et al. (2015)46 | 175 | 24 | ICD therapya, ventricular tachycardia / fibrillation | 61 | 28 | Both septal and lateral midwall LGE presence | 27.6 (7.18–106.3) | 23.1 (2.88–184.9) | 0.003 |
| Piers et al. (2015)47 | 87 | 28 | Ventricular tachycardia / fibrillation | 45 | 29 | Core extent per 10-g increase | 2.38 (1.34–4.22) | NR | 0.003 |
| Studies with mixed ischemic and nonischemic cardiomyopathy patients | |||||||||
| Fernandez-Armenta et al. (2012)48 | 78 (41/37)d | 9 | ICD therapya | 25 | 22 | LGE extent per 1% increase | 1.09 (1.05–1.14) | 1.1 (1.06–1.15) | <0.01 |
| Gao et al. (2012)49 | 124 (59/65)d | 18 | ICD therapya, sudden death, aSD | 21 | 26 | LGE extent per 10-g increase | 1.40 (1.21–1.62) | 1.38 (1.18–1.62) | <0.001 |
| Klem et al. (2012)29 | 137 (73/64)d | 25 | ICD therapy, MIb | 24 | 35 | LGE >5% | 4.76 (1.65–13.7) | 4.59 (1.79–11.8) | 0.004 |
| Mordi et al. (2014)50 | 157 (61/96)d | 20 | ICD therapya,b | 31 | 28 | LGE extent per 1% increase | 1.06 (1.04–1.09) | 1.04 (1.01–1.07) | 0.004 |
| Almehmadi et al. (2014)51 | 318 (149/169)d | 49 | ICD therapya, sudden death, aSD | 16 | 33 | Midwall LGE presence | 2.7 (1.5–5.0) | 2.4 (1.2–4.6) | 0.01 |
Only studies with evidence of a statistical analysis of the arrhythmic endpoint have been reported. AE, arrhythmic events; aSD, aborted sudden death; CI, confidence interval; CMR, cardiac magnetic resonance; F-U, mean follow-up; HR, hazard ratio; LGE, late gadolinium enhancement; NR, not reported.
aIncluding antitachycardia pacing.
bSecondary endpoint.
cOdds ratio (95% CI).
dIschemic/nonischemic cardiomyopathy patients.
Ventricular fibrosis was present in approximately 40% of patients with nonischemic cardiomyopathy, predominantly located within the myocardial wall (midwall fibrosis).53 By contrast, fibrosis was present in almost all ischemic cardiomyopathy patients, with a common pattern of core dense fibrosis within a heterogeneous peri-infarct zone (or gray zone) characterized by both viable and nonviable myocardium.52 In nonischemic cardiomyopathy patients, the presence/absence of fibrosis and midwall fibrosis were most widely used as indicators to differentiate patients at high versus low risk of arrhythmic events. In ischemic cardiomyopathy patients the problem is more complex: the majority of studies analyzing total LGE or gray zone extent reported a statistically significant dose-response effect for arrhythmic risk. The larger and more heterogeneous the scar was, the higher the probability of ventricular arrhythmias during follow-up. In the absence a definite cut-off value, the presence of a large versus small extent of ventricular fibrosis has been generally used as an indicator to differentiate patients at high versus low risk of arrhythmic events. However, different studies on this topic have applied a variety of analysis methods and diagnostic thresholds. Therefore, standardization of LGE-CMR could be of great importance to aid in the practical implementation of the LGE test for the stratification of ventricular arrhythmic risk.
T-wave alternans
The association of TWA with the risk of sudden arrhythmic death in ischemic and nonischemic cardiomyopathy patients is likely related to the ability of TWA to provide a quantitative assessment of the temporal and spatial heterogeneity of repolarization, which facilitates the occurrence of ventricular arrhythmias.54 The clinical utility of TWA in sudden arrhythmic death risk stratification was confirmed by numerous studies in patients with both chronic ischemic and nonischemic cardiomyopathy.30,55–63 In contrast to many studies with positive results on the utility of TWA in sudden arrhythmic death risk stratification, other studies have reported negative findings, such as the subanalysis of the SCD-HeFT study and the MASTER study.64,65 The negative results of these studies could be related in part to their methodology, because in both studies β-blocker therapy was discontinued before the test. The meta-analysis by Chan et al.66 highlighted the importance of TWA testing without discontinuing β-blocker therapy: NPV of the test with respect to sudden arrhythmic death was 98% in patients studied on a β-blocker and decreased to 91% in those studied after β-blocker discontinuation.
The Consensus Guideline by the International Society for Holter and Noninvasive Electrocardiology,54 published in 2011, stated that TWA provides valuable information regarding the risk of cardiovascular mortality and sudden arrhythmic death beyond that of standard clinical variables for cardiovascular diseases. In a recent multicenter study of 2883 patients with ischemic and nonischemic cardiomyopathy by Merchant et al.,60 the annualized risk of sudden arrhythmic death was 0.4% in patients with a negative TWA test result (0.9% in the subgroup of 1004 patients with ejection fraction ≤35%). Moreover, all meta-analyses66–71 have confirmed the predictive value of TWA with respect to ventricular arrhythmic events with a high NPV, thus allowing the identification of patients with a relatively low risk of sudden arrhythmic death (Table 2).
Table 2.
Meta-analyses on microvolt T-wave alternans testing for arrhythmic risk stratification in patients with with low left ventricular ejection fraction of ischemic and nonischemic etiology
| Meta-analysis | Studies, n | Patients, n | Relative risk (95% CI) | P | NPV (%) |
| Studies with only ischemic cardiomyopathy patients | |||||
| Chen et al. (2013)70 | 7 | 3385 | 1.65 (1.32–2.07) | <0.001 | NR |
| Studies with only nonischemic cardiomyopathy patients | |||||
| Golberger et al. (2014)71 | 12 | 1631 | 3.25 (2.04–5.16) | <0.001 | 97 |
| Studies with mixed ischemic and nonischemic cardiomyopathy patients | |||||
| Gehi et al. (2005)67 | 19 | 2608 | 3.77 (2.39–5.55) | NR | 97 |
| Chan et al. (2010)66 | 9 | 3939 | 1.95 (1.29–2.96) | 0.002 | NR |
| Calò et al. (2011)68 | 15 | 5681 | 2.40 (1.54–3.74) | NR | 95 |
| Gupta et al. (2012)69 | 20 | 5945 | 3.68 (2.23–6.07) | NR | 96 |
| Studies in which β-blockers were administered | |||||
| Chan et al. (2010)66 | 4 | 1277 | 5.39 (2.68–10.84) | <0.001 | 98 |
| Studies in which β-blockers were withheld | |||||
| Chan et al. (2010)66 | 5 | 2662 | 1.40 (1.06–1.84) | 0.02 | 91 |
CI, confidence interval; NPV, negative predictive value; NR, not reported.
