Abstract
Purpose of review
Human eosinophils were first identified and named by Paul Ehrlich in 1879 on the basis of the cell's granular uptake of eosin. Although eosinophils represent approximately 1% of peripheral blood leukocytes, they have the propensity to leave the blood stream and migrate into inflamed tissues. Eosinophils and their mediators are critical effectors to asthma and eosinophilic granulomatosis with polyangiitis (EGPA). Eosinophils are equipped with a large number of cell-surface receptors and produce specific cytokines and chemokines.
Recent findings
Eosinophils are the major source of interleukin-5 and highly express the interleukin-5Rα on their surface. Clinical trials evaluating monoclonal antibodies to interleukin-5 (mepolizumab and reslizumab) and its receptor interleukin-5Rα (benralizumab) have been or are underway in patients with eosinophilic asthma, EGPA and chronic obstructive pulmonary disease (COPD). Overall, targeting interleukin-5/interleukin-5Rα is associated with a marked decrease in blood and sputum eosinophilia, the number of exacerbations and improvement of some clinical parameters in adult patients with severe eosinophilic asthma. Pilot studies suggest that mepolizumab might be a glucocorticoid-sparing treatment in patients with EGPA. A preliminary study found that benralizumab did not reduce the exacerbations and did modify lung function in patients with eosinophilic COPD.
Summary
The review examines recent advances in the biology of eosinophils and how targeting the interleukin-5 pathway might offer benefit to some patients with severe asthma, EGPA, and COPD. Interleukin-5/interleukin-5Rα-targeted treatments offer promises to patients with eosinophilic respiratory disorders.
Keywords: asthma, benralizumab, chronic obstructive pulmonary disease, eosinophils, interleukin-5, mepolizumab, reslizumab
INTRODUCTION
Paul Ehrlich first named a bilobed nucleated cell as an ‘eosin’-‘ophil’ in 1879 on the basis of the cell's granular uptake of eosin [1,2] which binds to cationic proteins present in specific granules. Eosinophils represent approximately 1% of peripheral blood leukocytes and their differentiation and activation are mainly regulated by interleukin-5 [3]. One of their characteristics is the capacity to adhere to activated blood endothelial cells, leave the blood stream to migrate into inflamed tissues, and concentrate at the site of certain types of inflammation [4–6] and tumors [7]. These cells were soon found in airway tissue and sputum of patients with asthma [8▪]. Over the years, eosinophils were identified as a prominent cell type in certain forms of asthma [8▪] and eosinophilic vasculitis [4,9].
Asthma is a chronic disease of the airways characterized by inflammatory, functional, and structural changes responsible for variable bronchial hyperresponsiveness and reversible expiratory airway limitation [10]. Airway inflammation is central to disease pathophysiology through the release of several proinflammatory mediators and remodeling of the airway wall [11]. Varying combinations of these complex processes explain the different asthma phenotypes [10]. Most asthmas are associated with T helper type 2 (Th2) cell-dependent production of IgE and recruitment of eosinophils, mast cells, and basophils [11].
Eosinophilic granulomatosis with polyangiitis (EGPA) (formerly Churg–Strauss syndrome) is a systemic small-vessel vasculitis associated with asthma and eosinophilia [9]. EGPA commonly presents with an upper airway tract and lung involvement associated with persistent eosinophilia and upregulation of interleukin-5.
Chronic obstructive pulmonary disease (COPD), an inflammatory disease distinct from asthma, develops later in life, in smokers and is characterized by progressive irreversible airflow obstruction where a key role is played by CD8 T cells and neutrophils [12]. Acute exacerbations of COPD are usually associated with neutrophils, but can also present airway [13], sputum [14], or blood eosinophilia [15].
The review examines recent advances in the unique biology of the eosinophil, how dysregulated eosinophil functions promote different respiratory disorders, and how targeting the interleukin-5 pathway might offer clinical benefit to some patients with asthma, EGPA, and COPD.
Box 1.
no caption available
THE UNIQUE BIOLOGY OF THE EOSINOPHIL
Human eosinophils are equipped with a large number of cell-surface receptors [16▪,17,18] (Fig. 1). Human eosinophils can be distinguished from other granulocytes by certain surface receptors selectively expressed on eosinophils [interleukin-5Rα, CC-chemokine receptor 3 (CCR3), cysteinyl leukotriene type 1, α4β1 and α4β7 integrins]. The epidermal growth factor-like module containing mucin-like hormone receptor 1 (EMR1) appears truly eosinophil specific [17,20]. A variety of inhibitory receptors that regulate eosinophil survival and activation have also been described including Siglec-8, CD300a, killer activating receptors, potassium inwardly rectifying channel, and FcγRIIb [19].
FIGURE 1.
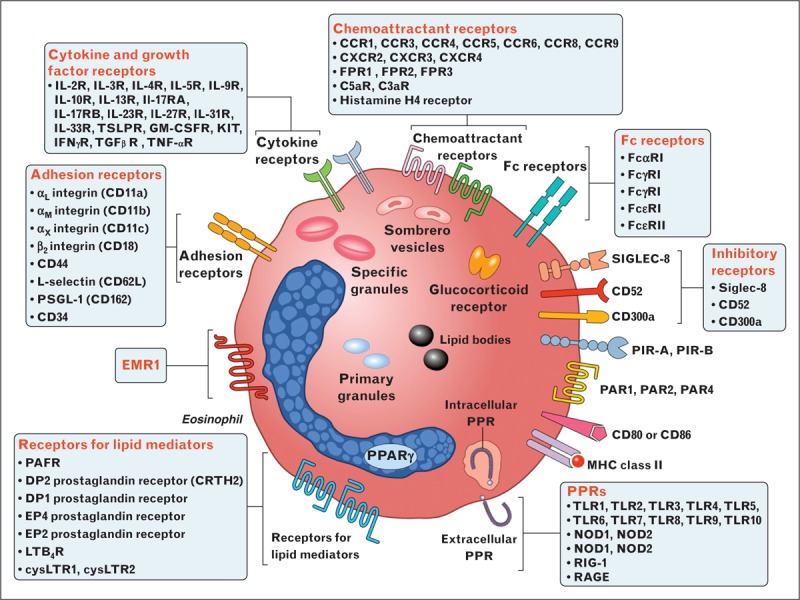
Human eosinophils display a wide spectrum of surface receptors that are important for their pleiotropic functions. Eosinophils express cell-surface receptors for cytokines and growth factors, chemokines, adhesion molecules, lipid mediators, chemoattractants, complement, immunoglobulins, Siglecs, histamine, PIRs, PARs, PPRs, CD40, CD80/CD86, and MHC class II. The epidermal growth factor-like module containing mucin-like hormone receptor 1 (EMR1) appears truly eosinophil specific [17]. Eosinophils contain the glucocorticoid receptor in high copy number [19]. The α variant of the glucocorticoid receptor is five-fold higher in eosinophils than in neutrophils making these cells highly susceptible to the therapeutic effects of glucocorticoids. Eosinophils contain specific granules containing several cationic proteins, primary granules, lipid bodies, and sombrero vesicles. CC, chemokine ligand; CCR, CC-chemokine receptor; CXCL, CXC-chemokine ligand; CXCR, CXC-chemokine receptor; PIRs, paired immunoglobulin-like receptors; PARs, proteinase-activated receptors; PPRs, pattern-recognition receptors.
Eosinophils contain intracellularly the α splice variant of the glucocorticoid receptor (GR-A) in high copy number [21]. The proapoptotic GR-A isoform is five-fold higher in eosinophils than in neutrophils making eosinophils highly susceptible (and the neutrophil much less) to therapeutic effects of glucocorticoids, such as apoptosis [22].
Eosinophil specific granules contain four cationic proteins, major basic proteins (MBP), eosinophil cationic protein (ECP), eosinophil-derived neurotoxin (EDN), and eosinophil peroxidase (EPX) which exhibit cytotoxic activity and can cause significant tissue damage [23]. Lipid mediators produced by eosinophils include leukotriene C4 (LTC4), platelet-activating factor (PAF), thromboxane B2 (TxB2), prostaglandin (PG)E1, and PGE2. Human eosinophils are a major source of a wide spectrum of cytokines, including interleukin-5, interleukin-4, interleukin-13, TGFβ, and IFNγ [24–27] (Fig. 2). They also produce a wide spectrum of immunologically active factors, including chemokines [28,29]. Thus, it is likely to predict that eosinophils are equipped to perform different functions such as tissue repair and remodeling, angiogenesis [30], clearance of parasites [31], metabolic homeostasis [32], and immune cell activation [16▪,33]. During their transit in the bloodstream and at sites of inflammatory/immune reactions, eosinophils interact with and modulate the functions of several cells of the innate and adaptive immune system [16▪,34,35] (Fig. 3).
FIGURE 2.
