Abstract
Outer membrane vesicles (OMVs) are released spontaneously during growth by many Gram‐negative bacteria. They present a range of surface antigens in a native conformation and have natural properties like immunogenicity, self‐adjuvation and uptake by immune cells which make them attractive for application as vaccines against pathogenic bacteria. In particular with Neisseria meningitidis, they have been investigated extensively and an OMV‐containing meningococcal vaccine has recently been approved by regulatory agencies. Genetic engineering of the OMV‐producing bacteria can be used to improve and expand their usefulness as vaccines. Recent work on meningitis B vaccines shows that OMVs can be modified, such as for lipopolysaccharide reactogenicity, to yield an OMV product that is safe and effective. The overexpression of crucial antigens or simultaneous expression of multiple antigenic variants as well as the expression of heterologous antigens enable expansion of their range of applications. In addition, modifications may increase the yield of OMV production and can be combined with specific production processes to obtain high amounts of well‐defined, stable and uniform OMV particle vaccine products. Further improvement can facilitate the development of OMVs as platform vaccine product for multiple applications.
Keywords: Lipopolysaccharide, Neisseria, Outer membrane vesicle, Pertussis, Vaccine
Short abstract
Outer membrane vesicles (OMVs) are released spontaneously during growth by many Gram‐negative bacteria. They present a range of surface antigens in a native conformation and have natural properties like immunogenicity, self‐adjuvation and uptake by immune cells which make them attractive for application as vaccines against pathogenic bacteria. In particular with Neisseria meningitidis, they have been investigated extensively and an OMV‐containing meningococcal vaccine has recently been approved by regulatory agencies. Genetic engineering of the OMV‐producing bacteria can be used to improve and expand their usefulness as vaccines. Recent work on meningitis B vaccines shows that OMVs can be modified, such as for lipopolysaccharide reactogenicity, to yield an OMV product that is safe and effective. The overexpression of crucial antigens or simultaneous expression of multiple antigenic variants as well as the expression of heterologous antigens enable expansion of their range of applications. In addition, modifications may increase the yield of OMV production and can be combined with specific production processes to obtain high amounts of well‐defined, stable and uniform OMV particle vaccine products. Further improvement can facilitate the development of OMVs as platform vaccine product for multiple applications.
Abbreviations:
D‐OMV, OMV extracted from cells with detergent; EMA, European Medicine Agency; FDA, Food and Drugs Administration (US); fetA, iron‐regulated OM protein of Neisseria ; fHbp, factor H binding protein; FliC, flagellin; GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; IM, inner (or cytoplasmic) membrane; LOS, lipo‐oligosaccharide; Lpp, Braun lipoprotein; LPS, lipopolysaccharide; MenB, Meningitis type B; MS, mass spectroscopy; NadA, Neisseria adhesine A; NHBA, neisserial heparin binding antigen; N‐OMV, OMV extracted from cells with detergent‐free methods; OM, outer membrane ; OmpA, outer membrane protein A; OMV, outer membrane vesicle; opC, opacity‐associated protein C; PAMPs, pathogen‐associated molecular patterns; PE, phosphatidylethanolamine; PG, peptidoglycan; PL, phospholipid; porA, porine A; PQS, Pseudomonas quinolone signal; S‐OMV, OMV spontaneously released by cells
1 Introduction
1.1 General
The release of various types of membrane vesicles is widespread, from prokaryotes to more complex eukaryotic cells [1]. It has been known for decades that Gram‐negative bacteria shed outer membrane vesicles (OMVs), but they are also released by other groups such as Gram‐positive bacteria [2, 3, 4], mycobacteria [5], and archaea [6]. There is increasing interest in vesicles and recruitment of the immune system for medical applications which could be linked to the finding that 60% of the available pharmaceutical drugs exert their effect through interaction with membrane proteins [7]. A few years after their discovery, research started to explore OMVs as vaccine product, in particular for the application against meningitis B disease. In 2013 a meningitis serogroup B vaccine, Bexsero, was approved by the EMA, which contains a bacterial OMV component [8]. This review will focus on bacterial outer membrane vesicles as platform technology for vaccines, giving an overview of current possibilities and limitations
1.2 OMV as vaccine
A classical human vaccine is a pharmaceutical product that stimulates the immune system to prevent pathogens from causing disease. To evoke a broad and long‐lasting immune response, involving both innate and adaptive immune systems, a vaccine product should resemble a pathogen without causing the associated disease (Fig. 1) [9, 10, 11, 12]. This implies that a vaccine product should have a proper size and should contain both PAMPs as well as pathogen specific antigens. In addition there are a number of properties the vaccine product should not have, because pathogens have developed a number of immune evasion strategies, such as creating enormous variety of certain surface components, mimicry with host components, production of proteases that degrade antibodies or developing biofilms.
Figure 1.
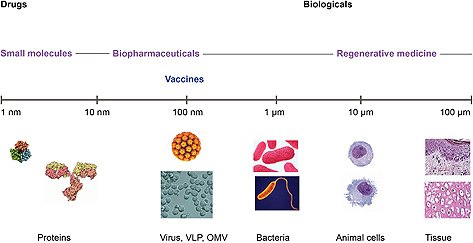
Vaccines as therapeutic products, positioned in complexity and size between well‐characterized recombinant proteins and less‐defined tissue products. For recombinant protein products, as monoclonal antibodies, the detailed molecular structure is usually known and there is scientific understanding on how active components of the product exert their therapeutic effect in humans (structure‐function relationship). Such detailed knowledge on a molecular level is not available for the regenerative medicine products, such as modified cartilage or skin tissue. Presently, vaccines seem to possess an in‐between position which affects the development strategy.
OMVs have a proper size (20–200 nm) to enable their entry into lymph vessels and uptake by antigen presenting cells [9]. They naturally contain components that stimulate humoral and cell‐mediated immune responses [13], since they resemble the bacterial antigenic surface of the pathogen.
The main challenges that may have to be addressed for OMV vaccine development include: (i) the high reactogenicity of PAMPs like LPS; (ii) low expression levels of relevant protective antigens; (iii) strain variation resulting in many subtypes of specific antigens, thus lower coverage; (iv) immuno‐dominant antigens that misdirect the immune response; and (v) molecules which are immunosuppressive or otherwise interfere with a protective immune response. Genetic engineering of the OMV‐producing strain can therefore be applied in many different ways to improve their vaccine application, by removing, adding, or altering OM proteins and other components.
2 Natural OMV
2.1 Composition of OMVs
Natural, or spontaneous, OMVs are spherical bi‐layered membrane structures with a diameter in the range of 20–250 nm, that are pinched off from the outer membrane of Gram‐negative bacteria [14, 15]. In some cases the diameter range is increased to 10–500 nm and may include various irregular shapes, similar to the variation observed for many enveloped viruses [15]. The OMV membrane contains phospholipids (PL) on the inside and LPS and PL on the outside, mixed with membrane proteins in various positions, largely reflecting the structure of the outer membrane [14, 16]. The lumen of the vesicle may contain various compounds from the periplasm or cytoplasm, such as proteins, RNA/DNA, and peptidoglycan (PG) [17, 18, 19, 20].
Emphasis of early research was on demonstrating that the OMVs indeed were formed from the outer membrane and not from IM or cytoplasmic components [16, 21], while current research is focusing more on which components from the cytoplasm, periplasm, or IM are directed towards the OMV as specific cargo and which OM proteins and lipids are included or excluded from the OMV [22, 23].
The used purification procedure for the OMVs as well as the use of specific mutant strains will affect the OMV composition. In addition the protein profile may change depending on the growth phase, media composition, or a specific stress signal that induces OMV formation [24, 25, 26].
2.1.1 Protein composition
The overall protein composition of purified OMVs was first analyzed with SDS‐PAGE which can show the most abundant membrane proteins based on their molecular weight, while ELISA, flow cytometry, and Western blot or mass spectrometry (MS) in combination with 1D or 2D‐gel electrophoreses can support identification of specific proteins. In proteomic studies of OMVs total numbers of identified proteins are in the range of 50–338, dependent on the technique and bacterium species, of which 40–80% was predicted to be OM protein [22, 23, 24, 27, 28, 29, 30, 31, 32].
The identified proteins can be grouped in three categories: the basic constituents of the outer membrane, the specific cargo proteins in the lumen, and unknown or contaminating proteins.
To the first category belong the major OM proteins such as porines, OM protein parts of larger transport systems, adhesins, enzymes such as phospholipases and proteases, and flagellum or pilus proteins. Adhesins mediate binding of OMVs to specific target cells [18, 33].
In OMVs of flagella‐containing bacteria, usually flagellin (FliC) is found, even when elaborate purification techniques are used that should exclude flagella as contaminants of the OMV preparation [34]. For Pseudomonas aeruginosa the most abundant OM proteins identified in the OMVs were porines OprF and OprH/OprG, and flagellin, each representing >20% of the total protein content [29]. Flagellin was identified as the major discriminating protein, highly present in OMVs but hardly at all in other fractions. In natural OMVs of Neisseria meningitidis a total of 155 proteins were identified, including the membrane antigens used for MenB vaccine development such as porine A (PorA), factor H binding protein (fHbp), neisserial adhesine A (NadA) and opacity‐associated protein C (Opc) [23]. The content of porines and anchor proteins, connecting OM to PG and IM, were reduced in OMVs compared to the OM fraction, while complement regulatory proteins, auto‐transporters, proteins involved in iron and zinc acquisition, and a two‐partner secretion system were enriched in the vesicles.
The second category of specific cargo proteins can include a number of toxins, such as cytolysin A of enterotoxic Escherichia coli [35, 36], cholera toxin of Vibrio cholerae [37], vacuolating cytotoxin (VacA) of Helicobacter pylori [38], and cytolethal distending toxin (CDT) from Campylobacter jejuni [39], and enzymes such as proteases and ureases. The last category of proteins consists of proteins from other cellular compartments, which are not expected to be normal OMV components, but this category is likely to become smaller with improved isolation methods. However, even in highly purified fractions of spontaneous natural OMVs, a number of cytoplasmic and IM proteins is usually found [40]. Common cytoplasmic proteins found in OMVs include elongation factors (EF‐Tu), enzymes (enolase, GADPH), chaperones (GroEL), and heat shock protein (DnaK) [24, 41]. These proteins have also been identified as immunogenic surface proteins [35, 42]. Their presence may be an artefact resulting from cell fractionation, but some cytoplasmic proteins have been identified as “moonlighting” proteins, that seem to perform additional functions beyond their original cytoplasmic activity [43]. Examples are the enzyme enolase that functions as a plasminogen binding protein, and EF‐Tu, that may also serve as adhesine for binding to host cells [43, 44]. However, surface associated cytoplasmic proteins were not found in recent proteomic studies of spontaneously released OMVs in Porphyromonas gingivalis [31], nor in an analysis of the OM proteasome in E. coli [45]. Though no true detergents were used, it cannot be excluded that the applied purification methods and washing steps with surface active components caused the loss of associated proteins.
2.1.2 Lipid components (phospholipids and LPS)
The phospholipid composition of OMVs in E. coli was shown to be comparable to the OM, with phosphatidylethanolamine (PE) as most abundant phospholipid and elevated levels of phosphatidylglycerol and lyso‐PE compared with the cytoplasmatic membrane [46]. With alternative techniques a similar composition of OM and OMVs with respect to phospholipid content was confirmed for H. pylori [22]. The phospholipid content of OMVs has been found to differ for various Gram‐negative bacteria. OMVs of N. meningitidis contained phoshatidylglycerol and PE as most abundant lipids [47]. In P. aeruginosa it was found that phosphatidylglycerol and stearic acid were present at higher levels in OMVs compared to the OM [26, 48], indicating a higher membrane rigidity of the vesicles. For H. pylori, PE, and to a lesser extent, cardiolipin were found as major lipid constituents, while elevated amounts of lyso‐PE were found in OM and OMV compared to the IM [22, 49].
In H. pylori, one of the few bacteria containing cholesterol in the membrane, this lipid component was estimated to constitute 10% of the total lipids [22]. The OM of Borrelia burgdorferi also contains both free cholesterol and cholesterol glycolipids, which is contributing to the formation of lipid rafts [50]. While phospholipids are present in the inner leaflet of the OM for E. coli and other Enterobacteria, it can be a component of the outer leaflet in other bacterial species. LPS, however, is exclusively located at the outside surface of the OM. OMVs of P. aeruginosa were found to contain exclusively B‐band LPS, while in the OM both A‐ and B‐band LPS are present [51].
