Abstract
Insect pests in stored wheat cause significant losses and play an important role in the dispersal of viable fungal spores of various species including aflatoxin producing Aspergillus parasiticus. The problem of insecticide resistance in stored insects and environmental hazards associated with fumigants and conventional grain protectants underscore the need to explore reduced risk insecticides to control stored insects with the ultimate effect on aflatoxin production. The purpose of this study was to investigate the insecticidal potential of four biorational insecticides: spinosad, thiamethoxam, imidacloprid and indoxacarb, on wheat grains artificially infested with Rhyzopertha dominica/Sitophilus oryzae and/or A. parasiticus spores, and the subsequent effect on aflatoxin production. Spinosad and thiamethoxam were the most effective insecticides against R. dominica compared to S. oryzae followed by imidacloprid. Spinosad applied at 0.25–1 ppm and thiamethoxam at 2 and 4 ppm concentrations resulted in complete mortality of R. dominica. However, indoxacarb was more toxic against S. oryzae compared to R. dominica. Wheat grains inoculated with R. dominica/S. oryzae +spores elicited higher aflatoxin levels than wheat grains inoculated with or without insecticide+spores. In all the treatment combinations containing insects, aflatoxin production was dependent on insects’ survival rate. In addition, thiamethoxam and imidacloprid had also a significant direct effect on reducing aflatoxin production. Aflatoxin levels were lower in the treatment combinations with any concentration of thiamethoxam/imidacloprid+spores as compared to wheat grains inoculated with spores only. Correlation analyses revealed highly significant and positive association between moisture contents/insect survival rate and production of aflatoxin levels, and insect survival rate and moisture contents of the wheat grains. In conclusion, the results of the present study provide baseline data on the use of biorational insecticides against R. dominica and S. oryzae and subsequent effect on aflatoxin production.
Keywords: Spinosad, Indoxacarb, Thiamethoxam, Imidacloprid, Stored insect pests, Aflatoxin, Mycotoxin, Tribolium, Rhyzopertha
Introduction
Aflatoxins are the group of structurally diverse mycotoxins that are mainly produced by Aspergillus flavus and A. parasiticus, both belonging to section Flavi (Bennett & Klich, 2003; Horn, 2007). These mycotoxins are recognized as immunosuppressive, carcinogenic, hepatotoxic, mutagenic and teratogenic (Patten, 1981), since they lead to serious human and animal health hazards, including acute and chronic liver diseases, tumor induction, reproductive disorders, genotoxicity and nephrotoxicity (Chao, Maxwell & Wong, 1991; Lacey, 1988; Desjardins et al., 2000). These mycotoxins are also known to contaminate more than 25% of the world stored grain cereal commodities of which more than 300 fungal metabolites have been reported to cause human and animal toxicity (Galvano et al., 2001).
Stored grain contaminations with insect pests and fungi are a serious problem resulting in more than 20% losses in overall production by decreasing seed germination and downgrading of grains (Philip & Throne, 2010). Factors like the temperature and humidity at postharvest stages play an important role in insect pest infestations, growth of toxigenic fungi and aflatoxin production in storage ecosystems. Contamination of stored grains with fungal spores is mainly a source of mycotoxins which usually results from stored insect pest infestations (Birck, Lorini & Scussel, 2006). Insects disseminate the fungal spores all over the grain bulk by their constant movement, which are carried on their body and/or deposited in insect frass (Santos & Mantovani, 1997). These insects break the seed coat as a natural barrier to fungus and provide an entry point for fungal infection (Setamau et al., 1998) which ultimately results in mycotoxin production. Therefore, strategies to control insects are needed. The most serious insect pests of stored grains that cause >20% postharvest losses in developing countries are: rice weevil (Sitophilus oryzae), red flour beetle (Tribolium castaneum), lesser grain borer (Rhyzopertha dominica), rusty grain beetle (Cryptolestes ferrugineus) and khapra beetle (Trogoderma granarium) (Philip & Throne, 2010).
