Abstract
IMPORTANCE
A major goal of translational neuroscience is to identify neural circuit abnormalities in neuropsychiatric disorders that can be studied in animal models to facilitate the development of new treatments. Oscillations in the gamma band (30–100 Hz) of the electroencephalogram have received considerable interest as the basic mechanisms underlying these oscillations are understood, and gamma abnormalities have been found in schizophrenia (SZ). Animal models of SZ based on hypofunction of the N-methyl-d-aspartate receptor (NMDAR) demonstrate increased spontaneous broadband gamma power, but this phenomenon has not been identified clearly in patients with SZ.
OBJECTIVE
To examine spontaneous gamma power and its relationship to evoked gamma oscillations in the auditory cortex of patients with SZ.
DESIGN, SETTING, AND PARTICIPANTS
We performed a cross-sectional study including 24 patients with chronic SZ and 24 matched healthy control participants at the Veterans Affairs Boston Healthcare System from January 1, 2009, through December 31, 2012. Electroencephalograms were obtained during auditory steady-state stimulation at multiple frequencies (20, 30, and 40 Hz) and during a resting state in 18 participants in each group.
MAIN OUTCOMES AND MEASURES
Electroencephalographic activity in the auditory cortex was estimated using dipole source localization. Auditory steady-state response (ASSR) measures included the phase-locking factor and evoked power. Spontaneous gamma power was measured as induced (non–phase-locked) gamma power in the ASSR data and as total gamma power in the resting-state data.
RESULTS
The ASSR phase-locking factor was reduced significantly in patients with SZ compared with controls for the 40-Hz stimulation (mean [SD], 0.075 [0.028] vs 0.113 [0.065]; F1,46 = 6.79[P = .012]) but not the 20- or the 30-Hz stimulation (0.042 [0.038] vs 0.043 [0.034]; F1,46 = 0.006 [P = .938] and 0.084 [0.040] vs 0.098 [0.050]; F1,46 = 1.605 [P = .212], respectively), repeating previous findings. The mean [SD] broadband-induced (30–100 Hz) gamma power was increased in patients with SZ compared with controls during steady-state stimulation (6.579 [3.783] vs 3.984 [1.843]; F1,46 = 9.128 [P = .004]; d = 0.87) but not during rest (0.006 [0.003] vs 0.005 [0.002]; F1,34 = 1.067 [P = .309]; d = 0.35). Induced gamma power in the left hemisphere of the patients with SZ during the 40-Hz stimulation was positively correlated with auditory hallucination symptoms (tangential, ρ = 0.587 [P = .031]; radial, ρ = 0.593 [P = .024]) and negatively correlated with the ASSR phase-locking factor (baseline: ρ = −0.572 [P = .024]; ASSR: ρ = −0.568 [P = .032]).
CONCLUSIONS AND RELEVANCE
Spontaneous gamma activity is increased during auditory steady-state stimulation in SZ, reflecting a disruption in the normal balance of excitation and inhibition. This phenomenon interacts with evoked oscillations, possibly contributing to the gamma ASSR deficit found in SZ. The similarity of increased spontaneous gamma power in SZ to the findings of increased spontaneous gamma power in animal models of NMDAR hypofunction suggests that spontaneous gamma power could serve as a biomarker for the integrity of NMDARs on parvalbumin-expressing inhibitory interneurons in humans and in animal models of neuropsychiatric disorders.
A major goal of translational neuroscience is to identify neural circuit abnormalities in neuropsychiatric disorders that can be studied in animal models to facilitate the development of new treatments.1 Oscillations in the gamma band (30–100 Hz) of the electroencephalogram (EEG) have received considerable interest in this effort because the basic mechanisms underlying these oscillations are understood2 and are believed to be conserved across species. Schizophrenia (SZ) is characterized by abnormalities in gamma oscillations elicited by a variety of stimuli and tasks,3,4 particularly deficits in the auditory steady-state response (ASSR) to gamma frequency stimulation.5 Dysfunctional gamma oscillations have been proposed to be caused by abnormalities in parvalbumin (PV)-expressing fast-spiking basket cells (PVBCs).6 The PVBCs are a critical element in neural circuits that generate gamma oscillaitons,7 and neuropathological studies have demonstrated abnormalities in PVBCs in SZ.6
Hypofunction of the N-methyl-d-aspartate receptor (NMDAR) has been proposed to be an important factor in the pathophysiological features of SZ.8–10 In awake and behaving rodents, NMDA Rantagonism induces behavioral and psychophysiological abnormalities resembling those found in SZ11 and increased spontaneous (non–stimulus-locked) broadband gamma activity.12,13 This effect may be caused by the reduction of NMDAR function on PVBCs because mice with genetic reductions of NMDAR function on PV-expressing interneurons also exhibit increased spontaneous gamma activity.14–17
For gamma oscillations to be useful as biomarkers of PVBC mediated inhibition, correspondences must be found between gamma alterations in neuropsychiatric disorders and in animal models of these disorders. However, most reported gamma abnormalities in patients with SZ are characterized by reduced power and/or phase synchronization, whereas spontaneous gamma power is increased in animal models of NMDAR hypofunction. Two studies suggest that the latter phenomenon also may occur in an awake and stimulated state in patients with SZ. Teale et al18 found that induced (non–phase-locked) 40-Hz power during the ASSR to 40-Hz stimuli was increased in patients with SZ compared with controls. Spencer19 reported increased baseline 40-Hz power during 40-Hz auditory steady-state stimulation in patients with SZ. However, no study, to our knowledge, has demonstrated an increase in spontaneous broadband gamma oscillations in SZ as in animal models of NMDAR hypofunction.
Herein we examine whether spontaneous gamma power in patients with chronic SZ differed from that of healthy control participants at rest and during auditory steady-state stimulation at multiple frequencies. We tested whether (1) broad-band spontaneous gamma power was increased in SZ, (2) spontaneous gamma power was related to deficits in stimulus-evoked activity in SZ, and (3) spontaneous gamma power was related to psychotic symptoms, such as hallucinations.
Methods
The institutional review boards of the Veterans Affairs Boston Healthcare System and Harvard Medical School approved this study. After a detailed description of the study, each participant gave written informed consent. Details of the participants’ demographic and clinical characteristics are given in eTable 1 and eTable 2 in the Supplement. Recruitment and characterization of participants, stimuli and procedures, EEG recording and processing methods, source localization, and spectral analyses are described in the eAppendix in the Supplement. The final sample of participants consisted of 24 controls and 24 patients with SZ who underwent EEG recording while listening to auditory steady-state stimuli (20, 30, and 40 Hz) and during rest (18 controls and 18 patients with SZ).