2.2 Function of OMVs
Several biological functions have been suggested for natural OMV release. One obvious possibility is that naturally occurring OMVs function as long distance delivery system of specific components [17, 41], protecting their cargo molecules from dilution and degradation. The transported components can be involved in acquiring nutrients, or help pathogens to create a suitable micro‐environment for survival or growth in a host by supporting transfer of various virulence factors [18, 52]. They can also function as decoys which bind and remove antibodies and other bactericidal components in e.g. serum, as suggested for OM blebs of Neisseria gonorrhoeae [53]. For a number of bacteria it has been shown that OMVs participate in the formation of biofilms and thereby increase survival in hosts [54], or in soil [55]. Production of OMVs has also been reported to aid in the bacterial defense against antibiotics, since OMV production seems to dilute the damaging effect of the membrane perturbing agent gentamycin [51]. Protection against bacteriophages is possible through capture and inactivation of virus particles [56].
The possibility of gene transfer between bacteria by OMVs of various bacteria was recently summarized [19], including the example of the transfer of antibiotic resistance by DNA in OMVs. The OMVs can contain plasmids, but also chromosomal DNA fragments or bacteriophage DNA [57].
Finally, there remains the possibility that OMVs are sometimes released for strictly physiological reasons, i.e. as a consequence of an imbalance between cell growth and outer membrane synthesis, resulting in release of excess membrane material as OMVs.
2.3 OMV formation
The biogenesis of OMVs can be described as a budding process creating vesicles with the outside of the outer membrane at the surface of the OMV [18, 38, 58]. No universal mechanism for OMV formation can be given, only a number of events that are involved. These include: (i) the breaking of connections between OM and Peptidoglycan (PG); (ii) the accumulation of components in the periplasmic space; and (iii) the activity of specific signal and effector molecules such as PQS (Pseudomonas quinolone signal) [59].
A number of mutations, especially those involving Braun lipoprotein (Lpp) [60], the Tol‐Pal system [61] of E. coli, and RmpM of N. meningitidis [62, 63], indicate the importance of losing anchoring structures for increased OMV production. In wild type E. coli the connections of OM with PG formed by Lpp are also in a dynamic equilibrium of breaking and binding [64]. Mutations affecting the enzymes involved in peptidoglycan degradation can also induce OMV production [65, 66]. A broad dissociation of connections between OM and PG layer does not seem to be an absolute requirement if accumulating components in the periplasm present an outward pressure [58, 67].
The initiation of the budding of an OMV by induction of membrane curvature has been associated with the lipid components of the membrane. Lysophospolipids, formed by one of the few enzymes present in the outer membrane, phospholipase‐A [68], and CL seem to be involved in increasing membrane curvature required to form vesicles [22]. The formation of lysophospholipids has also been suggested as stress signal and associated with destabilization of the membrane in H. pylori [69]. For E. coli, however, mutations involved in synthesis of LPS or PG did affect vesicle formation, but no correlation was found for OMV production and instability of the membrane [70]. PQS in P. aeruginosa was not just reported as an inducing signal but also as a direct effector for OMV formation [59, 71]. PQS has a higher affinity for LPS than for phospholipids and could thus contribute to membrane curvature. For P. aeruginosa it was suggested that the presence of B‐band type LPS in OMVs would add to the curvature of the vesicle by charge repulsion, because it is longer and more negatively charged than the A‐band LPS [72].
Current research does not support one particular mechanism for OMV formation but instead increases the number of possible mechanisms. During cell division, OMVs formed at septa are distinct from membrane vesicles formed along the cell body associated with local dissociation of the OM [73]. The septa OMVs were enriched with the TonB protein, while the cell body OMVs were enriched in flagellar proteins. The synthesis of FliC flagellum protein has been correlated with the formation of vesicles in E. coli, without yet presenting a mechanism for action [34]. The same holds true for outer membrane protein A (OmpA) that affects OMV formation in Acinetobacter baumannii [74] and V. cholerae [75, 76], although in that case the well‐known anchor function of OmpA proteins to PG may play a role.
2.3.1 Induction of OMV formation
In general a number of signals that induce OMV formation are associated with stress [77], such as temperature stress [32, 77, 78, 79], amino acid limitation [80, 81], antibiotics [51], or the absorption of phages [82]. For N. meningitidis it was shown that cysteine depletion causes oxidative stress which triggered OMV release [81]. Also a number of media components can induce OMV production such as hexadecane for Acinetobacter [83], glucose for Yersinia [32, 84] and sodium carbonate for V. cholerae [85]. In P. aeruginosa the signal molecule PQS promotes OMV release [59], or under anoxic conditions, when no PQS can be synthesized, the bactericidal toxin pyocin [86].
Most early publications connect OMV formation with cell growth, since bleb formation was not observed in the stationary phase [87, 88], and cells growing at exponential phase produced more OMVs [79, 89]. The pattern is now mixed, with higher release of OMVs during late culture phase reported for N. meningitidis [90] and Francisella [91], while Salmonella typhimurium [73, 92], Borrelia [93], and Gallibacterium [94] seem to produce more vesicles during exponential growth. For H. pylori the release of OMVs is inversely related to the cell growth and is also correlated with shape transitions of the bacterium from spiral to coccoid [22]. Legionella pneumophila forms OMVs during intracellular and extracellular growth and in all phases of the cell growth cycle [95]. In general OMVs seem to be constitutively released by Gram‐negative bacteria during all growth phases [96], also during normal stress‐free growth conditions [21, 97]. Enhanced OMV production can be induced by specific signals, such as limitation at the end of the growth phase [38, 78, 81].
2.3.2 Cargo selection
Differences found in protein composition between OMV and OM are used as indication for the existence of a specific mechanism for exclusion of certain membrane proteins during the formation of OMVs and the selection of specific cargo proteins to include in OMVs [41, 98]. A proteomic study analyzing differences in OM and OMV in Bacteroides fragilis identified 40 unique proteins in the OMVs, in particular glycosidases and proteases [99], illustrating that OMV formation is not only the non‐specific entrapment of periplasmic components in the lumen of the OMV [29, 59, 100]. The exact mechanism by which specific proteins are sorted to OMVs is not yet known [15, 18], but some possibilities have been reported.
For Lysobacter a model was described for the excretion of proteases by OMVs [101]. The bacteriolytic enzymes are transported to the periplasmic space via the Sec transport system, and those enzymes having a high affinity for association with the inner leaflet of the OM have a higher probability to be included in OMVs. In P. gingivalis virulence factors and proteins containing a C‐terminal secretion signal, that directs proteins to the cell surface via a new‐type secretion system, are enriched in OMVs [31]. In the same organism it was found that LPS is involved in a mechanism responsible for the sorting of virulence factors towards OMVs, while major OM proteins were excluded [102]. For V. cholerae the periplasmic protein DegP, which has both a chaperone and protease function, was shown to be involved in the selection of nine cargo proteins as OMV content [85]. The presence of cholesterol and lipid rafts in B. burgdorferi could indicate involvement of these microdomain membrane structures in biological sorting processes, as has been reported for eukaryotic systems [15, 50]. Finally, the PG anchor proteins may not be randomly distributed over the cell surface but instead concentrated in specific micro‐domains, which are than less likely to be released as OMVs [23]. Electron microscopy studies also point to the existence of substantial heterogeneity in OM structure within a single cell [103].
Besides proteins, OMVs can also transport PG, RNA and DNA. OMVs from N. gonorrhoeae, H. pylori, P. aeruginosa, and V. cholerae were shown to contain PG, which contributed to the innate immune response in host cells [104, 105, 106]. For P. aeruginosa and a number of other bacteria, DNA has been investigated as content of OMVs for transformation to other bacteria [18, 55, 78, 107]. For Haemophilus influenzae OMVs it was shown that 94% of the DNA was inside regular sized (20–200 nm) OMVs, while 6% was on the surface which has been associated with biofilm formation [108]. Presence of RNA in OMVs was indicated more than 25 years ago [109] and recently analyzed for E. coli [110].
3 OMVs vaccine development
3.1 Development of specific types of OMV vaccines
The development and use of OMVs as vaccine has received most attention for Meningitis type B (MenB). Thus far only MenB OMV vaccine products have been tested in clinical studies (Table 1), but the performance of OMVs for various other diseases has been tested in rodents (Table 2). For meningococcal OMV vaccines a number of basic variants have been developed. First, there are non‐recombinant, wild‐type OMVs, derived from a particular strain and therefore monovalent in specific antigen content, mainly produced for the control of MenB outbreaks caused by a single clone [111]. Various forms of recombinant and/or multivalent OMV vaccines have also been used and will be discussed below.
Table 1.
Overview Neisseria meningitidis type B or Neisseria lactamica OMV vaccines tested in clinical trials
| Type OMV | Active component | Dose | Adjuvants | Response | Additional info | Reference |
|---|---|---|---|---|---|---|
| D‐OMV/proteoliposome | Multiple, OMPs (PorA), Men C polysaccharide, LPS | 2 × IM 50 μg OMP 50 μg polysaccharide. 1% LPS | AlOH | Efficacy 83% | no severe or long lasting side effects VA‐Mengoc‐BC | [120] |
| D‐OMV | multiple | 2 × 25 μg | ALOH | Efficacy estimated at 57% | MenBvac Includes capsular B polysaccharide | [115, 119] |
| D‐OMV | Multiple | 2 × 25 or 50 μg IM | AlOH | Efficacy 73–85% | MeNZB | [111, 170] |
| D‐OMV | 3 × 45 or 90 μg PorA IM; 3 × 90 μg PorA | AlPO4 | Induction of bactericidal antibodies (SBA) | Hexamen, phase I and phase II study | [171, 172, 173] | |
| 6 PorA subtypes | ||||||
| N‐OMV | OpcA (+ multiple) | 3 × 25 or 50 μg IM | +/– AlOH | Induction of SBA | Modified LPS, Lpx L2 | [174] |
| N‐OMV | fHbp, LOS, OpcA, PorA | 3 × 10, 25, 50 or 75 μg IM | AlOH | Induction of SBA | Modified LPS, Lpx L1 | [175] |
| D‐OMV | NHBA, fHbp, NadA, PorA | 1–4 doses, IM 50 μg NhbA, 50 μg fHbp, 50 μg NadA, 25 μg OMV/PorA | AlOH | Induction (SBA) | Bexero, multiple clinical studies | [8] |
| Neisseria lactamica, D‐OMV | multiple | 3–4 doses 25 μg IM, | AlOH | High specific antibody response against N lactamica | Weak broad humoral response towards N. meningitidis | [124, 176] |
IM intramuscular
Table 2.
