Abstract
Objectives:
Cold plasma (CP) has been shown to be effective even against multiresistant microorganisms. As previous investigations on the effect of CP in root canals showed promising results, the aim of the present study was to analyze the bactericidal efficacy of CP in different depths of infected dentin.
Methods:
32 standardized root canals of human mandibular premolars were infected with Enterococcus faecalis and incubated for one week. Specimens were randomly selected for one of four disinfection methods: control (5mL NaCl), 5mL chlorhexidine (CHX), CP alone (CP), and a combination of 5mL CHX and cold plasma (CHX+CP). CHX was ultrasonically activated for 30s, while cold plasma was used for 60s in the root canals. Dentin samples at depths of 300, 500 and 800 µm were obtained and diluted serially. Colony forming units (CFUs) were counted on agar plates after 24h of incubation.
Results:
The highest overall logarithmic reduction factors (RF) were obtained from CHX+CP (log RF 3.56 p<0.01; Mann-Whitney U test), followed by CP (log RF 3.27 p<0.01) and CHX alone (log RF 2.65 p<0.01) related to the control. All disinfection methods showed significantly lower CFU counts compared to the control group in 300 µm and 800 µm (both p<0.01, Kruskal-Wallis test).
Discussion:
The adjuvant use of CP might be beneficial in highly infected root canals to improved disinfection. However, the disinfection effect against Enterococcus faecalis of CP is comparable to ultrasonically activated CHX.
Keywords: Cold plasma, dentinal tubules, disinfection, Enterococcus faecalis, penetration depth, tissue tolerable plasma.
INTRODUCTION
The aim of endodontic treatment is the elimination of bacteria in the root canal system [1]. Sodium hypochlorite and chlorhexidine (CHX) both are standard of care for the disinfection of root canals [2]. Nevertheless, complex root canal anatomies and inter-individual differences in dentinal tubule morphology potentially cause difficulties in sufficiently disinfecting all parts of the root canal system using these standard methods alone [3, 4].
As especially Enterococcus faecalis survives endodontic treatment in the depth of tubules and under postendodontic conditions, it is one of the main reasons for endodontic failure, even in cases with obviously lege artis accomplished root canal treatment [5, 6]. Multiresistant microorganisms are also a major problem in the treatment of infected wounds where cold plasma (CP) has recently been introduced. The antimicrobial effectiveness of CP against different pathogens, regardless of resistance patterns, has been proven in vitro in bacterial and fungal cultures [7-13] as well as in case reports and clinical studies involving animals and humans, the latter especially in dermatology and otorhinolaryngology [14-16]. Via diverse biochemical and physiological mechanisms, CP improves wound healing and tissue .regeneration in addition to its antimicrobial efficacy [17, 18]. No harm to viable tissues or altering effects on tissue function have been reported [19, 20].
Plasma is an ionized gas, carrying out synergetic antimicrobial effects through e.g. excited oxygen atoms, UV-radiation, and a light current flow [21]. Therefore, cold plasma could be a new approach for a more efficient disinfection of the root canal system. Previous studies have already examined the bactericidal efficiency against E. faecalis in biofilms [22, 23], in resin block models of root canals [24], and in different sections of root canals [25], but its efficacy at different depths of dentinal tubules has not been investigated yet.
The aim of the present study was to examine the bactericidal efficacy against E. faecalis in dentinal tubules using CP alone and combined with CHX at different depth layers of human dentinal tubules, hypothesizing that a combination of cold plasma and CHX is more effective than CHX alone.
MATERIAL AND METHODS
Human Dentin Specimen Preparation
For the intended approach, we modified certain parameters of the established in vitro model of Haapasalo [26]: 32 human mandibular premolars with straight roots and a round cross-section (no cracks, no caries, no obliteration, apical root canal sizes below 0.3 mm diameter) were selected. Subsequently, the apical part (3 mm) was cut off and the teeth were decoronated, resulting in 8 mm specimens. The minimum thickness at the apical end was 1 mm circularly around the root canal. To ensure a constant removal over the entire circumference later on, root canals were standardized using Gates-Glidden burs No. I (0.5 mm) for cylindrical preparation. Subsequently, the specimens were embedded in transparent acrylic resin (Technovit 4004, Heraeus Kulzer, Wehrheim, Germany) to simulate a closed root surface. Afterwards, the specimens were sealed at the apical and coronal surface with a dentin adhesive (Optibond FL, Kerr, Rastatt, Germany).