Synthesis
A subset of patients has a relatively low risk of sudden arrhythmic death despite having an ejection fraction of 35% or less (approximately 1–2% annualized risk of sudden arrhythmic death), who could be identified by LGE-CMR or TWA tests (Figs 1 and 2). In these patients, the appropriateness of ICD implantation could be discussed because of their lower chance to gain a meaningful benefit from ICD therapy, in spite of the exposition to its side effects.
Fig. 1.
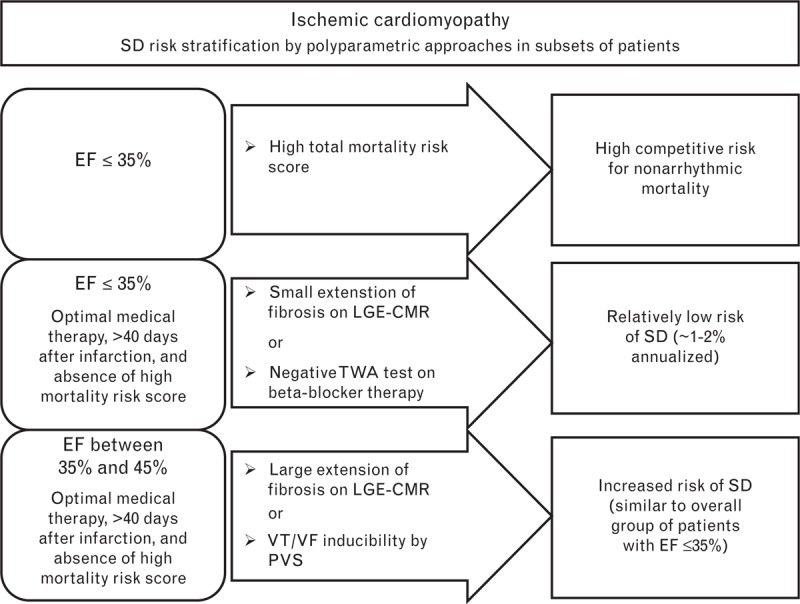
Sudden arrhythmic death risk stratification in patients with ischemic cardiomyopathy. The risk of sudden arrhythmic death was stratified according to ejection fraction, total mortality risk score, and the results of specific tests: late gadolinium enhancement cardiac magnetic resonance imaging (LGE-CMR) or the T-wave alternans (TWA) test in patients with ejection fraction of 35% or less, and LGE-CMR or programmed ventricular stimulation (PVS) in patients with ejection fraction between 35 and 45%. In ischemic cardiomyopathy, LGE is present in almost all patients; negative and positive LGE-CMR results are related to the presence of a small and large extent of ventricular fibrosis, respectively. The TWA test is considered negative only if it is performed under β-blocker therapy. The PVS test is considered positive if sustained ventricular tachycardia or ventricular fibrillation is inducible. EF, ejection fraction; LGE-CMR, late gadolinium enhancement cardiac magnetic resonance imaging; PVS, programmed ventricular stimulation; SD, sudden arrhythmic death; TWA, T-wave alternans; VF, ventricular fibrillation; VT, ventricular tachycardia.
Fig. 2.
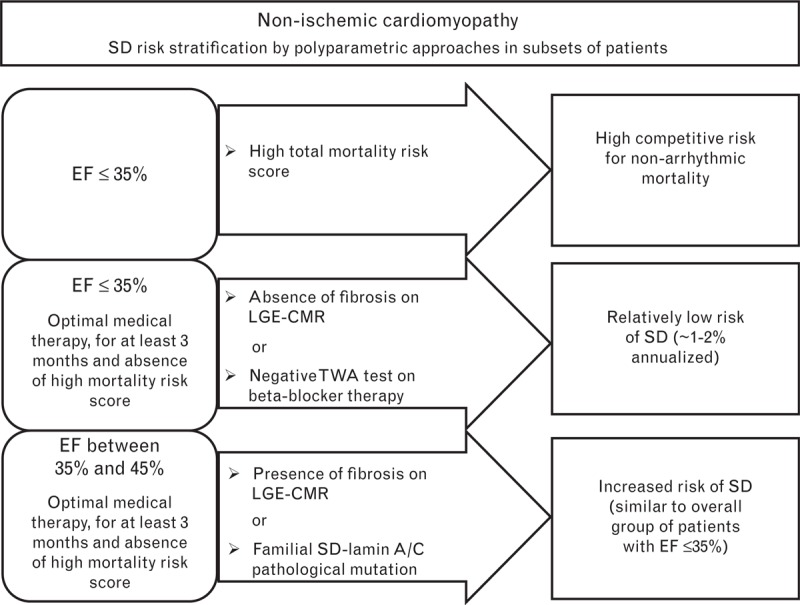
Sudden arrhythmic death risk stratification in patients with nonischemic cardiomyopathy. The risk of sudden arrhythmic death was stratified according to ejection fraction, risk score of total mortality, and the results of specific tests: late gadolinium enhancement cardiac magnetic resonance imaging (LGE-CMR) or the T-wave alternans (TWA) test in patients with ejection fraction of 35% or less, and LGE-CMR in patients with ejection fraction between 35 and 45%. In nonischemic cardiomyopathy, negative and positive LGE-CMR results are defined as the absence and presence of LGE, respectively. The TWA test is considered negative only if it is performed under β-blocker therapy. A genetic test is proposed in cases of familial dilated cardiomyopathy, in particular for the identification of a family history of sudden arrhythmic death and for the identification of a pathological mutation in lamin A/C, which both select patients at high risk of sudden arrhythmic death, even in the presence of only a moderately impaired ejection fraction. EF, ejection fraction; LGE-CMR, late gadolinium enhancement cardiac magnetic resonance imaging; SD, sudden arrhythmic death; TWA, T-wave alternans.