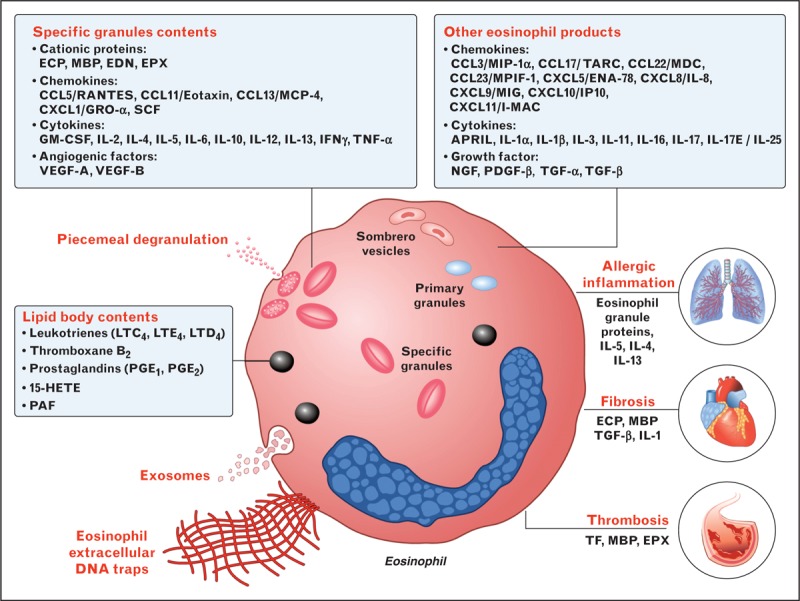
Eosinophils contain and/or release a wide array of preformed and de novo synthesized mediators important for their effector functions. Specific granules contain several cationic proteins, including MBP, ECP, EDN, and EPX. Eosinophils can degranulate by exocytosis or by piecemeal degranulation whereby individual granule contents are differentially secreted by activated eosinophils without disruption of the cell membrane. Sombrero vesicles are morphologically distinct vesicles that carry granules to the plasma membrane. Lipid bodies are structurally distinct sites within eosinophils that are responsible for synthesis of eicosanoid mediators of inflammation [26]. Eosinophils produce numerous chemokines, cytokines, growth and angiogenic factors that mediate allergic inflammation, fibrosis, and thrombosis. Eosinophils generate extracellular DNA traps [24] and secrete exosomes [27]. A nonexhaustive list of these products is shown in boxes. ECP, eosinophil cationic protein; EDN, eosinophil-derived neurotoxin; EPX, eosinophil peroxidase; MBP, major basic protein; PAF, platelet activating factor; PDGF, platelet-derived growth factor; SCF, stem cell factor; TF, tissue factor; TGF, transforming growth factor; VEGF, vascular endothelial growth factor.
FIGURE 3.
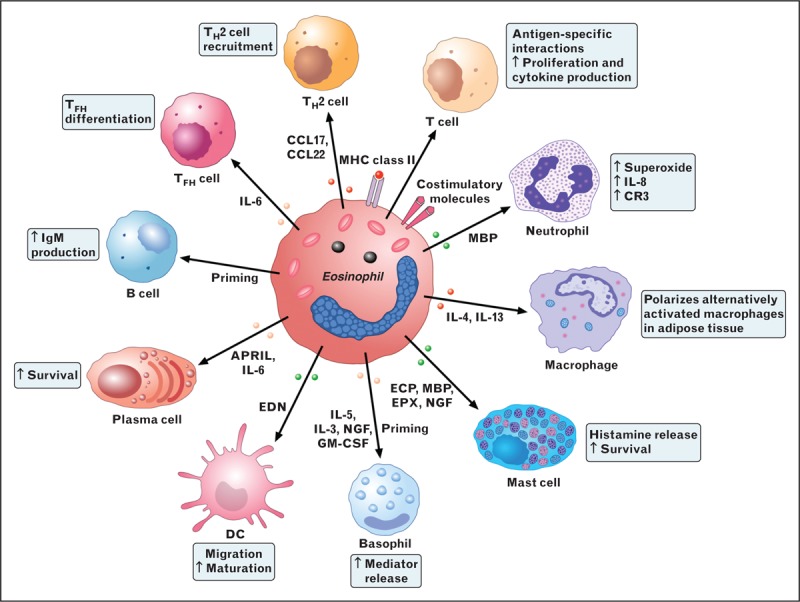
Eosinophils modulate the functions of a multitude of cells of the innate and adaptive immune system. Although not professional antigen-presenting cells (APC), eosinophils can express MHC class II and costimulatory molecules (CD80 or CD86), process antigens and stimulate T cells to proliferate and produce cytokines in an antigen-specific manner. Acting with dendritic cells (DCs), eosinophils regulate the recruitment of T helper 2 (Th2) cells in response to allergen sensitization by producing CCL17 and CCL22. Eosinophil can also favor T follicular helper (Tfh) cell differentiation through the production of interleukin-6 [35]. Eosinophils also prime B cells and sustain long-lived plasma cells in bone marrow via the production of APRIL and interleukin-6. Eosinophils stimulated by CpG DNA and by EDN promotes the maturation and activation of DCs. MBP activates neutrophils causing the release of superoxide and interleukin-8 and increases their expression of the cell-surface integrin complement receptor 3 (CR3). Eosinophils also maintain alternatively activated macrophages (M2 macrophages) by producing interleukin-4 and interleukin-13. MBP, ECP, and EPX activate basophils and mast cells, resulting in the release of histamine. Eosinophil granule proteins also activate platelets. Eosinophil-derived NGF primes human basophils and modulates several functions of mast cells.
Eosinophils and their mediators participate in the pathophysiology of a variety of diseases, including allergic asthma [10,36▪], EGPA [4,9], and cancer rejection [7]. However, current data suggest that deficiency of eosinophils in animals and humans appears to have no ill effects on normal health [37].
INTERLEUKIN-5 AND EOSINOPHILS
Interleukin-5 is a cytokine that belongs to the β common chain family [together with interleukin-3 and granulocyte-monocyte colony-stimulating factor (GM-CSF)] and binds an heterodimer receptor composed by the specific subunit interleukin-5Rα and common β subunit βc [3,38] (Fig. 4). Interleukin-5 plays a fundamental role in eosinophil differentiation in the bone marrow, recruitment and activation at sites of allergic inflammation [3]. Human eosinophils express about a three-fold higher level of interleukin-5Rα compared with basophils [39]. Th2 cells, mast cells, CD34+ progenitor cells, invariant natural killer T, group 2 innate lymphoid cells, and eosinophils themselves are major cellular source of interleukin-5 [40–42]. Group 2 ILCs are an important source of interleukin-5 contributing to tissue and blood eosinophilia [43]. Interestingly, blood eosinophils demonstrate circadian cycling and group 2 innate lymphoid cells control eosinophil number through the production of interleukin-5 [42]. Interleukin-5 modulates the differentiation and maturation of eosinophil in the bone marrow, their migration from blood to tissue sites [44], and the prevention of eosinophil apoptosis [45]. Interleukin-5 also appears to modulate the development and functions of human basophils and mast cells. Interleukin-5 enhances the release of mediators from human basophils [46] via the engagement of IL-5 receptor [42].
FIGURE 4.
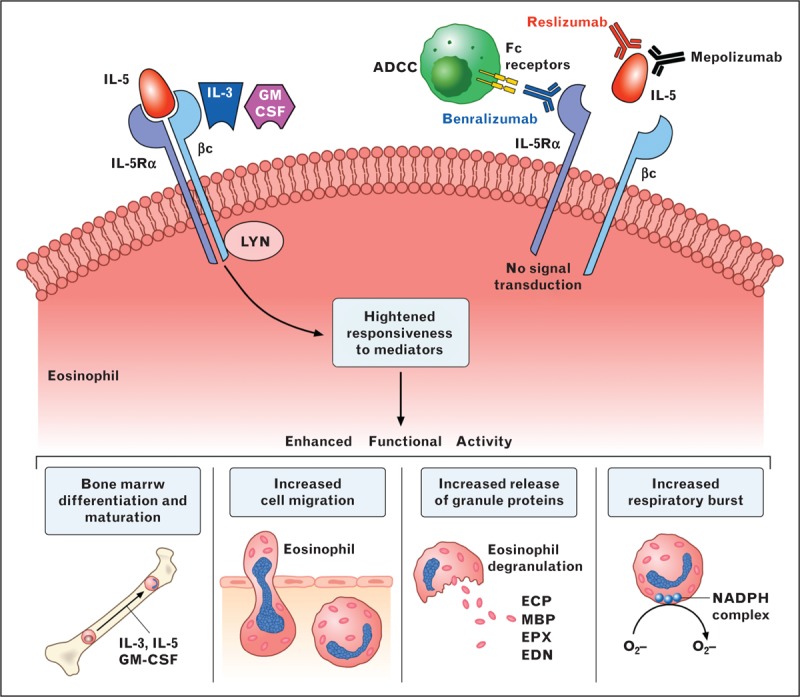
Interleukin-5 plays a fundamental role in the proliferation, maturation in the bone marrow, recruitment and activation at sites of allergic inflammation of eosinophils. The engagement of interleukin-5R through the interaction of interleukin-5 with interleukin-5αR and the βc subunit results in differentiation and maturation of eosinophils in the bone marrow, enhanced cell migration, release of granule proteins, and respiratory burst of eosinophils. Interleukin-3 and GM-CSF interact only with the βc subunit of interleukin-5R and enhance the functional response to stimuli of eosinophils (left side). Anti-interleukin-5 monoclonal antibodies (mepolizumab and reslizumab) bind to different epitopes of interleukin-5 blocking its ligation to interleukin-5Rα highly expressed on the human eosinophil membrane. Benralizumab is a humanized monoclonal antibody that binds to human interleukin-5Rα, resulting in inhibition of interleukin-5 activation. The latter approach also leads to antibody-dependent cellular cytotoxicity (ADDC) caused by Fc receptor binding on natural killer cells to the anti-interleukin-5Rα antibody on eosinophil (right side).