Bacteria Cultivation
Enterococcus faecalis (ATCC 29212, DSMZ, Braunschweig, Germany) cultures were obtained by cultivation for 24 hours at 37 °C on Columbia agar (Sifin, Berlin, Germany) plates under anaerobic conditions. Sterile brain-heart infusion (BHI, Sifin, Berlin, Germany) was subsequently inoculated with bacteria to approximately 5 × 105 cells/mL, confirmed by an optical density of 0.5 OD at 600 nm using a photometer (Novaspec II Visible Spectrophotometer, GE Healthcare, Solingen, Germany). Bacteria broth was stored with gas generating sachets (Oxoid AnaeroGen, Thermo Fisher Scientific, Waltham, USA) in anaerobic jars (Oxoid 2.5 Litre AnaeroJar™, Oxoid Ltd., Hampshire, UK) at 37 °C. Indicator stripes (Oxoid Anaerobic Indicator, Thermo Fisher Scientific, Waltham, USA) were used to confirm anaerobic conditions at all times.
Bacterial Colonization
Prior to incubation, specimens were irrigated with NaOCl 1% (Hedinger, Stuttgart, Germany), EDTA 17% (CanalPro EDTA, Coltene/Whaledent Inc., Cuyahoga Falls, USA) and Aqua dest. for four minutes, each with ultrasonic activation (Sonorex Super RK102H, Bandelin, Berlin, Germany). The specimens were subsequently autoclaved at 121 °C for 15 minutes. Before incubation, teeth were flushed, each with brain-heart infusion (BHI) using ultrasonic activation for 30 s to boost the presence of BHI in the tubules (Irri-Tip S, VDW, Munich, Germany). Then, the root canals were inoculated with E. faecalis cultures at an optical density of 0.5 OD and incubated in BHI broth for seven days. The medium was refreshed daily.
Antibacterial Treatment
Initially all specimens were flushed with 5 mL sterile NaCl 0.9% through the root canal using an endodontic needle. Bactericidal treatment was carried out after randomized assignment according to:
Control (n = 8): no additional treatment.
CHX (n = 8): 5 mL 2% CHX, 30 s ultrasonically enhanced irrigation.
CP (n = 8): 60 seconds CP treatment with a defined distance of 3 mm to the root canal.
CHX + CP (n = 8): 5 mL 2% CHX, 30 s ultrasonically enhanced irrigation followed by 60 s CP treatment.
As chlorhexidine digluconate (2%) is known to be the most effective irrigant against E. faecalis within a short time (30 s) [27], the specimens of the CHX group were flushed with 5 mL CHX 2% and simultaneously ultrasonically activated for 30 s (Irri-Tip S, VDW, Munich, Germany).
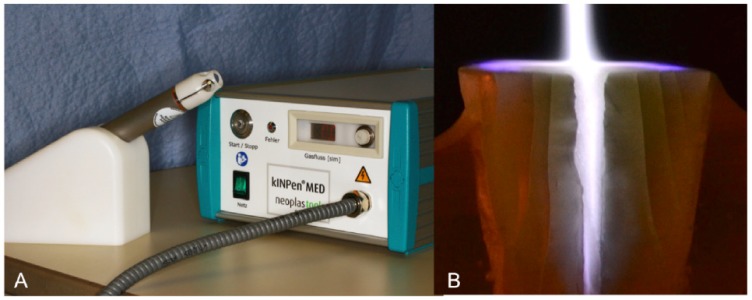
The KINPen Med® (neoplas tools, Greifswald, Germany) (Fig. 1) was used to generate the cold plasma. It consists of a control gear and a permanently attached handset. Argon, the operating gas, was set to 4.8 slm during application.
Fig. (1).
A: Cold plasma device kINPen Med (neoplas tools GmbH, Germany). B: Cold plasma jet in root canal: For visualization the specimen was separated at the height of the root canal.