Overview OMV vaccines tested in mice (or other rodents)
| Pathogen | disease | Type OMV | Active component | Dose | Adjuvants | Response | Additional info | Reference |
|---|---|---|---|---|---|---|---|---|
| Acinetobacter baumannii | Pneumonia, meningitis, sepsis | S‐OMV | Multiple | 2 × 50 μg IM | ALPhos | Humoral response (specific IgG and IgM) and protection in challenge study | Specific mouse model for sepsis | [177] |
| Bordetella pertussis | Whooping cough | D‐OMV (EDTA, sonication + DOC) | Multiple | 2 × 5 μg protein IP | AlOH IP; no IN | Protection against IN challenge (comparable to whole inactivated pathogen vaccine); Also innate | Reactogenicity: some loss in weight gain test (mice) | [132] |
| id | id | D‐OMV (sonication + DOC) | Multiple | 2 × 3 μg, 20 μg IN | No | Protection against IN challenge (both 3 and 20 μg) | LPS PagL modified; no weight loss; proper IL‐6 respons | [133] |
| Bordetella pertussis and B. parapertussis | id | D‐OMV | Multiple | 2 × 3 μg IN | AlOH | Protection against IN challenge | Formalin inactivated; LPS of parapertussis less reactogenic | [134] |
| Borrelia burgdorferi | Lyme | N‐OMV (vortexed, sucrose gradient) | Multiple, including DpbA, OspA and OspC | 4 × 60 μl OMV from 109 spirochetes IM and ID | No | Protection against challenge; various antibodies measured | Tested in rabbits, no LPS modification required | [168] |
| Brucella melitensis | Brucellosis | S‐OMV | Multiple | 2 × 5 μg IM | no | Protection against challenge (smooth 2 log; rough 3 log units) | cytokine expression of APC (DC) | [178] |
| Burkholderia mallei & pseudomallei | Melioidosis | S‐OMV | Multiple | 3 × 2.5 μg SC & IN | no | Protection against nasal challenge for SC; reduction of pathogen no elimination; cellular & humoral response | no LPS removal required IN not effective | [140] |
| Burkholderia pseudomallei | Melioidosis | S‐OMV (precipitation) | Multiple | 3 × 5 μg SC | no | Protection against challenge | Diverse bactericidal antibody response | [179] |
| Chlamydia muridarum antigen in E. coli | Chlamydia | S‐OMV | Serine protease HtrA (DegP) | 3 × 50 μg IM (total protein) | AlOH | Induction of neutralizing antibodies | Antigen coupled to E. coli OmpA; No modified LPS | [147] |
| Francisella (strains) | Tularemia | S‐OMV | multiple | 1 × 20 μg IN | no | Protection against challenge | No LPS reduction required | [141] |
| Helicobacter pylori and H. felis | Gastritis, ulcer, gastric | S‐OMV | multiple | 4 × 50 μg IG | Cholera toxin 10 μg | Protection against challenge cancer | Mucosal IgA ; no cross protection | [137, 180] |
| Klebsiella pneumoniae | Lung infections | S‐OMV | Multiple | 20 μg IT | NA | Cytokine induction; innate response. OMVs induce lung pathology in mouse pneumonia model | No damage in vitro cell culture | [181] |
| Neisseria meningitidis type B | Meningitis | N‐OMV | Multiple (iron limited culture) | 2 × 0.1 – 20 μg IN or IP | No. | Bactericidal antibodies (SBA) | IN requires 8–10 times higher dose than IP; bacteria warmed and sheared | [112] |
| id | id | S‐OMV/N‐OMV TFF | fHbp, (+ multiple) | 2 × 2.5 μg IP | AlOH | High antibody titers with bactercidal effect (SBA) | Bacteria killed with phenol; modified LPS | [129] |
| id | id | S‐OMV | Multiple (increased fHbp) | 3 × 0.2, 2, or 5 μg | AlOH | Induction bactericidal antibodies; cross protection | Engineered GMMA, modified LpxL1 | [128] |
| Neisseria lactamica | D‐OMV | Multiple | 2 × 10 μg IP | AlOH | Strain specific Bactericidal antibodies (SBA) | No Cross‐reactive SBA, but antisera did mediate opsonophagocytosis | [182] | |
| Porphy‐romonas gingivalis | Chronic periodontitis | S‐OMV (ammonium sulph precipitat) | Multiple | 2 × 100 μg SC | IFA | Protection against induction of lesions; IgG and IgM antibody response | Comparison with LPS, OMF | [135] |
| Pseudomonas aeruginosa | Lung infections | S‐OMV TFF | Multiple | 6 × 500 μg IN | No LPS) | Protection against challenge | potent innate immune response; induction of pro‐inflammatory cytokines; TLR‐4 | [138] |
| Salmonella typhimurium | Typhoid fever, gastroenteritis | S‐OMV (AmSulph precipitation) | Multiple | 100 μg IP, repeated | No (NA) | Stimulation of macrophages and dendritic cells; pro‐inflammatory cytokines | Innate response, involving but not only relying on TLR‐4 | [92] |
| Shigella | Shigellosis | Multi serotype | 4 × 50 μg oral | No (ref to LPS) | Transfer of passive immunity to offspring | [183] | ||
| Vibrio cholerae | Cholera | S‐OMV | Multiple | 3 × 25 μg IN, IG, 1 × 1 μg + 2 × 0.25 μg IP | No | Elevated Ig titer against OMV antigens; IgA by mucosal route (IN best); Protection against challenge in offspring (prevent colonization) | Long lasting (3 months) response | [136] |
| id | id | S‐OMV | Multiple | 3 × 25 μg | no | Protection against challenge in offspring (prevent colonization) | oral response less robust than intranasal | [139] |
IP, intraperitoneal; IM, intramuscular; ID, intradermal; IN, intranasal; IG, intragastric; IT, intratracheal; SC, subcutaneous; IFA, incomplete Freunds adjuvants ; APC, antigen presenting cell; DC, dendritic cell
Spontaneous – or natural – S‐OMVs are purified and concentrated from culture supernatant, by separating intact cells from the already formed OMVs. The detergent D‐OMVs, are extracted from cells with detergent which also reduces the content of reactogenic LPS. The term native N‐OMV is used for intact spontaneous OMVs from cell supernatant [40], as well as for OMVs that are generated from concentrated dead cells with non‐detergent cell disruption techniques [90, 112]. Here, native N‐OMV will be used for vesicles extracted from cells with detergent–free methods, in accordance with previous nomenclature, and to be able to clearly distinguish them from the wild‐type spontaneous OMVs and the detergent‐extracted OMVs.
3.1.1 OMV (MenB) historical overview
For several serogroups of N. meningitidis effective conjugate vaccines consisting of capsular polysaccharide coupled to a carrier protein, are on the market. However, for serogroup B this approach was not feasible because this polysaccharide has homology to molecular structures in the human brain, generating the risk of autoimmunity [113]. Therefore, MenB vaccine development has mostly focused on other approaches, and OMVs have been in the picture for quite a few years [111, 114, 115].
An antigen which has received considerable attention is the immuno‐dominant OM protein porin A (PorA), which is one of the major proteins in the meningococcal OM and a target for effective bactericidal antibodies, both in animal studies and in human sera [111]. A major challenge for PorA‐based vaccines is the high sequence variability, which necessitates the inclusion of a high number of PorA subtypes to generate sufficient coverage [115]. However, by judiciously combining PorA with another antigen like FetA a limited number could still provide adequate coverage [116].
The availability of meningococcal genome sequences has enabled the search for novel antigens which might be used for the “holy grail” of a universal MenB vaccine. With this so‐called reverse vaccinology concept, genes encoding potential surface components were expressed in E. coli and the proteins tested for immunogenicity [117]. This omics approach initially yielded five suitable OM antigens, which are formulated as three recombinant proteins: a fragment of NadA, a fusion protein containing NHBA, and a fusion protein containing fHbp. D‐OMVs, as used for the outbreak in New Zealand, are added to the MenB vaccine product as adjuvant but might also contribute to the protective effect through the presence of PorA. This vaccine, Bexsero, has been approved for human use by the EMA and the FDA.
Traditionally, meningococcal OMV vaccines have been prepared with the use of DOC detergent to release the vesicles [115, 118]. This approach gives high yields of OMV material with reduced LPS content, but has some disadvantages such as loss of important protective lipoprotein antigens which are more loosely associated with the OM, compromised vesicle integrity, and increase of contaminating IM and cytoplasmic components. D‐OMV vaccines have been used successfully to combat outbreaks of meningitis in Cuba, Norway and New Zealand where monovalent detergent‐extracted OMVs were prepared from a manufacturing strain, matching the circulating strains causing the epidemic [111]. The efficacy of these wild‐type OMV vaccines was estimated at 83% in Cuba, 57% to 87% in Norway and minimally 70% in New Zealand [111, 115, 119, 120, 121]. For the three outbreaks, summarizing findings for the application of 60 million doses of wild type OMVs have been published [111], showing a consistent pattern of moderate reactogenicity and safety. In the last large outbreak in New Zealand three doses of 25 μg OMV were required to reach a sufficient level of protection. The immune response to wild‐type OMV vaccines is, in particular in infants, largely directed towards PorA [115].
This limits the OMV approach to epidemic outbreaks caused by a single clone, and in the more common endemic situations where multiple strains are circulating it will be inadequate. In the Netherlands, a multivalent PorA‐based OMV vaccine has been developed based on genetically engineered strains in which multiple porA genes were inserted. A single dose of 7.5 or 15 μg of D‐OMVs containing six subtypes of PorA evoked an at least four‐fold increase in bactericidal antibodies directed against most of the included PorA types in approximately half the number of volunteers in a clinical phase I study [122, 123]. This Hexamen vaccine, given with alum as adjuvant, showed a good safety profile. In a study to evaluate the feasibility of OMVs from N. lactamica as protective agent against N. meningitidis B based upon cross protection, demonstrated that N. lactamica derived OMVs did cause opsonophagocytosis of N. meningitidis but did not a evoke a specific cross‐protective humoral response [124]. An overview of clinical studies with meningococcal OMV vaccines is given in Table 1.
3.1.2 MenB OMV vaccines, recent development and current status
As mentioned before, D‐OMVs are included in the first generation vaccine product – Bexsero (Novartis) – that has been approved for the market. Recent developments towards a second generation MenB OMV product rather aim at the use of recombinant N‐OMVs or S‐OMVs. These S‐OMVs contain genetically detoxified, and therefore, less reactogenic LPS, thus avoiding the use of detergent and preventing the loss of surface associated antigens [125, 126, 127, 128]. The used Neisseria strains are deficient in capsular polysaccharide and make penta‐acylated LpxL1 LPS which does not need detergent extraction because of its low endotoxic activity. The strongly reduced induction of IL‐6 in human monocytes by OMVs containing this mutant form of LPS is a good indication for low in vivo reactogenicity [125], as this so‐called MAT assay has already been accepted by the EMA as alternative indicator for in vivo pyrogenicity.
Whereas the immunodominant antigen in D‐OMVs is PorA, N‐OMVs give a broader response and increased coverage because additional antigens are present, such as the surface‐exposed lipoprotein factor H‐binding protein [30, 129]. In an analysis of the bactericidal antibody response to N‐OMVs in rabbits it was found that the strongest response was against PorA, NadA and LOS, and to a lesser extend to fHbp [130]. An N‐OMV vaccine containing modified LpxL1 LPS with PorA, fHbp and the conserved outer membrane protein OpcA as prime antigens, has been tested in a phase I clinical study, generating appropriate serum bactericidal assay (SBA) activity, while causing acceptable reactogenicity [126]. In Africa serogroup A is the main cause of meningococcal meningitis, though type W and X are increasing subtypes. Spontaneous S‐OMVs from a serogroup W strain with overexpressed fHbp have been suggested as a novel approach, since they were also effective against serogroup A and X strains [128, 131].
3.2 Other human OMV vaccines; engineered homologous
3.2.1 Bordetella pertussis
The pertussis vaccine is one of the classical vaccines that was introduced as an whole‐cell inactivated product. During the 1990's it was largely replaced by a purified subunit vaccine, in the developed world market. This acellular vaccine contains inactivated pertussis toxin, filamanentous haemagglutinine and pertactin, and in some cases fimbriae. While acellular vaccines are less reactogenic than the classical whole cell vaccines, they have failed to effectively reduce pertussis infections; indeed, an increase has been seen in many countries which switched to acellular vaccines. Reasons may include selection of strain variation and waning immunity due to a lower memory response. It has prompted the search for novel, more effective approaches to pertussis vaccination. One option is the use of OMVs from B. pertussis, because many of the known virulence factors are OM components or are at least associated with the OM. An OMV preparation, prepared by sonication of cells and treatment with DOC, combined with alum adjuvant provided protection against pertussis challenge in a mouse model [132], which was comparable to the effect of a whole‐cell vaccine. Another version of OMVs containing a PagL‐deacylated modified LPS showed both protection and a lower reactogenicity, the latter determined in vivo by both weight gain and cytokine induction [133]. Another interesting finding with B. parapertussis OMVs was their cross‐protection against both pertussis and parapertussis [134]. The findings of OMVs of B. pertussis in mouse studies are summarized in Table 2.
3.2.2 Other bacterial species
For a number of human pathogens OMVs have been investigated in animal models, but none has yet progressed to the stage of clinical trials. The findings of these OMV vaccines in tests in mice or rabbits are presented in Table 2 (containing additional references [177, 178, 179, 180, 181, 182, 183]). The OMVs are usually S‐OMVs from selected strains without specific increased or modified antigenic determinants. Most OMVs evoke a diverse antibody response, including different IgG subtypes and IgM [135] or IgA after mucosal administration [136, 137], as well as a cell‐mediated immune response [92, 138]. The antibody response can be directed towards proteins as well as towards the LPS, as determined for H. pylori [139]. The active dose applied that provides protection is in the range of 1 to 500 μg, in most cases without the addition of adjuvants. As mentioned before, for some strains the LPS is already of low toxicity and does not need to be modified or extracted. Different routes of administration have been explored, which is also connected to the specific disease to be prevented (as e.g. intragastric for H. pylori). Intranasal immunization with OMVs did in this case not provide a protective effect [140], but did work for V. cholerae [136], P. aeruginosa [138], and Francisella [141].