EOSINOPHILS AND INTERLEUKIN-5 IN ASTHMA
There is increasing evidence that eosinophilic inflammation of the lungs is a hallmark of eosinophilic asthma and has been associated with elevated levels of interleukin-5 in bronchial biopsies from asthmatic patients [47]. Moreover, interleukin-5 mRNA is upregulated in the bronchial mucosa upon allergen challenge [48] and interleukin-5 concentrations correlate with clinical features of asthma [49]. Eosinophils play a critical role in the pathogenesis and severity of asthma through the action of interleukin-5. In the asthmatic lung, T lymphocytes and group 2 ILCs are main sources of interleukin-5 with eosinophils and mast cells contributing to the level of this cytokine [43,50]. Interleukin-25 stimulates Th2 cells and group 2 ILCs to markedly increase the production of interleukin-5 [41,43]. The precise role of eosinophils as a prominent cell type in certain phenotypes of asthma was not firmly established until a number of clinical trials demonstrated that treatment with monoclonal antibodies against interleukin-5 significantly reduced the number of lung and blood eosinophils in patients with severe corticosteroid-resistant asthma [51▪▪,52–55,56▪▪,57]. Trials of therapeutics involving monoclonal antibodies to interleukin-5 and its receptor, interleukin-5Rα, and other approaches have been completed or are underway in patients with bronchial asthma, EGPA, and COPD.
CLINICAL TRIALS EVALUATING INTERLEUKIN-5 ANTAGONISM IN ASTHMA
Targeting interleukin-5 or interleukin-5Rα is an appealing approach to the treatment of patients with eosinophilic asthma. Anti-interleukin-5 monoclonal antibodies bind to interleukin-5 interfering with its ligation to interleukin-5-Rα expressed on the eosinophil and basophil membranes [3] (Fig. 4). Two different humanized anti-interleukin-5 monoclonal antibodies, mepolizumab (GlaxoSmithKline, Brentford, UK) and reslizumab (Teva Pharmaceuticals, Petha Tiqwa, Israel), have been developed and shown safety and efficacy in clinical trials for asthma.
Mepolizumab
Mepolizumab is a humanized monoclonal antibody (mAb) of the IgG1/k class which has been investigated for the treatment of asthma, atopic dermatitis, hypereosinophilic syndrome, eosinophilic esophagitis, nasal polyposis, and EGPA [51▪▪,53,55,56▪▪,57–59]. Table 1 summarizes the clinical trials evaluating the effects of mepolizumab in asthma [51▪▪,53,55,56▪▪,57,58,60]. An initial study [58] on the safety and efficacy of mepolizumab (750 mg intravenously (i.v.) every 4 weeks for 3 months) in 11 patients with mild asthma showed that the antibody was well tolerated and induced a decrease of blood eosinophils, but did not deplete airway or bone marrow eosinophils. The authors found no difference between mepolizumab and placebo on peak expiratory flow rate, airway hyperresponsiveness, or forced expired volume in one second (FEV1) in these patients with mild asthma. A subsequent larger study [53] in patients with refractory eosinophilic asthma confirmed that mepolizumab (750 mg i.v. every 4 weeks for 1 year) reduced blood and sputum eosinophils. This treatment reduced the number of severe exacerbations during the treatment and improved the quality of life score [Asthma Quality of Life Questionnaire (AQLQ)]. Also in this study, mepolizumab did not influence FEV1 and bronchial hyperreactivity. In a similar study [55] performed on a limited number of nine patients with prednisone-dependent asthma, mepolizumab (750 mg i.v. every 4 weeks for 5 months) had similar effects on blood and sputum eosinophils and reduced exacerbations. This study showed that mepolizumab allowed prednisone sparing in patients who had asthma with sputum eosinophilia. In the largest study ever undertaken (462 patients) in severe eosinophilic asthma, mepolizumab (75, 250, or 750 mg i.v. every 4 weeks for 13 infusions) reduced blood and sputum eosinophilia and exacerbations. Also in this study [57], FEV1, AQLQ, and Asthma Control Questionnaire (ACQ) scores were not modified by mepolizumab treatment.
Table 1.
Clinical trials of mepolizumab in asthma (anti-interleukin-5, IgG1 – Bosatria – GSK)
| First author/ref/year | Disease severity | No. of patients treated | Dosage/delivery | Outcome summary |
| Flood-Page et al. [58], 2003 | Mild asthma | 11 | 750 mg i.v. every 4 weeks for 3 months | ↓Blood Eos; ↓Airway Eos only by 50% = PEF, FEV1, bronchial hyperresponsiveness |
| Haldar et al. [53], 2009 | Eosinophilic asthma | 61 | 750 mg i.v. every 4 weeks for 1 year | ↓Blood + Sputum Eos; ↓Severe exacerbations; ↑QoL = FEVj, bronchial hyperreactivity |
| Nair et al. [55], 2009 | Prednisone-dependent asthma | 9 | 750 mg i.v. every 4 weeks for 5 months | ↓Blood + Sputum Eos; ↓Exacerbations; Prednisone sparing effect |
| Pavord et al. [57], 2012 | Severe eosinophilic asthma | 462 | 75–250–750 mg i.v. every 4 weeks for 13 infusions | ↓Blood + Sputum Eos; ↓Exacerbations = FEV1, AQLQ, and ACQ scores |
| Bel et al. [51▪▪], 2014 | Severe eosinophilic asthma | 135 | 100 mg s.c. every week for 20 weeks | Glucocorticoid sparing effect; ↓Exacerbations; Improvement ACQ-5 score |
| Ortega et al. [56▪▪], 2014 | Severe eosinophilic asthma | 385 | 75 mg i.v. or 100 mg s.c. every 4 weeks for 32 weeks | ↓Blood + Sputum Eos; ↓Exacerbations; ↑FEV1; ↑ACQ-5 score |
| Basu et al. [60], 2015 | Severe eosinophilic asthma | Healthcare resources and costs of mepolizumab versus placebo in a clinical trial (MENSA Study) |
Two recent studies have evaluated the efficacy and safety of mepolizumab administered subcutaneously (s.c.). In 135 patients with severe eosinophilic asthma, mepolizumab (100 mg s.c. every 4 weeks for 20 weeks) had a glucocorticoid-sparing effect and reduced exacerbations. The authors also reported a significant improvement in the ACQ-5 score [51▪▪]. In another study [56▪▪] in which 385 patients with severe eosinophilic asthma were treated with mepolizumab (75 mg i.v. or 100 mg s.c. every 4 weeks for 32 weeks), this treatment reduced blood eosinophils, the number of exacerbations, improved FEV1 and ACQ-5 score. The latter study has been subjected to additional analysis showing that mepolizumab treatment significantly reduced the cost of the treatment of these patients [60]. An open-label study evaluating the pharmacokinetics and pharmacodynamics of mepolizumab administered s.c. in children from 6 to 11 years of age with severe eosinophilic asthma is underway (NCT02377427). First, on November 2015 the US FDA and, then, on December 2, 2015 the European EMA approved mepolizumab as an add-on maintenance treatment for adults with severe eosinophilic asthma.
Reslizumab
Reslizumab (formerly SCH55700, Cinquil; Teva Pharmaceuticals) is a humanized anti-interleukin-5 mAb of the IgG4/k class in clinical development for the treatment of eosinophilic inflammatory disorders. Reslizumab has been evaluated in randomized controlled clinical trials in patients with asthma [52,54] (Table 2) and nasal polyps [61]. In 18 patients with severe asthma reslizumab (0.03–1 mg/kg i.v. in single dose) was safe and decreased blood eosinophils for at least 4 weeks [62]. In a more recent study [52], reslizumab administered in 53 adult patients with severe eosinophilic asthma (3 mg/kg i.v. every 4 weeks for 12 weeks) reduced blood and sputum eosinophils, improved airway function (FEV1), and increased ACQ score. The biological and clinical improvement was more marked in patients with nasal polyps that represented approximately 30% of patients with severe eosinophilic asthma. These findings have prompted multifaceted asthma studies that are currently underway. The US FDA Advisory Committee recommended approval for reslizumab on 11 December 2015 as an add-on maintenance treatment for adults with severe eosinophilic asthma.
