Abstract
Antenatal care (ANC) represents a delivery platform for a broad range of health services; however, these opportunities are insufficiently utilised. This review explores key barriers and enablers for successful integration of health s"ervices with ANC in different contexts. Data from peer‐reviewed and grey literature were organised using the SURE checklist. We identified 46 reports focusing on integration of HIV, tuberculosis, malaria, syphilis or nutrition services with ANC from Asia, Africa and the Pacific. Perspectives of service users and providers, social and political factors, and health system characteristics (such as resource availability and organisational structures) affected ease of integration.
Tweetable abstract
Health system factors, context and stakeholders must be considered for integrated antenatal care services.
Keywords: Antenatal care, integration, low and middle income countries
Tweetable abstract
Health system factors, context and stakeholders must be considered for integrated antenatal care services.
Introduction
For most women in low‐ and middle‐income countries (LMIC), antenatal care (ANC) plays a highly important dual role: not only does ANC provide effective interventions to reduce the risks associated with pregnancy and childbirth, it can also serve as a delivery platform for other health services.1 Particularly in settings where the prevalence of HIV/AIDS, sexually transmitted infections (STIs), tuberculosis (TB) and malaria is high, integrating services for these conditions with ANC can significantly expand their reach.2 In fact, the World Health Organization (WHO) identified integration of ANC with other health programmes as a key strategy for reducing missed opportunities for patient contact and improving maternal and child health (MCH).1, 3, 4 Evidence from the countries studied, however, suggests that in practice integrated delivery of ANC with other health services is not systematic or adequate and that opportunities for providing care for women are lost.5, 6
Several factors enable or hinder the integration of other health services with ANC. There may be barriers in the specific health system context where services are delivered: for example, lack of trained health workers may prevent delivery of a full range of services, even if these are prescribed by national health policies. Factors related to broader political and social context can present significant hurdles for effective integration. Hence, delivery of integrated care that combines ANC with other health services to expand coverage and improve health outcomes requires an understanding of the main barriers and enablers to be successful.
We present a comprehensive review of drivers for integration of health programmes with ANC. The review combines published peer‐reviewed literature with grey literature sources. We provide a synthesis of the key barriers and enablers to integration in different contexts, bringing together randomised controlled trials and other published studies on how integrated delivery of ANC with other health services affects MCH outcomes, user experience, service access and coverage, and programme efficiency when compared with ‘routine’ models of care in which the same services were delivered separately.
Methods
Defining ‘integration’
For the purposes of this review, we considered any study that described a change from ‘routine practice’ with the intention of integrating provision of ANC services with other health services. Integrated service provision models include co‐location of services, using a single point of access; collaboration between different service providers involved in a woman's care (e.g. in integrated care teams) or a well‐organised referral system, with follow up and feedback among different service providers.
Search strategy
The search for relevant studies included both electronic database searches for peer‐reviewed literature and conference proceedings as well as online searches for grey literature to identify qualitative evidence on the barriers and enablers for successful integration of health services with ANC.
The databases searched included the Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (Cochrane Reviews), Cochrane Database of Abstracts of Reviews of Effects (Other Reviews), MEDLINE, EMBASE, CINAHL Plus, Global Health and POPLINE. We used a comprehensive search strategy with no language or publication date restrictions. The search string for MEDLINE, which was tailored to each of the databases, is provided in Appendix S1.
For grey literature, we searched clinical trial registers [ClinicalTrials.gov and Current Controlled Trials (ISRCTN)], electronic databases for grey literature [GreyNet International, The New York Academy of Medicine (NYAM) Grey Literature Report] and online directories and websites of organisations involved in MCH issues or global health. These included: WHO and WHOLIS; Maternal Health Task Force; Implementing Best Practice Initiative; World Bank; Count Down to 2015; Women Deliver; Catalyst Consortium; CEDPA; DFID; EngenderHealth; FHI; ICRW; INFO Project; IGWG; IPPF; IntraHealth International; Global Fund; JHPIEGO; JHUCCP; John Snow Inc.; Management Sciences for Health; Partners in Population and Development; PATH; Pathfinder International; PEPFAR; Pop Council; PSI; UNFAO; UNFPA and UNAIDS. The search string used in grey literature sources was ‘integrat* (e.g. integration, integrated, integrating) AND (maternal OR neonatal OR pregnancy OR mother OR infant or baby)’. There were no date restrictions. The electronic database search was completed in February 2014 and the grey literature search in March 2014.
Selection and data extraction
This review followed a comprehensive selection process. In the first stage, the authors (T.dJ, E.A.) evaluated publications for their potential relevance based on titles. Any title judged as potentially relevant by either of the authors was next assessed for eligibility on the basis of the abstract. Due to the large number of abstracts, those on which the authors disagreed were independently reviewed by a third author (I.G.U.) who decided on its inclusion into the third round of screening, where two authors (T.dJ., E.A.) reviewed the full text of each retained publication and noted whether the study reported on an integrated service into ANC. In the final stage, one author (I.G.U.) screened all studies that focused on integration of other health services into ANC regardless of any study design considerations. Studies discussing any qualitative evidence on enablers and barriers for implementation were eligible for inclusion in this review. We extracted basic data from studies to a standardised form (including title, author, year of publication, country, setting, funding). We used the SURE checklist7 to facilitate data extraction on barriers and enablers, organise emerging findings and identify gaps in knowledge.8 The SURE checklist was developed to identify barriers to implementing health systems changes and has been used to evaluate the evidence on factors affecting implementation of large‐scale programmes aimed at scaling up human resources for maternal and newborn health.8 The factors are grouped in five main categories: (1) recipients of care, (2) providers of care, (3) other stakeholders, (4) health system constraints, and (5) social and political constraints), with potential barriers and enablers identified in each category.