4 Heterologous vaccine OMV
Genetic engineering can be used to introduce additional antigens into OMVs. First, antigens which are normally released from the cell surface by proteolytic processing can be retained by making mutants in which this is prevented. Second, novel antigens can be introduced in OMVs by targeting them to the outer membrane. Autotransporters have been used most often for such surface display, e.g. the Hbp protein from E. coli which was successfully applied for surface expression of Mycobacterium tuberculosis antigens in Salmonella [142]. While effective, this approach seems to be limited to relatively small fragments of foreign antigens. Surface display of larger domains is more challenging and may require other approaches, e.g. based on lipoprotein export systems [143]. Kesty and Kuehn tested E. coli OMVs as delivery system for heterologous expressed proteins [100]. First, the Ail protein from Y. enterocolitica was used to produce OMVs with a heterologous adhesin in the membrane, thereby stimulating their uptake by human colon cells. In addition, as an example of a heterologously expressed protein, GFP could be directed into the lumen of these OMVs. Foreign antigens may be targeted to the periplasm and when retained in OMVs, provide an alternative for surface display [144]. Remarkably, there are very little published data on the need for surface exposure in OMVs versus only the presence within the vesicles; this aspect deserves more attention. Finally, there is the possibility to add external antigens to OMVs in such a form that binding to specific outer membrane components is facilitated, circumventing the problems inherent in heterologous expression [145]. Several examples have been published where OMVs carrying heterologous antigens confer protective immunity against the target pathogen. A surface protein of the Gram‐positive pathogen Streptococcus pneumoniae, PspA, could be expressed in Salmonella enterica and directed to the periplasm, and collected in OMVs [146]. The OMVs containing PspA in the lumen of the vesicles protected intranasally immunized mice against a challenge with S. pneumoniae, while control mice receiving purified PspA or normal S. enterica OMVs were not protected. To generate an OMV‐based vaccine against Chlamydia the serine protease HtrA (degP) was coupled to the OmpA leader sequence of a high‐blebbing tolR deletion mutant E. coli [147]. The resulting recombinant heterologous Chlamydia HtrA‐E. coli OMVs were able to induce neutralizing antibodies in an in vitro infectivity assay, while recombinant purified HtrA did not generate such a response.
5 OMV and innate response
As bacteria‐derived products, OMVs will naturally contain a variety of molecules which can interact with the innate immune system. In the first place, LPS will be present as a major component of the outer leaflet of the outer membrane. It is a very potent activator of immune cells such as monocytes/macrophages, through specific recognition by the TLR4/MD2 receptor which results in triggering of NF‐kB and IRF3 mediated gene expression. The resulting activation is important in activating and directing the adaptive immune response, which is highly advantageous for vaccine applications of OMVs [148]. On the other hand, this inflammatory activation can also result in high vaccine reactogenicity. The use of mutants with defects in lipid‐A biosynthesis seems to provide a balanced response, generating sufficient adjuvant activity but preventing harsh side effects [63]. Inactivation of msbA in E. coli, or lpxL1 in N. meningitidis, results in loss of a secondary acyl chain in lipid A which leads to strongly reduced capacity to activate TLR4/MD2 [125, 149], enabling the development of a detergent‐free production process. Further fine‐tuning of the lipid‐A structure is possible by introducing lipid A modifying enzymes such as LpxE or PagL, leading to dephosphorylation or deacylation, respectively. In this way, LPS forms can be obtained with an optimal ratio between adjuvant activity and reactogenicity, e.g. by a bias towards the TRIF signaling pathway [150, 151]. In addition to LPS, OMVs always contain lipoproteins which are also activators of innate immunity through their recognition by TLR2 [152, 153]. This is more difficult to modify than LPS‐mediated activation because many different lipoproteins are present in OMVs, and their contribution to adjuvant activity and reactogenicity is therefore less well characterized. Also, downstream signaling pathways differ for TLR4 and TLR2 which may result in different immune bias. Nevertheless, TLR2 does also undoubtedly play an important role in OMV‐mediated activation of the immune response [154, 155]. Finally, recognition of various receptors on antigen‐presenting cells by surface components of OMVs can interfere positively or negatively with the immune response, and this can vary depending on the particulars of the pathogen used for OMV preparation. For instance, Opa proteins in the outer membrane of pathogenic Neisseria have been reported to lead to immunosuppression by their interaction with CEACAM receptors in human immune cells, and in vivo experiments with transgenic mice showed that this led to a reduced Opa‐specific antibody response [156]. Some LPS glycoforms can bind to receptors on dendritic cells, e.g. LgtB LPS from N. meningitidis to DC‐SIGN, leading to a Th1‐biased immune response [157]. Clearly, because many surface molecules of pathogenic bacteria have evolved to interact with the host immune system in a particular way, many further possibilities for fine‐tuning the use of OMVs as vaccines, are possible.
6 OMV process development
The design of a process to obtain a biopharmaceutical product starts with defining the target product profile (TPP), and the methods to measure this. Based on the TPP a suitable expression system can be determined to produce an OMV vaccine. For most bacterial vaccines this will include cultivation of a pathogenic bacterium, either genetically modified or not.
From the selected strains a master and a working cell bank (MCB, WCB) is generated that serves as a stable source for a manufacturing process. The standard vaccine production starts with the thawing of a vial from the WCB for an expansion culture, followed by the production fermentation, the down‐stream purification process (DSP) and the final filling of a – often combination or multivalent – vaccine product. During all steps of the manufacturing, in‐process controls will be taken to assess that the intermediate product meets pre‐set quality criteria. General objectives include avoiding animal‐derived components, chemically defined culture medium, and feasible sterile filtration of vaccine product. For the process development it makes a considerable difference whether an S‐OMV has to be produced from the supernatant of the fermentation harvest, or that a native N‐OMV has to be extracted from cells with detergent‐free or detergent containing techniques.
6.1 Strain development
For some applications wild‐type bacterial strains can be used directly to produce an OMV vaccine (Table 2), but in most cases, genetic engineering of the OMV‐generating bacteria is required to improve the vaccine product. Such modifications can include: (i) modifying the LPS biosynthesis pathway in order to obtain less endotoxic and reactogenic variants; (ii) overexpression of crucial antigens; (iii) simultaneous expression of multiple antigenic variants; (iv) outer membrane retention of normally secreted antigens; (v) increased OMV production by removing outer membrane anchor proteins; (vi) removal of immune‐modulating components which may trigger the wrong type of immune response; and (vii) expression of heterologous antigens from other pathogens than the host OMV producing strain.
An often used genetic modification of production strains is to delete genes encoding anchor proteins between OM and PG in order to increase vesicle formation. This can be done by inactivating the OmpA homologues which are commonly found in Gram‐negative bacteria, e.g. the RmpM protein in Neisseria [62, 63]. Other mutations which can have the same effect target the tol‐pal system [61], PG biosynthesis [66], or the cell envelope stress response [77]. However, while there are many reports on low versus high “blebbing” strains and conditions, very little comparative data have been published on OMV quantities produced by specific bacteria. Another crucial modification for most species is the reduction of LPS reactogenicity, for instance by inactivation of lpxL1 in Neisseria [125] or msbB in E. coli or Salmonella [158]. A third standard genetic modification is the introduction of additional antigens or the upregulation of crucial protective antigens. Examples for Neisseria are PorA and fHbp. Since PorA is a highly variable antigen, multiple antigenic variants are required to confer a sufficiently broad protection, and this has been achieved by constructing strains with multiple chromosomal copies [125]. For fHbp, it has been shown that induction of bactericidal antibodies is directly related to its expression level, therefore up‐regulation of expression by altering the promoter has been used [129].
Bacterial strains without capsular polysaccharide are generally preferred since these structures can interfere with the vesicle purification, as found for Klebsiella [159]. Since capsular polysaccharide is an essential virulence factor for many pathogenic bacteria, its removal has the additional benefit of rendering the production strains safer to handle. Many outer membrane antigens function by binding to some host receptor or ligand, and it has recently been found that in such cases their immunogenicity is reduced by this interaction [127, 156, 160]. By using mutants, which no longer bind their natural human ligand through small alterations in the binding site, one can increase their protective capacity.
6.2 Techniques USP
The start of the upstream process (USP), the expansion pre‐culture of bacteria, is generally performed in shaker flasks. For the MCB it could be chosen to prepare a lyophilized seed lot, since secured storage and initial slow growth in agar plate culture is more important than fast expansion. When sufficient inoculum has been generated in expansion cultures, a production fermentation can be started. The standard upstream process for bacterial vaccines contains a fermentation in a stirred tank reactor to yield a crude product [161]. For larger scale processes a first reactor vessel can be used to inoculate the larger production reactor.
Medium composition can be of influence on formation of OMVs by bacteria, as mentioned by the signals that can induce OMV formation (section 2.3.1), which is also illustrated by the difference of OMV yield in different media for Francisella tularensis [91]. Cell densities reached during production fermentations to produce OMV vaccines have been reported as an OD of 6 [162] and 9 [163] at 60 to 1200 L scale for N. meningitidis B strains, and an OD of 30–45 was reported for a high blebbing mutant of Shigella sonnei in a 5 L stirred bioreactor [164]. The yield of OMV corresponding to these fermentations were 40 mg/L for N. meningitidis strains [165] and 100 mg/L for the S. sonnei strain [164]. A challenge for the scale‐up of the fermentation process is to find alternatives for the use of antifoam, which is the standard method to prevent excessive foaming that is the result of the required aeration at higher cell densities. Most antifoams are not compatible with an OMV production process, because the surfactants can affect OMV integrity or will interfere with the OMV purification. Therefore, alternative techniques for mechanical foam breaking have been introduced as part of the scale‐up of the fermentation process [162].
The harvest of S‐OMVs of P. aeruginosa should be scheduled during the exponential phase, because protease synthesis can damage the S‐OMV composition during stationary phase [159], and the appearance of irregular membrane structures has been reported for H. pylori during stationary phase [38]. For N. meningitidis it seems beneficial for D‐OMVs and N‐OMVs yield to maintain the cell culture to end of the exponential phase [90, 163], though this has to be optimized versus the increasing level of contaminants such as DNA.
6.3 Techniques DSP OMV
6.3.1 Techniques DSP for S‐OMV
For the primary recovery of the S‐OMV product from cell suspension, the standard technique to separate bacteria from vesicles, is to apply low speed centrifugation (2000–10 000 xg). In some cases the separation of OMVs from intact bacteria or from smaller soluble components is performed by tangential flow microfiltration, using 10 kDa [50] or 50–100 kDa filters [138, 166] or a combination of 0.2 and 0.1 μm filters [163]. The separation of S‐OMVs from contaminants is combined with a filtration step through a 0.22 or 0.45 μm dead‐end filter to remove larger debris fragments. The S‐OMVs are collected with an ultracentrifugation (UC) step at 50 000–200 000 xg, which can be combined with density gradient UC [158, 159]. As alternative concentration step precipitation with ammonium sulfate can be performed [93, 158], followed by differential centrifugation.
Common contaminants during purification of S‐OMVs include pili, flagella and soluble components, and as for all DSP processes the steps to yield a higher purified OMV fraction will reduce the overall yield [159]. Many small scale purification steps for S‐OMVs are executed at 4°C, or with the addition of protease inhibitors, but for large‐scale applications this is usually avoided because of costs.
Purified OMVs are mostly resuspended in PBS and frozen at ‐20 to ‐80°C. The storage of S‐OMVs from H. pylori at ‐20°C did not change the structure of the vesicles [28].
6.3.2 Techniques DSP for N‐OMV and D‐OMV
For extracted vesicles the supernatant of the fermentation harvest containing spontaneous vesicles is discarded, and usually the cells are concentrated with centrifugation or tangential flow microfiltration. Subsequently, extraction of OMVs can be performed by detergent, with DOC as most frequently applied component to generate D‐OMVs [63, 115]. As mild chemical alternative to detergents EDTA [94, 163] has been tested, and sonication as a mechanical vesicle releasing technique [167] and vortexing [168] has been used to prepare an N‐OMV fraction. For Neisseria a combination of detergent‐free techniques [112, 130] has been performed to obtain N‐OMVs.