Patients with ejection fraction 35% or less and high competitive risk of death due to evolution of heart failure or noncardiac causes
Among patients with low left ventricular ejection fraction, a subset has a high risk of death due to heart failure evolution or noncardiac conditions. Existing guidelines do not recommend ICD implantation in patients with NYHA functional class IV, high 1-year total mortality, or relevant comorbidities.7,10 However, these criteria seem too generic to identify patients who are ‘too sick’ for ICD implantation. Therefore, a polyparametric approach was suggested to develop some risk prediction scores for total mortality. Prognostic scores allow an objective evaluation of patient risk, but for several reasons (historical cohorts with different treatments, heterogeneous methodological approach, case selection) lack optimal discriminatory power and calibration in real-world applications.72 Nevertheless, increasing age, comorbidity burden, and life expectancy should be taken into account in the decision-making process for ICD implantation. The frequent finding of 1-year mortality greater than 20–25% in elderly patients with multiple comorbidities, with a proportion of sudden arrhythmic death usually <20–25%, is a convincing illustration of the general lack of efficacy of ICD implantation in this cohort of patients.72–75
The MADIT-II trial provided a classic example of this subset of patients: a posthoc analysis of the presence or absence of five risk factors associated with ejection fraction (NYHA class >II, age >70 years, blood urea nitrogen >26 mg/dl, QRS duration >0.120 s, and atrial fibrillation) demonstrated that it was possible to identify patients with largely different mortality risk. In patients with a score of at least 3 using this polyparametric approach, no benefit of ICD implantation was observed, even over a prolonged follow-up period of up to 8 years.74
Many other scores reflecting risk of death have been developed.73,75–80 Levy et al.73 applied the Seattle Heart Failure Model score, previously tested in patients with heart failure, to patients of the SCD-HeFT trial. They found that patients with an annual risk of death more than 20% did not receive any benefit from ICD implantation. Recently, Zhang et al.78 performed the prospective PROSE-ICD trial in a population of 1189 patients and validated a score based on six clinical parameters (age ≥75 years, NYHA class III/IV, atrial fibrillation, glomerular filtration rate <30 ml/min, diabetes, and use of diuretics) in addition to three biomarkers (tumour necrosis factor α receptor II, pro–brain natriuretic peptide and cardiac troponin T), which allowed the identification of patients with a high probability of early total mortality [area under the curve for prediction of 1-year mortality 0.82 (95% CI: 0.76–0.88)] but not of sudden arrhythmic death. Thus, the score was able to identify patients with a low probability of receiving a benefit from ICD therapy (those with a score >4) with high accuracy. Senni et al.77 validated another score in a cohort of 6274 patients with heart failure treated at 24 European Departments of Cardiology and Internal Medicine. In this study, in addition to age and severity of heart failure (blood pressure, NYHA class, and ejection fraction), the presence of aortic stenosis, atrial fibrillation, prescription of validated drugs for heart failure, and a number of comorbidities were combined to form the prognostic score. In patients identified by the score with an annualized mortality risk of at least 20%, the efficacy of ICD therapy was not clear.
Synthesis
Determining what risk score to use and what cut-off points should be applied is challenging. Nevertheless, it would be useful to obtain a score of total mortality (better defined by a multidisciplinary group) in the majority of patients prior to ICD implantation for primary prevention of sudden arrhythmic death, and possibly discuss the appropriateness of ICD therapy in those with a high score for nonarrhythmic death (Figs 1 and 2).
Patients with ejection fraction between 35 and 45% and a relatively high risk of sudden arrhythmic death
Patients with an ejection fraction between 35 and 45% do not have an indication for ICD implantation for primary prevention of sudden arrhythmic death according to the current guidelines.7,10 However, this patient subset is also a heterogeneous group, with largely different levels of sudden arrhythmic death risk. To date, the most useful techniques for identifying patients at relatively high risk of sudden arrhythmic death seem to be LGE-CMR, programmed ventricular stimulation (PVS), autonomic tone, and genetic testing.
Late gadolinium enhancement cardiac magnetic resonance
Based on the data previously reported in the text and Table 1, nonischemic cardiomyopathy patients with the presence of ventricular fibrosis confirmed by LGE-CMR and ischemic cardiomyopathy patients with a large fibrotic area could represent a subset of patients at relatively high risk of sudden arrhythmic death, despite having an ejection fraction between 35 and 45%.
Programmed ventricular stimulation
In patients with postinfarction cardiomyopathy, the inducibility of ventricular tachyarrhythmias by PVS seems to be related to the presence of a reentry circuit.81,82 Based on the results of the MUSTT trial83,84 and subsequent studies,58,85,86 the predictive ability of PVS with respect to sudden arrhythmic death was particularly high in patients with an ejection fraction between 30 and 40%. The ACCF/AHA/HRS 2012 guidelines for device-based treatment of cardiac rhythm abnormalities7 recommend ICD implantation (Class I recommendation, level of evidence B) in chronic postinfarction patients with an ejection fraction of 40% or less, nonsustained ventricular tachycardia, and inducibility of sustained ventricular tachyarrhythmias by PVS. Unfortunately, the clinical use of PVS is progressively declining.86
Autonomic tone
Numerous clinical observations and experimental studies have shown that alterations in the autonomic nervous system and in particular sympathetic activation and reduced vagal modulation have an important proarrhythmic effect and may facilitate the onset of ventricular tachycardia/fibrillation, in particular in ischemic heart disease.20,87 Even in the investigation of autonomic tone, the usefulness of the combination of more tests has been confirmed in several studies.88–91 In the REFINE study,88 322 patients with ischemic heart disease were studied at different intervals from infarction (2–4 weeks and 10–14 weeks). In the analysis carried out at 10–14 weeks after infarction, the predictive value of depressed HRT (HR 2.91; 95% CI: 1.13–7.48) increased if the test was associated with an abnormal TWA test (HR 4.18; 95% CI: 2.06–8.32) with respect to a combined endpoint of cardiac death and arrhythmic events. The further addition of ejection fraction of less than 50% increased the HR to 6.22 (95% CI: 2.88–13.42). The ongoing REFINE-ICD study described above was designed on the basis of these results.