Control of these insects is of prime importance as they have the ability to carry and transmit spores of aflatoxigenic Aspergillus spp. internally (Barra, Nesci & Etcheverry, 2013). Current control measures for these insects rely on the extensive use of fumigants and conventional insecticides that have created some drawbacks like increased insect resistance (Gurusubramanian et al., 2008), primary pest resurgence (Hazarika, Bhuyan & Hararika, 2009), secondary pest outbreak (Cranham, 1996), and their use as grain protectants is being reconsidered for their effect on health and environmental safety (Vayias et al., 2010). Some of the conventional insecticides have also been reported to inhibit the production of aflatoxins in storage ecosystems. Dichlorvos (an organophosphate) was the first conventional insecticide reported to be effective in the inhibition of aflatoxin production in different stored commodities like corn, rice, wheat and peanuts (Rao & Hareim, 1972). Thereafter, insecticides from different classes like naled, pyrethrum, sevin, malathion, diazinon (Draughon & Ayres, 1981) and carbaryl (D’Mello et al., 1998) were reported to significantly inhibit the production of aflatoxins. However, recent reports on drawbacks (see above) of conventional insecticides raised questions about their use in the future. Therefore, there is a need to explore reduced risk or biorational insecticides with minimal environmental effect and mammalian toxicity for judicious insect pest and aflatoxin management.
Biorational insecticides are usually target specific new insecticides with low mammalian toxicity. Some of the biorational insecticides like spinosad, thiamethoxam have been proved as potential grain protectants against stored insect pests in different parts of the world (Vayias et al., 2010; Arthur, Yue & Wilde, 2004). However, such studies are rare at the Pakistan level. Therefore, in the present study, our aims were to evaluate the toxicity of spinosad, thiamethoxam, imidacloprid and indoxacarb against two major pests of stored wheat R. dominica and S. oryzae, and the subsequent effect on aflatoxin production in wheat grains. The results would be helpful in the management of insects and aflatoxins in stored commodities.
Materials and Methods
Commodities, formulations and fungal cultures
Clean and infestation free wheat grains (var. Seher-06) were used in the present study. The grains were surface sterilized with a 0.5% sodium hypochlorite solution for 2 min and washed with sterile distilled water before starting the experiments. The insecticide formulations were spinosad (Tracer® 24% active ingredients [IA]; Arysta Life Sciences, Karachi, Pakistan), thiamethoxam (Actara® 25% [AI]; Syngenta, Pakistan), imidacloprid (Confidor® 20% [AI]; Bayer Crop Sciences, Karachi, Pakistan) and indoxacarb (Steward 15%, DuPont, Pakistan). The Aspergillus parasiticus culture was obtained from the First Fungus Culture Bank, Pakistan (FFCB), and used in bioassays.
Insects
Healthy cultures of R. dominica and S. oryzae adults were collected from grain market, Lahore (31.5497°N, 74.3436°E), and reared on whole wheat in the laboratory at 26 ± 1 °C and 70 ± 5%. Adults of both species used in bioassays were mixed sex and 2-3-weeks-old (Athanassiou et al., 2011).
Grain treatment and Bioassays
Insecticidal bioassays were done by following the methodology of Athanassiou et al. (2011) with some modifications. Briefly, insecticides were applied as a solution diluted with distilled water. Individual replicate lots of 200 g of wheat grains were placed in 0.5 liter glass jars for different treatment combinations (Table 1). To achieve the desired insecticide concentrations, 2 mL solution of a respective insecticide was prepared and applied to the 200 g grains. In order to maximize insecticide distribution, the jar was shaken manually for 5 min (Athanassiou et al., 2011; Krishnamurthy, Shashikala & Naik, 2008). The treatment combinations 5 and 6 (without any insecticide) were treated with distilled water. The jars were left in the laboratory for 24 h at 25 °C and complete darkness to dry. After 24 h, the jars with and without insecticide treated wheat grains were inoculated with 0.5 ml spore suspension (104 spores/ml) of A. parasiticus, except the treatment combination 6 (i.e., wheat only). Twenty adults of R. dominica (arena “A”) or S. oryzae (arena “B”) were introduced in each jar. The insects were disinfected with 0.5% sodium hypochlorite for 2 min and washed with sterile distilled water before entering into the jars (Ferreira-Castro et al., 2012). To check the direct effect of insecticides on aflatoxin production, the same procedure was repeated, but without introducing insects in treated jars (treatment combination 7–9). The treated jars were closed with muslin cloth and maintained in the laboratory at 26 ± 1 °C and 70 ± 5%. The entire procedure was replicated five times by preparing new lots of treated wheat for each replicate of the respective treatment combination. After 14 days, the jars were opened for the determination of insects’ mortality, aflatoxin levels and moisture contents. The moisture contents were determined by following the procedure of AACC (2000) method No. 44-15.