The further DSP to purify D‐OMVs and N‐OMVs make use of the same UC and TFF techniques as applied for the S‐OMVs. Long term stability in liquid solution at 4°C has been demonstrated for D‐OMVs and N‐OMVs [115, 133, 165].
7 Outlook
The development of vaccine products is slowly advancing from undefined whole inactivated or attenuated pathogens towards better characterized subunit vaccine products, resembling pathogen‐like structures. OMVs contain a number of features necessary for an effective vaccine product: a native configuration for membrane surface antigens to elicit a humoral response [13], the potential to evoke a T‐cell‐mediated immune response, the presence of several PAMPs to trigger the innate immune response, and a size that is appropriate for efficient processing by antigen‐presenting cells [9, 10, 11, 12]. The successful application of OMVs as platform structure for vaccines includes the optimization of the appropriate innate and adaptive immune responses by either removing or including specific components, which has to be individually evaluated for each disease application. A crucial but still unresolved issue is whether the antigens have to be surface exposed or merely present in the OMV. The example of MenB shows that an OMV can be modified, such as for LPS reactogenicity, to yield a ready to use OMV product that is safe and effective as demonstrated in SBA scores. Development of a target manufacturing process shows that high yield of a stable, sterile product of constant quality can be produced, according to state‐of‐the‐art conditions [164, 165].
With the given advantages, and the expected further decrease of the blind‐spots with respect to unknown host‐pathogen effects, the development of OMVs as platform vaccine is expected to enable a well‐controlled development for new vaccine products based upon scientific understanding of product and process, in line with a structural vaccinology approach [169].
Biographical Information
Leo van der Pol is principal scientist at Intravacc, the Institute for Translational Vaccinology in Bilthoven, The Netherlands. From 2005 to 2013 he was Head of Process Development at the NVI (Netherlands Vaccine Institute). Before this he was senior scientist at Contract Manufacturing Company DSM Biologics, involved in process development, scale‐up, process validation, and trouble shooting. During this 18 years of CMO experience, process development was also performed for various biopharmaceutical products. Leo van der Pol holds a PhD in Bioprocess technology from the Wageningen University & Reseach Centre (WUR). Recent research focuses on the development of new platform‐technology for vaccines.

Michiel Stork studied molecular microbiology in Utrecht and subsequently worked for six years at the Oregon health and Sciences University in Portland Oregon on the iron uptake of pathogenic Vibrio species. After returning to the Netherlands he received his PhD in molecular microbiology from the Vrije Universiteit in Amsterdam in 2006. After a postdoctoral fellowship at the Utrecht University where he worked on a vaccine against Neisseria meningitidis he moved to Intravacc where he currently leads the process development group for bacterial vaccines.

Peter van der Ley studied Biology at Utrecht University. He got his PhD degree in Molecular Microbiology from Utrecht University in 1987. He next went to the Rocky Mountain Laboratories (NIH) in Hamilton, Montana, USA, where he worked on antigenic variation in Neisseria gonorrhoeae. In 1988, he returned to the Netherlands to work at the RIVM on vaccine development against Neisseria meningitidis. He has continued to work in this lab, expanding his research into projects on pertussis, tuberculosis and innate immunity. Two short spells abroad were spent in Oxford and at the NIH. He has (co)‐authored ca. 120 scientific publications.

Acknowledgements
The authors declare no financial or commercial conflict of interest.
References
- 1. Deatherage, B. L. , Cookson, B. T. , Membrane vesicle release in bacteria, eukaryotes, and archea: A conserved yet underappreciated aspect of microbial life. Infect. Immun. 2012, 80, 1948–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dorward, D. W. , Garon, C. F. , DNA is packaged within membrane derived vesicles of gram‐negative but not gram‐positive bacteria. Appl. Environ. Microbiol. 1990, 56, 1960–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rivera, J. , Cordero, R. J. , Nakouzi, A. S. , Frases, S. et al., Bacillus anthracis produces membrane‐derived vesicles containing biologically active toxins. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 19002–19007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee, E. Y. , Choi, D. Y. , Kim, D. K. , Kim, J. W. et al., Gram‐positive bacteria produce membrane vesicles: Proteomics‐based characterization of Staphylococcus aureus‐derived membrane vesicles. Proteomics 2009, 9, 5425–5436. [DOI] [PubMed] [Google Scholar]
- 5. Prados‐Rosales, R. , Baena, A. , Martinez, L. R. , Luque‐Garcia, J. et al., Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2‐dependent manner in mice. J. Clin. Invest. 2011, 121, 1471–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ellen, A. F. , Albers, S‐V. , Huibers, W. , Pitcher, A. et al., Proteomic analysis of secreted membrane vesicles of archaeal Sulfolobus species reveals the presence of endosome sorting complex components. Extremophiles 2009, 13, 67–79. [DOI] [PubMed] [Google Scholar]
- 7. Hopkins, A. L. , Groom, C. R. , The druggable genome. Nat. Rev. Drug. Discov. 2002, 1, 727–730. [DOI] [PubMed] [Google Scholar]
- 8. O'Ryan, M. , Stoddard, J. , Toneatto, D. , Wassil, J. , Dull, P. M. , A multi‐component meningococcal serogroup B vaccine (4CMenB): The clinical development program. Drugs 2014, 74, 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bachmann, M. F. , Jennings, G. T. , Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [DOI] [PubMed] [Google Scholar]
- 10. Zepp, F. , Principles of vaccine design – lessons from nature. Vaccine 2010, C14–C24. [DOI] [PubMed] [Google Scholar]
- 11. Singh, M. , Chakrapani, A. , O'Hagan, D. , Nanoparticles and microparticles as vaccine‐delivery systems. Expert Rev. Vaccines 2007, 6, 797–808. [DOI] [PubMed] [Google Scholar]
- 12. Scheerlinck, J. P. , Greenwood, D. L. , Virus‐sized vaccine delivery systems. Drug Discov. Today 2008, 882–887. [DOI] [PubMed] [Google Scholar]
- 13. Ellis, T. N. , Kuehn, M. J. , Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuehn, M. J. , Kesty, N. C. , Bacterial outer membrane vesicles and the host‐pathogen interaction. Genes Dev. 2005, 19, 2645–2655. [DOI] [PubMed] [Google Scholar]
- 15. Kulp, A. , Kuehn, M. J. , Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beveridge, T. J. , Structures of gram‐negative cell walls and their derived membrane vesicles. J. Bacteriol. 1999, 181, 4725–4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mashburn‐Warren, L. M. , Whiteley, M. , Special delivery: Vesicle trafficking in prokaryotes. Mol. Microbiol. 2006, 61, 839–846. [DOI] [PubMed] [Google Scholar]
- 18. Bonnington, K. E. , Kuehn, M. J. , Protein selection and export via outer membrane vesicles. Biochim. Biophys. Acta 2014, 1843, 1612–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fulsundar, S. , Harms, K. , Flaten, G. E. , Johnsen, P. J. et al., Gene transfer potential of outer membrane vesicles of Acinetobacter baylyi and effects of stress on vesiculation. Appl. Environ. Microbiol. 2014, 80, 3469–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaparakis, M. , Turnbull, L. , Carneiro, L. , Firth, S. et al., Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell Microbiol. 2010, 12, 372–385. [DOI] [PubMed] [Google Scholar]
- 21. Wensink, J. , Witholt, B. , Outer‐membrane vesicles released by normally growing Escherichia coli contain very little lipoprotein. Eur. J. Biochem. 1981, 116, 331–335. [DOI] [PubMed] [Google Scholar]
- 22. Olofsson, A. , Vallström, A. , Petzold, K. , Tegtmeyer, N. et al., Biochemical and functional characterization of Helicobacter pylori vesicles. Mol. Microbiol. 2010, 77, 1539–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lappann, M. , Otto, A. , Becher, D. , Ulrich Vogel, U. , Comparative Proteome Analysis of Spontaneous Outer Membrane Vesicles and Purified Outer Membranes of Neisseria meningitidis. J. Bacteriol. 2013, 195, 4425–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berlanda Scorza, F. , Doro, F. , Rodriguez‐Ortega, M. J. , Stella, M. et al., Proteomics characterization of outer membrane vesicles from the extraintestinal pathogenic Escherichia coli DeltatolR IHE3034 mutant. Mol. Cell. Proteomics 2008, 7, 473–485. [DOI] [PubMed] [Google Scholar]
- 25. Tsolakos, N. , Lie, K. , Bolstad, K. , Maslend, S. et al., Characterization of meningococcal serogroup B outer membrane vesicle vaccines from strain 44/76 after growth in different media. Vaccine 2010, 28, 3211–3218. [DOI] [PubMed] [Google Scholar]
- 26. Baumgarten, T. , Sperling, S. , Seifert, J. , von Bergen, M. et al., Membrane vesicle formation as a multiple‐stress response mechanism enhances Pseudomonas putida DOT‐T1E cell surface hydrophobicity and biofilm formation. Appl. Environ. Microbiol. 2012, 78, 6217–6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferrari, G. , Garaguso, I. , Adu‐Bobie, J. , Doro, F. et al., Outer membrane vesicles from group B Neisseria meningitidis delta gna33 mutant: Proteomic and immunological comparison with detergent‐derived outer membrane vesicles. Proteomics 2006, 6, 1856–1866. [DOI] [PubMed] [Google Scholar]
- 28. Mullaney, E. , Brown, P. A. , Smith, S. M. , Botting, C. H. et al., Proteomic and functional characterization of the outer membrane vesicles from the gastric pathogen Helicobacter pylori. Proteomics Clin. Appl. 2009, 3, 785–796. [DOI] [PubMed] [Google Scholar]
- 29. Choi, D. S. , Kim, D. K. , Choi, S. J. , Lee, J. et al., Proteomic analysis of outer membrane vesicles derived from Pseudomonas aeruginosa. Proteomics 2011, 11, 3424–3429. [DOI] [PubMed] [Google Scholar]
- 30. van de Waterbeemd, B. , Mommen, G. P. M. , Pennink, J. L. A. , Eppink, M. H. et. al. , Quantitative proteomics reveals distinct differences in the protein content of outer membrane vesicle vaccines. J. Proteome Res. 2013, 12, 1898–1908. [DOI] [PubMed] [Google Scholar]
- 31. Veith, P. D. , Chen, Y. Y. , Gorasia, D. G. , Chen, D. et al., Porphyromonas gingivalis outer membrane vesicles exclusively contain outer membrane and periplasmic proteins and carry cargo enriched with virulence factors. J. Proteome Res. 2014, 13, 2420–2432. [DOI] [PubMed] [Google Scholar]
- 32. Eddy, J. L. , Gielda, L. M. , Caulfield, A. J. , Rangel, S. M. , Lathem, W. W. , Production of outer membrane vesicles by the plague pathogen Yersinia pestis. PLoS One 2014, 9, e107002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manabe, T. , Kato, M. , Ueno, T. , Kawasaki, K. , Flagella proteins contribute to the production of outer membrane vesicles from Escherichia coli W3110. Biochem. Biophys. Res. Commun. 2013, 441, 151–156. [DOI] [PubMed] [Google Scholar]
- 34. Parker, H. , Keenan, J. I. , Composition and function of Helicobacter pylori outer membrane vesicles. Microbes Infect. 2012, 14, 9–16. [DOI] [PubMed] [Google Scholar]
- 35. Horstman, A. L. , Kuehn, M. J. , Enterotoxigenic Escherichia coli secretes active heat‐labile enterotoxin via outer membrane vesicles. J. Biol. Chem. 2000, 275, 12489–12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wai, S. N. , Lindmark, B. , Soderblom, T. , Takade, A. et al., Vesicle‐mediated export and assembly of pore‐forming oligomers of the enterobacterial ClyA cytotoxin. Cell 2003, 115, 25–35. [DOI] [PubMed] [Google Scholar]
