Abstract
The maximum age of patients receiving allogeneic hematopoietic stem cell transplantation (alloHCT) has been moving up over time. However, the availability of a suitable HLA-matched sibling donor may limit access of this patient population to alloHCT. We retrospectively investigated the outcomes of umbilical cord blood transplantation (UCBT) after reduced-intensity conditioning regimens in patients aged ≥70 years with myelodysplastic syndrome (MDS) and acute myelogenous leukemia (AML) between 2010 and 2014. During this period 70 patients with AML/MDS were referred to our center for alloHCT consideration. Twenty-two patients (33%) received alloHCT: 10 UCBT, 9 HLA full-matched sibling donor transplantation, 2 haploidentical alloHCT, and 1 unrelated donor alloHCT. In UCBT, cumulative incidences of nonrelapse mortality and relapse were 20% and 30% at 2 years, respectively. The cumulative incidence of acute graft-versus-host disease (GVHD) at day +100 and chronic GVHD at 2 years was 10%. Seven patients had viral reactivation/infections. Rates of overall survival and disease-free survival were 60% and 50% at 2 years, respectively. Moreover, these outcomes seemed to be similar to that of patients aged 60 to 69 years receiving UCBT (n = 60) and patients aged ≥70 years receiving HLA full-matched sibling donor transplantation (n = 9). These results suggest that UCBT is feasible in selected AML/MDS patients aged ≥70 years. In fact, UCBT shortens the required time for an unrelated donor search and thus increases the chance of proceeding with alloHCT, which might contribute to higher rates of alloHCT in the referral group. Outcomes of UCBT are promising; however, larger studies with a longer follow-up are needed.
Keywords: Umbilical cord blood transplantation, acute myelogenous leukemia, myelodysplastic syndrome, old
Introduction
AML and MDS are more frequent in older patients, and elderly patients have poor survival compared to younger patients.1 Old AML patients who are treated may have better outcomes;1 however even in patients who achieve complete remission, their overall survival (OS) can still be poor.2 Allogeneic hematopoietic cell transplant (alloHCT) may improve outcomes in older patients with AML and MDS in CR.3-5 However, traditionally-set age limits, increased risk of non-relapse mortality (NRM) with myeloablative (MA) conditioning regimen and less frequent availability of a suitable HLA-matched sibling or unrelated donor may preclude this treatment option from the elderly population.
A study from the Center for International Blood & Marrow Transplant Research (CIBMTR) suggested that older patients (≥60 years) with AML/MDS had similar NRM and OS than younger age groups.6 Moreover, a recent study showed that alloHCT from matched sibling donor (MSD) or unrelated donors (URD) is feasible and reasonably safe in a selected very old (≥70 years) population.7 We have previously reported the feasibility of UCB as an alternative donor source using RIC in patients aged 55-70.8 In this current study, we evaluated our experience with the outcomes of UCB in AML/MDS patients≥ 70 years, focusing on early NRM. We looked at GVHD/relapse free survival (GRFS) and the survival out of hospital (ie, days alive and out of hospital, DAOH) as surrogate markers for quality of life.
Pateints and Methods
We searched the bone marrow transplantation database to identify patients aged 70 years who received UCBT for AML and MDS between January 1, 2010 and December 31, 2014 at the University of Minnesota. Moreover, to compare the outcomes of these patients, we also collected data on AML/MDS patients aged 60 to 69 years receiving UCBT and AML/MDS patients aged 70 years receiving HLA full MSD transplantation. To understand better the selection/screening process of these patients, all patients aged 70 years referred to our center for alloHCT consideration were evaluated in this period. Patients between ages 55 and 75 years were eligible for RIC alloHCT if the following criteria were fulfilled: blast counts < 5% and no morphologic evidence of AML, < 3, corrected carbon monoxide diffusing capacity 40% of predicted, left ventricular ejection fraction 35%, creatinine < 2 mg/dL or creatinine clearance 40 mL/min, serum total bilirubin < 2.5 mg/dL, and alanine and aspartate aminotransferases and alkaline phosphatase < 5 times normal. Any deviation from the study protocol for selection process to include a patient requires an approval of local institutional review board after the treating transplant physician and principal