Table 1. Treatment combinations used in different experiments.
| Insecticide | Treatment | Combination |
|---|---|---|
| Spinosad | 1 | 0.25 ppm + R. dominica OR S. oryzae + spores |
| 2 | 0.5 ppm + R. dominica OR S. oryzae + spores | |
| 3 | 1 ppm + R. dominica OR S. oryzae + spores | |
| 4 | R. dominica OR S. oryzae + spores | |
| 5 | Spores only | |
| 6 | Wheat only | |
| 7 | 0.25 ppm + spores | |
| 8 | 0.5 ppm + spores | |
| 9 | 1 ppm + spores | |
| Imidacloprid, Thiamethoxam or Indoxacarb | 1 | 1 ppm + R. dominica OR S. oryzae + spores |
| 2 | 2 ppm + R. dominica OR S. oryzae + spores | |
| 3 | 4 ppm + R. dominica OR S. oryzae + spores | |
| 4 | R. dominica OR S. oryzae + spores | |
| 5 | Spores only | |
| 6 | Wheat only | |
| 7 | 1 ppm + spores | |
| 8 | 2 ppm + spores | |
| 9 | 4 ppm + spores |
Aflatoxin content was determined by following the methodology described by Beti, Phillips & Smalley (1995), using an indirect competitive enzyme linked immunosorbent assay (ELISA) method.
Data analysis
Percent mortality and aflatoxin contents in different treatments were analyzed using a one-way analysis of variance (ANOVA) with Statistix 8.1 software (Analytical Software, 2005). Means were compared by Tukey’s Honestly Significant Difference (HSD) test, at 0.05 probability. Correlation analysis was used to study the relationship among aflatoxin content, grain moisture content and insects’ survival rate.
Results
Insects’ mortality
In the case of spinosad, mortality for R. dominica was higher than for S. oryzae. Rhyzopertha dominica showed 100% mortality at the spinosad concentrations of 0.25–1 mg/kg (F = 3,610; df = 3, 16; p < 0.01) (Fig. 1A). However, S. oryzae showed the highest mortality at the concentration 1 ppm followed by 0.5 and 0.25 ppm (F = 269; df = 3, 16; p < 0.01) (Fig. 1B).
Figure 1. (A) and (B) (Spinosad).

Effect of different treatment combinations on mortality of R. dominica (1A) or S. oryzae (1B), grain moisture contents (primary axis) and aflatoxin production levels (secondary axis). Data bars or lines with different letters are significantly different; in all 369 cases df = 3, 16, Tukey’s HSD test at p < 0.01.
Rhyzopertha dominica showed complete mortality on wheat treated with thiamethoxam at concentrations 2 and 4 ppm (F = 1,170; df = 3, 16; p < 0.01) (Fig. 2A). Whereas, S. oryzae showed complete mortality only at 4 ppm (F = 1,036; df = 3, 16; p < 0.01) (Fig. 2B).
Figure 2. (A) and (B) (Thiamethoxam).

Effect of different treatment combinations on mortality of R. dominica (2A) or S. oryzae (2B), grain moisture contents (primary axis) and aflatoxin production levels (secondary axis). Data bars or lines with different letters are significantly different; in all cases df = 3, 16, Tukey’s HSD test at p < 0.01.
In the case of imidacloprid, mortality for R. dominica was higher than for S. oryzae. R. dominica showed 93% mortality at the highest concentration 4 ppm (F = 473; df = 3, 16; p < 0.01) (Fig. 3A). Whereas, S. oryzae showed 36% mortality at the highest concentration 4 ppm (F = 88.0; df = 3, 16; p < 0.01) (Fig. 3B).
Figure 3. (A) and (B) (Imidacloprid).