In patients with moderately depressed ejection fraction, reduced HRT (especially when combined with a positive TWA test) appears to identify a subgroup of patients at relatively high risk of sudden arrhythmic death. However, it may be appropriate to await the results of the randomized REFINE-ICD trial before using HRT for the evaluation of possible ICD implantation.
Genetic testing
The combination of ejection fraction with genetic analysis can also contribute to sudden arrhythmic death risk stratification.92,93 Both familial history and genotyping may aid in diagnostic and prognostic classification in patients with familial dilated cardiomyopathy, particularly for the identification of a family history of sudden arrhythmic death8,94 and the identification of a pathological mutation in lamin A/C,95–98 which both indicate patients at high risk of sudden arrhythmic death even in the presence of only a moderately depressed ejection fraction (Fig. 2). In the recent ESC guidelines,10 an ICD should be considered (Class IIa recommendation, level of evidence B) in patients with dilated cardiomyopathy, a confirmed disease-causing lamin A/C mutation, and clinical risk factors (nonsustained ventricular tachycardia, ejection fraction <45%, male sex, and nonmissense mutations).
Synthesis
Despite having an ejection fraction between 35 and 45%, a subset of patients has a relatively high risk of sudden arrhythmic death similar to the overall group of patients with ejection fraction of 35 or less and could be identified by LGE-CMR in cases of both ischemic and nonischemic cardiomyopathy, and by PVS in ischemic cardiomyopathy (Figs 1 and 2). Moreover, the combination of ejection fraction with genetic testing in nonischemic cardiomyopathy patients could contribute to identifying those with a relatively high risk of sudden arrhythmic death. In patients with a relatively high arrhythmic risk, ICD therapy could be critically evaluated.
Clinical considerations
In the described subsets of patients, until a more thorough evaluation method is included in the guidelines for sudden arrhythmic death risk stratification, the choice of ICD therapy could be critically evaluated using a polyparametric analysis, as outlined above. The conclusions and uncertainties resulting from the polyparametric analysis could be discussed with the patient to allow a truly ‘informed’ consensus. The emerging concept of ‘sharing the work’ or ‘healthcare co-production,’ which involves discussion between the patient and doctor, could be used in ICD therapy decisions.
In addition, it is important to recognize the side effects of ICD implantation, which are not trivial. In a recently published registry of patients who underwent ICD (n = 1729) or CRT-D (n = 1326),6 the 12-year cumulative incidence of adverse events was 20% (95% CI: 18–22%) for inappropriate shock, 6% (95% CI: 5–8%) for device-related infection, and 17% (95% CI: 14–21%) for lead failure. In the Danish registry,14 complications following implantable electronic device treatment were more frequent than generally acknowledged: at 6 months, major complications occurred in 5.6% (95% CI: 5.0–6.1%) of patients, and any complication (major and minor) in 9.5% (95% CI: 8.7–10.2%). Both patient- (particularly female, age, and underweight) and procedure-related predictors (complexity and annual center volume of procedures) may identify patients at particularly high risk of complications.14 This information should be taken into account when establishing individual patient treatment plans, but it is not included in our algorithm, which is mainly aimed at arrhythmic risk stratification. Recently a pooled analysis of IDE study and EFFORTLESS Registry provided evidence for the safety and efficacy of the totally subcutaneous implantable defibrillator.99 This technological innovation, that is less affected by transvenous technology-related complications, will help address some of the current problems regarding ICD therapy, but it is unlikely to significantly affect clinical indications. In addition, ICD is an expensive device that requires frequent follow-up monitoring. Although not all data are in agreement,100 ICD implantation following the current guidelines is considered cost-effective.101 However, if a more complex strategy of sudden arrhythmic death risk stratification could improve the appropriateness of ICD implantation, it would lead to a further significant advantage in both cost/benefit and risk/benefit ratios. The modest increase in costs due to a polyparametric approach to risk stratification would be largely outweighed by the improvement in the appropriateness of ICD implantation.
Finally, it should be recognized that the definition of risk is not static but, rather, continues to change over time, necessitating periodic reassessment to determine significant variations in the patient's clinical status. No single arrhythmic risk stratification test should be considered permanently valid. However, the exact timing of the periodic reassessment of such tests is not known and will be varying according to not only the clinical aspects of the patient but also the complexity, availability, biological impact, and economic costs of the method used.
This point-of-view article does not intend to question actual guidelines for sudden arrhythmic death risk stratification, but rather to highlight the potential additive value of a new polyparametric approach to the risk stratification of specific groups of patients with low left ventricular ejection fraction, supported by highly significant published data derived from nonrandomized studies. We believe that this working hypothesis to aid in the decision-making process could be appropriate and implemented in the future. In the absence of randomized trials, the polyparametric approach could be further investigated by prospective trials, high-quality registries, and eventually by the new system of performing registry-based randomized trials that may be another alternative to obtaining high-quality clinical data at lower cost.102
Acknowledgement
This paper is partially based on a prior publication in Giornale Italiano di Cardiologia, 2015: 16: 651-666 (http://www.giornaledicardiologia.it/articoli.php?archivio=yes&vol_id=2066&id=22442), and is reproduced with the kind permission of the publisher Il Pensiero Scientifico Editore.
There are no conflicts of interest.