investigator of RIC alloHCT protocols agree. Data for MSD and elderly adult patients were collected prospectively through our bone marrow transplant database. Patients gave consent and were treated according to protocols approved by the University of Minnesota institutional review board. The RIC regimen included cyclophosphamide (50 mg/kg i.v. on day 6), fludarabine (30 to 40 mg/m2 i.v. daily from days -6 through -2), and total body irradiation (200 cGy on day -1). Equine antithymocyte globulin (ATG) 15 mg/kg i.v. every 12 hours for 6 doses was added for a subgroup of patients who had received no chemotherapy within 3 months of alloHCT. GVHD prophylaxis included cyclosporine and mycophenolate mofetil or, recently, sirolimus and mycophenolate mofetil. Mycophenolate mofetil was discontinued on day þ30. UCBT grafts were matched at 4 to 6 of 6 HLA-A, -B (antigen level), and -DRB1 (allele level) to the recipient and in patients receiving 2 UCB units were similarly matched to each other [10]. A target dose of cryopreserved total nucleated cells (TNCs) for single UCBT was 2.5 × 107/kg, whereas in double UCBT each individual unit can have cryopreserved TNCs < 2.5 × 107 /kg, but the sum of both cryopreserved units is 2.5 × 107 /kg. Neutrophil engraftment was defined as an absolute neutrophil count > .5 × 109 /L on 3 consecutive days without granulocyte growth factor. Platelet engraftment was defined as a platelet count > 20 × 109 /L on 3 consecutive days without platelet transfusion for the previous 7 days. Statistics Cumulative incidence was used to estimate relapse and GVHD, treating nonevent death as a competing risk. Cumulative incidence was similarly used to estimate NRM, treating relapse as a competing risk. Kaplan-Meier curves were used to estimate the probability of survival and disease-free survival (DFS) through 2 years post-transplant. Analyses were performed using SAS 9.3 (SAS Institute, Inc., Cary, NC).
Results
Patient Characteristics
Sixty-six patients aged 70 years were referred for alloHCT consideration. Twenty-four patients were ineligible for RIC alloHCT by the selection criteria, 10 patients declined alloHCT, and 10 patients died of disease progression or a complication of chemotherapy to meet eligibility criteria. Twenty-two patients (33%) received alloHCT: 10 UCBT, 9 HLA full MSD transplantation, 2 haploidentical alloHCT, and 1 URD alloHCT. Characteristics of UCBT and MSD transplant patients are given in Table 1. (Characteristics of patients between ages 60 to 70 years receiving UCBT are also given in Table 1.) Most patients had European LeukemiaNet [11] intermediate cytogenetics risk-1 AML (n = 6). All 3 patients with MDS had
Table1. Characteristics of patients with MDS/AML receiving UCB transplantation.
| UCB 60-64 years | UCB 65-69 years | UCB≥70years | Sibling≥70years | ||
|---|---|---|---|---|---|
| Patient (n) | 30 | 30 | 10 | 9 | |
| Age (years) | Median (range) | 61 (60-64) | 67 (65-69) | 71 (70-73) | 71 (70-74) |
| Male/Female (n) | 19/11 | 20/10 | 5/5 | 6/3 | |
| AML/MDS (n) | 23/7 | 23/7 | 7/3 | 6/3 | |
| IPSS-R score at Dx for MDS | Low | 3 | 5 | 0 | 2 |
| Intermediate | 2 | 0 | 2 | 0 | |
| High | 2 | 2 | 1 | 1 | |
| Disease Stage at alloHCT for AML | CR1, diagnosis to HCT < 6 mos | 14 | 15 | 2 | 1 |
| CR1, diagnosis to HCT ≥ 6 months | 1 | 2 | 3 | 2 | |
| CR2+, remission< 1 year | 3 | 1 | 0 | 2 | |
| CR2+, remission ≥ 1 year | 5 | 5 | 2 | 1 | |
| Time from Dx to AlloHCT (days) | Median (range) | 142 (69-11300) | 142 (62-3195) | 270 (103-958) | 260 (70-787) |
| ELN Cytogenetic Risk for AML | Favorable | 1 | 1 | 1 | 0 |
| Intermediate-1 | 6 | 9 | 6 | 4 | |
| Intermediate-2 | 6 | 6 | 0 | 0 | |
| Adverse | 10 | 7 | 0 | 2 | |
| Recipient CMV | Positive | 15 | 18 | 6 | 7 |
| HCT-CI score (n) | 0 | 11 | 6 | 3 | 3 |
| 1-2 | 8 | 9 | 5 | 2 | |
| ≥2 | 11 | 15 | 2 | 4 | |
| Conditioning | Cy/Flu/TBI | 23 | 23 | 4 | 5 |
| Cy/Flu/TBI/ATG | 7 | 7 | 6 | 4 | |
| GvHD Prophylaxis | CSA/MMF | 23 | 21 | 7 | 9 |
| Sirolimus/MMF | 7 | 9 | 3 | 0 |
Engraftment, Immune reconstitution and infections
Median time to neutrophil engraftment was 21 days (range, 13-38 days) (Table 2) in nine patients. One patient died before engraftment due to encephalopathy. Median time to platelet engraftment was 35 days. Of the eight surviving patients, six had a donor chimerism of ≥85% at day+100. Quantitative immune reconstitution data at +100 days were available in five patients: the number of total and subset of T cells was decreased, but the counts of B cells and NK cells were within normal range (Table 2).