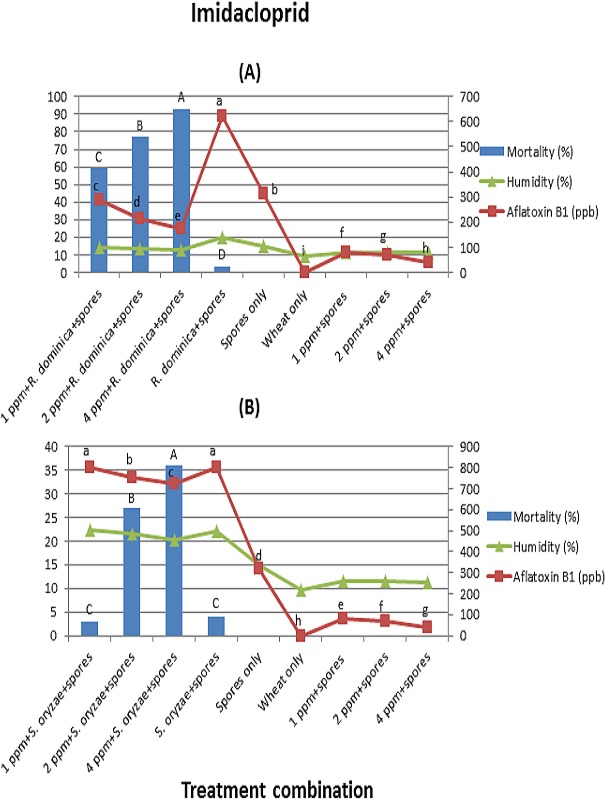
Effect of different treatment combinations on mortality of R. dominica (3A) or S. oryzae (3B), grain moisture contents (primary axis) and aflatoxin production levels (secondary axis). Data bars or lines with different letters are significantly different; in all cases df = 3, 16, Tukey’s HSD test at p < 0.01.
For indoxacarb, in contrast, mortality for R. dominica was lower than for S. oryzae. R. dominica showed 97% mortality at the highest concentration 4 ppm (F = 302; df = 3, 16; p < 0.01) (Fig. 4A). However, S. oryzae showed complete mortality at 2 and 4 ppm (F = 437; df = 3, 16; p < 0.01) (Fig. 4B).
Figure 4. (A) and (B) (Indoxacarb).

Effect of different treatment combinations on mortality of R. dominica (4A) or S. oryzae (4B), grain moisture contents (primary axis) and aflatoxin production levels (secondary axis). Data bars or lines with different letters are significantly different; in all cases df = 3, 16, Tukey’s HSD test at p < 0.01.
Aflatoxin B1 production
Wheat grains inoculated with R. dominica/S. oryzae + spores elicited higher aflatoxin levels than wheat grains inoculated with any concentration of spinosad + spores. In the case of concentrations of spinosad + spores + R. dominica/S. oryzae, aflatoxin production was dependent on insect survival rate. In the case of R. dominica, where 100% mortality was observed at all the concentrations of spinosad, aflatoxin production was lower as compared to the treatment combination where wheat grains were inoculated with R. dominica + spores. The same trend was observed in the treatment combinations where S. oryzae were inoculated. Spinosad concentrations seem to have no direct effect on aflatoxin production. For instance, there was no statistical difference between concentrations of spinosad + spores and wheat grains inoculated with spores only in both arenas. The average moisture contents in the wheat grain were ranged between 10–19% and 9.5–21% in arena A and B, respectively. The moisture contents were higher where survival percentage of insects was high compared to those treatments where the survival percentage was low or no insects inoculated (Figs. 1A and 1B).
In the case of thiamethoxam and imidacloprid, wheat grains inoculated with R. dominica/S. oryzae + spores elicited higher aflatoxin levels than the rest of the treatment combinations. In the case of treatment combination containing insects in both arenas, aflatoxin levels were dependent on insect survival rate. However, thiamethoxam and imidacloprid concentrations had also a significant direct effect on reducing aflatoxin production. Aflatoxin levels in both arenas were lower in the treatment combinations with any concentration of thiamethoxam/imidacloprid + spores as compared to wheat grains inoculated with spores only. Moreover, aflatoxin levels were reduced with increasing concentrations of both insecticides. The average moisture contents in the wheat grain were ranged between 9.5–20.1% and 9.7–22.25% in arena A and B, respectively.
Similar to previous experiments, wheat grains inoculated with R. dominica/S. oryzae + spores elicited higher aflatoxin levels than the rest of the treatment combinations, and the levels were dependent on insects’ survival rate. However, in contrast to thiamethoxam and imidacloprid, indoxacarb did not have a significant direct effect on reducing aflatoxin levels when compared to the treatments where wheat grains were inoculated with spores only. The average moisture contents of wheat grains were ranged between 9.5–19.7% and 9.5–22.3% in arena A and B, respectively (Figs. 4A and 4B).
Correlation analyses
Correlation analyses revealed highly significant and positive association between moisture contents/insect survival rate and production of aflatoxin levels, and insect survival rate and moisture contents of the wheat grains (Table 2).
Table 2. Correlation analyses among insects’ mortality, grain moisture contents and aflatoxin production in different treatment combinations.
| Aflatoxin | Moisture content | |
|---|---|---|
| Moisture content | 0.99** | – |
| Ryzopertha dominica | 0.91** | 0.92** |
| Sitophilus oryzae | 0.84** | 0.86** |
Notes.
p < 0.01.