Table 2.
Clinical trials of reslizumab in asthma (SCH55700 – anti-interleukin-5, IgG4 – Cephalon Inc.)
| First author/ref/year | Disease severity | No. of patients treated | Dosage/delivery | Outcome summary |
| Kips et al. [54], 2003 | Severe asthmatics | 18 | 0.03–1 mg/kg i.v. single dose | Safe; ↓Blood Eos |
| Castro et al. [52], 2011 | Severe eosinophilic asthma | 53 | 3 mg/kg i.v. every 4 weeks for 12 weeks | ↓Blood Eos; ↑FEV1; ↑ACQ-5 score; Particularly in patients with nasal polyps ±30% patients had nasal polyps |
Benralizumab
Benralizumab (formerly MEDI-563; MedImmune-AstraZeneca, London, UK) is a humanized mAb of the IgG1/k class that binds to human interleukin-5Rα, resulting in inhibition of interleukin-5 receptor activation. Benralizumab is not fucosylated and this enhances its binding to FcγRIIIa, leading to enhanced antibody-dependent cell-mediated cytotoxicity [3] (Fig. 4). Benralizumab binds with high affinity to the D1 domain of interleukin-5Rα on human eosinophils and basophils. Interestingly, human eosinophils express about a three-fold higher levels of interleukin-Rα compared with basophils [39]. In the latter study, it has been demonstrated that benralizumab induces eosinophil and basophil apoptosis mediated by antibody-dependent cell-mediated cytotoxicity.
Table 3 summarizes the clinical trials evaluating the effects of benralizumab in asthma [63,64▪▪,65,66]. The first study in 44 adult patients with mild atopic asthma a single dose of i.v. benralizumab (0.03–3– mg/kg) reduced blood eosinophils. Eosinopenia lasted 8–12 weeks. Benralizumab was associated with a transient, mild decrease of white blood cells and an increased of C-reactive protein (± 5.5-fold), interleukin-6 and creatine phosphokinase of peripheral muscular origin [63]. In another study of 26 adult patients with eosinophilic asthma, single-dose i.v. (1 mg/kg) and 3 s.c. doses (1 mg or 200 mg every month for 3 months) reduced eosinophil counts in blood sputum and airway mucosa/submucosa. Interestingly, also the number of basophils, which expressed interleukin-5Rα [39], markedly decreased [65].
Table 3.
Clinical trials of benralizumab in asthma (MEDI-563, Anti-interleukin-5a, IgG1 – Medimmune)
| First author/ref/year | Disease severity | No. of patients treated | Dosage/delivery | Outcome summary |
| Busse et al. [63], 2010 | Mild atopic asthma | 44 | 0.0003–3 mg/kg i.v. single dose | ↓Blood Eos at dose 0.03–3 mg; Eosinopenia lasted 8–12 weeks |
| Transient, mild decrease in WBC | ||||
| CRP increased ±5.5-fold | ||||
| Interleukin-6 increased | ||||
| CPK of peripheral muscular origin increased | ||||
| Laviolette et al. [65], 2013 | Eosinophilic asthma | 26 | 1 mg/kg i.v.; 100 mg s.c. every month for 3 doses; 200 mg s.c. every month for 3 doses | ↓Eos in blood, sputum and bronchial mucosa; ↓Basophils; Nasopharingitis 25%; Headache 25%; Nausea 22% |
| Castro et al. [64▪▪], 2014 | Eosinophilic asthma | 384 | 2–20–200 mg 2 s.c. every 4 weeks for the first 3 doses, then every 8 weeks for 1 year | 20 mg and 100 mg↓ asthma; Exacerbation = FEV1? |
| Nowak et al. [66], 2015 | Asthma after acute attack | 72 | Single dose 0.3 mg/kg i.v. 1 mg/kg i.v. Evaluated up to 6 months | ↓Blood Eos; ↓Exacerbations |
In a phase 2b study, benralizumab was administered to 385 adult patients with eosinophilic, uncontrolled asthma. In asthmatics receiving benralizumab (20 and 100 mg two s.c. injections every 4 weeks for a total of 12 months) there were fewer exacerbations. The higher 100 mg dose of benralizumab also improved lung function, asthma control, and mean ACQ-6 score compared with placebo [64▪▪]. This study has provided useful information to design phase 3 studies underway in patients with moderate or severe asthma with peripheral eosinophil count of at least 300 cells/μl. In a recent study [66], a single dose of benralizumab (0.3 and 1 mg/kg i.v.) was administered to 72 adult patients with severe asthma resulting in emergency department visit with the assumption that these patients are at increased risk for exacerbations. A single dose of benralizumab reduced blood eosinophils and the exacerbations during the following 3 months.
CLINICAL TRIALS EVALUATING ANTISENSE OLIGONUCLEOTIDES IN ASTHMA
Antisense oligonucleotides can be used as a therapeutic strategy to down-regulate the transcription of specific proteins [67]. TPI ASM8 (Topigen Pharmaceuticals, Montreal, Canada) is a mixture of two modified phosphorothioate antisense oligonucleotides, one directed against the human βc chain shared by the interleukin-3, interleukin-5, and GM-CSF receptors, and the other directed against the chemokine receptor CCR3 present on human eosinophils, basophils [68], and mast cells [69]. With this broad spectrum of activity, it was hoped that TPI ASM8 may provide more complete inhibition of eosinophilic influx than agents targeting interleukin-5 alone. Inhalation of TPI ASM8 reduced eosinophils in sputum and attenuated the allergen-induced airway responses in subjects with mild asthma [70]. A subsequent study evaluated the dose-response effects of TPI ASM8 in mild asthmatics. It found that TPI ASM8 was safe and well tolerated at all doses and inhibited eosinophil influx in sputum and ECP after allergen challenge. Moreover, the oligonucleotides attenuated early and late responses to allergen and improved airway hyperresponsiveness to methacholine [71]. In another study [72], TPI ASM8 reduced allergen-induced sputum eosinophils, the early and late asthmatics responses and the number of eosinophil progenitors CD34+ interleukin-5αR+ in mild asthmatics. Although therapy with this novel multitarget approach appears safe and promising, TPI ASM8 seems to have been discontinued.
CLINICAL TRIALS EVALUATING INTERLEUKIN-5 ANTAGONISM IN EOSINOPHILIC GRANULOMATOSIS WITH POLYANGIITIS
In 1951, J. Churg and L. Strauss [73] first described a form of systemic vasculitis occurring exclusively among patients with asthma and intense tissue eosinophils. This condition, called ‘Churg–Strauss syndrome’ for many years, has now been recognized as EGPA [9]. EGPA commonly presents with upper airway tract and lung involvement, cardiac and skin lesions. Although the pathogenesis of EGPA is multifactorial, the disease has a genetic background and can presumably be triggered by exposure to allergens or drugs [9]. The asthmatic and eosinophilic components suggest an activated Th2 imbalance [74]. Interleukin-5 appears to be upregulated in active EGPA [74]. Eosinophils are increased both in peripheral blood and tissue lesions. Eotaxin-3, produced by epithelial and endothelial cells, might contribute to tissue influx of eosinophils [75]. Activated eosinophils release cationic proteins, thereby contributing to tissue damage. Moreover, eosinophils in EGPA produce interleukin-25, which induces Th2 responses, thereby maintaining a vicious circle [76].
There is no consensus regarding the use of remission induction and remission maintenance therapeutic approach in patients with different forms of EGPA. Asthma is a well established and prominent clinical hallmark of EGPA. In a series of 383 patients, about 90% had asthma at EGPA diagnosis, with a mean onset of asthma to EGPA onset interval of 9 years [77]. In a series of 22 patients, the majority had severe or moderate asthma onset, and the condition was poorly controlled in 95% [78]. Cardiac involvement occurs in the majority of EGPA cases [77] and represents the major cause of early death and poor long-term prognosis. Eosinophil cationic proteins can activate human cardiac mast cells to induce the release of fibrogenic and vasoactive mediators [34,79].
A pilot study [80] tested the safety and efficacy of mepolizumab in seven steroid-dependent EGPA patients unable to taper prednisone below 10 mg daily. The patients received 4 monthly infusions of mepolizumab (750 mg each) on top of their therapy. Most patients were able to taper their prednisone dose, achieved a better control of the disease and a reduction in peripheral eosinophil count. Interestingly, the lack of improvement in pulmonary function despite reduced peripheral blood eosinophil count suggests that other variables contribute to EGPA airway disease. Finally, relapses were the rule following treatment discontinuation. Another pilot study [81] in refractory/relapsing EGPA patients confirmed the glucocorticoid-sparing property of mepolizumab in a series of eight out 10 patients. These results suggest that adjunct therapy with mepolizumab might be a glucocorticoid-sparing treatment option in patients with EGPA and reiterates the role of eosinophil in this eosinophilic vasculitis. Several double-blind placebo-controlled clinical trials are underway (NCT00716651 and NCT02020889).