Results
Search results
Among 6416 unique citations retrieved from electronic databases, 922 titles were considered potentially relevant. Of these citations, 842 included abstracts that were subsequently reviewed. Among the abstracts, 120 were considered potentially relevant. For an additional 80 citations, no abstracts were available. These citations were all carried forward to the next stage of the screening process, in which the full text of the potentially eligible studies was reviewed. We retrieved the full text for 177 of 200 citations, among which 78 reported on ANC programmes involving integration of other health services. Only 29 of the 78 citations discussed implementation enablers and barriers to integration of services, and were considered eligible for inclusion in this review. A separate search of databases of grey literature and trial databases yielded 19 more potentially relevant reports from NYAM, three randomised controlled trials (RCTs) from ISRCTN and five RCT studies from ClinicalTrials.gov. None of these studies included information on enablers or barriers to integration of health services into ANC programmes. Thirteen relevant reports were identified from the websites of organisations involved in MCH issues and global health9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, and four additional reports were identified through citation tracking.22, 23, 24, 25 In total, we thus identified 46 eligible reports (29 from peer‐reviewed and 17 from grey literature) for which we have here summarised the barriers and enablers to integration (Figure 1).
Figure 1.
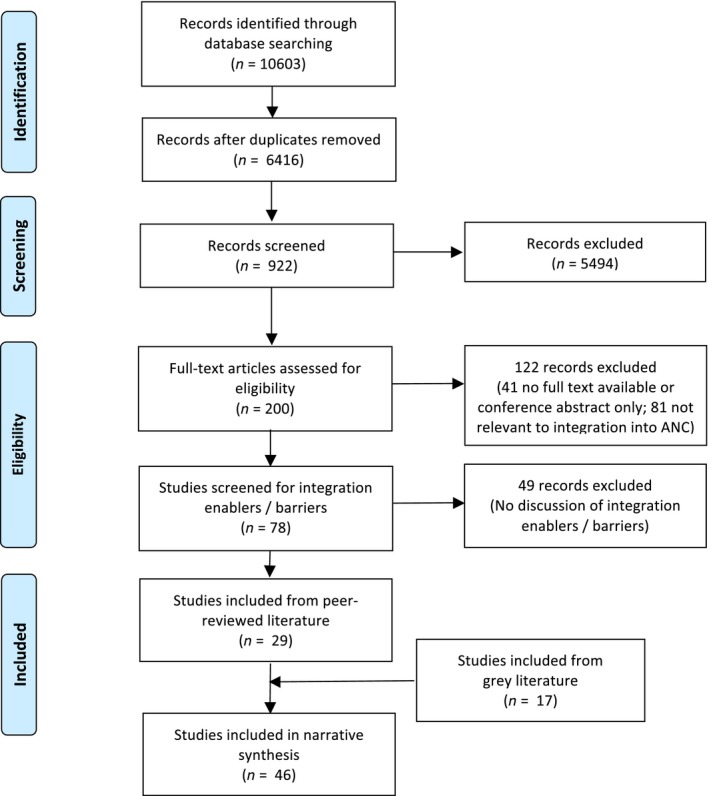
PRISMA flow diagram showing number of articles at each stage of the review.
The included reports cover programmes from Africa (Botswana26, Cameroon27, Cote d'Ivoire28, 29, Ethiopia30, Kenya18, 24, 31, 32, 33, Malawi30, 34, Mozambique35, 36, Rwanda10, 37, South Africa25, 38, 39, 40, 41, 42, 43, Swaziland44, Tanzania21, 23, 24, 45, 46, Uganda24, 47, 48, Zambia24, 37, 49, Zimbabwe50 and Sub‐Saharan Africa in general15, 16), Asia (China51, Mongolia52, Thailand53), Europe (Ukraine54) and the Pacific (Fiji13). The majority of the emerging evidence relates to integration of HIV services, mainly prevention of mother‐to‐child transmission (PMTCT) of HIV10, 11, 12, 13, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 38, 40, 42, 43, 44, 46, 47, 48, 50, 51, 53, 54, one study is on the integration of PMTCT and TB services39, one on malaria15, five on syphilis40, 42, 45, 49, 52 and two on integration of nutrition services.9, 20 We also include guidance from the Global Fund to Fight AIDS, Tuberculosis and Malaria on how to integrate maternal, newborn and child health interventions with HIV, tuberculosis and malaria programmes.14
Factors affecting integration of ANC with other health services
Recipients of care
Limited evidence from quantitative evaluations of integrated services suggests that integration can lead to improved uptake, earlier initiation of treatment and lower loss‐to‐follow up for women who require treatment.21, 24 These evaluations suggest that integrated programmes could provide an entry point for testing and counselling for other family members as well.21, 24 Participation of male partners has been low in PMTCT programmes, and innovative solutions for community mobilisation might be needed.47, 50 One specific strategy adopted in Fiji to incorporate partners into HIV care and reproductive health decision‐making was to distribute a ‘men's pack’ for all women in the antenatal programme, containing information, communication and education materials.13 In Cameroon, providers discussed culturally appropriate ways for mothers to present HIV test results to their spouses and strongly encouraged spouses to attend the first antenatal visit.27 Targeted invitation letters for partners were used in Zambia49 and Uganda.48 However, women may be reluctant to take up additional tests, particularly when their partners are involved. In South Africa, where TB screening is recommended as a routine component of ANC, the uptake has been lower than expected. A study by Kali et al.16, 55 suggests that pregnant women, particularly those recently diagnosed with HIV, were not willing to be screened for TB in order to avoid a positive diagnosis. Similarly, in Cote d'Ivoire, HIV‐infected women who lived with a partner showed poorer uptake of PMTCT.29 Couple‐counselling sessions, in particular those that stress the expected benefits for their children, could facilitate the uptake of PMTCT.37, 48
There is limited evidence on the perspectives and experiences of women and their partners of integrated ANC services. In a pilot programme of ART initiation in pregnancy in South Africa, most women appreciated rapid access to ART and found the provided counselling useful.38 HIV‐positive women in rural Kenya preferred an integrated ANC‐HIV model for its increased confidentiality (women were not readily identifiable as attending an HIV service), the ease of receiving comprehensive care in a single visit, and improved provider–patient relationships.31, 32 In Mongolia, patients preferred receiving syphilis testing in the same place as ANC, allowing them to get same‐day results and receive counselling and treatment from ANC providers.52 Similar results were reported in various other settings: coverage was lower for integrated services that were provided at different locations or those not provided on the same day, resulting in delayed testing and treatment initiation.33, 34, 40, 41, 42, 45
Providers of care