Discussion
The results of the present study revealed varying level of toxicity of insecticides against R. dominica and S. oryzae. The results also suggest that control of these insects may help limit the production of aflatoxins in stored wheat.
Overall, spinosad was the most effective insecticide against R. dominica compared to S. oryzae. Spinosad applied at 0.25, 0.5 and 1 ppm concentrations resulted in complete mortality of R. dominica. In contrast, S. oryzae showed 94% mortality at the highest concentration of spinosad tested. Spinosad is a relatively new insecticide of low mammalian toxicity and has a broad spectrum of target species, including stored insect pests (Athanassiou, Kavallieratos & Chintzoglou, 2008), and has been registered for use against stored insect pests in the United States (Athanassiou et al., 2011). The findings of spinosad toxicity against tested insect species are in agreement with a number of previous reports (Athanassiou et al., 2011; Athanassiou, Kavallieratos & Chintzoglou, 2008; Athanassiou et al., 2008; Fang, Subramanyam & Arthur, 2002; Nayak, Daglish & Byrne, 2005). It seems that, if R. dominica is the sole species in stored commodities, spinosad may be as an effective grain protectant.
In the case of thiamethoxam, R. dominica showed complete mortality at concentrations 2 and 4 ppm, whereas S. oryzae showed complete mortality at only 4 ppm. Thiamethoxam is a neonicotinoid and a relatively new insecticide of low mammalian toxicity and does not produce mutagenic and/or teratogenic effects (Arthur, Yue & Wilde, 2004). It has been extensively used as a seed coat treatment with satisfactory results against a wide range of pests (Khan et al., 2015). Thiamethoxam was the first time evaluated as a grain protectant by Arthur, Yue & Wilde (2004) and found very effective against R. dominica and S. oryzae. It was further reported that the mortality of S. oryzae and R. dominica increased with time and reached approximately 100% after 6 d of exposure to thiamethoxam. But in our study, S. oryzae, showed less than 100% mortality at 2 ppm after 14 d of exposure. This difference could be due to the different origin of S. oryzae strains.
Imidacloprid is also a neonicotinoid with low mammalian toxicity and has been used as a seed treatment for the management of a number of sucking insect pests (Khan et al., 2015). Recently, Daglish & Nayak (2012) investigated the potential of imidacloprid against different beetle pests of stored grains. They reported that the toxicity of imidacloprid was species and dose dependent, and that R. dominica was more susceptible to imidacloprid than S. oryzae. The results of the present study with respect to imidacloprid also revealed that the mortality for R. dominica was higher than for S. oryzae. Rhyzopertha dominica showed up to 94% mortality at the highest concentration 4 ppm as compared to 39% mortality for S. oryzae.
Indoxacarb belongs to an oxadiazine insecticide group and has strong potential against different insect pests (Khan, Shad & Akram, 2013). In the present study mortality for R. dominica was lower than for S. oryzae, which is in agreement with Daglish & Nayak (2012) who evaluated indoxacarb potential as a grain protectant first time.
Concerning the aflatoxin production in different treatment combinations, production of aflatoxin was directly related to survival rate of both insect species. Moreover, a highly significant and positive association was observed between moisture contents/insect survival rate and production of aflatoxin levels, and insect survival rate and moisture contents of wheat grains. There was an indirect effect of spinosad and indoxacarb on the inhibition aflatoxin production. These insecticides caused mortality of the insect species which might result in the reduction of aflatoxin production. One most probable reason could be that increased mortality of insects resulted in the reduced insects’ movement, humidity and seed coat damage which ultimately affect fungal infection and aflatoxin production. However, thiamthoxam and imidacloprid also showed a direct effect on the inhibition of aflatoxin production in the treated grains. After the harvest of the crops, temperature and humidity play an important role in the growth of toxigenic fungi, aflatoxin production and insects in the storage ecosystem (Nesci, Barra & Etcheverry, 2011). Stored insect pests in the granary ecosystem move for breeding and feeding purposes from one point another and contribute the dispersal of viable spores of fungi of various species, including A. parasiticus, which are carried on the insect body surface or deposited in their frass (Nesci, Barra & Etcheverry, 2011). During feeding in stored commodities, insects break the seed coat of grains, which is a natural barrier to fungal growth, and ultimately promote the easy spread of mycotoxin producing fungal spores (Setamau et al., 1998). Hence, in the present study aflatoxin production was more in those treatments where the survival rate of insects was high, most probably due to a high rate of feeding and movement activities. Dispersal of fungal spores usually depends on the type of insect species present in storage commodities (Lamboni & Hell, 2009). During storage, the invasion by a variety of insects causes losses to storage grains and act as mechanical vectors of viable fungal spores (Ferreira-Castro et al., 2012). If storage grains left untreated, insects within the granary ecosystem can create local conditions of humidity and temperature that favor the rapid growth of fungi, resulting in deterioration of the grain mass and production of mycotoxins (Sinha & Sinha, 1992). The results of the correlation in the present study are in accordance with those of Beti et al. (Daglish & Nayak, 2012) who reported a highly significant and positive association between moisture contents/insect survival rate and production of aflatoxin levels, and insect survival rate and moisture contents of the wheat grains.