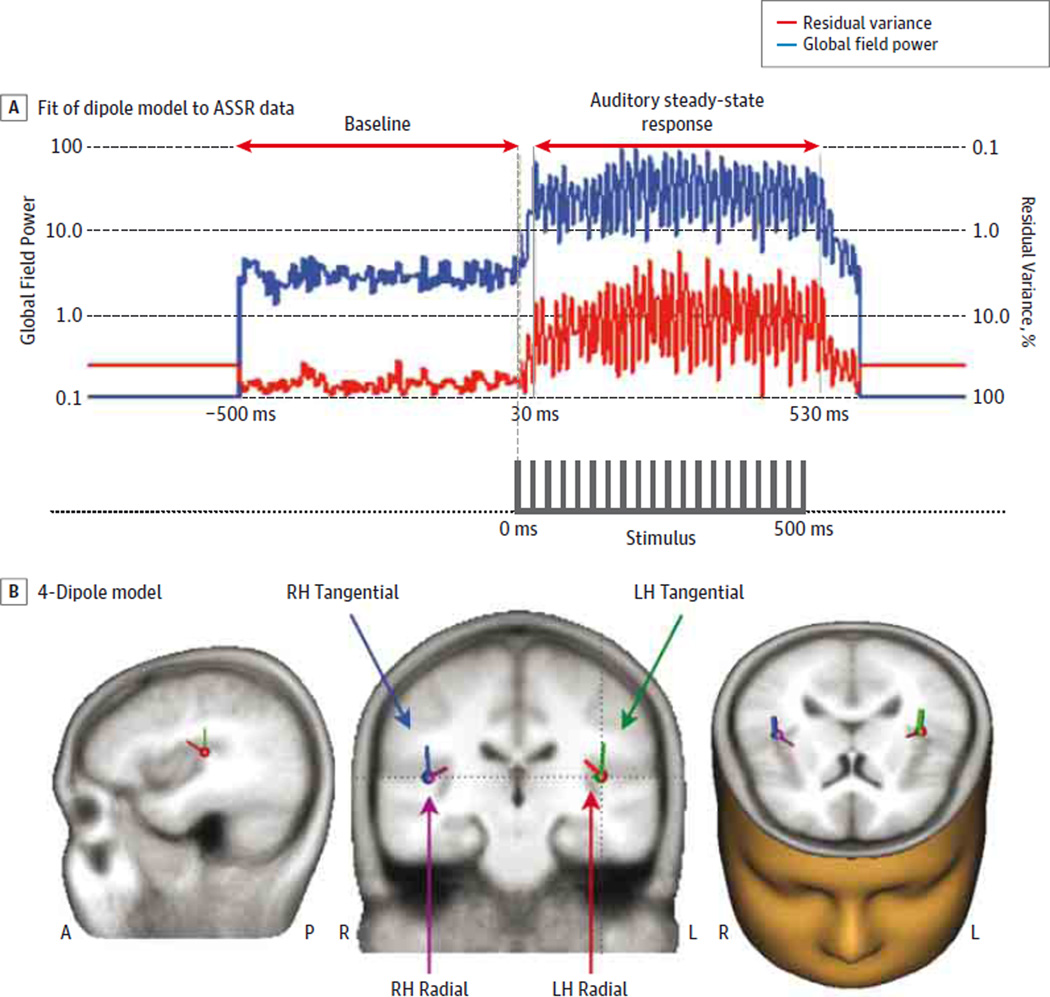
The ASSR sources were localized with a 4-dipolemodelconsisting of tangential and radial pairs of dipoles in the superior temporal plane of each hemisphere (Figure 1). This dipole model was applied to the induced power and resting EEG data. The ASSR was measured with the phase-locking factor (PLF) and evoked power. Spontaneous gamma activity in the ASSR data was measured as induced power during the baseline (−500 to 0milliseconds) and the ASSR(30–530 milliseconds) periods. We calculated the mean PLF and evoked power within the time window(30–530 milliseconds) and the frequencies (20Hz: 20–22 Hz; 30 Hz: 30–33 Hz; and 40 Hz: 40–44 Hz) at which the ASSR was maximal. The frequency range of 30 to 100 Hz was used for the baseline, ASSR-induced, and resting-state power analyses (excluding 60 Hz owing to power line artifacts). Corrections for multiple tests used the Bonferroni method.
Figure 1. Electrophysiological Assessment of the Primary Auditory Cortex.
A, Global field power of the 40-Hz auditory steady-state response (ASSR) and the percentage of residual variance (RV) of the dipole model from the scalp electrodes in healthy control participants. Arrows indicate analyzed ranges for baseline activity (−500 to 0 ms) and ASSR (30 to 530 ms). The 40-Hz ASSR stimuli (click-trains) are indicated at the bottom. B, The 4-dipole models of the ASSR are shown (middle schematic) in the magnetic resonance imaging template from BESA GmbH. The dotted lines in the coronal section correspond to the sagittal (left side) and axial (right side) sections. Corresponding dipole colors are green for the left hemisphere (LH) tangential, blue for the right hemisphere (RH) tangential, red for the LH radial, and purple for the RH radial. A indicates anterior; P, posterior.
For statistical analysis of ASSR PLF and evoked power data, analyses of variance used the following design: group (SZ/controls) × stimulation frequency (20-Hz/30-Hz/40-Hz) × hemisphere (left/right) × dipole (tangential/radial). Induced γ analyses also included the factor time period (pre-stimulus baseline/period of the ASSR). For resting-state γ data, analyses of variance used the following design: group (SZ/controls) × hemisphere (left/right) × dipole (tangential/ radial). The Greenhouse-Geisser correction for inhomogeneity of variance20 was applied for factors with more than 2 levels and is reflected in the reported P values.
Effect sizes are expressed as Cohen d. The nonparametric Spearman ρ was used for correlation analyses. All statistical tests were 2-tailed with α = 05.