References
- 1.Disertori M, Gulizia MM, Casolo G, et al. Remarks on polyparametric assessment of sudden death risk for primary prevention ICD implantation in patients with left ventricular dysfunction of ischemic and non ischemic etiology. Italian Association of Hospital Cardiologists (ANMCO) Experts Position Paper. G Ital Cardiol 2015; 16:651–666. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883. [DOI] [PubMed] [Google Scholar]
- 3.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352:225–237. [DOI] [PubMed] [Google Scholar]
- 4.Al-Khatib SM, Hellkamp A, Bardy GH, et al. Survival of patients receiving a primary prevention implantable cardioverter-defibrillator in clinical practice vs clinical trials. JAMA 2013; 309:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Proclemer A, Muser D, Campana A, et al. Indication to cardioverter-defibrillator therapy and outcome in real world primary prevention. Data from the IRIDE (Italian registry of prophylactic implantation of defibrillators) study. Int J Cardiol 2013; 168:1416–1421. [DOI] [PubMed] [Google Scholar]
- 6.Van der Heijden AC, Borleffs CJ, Buiten MS, et al. The clinical course of patients with implantable cardioverter-defibrillators: extended experience on clinical outcome, device replacements, and device-related complications. Heart Rhythm 2015; 12:1169–1176. [DOI] [PubMed] [Google Scholar]
- 7.Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2013; 127:e283–e352. [DOI] [PubMed] [Google Scholar]
- 8.Russo AM, Stainback RF, Bailey SR, et al. ACCF/HRS/AHA/ASE/HFSA/SCAI//S CCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Heart Rhythm Society, American Heart association, American Society of Echocardiography, Heart Failure Society of America, Society of Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol 2013; 61:1318–1368. [DOI] [PubMed] [Google Scholar]
- 9.Lip GY, Heinzel FR, Gaita F, et al. European Heart Rhythm Association/Heart Failure Association joint consensus document on arrhythmias in heart failure, endorsed by the Heart Rhythm Society and the Asian Pacific Heart Rhythm Society. Eur J Heart Fail 2015; 17:848–874. [DOI] [PubMed] [Google Scholar]
- 10.Priori SG, Blomstrom-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2015; 36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 11.Hartikainen JE, Malik M, Staunton A, Poloniecki J, Camm AJ. Distinction between arrhythmic and nonarrhythmic death after acute myocardial infarction based on heart rate variability, signal-averaged electrocardiogram, ventricular arrhythmias and left ventricular ejection fraction. J Am Coll Cardiol 1996; 28:296–304. [DOI] [PubMed] [Google Scholar]
- 12.Weeke P, Johansen JB, Jorgensen OD, et al. Mortality and appropriate and inappropriate therapy in patients with ischaemic heart disease and implanted cardioverter-defibrillators for primary prevention: data from the Danish ICD Register. Europace 2013; 15:1150–1157. [DOI] [PubMed] [Google Scholar]
- 13.Sabbag A, Suleiman M, Laish-Farkash A, et al. Contemporary rates of appropriate shock therapy in patients who receive implantable device therapy in a real world setting. Heart Rhythm 2015; 12:2426–2433. [DOI] [PubMed] [Google Scholar]
- 14.Kirkfeldt RE, Johansen JB, Nohr EA, Jorgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Demark. Eur Heart J 2014; 35:1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Maria E, Diemberg I, Vassallo P, et al. Prevention of infection in cardiovascular implantable electronic devices beyond the antibiotic agent. J Cardiovasc Med 2014; 15:554–564. [DOI] [PubMed] [Google Scholar]
- 16.Niemeijer MN, Van den Berg ME, Leening MJ, et al. Declining incidence of sudden cardiac death from 1990–2010 in a general middle-aged and elderly population: the Rotterdam Study. Heart Rhythm 2015; 12:123–129. [DOI] [PubMed] [Google Scholar]
- 17.Marcantoni L, Toselli T, Urso G, Pratola C, Ceconi C, Bertini M. Impact of remote monitoring on the management of arrhythmias in patients with implantable cardioverter-defibrillator. J Cardiovasc Med 2015; 16:775–781. [DOI] [PubMed] [Google Scholar]
- 18.Gorgels AP, Gijsbers C, De Vreed-Swagemakers J, Lousberg A, Wellens HJ. Out-of-hospital cardiac arrest – the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J 2003; 24:1204–1209. [DOI] [PubMed] [Google Scholar]
- 19.Narayanan K, Reinier K, Uy-Evanado A, et al. Frequency and determinants of implantable cardioverter defibrillator deployment among primary prevention candidates with subsequent sudden cardiac arrest in the community. Circulation 2013; 128:1733–1738. [DOI] [PubMed] [Google Scholar]
- 20.Wellens HJ, Schwartz PJ, Lindemans FW, et al. Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J 2014; 35:1642–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theuns DA, Smith T, Hunink MG, Bardy GH, Jordaens L. Effectiveness of prophylactic implantation of cardioverter-defibrillators without cardiac resynchronization therapy in patients with ischaemic or nonischaemic heart disease: a systematic review and meta-analysis. Europace 2010; 12:1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberger JJ, Buxton AE, Cain M, et al. Risk stratification for arrhythmic sudden cardiac death. Identifying the roadblocks. Circulation 2011; 123:2423–2430. [DOI] [PubMed] [Google Scholar]
- 23.Kadish AH, Bello D, Finn JP, et al. Rationale and design for the defibrillators to reduce risk by magnetic resonance imaging evaluation (DETERMINE) trial. J Cardiovasc Electrophysiol 2009; 20:982–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golberger JJ, Basu A, Boineau R, et al. Risk stratification for sudden cardiac death. A plan for the future. Circulation 2014; 129:516–526. [DOI] [PubMed] [Google Scholar]
- 25.Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014; 35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 26.Bailey JJ, Berson AS, Handelsman H, Hodges M. Utility of current risk stratification tests for predicting major arrhythmic events after myocardial infarction. J Am Coll Cardiol 2001; 38:1902–1911. [DOI] [PubMed] [Google Scholar]
- 27.Buxton AE, Lee KL, Hafley GE, et al. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol 2007; 50:1150–1157. [DOI] [PubMed] [Google Scholar]