Since the insecticides tested in the present study are not recommended yet as grain protectants in Pakistan, further studies should be conducted to assess their long term efficacy or persistence against different stored pests across different stored grain commodities. Spinosad and thiamethoxam proved very effective against R. dominica. In granary systems where R. dominica is the sole problem, spinosad and thiamethoxam may provide adequate protection. However, tested insecticides except indoxacarb did not provide adequate control of S. oryzae. Therefore, in storage bins where both insect species coexist, there is a need to evaluate other insecticides or insecticide combinations which can provide adequate protection against insect pests and subsequent fungal infection. In conclusion, keeping in view the role of stored insects in aflatoxin production, the results of the present study provide baseline data on the use of biorational insecticides against R. dominica and S. oryzae and the subsequent effect on the inhibition of aflatoxin production. Whether this is a practical solution in granary systems for a long time needs to be further characterized in future studies.
Supplemental Information
Acknowledgments
Sincere thanks to the University of the Punjab, Lahore, for providing assistance in publication fee of this work. An earlier version of this article is online as PeerJ PrePrint doi:10.7287/peerj.preprints.1117v1.
Funding Statement
The authors received compensation from the University of the Punjab, Lahore, for the publication of this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Tiyyabah Khan and Hafiz Azhar Ali Khan conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Ahmad Ali Shahid conceived and designed the experiments, contributed reagents/materials/analysis tools, prepared figures and/or tables, reviewed drafts of the paper.
Data Availability
The following information was supplied regarding data availability:
Raw data has been provided in the Supplemental Information.
References
- Analytical Software (2005).Analytical Software . Statistix Version 8: 1: User’s manual. Tallahassee: Analytical Software; 2005. [Google Scholar]
- Arthur, Yue & Wilde (2004).Arthur FH, Yue B, Wilde GE. Susceptibility of stored-product beetles on wheat and maize treated with thiamethoxam: effects of concentration, exposure interval, and temperature. Journal of Stored Products Research. 2004;40:527–546. doi: 10.1016/j.jspr.2003.08.001. [DOI] [Google Scholar]
- Athanassiou et al. (2011).Athanassiou CG, Arthur FH, Kavallieratos NG, Throne JE. Efficacy of spinosad and methoprene, applied alone or in combination, against six stored-product insect species. Journal of Pest Science. 2011;84:61–67. doi: 10.1007/s10340-010-0326-1. [DOI] [Google Scholar]
- Athanassiou, Kavallieratos & Chintzoglou (2008).Athanassiou CG, Kavallieratos NG, Chintzoglou GJ. Effectiveness of spinosad dust against different European populations of the confused flour beetle, Tribolium confusum Jacquelin du Val. Journal of Stored Products Research. 2008;44:47–51. doi: 10.1016/j.jspr.2007.05.001. [DOI] [Google Scholar]
- Athanassiou et al. (2008).Athanassiou CG, Kavallieratos NG, Yiatilis AE, Vayias BJ, Mavrotas CS, Tomanovic Z. Influence of temperature and humidity on the efficacy of spinosad against four stored grain beetle species. Journal of Insect Science. 2008;8:60. doi: 10.1673/031.008.6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra, Nesci & Etcheverry (2013).Barra P, Nesci A, Etcheverry M. In vitro compatibility of natural and food grade fungicide and insecticide substances with Purpureocillium lilacinum and their effect against Aspergillus flavus. Journal of Stored Products Research. 2013;54:67–73. doi: 10.1016/j.jspr.2013.06.002. [DOI] [Google Scholar]