Dentin Sampling
After treatment of each specimen, samples from defined dentin layers were harvested stepwise using different burs and transferred into two 2 mL-Eppendorf-vessel containing 1 mL sterile BHI:
Circular removal of 300 µm dentin with a Peeso reamer No. III (1.1 mm)
Circular removal to 500 µm with a Peeso reamer No. V (1.5 mm)
Circular removal to 800 µm with a round bur 021 (Hager & Meisinger, Neuss, Germany).
To prevent heating, rotation was limited to 6.000 U/min using an implant motor (OsseoSet, Nobel Biocare, Zurich, Switzerland) and a reducing contra-angle handpiece.
Then, 100 µL from the jars were transferred into another Eppendorf vessel containing 900 µL BHI for dilution of
1:1,00. The first two layers of the control group were additionally diluted to 1:1,000. 100 µL of the obtained dilution were spread on a Columbia agar plate and a CFU count was performed after 24 h of incubation under anaerobic conditions. The experiments were repeated three times. Gram staining and a biochemical identification system (API Rapid ID32 Strep, bioMérieux, Nürtingen, Germany) were used to confirm the identity of the microorganisms growing on Columbia agar plates as being E. faecalis.
Scanning Electron Microscopy (SEM)
Two additionally infected specimens were chosen for scanning electron microscopy. The root canals were split along the longitudinal axis and immersed in acetone for one second. Subsequently, the specimens were prepared for SEM with gold particles. Scanning electron microscope (CamScan Maxim 2040S, CamScan Electron Optics, Cambridgeshire, UK) revealed a dense biofilm and an adherence of bacteria in the tubules (Fig. 2). Preliminary tests showed bacterial penetration depths of up to 800 µm. As the present study intended to investigate the efficacy of bactericidal agents providing no biofilm removal SEM was limited to confirm biofilm formation ahead of treatment.
Fig. (2).
SEM of infected dentinal tubules.
Statistical Analysis
The median values of counted CFU/mL were determined and logarithmized. Subsequently, the reduction factors were calculated according to the formula: log RF = log(control layer) − log(disinfected layer) [28].
For statistical analysis, SPSS Statistics 21 (IBM, Armonk, USA) was used. The Kruskal-Wallis and Mann-Whitney U tests were performed to identify significances among different disinfection methods and investigated dentin layers.
RESULTS
Overall Disinfection
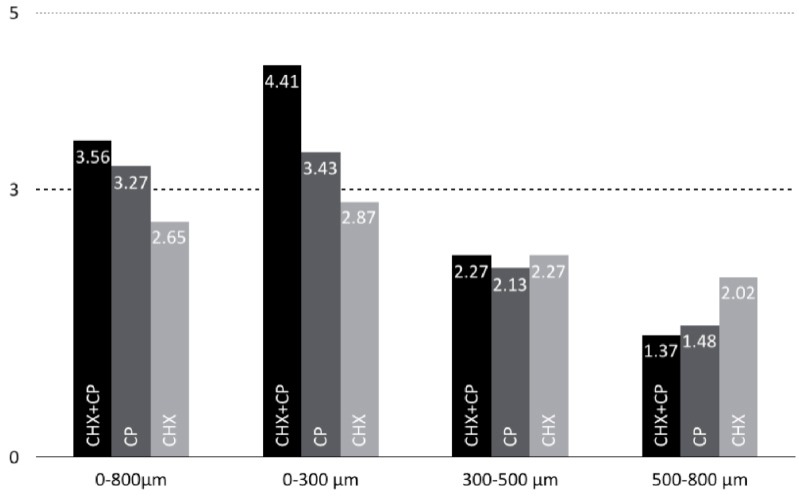
All tested disinfection methods were significantly more effective than the control group (p<0.01, Mann-Whitney U test) within the examined depth of 0-800 µm (Table 1). CHX + CP showed the highest CFU number reduction (3.56, p<0.01), followed by CP (3.27, p<0.01) and CHX (2.65, p<0.01) (Fig. 3). Comparing the results of the different disinfection methods among each other, CHX + CP was significantly more effective than CHX alone (p<0.01). There were no significant differences between the other methods.