- 28.Disertori M, Quintarelli S, Mazzola S, Favalli V, Narula N, Arbustini E. The need to modify patient selection to improve the benefits of implantable cardioverter-defibrillator for primary prevention of sudden death in nonischaemic dilated cardiomyopathy. Europace 2013; 15:1693–1701. [DOI] [PubMed] [Google Scholar]
- 29.Klem I, Weinsaft JW, Bahnson TD, et al. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol 2012; 60:408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merchant FM, Zheng H, Bigger T, et al. A combined anatomic and electrophysiologic substrate based approach for sudden cardiac death risk stratification. Am Heart J 2013; 166:744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu TJ, Ong JJ, Hwang C, et al. Characteristics of wave fronts during ventricular fibrillation in human hearts with dilated cardiomyopathy: role of increased fibrosis in the generation of reentry. J Am Coll Cardiol 1998; 32:187–196. [DOI] [PubMed] [Google Scholar]
- 32.Bello D, Fieno DS, Kim RJ, et al. Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J Am Coll Cardiol 2005; 45:1104–1108. [DOI] [PubMed] [Google Scholar]
- 33.Bogun FM, Desjardins B, Good E, et al. Delayed-enhanced magnetic resonance imaging in nonischemic cardiomyopathy: utility for identifying the ventricular arrhythmia substrate. J Am Coll Cardiol 2009; 53:1138–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roes SD, Borleffs CJ, Van der Geest RJ, et al. Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ Cardiovasc Imaging 2009; 2:183–190. [DOI] [PubMed] [Google Scholar]
- 35.Scott PA, Morgan JM, Carroll N, et al. The extent of left ventricular scar quantified by late gadolinium enhancement MRI is associated with spontaneous ventricular arrhythmias in patients with coronary artery disease and implantable cardioverter-defibrillators. Circ Arrhythm Electrophysiol 2011; 4:324–330. [DOI] [PubMed] [Google Scholar]
- 36.Alexandre J, Saloux E, Dugué AE, et al. Scar extent evaluated by late gadolinium enhancement CMR: a powerful predictor of long term appropriate ICD therapy in patients with coronary artery disease. J Cardiovasc Magn Reson 2013; 15:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demirel F, Adiyaman A, Timmer JR, et al. Myocardial scar characteristics based on cardiac magnetic resonance imaging is associated with ventricular tachyarrhythmia in patients with ischemic cardiomyopathy. Int J Cardiol 2014; 177:392–399. [DOI] [PubMed] [Google Scholar]
- 38.Zeidan-Shwiri T, Yang Y, Lashevsky I, et al. Magnetic resonance estimates of the extent and heterogeneity of scar tissue in ICD patients with ischemic cardiomyopathy predict ventricular arrhythmia. Heart Rhythm 2015; 12:802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Assomull RG, Prasad SK, Lyne J, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol 2006; 48:1977–1985. [DOI] [PubMed] [Google Scholar]
- 40.Iles L, Pfluger H, Lefkovits L, et al. Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter-defibrillators for primary prevention of sudden cardiac death. J Am Coll Cardiol 2011; 57:821–828. [DOI] [PubMed] [Google Scholar]
- 41.Leyva F, Taylor RJ, Foley PW, et al. Left ventricular midwall fibrosis as a predictor of mortality and morbidity after cardiac resynchronization therapy in patients with nonischemic cardiomyopathy. J Am Coll Cardiol 2012; 60:1659–1667. [DOI] [PubMed] [Google Scholar]
- 42.Gulati A, Jabbour A, Ismail TF, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013; 309:896–908. [DOI] [PubMed] [Google Scholar]
- 43.Neilan TG, Coelho-Filho OR, Danik SB, et al. CMR quantification of myocardial scar provides additive prognostic information in nonischemic cardiomyopathy. J Am Coll Cardiol Img 2013; 9:944–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perazzolo MM, De Lazzari M, Zorzi A, et al. Impact of the presence and amount of myocardial fibrosis by cardiac magnetic resonance on arrhythmic outcome and sudden cardiac death in nonischemic dilated cardiomyopathy. Heart Rhythm 2014; 11:856–863. [DOI] [PubMed] [Google Scholar]
- 45.Masci PG, Doulaptsis C, Bertella E, et al. Incremental prognostic value of myocardial fibrosis in patients with nonischemic cardiomyopathy without congestive heart failure. Circ Heart Fail 2014; 7:448–456. [DOI] [PubMed] [Google Scholar]
- 46.Chimura M, Kiuchi K, Okajima K, et al. Distribution of ventricular fibrosis associated with life threatening ventricular tachyarrhythmias in patients with nonischemic dilated cardiomyopathy. J Cardiovasc Electrophysiol 2015; 26:1239–1246. [DOI] [PubMed] [Google Scholar]
- 47.Piers SR, Everaerts K, Van der Geest RJ, et al. Myocardial scar predicts monomorphic ventricular tachycardia but not polymorphic ventricular tachycardia or ventricular fibrillation in nonischemic dilated cardiomyopathy. Heart Rhythm 2015; 12:2106–2114. [DOI] [PubMed] [Google Scholar]
- 48.Fernandez-Armenta J, Berruezo A, Mont L, et al. Use of myocardial scar characterization to predict ventricular arrhythmia in cardiac resynchronization therapy. Europace 2012; 14:1578–1586. [DOI] [PubMed] [Google Scholar]
- 49.Gao P, Yee R, Gula L, et al. Prediction of arrhythmic events in ischemic and dilated cardiomyopathy patients referred for implantable cardiac defibrillator: evaluation of multiple scar quantification measures for Late Gadolinium Enhancement Magnetic Resonance Imaging. Circ Cardiovasc Imaging 2012; 5:448–456. [DOI] [PubMed] [Google Scholar]
- 50.Mordi I, Jhund PS, Gardner RS, et al. LGE and NT-proBNP identify low risk of death or arrhythmic events in patients with primary prevention ICDs. J Am Coll Cardiol Img 2014; 7:561–569. [DOI] [PubMed] [Google Scholar]
- 51.Almehmadi F, Joncas SX, Nevis I, et al. Prevalence of myocardial fibrosis patterns in patients with systolic dysfunction: prognostic significance for the prediction of sudden cardiac arrest or appropriate implantable cardiac defibrillator therapy. Circ Cardiovasc Imaging 2014; 7:593–600. [DOI] [PubMed] [Google Scholar]