Training and clear care guidelines are essential for providing knowledge and skills to deliver integrated services. This training should be provided consistently to all health workers providing ANC. For integrated PMTCT, training in the recognition and staging of HIV infection can allow care and treatment of HIV‐infected pregnant women to occur within comprehensive MCH services and reduce time‐to‐treatment initiation for the HIV‐infected mother.56, 57 In Malawi, some ANC providers were trained for counselling but not for HIV testing, necessitating referrals to other providers for testing and resulting in lower rates of HIV testing due to service inefficiencies.30 Lack of training was identified as a barrier to delivering an integrated TB‐ PMTCT‐ANC programme in South Africa, where not all healthcare workers were trained in all care aspects, contributing to poor TB case‐finding among pregnant women. Referrals to services provided in different locations also led to inefficiencies and poor uptake.39 Introduction of new skills could be done using a step‐wise approach: in Rwanda, a basic initial training was enhanced with additional training, as new services were gradually introduced.10 In an integrated ANC delivery model for HIV‐positive infants in Mozambique, staff did not feel that lack of technical knowledge was an impeding factor in their daily work but they appreciated supervisory visits, which provided an opportunity to clarify doubts and receive updated information. Staff also highly valued the regular interest in their work and conditions.35
Where guidelines are not available, there is a risk of sub‐optimal service provision. Evidence from multiple country programmes suggest that with integrated malaria services, providers are often not clear about how to deliver intermittent preventative treatment of malaria in pregnancy (IPTp) for specific cases (such as HIV‐positive women) and how to address potential side effects. Given the uncertainty about the care pathways and fear of doing harm, healthcare providers providing integrated malaria services opt for ‘doing nothing’ rather than intervene and risk making an error, leading to lower coverage of IPTp. To overcome this barrier, in Kenya a memo from a senior health official was combined with explanation of the guidelines for follow‐up supervision visits to boost the uptake of IPTp in some districts.15
Integrated services, by design, require health workers to assume additional tasks. These additional tasks may lead to increased workload and longer wait times, making health workers reluctant to implement them. In Malawi, clients and providers both voiced concerns about the acceptability of TB screening and treatment as part of ANC, due to a possible rise in workloads and the consequences thereof.16 It can be difficult to ask providers to take on additional tasks without extra compensation, unless it is made clear the extra services are now to be considered part of routine ANC services. New responsibilities without adequate compensation are likely to cause poor morale among providers.16, 43 Some studies suggest that poor morale, lack of motivation and tensions among providers may be higher in large urban health facilities with greater specialisation of services, as different departments and cadres of providers may be more reluctant to share or relinquish authority. By contrast, rural providers who make their own treatment and care decisions have fewer problems adapting the organisation of services to respond better to client needs.24 In Kenya, providers at smaller health facilities felt that integration of HIV and ANC services in one location had simplified their work, reducing the need to escort the patient to another section of the facility where the HIV documentation and medications were available.32
Integration of new services could potentially motivate health providers, as they feel empowered by being able to deliver more effective and potentially life‐saving services and simultaneously learn how to protect themselves. In Mozambique, for one‐stop integrated MCH services (including ANC) for HIV‐infected infants, staff reported high job satisfaction and a feeling of increased effectiveness, with less time spent on administrative tasks.35 In Kenya, providers at integrated sites also noted that they felt more motivated, intellectually challenged, and satisfied with their work as a result of the additional training and new knowledge gained.32
In the USAID Horizons Program, healthcare providers in Kenya, Zambia, Zimbabwe and Uganda were trained in multiple service components such as PMCT, rapid HIV tests, VCT, infant feeding, and couples counselling. These training programmes had important positive effects on the attitudes of health workers and on reducing stigma attached to HIV‐positive women.24 The motivation from being able to provide integrated PMCT services had encouraged many staff to put extra effort into the care offered to their clients, for example by providing after‐hours counselling and support to mothers living with HIV.24
Health system constraints
Integration of services does not take place in a vacuum, but is affected by many health system factors.
Accessibility and utilisation of health services
One of the prerequisites for effective use of ANC as a delivery platform for other health services is the level of ANC coverage. Despite encouraging progress, not all countries have reached ANC coverage targets set nationally or internationally and urban–rural inequities persist.58 In Ethiopia, for example, the ACCESS project showed that only 28% of pregnant women attended ANC at least once in their pregnancy, thus presenting a significant barrier to reaching these women with integrated PMTCT services30. By contrast, in Thailand the national scaling‐up of PMTCT services in ANC was facilitated by established ANC system structures.53 Moreover, not only the uptake but also the timing of ANC visits is important. For instance, although in Fiji nearly all pregnant women have at least one ANC visit, many of them do not attend until late in their pregnancy, significantly reducing the potential success of adding PMTCT services to the ANC encounter.13
Organisation of health services also matters. For PMTCT programmes at district hospitals in rural Zimbabwe and Malawi, follow up of mothers and children during the antenatal and postnatal period was hampered by centralisation of activities and poor access to health facilities.34, 50 To address these challenges, measures were taken to extend PMTCT activities to lower level health facilities, strengthen community outreach programmes and improve geographical access.