Table 2. Outcomes of patients with MDS/AML after UCB transplantation.
| Patient (n) | 10 | |
| Donor Chimerism at 100days | <85% | 2 |
| ≥85% | 6 | |
| Neutrophil engraftment (days) | Median and (range) | 20 (13-38) |
| Platelet engraftment (days) | Median and (range) | 35 (24-64) |
| Immune reconstitution at day 100 | CD4+ cells (normal, 441 - 2156 cells/uL) | 132 |
| CD8+ cells (normal, 125 - 1312 cells/uL) | 61.5 | |
| CD3 + cells (normal,603 - 2990 cells/uL) | 230 | |
| CD19 + cells (normal, 107 - 698 cells/uL) | 591 | |
| CD16+56+ cells (normal, 95 - 640 cells/uL) | 266.5 | |
| Infections | CMV reactivation | 4 |
| HHV 6 encephalitis | 1 | |
| BK Virus Cystitis | 1 | |
| EBV reactivation | 1 | |
| HHV6 Viremia | 1 | |
| aGVHD (n) | II-IV | 1 |
| III-IV | 1 | |
| cGVHD (n) | Extensive | 1 |
| Limited | 0 | |
| Relapse (n) | 4 | |
| GRFS (days) | Median and (range) | 110 (25-946) |
| DAOH in first 180 days (days) | Median and (range) | 145 (0-160) |
| Death (n) | 5 | |
| Cause of Death (n) | Disease progression | 3 |
| HHV6 encephalopathy | 1 | |
| Encephalopathy | 1 |
Seven patients had eight viral reactivations/infections (Table 2) as follow: Four CMV reactivations (one had CMV pneumonia), one HHV6 encephalitis, and one HHV6 viremia. One patient also had EBV reactivation associated with post-transplant lymphoproliferative disorder (PTLD) and BK virus-associated hemorrhagic cystitis. Four of these seven patients received ATG as a part of conditioning regimen.
Outcomes, including GVHD, Relapse, NRM and OS
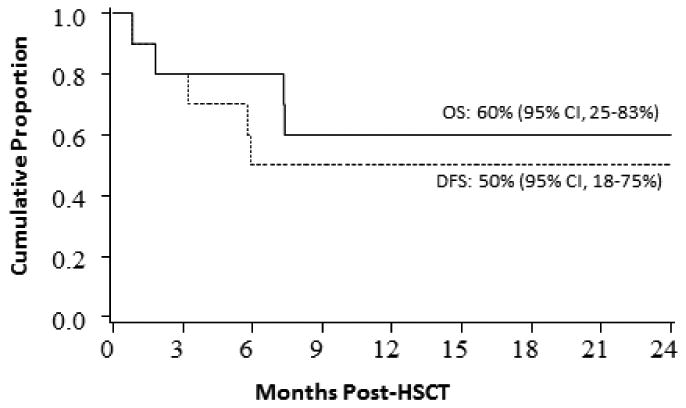
The cumulative incidence of NRM and relapse at 2 years was 20% (95% CI, 1-44%) and 30% (95% CI, 2-58%), respectively (Figure 1). For the MSD recipients, NRM and relapse was 24% (95% CI, 0-52%) and 49% (95% CI, 16-65%) at 2 years. Three of four UCB recipients who relapsed (two had AML >CR1 at UCB transplantation and two had MDS) died of disease progression. Mortality occurred in two more patients, both of whom had >low risk HCT-CI and died of encephalopathy (one HHV6 encephalopathy and one an encephalopathy of unknown etiology). The probability of estimated DFS and OS in UCB recipients was 50% (95% CI, 18-75%) and 60% (95% CI, 25-83%) respectively at 2 years (Figure 2). DFS and OS at 2 years were 27% (95% CI, 4-58%) and 55% (95% CI, 14-83%) for MSD transplant respectively.