Results
ASSR PLF and Evoked Power
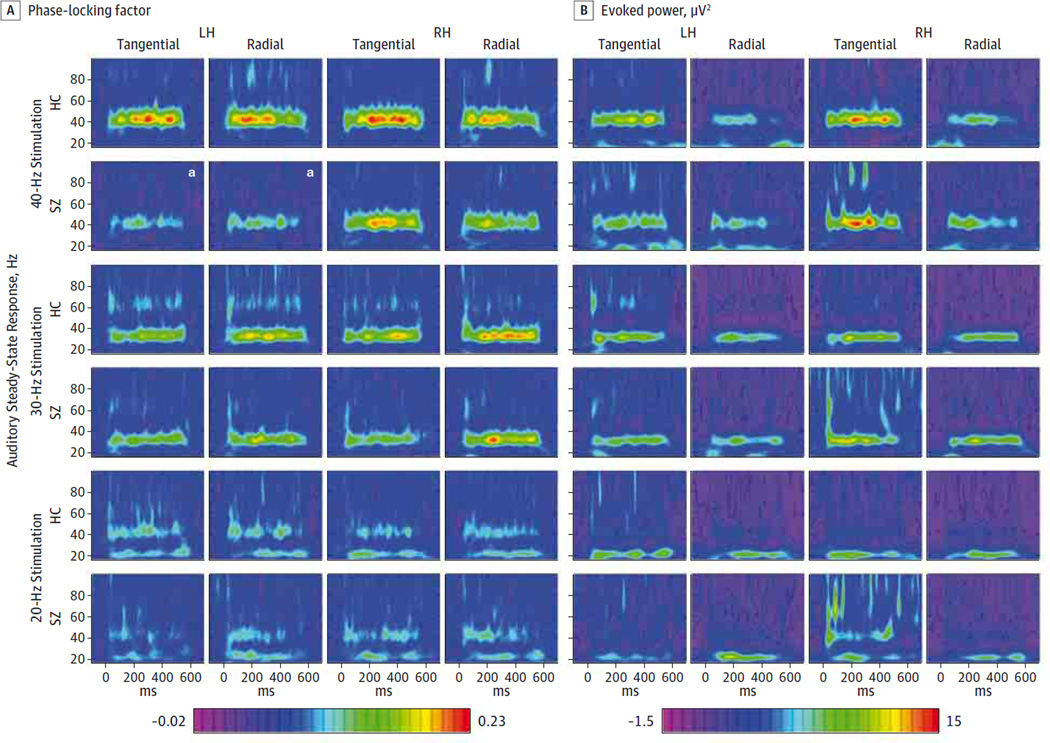
The ASSR PLF was reduced in patients with SZ compared with controls overall (mean [SD], 0.067 [0.024] vs 0.085 [0.034]; F1,46 = 4.36 [P = .042]) (Figure 2). This deficit varied between stimulation frequencies and hemispheres (group × frequency × hemisphere interaction: F1,46 = 3.36[P = .039]). The ASSR PLF did not differ between groups for the 20-Hz (mean [SD], 0.042 [0.038] vs 0.043 [0.034]; F1,46 = 0.006 [P = .938]) and 30-Hz (0.084 [0.040] vs 0.099 [0.050]; F1,46 = 1.605 [P = .212]) conditions. In the 40-Hz condition, we found a significant main effect of group (mean [SD], 0.075 [0.028] vs 0.113 [0.065]; F1,46 = 6.79[P = .012]) and a significant group × hemisphere interaction (F1,46 = 4.31 [P = .043]). The ASSR PLF was reduced in patients with SZ compared with controls for the left hemisphere dipoles (mean [SD], 0.057 [0.037] vs 0.110 [0.065]; t46 = 3.46 [P = .002, corrected]; d = 1.00) but not for the right hemisphere dipoles (0.093 [0.045] vs 0.115 [0.072]; t46 = 1.31 [P = .396, corrected]; d = 0.38). The PLF was reduced in patients with SZ for the left hemisphere radial (mean [SD], 0.056 [0.041] vs 0.111 [0.077]; t46 = 3.07, P = .007 corrected, d = 0.89) and tangential dipoles (0.059 [0.056] vs0.110 [0.078]; t46 = 2.62 [P = .02, corrected]; d = 0.76). The ASSR-evoked power did not differ between groups (mean [SD], 5.235 [3.243] vs 5.51 [2.923]; F1,46 = 0.096 [P = .758]) (Figure 2), and we found no significant interactions involving the factor group (P > .292 for all).
Figure 2. Time Frequency Maps of Evoked Gamma Oscillations in the Auditory Cortex.
Time frequency maps of cortical dipole source activities for the 20-, 30-, and 40-Hz auditory steady-state response (ASSR) for healthy control participants (HC) and patients with schizophrenia (SZ). A, Color scale indicates phase-locking factor (PLF) values. B, Color scale indicates power values inmicrovolts squared. RH indicates right hemisphere.
a Left hemisphere (LH) tangential and radial ASSR PLF during the 40-Hz stimulation were significantly reduced in patients with SZ.