Table 1.
log(CFU/mL) in the 0-800 µm layer of every specimen; standard deviation, median and mean are specified below.
| Specimen | Control | CP | CHX | CHX + CP |
|---|---|---|---|---|
| 1 | 6.52 | 3.40 | 2.78 | 3.26 |
| 2 | 6.00 | 4.13 | 4.17 | 2.70 |
| 3 | 6.72 | 3.38 | 4.87 | 2.78 |
| 4 | 6.82 | 3.66 | 4.74 | 2.70 |
| 5 | 6.81 | 3.43 | 3.99 | 3.96 |
| 6 | 8.02 | 4.12 | 4.16 | 3.86 |
| 7 | 7.01 | 2.78 | 3.34 | 3.72 |
| 8 | 8.00 | 4.53 | 4.19 | 3.26 |
| SD | 0.70 | 0.56 | 0.69 | 0.52 |
| Median | 6.82 | 3.55 | 4.16 | 3.26 |
| Mean | 6.99 | 3.68 | 4.03 | 3.28 |
Fig. (3).
Logarithmic reduction factors (log RF) of the different layers and disinfection methods.
Layer-Depending Disinfection
All tested disinfection methods were significantly more effective than the control group (p<0.01, Mann-Whitney U test) within 0-300 µm and 500-800 µm, respectively. Regarding bactericidal efficacy of the investigated agents in different layers, the results varied (Fig. 3): the combination of CHX and CP was significantly more effective than the other groups (p<0.01) in the superficial layer (0-300 µm), while CHX and CHX + CP were similarly effective in the second layer (300-500 µm) (p>0.05).
To quantify differences between the investigated dentin layers and applied methods, respective differences from the control were calculated in log(CFU/mL). CHX + CP were the most effective bactericidal methods in the 0-300 µm layer. The comparison with CHX (p<0.01) and CP (p<0.05) showed significantly higher CFU number reductions. All other differences were not significant.
DISCUSSION
The presented approach intended to investigate the bactericidal efficacy of CP in different infected dentin layers of human premolar root canals in vitro. The findings of the study indicate that the adjuvant usage of CP might be beneficial regarding its antibacterial effects being present in up to 800 µm. Confirming the initial hypothesis, a combination of CHX and CP was more effective, at least in overall disinfection and within the 0-300 µm layer compared to CP or CHX alone (Fig. 3). Disinfection methods involving plasma showed log RFs above 3.0, while CHX only achieved values below 3.0. Based on the assumption that log RF values between 3.0 to 4.0 would be sufficient [29], CP appears to be a promising approach to overcome the impairments in endodontic treatment related to resistant pathogens, such as E. faecalis. Nevertheless, the obtained results varied markedly with regard to the extent of CFU number reduction in different dentin layers. Despite that, the retrieved reduction factors of CP alone and combined with CHX are remarkable as the first 0-300 µm of our specimens showed a 43-fold higher CFU number compared to the second dentin layer. This is in good accordance with findings that have been reported earlier where bacterial contamination in bovine root canals in vitro was the highest at a depth of 100-200 µm [30]. Hence, bactericidal effects of CP were above all other tested agents within the most severely contaminated superficial dentin layer. In contrast, at a depth of 300-500 µm, no significant differences between the examined disinfection procedures were found. Furthermore, the results retrieved from the deepest layer (500-800 µm) using a combination of CHX and CP should have been at least similar to CHX alone, as the application of CHX was the same in both groups. This was not found in our study. Assumedly, an interaction of CP with CHX resulting in a diminished efficacy is unlikely regarding our recent knowledge about the modes of action of CP [31, 32]. Both provide independent mechanisms, so it is unlikely that the application of CP has influence on the effect of CHX and vice versa. The most efficient effect of disinfection in the first layer (0-300 µm) obtained from the combination of CHX and CP supports this thesis. The lower reduction factor of the combination of CHX and CP compared to CHX alone in the deepest layer (500-800 µm) might be based on different anatomical variants in the depth of the dentinal tubules of the specimens [4, 33].