- Bennett & Klich (2003).Bennett JW, Klich M. Mycotoxins. Clinical Microbiology Reviews. 2003;16:479–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beti, Phillips & Smalley (1995).Beti JA, Phillips TW, Smalley EB. Effects of maize weevils (Coleoptera: Curculionidae) on production of aflatoxin B1 by Aspergillus flavusin stored corn. Journal of Economic Entomology. 1995;88:1776–1782. doi: 10.1093/jee/88.6.1776. [DOI] [PubMed] [Google Scholar]
- Birck, Lorini & Scussel (2006).Birck NMM, Lorini I, Scussel VM. Fungus and mycotoxins in wheat grain at post harvest. 9th international working conference on stored product protection; 2006. pp. 198–205. Available at http://bru.gmprc.ksu.edu/proj/iwcspp/pdf2/9/6281.pdf . [Google Scholar]
- Chao, Maxwell & Wong (1991).Chao TC, Maxwell SM, Wong SY. An outbreak of aflatoxicosis and boric acid poisoning in Malaysia: a clinicopathological study. Journal of Pathology. 1991;164:225–233. doi: 10.1002/path.1711640307. [DOI] [PubMed] [Google Scholar]
- Cranham (1996).Cranham JE. Tea pest and their control. Annual Review of Entomology. 1996;11:419–514. [Google Scholar]
- Daglish & Nayak (2012).Daglish GJ, Nayak MK. Potential of the neonicotinoid imidacloprid and the oxadiazine indoxacarb for controlling five coleopteran pests of stored grain. Insect Science. 2012;19:96–101. doi: 10.1111/j.1744-7917.2011.01430.x. [DOI] [Google Scholar]
- Desjardins et al. (2000).Desjardins AE, Manandhar G, Plattner RD, Maragos CM, Shrestha K, McCormick SP. Occurrence of Fusarium species and mycotoxins in Nepalese maize and wheat and the effect traditional processing method on mycotoxin levels. Journal of Agricultural and Food Chemistry. 2000;48:1377–1383. doi: 10.1021/jf991022b. [DOI] [PubMed] [Google Scholar]
- D’Mello et al. (1998).D’Mello JPF, Macdonald AMC, Postel D, Dijksma WTP, Dujardin A, Placinta CM. Pesticide use and mycotoxin production in fusarium and aspergillus phytopathogens. European Journal of Plant Pathology. 1998;104:741–751. doi: 10.1023/A:1008621505708. [DOI] [Google Scholar]
- Draughon & Ayres (1981).Draughon FA, Ayres JC. Inhibition of aflatoxin production by selected insecticides. Applied and Environmental Microbiology. 1981;41:972–976. doi: 10.1128/aem.41.4.972-976.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, Subramanyam & Arthur (2002).Fang L, Subramanyam BH, Arthur FH. Effectiveness of spinosad on four classes of wheat against five stored product insects. Journal of Economic Entomology. 2002;95:640–650. doi: 10.1603/0022-0493-95.3.640. [DOI] [PubMed] [Google Scholar]
- Ferreira-Castro et al. (2012).Ferreira-Castro FL, Potenza MR, Rocha LO, Correa B. Interaction between toxigenic fungi and weevils in corn grain samples. Food Control. 2012;26:594–600. doi: 10.1016/j.foodcont.2012.02.016. [DOI] [Google Scholar]
- Galvano et al. (2001).Galvano F, Piva A, Ritieni A, Galvano G. Dietary strategies to counteract the effect of mycotoxins: a review. Journal of Food Protection. 2001;64:120–131. doi: 10.4315/0362-028x-64.1.120. [DOI] [PubMed] [Google Scholar]
- Gurusubramanian et al. (2008).Gurusubramanian G, Rahman A, Sarmah M, Roy S, Bora S. Pesticide usage pattern in tea ecosystem, their retrospects and alternative measures. Journal of Environmental Biology. 2008;29:813–826. [PubMed] [Google Scholar]
- Bhuyan & Hararika (2009).Hazarika LK, Bhuyan M, Hararika BN. Insect pest of tea and their management. Annual Review of Entomology. 2009;54:267–284. doi: 10.1146/annurev.ento.53.103106.093359. [DOI] [PubMed] [Google Scholar]
- Horn (2007).Horn BW. Biodiversity of Aspergillus section Flavi in the United states; a review. Food Additives and Contaminants. 2007;24:1088–1101. doi: 10.1080/02652030701510012. [DOI] [PubMed] [Google Scholar]