ASSR-Induced Gamma Power
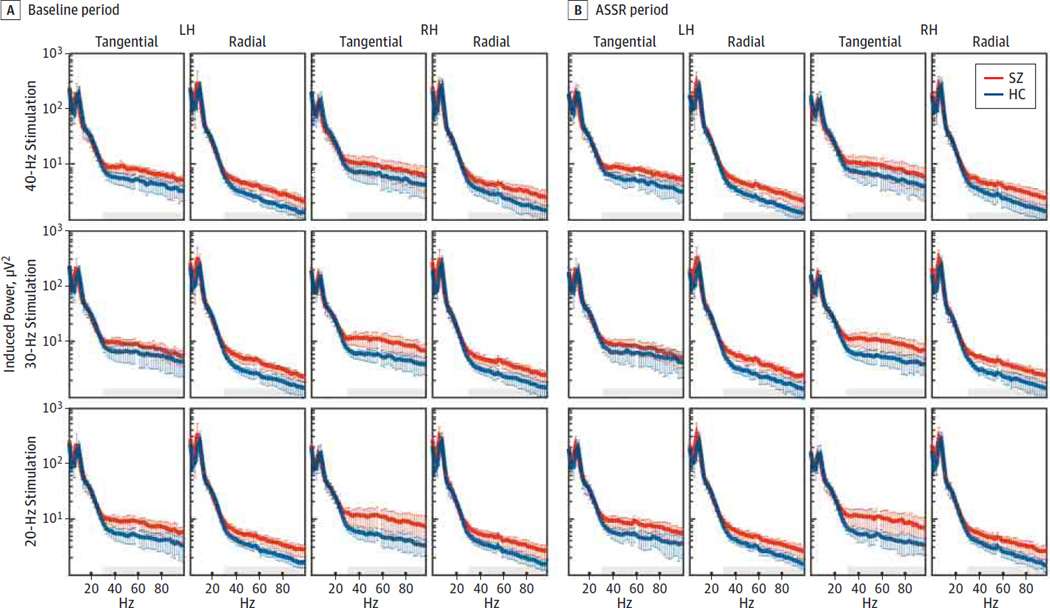
The induced power spectra are shown in Figure 3. In the pre-stimulus baseline (−500 to 0milliseconds) and ASSR (30–530 milliseconds) periods, the patients with SZ showed overall increased induced gamma power compared with the controls (6.579[3.783] vs 3.984[1.843];F1,46 = 9.128 [P = .004];d = 0.89). This effect varied among time range (baseline to ASSR), stimulation frequencies, and hemispheres (group × range × frequency × hemisphere interaction:F1,46 = 4.934[P = .03]). In the baseline period, the patients with SZ had increased induced gamma power compared with the controls (6.622 [3.765] vs 4.045 [1.933]; F1,46 = 8.895 [P = .005]; d = 0.88), and this effect also varied between stimulation frequencies and hemispheres (group × frequency × hemisphere interaction: F1,46 = 6.015 [P = .006]). Follow-up analyses of variance found that induced gamma power was increased in the patients with SZ compared with the controls in the 20-Hz (6.940 [4.755] vs 4.015 [2.205];F1,46 = 7.475 [P = .009];d = 0.79)and30-Hz(6.668 [3.657] vs 4.211 [2.775]; F1,46 = 6.876 [P = .012]; d = 0.76) conditions. For the 40-Hz stimulation, induced gamma power was increased in the patients with SZ compared with the controls (6.256 [4.501]vs 3.909[2.205];F1,46 = 5.250 [P = .027];d = 0.66), and this effect differed according to hemisphere (group × hemisphere interaction: F1,46 = 4.078 [P = .048]). Induced gamma power was increased in patients with SZ in the left hemisphere dipoles (6.368 [5.063] vs 3.128 [1.419]; t46 = −3.019 [P = .008, corrected]; d = 0.89) but not the right hemisphere dipoles (6.144 [4.449] vs 4.689 [3.508]; t46 = −1.258 [P = .43, corrected]; d = 0.36). Thus, in the baseline period, induced gamma power was increased in the patients with SZ in both hemispheres for the 20- and 30-Hz stimulations and in the left hemisphere only for the 40-Hz stimulation.
Figure 3. Induced Power Spectra in the Auditory Cortex.
Induced power spectra of dipole-source activities for each stimulation frequency for healthy control participants (HC) and patients with schizophrenia (SZ) are plotted. ASSR indicates auditory steady-state response period; LH, left hemisphere; RH, right hemisphere; shaded color bands, 95%CIs; and gray areas, gamma band (30–100 Hz).
During the ASSR period, induced gamma power was increased in patients with SZ compared with controls (6.536 [3.828] vs 3.923 [1.772]; F1,46 = 9.539 [P = .003]; d = 0.89). Although this effect did not vary between stimulation frequencies and hemispheres (group × frequency × hemisphere interaction: F1,46 = 1.528 [P = .22]), exploratory post hoc tests found that induced gamma power during the ASSR period was larger in the left hemisphere dipoles (5.896 [4.180] vs 3.501 [1.909]; t46 = −2.854 [P = .036, corrected]; d = 0.75) than in the right hemisphere dipoles (6.716 [5.811] vs 4.181 [2.675]; t46 = −1.941 [P = .35, corrected]; d = 0.56) in the patients with SZ.
Resting-State Gamma Power
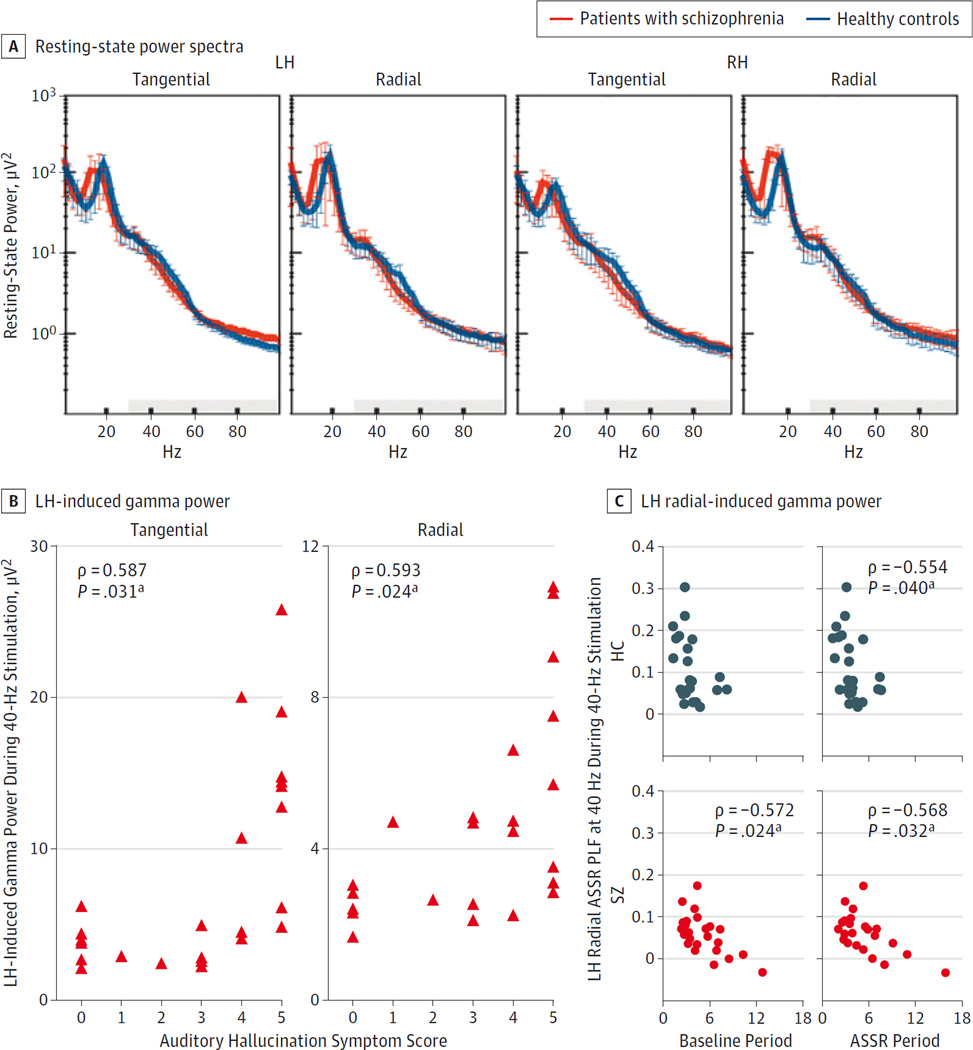
In contrast to induced gamma power during the ASSR task, we found no significant overall group difference in resting-state gamma power (0.006 [0.003] vs 0.005 [0.002]; F1,34 = 1.067 [P = .309]; d = 0.35) (Figure 4A) and no significant interactions of group with other factors (Fs < 1.674[Ps > .204]).Resting gamma power was not correlated with induced gamma power in controls or in the patients with SZ (eAppendix in the Supplement).