We suppose that the effect of CHX depends on multiple influencing factors, inter alia from dentinal tubule diameter and therefore varies markedly. The effect of deep disinfection depends not only on the applied method, but also on inter-individual differences in the anatomy [4, 34]. It is likely that tubular sclerosis is the main causative of diminishing the deep penetration of liquids [3].
It can be assumed that CP is foremost effective in superficial dentin but overall additional CFU number reduction within a depth of up to 800 µm can be achieved through its adjuvant use. Nevertheless, we suggest that CP should be applied in combination instead of alone as CHX was the most effective agent in deep dentin. A combination of CP with other aseptic solutions was also found to be a beneficial approach in dermatology [32].
Some limitations to the transferability of this study have to be discussed. The selection of specimens only included straight and round root canals. These root canals were prepared to a diameter of 0.5 mm and subsequently treated with EDTA under ultrasonic activation for four minutes. EDTA may have opened dentinal tubules to facilitate the penetration of CHX, which was additionally activated ultrasonically. In clinical practice, such an ideal situation in terms of, for example, narrower and slightly curved canals being present will not be achievable in all cases. For CP irradiation dentinal tubules might not have to be as wide as it is necessary for liquid penetration, so CP might be even more and CHX less effective in vivo than in vitro. Thus, CHX alone might not be more effective than CP alone or CP + CHX within 300-800 µm. Furthermore, CP is bactericidal and fungicidal against a broad spectrum of microorganisms, regardless of resistance patterns [21]. This might be especially relevant for infected root canals as a multi-species flora is frequently present [35, 36]. The reported surface modification of dentin suggests, that root canal fillings might adhere more sufficiently [23, 33]. Nonetheless, further research regarding transferability to in vivo should be performed, as the clinical significance of in vitro findings concerning CP remains somewhat uncertain. To investigate the influence of additional CP irradiation on relevant outcome parameters clinical studies need to be conducted.
In the future, CP might complement the established therapy methods in dentistry. Additional effects of CP were identified, such as bactericidal efficacy on dental implants [37], the improvement of dentin bonding [33], and of the adhesion of composite resin to dental ceramics [38], and the disinfection of acrylic resins to prevent oral candidiasis [39]. Hence, the use of CP might not only be limited to disinfection. Thus, the high costs of a CP device could amortize according to its broad spectrum scope of clinical applications. However, devices specially designed for a use in dentistry are not available to date, but currently being developed. Thus, we actually recommend dental practitioners to wait until more data as well as devices meeting all needs of dental clinicians are available.
CONCLUSION
A combination of CP and ultrasonically activated CHX is most promising for reducing the therapeutic failure rate as CHX is known to be effective against E. faecalis, a potential causative of postendodontic complications such as persisting infection. Our results support this thesis, as a combination of both agents showed the highest overall CFU reduction, as hypothesized. Nevertheless, adjuvant CP irradiation did not significantly improve the bactericidal efficacy compared to CHX alone within the 300-500 µm layer, and CP + CHX were less effective than CHX alone within 500-800 µm. Summarizing, the bactericidal efficacy of CP alone was comparable to ultrasonically activated CHX. Based on the results obtained, CP could be an effective agent, adjuvantly administered with rinsing agents, in antibacterial therapy of infected root canals.