Human resources
Addition of new services to the ANC package places demands on the health workers responsible for their delivery. Unfortunately, in many LMICs lack of human resources across all cadres of health workers is a major bottleneck to service delivery.59, 60 The health workforce crisis is exacerbated by well‐intended, but ultimately harmful incentive policies that have prompted migration of personnel from reproductive health and family planning programmes to priority programmes, such as those for HIV/AIDS.61 An adverse consequence of this internal workforce shift is that health workers who continue to work in ANC and MCH clinics are oftentimes underpaid and overworked. Increases in workload from new tasks, usually accompanied by training, combined with existing shortages and high turn‐over of staff have been highlighted as hurdles to scale‐up of integrated HIV, TB and syphilis services in, among others, Fiji, Uganda, Zambia, South Africa, Swaziland, Mozambique, Uganda and Kenya.13, 16, 24, 25, 35, 36, 44, 45, 48 A study from Tanzania on the impact of integrating and scaling‐up PMTCT on workload showed that integration could be done with existing staff, though this required adjustments in staff productivity and their distribution.46
Reluctant to further increase the burden on frontline staff, some countries have sought new approaches to health workforce management, by shifting tasks and engaging new cadres of health workers from the community. In Ethiopia, for example, Health Extension Workers, who were providing a core set of MCH services, were trained also to provide PMTCT measures.30 In South Africa the task of providing VCT to pregnant women was given to lay counsellors.62 In Cameroon, trained birth attendants provided confidential HIV counselling and testing, allowing for expansion of PMTCT to rural primary health centres.27 This resulted in higher acceptance rates by village women, and improved patient education and follow‐up. Although these strategies have improved access to these important services, they risk perpetuating the divide between ANC and other health services and can create confusion about allocation of responsibilities.21, 62
Supervision, management and leadership
Clarity on responsibilities for supervision and management of integrated services is vital to ensure quality of care. The ACCESS programme in Kenya found that, although integrated ANC‐PMTCT services had been introduced in over 3000 facilities, supervision was still managed by separate HIV and reproductive health teams, resulting in a lack of information about the overall quality of services.30 This misalignment of supervisory roles was subsequently addressed by the joint development of an integrated supervision tool. In South Africa, programmes to integrate TB and PMTCT services also highlighted that, where facilities had separate TB and HIV management guidelines, lack of leadership in the coordination and the supervision of the implementation of joint guidelines were detrimental to the success of integration.39
Effective operational management, service delivery, supervision and accountability arrangements are particularly complex in relation to HIV‐related services, where multiple agencies may be involved in financing, planning, service delivery, monitoring and evaluation. All too often each agency has their own systems and resources supporting different interventions, as illustrated by an assessment of the Horizon programme in Zambia, where ‘the Voluntary Counselling and Testing Programme provides VCT, the MTCT Working Group provides ARVs and infant formula, and the District Health Management Team dispenses haemoglobin and iron supplements’.24 Challenges to comprehensive integration were reported in South Africa, where PMTCT was administered as a vertical programme, managed by the national HIV coordinating body.21, 62
Financial resources
‘Verticalisation’ of health care delivery, resulting in a proliferation of management structures and financing mechanisms, can pose a significant obstacle to service integration.16, 21 For example, in Tanzania vertically organised remuneration systems for HIV programmes was detrimental to the implementation of integrated PMTCT and MCH services23, which required ‘horizontal integration’ of financial and human resources. In other settings, integration of new services, such as malaria treatment and prevention, with ANC have been hampered, as these were not accompanied by sufficient additional funding.15
Information systems
To track performance of newly integrated services, information systems for monitoring and evaluation need to be in place for routine recording and reporting of activities, outputs and outcomes. Although providers at integrated HIV services sites in Kenya observed that integration increased the amount of time spent on recordkeeping, it also led to easier and more accurate reporting of data.32 Furthermore, tracking performance of integrated services requires updating of existing ANC supervision checklists and record cards with suitable indicators to ensure capture of relevant data.24 For example, under the ACCESS programme in Malawi, integration of PMTCT interventions with existing MNCH services required an update of facility performance indicators and quality standards.30
Procurement and distribution systems
An important health system constraint affecting the success of integrated services is the availability and uninterrupted delivery of commodities and medicines. Unavailability of commodities and irregular supply of essential consumables and drugs were found to be major barriers to uptake of integrated HIV25, 35, 44, syphilis45 and malaria services15.
Social and political constraints
Aside from health system constraints, the wider socio‐political context in which the system operates can enable or hinder service integration. In particular, maternal health touches upon fundamental issues of women's rights and religious beliefs that may be contested in different settings. For example, integration of modern contraceptive methods with the ANC package may encounter resistance from faith‐based organisations (FBOs) opposed to the use of such methods, requiring collaboration of all parties to overcome resistance or find creative solutions. In Rwanda, where FBOs provide an important share of health services, the Ministry of Health devised a creative solution by establishing ‘secondary health posts’ adjoining the FBO‐operated facilities, where clients who opt for modern family planning methods may be referred for family planning counselling and to receive health products.10
Belief systems and ideologies are often reflected in a country's legal framework and policies, and affect how services are delivered. For instance, integrating HIV screening into the routine prenatal test panel may require a shift from a so‐called ‘opt‐in’ to an ‘opt‐out’ strategy.13, 26, 63, 64 However, not all countries have policies that allow opt‐out testing, requiring policy changes to enable integrated service delivery.