Figure 4. Resting-State Power and Correlations With Induced Gamma Power.
A, Resting-state power spectra of dipole-source activities for healthy control participants and patients with schizophrenia. Shaded color bands indicate 95%CIs; gray areas, gamma band (30–100 Hz). B, Correlations between the auditory hallucination symptom score (0 indicates no history; 5, a high propensity for experiencing auditory hallucinations) and induced gamma power in the left hemisphere (LH) during 40-Hz stimulation in patients with schizophrenia. C, Correlations between induced gamma power in the radial LH (baseline and auditory steady-state response [ASSR] periods) at 40 Hz and the radial LH ASSR phase-locking factor (PLF) at 40 Hz during the 40-Hz stimulation.
a Only correlations with Bonferroni-corrected P < .05 are indicated.
Correlations Between Induced Gamma Power and Auditory Hallucination Symptoms
Previous investigations21,22 found positive correlations between auditory hallucination symptom ratings and gamma ASSR PLF. Therefore, we examined whether the 40-Hz ASSR PLF in the left hemisphere, induced gamma power, and resting-state gamma power in the patients with SZ were correlated with the auditory hallucinations rating in the Scale for the Assessment of Positive Symptoms.23 The 40-Hz ASSR PLF was not correlated with auditory hallucinations (left hemisphere tangential: ρ = 0.225 [P = .29, uncorrected]; left hemisphere radial: ρ = −0.246 [P = .25, uncorrected]). The mean induced gamma power was calculated for the baseline and ASSR periods and tested for each hemisphere, dipole, and stimulation frequency (Bonferroni correction: 2 hemispheres × 2 dipoles × 3 frequencies). The left hemisphere tangential and radial dipole-induced gamma power during the 40-Hz stimulation were positively correlated with auditory hallucinations (ρ = 0.587 [P = .031] and ρ = 0.593 [P = .024], respectively). No other correlations were significant (−0.056 < ρ < 0.401, [P > .624]) (Figure 4B). Resting gamma power was not correlated with auditory hallucinations (ρ < 0.334 [P > .175, uncorrected]).
Correlations Between Induced Gamma Power and ASSR PLF/Evoked Power at 40 Hz
To test whether increased induced gamma power was related to the 40-Hz ASSR PLF deficit in SZ, induced gamma power at 40 Hz was correlated with the 40-Hz ASSR PLF for each dipole in the baseline and ASSR periods. In general, the PLF tended to show negative correlations with prestimulus- and poststimulus-induced gamma power in both groups. After Bonferroni correction (2 dipoles × 2 hemispheres × 2 subject groups × 2 time periods), the only significant correlations between induced gamma power and the PLF were for the left hemisphere radial dipole in the baseline and ASSR periods in the patients with SZ (baseline: ρ = −0.572 [P = .024]; ASSR: ρ = −0.568 [P = .032]) and in the ASSR period in the controls (ρ = −0.554 [P = .040]) (Figure 4C).
For the 40-Hz ASSR-evoked power, we found no correlations with the 40-Hz induced gamma power in the controls (−0.088 < ρ < 0.261[P > .218, uncorrected]). In the patients with SZ, the correlations were all positive, but none remained significant after Bonferroni correction (as for the PLF above) (0.070 < ρ < 0.560 [P > .064]).
Correlations Between Gamma Measures and Antipsychotic Medication Dosage
No correlations with antipsychotic dosage were found. Details are provided in the eAppendix in the Supplement.
Discussion
Gamma ASSR Deficit in SZ
Consistent with previous studies,21,24,25 the ASSR PLF was reduced for the 40-Hz stimulation in the patients with SZ compared with the controls but was not reduced or was less affected at lower stimulation frequencies. Thus, the basic finding of a deficit in gamma frequency synchronization in the auditory cortex in SZ was reproduced here. The 40-Hz ASSR PLF deficit was lateralized to the left hemisphere, similar to some previous findings.21,26 However, the40-HzASSR-evokedpower was not decreased in SZ, in contrast to most reports.18,21,22,24,25 The reason for this discrepancy is not clear, but similar patterns of gamma PLF deficits without evoked power deficits in SZ have been reported.27,28 One explanation is that the factors involved in increasing spontaneous gamma power might also increase evoked gamma power and counteract an evoked power reduction. Lazarewicz et al12 found that ketamine increased spontaneous gamma and evoked gamma power to tones in rats, and Spencer19 reported a correlation between baseline power and ASSR-evoked power. Providing some support for this hypothesis, the 40-Hz ASSR-evoked power and induced gamma power in SZ tended to be positively correlated here, but none of the correlations reached significance after strict Bonferroni correction.
Increased Spontaneous Gamma Activity in SZ
We measured spontaneous gamma activity as induced gamma power in the ASSR task and total gamma power during rest. We found that broadband (30–100 Hz) spontaneous gamma power was increased overall in the patients with SZ compared with the controls in the baseline and ASSR periods regardless of the frequency of steady-state stimulation. This study thus confirms and extends the earlier reports of increased spontaneous gamma power during the 40-Hz auditory steady-state stimulation in SZ. Spencer19 found that total 40-Hz power was increased in the left auditory cortex during the baseline period, with a trend for increased broadband gamma power. Teale et al18 found increased 40-Hz induced power during the 40-Hz ASSR. In the present study, with a larger sample size than that of Spencer,19 we found that increased spontaneous gamma activity was a broadband phenomenon and not specific to the stimulation frequency. Furthermore, spontaneous gamma activity was increased during the baseline and ASSR periods (and was highly correlated between these periods; see the eAppendix in the Supplement).