- 52.Scott PA, Rosengarten JA, Curzen NP, Morgan JM. Late gadolinium enhancement cardiac magnetic resonance imaging for the prediction of ventricular tachyarrhythmic events: a meta-analysis. Eur J Heart Fail 2013; 15:1019. [DOI] [PubMed] [Google Scholar]
- 53.Kuruvilla S, Adenaw N, Katwal AB, Lipinski MJ, Kramer CM, Salerno M. Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: a systematic review and meta-analysis. Circ Cardiovasc Imaging 2014; 7:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verrier RL, Klingenheben T, Malik M, et al. Microvolt T-wave alternans, physiological basis, methods of measurement, and clinical utility--Consensus Guideline by International Society for Holter and Noninvasive Electrocardiology. J Am Coll Cardiol 2011; 58:1309–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hohnloser SH, Klingenheben T, Bloomfield D, Dabbous O, Cohen RJ. Usefulness of microvolt T-wave alternans for prediction of ventricular tachyarrhythmic events in patients with dilated cardiomyopathy: results from a prospective observational study. J Am Coll Cardiol 2003; 41:2220–2224. [DOI] [PubMed] [Google Scholar]
- 56.Salerno-Uriarte JA, De Ferrari GM, Klersy C, et al. Prognostic value of T-wave alternans in patients with heart failure due to nonischemic cardiomyopathy. Results of the ALPHA Study. J Am Coll Cardiol 2007; 50:1896–1904. [DOI] [PubMed] [Google Scholar]
- 57.Stein PK, Sanghavi D, Domitrovich P, Mackey RA, Deedwania P. Ambulatory ECG-based T-wave alternans predicts sudden cardiac death in high-risk post-MI patients with left ventricular dysfunction in the EPHESUS study. J Cardiovasc Electrophysiol 2008; 19:1037–1042. [DOI] [PubMed] [Google Scholar]
- 58.Costantini O, Hohnloser SH, Kirk MM, et al. The ABCD (Alternans Before Cardioverter Defibrillator) trial. Strategies using T-wave alternans to improve efficiency of sudden cardiac death prevention. J Am Coll Cardiol 2009; 53:471–479. [DOI] [PubMed] [Google Scholar]
- 59.Shizuta S, Ando K, Nobuyoshi M, et al. Prognostic utility of T-wave alternans in a real-world population of patients with left ventricular dysfunction: the PREVENT-SCD study. Clin Res Cardiol 2012; 101:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merchant FM, Ikeda T, Pedretti RF, et al. Clinical utility of microvolt T-wave alternans testing in identifying patients at high or low risk of sudden cardiac death. Heart Rhythm 2012; 9:1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monasterio V, Laguna P, Cygankiewicz I, et al. Average T-wave alternans activity in ambulatory ECG records predicts sudden cardiac death in patients with chronic heart failure. Heart Rhythm 2012; 9:383–389. [DOI] [PubMed] [Google Scholar]
- 62.Hoshida K, Miwa Y, Miyakoshi M, et al. Simultaneous assessment of T-wave alternans and heart rate turbulence on holter electrocardiograms as predictors for serious cardiac events in patients after myocardial infarction. Circ J 2013; 77:432–438. [DOI] [PubMed] [Google Scholar]
- 63.Merchant FM, Salerno-Uriarte JA, Caravati F, et al. Prospective use of microvolt T-wave alternans testing to guide primary prevention implantable cardioverter defibrillator therapy. Circ J 2015; 79:1912–1919. [DOI] [PubMed] [Google Scholar]
- 64.Gold MR, Ip JH, Costantini O, et al. Role of microvolt T-wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: primary results from the T-wave alternans Sudden Cardiac Death in Heart Failure Trial substudy. Circulation 2008; 118:2022–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chow T, Kereiakes DJ, Onufer J, et al. Does microvolt T-wave alternans testing predict ventricular tachyarrhythmias in patients with ischemic cardiomyopathy and prophylactic defibrillators? The MASTER (Microvolt T- Wave Alternans Testing for Risk Stratification of Post-Myocardial Infarction Patients) trial. J Am Coll Cardiol 2008; 52:1607–1615. [DOI] [PubMed] [Google Scholar]
- 66.Chan PS, Gold MR, Nallamothu BK. Do beta-blockers impact microvolt T-wave alternans testing in patients at risk for ventricular arrhythmias? A meta-analysis. J Cardiovasc Electrophysiol 2010; 21:1009–1014. [DOI] [PubMed] [Google Scholar]
- 67.Gehi AK, Stein RH, Metz LD, Gomes JA. Microvolt T-wave alternans for risk stratification of ventricular tachyarrhythmic events. A meta-analysis. J Am Coll Cardiol 2005; 46:75–82. [DOI] [PubMed] [Google Scholar]
- 68.Calò L, De Santo T, Nuccio F, et al. Predictive value of microvolt T-wave alternans for cardiac death or ventricular tachyarrhythmic events in ischemic and nonischemic cardiomyopathy patients: a meta-analysis. Ann Noninvasive Electrocardiol 2011; 16:388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gupta A, Hoang DD, Karliner L, et al. Ability of microvolt T-wave alternans to modify risk assessment of ventricular tachyarrhythmic events: a meta-analysis. Am Heart J 2012; 163:354–364. [DOI] [PubMed] [Google Scholar]
- 70.Chen Z, Shi Y, Hou X, Xu S, Zou J. Microvolt T-wave alternans for risk stratification of cardiac events in ischemic cardiomyopathy: a meta-analysis. Int J Cardiol 2013; 167:2061–2065. [DOI] [PubMed] [Google Scholar]
- 71.Golberger JJ, Subacius H, Patel T, Cunnane R, Kadish AH. Sudden cardiac death risk stratification in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol 2014; 63:1879–1889. [DOI] [PubMed] [Google Scholar]
- 72.Alba AC, Agoritsas T, Jankowski M, et al. Risk prediction model for mortality in ambulatory patients with heart failure: a systematic review. Circ Heart Fail 2013; 6:881–889. [DOI] [PubMed] [Google Scholar]
- 73.Levy WC, Lee KL, Hellkamp AS, et al. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation 2009; 120:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol 2012; 59:2075–2079. [DOI] [PubMed] [Google Scholar]
- 75.Steinberg BA, Al-Khatib SM, Edwards R, et al. Outcomes of implantable cardioverter-defibrillator use in patients with comorbidities. Results from a combined analysis of 4 randomized clinical trials. J Am Coll Cardiol HF 2014; 2:623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barlera S, Tavazzi L, Franzosi MG, et al. Predictors of mortality in 6975 patients with chronic heart failure in the Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico-Heart Failure trial: proposal for a nomogram. Circ Heart Fail 2013; 6:31–39. [DOI] [PubMed] [Google Scholar]