Lastly, strong leadership and coordinated efforts of key government agencies, NGOs and donors are critical to the success of uptake and scale up of integrated programmes.26, 51, 54
Discussion and conclusion
Antenatal care provides an important platform for the delivery of additional health services to women. The rationale for integration is to improve user access to health services across the care continuum to reduce the high levels of ‘loss to follow up’ from first contact with ANC through to postnatal care, help expand coverage and increase the uptake and utilisation of essential services for women and children. Furthermore, integration can reduce programme costs through more synergistic use of human and financial resources.65
Benefits of integrating antenatal care with other service can be realised if the services provided, health system design and socio‐political context are carefully considered in the integration process, which requires involvement of all stakeholders. These factors range from those directly concerning the providers and recipients of health services to health system constraints, as well as wider socio‐political environment in which ANC services are delivered.
Provision of multiple services during a single point of contact requires that healthcare providers be sufficiently trained in all aspects of the services concerned to ensure good quality care However, injudicious integration can have undesirable consequences for already constrained and overloaded health systems.66 For example, in resource‐constrained health systems, training for integration can take away health staff from frontline services.67 Furthermore, provision of multiple services could stretch the limited capacity, leading to longer waiting times and hindering access for women who have to travel far to reach health facilities. In an attempt to reduce workload, providers may reduce the time spent on consultations, thus compromising service quality. Hence integration needs to be carefully planned and implemented.
Health system factors also influence integration of ANC with other services and may require restructuring of existing management, and financing, as well as monitoring and evaluation systems. Hence, the content and complexity of such a service package should be informed by the local health system capacity and the broader contextual factors. While integrated service delivery offers an opportunity for improved access, efficiency and outcomes, in practice implementation has been challenging. To increase chances of success, it is essential to ensure that all parties are involved in the conception, design and implementation of integrated services to create a sense of commitment to and responsibility and ensure ownership.
This study is limited by significant heterogeneity in the studies published as grey literature, as well as by the small number of rigorous studies identified. Notwithstanding these limitations, we draw on reported experiences from the field to highlight several critical factors that enable or hinder in different contexts successful programme integration which could help improve MCH outcomes through holistic provision of quality care, rather than narrow interventions, which is critical for improving for MNCH outcomes.24
The review shows a large evidence gap on the factors influencing integration of health services with ANC and the potential benefits. There is a clear need for more rigorously conducted studies, ideally involving comparison between different service delivery models with random allocation. However, additional quasi‐experimental studies, and demonstration projects, complemented by modelling studies, could also provide valuable insights in this area and in particular should help in understanding the role of contextual factors in achieving specific outcomes.
Disclosure of interests
None declared. Completed disclosure of interests form available to view online as supporting information.
Details of ethics approval
None required.
Contribution to authorship
T.dJ. and I.G.U. were responsible for development of the methodology, all stages of data collection and analysis, and reporting. E.A. and J.Z. contributed to data collection, screening of papers and data extraction. R.A. was responsible for the conception of the study and contributed to drafting and finalisation of the manuscript. All authors take responsibility for this study and its findings.
Funding
This study was supported by a grant from the Bill & Melinda Gates Foundation.
Supporting information
Appendix S1. Medline Search Strategy.
Acknowledgements
None.
de Jongh TE, Gurol‐Urganci I, Allen E, Jiayue Zhu N, Atun R. Barriers and enablers to integrating maternal and child health services to antenatal care in low and middle income countries. BJOG 2016;123:549–557.
Linked article This article is commented on by N van den Broek, p. 558 in this issue. To view this mini commentary visit http://dx.doi.org/10.1111/1471-0528.13937.
References
- 1. Lincetto O, Mothebesoane‐Anoh S, Gomez P, Munjanja S. Antenatal Care In: Lawn J, Kerber K, eds. Opportunities for Africa's Newborns: Practical Data, Policy, and Programmatic Support for Newborn Care in Africa. Cape Town: Partnership for Maternal, Newborn and Child Health; 2006. pp. 51–62. [Google Scholar]
- 2. USAID . ACCESS Program Update: Focused Antenatal Care‐Achieving Results in Antenatal Care: Improving Maternal and Newborn Outcomes through Integration of Services. Washington, DC, USAID, 2008. [Google Scholar]
- 3. Warren C, Daly P, Toure L, Mongi P. Postnatalcare In: Lawn J, Kerber K, eds. Opportunities for Africa's Newborns: Practical Data, Policy and Programmatic Support for Newborn Care in Africa. Cape Town: Partnership for Maternal, Newborn and Child Health, 2008; pp. 79–90. [Google Scholar]
- 4. Kerber KJ, de Graft‐Johnson JE, Bhutta ZA, Okong P, Starrs A, Lawn JE. Continuum of care for maternal, newborn, and child health: from slogan to service delivery. Lancet 2007;370:1358–69. [DOI] [PubMed] [Google Scholar]
- 5. WHO . Global Health Observatory Data Repository: Maternal and reproductive health [Internet]. 2013. Available from: [ http://apps.who.int/gho/data/node.main.REPWOMEN39?lang=en] Accessed 31 December 2015.
- 6. Tinker A, ten Hoope‐Bender P, Azfar S, Bustreo F, Bell R. A continuum of care to save newborn lives. Lancet 2005;365:822–5. [DOI] [PubMed] [Google Scholar]
- 7. The SURE Collaboration . SURE Guides for Preparing and Using Evidence‐Based Policy Briefs: 5. Identifying and addressing barriers to implementing policy options. Version 2.1. [Internet]. 2011. Available from: [ www.evipnet.org/sure] Accessed 31 December 2015.