CLINICAL TRIAL EVALUATING INTERLEUKIN-5 ANTAGONISM IN CHRONIC OBSTRUCTIVE PULMONARY DISEASE
COPD is a major cause of morbidity and mortality throughout the world that is primarily caused by tobacco smoking and indoor/outdoor pollution. It is characterized by irreversible, progressive airflow obstruction [82]. Chronic inflammation in the airways is mainly caused by CD8+ T cells, macrophages, and neutrophils [12]. Approximately 20% of patients with COPD without asthma or atopy have persistent circulating and airway eosinophilia associated with an increased risk of exacerbations [14,83]. Importantly, asthma attacks with eosinophils predict mortality in COPD patients [15]. Inhaled glucocorticoids are the key drug to prevent exacerbations in severe COPD where eosinophilia is present [12].
Although asthma and COPD in their typical forms are distinct clinical entities, some patients have features of both diseases. Their condition is now called asthma-COPD overlap syndrome (ACOS) [84,85]. ACOS is still poorly understood and presumably includes several phenotypes necessitating different treatments [86,87].
A phase 2 trial tested the safety and efficacy of benralizumab in eosinophilic COPD (sputum eosinophils >3%) [88▪]. In patients with COPD benralizumab did not reduce the exacerbations and did not modify lung function. However, it was noticed a trend toward an improvement in FEV1 and exacerbations in patients with a baseline blood eosinophils greater than 200 cells/μl and treated with benralizumab. Additional studies are needed to evaluate the safety and efficacy of interleukin-5-targeted therapy in different forms of COPD, including ACOS.
CONCLUSION
In several studies the administration of mepolizumab has been found to be well tolerated in adult patients with eosinophilic asthma [51▪▪,53,55,56▪▪,57] and EGPA [81,89] for periods of 3 months to approximately 1 year. Recent evidence demonstrates that eosinophils play a major role in cancer rejection [7] and several hematologic and tissue cancers can be associated with eosinophilia [90]. Moreover, it has been suggested that ‘targeted antieosinophilic strategies may unmask or even accelerate progression’ of certain tumors in few patients with hypereosinophilic syndrome [91]. Therefore, long-term studies should evaluate the safety of targeted antieosinophilic strategies.
The success of novel biological agents in general, and in particular for interleukin-5 pathway inhibition, in asthma largely depends on the ability to select the appropriate patients. Ideally, patients should be selected by an easily measurable biomarker. The blood and/or sputum eosinophil count appears to be closely associated with a clinical response to interleukin-5 pathway inhibition in adult patients with eosinophilic asthma [51▪▪,55,56▪▪,57]. It is unclear whether eosinophilia is a useful biomarker to predict the efficacy of interleukin-5R targeting in patients with eosinophilic COPD. Other biomarkers in asthma might include activated eosinophil surface phenotype as detected by flow cytometry [92], elevated levels of blood and/or sputum interleukin-5 [93], soluble interleukin-5Rα [94], EMR1 [21], and soluble Siglec-8 [95]. We would like to suggest that the combined use of multiple biomarkers might be a better strategy to select asthmatic, COPD and ACOS patients responsive to interleukin-5 pathway inhibitors. The use of multiple inflammatory biomarkers has been already shown to improve the prediction of risk for cardiovascular disease [96].
It has been suggested that using supervised cluster analysis can help to select specific patients characteristics and therapeutic response to mepolizumab [97▪]. It is likely that in the future, biologic samples (e.g. blood cells, tissue biopsy, or sputum) from patients with eosinophilic respiratory disorders will be analyzed (e.g. biomarkers, transcriptomes, genes, microRNA, and others) for the purpose of phenotyping patients to tailor their treatment.
Severe eosinophilic asthma can occur in children [36▪]. Pharmacologic and biologic treatment of different forms of asthma can differ in children when compared with adults also because of distinct pathogenetic mechanisms [36▪], comorbidities (e.g. cardiovascular involvement) and pharmacokinetics/pharmacodynamics [98]. An open-label study is underway to characterize the pharmacokinetics/pharmacodynamics of mepolizumab administered s.c. in children from 6 to 11 years of age with severe eosinophilic asthma (NCT02377427). This study will also provide information whether eosinophilia is a useful biomarker to predict the efficacy of interleukin-5 targeting in children with eosinophilic asthma.
Omalizumab is approved in the treatment of adults and adolescents with severe asthma. A comparison of the efficacy and cost-effectiveness of omalizumab versus interleukin-5 pathway inhibitors in the treatment of adults and adolescents with severe asthma is needed in populations eligible to both the biologics.
Benralizumab binds with high affinity to the D1 domain of interleukin-5Rα present on both human eosinophils and basophils. Although eosinophils express about three-fold higher level of interleukin-5Rα compared with basophils, benralizumab induces apoptosis of both eosinophils and basophils) [39]. Thus, the possibility exists that benralizumab might have a more articulated effect compared with the monoclonal antibodies anti-interleukin-5. In addition, given the relevance of basophils and their mediators (e.g. interleukin-4, interleukin-3, leukotrienes, VEGFs, and others) in the pathogenesis of allergic disorders [99–101] it is possible that some of the clinical benefits of benralizumab in asthma might be because of its modulatory effect on these cells.
Asthma and COPD are distinct chronic inflammatory respiratory disease characterized by some similarities and many striking pathophysiological differences [84–86]. A total of 20–30% of patients with COPD without history of asthma have blood and tissue eosinophilia associated with increased risk of exacerbations [13,15,83]. In a phase 2 clinical trial, benralizumab did not reduce the exacerbation rate in eosinophilic COPD [88▪]. Further studies should focus on selected populations of COPD, including ACOS.
Asthma is a prominent feature of EGPA and there is no consensus on the use of remission induction and remission maintenance of EGPA. Preliminary evidence suggested that mepolizumab is safe in patients with EGPA enabling glucocorticoid tapering without modifying lung function [89]. Interestingly, in a pilot study [102] we have found that omalizumab has a glucocorticoid-sparing effect while decreasing blood eosinophils and improving lung function in EGPA patients.
In conclusion, targeted therapies with anti-interleukin-5 or anti-interleukin-5Rα seem safe and promising in short-term and medium-term treatment of selected adult patients with severe eosinophilic asthma. The long-term safety of these agents is an important issue that needs to be addressed also in the light of recent evidence of antitumor activity of eosinophils. Identification of novel biomarkers, in addition to sputum and blood eosinophilia, will allow a more selective identification of patients responsive to these treatments. Ongoing studies will provide information whether interleukin-5/interleukin-5Rα inhibition is safe and efficacious in children with eosinophilic asthma and selected patients with EGPA or COPD.
Several biologics, small molecules, and a GATA binding protein 3 (GATA3)-specific DNA enzyme [103▪] are advancing in clinical trials that would meet the criteria referred to as personalized or precision medicine treatment for patients with eosinophilic respiratory disorders (Table 4) [104–121]. Excitement is growing that within the next few years several biologics specifically targeting interleukin-5 pathway may become approved for clinical use in selected patients with eosinophilic inflammation. This will possibly happen when a wider range of specific biomarkers will lead us to a more precise identification of patients eligible for treatment with these biologic drugs [122▪].
Table 4.
Examples of agents targeting directly or indirectly human eosinophils in preclinical or clinical development
| Strategy | Target | Drug | Antieosinophil effects | References |
| Cell-surface protein | Siglec-8 | Anti-Siglec-8 monoclonal antibody | Apoptosis | Nutku et al. [104]; Bochner et al. [105] |
| CD172a | Inhibitor of signaling | Verjan Garcia et al. [106] | ||
| CD300a | Activation of inhibitory receptor | Munitz et al. [19] | ||
| Immunoglobulin -like receptor B | Munitz et al. [107] | |||
| α4β1, α4β7 | Natalizumab | Increase blood eosinophils and inhibits their tissue accumulation | Abbas et al. [108] | |
| α4β7 integrin | Vedolizumab | No effect | Soler et al. [109] | |
| α4β7, αEβ7 | Etrolizumab | Unknown | ||
| CCR3 | GW766944 | Block chemokine-induced eosinophils in vitro; no effect in vivo | Neighbour et al. [110] | |
| CD52 | Alemtuzumab | Deplete eosinophils in vivo | Wechsler et al. [25] | |
| CD131 | CSL311 | Unknown | ||
| CRTH2 | 0C000459 | Reduces tissue eosinophils | ||
| ACT-453859 | CRTH2 blockade | Gehin et al. [111] | ||
| EMR1 | Afucosylated anti EMR1 monoclonal antibody | Deplete primate eosinophils | Legrand et al. [20] | |
| Interleukin-4Rα | Dupilumab | Reduces airway eosinophils | Wenzel et al. [112] | |
| Interleukin-4Rα | AMG-317 | Does not reduce airway eosinophils | Corren et al. [113] | |
| H4 Receptor | UR-63325 JNJ 28610244 | Salcedo et al. [114]; Dib et al. [115] | ||
| Soluble mediator antagonist | Eotaxin-1 | Bertilimumab | Inhibits Eotaxin-1 mediated eosinophil activation in vitro | Ding et al. [116] |
| IgE | Omalizumab | Reduces eosinophils at sites of allergic inflammation and peripheral blood | Detoraki et al. [102] | |
| Interleukin-4 | Altrakincept; Pascolizumab; Pitrakinra | Reduce eosinophils at sites of allergic inflammation | Borish et al. [117]; Hart et al. [118] | |
| Interleukin-13 | Tralokinumab; Lebrikizumab; Anrukinzumab; RPC4046; QAX576 | Reduce eosinophils in blood and at sites of allergic inflammation | Blanchard et al. [119]; Maselli et al. [120] | |
| TSLP | AMG157 | Reduce eosinophils in blood and at sites of allergic inflammation | Gauvreau et al. [121] | |
| Transcription factor | GATA3 | SB010 | Reduce interleukin-5 and late asthmatic response after allergen challenge | Krug et al. [103▪] |
Acknowledgements
We apologize to the many authors who have contributed importantly to this field and whose work has not been cited due to space limitations.