- 37. Chatterjee, D. , Chaudhuri, K. , Association of cholera toxin with Vibrio cholerae outer membrane vesicles which are internalized by human intestinal epithelial cells. FEBS Lett. 2011, 585, 1357–1362. [DOI] [PubMed] [Google Scholar]
- 38. Fiocca, R. , Necchi, V. , Sommi, P. , Ricci, V. et al., Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J. Pathol. 1999, 188, 220–226. [DOI] [PubMed] [Google Scholar]
- 39. Lindmark, B. , Rompikuntal, P. K. , Vaitkevicius, K. , Song, T. et al., Outer membrane vesicle‐mediated release of cytolethal distending toxin (CDT) from Campylobacter jejuni. BMC Microbiol. 2009, 9, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee, E. Y. , Bang, J. Y. , Park, G. W. , Choi, D. S. et al., Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics 2007, 7, 3143–3153. [DOI] [PubMed] [Google Scholar]
- 41. Lee, E. Y. , Choi, D. S. , Kim, K. P. , Gho, Y. S. , Proteomics in gram‐negative bacterial outer membrane vesicles. Mass Spectrom. Rev. 2008, 27, 535–555. [DOI] [PubMed] [Google Scholar]
- 42. Dennehy, R. , McClean, S. , Immunoproteomics: The key to discovery of new vaccine antigens against bacterial respiratory infections. Curr. Protein Pept. Sci. 2012, 13, 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Henderson, B. , Martin, A. , Bacterial virulence in the moonlight: Multitasking bacterial moonlighting proteins are virulence determinants in infectious disease. Infect. Immun. 2011, 79, 3476–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Toledo, A. , Coleman, J. L. , Kuhlow, C. J. , Crowley, J. T. , Benach, J. L. , The enolase of Borrelia burgdorferi is a plasminogen receptor released in outer membrane vesicles. Infect. Immun. 2012, 80, 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Molloy, M. P. , Herbert, B. R. , Slade, M. B. , Rabilloud, T. et al., Proteomic analysis of the Escherichia coli outer membrane. Eur. J. Biochem. 2000, 267, 2871–2881. [DOI] [PubMed] [Google Scholar]
- 46. Hoekstra, D. , van der Laan, J. W. , de Leij, L. , Witholt, B. , Release of outer membrane fragments from normally growing Escherichia coli. Biochim. Biophys. Acta 1976, 455, 889–899. [DOI] [PubMed] [Google Scholar]
- 47. Post, D. M. , Zhang, D. , Eastvold, J. S. , Teghanemt, A. et al., Biochemical and functional characterization of membrane blebs purified from Neisseria meningitidis serogroup B. J. Biol. Chem. 2005, 80, 38383–38394. [DOI] [PubMed] [Google Scholar]
- 48. Tashiro, Y. , Inagaki, A. , Shimizu, M. , Ichikawa, S. et al., Characterization of phospholipids in membrane vesicles derived from Pseudomonas aeruginosa. Biosci. Biotechnol. Biochem. 2011, 75, 605–607. [DOI] [PubMed] [Google Scholar]
- 49. Shimomura, H. , Hayashi, S. , Yokota, K. , Oguma, K. , Hirai, Y. , Alteration in the composition of cholesteryl glucosides and other lipids in Helicobacter pylori undergoing morphological change from spiral to coccoid form. FEMS Microbiol. Lett. 2004, 237, 407–413. [DOI] [PubMed] [Google Scholar]
- 50. LaRocca, T. J. , Crowley, J. T. , Cusack, B. J. , Pathak, P. et al., Cholesterol lipids of Borrelia burgdorferi form lipid rafts and are required for the bactericidal activity of a complement‐independent antibody. Cell Host Microbe 2010, 8, 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kadurugamuwa, J. L. , Beveridge, T. J. , Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: A novel mechanism ofenzyme secretion. J. Bacteriol. 1995, 177, 3998–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Unal, C. M. , Schaar, V. , Riesbeck, K. , Bacterial outer membrane vesicles in disease and preventive medicine. Semin. Immunopathol. 2011, 33, 395–408. [DOI] [PubMed] [Google Scholar]
- 53. Pettit, R. K. , Judd, R. C. , The interaction of naturally elaborated blebs from serum‐susceptible and serum‐resistant strains of Neisseria gonorrhoeae with normal human serum. Mol. Microbiol. 1992, 6, 729–734. [DOI] [PubMed] [Google Scholar]
- 54. Schooling, S. R. , Beveridge, T. J. , Membrane vesicles: An overlooked component of the matrices of biofilms J. Bacteriol. 2006, 188, 5945–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Palsdottir, H. , Remis, J. P. , Schaudinn, C. , O'Toole, E. et al., Three‐dimensional macromolecular organization of cryofixed Myxococcus xanthus biofilms as revealed by electron microscopic tomography. J. Bacteriol. 2009, 191, 2077–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Manning, A. J. , Kuehn, M. J. , Contribution of bacterial outer membrane vesicles to innate bacterial defence. BMC Microbiol. 2011, 11, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Renelli, M. , Matias, V. , Lo, R. Y. , Beveridge, T. J. , DNA‐containing membrane vesicles of Pseudomonas aeruginosa PAO1 and their genetic transformation potential. Microbiology 2004, 150, 2161–2169. [DOI] [PubMed] [Google Scholar]
- 58. Schwechheimer, C. , Sullivan, C. J. , Kuehn, M. J. , Envelope control of outer membrane vesicle production in Gram‐negative bacteria. Biochemistry 2013, 52, 3031–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Scherzer, J. W. , Whiteley, M. , A bilayer‐couple model of outer membrane vesicle biosynthesis. mBio 2012, 3, e00297–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sonntag, I. , Schwarz, H. , Hirota, Y. , Henning, U. , Escherichia coli: Multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J. Bacteriol. 1978, 136, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bernadac, A. , Gavioli, M. , Lazzaroni, J. C. , Raina, S. , Lloubès, R. , Escherichia coli tol‐pal mutants form outer membrane vesicles. J. Bacteriol. 1998, 180, 4872–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Steeghs, L. , Berns, M. , ten Hove, J. , de Jong, A. et al., Expression of foreign LpxA acyltransferases in Neisseria meningitidis results in modified lipid A with reduced toxicity and retained adjuvant activity. Cell. Microbiol. 2002, 4, 599–611. [DOI] [PubMed] [Google Scholar]
- 63. van de Waterbeemd, B. , Streefland, M. , van der Ley, P. , Zomer, B. et al., Improved OMV vaccine against Neisseria meningitidis using genetically engineered strains and a detergent‐free purification process. Vaccine 2010, 28, 4810–4816. [DOI] [PubMed] [Google Scholar]
- 64. Vollmer, W. , Bertsche, U. , Murein (peptidoglycan) structure, architecture and biosynthesis in Escherichia coli. Biochim. Biophys. Acta 2008, 1778, 1714–1734. [DOI] [PubMed] [Google Scholar]
- 65. Hayashi, J. , Hamada, N. , Kuramitsu, H. K. , The autolysin of Porphyromonas gingivalis is involved in outer membrane vesicle release. FEMS Microbiol. Lett. 2002, 216, 217–222. [DOI] [PubMed] [Google Scholar]
- 66. Adu‐Bobie, J. , Lupetti, P. , Brunelli, B. , Granoff, D. et al., GNA33 of Neisseria meningitidis is a lipoprotein required for cell separation, membrane architecture, and virulence. Infect. Immun. 2004, 72, 1914–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Schwechheimer, C. , Kulp, A. , Kuehn, M. J. , Modulation of bacterial outer membrane vesicle production by envelope structure and content. BMC Microbiol. 2014, 14, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bos, M. P. , Tefsen, B. , Voet, P. , Weynants, V. et al., Function of neisserial outer membrane phospholipase A in autolysis and assessment of its vaccine potential. Infect. Immun. 2005, 73, 2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tannaes, T. , Grav, H. J. , and Bukholm, G. , Lipid profiles of Helicobacter pylori colony variants. APMIS 2000, 349–356. [DOI] [PubMed] [Google Scholar]
- 70. McBroom, A. J. , Johnson, A. P. , Vemulapalli, S. , Kuehn, M. J. , Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J. Bacteriol. 2006, 188, 5385–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mashburn‐Warren, L. , Howe, J. , Garidel, P. , Richter, W. et al., Interaction of quorum signals with outer membrane lipids: Insights into prokaryotic membrane vesicle formation. Mol. Microbiol. 2008, 69, 491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kadurugamuwa, J. L. , Beveridge, T. J. , Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: Conceptually new antibiotics. J. Bacteriol. 1996, 178, 2767–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Deatherage, B. L. , Cano Lara, J. , Bergsbaken, T. , Rassoulian Barrett, S. L. et al., Biogenesis of bacterial membrane vesicles. Mol. Microbiol. 2009, 72, 1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Moon, D. C. , Choi, C. H. , Lee, J. H. , Choi, C. W. et al., Acinetobacter baumannii outer membrane protein A modulates the biogenesis of outer membrane vesicles. J. Microbiol. 2012, 50, 155–160. [DOI] [PubMed] [Google Scholar]
- 75. Song, T. , Mika, F. , Lindmark, B. , Liu, Z. et al., “A new Vibrio cholera sRNA modulates colonization and affects release of outer membrane vesicles, ” Mol. Microbiol. 2008, 70, 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Valeru, S. P. , Shanan, S. , Alossimi, H. , Saeed, A. et al., Lack of outer membrane protein A enhances the release of outer membrane vesicles and survival of Vibrio cholerae and suppresses viability of Acanthamoeba castellanii. Int. J. Microbiol. 2014, 610190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. McBroom, A. J. , Kuehn, M. J. , Release of outer membrane vesicles by Gram‐negative bacteria is a novel envelope stress response. Mol. Microbiol. 2007, 63, 545–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hagemann, S. , Stöger, L. , Kappelmann, M. , Hassl, I. et al., DNA‐bearing membrane vesicles produced by Ahrensia kielensis and Pseudoalteromonas marina. J. Basic Microbiol. 2013, 53, 1–11. [DOI] [PubMed] [Google Scholar]
- 79. Katsui, N. , Tsuchido, T. , Hiramatsu, R. , Fujikawa, S. et al., Heat‐induced blebbing and vesiculation of the outer membrane of Escherichia coli. J. Bacteriol. 1982, 151, 1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Knox, K. W. , Vesk, M. , Work, E. , Relation between excreted lipopolysaccharide complexes and surface structures of a lysine‐limited culture of Escherichia coli. J. Bacteriol. 1966, 92, 1206–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. van de Waterbeemd, B. , Zomer, G. , van den IJssel, J. , van Keulen, L. et al., Cysteine depletion causes oxidative stress and triggers outer membrane vesicle release by Neisseria meningitidis; Implications for vaccine development. PLoS One 2013, 8, e54314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Loeb, M. R. , Kilner, J. , Release of a special fraction of the outer membrane from both growing and phage T4‐infected Escherichia coli B. Biochim. Biophys. Acta 1978, 514, 117–127. [DOI] [PubMed] [Google Scholar]
- 83. Borneleit, P. , Hermsdorf, T. , Claus, R. , Walther, P. , Kleber, H. P. , Effect of hexadecane‐induced vesiculation on the outer membrane of Acinetobacter calcoaceticus. Gen. Microbiol. 1988, 134, 1983–1992. [DOI] [PubMed] [Google Scholar]
- 84. Kolodziejek, A. M. , Caplan, A. B. , Bohach, G. A. , Paszczynski, A. J. et al., Physiological levels of glucose induce membrane vesicle secretion and affect the lipid and protein composition of Yersinia pestis cell surfaces. Appl. Environ. Microbiol. 2013, 79, 4509–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Altindis, E. , Fu, Y. , Mekalanos, J. J. , Proteomic analysis of Vibiro cholerae outer membrane vesicles. Proc. Natl. Acad. Sci. U.S.A. 2014, E1546–E1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Toyofuku, M. , Zhou, S. , Sawada, I. , Takaya, N. et al., Membrane vesicle formation is associated with pyocin production under denitrifying conditions in Pseudomonas aeruginosa PAO1. Environ. Microbiol. 2013, 16, 2927–2938. [DOI] [PubMed] [Google Scholar]
- 87. Chatterjee, S. N. , Das, J. , Electron microscopic observations on the excretion of cell‐wall material by Vibrio cholerae. J. Gen. Microbiol. 1967, 49, 1–11. [DOI] [PubMed] [Google Scholar]