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Chavez de Paz L.E. Redefining the persistent infection in root canals: possible role of biofilm communities. J. Endod. 2007;33(6):652–662. doi: 10.1016/j.joen.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Vianna M.E., Gomes B.P. Efficacy of sodium hypochlorite combined with chlorhexidine against Enterococcus faecalis in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009;107(4):585–589. doi: 10.1016/j.tripleo.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Paqué F., Luder H.U., Sener B., Zehnder M. Tubular sclerosis rather than the smear layer impedes dye penetration into the dentine of endodontically instrumented root canals. Int. Endod. J. 2006;39(1):18–25. doi: 10.1111/j.1365-2591.2005.01042.x. [DOI] [PubMed] [Google Scholar]
- 4.Lo Giudice G, Cutroneo G, Centofanti A, et al. Dentin morphology of root canal surface: a quantitative evaluation based on a scanning electronic microscopy study. Biomed Res Int. 2015 doi: 10.1155/2015/164065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George S., Kishen A., Song K.P. The role of environmental changes on monospecies biofilm formation on root canal wall by Enterococcus faecalis. J. Endod. 2005;31(12):867–872. doi: 10.1097/01.don.0000164855.98346.fc. [DOI] [PubMed] [Google Scholar]
- 6.Peciuliene V., Reynaud A.H., Balciuniene I., Haapasalo M. Isolation of yeasts and enteric bacteria in root-filled teeth with chronic apical periodontitis. Int. Endod. J. 2001;34(6):429–434. doi: 10.1046/j.1365-2591.2001.00411.x. [DOI] [PubMed] [Google Scholar]
- 7.Matthes R., Bender C., Schlüter R., Koban I., Bussiahn R., Reuter S., Lademann J., Weltmann K.D., Kramer A. Antimicrobial efficacy of two surface barrier discharges with air plasma against in vitro biofilms. PLoS One. 2013;8(7):e70462. doi: 10.1371/journal.pone.0070462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthes R., Bekeschus S., Bender C., Koban I., Hübner N.O., Kramer A. Pilot-study on the influence of carrier gas and plasma application (open resp. delimited) modifications on physical plasma and its antimicrobial effect against Pseudomonas aeruginosa and Staphylococcus aureus. GMS Krankenhhyg. Interdiszip. 2012;7(1):Doc02. doi: 10.3205/dgkh000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lademann O., Kramer A., Richter H., Patzelt A., Meinke M.C., Czaika V., Weltmann K.D., Hartmann B., Koch S. Skin disinfection by plasma-tissue interaction: comparison of the effectivity of tissue-tolerable plasma and a standard antiseptic. Skin Pharmacol. Physiol. 2011;24(5):284–288. doi: 10.1159/000329913. [DOI] [PubMed] [Google Scholar]
- 10.Klämpfl T.G., Isbary G., Shimizu T., Li Y.F., Zimmermann J.L., Stolz W., Schlegel J., Morfill G.E., Schmidt H.U. Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Appl. Environ. Microbiol. 2012;78(15):5077–5082. doi: 10.1128/AEM.00583-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hübner N.O., Matthes R., Koban I., Rändler C., Müller G., Bender C., Kindel E., Kocher T., Kramer A. Efficacy of chlorhexidine, polihexanide and tissue-tolerable plasma against Pseudomonas aeruginosa biofilms grown on polystyrene and silicone materials. Skin Pharmacol. Physiol. 2010;23(Suppl.):28–34. doi: 10.1159/000318265. [DOI] [PubMed] [Google Scholar]
- 12.Hammann A., Huebner N.O., Bender C., Ekkernkamp A., Hartmann B., Hinz P., Kindel E., Koban I., Koch S., Kohlmann T., Lademann J., Matthes R., Müller G., Titze R., Weltmann K.D., Kramer A. Antiseptic efficacy and tolerance of tissue-tolerable plasma compared with two wound antiseptics on artificially bacterially contaminated eyes from commercially slaughtered pigs. Skin Pharmacol. Physiol. 2010;23(6):328–332. doi: 10.1159/000314724. [DOI] [PubMed] [Google Scholar]