Financial support and sponsorship
The work was supported in part by grants from Regione Campania CISI-Lab Project, CRèME Project, TIMING Project, and Associazione Ricerca Malattie Allergiche e Immunologiche (ARMIA).
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Ehrlich P. Beiträge zur Kenntnis der granulirten Bindegewbszellen und der ecosinophilen Leukocythen. Arch Anat Physiol (Leipzig) 1879; 3:166–169. [Google Scholar]
- 2.Ehrlich P. Ueber die spezifischen granulationen des blutes. Arch Anat Physiol (Leipzig) 1879. 571–579. [Google Scholar]
- 3.Broughton SE, Nero TL, Dhagat U, et al. The betac receptor family: structural insights and their functional implications. Cytokine 2015; 74:247–258. [DOI] [PubMed] [Google Scholar]
- 4.Khoury P, Grayson PC, Klion AD. Eosinophils in vasculitis: characteristics and roles in pathogenesis. Nat Rev Rheumatol 2014; 10:474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landolina N, Gangwar RS, Levi-Schaffer F. Mast cells’ integrated actions with eosinophils and fibroblasts in allergic inflammation: implications for therapy. Adv Immunol 2015; 125:41–85. [DOI] [PubMed] [Google Scholar]
- 6.Mehta P, Furuta GT. Eosinophils in gastrointestinal disorders: eosinophilic gastrointestinal diseases, celiac disease, inflammatory bowel diseases, and parasitic infections. Immunol Allergy Clin North Am 2015; 35:413–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carretero R, Sektioglu IM, Garbi N, et al. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol 2015; 16:609–617. [DOI] [PubMed] [Google Scholar]
- 8▪.Kay AB. The early history of the eosinophil. Clin Exp Allergy 2015; 45:575–582. [DOI] [PubMed] [Google Scholar]; It is a comprehensive overview on the role of eosinophils in respiratory diseases.
- 9.Vaglio A, Buzio C, Zwerina J. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): state of the art. Allergy 2013; 68:261–273. [DOI] [PubMed] [Google Scholar]
- 10.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 2012; 18:716–725. [DOI] [PubMed] [Google Scholar]
- 11.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med 2012; 18:673–683. [DOI] [PubMed] [Google Scholar]
- 12.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/. [Google Scholar]
- 13.Saetta M, Di Stefano A, Maestrelli P, et al. Airway eosinophilia in chronic bronchitis during exacerbations. Am J Respir Crit Care Med 1994; 150:1646–1652. [DOI] [PubMed] [Google Scholar]
- 14.Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med 2011; 184:662–671. [DOI] [PubMed] [Google Scholar]
- 15.Hospers JJ, Schouten JP, Weiss ST, et al. Asthma attacks with eosinophilia predict mortality from chronic obstructive pulmonary disease in a general population sample. Am J Respir Crit Care Med 1999; 160:1869–1874. [DOI] [PubMed] [Google Scholar]
- 16▪.Bochner BS. Novel therapies for eosinophilic disorders. Immunol Allergy Clin North Am 2015; 35:577–598. [DOI] [PMC free article] [PubMed] [Google Scholar]; The article provides insights about the mechanisms to block eosinophil damage in humans.
- 17.Hamann J, Koning N, Pouwels W, et al. EMR1, the human homolog of F4/80, is an eosinophil-specific receptor. Eur J Immunol 2007; 37:2797–2802. [DOI] [PubMed] [Google Scholar]
- 18.Travers J, Rothenberg ME. Eosinophils in mucosal immune responses. Mucosal Immunol 2015; 8:464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munitz A, Bachelet I, Eliashar R, et al. The inhibitory receptor IRp60 (CD300a) suppresses the effects of IL-5, GM-CSF, and eotaxin on human peripheral blood eosinophils. Blood 2006; 107:1996–2003. [DOI] [PubMed] [Google Scholar]
- 20.Legrand F, Tomasevic N, Simakova O, et al. The eosinophil surface receptor epidermal growth factor-like module containing mucin-like hormone receptor 1 (EMR1): a novel therapeutic target for eosinophilic disorders. J Allergy Clin Immunol 2014; 133:1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pujols L, Mullol J, Roca-Ferrer J, et al. Expression of glucocorticoid receptor alpha- and beta-isoforms in human cells and tissues. Am J Physiol Cell Physiol 2002; 283:C1324–C1331. [DOI] [PubMed] [Google Scholar]
- 22.Hsu J, Saltoun CA, Avila PC. Advances in upper airway diseases and allergen immunotherapy in 2011. J Allergy Clin Immunol 2012; 129:646–652. [DOI] [PubMed] [Google Scholar]
- 23.Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol 2000; 105:651–663. [DOI] [PubMed] [Google Scholar]
- 24.Dworski R, Simon HU, Hoskins A, et al. Eosinophil and neutrophil extracellular DNA traps in human allergic asthmatic airways. J Allergy Clin Immunol 2011; 127:1260–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wechsler ME, Fulkerson PC, Bochner BS, et al. Novel targeted therapies for eosinophilic disorders. J Allergy Clin Immunol 2012; 130:563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melo RC, Weller PF. Unraveling the complexity of lipid body organelles in human eosinophils. J Leukoc Biol 2014; 96:703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazzeo C, Canas JA, Zafra MP, et al. Exosome secretion by eosinophils: a possible role in asthma pathogenesis. J Allergy Clin Immunol 2015; 135:1603–1613. [DOI] [PubMed] [Google Scholar]
- 28.Kita H. Eosinophils: multifaceted biological properties and roles in health and disease. Immunol Rev 2011; 242:161–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol 2013; 13:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nissim Ben Efraim AH, Levi-Schaffer F. Marone G, Granata F. Roles of eosinophils in the modulation of angiogenesis. Angiogenesis, lymphangiogenesis and clinical implications. Chem Immunol Allergy, Basel: Karger; 2014. 138–154. [DOI] [PubMed] [Google Scholar]
- 31.Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol 2004; 113:30–37. [DOI] [PubMed] [Google Scholar]
- 32.Wu D, Molofsky AB, Liang HE, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 2011; 332:243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu VT, Frohlich A, Steinhauser G, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol 2011; 12:151–159. [DOI] [PubMed] [Google Scholar]
- 34.Patella V, de Crescenzo G, Marino I, et al. Eosinophil granule proteins activate human heart mast cells. J Immunol 1996; 157:1219–1225. [PubMed] [Google Scholar]
- 35.Varricchi G, Harker J, Borriello F, et al. T follicular helper (Tfh) cells in normal immune responses and in allergic disorders. Allergy 2015; (in press). [DOI] [PubMed] [Google Scholar]
- 36▪.Saglani S, Lloyd CM. Eosinophils in the pathogenesis of paediatric severe asthma. Curr Opin Allergy Clin Immunol 2014; 14:143–148. [DOI] [PubMed] [Google Scholar]; The study explores the role of eosinophils in severe asthma in children: a relevant problem in clinical practice, where the use of biologic treatments is becoming a real need.