- 88. Devoe, I. W. , Gilchrist, J. E. , Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J. Exp. Med. 1973, 138, 1156–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mug‐Opstelten, D. , Witholt, B. , Preferential release of new outer membrane fragments by exponentially growing Escherichia coli. Biochim. Biophys. Acta 1978, 508, 287–295. [DOI] [PubMed] [Google Scholar]
- 90. Santos, S. , Arauz, L. J. , Baruque‐Ramos, J. , Lebrun, I. et al., Outer membrane vesicles (OMV) production of Neisseria meningitidis serogroup B in batch process. Vaccine 2012, 30, 6064–6069. [DOI] [PubMed] [Google Scholar]
- 91. McCaig, W. D. , Koller, A. , Thanassi, D. G. , Production of outer membrane vesicles and outer membrane tubes by Francisella novicida. J. Bacteriol. 2013, 195, 1120–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Alaniz, R. C. , Deatherage, B. L. , Lara, J. C. , Cookson, B. T. , Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J. Immunol. 2007, 179, 7692–7701. [DOI] [PubMed] [Google Scholar]
- 93. Charon, N. W. , Goldstein, S. F. , Marko, M. , Hsieh, C. et al., The flat‐ribbon configuration of the periplasmic flagella of Borrelia burgdorferi and its relationship to motility and morphology. J. Bacteriol. 2009, 191, 600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bager, R. J. , Persson, G. , Nesta, B. , Soriani, M. et al., Outer membrane vesicles reflect environmental cues in Gallibacterium anatis. Veter. Microbiol. 2013, 167, 565–572. [DOI] [PubMed] [Google Scholar]
- 95. Galka, F. , Wai, S. N. , Kusch, H. , Engelmann, S. et al., Proteomic characterization of the whole secretome of Legionella pneumophila and functional analysis of outer membrane vesicles. Infect. Immun. 2008, 76, 1825–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Manning, A. J. , Kuehn, M. J. , Functional advantages conferred by extracellular prokaryotic membrane vesicles. J. Mol. Microbiol. Biotechnol. 2013, 23, 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kadurugamuwa, J. L. , Beveridge, T. J. , Natural release of virulence factors in membrane vesicles by Pseudomonas aeruginosa and the effect of aminoglycoside antibiotics on their release. J. Antimicrob. Chemother. 1997, 40, 615–621. [DOI] [PubMed] [Google Scholar]
- 98. Aguilera, L. , Toloza, L. , Gimenez, R. , Odena, A. et al., Proteomic analysis of outer membrane vesicles from the probiotic strain Escherichia coli Nissle 1917. Proteomics 2014, 14, 222–229. [DOI] [PubMed] [Google Scholar]
- 99. Elhenawy, W. , Debelyy, M. O. , Feldman, M. F. , Preferential packing of acidic glycosidases and proteases into Bacteroides outer membrane vesicles. Mbio 2014, 5, e00909–e00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kesty, N. C. , Kuehn, M. J. , Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. J. Biol. Chem. 2004, 279, 2069–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Vasilyeva, N. V. , Tsfasman, I. M. , Kudryakova, I. V. , Suzina, N. E. et al., The role of membrane vesicles in secretion of Lysobacter sp. bacteriolytic enzymes. J. Mol. Microbiol. Biotechnol. 2013, 23, 142–151. [DOI] [PubMed] [Google Scholar]
- 102. Haurat, M. F. , Aduse‐Opoku, J. , Rangarajan, M. , Dorobantu, L. et al., Selective sorting of cargo proteins into bacterial membrane vesicles. J. Biol. Chem. 2011, 286, 1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jaroslawski, S. , Duquesne, K. , Sturgis, J. N. , Scheuring, S. , High resolution architecture of the outer membrane of the Gram‐negative bacteria Roseobacter denitrificans. Mol. Microbiol. 2009, 74, 1211–1222. [DOI] [PubMed] [Google Scholar]
- 104. Bielig, H. , Dongre, M. , Zurek, B. , Wai, S. N. , Kufer, T. A. , A role for quorum sensing in regulating innate immune responses mediated by Vibrio cholerae outer membrane vesicles (OMVs). Gut Microbes 2011, 2, 274–279. [DOI] [PubMed] [Google Scholar]
- 105. Chatterjee, D. , Chaudhuri, K. , Vibrio cholerae O395 outer membrane vesicles modulate intestinal epithelial cells in a NOD1 protein‐dependent manner and induce dendritic cell‐mediated Th2/Th17 cell responses. J. Biol. Chem. 2013, 288, 4299–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yaron, S. , Kolling, G. L. , Simon, L. , Matthews, K. R. , Vesicle‐mediated transfer of virulence genes from Escherichia coli O157:H7 to other enteric bacteria. Appl. Environ. Microbiol. 2000, 66, 4414–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dorward, D. W. , Garon, C. F. , Judd, R. C. , Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J. Bacteriol. 1989, 171, 2499–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sharpe, S. W. , Kuehn, M. J. , Mason, K. M. , Elicitation of epithelial cell‐derived immune effectors by outer membrane vesicles of nontypeable Haemophilus influenzae. Infect. Immun. 2011, 79, 4361–4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Dorward, D. W. , Garon, C. F. , Judd, R. C. , Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J. Bacteriol. 1989, 171, 2499–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ghosal, A., Upadhyaya, B. B., Fritz, J. V., Heintz‐Buschart, A. et al., The extracellular RNA complement of Escherichia coli. Microbiology Open 2015, DOI: 10.1002/mbo3.235. [DOI] [PMC free article] [PubMed]
- 111. Holst, J. , Oster, P. , Arnold, R. , Tatley, M. V. et al., Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): Lessons from past programs and implications for the future. Hum. Vaccin. Immunother. 2013, 9, 1241–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Saunders, N. B. , Shoemaker, D. R. , Brandt, B. L. , Moran, E. E. et al., Immunogenicity of intranasally administered meningococcal native outer membrane vesicles in mice. Infect. Immun. 1999, 67, 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Finne, J. , Leinonen, M. , Makela, P. H. , Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet 1983, 2, 355–357. [DOI] [PubMed] [Google Scholar]
- 114. Granoff, D. M., Pelton, S., Harrison, L. H., Meningococcal vaccines, in: Plotkin, S. A., Orenstein, W. A., Offit, P. A. (Eds.), Vaccines 6th Edn., Elsevier Health Sciences 2013, pp. 388–418.
- 115. Holst, J. , Martin, D. , Arnold, R. , Huergo, C. C. et al., Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 2009, B3–B12. [DOI] [PubMed] [Google Scholar]
- 116. Urwin, R. , Russell, J. E. , Thompson, E. A. , Holmes, E. C. et al., Distribution of surface protein variants among hyperinvasive meningococci: Implications for vaccine design. Infect. Immun. 2004, 72, 5955–5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Rappuoli, R. , Reversevaccinology. Curr. Opin. Microbiol. 2000, 3, 445–450. [DOI] [PubMed] [Google Scholar]
- 118. Acevedo, R. , Fernández, S. , Zayas, C. , Acosta, A. et al., Bacterial outer membrane vesicles and vaccine applications. Front. Immunol. 2014, 5, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bjune, G. , Høiby, E. A. , Grønnesby, J. K. , Arnesen, O. et al., Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 1991, 338, 1093–1096. [DOI] [PubMed] [Google Scholar]
- 120. Sierra, G. V. , Campa, H. C. , Varcacel, N. M. , Garcia, I. L. et al., Vaccine against group B Neisseria meningitidis: Protection trial and mass vaccination results in Cuba. NIPH Ann. 1991, 14, 195–210. [PubMed] [Google Scholar]
- 121. Arnold, R., Galloway, Y., McNicholas, A., O'Hallahan, J., Effectiveness of a vaccination programme for an epidemic of meningococcal B in New Zealand. Vaccine 2011, 29, 7100–7106. [DOI] [PubMed]
- 122. Claassen, I. , Meylis, J. , van der Ley, P. , Peeters, C. et al., Production, characterization and control of a Neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine 1996, 14, 1001–1008. [DOI] [PubMed] [Google Scholar]
- 123. Peeters, C. C. , Rümke, H. C. , Sundermann, L. C. , Rouppe van der Voort, E. M. et al., Phase I clinical trial with a hexavalent PorA containing meningococcal outer membrane vesicle vaccine. Vaccine 1996, 14, 1009–1015. [DOI] [PubMed] [Google Scholar]
- 124. Evans, C. M. , Pratt, C. B. , Matheson, M. , Vaughan, T. E. et al., Nasopharyngeal colonization by Neisseria lactamica and induction of protective immunity against Neisseria meningitides. Clin. Infect. Dis. 2011, 52, 70–77. [DOI] [PubMed] [Google Scholar]
- 125. van der Ley, P. , Steeghs, L. , Hamstra, H. J. , ten Hove, J. et al., Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: Influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect. Immun. 2001, 69, 5981–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Keiser, P. B. , Biggs‐Cicatelli, S. , Moran, E. E. , Schmiel, D. H. et al., A phase 1 study of a meningococcal native outer membrane vesicle vaccine made from a group B strain with deleted lpxL1 and synX, over‐expressed factor H binding protein, two PorAs and stabilized OpcA expression. Vaccine 2011, 29, 1413–1420. [DOI] [PubMed] [Google Scholar]
- 127. Costa, I. , Pajon, R. , Granoff, D. M. , Human factor H (FH) impairs protective meningococcal anti‐FHbp antibody responses and the antibodies enhance FH binding. MBio 2014, 5, e01625–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Koeberling, O. , Ispasanie, E. , Hauser, J. , Rossi, O. et al., A broadly‐protective vaccine against meningococcal disease in sub‐Saharan Africa based on generalized modules for membrane antigens (GMMA). Vaccine 2014, 32, 2688–2695. [DOI] [PubMed] [Google Scholar]
- 129. Koeberling, O. , Delany, I. , Granoff, D. M. , A critical threshold of meningococcal factor H binding protein expression is required for increased breadth of protective antibodies elicited by native outer membrane vesicle vaccines. Clin. Vaccine Immunol. 2011, 18, 736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Moran, E. , Burden, R. , Labrie III, J. E. , Wen, Z. et al., Analysis of the Bactericidal Response to an Experimental Neisseria meningitidis Vesicle Vaccine. Clin. Vaccine Immunol. 2012, 19, 659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Beernink, P. T. , Caugant, D. A. , Welsch, J. A. , Koeberling, O. , Granoff, D. M. , Meningococcal factor H–binding protein variants expressed by epidemic capsular group A, W‐135, and X strains from Africa. J. Infect. Dis. 2009, 199, 1360–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Roberts, R. , Moreno, G. , Bottero, D. , Gaillard, M. E. et al., Outer membrane vesicles as acellular vaccine against pertussis. Vaccine 2008, 26, 4639–4646. [DOI] [PubMed] [Google Scholar]
- 133. Asensio, C. J. , Gaillard, M. E. , Moreno, G. , Bottero, D. et al., Outer membrane vesicles obtained from Bordetella pertussisTohama expressing the lipid A deacylase PagL as a novel acellular vaccine candidate. Vaccine 2011, 29, 1649–1656. [DOI] [PubMed] [Google Scholar]
- 134. Bottero, D. , Gaillard, M. E. , Errea, A. , Moreno, G. et al., Outer membrane vesicles derived from Bordetella parapertussis as an acellular vaccine against Bordetella parapertussis and Bordetella pertussis infection. Vaccine 2013, 31, 5262–5268. [DOI] [PubMed] [Google Scholar]
- 135. Kesavalu, L. , Ebersole, J. L. , Machen, R. L. , Holt, S. C. , Porphyromonas gingivalis virulence in mice: Induction of immunity to bacterial components. Infect. Immun. 1992, 60, 1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Schild, S. , Nelson, E. J. , Camilli, A. , Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice. Infect. Immun. 2008, 76, 4554–4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Keenan, J. I. , Allardyce, R. A. , Bagshaw, P. F. , Lack of protection following immunisation with H. pylori outer membrane vesicles highlights antigenic differences between H. felis and H. pylori. FEMS Microbiol. Lett. 1998, 161, 21–27. [DOI] [PubMed] [Google Scholar]