- 77.Senni M, Parrella P, De Maria R, et al. Predicting heart failure outcome from cardiac and comorbid conditions: the 3C-HF score. Int J Cardiol 2013; 163:206–211. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y, Guallar E, Blasco-Colmenares E, et al. Clinical and serum-based markers are associated with death within 1 year of de novo implant in primary prevention ICD recipients. Heart Rhythm 2015; 12:360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shadman R, Poole JE, Dardas TF, et al. A novel method to predict the proportional risk of sudden cardiac death in heart failure: derivation of the Seattle Proportional Risk Model. Heart Rhythm 2015; 12:2069–2077. [DOI] [PubMed] [Google Scholar]
- 80.Lee DS, Hardy J, Yee R, et al. Clinical risk stratification for primary prevention implantable cardioverter defibrillators. Circ Heart Fail 2015; 8:927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Buxton AE, Waxman HL, Marchlinski FE, Untereker WJ, Waspe LE, Josephson ME. Role of triple extrastimuli during elctrophysiologic study of patients with documented sustained ventricular tachyarrhythmias. Circulation 1984; 69:532–540. [DOI] [PubMed] [Google Scholar]
- 82.Zoni-Berisso M, Molini D, Mela GS, Vecchio C. Value of programmed ventricular stimulation in predicting sudden death and sustained ventricular tachycardia in survivors of myocardial infarction. Am J Cardiol 1996; 77:673–680. [DOI] [PubMed] [Google Scholar]
- 83.Buxton AE, Lee KL, DiCarlo L, et al. Electrophysiologic testing to identify patients with coronary artery disease who are at high risk for sudden death. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med 2000; 342:1937–1945. [DOI] [PubMed] [Google Scholar]
- 84.Buxton AE, Lee KL, Hafley GE, et al. Relation of ejection fraction and inducible ventricular tachycardia to mode of death in patients with coronary artery disease: an analysis of patients enrolled in the Multicenter UnSustained Tachycardia Trial. Circulation 2002; 106:2466–2472. [DOI] [PubMed] [Google Scholar]
- 85.De Ferrari GM, Rordorf R, Frattini F, Petracci B, De Filippo P, Landolina M. Predictive value of programmed ventricular stimulation in patients with ischaemic cardiomyopathy: implications for the selection of candidates for an implantable defibrillator. Europace 2007; 9:1151–1157. [DOI] [PubMed] [Google Scholar]
- 86.Buxton AE. Programmed ventricular stimulation: not dead. Circulation 2014; 129:831–833. [DOI] [PubMed] [Google Scholar]
- 87.La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 1998; 351:478–484. [DOI] [PubMed] [Google Scholar]
- 88.Exner DV, Kavanagh KM, Slawnych MP, et al. Noninvasive risk assessment early after a myocardial infarction. The REFINE study. J Am Coll Cardiol 2007; 50:2275–2284. [DOI] [PubMed] [Google Scholar]
- 89.Bauer A, Barthel P, Schneider R, et al. Improved stratification of autonomic regulation for prediction in postinfarction patients with preserved left ventricular function (ISAR-Risk). Eur Heart J 2009; 30:576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huikuri HV, Exner DV, Kavanagh KM, et al. Attenuated recovery of heart rate turbulence early after myocardial infarction identifies patients at high risk for fatal or near-fatal arrhythmic events. Heart Rhythm 2010; 7:229–235. [DOI] [PubMed] [Google Scholar]
- 91.La Rovere MT, Pinna GD, Maestri R, et al. Autonomic markers and cardiovascular and arrhythmic events in heart failure patients: still a place in prognostication? Data from the GISSI-HF trail. Eur J Heart Fail 2012; 14:1410–1419. [DOI] [PubMed] [Google Scholar]
- 92.Hershberger RE, Lindenfeld J, Mestroni L, Seidman CE, Taylor MR, Towbin JA. Genetic evaluation of cardiomyopathy – A Heart Failure Society of America practice guideline. J Card Fail 2009; 15:83–97. [DOI] [PubMed] [Google Scholar]
- 93.Ganesh SK, Arnett DK, Assimes TL, et al. Genetics and genomics for the prevention and treatment of cardiovascular disease: update: a scientific statement from the American Heart Association. Circulation 2013; 128:2813–2851. [DOI] [PubMed] [Google Scholar]
- 94.Kusumoto FM, Calkins H, Boehmer J, et al. HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. Circulation 2014; 130:94–125. [DOI] [PubMed] [Google Scholar]
- 95.Taylor MR, Fain PR, Sinagra G, et al. Familial Dilated Cardiomyopathy Registry Research Group. Natural history of dilated cardiomyopathy due to lamin A/C gene mutations. J Am Coll Cardiol 2003; 41:771–780. [DOI] [PubMed] [Google Scholar]
- 96.Meune C, Van Berlo JH, Anselme F, Bonne G, Pinto YM, Duboc D. Primary prevention of sudden death in patients with lamin A/C gene mutations. N Engl J Med 2006; 354:209–210. [DOI] [PubMed] [Google Scholar]
- 97.Pasotti M, Klersy C, Pilotto A, et al. Long-term outcome and risk stratification in dilated cardiolaminopathies. J Am Coll Cardiol 2008; 52:1250–1260. [DOI] [PubMed] [Google Scholar]
- 98.Van Rijsingen IA, Arbustini E, Elliott PM, et al. Risk factors for malignant ventricular arrhythmias in lamin A/C mutation carriers. A European cohort study. J Am Coll Cardiol 2012; 59:493–500. [DOI] [PubMed] [Google Scholar]
- 99.Burke MC, Gold MR, Knight BP, et al. Safety and efficacy of the totally subcutaneous implantable defibrillator: a 2-year results from a pooled analysis of the IDE Study and EFFORTLESS Registry. J Am Coll Cardiol 2015; 65:1605–1615. [DOI] [PubMed] [Google Scholar]
- 100.Tung R, Zimetbaum P, Josephson ME. A critical appraisal of implantable cardioverter-defibrillator therapy for the prevention of sudden cardiac death. J Am Coll Cardiol 2008; 52:1111–1121. [DOI] [PubMed] [Google Scholar]
- 101.Smith T, Jordaens L, Theuns DA, Van Dessel PF, Wilde AA, Hunink MG. The cost-effectiveness of primary prophylactic implantable defibrillator therapy in patients with ischaemic and nonischaemic heart disease: a European analysis. Eur Heart J 2013; 34:211–219. [DOI] [PubMed] [Google Scholar]
- 102.Lauer MS, D’Agostino R. The randomized registry trial – the next disruptive technology in clinical research? N Engl J Med 2013; 369:1579–1581. [DOI] [PubMed] [Google Scholar]