However, spontaneous gamma power was not increased in SZ during rest. Although the effect size (d = 0.35) suggests a weak effect that could become significant with a larger sample size, a recent study29 with a very large sample failed to find increased gamma power during rest. Furthermore, we did not find a correlation between resting and induced gamma power. This dissociation may have been found because cortical gamma activity is increased during wakefulness compared with rest, an effect that involves activation of the mesencephalic reticular formation and cholinergic input to the cortex from the basal forebrain.30,31 Increased spontaneous gamma activity in SZ may require this cholinergic input to occur or to be amplified to a detectable level.
An important question is whether increased spontaneous gamma activity in SZ occurred in the same or in different neural circuitry as that which generates the ASSR. As noted above, the 40-Hz ASSR PLF was decreased in the left hemisphere of the patients with SZ without a concomitant decrease in evoked power, and the typical correlation between ASSR-evoked power and the PLF was absent in the patients with SZ in the left hemisphere during the 40-Hz stimulation. One explanation for this pattern of results is that increased spontaneous gamma activity occurred in the same circuit that generates the ASSR, but the higher level of intrinsic activity prevented the circuit from synchronizing to the steady-state stimulus. However, if this were the case, increased spontaneous gamma power should have been correlated with the PLF and evoked power reductions, but we did not observe this pattern. Another possibility is that the reduction of the 40-HzASSR PLF in the left hemisphere of the patients with SZ could have resulted from an increase in overlapping spontaneous gamma power, which could have disrupted the PLF measure without altering the actual ASSR. In signal averaging, as the power of background noise increases, the evoked power of a signal decreases. However, the 40-Hz ASSR-evoked power was not reduced in the SZ group, just the PLF. Evoked power and PLF are normally highly correlated, although this relationship was disrupted in the SZ group in some conditions (see the eAppendix in the Supplement and Spencer et al22). Additional investigations will be needed to determine which of these explanations is correct.
Although our results suggest that increased spontaneous gamma power in SZ may be present in the auditory cortex, the full set of sources of this effect could extend outside of this region. Distributed source modeling will be necessary to determine the full anatomical extent of the generators of this phenomenon in the brain. Also, given the limited spatial resolution of the present dipole localization method, we cannot conclude that spontaneous gamma power is increased in exactly the same region of the cortex as that generating the ASSR. The region could be nearby or overlapping.
The broadband nature of the increased spontaneous gamma effect raises the question of whether true oscillatory activity was affected. Rather, this effect could represent an increase in random noise in the gamma band.32,33 A resolution of this question will require detailed analyses of the dynamics of the spontaneous gamma activity.
Increased Intrinsic Neural Activity in SZ
In SZ, induced gamma power in left hemisphere sources was positively correlated with auditory hallucination symptoms for the 40-Hz but not for the other stimulation frequencies. This finding is consistent with many reports of relationships between auditory hallucinations and left auditory cortex structure and function.34–36 Thus, greater gamma power was associated with an increase in this aspect of psychosis. The direction of this abnormality goes against the common assumption that SZ should be associated only with decreased gamma-band activity.37 In some previous studies,21,22,38,39 positive correlations between the PLF of particular beta and gamma oscillations and psychotic symptoms, including hallucinations, have been found despite sometimes finding group-level reductions in the PLF21,38 (although we did not replicate previous findings21,22,37 of correlations between auditory hallucination symptoms and the ASSR PLF here). These findings led to a proposal that psychosis could involve increased high-frequency oscillatory activity.39 The present findings support this hypothesis and complement reports of increased intrinsic neural activity40–43 and cortical excitability44 in particular brain regions in SZ.
Possible Confounds by Artifacts
The high- and low-frequency bands of the EEG are susceptible to particular types of physiological artifacts, such as electromyographic45 and electro-oculographic activity,46 which can differ between task conditions or participant groups and thus masquerade as experimentally induced oscillation effects.47 These confounds appear to affect only measures of non–phase-locked activity. Thus, increased spontaneous gamma power in SZ simply could have been caused by a greater degree of artifact contamination in the patients’ EEGs. We attempted to minimize this possibility by (1) identifying artifacts in the scalp EEGs and removing them using independent component analysis, the present state-of-the-art method for artifact removal, and (2) using source localization as a spatial filter to focus on auditory cortex activity, a method that also reduces contamination by noise at the scalp electrode level.48 (Previous investigations of EEG noise in SZ32,33 have not used these methods to reduce contamination by artifacts, so the validity of their findings is not clear.) Although the possibility of residual noise cannot be excluded, the increased spontaneous gamma effect was modulated by the factors of stimulation frequency, period, and hemisphere. Furthermore, spontaneous gamma power was correlated with auditory hallucination symptoms only in the left hemisphere for the 40-Hz stimulation. Thus, artifacts are unlikely to explain the pattern of increased spontaneous gamma effects found here.
Neural Circuit Basis of Increased Spontaneous Gamma Power
One explanation for increased induced gamma power in SZ is an alteration in the balance of excitation and inhibition49 in the auditory cortex via the disinhibition of pyramidal cells by reduced PVBC activity. Such disinhibition could come about by a reduction in PVBC firing from reduced function of NMDARs on PVBCs because NMDAR hypofunction is implicated in SZ,8–10,50 and evidence of reduced expression of NMDARs on PV-expressing inhibitory interneurons in SZ exists.51 In rodents, pyramidal cell excitability52,53 and spontaneous gamma power12,13,54,55 are increased by NMDAR antagonists. The genetic reduction of NMDAR function on PV-expressing interneurons also increases excitability56 and spontaneous gamma power.14–17 Likewise, in humans, ketamine increased the power of an auditory-evoked gamma oscillation57 and motor cortex excitability.58 Computational modeling suggests that a reduction of NMDAR input to fast-spiking interneurons could increase gamma power by improving the ability of interneurons to track fast inputs.59,60 We note that increased activity could also result from other mechanisms, as demonstrated by studies involving the global downregulation of NMDAR subunit NR1 expression,61 deletion of the neuregulin receptor ErbB4 on PV-expressing interneurons,62 15q13.3 microdeletion,63 and dopamine D4 receptor agonism.64,65
Translational Implications and Caveats
These results demonstrate that induced gamma activity is increased in patients with chronic SZ during awake listening to steady-state stimuli but not during rest. The similarity of this induced gamma effect to the findings of increased spontaneous gamma activity in animal models of NMDAR hypofunction suggests that induced gamma activity could serve as a biomarker for the integrity of NMDARs on PVBCs in humans and in animal models of neuropsychiatric disorders (although other mechanisms could be involved, as noted above). Because induced gamma activity was related to auditory hallucination symptoms, the reduction of induced gamma activity by increasing PVBC inhibitory output could lead to the amelioration of psychotic symptoms.49
Some caveats of this study should be noted. First, the patients with SZ had used atypical antipsychotics for many years, as in most studies of SZ, so the degree to which medication influences the present findings can be accounted for by correlating effects with chlorpromazine equivalents is unknown. Second, the active behavioral state of rodents in most of the model studies of NMDAR hypofunction differed from the passive listening state of the human participants during the ASSR recording. The degree to which this difference in state might influence spontaneous gamma activity is unknown.