- 13.Bender C, Kramer A. Efficacy of Tissue Tolerable Plasma (TTP) against Ixodes ricinus. . GMS Hyg Infect Control. 2014;9(1 Doc04.) doi: 10.3205/dgkh000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lademann J., Richter H., Schanzer S., Patzelt A., Thiede G., Kramer A., Weltmann K.D., Hartmann B., Lange-Asschenfeldt B. Comparison of the antiseptic efficacy of tissue-tolerable plasma and an octenidine hydrochloride-based wound antiseptic on human skin. Skin Pharmacol. Physiol. 2012;25(2):100–106. doi: 10.1159/000335558. [DOI] [PubMed] [Google Scholar]
- 15.Klebes M., Ulrich C., Kluschke F., Patzelt A., Vandersee S., Richter H., Bob A., von Hutten J., Krediet J.T., Kramer A., Lademann J., Lange-Asschenfeld B. Combined antibacterial effects of tissue-tolerable plasma and a modern conventional liquid antiseptic on chronic wound treatment. J. Biophotonics. 2015;8(5):382–391. doi: 10.1002/jbio.201400007. [DOI] [PubMed] [Google Scholar]
- 16.Isbary G., Shimizu T., Zimmermann J.L., Thomas H.M., Morfill G.E., Stolz W. Cold atmospheric plasma for local infection control and subsequent pain reduction in a patient with chronic post-operative ear infection. New Microbes New Infect. 2013;1(3):41–43. doi: 10.1002/2052-2975.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinlin J., Isbary G., Stolz W., Morfill G., Landthaler M., Shimizu T., Steffes B., Nosenko T., Zimmermann J., Karrer S. Plasma applications in medicine with a special focus on dermatology. J. Eur. Acad. Dermatol. Venereol. 2011;25(1):1–11. doi: 10.1111/j.1468-3083.2010.03702.x. [DOI] [PubMed] [Google Scholar]
- 18.Arndt S., Landthaler M., Zimmermann J.L., Unger P., Wacker E., Shimizu T., Li Y.F., Morfill G.E., Bosserhoff A.K., Karrer S. Effects of cold atmospheric plasma (CAP) on ß-defensins, inflammatory cytokines, and apoptosis-related molecules in keratinocytes in vitro and in vivo. PLoS One. 2015;10(3):e0120041. doi: 10.1371/journal.pone.0120041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lademann J., Richter H., Alborova A., Humme D., Patzelt A., Kramer A., Weltmann K.D., Hartmann B., Ottomann C., Fluhr J.W., Hinz P., Hübner G., Lademann O. Risk assessment of the application of a plasma jet in dermatology. J. Biomed. Opt. 2009;14(5):054025. doi: 10.1117/1.3247156. [DOI] [PubMed] [Google Scholar]
- 20.Fluhr J.W., Sassning S., Lademann O., Darvin M.E., Schanzer S., Kramer A., Richter H., Sterry W., Lademann J. In vivo skin treatment with tissue-tolerable plasma influences skin physiology and antioxidant profile in human stratum corneum. Exp. Dermatol. 2012;21(2):130–134. doi: 10.1111/j.1600-0625.2011.01411.x. [DOI] [PubMed] [Google Scholar]
- 21.Ehlbeck J., Brandenburg R., von Woedtke T., Krohmann U., Stieber M., Weltmann K.D. PLASMOSE - antimicrobial effects of modular atmospheric plasma sources. GMS Krankenhhyg. Interdiszip. 2008;3(1):Doc14. [PMC free article] [PubMed] [Google Scholar]
- 22.Du T., Ma J., Yang P., Xiong Z., Lu X., Cao Y. Evaluation of antibacterial effects by atmospheric pressure nonequilibrium plasmas against Enterococcus faecalis biofilms in vitro. J. Endod. 2012;38(4):545–549. doi: 10.1016/j.joen.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Li Y., Sun K., Ye G., Liang Y., Pan H., Wang G., Zhao Y., Pan J., Zhang J., Fang J. Evaluation of cold plasma treatment and safety in disinfecting 3-week root canal Enterococcus faecalis biofilm In Vitro. J. Endod. 2015;41(8):1325–1330. doi: 10.1016/j.joen.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Jablonowski L., Koban I., Berg M., et al. Elimination of E. faecalis by a new non-thermal atmospheric pressure plasma handheld device for endodontic treatment. A preliminary investigation. Plasma Process. Polym. 2013;10:499–505. doi: 10.1002/ppap.201200156. [DOI] [Google Scholar]
- 25.Üreyen Kaya B., Kececi A.D., Güldaş H.E., Çetin E.S., Öztürk T., Öksuz L., Bozduman F. Efficacy of endodontic applications of ozone and low-temperature atmospheric pressure plasma on root canals infected with Enterococcus faecalis. Lett. Appl. Microbiol. 2014;58(1):8–15. doi: 10.1111/lam.12148. [DOI] [PubMed] [Google Scholar]