Figure 1. Relapase/NRM patients with AML/MDS after UCB transplantation.
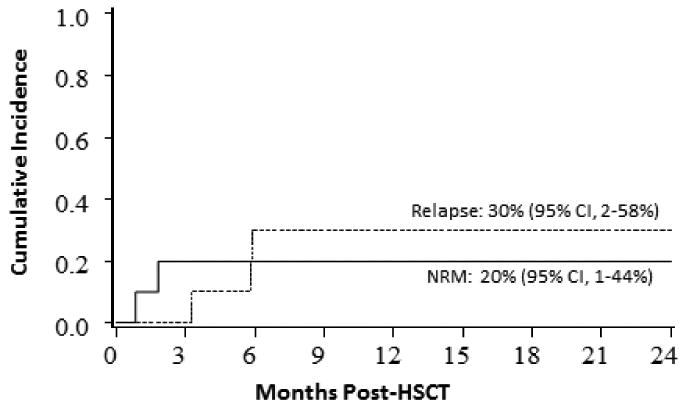
Figure 2. DFS/OS in patients with AML/MDS after UCB transplantation.
NRM, relapse, DFS, and OS at 2-years were 32% (95% CI, 15-49%) and 28% (95% CI, 11-40%), 40% (95% CI, 22-58%), and 43% (95% CI, 24-61%) for the 60-64 year UCB transplantation group, respectively. These rates were 21% (95% CI, 6-36%) and 46% (95% CI, 26-66%), 33% (95% CI, 17-51%), and 42% (95% CI, 24-59%) for the 65-69 year UCB transplantation group, respectively.
GVHD, GRFS and DAOH
The cumulative incidence of aGVHD grade III-IV was 10% at day+100 and of extensive cGVHD was 10% at 2 years (Table 2). GRFS12 was 30% at 6 month and 20% at 12 months. Beside initial hospitalization for transplantation, three patients required re-hospitalization or rehabilitation stay. Within the first 180 days after UCB transplantation, the median time of DAOH was 145 days (range, 0-160 days). One patient developed and fully recovered from congestive heart failure. One patient had bilateral hip arthroplasty for femoral neck fractures. Four patients are in remission, off immunosuppressive medications, and followed every 2-4 months at the clinic. These patients continue to have normal, independent daily activities with a KPS of>80%.
Discussion
In this study, although it is a small cohort of UCB recipients with a shorter duration of follow up, we observed several important findings. First, NRM was acceptable for this population and was relatively similar to MSD recipients. Moreover, the outcomes of these patients are similar to “relatively younger” old adult patients receiving UCB or other donor transplantation as is shown in the study and in our prior study.8 Low HCT-CI score seems to be associated with improved survival. Low incidences of aGVHD and cGVHD, in part due to 60% of patients receiving ATG, were encouraging in comparison to UCB transplantation in younger populations.8, 9 Brunner et al also reported low GVHD rates in a similarly old population.7
Viral reactivations/infections were common in the study. Our patients had markedly suppressed early T-cell immunity, which most likely increased the risk for the viral infections. Most of these patients also received ATG in the conditioning regimens. ATG use13 and UCB transplantation14 were reported to be associated with delayed T-cell recovery, and thus increased viral infections.13, 15
Outcomes of elder patients were similar, and perhaps were not inferior to previous studies that of younger adult patients receiving UCB, sibling or unrelated donor alloHCT.4,6,8,9 Current results were further supported by our previous studies where UCB had similar outcomes compared to other graft sources in older patients with AML in CR.16, 17 Brunner et al had also reported had also reported similar survival rates in patients ≥70 years (39% at 2 years).7 Moreover, most patients were not only alive but also spent the majority of their time away from hospital in the first 6 months in our study. Their survival was encouraging compared to poor survival of very old AML patients receiving supportive care or intensive therapy but no AlloHCT.1,2
These results support to the use of UCB as a plausible alternative donor source for selected very old patients with AML/MDS. Although this is a selected group, our selection criteria of ≥70 years are not different from (i.e., more stringent than) the ones of younger old patients (55-69 years). In addition, a relatively high proportion (1/3) of the referred patients ≥70 years actually received alloHCT. UCB may, in fact, have an advantage of shortening required time from an URD search and thus increases the chance of proceeding with alloHCT.18
Highlights.