- 8. WHO . WHO Recommendations: Optimizing Health Worker Roles to Improve Access to Key Maternal and Newborn Health Interventions Through Task Shifting [Internet]. Geneva: WHO, 2012. Available from: [ http://apps.who.int/iris/bitstream/10665/77764/1/9789241504843_eng.pdf] Accessed 7 July 2014. [PubMed] [Google Scholar]
- 9. The World Bank . The Bangladesh Integrated Nutrition Project: Effectiveness and Lessons. Dhaka, The World Bank, 2005. Report No.: 8. [Google Scholar]
- 10. Government of Rwanda, IPPF, UNAIDS, UNFPA, WHO . Linking Sexual and Reproductive Health and HIV/AIDS, Gateways to Integration: A Case Study from Rwanda. London: Government of Rwanda, IPPF, UNAIDS, UNFPA, WHO, 2013. [Google Scholar]
- 11. UNAIDS, World Health Organization . Technical Guidance Note for Global Fund HIV Proposals: Prevention of Mother‐To‐Child Transmission of HIV. Geneva: WHO, 2011. [Google Scholar]
- 12. UNAIDS . Global Plan Towards the Elimination of New HIV Infections and Keeping their Mothers Alive 2011–2015. Geneva: UNAIDS, 2011. [Google Scholar]
- 13. Fiji Islands Ministry of Health . Global AIDS Report 2012: Fiji Islands [Internet]. 2012. Available from: [ http://www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2012countries/ce_FJ_Narrative_Report.pdf] Accessed 31 December 2015.
- 14. The Global Fund to Fight AIDS Tuberculosis and Malaria . Strengthening Maternal, Newborn and Child Health Interventions. 2013. Geneva: The Global Fund to Fight AIDS Tuberculosis and Malaria, 2013. [Google Scholar]
- 15. Maternal Health Task Force . Malaria in Pregnancy: Bringing the maternal health and malaria communities together. Meeting Report. Istanbul; 2012. [Google Scholar]
- 16. Kendall T, Danel I. Research and Evaluation Agenda for Maternal Health and HIV in Sub‐Saharan Africa [Internet]. Boston, MA; 2014. Report No.: No. 1. Available from: [ http://www.mhtf.org] Accessed 31 December 2015.
- 17. Harvard School of Public Health, Maternal Health Task Force, Centers for Disease Control and Prevention . Technical Summary: Maternal Mortality and HIV (revised) [Internet]. 2014. Available from: [ http://www.mhtf.org/maternal-health-and-hiv/] Accessed 31 December 2015.
- 18. Pathfinder International . Moving Forward: Pathfinder International's Contribution to the Global HIV and AIDS Response. 2009. Watertown, MA: Pathfinder International, 2009.
- 19. Israel E, Kroeger M. Integrating Prevention of Mother‐To‐Child HIV Transmission into Existing Maternal, Child, and Reproductive Health Programs. Watertown, MA; 2003. Report No. 3. [Google Scholar]
- 20. USAID, University of California San Francisco, Cochrane HIV/AIDS Group . Integration of Maternal, Neonatal, and Child Health and Nutrition, Family Planning, and HIV: Current Evidence and Practice—Presentation of Findings. Washington, DC: USAID, 2011. [Google Scholar]
- 21. Stone‐Jimenez M, Ojikutu B, Diese M, Blazer C. Technical Brief: Integrating Prevention of Mother‐to‐Child Transmission of HIV Interventions with Maternal, Newborn and Child Health Services. Arlington, VA; 2011.
- 22. PMTCT Working Group . Integration Prevention of Mother to Child Transmission of HIV/AIDS, Protocol Guidelines. 2003.
- 23. Evjen‐Olsen B, Olsen OE, leKvå G . Achieving progress in maternal and neonatal health through integrated and comprehensive healthcare services—experiences from a programme in northern Tanzania. Int J Equity Health [Internet]. 2009 Jan; 8: 27. Available from: [ http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2725038&tool=pmcentrez&rendertype=abstract] Accessed 20 June 2014. [DOI] [PMC free article] [PubMed]
- 24. Rutenberg N, Kalibala S, Mwai C, Rosen J. Integrating HIV Prevention and Care into Maternal and Child Health Care Settings: Lessons Learned from Horizons Studies. New York: The Population Council Inc., 2002. [Google Scholar]
- 25. Chege JN, Askew I, Mosery N, Ndube‐Nxumalo M, Kunene B, Beksinska M. Feasibility of introducing a comprehensive integrated package of antenatal care services in rural public clinics in South Africa. 2005; (August). Available from: [ http://pdf.dec.org/pdf_docs/PNADD878.pdf] Accessed 31 December 2015. [Google Scholar]
- 26. Creek TL, Ntumy R, Seipone K, Smith M, Mogodi M, Smit M, et al. Successful introduction of routine opt‐out HIV testing in antenatal care in Botswana. J Acquir Immune Defic Syndr 2007;45:102–7. [DOI] [PubMed] [Google Scholar]
- 27. Welty TK, Bulterys M, Welty ER, Tih PM, Ndikintum G, Nkuoh G, et al. Integrating prevention of mother‐to‐child HIV transmission into routine antenatal care: the key to program expansion in Cameroon. J Acquir Immune Defic Syndr 2005;40:486–93. [DOI] [PubMed] [Google Scholar]
- 28. Msellati P, Hingst G, Kaba F, Vino I, Welffens‐Ekra C, Dabis F. Operational issues in preventing mother‐to‐child transmission of HIV‐1 in Abidjan, Côte d'Ivoire, 1998–99. Bull World Health Org 2001;79:641–7. [PMC free article] [PubMed] [Google Scholar]
- 29. Ekouevi DK, Leroy V, Viho I, Bequet L, Horo A, Rouet F, et al. Acceptability and uptake of a package to prevent mother‐to‐Child transmission using rapid HIV testing in Abidjan, Cote d'Ivoire [Internet]. AIDS 2004. p. 697–700. Available from: [ http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed6&NEWS=N&AN=2004141149] Accessed 31 December 2015. [DOI] [PubMed]
- 30. Jhpiego . ACCESS End of Project Report: Strengthening the Integration of PMTCT within MNCH Services [Internet]. 2010. Available from: [ http://www.mchip.net/sites/default/files/g_Strengthening_Integration_of_PMTCT.pdf] Accessed 31 December 2015.