- 26.Haapasalo M., Orstavik D. In vitro infection and disinfection of dentinal tubules. J. Dent. Res. 1987;66(8):1375–1379. doi: 10.1177/00220345870660081801. [DOI] [PubMed] [Google Scholar]
- 27.Gomes B.P., Ferraz C.C., Vianna M.E., Berber V.B., Teixeira F.B., Souza-Filho F.J. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int. Endod. J. 2001;34(6):424–428. doi: 10.1046/j.1365-2591.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- 28.Holler C., Martiny H., Christiansen B., Ruden H., Gundermann K.O. The efficacy of low temperature plasma (LTP) sterilization, a new sterilization technique. Zentralblatt fur Hygiene und Umweltmedizin. Int J Hyg Environ Med. 1993;194(4):380–391. [PubMed] [Google Scholar]
- 29.Sarkis S., Richard L., Mulvenna V., Poon R. Guidelines for validating treatment processes for pathogen reduction. Australia. Australia: Department of Health; 2013. pp. 1–80. [Google Scholar]
- 30.Assouline L.S., Fuss Z., Mazor Y., Weiss E.I. Bacterial penetration and proliferation in root canal dentinal tubules after applying dentin adhesives in vitro. J. Endod. 2001;27(6):398–400. doi: 10.1097/00004770-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Lackmann J.W., Bandow J.E. Inactivation of microbes and macromolecules by atmospheric-pressure plasma jets. Appl. Microbiol. Biotechnol. 2014;98(14):6205–6213. doi: 10.1007/s00253-014-5781-9. [DOI] [PubMed] [Google Scholar]
- 32.Ulrich C., Kluschke F., Patzelt A., Vandersee S., Czaika V.A., Richter H., Bob A., Hutten Jv., Painsi C., Hüge R., Kramer A., Assadian O., Lademann J., Lange-Asschenfeldt B. Clinical use of cold atmospheric pressure argon plasma in chronic leg ulcers: A pilot study. J. Wound Care. 2015;24(5):196–, 198-200, 202-203. doi: 10.12968/jowc.2015.24.5.196. [DOI] [PubMed] [Google Scholar]
- 33.Dong X., Ritts A.C., Staller C., Yu Q., Chen M., Wang Y. Evaluation of plasma treatment effects on improving adhesive-dentin bonding by using the same tooth controls and varying cross-sectional surface areas. Eur. J. Oral Sci. 2013;121(4):355–362. doi: 10.1111/eos.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivancik J., Arola D.D. The importance of microstructural variations on the fracture toughness of human dentin. Biomaterials. 2013;34(4):864–874. doi: 10.1016/j.biomaterials.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tennert C., Fuhrmann M., Wittmer A., Karygianni L., Altenburger M.J., Pelz K., Hellwig E., Al-Ahmad A. New bacterial composition in primary and persistent/secondary endodontic infections with respect to clinical and radiographic findings. J. Endod. 2014;40(5):670–677. doi: 10.1016/j.joen.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Hancock H.H., III, Sigurdsson A., Trope M., Moiseiwitsch J. Bacteria isolated after unsuccessful endodontic treatment in a North American population. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001;91(5):579–586. doi: 10.1067/moe.2001.113587. [DOI] [PubMed] [Google Scholar]
- 37.Preissner S., Wirtz H.C., Tietz A.K., Abu-Sirhan S., Herbst S.R., Hartwig S., Pierdzioch P., Schmidt-Westhausen A.M., Dommisch H., Hertel M. Bactericidal efficacy of tissue tolerable plasma on microrough titanium dental implants: An in-vitro-study. J. Biophotonics. 2015 doi: 10.1002/jbio.201500189. [Epub Ahead of Print]. [DOI] [PubMed] [Google Scholar]
- 38.Han G.J., Chung S.N., Chun B.H., Kim C.K., Oh K.H., Cho B.H. Effect of the applied power of atmospheric pressure plasma on the adhesion of composite resin to dental ceramic. J. Adhes. Dent. 2012;14(5):461–469. doi: 10.3290/j.jad.a25688. [DOI] [PubMed] [Google Scholar]
- 39.Pan H., Wang G., Pan J., Ye G., Sun K., Zhang J., Wang J. Cold plasma-induced surface modification of heat-polymerized acrylic resin and prevention of early adherence of Candida albicans. Dent. Mater. J. 2015;34(4):529–536. doi: 10.4012/dmj.2015-035. [DOI] [PubMed] [Google Scholar]