Umbilical cord blood (UCB) transplantation is feasible in patients with myelodysplastic syndrome (MDS) and acute myelogenous leukemia (AML) ≥ 70 years.
These outcomes seem similar to that of younger old patients (60-69 years) receiving UCB transplantation and patients ≥ 70 years receiving HLA full-matched sibling donor transplantation.
Viral reactivations/infections are common, in part due to ATG use in conditioning regimen
Non-relapse mortality, acute and chronic GVHD are low
UCB shortens required time for URD search and thus increases the chance of proceeding with alloHCT, which might contribute to higher rates of alloHCT (1/3)
Footnotes
Conflict of Interest: These authors no conflict to disclose
References
- 1.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population- based study. Haematologica. 2012;97:1916–1924. doi: 10.3324/haematol.2012.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantarjian H, Ravandi F, O'Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116:4422–4429. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farag SS, Maharry K, Zhang MJ, et al. Comparison of Reduced-Intensity Hematopoietic Cell Transplantation with Chemotherapy in Patients Age 60-70 Years with Acute Myelogenous Leukemia in First Remission. Biol Blood Marrow Tr. 2011;17:1796–1803. doi: 10.1016/j.bbmt.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurosawa S, Yamaguchi T, Uchida N, et al. Comparison of Allogeneic Hematopoietic Cell Transplantation and Chemotherapy in Elderly Patients with Non-M3 Acute Myelogenous Leukemia in First Complete Remission. Biol Blood Marrow Tr. 2011;17:401–411. doi: 10.1016/j.bbmt.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Ustun C, Lazarus HM, Weisdorf D. To transplant or not: a dilemma for treatment of elderly AML patients in the twenty-first century. Bone Marrow Transplant. 2013;48:1497–1505. doi: 10.1038/bmt.2013.67. [DOI] [PubMed] [Google Scholar]
- 6.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of Age on Outcome of Reduced-Intensity Hematopoietic Cell Transplantation for Older Patients With Acute Myeloid Leukemia in First Complete Remission or With Myelodysplastic Syndrome. J Clin Oncol. 2010;28:1878–1887. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunner AM, Kim HT, Coughlin E, et al. Outcomes in Patients Age 70 or Older Undergoing Allogeneic Hematopoietic Stem Cell Transplantation for Hematologic Malignancies. Biol Blood Marrow Tr. 2013;19:1374–1380. doi: 10.1016/j.bbmt.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Majhail NS, Brunstein CG, Shanley R, et al. Reduced-intensity hematopoietic cell transplantation in older patients with AML/MDS: umbilical cord blood is a feasible option for patients without HLA-matched sibling donors. Bone Marrow Transplant. 2012;47:494–498. doi: 10.1038/bmt.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mrozek K, Marcucci G, Nicolet D, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30:4515–4523. doi: 10.1200/JCO.2012.43.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–1338. doi: 10.1182/blood-2014-10-609032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindemans CA, Chiesa R, Amrolia PJ, et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood. 2014;123:126–132. doi: 10.1182/blood-2013-05-502385. [DOI] [PubMed] [Google Scholar]
- 14.Kanda J, Chiou LW, Szabolcs P, et al. Immune recovery in adult patients after myeloablative dual umbilical cord blood, matched sibling, and matched unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:1664–1676. doi: 10.1016/j.bbmt.2012.06.005. e1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Illiaquer M, Imbert-Marcille BM, Planche L, et al. Prospective Comparison Of Five Viral Infections (HHV6, CMV, EBV, ADV, BKv) After Umbilical Cord Blood (UCB) Vs PBSC Reduced-Intensity Conditioning Allogeneic Stem Cell Transplantation (allo-SCT) In Adults. Blood. 2013;122 [Google Scholar]
- 16.Weisdorf D, Eapen M, Ruggeri A, et al. Alternative donor transplantation for older patients with acute myeloid leukemia in first complete remission: a center for international blood and marrow transplant research-eurocord analysis. Biol Blood Marrow Transplant. 2014;20:816–822. doi: 10.1016/j.bbmt.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warlick ED, Peffault de Latour R, Shanley R, et al. Allogeneic hematopoietic cell transplantation outcomes in acute myeloid leukemia: similar outcomes regardless of donor type. Biol Blood Marrow Transplant. 2015;21:357–363. doi: 10.1016/j.bbmt.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estey E, de Lima M, Tibes R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS) Blood. 2007;109:1395–1400. doi: 10.1182/blood-2006-05-021907. [DOI] [PubMed] [Google Scholar]