- Khan et al. (2015).Khan HAA, Akram W, Iqbal J, Naeem-Ullah U. Thiamethoxam resistance in the house fly, Musca domestica L.: current status, resistance selection, cross-resistance potential and possible biochemical mechanisms. PLoS ONE. 2015;10(5):e1665. doi: 10.1371/journal.pone.0125850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, Shad & Akram (2013).Khan HAA, Shad SA, Akram W. Resistance to new chemical insecticides in the house fly, Musca domestica L., from dairies in Punjab, Pakistan. Parasitology Research. 2013;112:2049–2054. doi: 10.1007/s00436-013-3365-8. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy, Shashikala & Naik (2008).Krishnamurthy YL, Shashikala JB, Naik S. Antifungal potential of some natural products against Aspergillus flavus in soybean seeds during storage. Journal of Stored Products Research. 2008;44:305–309. doi: 10.1016/j.jspr.2008.03.001. [DOI] [Google Scholar]
- Lacey (1988).Lacey J. The microbiology of cereal grains from areas of Iran with a high incidence of oesophageal cancer. Journal of Stored Products Research. 1988;24:39–50. doi: 10.1016/0022-474X(88)90007-0. [DOI] [Google Scholar]
- Lamboni & Hell (2009).Lamboni Y, Hell K. Propagation of mycotoxigenic fungi in maize stores by post-harvest insects. International Journal of Tropical Insect Science. 2009;29:31–39. doi: 10.1017/S1742758409391511. [DOI] [Google Scholar]
- Nayak, Daglish & Byrne (2005).Nayak MK, Daglish GJ, Byrne VS. Effectiveness of spinosad as a grain protectant against resistant beetle and psocid pests of stored grain in Australia. Journal of Stored Products Research. 2005;41:455–467. doi: 10.1016/j.jspr.2004.07.002. [DOI] [Google Scholar]
- Nesci, Barra & Etcheverry (2011).Nesci A, Barra P, Etcheverry M. Integrated management of insect vectors of Aspergillus flavus in stored maize, using synthetic antioxidants and natural phytochemicals. Journal of Stored Products Research. 2011;47:231–237. doi: 10.1016/j.jspr.2011.03.003. [DOI] [Google Scholar]
- Patten (1981).Patten RC. Aflatoxins and disease. American Journal of Tropical Medicine and Hygiene. 1981;30:422–425. doi: 10.4269/ajtmh.1981.30.422. [DOI] [PubMed] [Google Scholar]
- Philip & Throne (2010).Philip TW, Throne JE. Biorational approaches for managing stored-product insect. Annual Review of Entomology. 2010;55:375–39. doi: 10.1146/annurev.ento.54.110807.090451. [DOI] [PubMed] [Google Scholar]
- Rao & Hareim (1972).Rao HRG, Hareim PK. Dichlorvos as an inhibitor of aflatoxin production on wheat, corn, rice and peanuts. Journal of Economic Entomology. 1972;65:988–990. doi: 10.1093/jee/65.4.988. [DOI] [Google Scholar]
- Santos & Mantovani (1997).Santos JP, Mantovani EC. Perdas de grãos do milho pré-colheita, colheita, transporte e armazenamento. Sete Lagoas: Embrapa/CNPMS; 1997. 6p. [Google Scholar]
- Setamau et al. (1998).Setamau M, Cardwell KF, Schulthess F, Hell K. Effect of insect damage to maize ears, with special refrence to Mussidia nigrivenella (Lepidoptera: Pyralydae) on Aspergillus flavus (Deuteromycetes: Monoliales) infection and aflatoxin production in maize before harvest in the Repuplic of Benin. Journal of Economic Entomology. 1998;91:433–439. doi: 10.1093/jee/91.2.433. [DOI] [Google Scholar]
- Sinha & Sinha (1992).Sinha KK, Sinha AK. Effect of Sitophilus oryzae infestation on Aspergillus flavus infection and aflatoxin contamination in stored wheat. Journal of Stored Products Research. 1992;28:211–219. doi: 10.1016/0022-474X(92)90043-P. [DOI] [Google Scholar]
- Vayias et al. (2010).Vayias BJ, Athanassiou CG, Milonas DN, Mavrotas C. Persistence and efficacy of spinosad on wheat, maize and barley grains against four major stored product pests. Crop Protection. 2010;29:496–505. doi: 10.1016/j.cropro.2009.12.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
Raw data has been provided in the Supplemental Information.