- 31. Vo BN, Cohen CR, Smith RM, Bukusi EA, Onono MA, Schwartz K, et al. Patient satisfaction with integrated HIV and antenatal care services in rural Kenya. AIDS Care 2012;24:1442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Winestone L, Bukusi E, Cohen C. Acceptability and feasibility of integration of HIV care services into antenatal clinics in rural Kenya: a qualitative provider interview study. Glob Public Health 2012;7:149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ong'ech J, Hoffman H, Kose J. Provision of Services and Care for HIV‐Exposed Infants: a comparison of maternal and child health clinic and HIV comprehensive care clinic models. J Acquir Immune Defic Syndr 2012;61:83–9. [DOI] [PubMed] [Google Scholar]
- 34. Manzi M, Zachariah R, Teck R, Buhendwa L, Kazima J, Bakali E, et al. High acceptability of voluntary counselling and HIV‐testing but unacceptable loss to follow up in a prevention of mother‐to‐child HIV transmission programme in rural Malawi: scaling‐up requires a different way of acting. Trop Med Int Health 2005;10:1242–50. [DOI] [PubMed] [Google Scholar]
- 35. Geelhoed D, Lafort Y, Chissale É, Candrinho B, Degomme O. Integrated maternal and child health services in Mozambique: structural health system limitations overshadow its effect on follow‐up of HIV‐exposed infants. BMC Health Serv Res 2013;13:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pfeiffer J, Montoya P. Integration of HIV/AIDS services into African primary health care: lessons learned for health system strengthening in Mozambique—a case study. J Int AIDS Soc 2010;13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Conkling M, Shutes EL, Karita E, Chomba E, Tichacek A, Sinkala M, et al. Couples' voluntary counselling and testing and nevirapine use in antenatal clinics in two African capitals: a prospective cohort study. J Int AIDS Soc 2010;13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Myer L, Zulliger R, Black S, Pienaar D, Bekker L‐G. Pilot programme for the rapid initiation of antiretroviral therapy in pregnancy in Cape Town, South Africa. AIDS Care 2012;94:986–92. [DOI] [PubMed] [Google Scholar]
- 39. Uwimana J, Jackson D. Integration of tuberculosis and prevention of mother‐to‐child transmission of HIV programmes in South Africa. Int J Tuberc Lung Dis. 2013;17:1285–90. [DOI] [PubMed] [Google Scholar]
- 40. Dinh T‐H, Kamb ML, Msimang V, Likibi M, Molebatsi T, Goldman T, et al. Integration of preventing mother‐to‐child transmission of HIV and syphilis testing and treatment in antenatal care services in the Northern Cape and Gauteng provinces. South Africa. Sex Transm Dis 2013;40:846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stinson K, Jennings K, Myer L. Integration of antiretroviral therapy services into antenatal care increases treatment initiation during pregnancy: a cohort study. PLoS ONE 2013;8:e63328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bronzan RN, Mwesigwa‐Kayongo DC, Narkunas D, Schmid GP, Neilsen G, Ballard RC, et al. On‐site rapid antenatal syphilis screening with an immunochromatographic strip improves case detection and treatment in rural South African clinics. Sex Transm Dis 2007;34(7 Suppl):S55–60. [DOI] [PubMed] [Google Scholar]
- 43. Gounder CR, Wada NI, Kensler C, Violari A, McIntyre J, Chaisson RE, et al. Active tuberculosis case‐finding among pregnant women presenting to antenatal clinics in Soweto, South Africa. J Acquir Immune Defic Syndr 2011;57:e77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bancheno WM, Mwanyumba F, Mareverwa J. Outcomes and challenges of scaling up comprehensive PMTCT services in rural Swaziland. Southern Africa. AIDS Care 2010;22:1130–5. [DOI] [PubMed] [Google Scholar]
- 45. Watson‐Jones D, Oliff M, Terris‐Prestholt F, Changalucha J, Gumodoka B, Mayaud P, et al. Antenatal syphilis screening in sub‐Saharan Africa: lessons learned from Tanzania. Trop Med Int Health 2005;10:934–43. [DOI] [PubMed] [Google Scholar]
- 46. Simba D, Kamwela J, Mpembeni R, Msamanga G. The impact of scaling‐up prevention of mother‐to‐child transmission (PMTCT) of HIV infection on the human resource requirement: the need to go beyond numbers. Int J Health Plann Manage 2010;25:17–29. [DOI] [PubMed] [Google Scholar]
- 47. Kim LH, Arinaitwe E, Nzarubara B, Kamya MR, Clark TD, Okong P, et al. Acceptability and feasibility of serial HIV antibody testing during pregnancy/postpartum and male partner testing in Tororo. Uganda. AIDS Care 2014;26:360–6. [DOI] [PubMed] [Google Scholar]
- 48. Homsy J, Kalamya JN, Obonyo J, Ojwang J, Mugumya R, Opio C, et al. Routine intrapartum HIV counseling and testing for prevention of mother‐to‐child transmission of HIV in a rural Ugandan hospital. J Acquir Immune Defic Syndr 2006;42:149–54. [DOI] [PubMed] [Google Scholar]
- 49. Strasser S, Bitarakwate E, Gill M, Hoffman HJ, Musana O, Phiri A, et al. Introduction of rapid syphilis testing within prevention of mother‐to‐child transmission of HIV programs in Uganda and Zambia: a field acceptability and feasibility study. J Acquir Immune Defic Syndr 2012;61:e40–6. [DOI] [PubMed] [Google Scholar]