- 37.Gleich GJ, Klion AD, Lee JJ, et al. The consequences of not having eosinophils. Allergy 2013; 68:829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosas M, Dijkers PF, Lindemans CL, et al. IL-5-mediated eosinophil survival requires inhibition of GSK-3 and correlates with beta-catenin relocalization. J Leukoc Biol 2006; 80:186–195. [DOI] [PubMed] [Google Scholar]
- 39.Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol 2010; 125:1344–1353. [DOI] [PubMed] [Google Scholar]
- 40.Phillips C, Coward WR, Pritchard DI, et al. Basophils express a type 2 cytokine profile on exposure to proteases from helminths and house dust mites. J Leukoc Biol 2003; 73:165–171. [DOI] [PubMed] [Google Scholar]
- 41.Fallon PG, Ballantyne SJ, Mangan NE, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med 2006; 203:1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nussbaum JC, Van Dyken SJ, von Moltke J, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 2013; 502:245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeKruyff RH, Yu S, Kim HY, et al. Innate immunity in the lung regulates the development of asthma. Immunol Rev 2014; 260:235–248. [DOI] [PubMed] [Google Scholar]
- 44.Shahabuddin S, Ponath P, Schleimer RP. Migration of eosinophils across endothelial cell monolayers: interactions among IL-5, endothelial-activating cytokines, and C-C chemokines. J Immunol 2000; 164:3847–3854. [DOI] [PubMed] [Google Scholar]
- 45.Ochiai K, Kagami M, Matsumura R, et al. IL-5 but not interferon-gamma (IFN-gamma) inhibits eosinophil apoptosis by up-regulation of bcl-2 expression. Clin Exp Immunol 1997; 107:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dahinden CA, Rihs S, Ochsensberger B. Regulation of cytokine expression by human blood basophils. Int Arch Allergy Immunol 1997; 113:134–137. [DOI] [PubMed] [Google Scholar]
- 47.Hamid Q, Azzawi M, Ying S, et al. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J Clin Invest 1991; 87:1541–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson D, Hamid Q, Bentley A, et al. Activation of CD4+ T cells, increased Th2-type cytokine mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. J Allergy Clin Immunol 1993; 92:313–324. [DOI] [PubMed] [Google Scholar]
- 49.Humbert M, Corrigan CJ, Kimmitt P, et al. Relationship between IL-4 and IL-5 mRNA expression and disease severity in atopic asthma. Am J Respir Crit Care Med 1997; 156:704–708. [DOI] [PubMed] [Google Scholar]
- 50.Ying S, Meng Q, Zeibecoglou K, et al. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and C-C chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (Intrinsic) asthmatics. J Immunol 1999; 163:6321–6329. [PubMed] [Google Scholar]
- 51▪▪.Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med 2014; 371:1189–1197. [DOI] [PubMed] [Google Scholar]; The study reports the results of the SIRIUS study, having as its primary outcome the steroid-sparing effect of mepolizumab in severe asthma treatment in adults.
- 52.Castro M, Mathur S, Hargreave F, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med 2011; 184:1125–1132. [DOI] [PubMed] [Google Scholar]
- 53.Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med 2009; 360:973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kips JC, O’Connor BJ, Langley SJ, et al. Effect of SCH55700, a humanized antihuman interleukin-5 antibody, in severe persistent asthma: a pilot study. Am J Respir Crit Care Med 2003; 167:1655–1659. [DOI] [PubMed] [Google Scholar]
- 55.Nair P, Pizzichini MM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med 2009; 360:985–993. [DOI] [PubMed] [Google Scholar]
- 56▪▪.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014; 371:1198–1207. [DOI] [PubMed] [Google Scholar]; The study reports the results of the MENSA study, having as its primary outcome the effect of mepolizumab on the rate of exacerbations in severe asthma treatment in over 500 adults.
- 57.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012; 380:651–659. [DOI] [PubMed] [Google Scholar]
- 58.Flood-Page PT, Menzies-Gow AN, Kay AB, et al. Eosinophil's role remains uncertain as antiinterleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med 2003; 167:199–204. [DOI] [PubMed] [Google Scholar]
- 59.Verzegnassi F. Hypereosinophilic syndrome and mepolizumab. N Engl J Med 2008; 358:2838–2840. [PubMed] [Google Scholar]
- 60.Basu A, Dalal A, Canonica GW, et al. The use of mepolizumab: healthcare resources and costs versus placebo in a clinical trial setting. Value Health 2015; (in press). [Google Scholar]
- 61.Gevaert P, Lang-Loidolt D, Lackner A, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol 2006; 118:1133–1141. [DOI] [PubMed] [Google Scholar]
- 62.Wang YH, Liu YJ. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin Exp Allergy 2009; 39:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Busse WW, Katial R, Gossage D, et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor alpha antibody, in a phase I study of subjects with mild asthma. J Allergy Clin Immunol 2010; 125:1237–1244. [DOI] [PubMed] [Google Scholar]
- 64▪▪.Castro M, Wenzel SE, Bleecker ER, et al. Benralizumab, an antiinterleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med 2014; 2:879–890. [DOI] [PubMed] [Google Scholar]; The study reports the results of a randomized, controlled, double-blind, dose-ranging phase 2b study in severe asthma treatment, having as primary outcome the effect of benralizumab on the annual exacerbation rate in eosinophilic individuals after 1-year follow-up.
- 65.Laviolette M, Gossage DL, Gauvreau G, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol 2013; 132:1086–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nowak RM, Parker JM, Silverman RA, et al. A randomized trial of benralizumab, an antiinterleukin 5 receptor alpha monoclonal antibody, after acute asthma. Am J Emerg Med 2015; 33:14–20. [DOI] [PubMed] [Google Scholar]
- 67.McClorey G, Wood MJ. An overview of the clinical application of antisense oligonucleotides for RNA-targeting therapies. Curr Opin Pharmacol 2015; 24:52–58. [DOI] [PubMed] [Google Scholar]
- 68.Uguccioni M, Mackay CR, Ochensberger B, et al. High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP-4, and other chemokines. J Clin Invest 1997; 100:1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Romagnani P, De Paulis A, Beltrame C, et al. Tryptase-chymase double-positive human mast cells express the eotaxin receptor CCR3 and are attracted by CCR3-binding chemokines. Am J Pathol 1999; 155:1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gauvreau GM, Boulet LP, Cockcroft DW, et al. Antisense therapy against CCR3 and the common beta chain attenuates allergen-induced eosinophilic responses. Am J Respir Crit Care Med 2008; 177:952–958. [DOI] [PubMed] [Google Scholar]
- 71.Gauvreau GM, Pageau R, Seguin R, et al. Dose-response effects of TPI ASM8 in asthmatics after allergen. Allergy 2011; 66:1242–1248. [DOI] [PubMed] [Google Scholar]
- 72.Imaoka H, Campbell H, Babirad I, et al. TPI ASM8 reduces eosinophil progenitors in sputum after allergen challenge. Clin Exp Allergy 2011; 41:1740–1746. [DOI] [PubMed] [Google Scholar]
- 73.Churg J, Strauss L. Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am J Pathol 1951; 27:277–301. [PMC free article] [PubMed] [Google Scholar]
- 74.Jakiela B, Szczeklik W, Plutecka H, et al. Increased production of IL-5 and dominant Th2-type response in airways of Churg-Strauss syndrome patients. Rheumatology (Oxford) 2012; 51:1887–1893. [DOI] [PubMed] [Google Scholar]
- 75.Zwerina J, Bach C, Martorana D, et al. Eotaxin-3 in Churg–Strauss syndrome: a clinical and immunogenetic study. Rheumatology (Oxford) 2011; 50:1823–1827. [DOI] [PubMed] [Google Scholar]
- 76.Terrier B, Bieche I, Maisonobe T, et al. Interleukin-25: a cytokine linking eosinophils and adaptive immunity in Churg–Strauss syndrome. Blood 2010; 116:4523–4531. [DOI] [PubMed] [Google Scholar]
- 77.Comarmond C, Pagnoux C, Khellaf M, et al. Eosinophilic granulomatosis with polyangiitis (Churg–Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum 2013; 65:270–281. [DOI] [PubMed] [Google Scholar]
- 78.Szczeklik W, Sokolowska BM, Zuk J, et al. The course of asthma in Churg–Strauss syndrome. J Asthma 2011; 48:183–187. [DOI] [PubMed] [Google Scholar]
- 79.Marone G, Patella V, de Crescenzo G, et al. Immunological interactions between human eosinophils and cardiac mast cells. Chem Immunol 2000; 76:118–133. [DOI] [PubMed] [Google Scholar]
- 80.Kahn JE, Grandpeix-Guyodo C, Marroun I, et al. Sustained response to mepolizumab in refractory Churg–Strauss syndrome. J Allergy Clin Immunol 2010; 125:267–270. [DOI] [PubMed] [Google Scholar]
- 81.Moosig F, Gross WL, Herrmann K, et al. Targeting interleukin-5 in refractory and relapsing Churg–Strauss syndrome. Ann Intern Med 2011; 155:341–343. [DOI] [PubMed] [Google Scholar]
- 82.Bateman ED, Reddel HK, van Zyl-Smit RN, et al. The asthma-COPD overlap syndrome: towards a revised taxonomy of chronic airways diseases? Lancet Respir Med 2015; 3:719–728. [DOI] [PubMed] [Google Scholar]
- 83.Bafadhel M, Davies L, Calverley PM, et al. Blood eosinophil guided prednisolone therapy for exacerbations of COPD: a further analysis. Eur Respir J 2014; 44:789–791. [DOI] [PubMed] [Google Scholar]
- 84.Barrecheguren M, Esquinas C, Miravitlles M. The asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challenges. Curr Opin Pulm Med 2015; 21:74–79. [DOI] [PubMed] [Google Scholar]
- 85.Postma DS, Rabe KF. The asthma-COPD overlap syndrome. N Engl J Med 2015; 373:1241–1249. [DOI] [PubMed] [Google Scholar]
- 86.Barnes PJ. Therapeutic approaches to asthma-chronic obstructive pulmonary disease overlap syndromes. J Allergy Clin Immunol 2015; 136:531–545. [DOI] [PubMed] [Google Scholar]
- 87.Reddel HK. Treatment of overlapping asthma-chronic obstructive pulmonary disease: can guidelines contribute in an evidence-free zone? J Allergy Clin Immunol 2015; 136:546–552. [DOI] [PubMed] [Google Scholar]
- 88▪.Brightling CE, Bleecker ER, Panettieri RA, Jr, et al. Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: a randomised, double-blind, placebo-controlled, phase 2a study. Lancet Respir Med 2014; 2:891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study reports the results of a randomized, controlled, double-blind phase 2 study, in COPD treatment, having as its primary outcome the effect of benralizumab on acute exacerbations of COPD in patients with eosinophilia and COPD.