- 138. Zhou, K. , Deng, X. , He, C. , Yue, B. , Wu, M. , Pseudomonas aeruginosa outer membrane vesicles modulate host immune responses by targeting the Toll‐like receptor 4 signaling pathway. Infect. Immun. 2013, 81, 4509–4518. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 139. Bishop, A. L. , Schild, S. , Patimalla, B. , Klein, B. , Camilli, A. , Mucosal immunization with Vibrio cholerae outer membrane vesicles provides maternal protection mediated by antilipopolysaccharide antibodies that inhibit bacterial motility. Infect. Immun. 2010, 78, 4402–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Nieves, W. , Asakrah, S. , Qazi, O. , Brown, K. A. et al., A naturally derived outer membrane vesicle vaccine protects against lethal pulmonary Burkholderia pseudomallei infection. Vaccine 2011, 29, 8381–8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Pierson, T. , Matrakas, D. , Taylor, Y. U. , Manyam, G. et al., Proteomic characterization and functional analysis of outer membrane vesicles of Francisella novicida suggests possible role in virulence and use as a vaccine. J. Proteome Res. 2011, 10, 954–967. [DOI] [PubMed] [Google Scholar]
- 142. Jong, W. , Daleke‐Schermerhorn, M. H. , Vikström, D. , Ten Hagen‐Jongman, C. M. et al., An autotransporter display platform for the development of multivalent recombinant bacterial vector vaccines. Microb. Cell Fact. 2014, 13, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Basto, A. P. , Piedade, J. , Ramalho, R. , Alves, S. et al., A new cloning system based on the OprI lipoprotein for the production of recombinant bacterial cell wall‐derived immunogenic formulations. J. Biotechnol. 2012, 157, 50–63. [DOI] [PubMed] [Google Scholar]
- 144. Fantappiè, L. , de Santis, M. , Chiarot, E. , Carboni, F. et al., Antibody‐mediated immunity induced by engineered Escherichia coli OMVs carrying heterologous antigens in their lumen. J. Extracell. Vesicles 2014, 3, 24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Park, M. , Sun, Q. , Liu, F. , DeLisa, M. P. , Chen, W. , Positional Assembly of enzymes on bacterial outer membrane vesicles for cascade reactions. PLoS One 2014, 9, e97103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Muralinath, M. , Kuehn, M. J. , Immunization with Salmonella enterica serovar typhimurium‐derived outer membrane vesicles delivering the pneumococcal protein PspA confers protection against challenge with Streptococcus pneumoniae. Infect. Immun. 2011, 79, 887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Bartolini, E. , Ianni, E. , Frigimelica, E. , Petracca, R. et al., Recombinant outer membrane vesicles carrying Chlamydia muridarum HtrA induce antibodies that neutralize chlamydial infection in vitro. J. Extracell. Vesicles 2013, 2, 20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Maisonneuve, C. , Bertholet, S. , Philpott, D. J. , de Gregorio, E. , Unleashing the potential of NOD‐ and Toll‐like agonists as vaccine adjuvants. Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 12294–12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Somerville, J. E. Jr , Cassiano, L. , Bainbridge, B. , Cunningham, M. D. , Darveau, R. P. , A novel Escherichia coli lipid A mutant that produces an antiinflammatory lipopolysaccharide. J. Clin. Invest. 1996, 97, 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Mata‐Haro, V. , Cekic, C. , Martin, M. , Chilton, P. M. et al., The vaccine adjuvant monophosphoryl lipid A as a TRIF‐biased agonist of TLR4. Science 2007, 316, 1628–1632. [DOI] [PubMed] [Google Scholar]
- 151. Pupo, E. , Hamstra, H. J. , Meiring, H. , van der Ley, P. , Lipopolysaccharide engineering in Neisseria meningitidis: Structural analysis of different pentaacyl lipid A mutants and comparison of their modified agonist properties. J. Biol. Chem. 2014, 289, 8668–8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Jin, M. S. , Kim, S. E. , Heo, J. Y. , Lee, M. E. et al., Crystal structure of the TLR1‐TLR2 heterodimer induced by binding of a tri‐acylated lipopeptide. Cell 2007, 130, 1071–1082. [DOI] [PubMed] [Google Scholar]
- 153. Kovacs‐Simon, A. , Titball, R. W. , Michell, S. L. , Lipoproteins of bacterial pathogens. Infect. Immun. 2011, 79, 548–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Fransen, F. , Stenger, R. M. , Poelen, M. C. , van Dijken, H. H. et al., Differential effect of TLR2 and TLR4 on the immune response after immunization with a vaccine against Neisseria meningitidis or Bordetella pertussis. PLoS One 2010, 5, e15692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Li, J. , Lee, D. S. , Madrenas, J. , Evolving bacterial envelopes and plasticity of TLR2‐dependent responses: Basic research and translational opportunities. Front. Immunol. 2013, 4, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Zariri, A. , van Dijken, H. , Hamstra, H. J. , van der Flier, M. et al., Expression of human CEACAM1 in transgenic mice limits the Opa‐specific immune response against meningococcal outer membrane vesicles. Vaccine 2013, 31, 5585–5593. [DOI] [PubMed] [Google Scholar]
- 157. Steeghs, L. , van Vliet, S. J. , Uronen‐Hansson, H. , van Mourik, A. et al., Neisseria meningitidis expressing lgtB lipopolysaccharide targets DC‐SIGN and modulates dendritic cell function. Cell. Microbiol. 2006, 8, 316–325. [DOI] [PubMed] [Google Scholar]
- 158. Kim, S‐H. , Kim, K‐S. , Lee, S‐R. , Kim, E. et al., Structural modifications of outer membrane vesicles to refine them as vaccine delivery vesicles. Biochim. Biophys. Acta 2009, 1788, 2150–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Chutkan, H., MacDonald, I., Manning, A., Kuehn, M. J., Quantitative and qualitative preparations of bacterial outer membrane vesicles, in: Delcour, A. H. (Ed.), Bacterial Cell Surfaces: Methods and Protocols, Methods in Molecular Biology, Springer, New York 2013, pp. 259–272. [DOI] [PMC free article] [PubMed]
- 160. Frandoloso, R. , Martínez‐Martínez, S. , Calmettes, C. , Fegan, J. et al., Nonbinding site‐directed mutants of transferrin binding protein B exhibit enhanced immunogenicity and protective capabilities. Infect. Immun. 2015, 83, 1030–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Josefsberg, J. O. , Buckland, B. , Vaccine process technology. Biotechnol. Bioeng. 2012, 109, 1443–1460. [DOI] [PubMed] [Google Scholar]
- 162. Baart, G. J. E. , de Jong, G. , Philippi, M. , van 't Riet, K. et al., Scale‐up of bulk production of vaccine against meningococcal disease. Vaccine 2007, 25, 6399–6408. [DOI] [PubMed] [Google Scholar]
- 163. van de Waterbeemd, B. , Streefland, M. , van Keulen, L. , van den IJssel, J. et al., Identification and optimization of critical process parameters for the production of nOMV vaccine against Neisseria meningitidis. Vaccine 2012, 30, 3683–3690. [DOI] [PubMed] [Google Scholar]
- 164. Berlanda Scorza, F. , Colucci, A. M. , Maggiore, L. , Sanzone, S. et al., High yield production process for Shigella outer membrane particles. PLoS One 2012, 7, e35616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. van de Waterbeemd, B. , Zomer, B. , Kaaijk, P. , Ruiterkamp, N. et al., Improved production process for native outer membrane vesicle vaccine against Neisseria meningitidis. PLoS One 2013, e65157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ellis, T. N. , Leiman, S. A. , Kuehn, M. J. , Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect. Immun. 2010, 78, 3822–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Donato, G. M. , Goldsmith, C. S. , Paddock, C. D. , Eby, J. C. et al., Delivery of Bordetella pertussis adenylate cyclase toxin to target cells via outer membrane vesicles. FEBS Lett. 2012, 586, 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Shang, E. S. , Champion, C. I. , Wu, X. Y. , Skare, J. T. et al., Comparison of protection in rabbits against host‐adapted and cultivated Borrelia burgdorferi following infection‐derived immunity or immunization with outer membrane vesicles or outer surface protein A. Infect. Immun. 2000, 68, 4189–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Dormitzer, P. R. , Grandi, G. , Rappuoli, R. , Structural vaccinology starts to deliver. Nat. Rev. Microbiol. 2012, 10, 807–813. [DOI] [PubMed] [Google Scholar]
- 170. Thornton, V. , Lennon, D. , Rasanathan, K. , O'Hallahan, J. et al., Safety and immunogenicity of New Zealand strain meningococcal serogroup B OMV vaccine in healthy adults: Beginning of epidemic control. Vaccine 2006, 24, 1395–1400. [DOI] [PubMed] [Google Scholar]
- 171. de Kleijn, E. D. , de Groot, R. , Labadie, J. , Lafeber, A. B. et al., Immunogenicity and safety of a hexavalent meningococcal outer‐membrane‐vesicle vaccine in children of 2±3 and 7±8 years of age. Vaccine 2000, 18, 1456–1466. [DOI] [PubMed] [Google Scholar]
- 172. de Kleijn, E. D. , van Eijndhoven, L. , Vermont, C. , Kuipers, B. et al., Serum bactericidal activity and isotype distribution of antibodies in toddlers and schoolchildren after vaccination with RIVM hexavalent PorA vesicle vaccine. Vaccine 2002, 20, 352–358. [DOI] [PubMed] [Google Scholar]
- 173. Martin, S. , Sadler, F. , Borrow, R. , Dawson, M. et al., IgG antibody subclass responses determined by immunoblot in infants' sera following vaccination with a meningococcal recombinant hexavalent PorA OMV vaccine. Vaccine 2001, 19, 4404–4408. [DOI] [PubMed] [Google Scholar]
- 174. Keiser, P. B. , Gibbs, B. T. , Coster, T. S. , Moran, E. E. et al., A phase 1 study of a group B meningococcal native outer membrane vesicle vaccine made from a strain with deleted lpxL2 and synX and stable expression of opcA. Vaccine 2010, 28, 6970–6976. [DOI] [PubMed] [Google Scholar]
- 175. Keiser, P. B. , Biggs‐Cicatelli, S. , Moran, E. E. , Schmiel, D. H. et al., A phase 1 study of a meningococcal native outer membrane vesicle vaccine made from a group B strain with deleted lpxL1 and synX, over‐expressed factor H binding protein, two PorAs and stabilized OpcA expression. Vaccine 2011, 29, 1413–1420. [DOI] [PubMed] [Google Scholar]
- 176. Gorringe, A. R. , Taylor, S. , Brookes, C. , Matheson, M. et al., Phase I safety and immunogenicity study of a candidate meningococcal disease vaccine based on Neisseria lactamica outer membrane vesicles. Clin. Vaccine Immunol. 2009, 16, 1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. McConnell, M.J. , Rumbo, C. , Bou, G. , Pachón, J. , Outer membrane vesicles as an acellular vaccine against Acinetobacter baumannii. Vaccine 2011, 29, 5705–5710. [DOI] [PubMed] [Google Scholar]
- 178. Avila‐Calderon, E. D. , Lopez‐Merino, A. , Jain, N. , Peralta, H. et al., Characterization of outer membrane vesicles from Brucella melitensis and protection induced in mice. Clin. Dev. Immunol. 2012, 2012, 352493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Nieves, W. , Petersen, H. , Judy, B. M. , Blumentritt, C. A. et al., A Burkholderia pseudomallei outer membrane vesicle vaccine provides protection against lethal sepsis. Clin. Vaccine Immunol. 2014, 21, 747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Keenan, J. , Day, T. , Neal, S. , Cook, B. et al., A role for the bacterial outer membrane in the pathogenesis of Helicobacter pylori infection. FEMS Microbiol. Lett. 2000, 182, 259–264. [DOI] [PubMed] [Google Scholar]
- 181. Lee, J. C. , Lee, E. J. , Lee, J. H. , Jun, S. H. et al., Klebsiella pneumoniae secretes outer membrane vesicles that induce the innate immune response. FEMS Microbiol Lett. 2012, 331, 17–24. [DOI] [PubMed] [Google Scholar]
- 182. Finney, M. , Vaughan, T. , Taylor, S. , Hudson, M. J. et al., Characterization of the key antigenic components and pre‐clinical immune responses to a meningococcal disease vaccine based on Neisseria lactamica outer membrane vesicles. Hum. Vaccin. 2008, 4, 23–30. [DOI] [PubMed] [Google Scholar]
- 183. Mitra, S. , Chakrabarti, M. K. , Koley, H. , Multi‐serotype outer membrane vesicles of Shigellae confer passive protection to the neonatal mice against shigellosis. Vaccine 2013, 31, 3163–3173. [DOI] [PubMed] [Google Scholar]