- 50. Perez F, Orne‐Gliemann J, Mukotekwa T, Miller A, Glenshaw M, Mahomva A, et al. Prevention of mother to child transmission of HIV: evaluation of a pilot programme in a district hospital in rural Zimbabwe. BMJ Br Med J 2004;329:1147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Song J, Feng T, Bulterys M, Zhang D, Korhonen C, Shi X, et al. An integrated city‐driven perinatal HIV prevention program covering 1.8 million pregnant women in Shenzhen, China, 2000 to 2010. Sex Transm Dis 2013;40:329–34. [DOI] [PubMed] [Google Scholar]
- 52. Munkhuu B, Liabsuetrakul T, Chongsuvivatwong V, McNeil E, Janchiv R. One‐stop service for antenatal syphilis screening and prevention of congenital syphilis in Ulaanbaatar, Mongolia: a cluster randomized trial. Sex Transm Dis 2009;36:714–20. [DOI] [PubMed] [Google Scholar]
- 53. Amornwichet P, Teeraratkul A, Simonds RJ, Naiwatanakul T, Chantharojwong N, Culnane M, et al. Preventing mother‐to‐child HIV transmission: the first year of Thailand's national program. JAMA 2002;288:245–8. [DOI] [PubMed] [Google Scholar]
- 54. Malyuta R, Newell M‐L, Ostergren M, Thorne C, Zhilka N. Prevention of mother‐to‐child transmission of HIV infection: Ukraine experience to date. Eur J Public Health 2006;16:123–7. [DOI] [PubMed] [Google Scholar]
- 55. Kali PB, Gray GE, Violari A, Chaisson RE, McIntyre JA, Martinson NA. Combining PMTCT with active case finding for tuberculosis. J Acquir Immune Defic Syndr [Internet] 2006;42:379–81. [DOI] [PubMed] [Google Scholar]
- 56. Van Der Merwe K, Chersich MF, Technau K, Umurungi Y, Conradie F, Coovadia A. Integration of antiretroviral treatment within antenatal care in Gauteng Province, South Africa. J Acquir Immune Defic Syndr 2006;43:577–81. [DOI] [PubMed] [Google Scholar]
- 57. Ginsburg AS, Hoblitzelle CW, Sripipatana TL, Wilfert CM. Provision of care following prevention of mother‐to‐child HIV transmission services in resource‐limited settings. AIDS 2007;21:2529–32. [DOI] [PubMed] [Google Scholar]
- 58. Bhutta ZA, Chopra M, Axelson H, Berman P, Boerma T, Bryce J, et al. Countdown to 2015 decade report (2000–10): taking stock of maternal, newborn, and child survival. Lancet 2010;375:2032–44. [DOI] [PubMed] [Google Scholar]
- 59. Ten Hoope‐Bender P, Liljestrand J, MacDonagh S. Human resources and access to maternal health care. Int J Gynaecol Obstet [Internet] 2006;94:226–33. [DOI] [PubMed] [Google Scholar]
- 60. Sripipatana T, Spensley A, Miller A, McIntyre J, Sangiwa G, Sawe F, et al. Site‐specific interventions to improve prevention of mother‐to‐child transmission of human immunodeficiency virus programs in less developed settings. Am J Obstet Gynecol 2007;197(3 Suppl):S107–12. [DOI] [PubMed] [Google Scholar]
- 61. Biesma RG, Brugha R, Harmer A, Walsh A, Spicer N, Walt G. The effects of global health initiatives on country health systems: a review of the evidence from HIV/AIDS control. Health Policy Plan [Internet] 2009;24:239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Horwood C, Haskins L, Vermaak K, Phakathi S, Subbaye R, Doherty T. Prevention of mother to child transmission of HIV (PMTCT) programme in KwaZulu‐Natal, South Africa: an evaluation of PMTCT implementation and integration into routine maternal, child and women's health services. Trop Med Int Heal [Internet] 2010;15:992–9. [DOI] [PubMed] [Google Scholar]
- 63. Samuel NM, Srijayanth P, Dharmarajan S, Bethel J, Van Hook H, Jacob M, et al. Acceptance of HIV‐1 education & voluntary counselling/testing by & seroprevalence of HIV‐1 among, pregnant women in rural south India. Indian J Med Res 2007;125:49–64. [PubMed] [Google Scholar]
- 64. Byamugisha R, Tylleskar T, Kagawa MN, Onyango S, Karamagi CA, Tumwine JK. Dramatic and sustained increase in HIV‐testing rates among antenatal attendees in Eastern Uganda after a policy change from voluntary counselling and testing to routine counselling and testing for HIV: a retrospective analysis of hospital records, 2002–20. BMC Health Serv Res 2010;10:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tudor CL, Brusamento S, Elmoniry H, van Velthoven MHMMT, Pape UJ, Welch V, et al. The uptake of integrated perinatal prevention of mother‐to‐child HIV transmission programs in low‐ and middle‐income countries: a systematic review. PLoS ONE 2013;8:e56550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Atun R, Jaffar S, Nishtar S, Knaul FM, Barreto ML, Nyirenda M, et al. Improving responsiveness of health systems to non‐communicable diseases. Lancet 2013;381:690–7. [DOI] [PubMed] [Google Scholar]
- 67. Manzi F, Schellenberg JA, Hutton G, Wyss K, Mbuya C, Shirima K, et al. Human resources for health care delivery in Tanzania: a multifaceted problem. Hum Resour Health 2012;10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Medline Search Strategy.