- 89.Kim S, Marigowda G, Oren E, et al. Mepolizumab as a steroid-sparing treatment option in patients with Churg–Strauss syndrome. J Allergy Clin Immunol 2010; 125:1336–1343. [DOI] [PubMed] [Google Scholar]
- 90.Simson L, Ellyard JI, Dent LA, et al. Regulation of carcinogenesis by IL-5 and CCL11: a potential role for eosinophils in tumor immune surveillance. J Immunol 2007; 178:4222–4229. [DOI] [PubMed] [Google Scholar]
- 91.Roufosse F, de Lavareille A, Schandene L, et al. Mepolizumab as a corticosteroid-sparing agent in lymphocytic variant hypereosinophilic syndrome. J Allergy Clin Immunol 2010; 126:828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bochner BS. Systemic activation of basophils and eosinophils: markers and consequences. J Allergy Clin Immunol 2000; 106:S292–S302. [DOI] [PubMed] [Google Scholar]
- 93.Simon HU, Plotz SG, Dummer R, et al. Abnormal clones of T cells producing interleukin-5 in idiopathic eosinophilia. N Engl J Med 1999; 341:1112–1120. [DOI] [PubMed] [Google Scholar]
- 94.Wilson TM, Maric I, Shukla J, et al. IL-5 receptor alpha levels in patients with marked eosinophilia or mastocytosis. J Allergy Clin Immunol 2011; 128:1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Na HJ, Hamilton RG, Klion AD, et al. Biomarkers of eosinophil involvement in allergic and eosinophilic diseases: review of phenotypic and serum markers including a novel assay to quantify levels of soluble Siglec-8. J Immunol Methods 2012; 383:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zethelius B, Berglund L, Sundstrom J, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med 2008; 358:2107–2116. [DOI] [PubMed] [Google Scholar]
- 97▪.Ortega H, Li H, Suruki R, et al. Cluster analysis and characterization of response to mepolizumab. A step closer to personalized medicine for patients with severe asthma. Ann Am Thorac Soc 2014; 11:1011–1017. [DOI] [PubMed] [Google Scholar]; It is a statistical analysis study with intent to provide/suggest the best predictive biomarkers of response in clinical practice.
- 98.Anderson WC, 3rd, Szefler SJ. New and future strategies to improve asthma control in children. J Allergy Clin Immunol 2015; 136:848–859. [DOI] [PubMed] [Google Scholar]
- 99.Borriello F, Granata F, Marone G. Basophils and skin disorders. J Invest Dermatol 2014; 134:1202–1210. [DOI] [PubMed] [Google Scholar]
- 100.Marone G, Galli SJ, Kitamura Y. Probing the roles of mast cells and basophils in natural and acquired immunity, physiology and disease. Trends Immunol 2002; 23:425–427. [DOI] [PubMed] [Google Scholar]
- 101.Varricchi G, Granata F, Loffredo S, et al. Angiogenesis and lymphangiogenesis in inflammatory skin disorders. J Am Acad Dermatol 2015; 73:144–153. [DOI] [PubMed] [Google Scholar]
- 102.Detoraki A, Di Capua L, Varricchi G, et al. Omalizumab in patients with eosinophilic granulomatosis with polyangiitis: a 36-month follow-up study. J Asthma 2015. 1–6. [DOI] [PubMed] [Google Scholar]
- 103▪.Krug N, Hohlfeld JM, Kirsten AM, et al. Allergen-induced asthmatic responses modified by a GATA3-specific DNAzyme. N Engl J Med 2015; 372:1987–1995. [DOI] [PubMed] [Google Scholar]; The study highlights a new possible intervention allergic asthma using as a model the allergen challenge test.
- 104.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood 2003; 101:5014–5020. [DOI] [PubMed] [Google Scholar]
- 105.Bochner BS. Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin Exp Allergy 2009; 39:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Verjan Garcia N, Umemoto E, Saito Y, et al. SIRPalpha/CD172a regulates eosinophil homeostasis. J Immunol 2011; 187:2268–2277. [DOI] [PubMed] [Google Scholar]
- 107.Munitz A, McBride ML, Bernstein JS, Rothenberg ME. A dual activation and inhibition role for the paired immunoglobulin-like receptor B in eosinophils. Blood 2008; 111:5694–5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abbas M, Lalive PH, Chofflon M, et al. Hypereosinophilia in patients with multiple sclerosis treated with natalizumab. Neurology 2011; 77:1561–1564. [DOI] [PubMed] [Google Scholar]
- 109.Soler D, Chapman T, Yang LL, et al. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther 2009; 330:864–875. [DOI] [PubMed] [Google Scholar]
- 110.Neighbour H, Boulet LP, Lemiere C, et al. Safety and efficacy of an oral CCR3 antagonist in patients with asthma and eosinophilic bronchitis: a randomized, placebo-controlled clinical trial. Clin Exp Allergy 2014; 44:508–516. [DOI] [PubMed] [Google Scholar]
- 111.Gehin M, Strasser DS, Zisowsky J, et al. A novel CRTH2 antagonist: Single- and multiple-dose tolerability, pharmacokinetics, and pharmacodynamics of ACT-453859 in healthy subjects. J Clin Pharmacol 2015; 55:787–797. [DOI] [PubMed] [Google Scholar]
- 112.Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med 2013; 368:2455–2466. [DOI] [PubMed] [Google Scholar]
- 113.Corren J, Busse W, Meltzer EO, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am J Respir Crit Care Med 2010; 181:788–796. [DOI] [PubMed] [Google Scholar]
- 114.Salcedo C, Pontes C, Merlos M. Is the H4 receptor a new drug target for allergies and asthma? Front Biosci (Elite Ed) 2013; 5:178–187. [DOI] [PubMed] [Google Scholar]
- 115.Dib K, Perecko T, Jenei V, et al. The histamine H4 receptor is a potent inhibitor of adhesion-dependent degranulation in human neutrophils. J Leukoc Biol 2014; 96:411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ding C, Li J, Zhang X. Bertilimumab Cambridge Antibody Technology Group. Curr Opin Investig Drugs 2004; 5:1213–1218. [PubMed] [Google Scholar]
- 117.Borish LC, Nelson HS, Lanz MJ, et al. Interleukin-4 receptor in moderate atopic asthma. A phase I/II randomized, placebo-controlled trial. Am J Respir Crit Care Med 1999; 160:1816–1823. [DOI] [PubMed] [Google Scholar]
- 118.Hart TK, Blackburn MN, Brigham-Burke M, et al. Preclinical efficacy and safety of pascolizumab (SB 240683): a humanized anti-interleukin-4 antibody with therapeutic potential in asthma. Clin Exp Immunol 2002; 130:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Blanchard C, Mishra A, Saito-Akei H, et al. Inhibition of human interleukin-13-induced respiratory and oesophageal inflammation by anti-human-interleukin-13 antibody (CAT-354). Clin Exp Allergy 2005; 35:1096–1103. [DOI] [PubMed] [Google Scholar]
- 120.Maselli DJ, Keyt H, Rogers L. Profile of lebrikizumab and its potential in the treatment of asthma. J Asthma Allergy 2015; 8:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gauvreau GM, O’Byrne PM, Boulet LP, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med 2014; 370:2102–2110. [DOI] [PubMed] [Google Scholar]
- 122▪.Chiappori A, De Ferrari L, Folli C, et al. Biomarkers and severe asthma: a critical appraisal. Clin Mol Allergy 2015; 13:20. [DOI] [PMC free article] [PubMed] [Google Scholar]; The latest comprehensive review on biomarkers, their potential meaning and possible use to identify responders to biologics in clinical practice.