Conclusions
We found that spontaneous gamma activity is increased during auditory steady-state stimulation in patients with SZ, reflecting adisruption in the normal balance of excitation and inhibition. This phenomenon interacts with evoked oscillations, possibly contributing to the gamma ASSR deficit found in SZ. The similarity of increased spontaneous gamma power in SZ to the findings of increased spontaneous gamma power in animal models of NMDAR hypofunction suggests that spontaneous gamma power could serve as a biomarker for the integrity of NMDARs on PV-expressing inhibitory interneurons in humans and in animal models of neuropsychiatric disorders.
Supplementary Material
Acknowledgments
Dr Spencer reports receiving consultation fees from Galenea Corp and Bristol-Myers Squibb in the past 3 years.
Funding/Support: This work was supported in part by VA Merit CX000154 from the US Department of Veterans Affairs (Dr Spencer); grants R01MH080187 and R01MH093450 from the National Institutes of Health (Dr Spencer); by Strategic Young Researcher Overseas Visits Program for Accelerating Brain Circulation S2208 (Drs Kanba and Onitsuka) and Grant-in-Aid for Young Scientists B 22791129 (Dr Hirano) from the Japan Society for the Promotion of Science; and by the Fund for Pharmacopsychiatry Research from the Senshin Medical Research Foundation (Dr Hirano).
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We thank all the participants for their contribution to this research. Elizabeth Thompson, BA, and Israel Molina, BS, Veterans Affairs Boston Healthcare System and Harvard Medical School, provided help as paid research assistants. John H. Krystal, MD, Yale University School of Medicine and Veterans Affairs Connecticut Healthcare System, provided comments on this work, for which he received no financial compensation.
Footnotes
Supplemental content at jamapsychiatry.com
Author Contributions: Drs Hirano and Spencer had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Hirano, Onitsuka, Spencer.
Acquisition, analysis, or interpretation of data: Hirano, Oribe, Kanba, Nestor, Spencer.
Drafting of the manuscript: Hirano, Oribe, Onitsuka, Spencer.
Critical revision of the manuscript for important intellectual content: Hirano, Kanba, Nestor, Spencer.
Statistical analysis: Hirano, Oribe, Spencer.
Obtained funding: Hirano, Kanba, Spencer.
Administrative, technical, or material support: Hirano, Kanba, Spencer.
Study supervision: Kanba, Onitsuka, Spencer.
Conflict of Interest Disclosures: No other disclosures were reported.
REFERENCES
- 1.Markou A, Chiamulera C, Geyer MA, Tricklebank M, Steckler T. Removing obstacles in neuroscience drug discovery: the future path for animal models. Neuropsychopharmacology. 2009;34(1):74–89. doi: 10.1038/npp.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buzsáki G, Wang X-J. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35(1):203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11(2):100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 4.Woo T-UW, Spencer K, McCarley RW. Gamma oscillation deficits and the onset and early progression of schizophrenia. Harv Rev Psychiatry. 2010;18(3):173–189. doi: 10.3109/10673221003747609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner CA, Krishnan GP, Vohs JL, et al. Steady state responses: electrophysiological assessment of sensory function in schizophrenia. Schizophr Bull. 2009;35(6):1065–1077. doi: 10.1093/schbul/sbp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35(1):57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459(7247):698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 2003;169(3–4):215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- 9.Moghaddam B, Krystal JH. Capturing the angel in “angel dust”: twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophr Bull. 2012;38(5):942–949. doi: 10.1093/schbul/sbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26(4–6):365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- 11.Amann LC, Gandal MJ, Halene TB, et al. Mouse behavioral endophenotypes for schizophrenia. Brain Res Bull. 2010;83(3–4):147–161. doi: 10.1016/j.brainresbull.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ. Ketamine modulates theta and gamma oscillations. J Cogn Neurosci. 2010;22(7):1452–1464. doi: 10.1162/jocn.2009.21305. [DOI] [PubMed] [Google Scholar]
- 13.Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry. 2008;63(8):730–735. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Carlén M, Meletis K, Siegle JH, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2012;17(5):537–548. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68(3):557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Billingslea EN, Tatard-Leitman VM, Anguiano J, et al. Parvalbumin cell ablation of NMDA-R1 causes increased resting network excitability with associated social and self-care deficits. Neuropsychopharmacology. 2014;39(7):1603–1613. doi: 10.1038/npp.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakao K, Nakazawa K. Brain state-dependent abnormal LFP activity in the auditory cortex of a schizophrenia mouse model. Front Neurosci. 2014;8:168. doi: 10.3389/fnins.2014.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teale P, Collins D, Maharajh K, Rojas DC, Kronberg E, Reite M. Cortical source estimates of gamma band amplitude and phase are different in schizophrenia. Neuroimage. 2008;42(4):1481–1489. doi: 10.1016/j.neuroimage.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spencer KM. Baseline gamma power during auditory steady-state stimulation in schizophrenia. Front Hum Neurosci. 2011;5:190. doi: 10.3389/fnhum.2011.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keselman HJ, Rogan JC. Repeated measures F tests and psychophysiological research: controlling the number of false positives. Psychophysiology. 1980;17(5):499–503. doi: 10.1111/j.1469-8986.1980.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 21.Spencer KM, Salisbury DF, Shenton ME, McCarley RW. γ-Band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64(5):369–375. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, McCarley RW. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009;10(1):85. doi: 10.1186/1471-2202-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa; 1984. [Google Scholar]
- 24.Light GA, Hsu JL, Hsieh MH, et al. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60(11):1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 25.Krishnan GP, Hetrick WP, Brenner CA, Shekhar A, Steffen AN, O’Donnell BF. Steady state and induced auditory gamma deficits in schizophrenia. Neuroimage. 2009;47(4):1711–1719. doi: 10.1016/j.neuroimage.2009.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edgar JC, Chen Y-H, Lanza M, et al. Cortical thickness as a contributor to abnormal oscillations in schizophrenia? NeuroImage Clinl. 2014;4:122–129. doi: 10.1016/j.nicl.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirihara K, Rissling AJ, Swerdlow NR, Braff DL, Light GA. Hierarchical organization of gamma and theta oscillatory dynamics in schizophrenia. Biol Psychiatry. 2012;71(10):873–880. doi: 10.1016/j.biopsych.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spencer KM, Niznikiewicz MA, Shenton ME, McCarley RW. Sensory-evoked gamma oscillations in chronic schizophrenia. Biol Psychiatry. 2008;63(8):744–747. doi: 10.1016/j.biopsych.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayanan B, O’Neil K, Berwise C, et al. Resting state electroencephalogram oscillatory abnormalities in schizophrenia and psychotic bipolar patients and their relatives from the Bipolar and Schizophrenia Network on Intermediate Phenotypes Study. Biol Psychiatry. 2014;76(6):456–465. doi: 10.1016/j.biopsych.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez R, Kallenbach U, Singer W, Munk MHJ. Short- and long-term effects of cholinergic modulation on gamma oscillations and response synchronization in the visual cortex. J Neurosci. 2004;24(46):10369–10378. doi: 10.1523/JNEUROSCI.1839-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steriade M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience. 2000;101(2):243–276. doi: 10.1016/s0306-4522(00)00353-5. [DOI] [PubMed] [Google Scholar]
- 32.Winterer G, Ziller M, Dorn H, et al. Schizophrenia: reduced signal-to-noise ratio and impaired phase-locking during information processing. Clin Neurophysiol. 2000;111(5):837–849. doi: 10.1016/s1388-2457(99)00322-3. [DOI] [PubMed] [Google Scholar]
- 33.Winterer G, Coppola R, Goldberg TE, et al. Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. Am J Psychiatry. 2004;161(3):490–500. doi: 10.1176/appi.ajp.161.3.490. [DOI] [PubMed] [Google Scholar]
- 34.Ford JM, Dierks T, Fisher DJ, et al. Neurophysiological studies of auditory verbal hallucinations. Schizophr Bull. 2012;38(4):715–723. doi: 10.1093/schbul/sbs009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hugdahl K, Løberg EM, Nygård M. Left temporal lobe structural and functional abnormality underlying auditory hallucinations in schizophrenia. Front Neurosci. 2009;3(1):34–45. doi: 10.3389/neuro.01.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Modinos G, Costafreda SG, van Tol M-J, McGuire PK, Aleman A, Allen P. Neuroanatomy of auditory verbal hallucinations in schizophrenia: a quantitative meta-analysis of voxel-based morphometry studies. Cortex. 2013;49(4):1046–1055. doi: 10.1016/j.cortex.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Lisman JE, Coyle JT, Green RW, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31(5):234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulert C, Kirsch V, Pascual-Marqui R, McCarley RW, Spencer KM. Long-range synchrony of γ oscillations and auditory hallucination symptoms in schizophrenia. Int J Psychophysiol. 2011;79(1):55–63. doi: 10.1016/j.ijpsycho.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spencer KM, Nestor PG, Perlmutter R, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101(49):17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heckers S, Rauch SL, Goff D, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1(4):318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 41.Schobel SA, Chaudhury NH, Khan UA, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78(1):81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang GJ, Murray JD, Repovs G, et al. Altered global brain signal in schizophrenia. Proc Natl Acad Sci U S A. 2014;111(20):7438–7443. doi: 10.1073/pnas.1405289111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffman RE, Cavus I. Slow transcranial magnetic stimulation, long-term depotentiation, and brain hyperexcitability disorders. Am J Psychiatry. 2002;159(7):1093–1102. doi: 10.1176/appi.ajp.159.7.1093. [DOI] [PubMed] [Google Scholar]
- 45.Shackman AJ, McMenamin BW, Maxwell JS, Greischar LL, Davidson RJ. Identifying robust and sensitive frequency bands for interrogating neural oscillations. Neuroimage. 2010;51(4):1319–1333. doi: 10.1016/j.neuroimage.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keren AS, Yuval-Greenberg S, Deouell LY. Saccadic spike potentials in gamma-band EEG: characterization, detection and suppression. Neuroimage. 2010;49(3):2248–2263. doi: 10.1016/j.neuroimage.2009.10.057. [DOI] [PubMed] [Google Scholar]
- 47.Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58(3):429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 48.Hipp JF, Siegel M. Dissociating neuronal gamma-band activity from cranial and ocular muscle activity in EEG. Front Hum Neurosci. 2013;7:338. doi: 10.3389/fnhum.2013.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yizhar O, Fenno LE, Prigge M, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477(7363):171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37(1):4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bitanihirwe BKY, Lim MP, Kelley JF, Kaneko T, Woo TUW. Glutamatergic deficits and parvalbumin-containing inhibitory neurons in the prefrontal cortex in schizophrenia. BMC Psychiatry. 2009;9:71. doi: 10.1186/1471-244X-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27(43):11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Behrens MM, Lisman JE. Prolonged exposure to NMDAR antagonist suppresses inhibitory synaptic transmission in prefrontal cortex. J Neurophysiol. 2008;100(2):959–965. doi: 10.1152/jn.00079.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kocsis B. Differential role of NR2A and NR2B subunits in N-methyl-d-aspartate receptor antagonist–induced aberrant cortical gamma oscillations. Biol Psychiatry. 2012;71(11):987–995. doi: 10.1016/j.biopsych.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wood J, Kim Y, Moghaddam B. Disruption of prefrontal cortex large scale neuronal activity by different classes of psychotomimetic drugs. J Neurosci. 2012;32(9):3022–3031. doi: 10.1523/JNEUROSCI.6377-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belforte JE, Zsiros V, Sklar ER, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13(1):76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hong LE, Summerfelt A, Buchanan RW, et al. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology. 2010;35(3):632–640. doi: 10.1038/npp.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Lazzaro V, Oliviero A, Profice P, et al. Ketamine increases human motor cortex excitability to transcranial magnetic stimulation. J Physiol. 2003;547(pt 2):485–496. doi: 10.1113/jphysiol.2002.030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rotaru DC, Yoshino H, Lewis DA, Ermentrout GB, Gonzalez-Burgos G. Glutamate receptor subtypes mediating synaptic activation of prefrontal cortex neurons: relevance for schizophrenia. J Neurosci. 2011;31(1):142–156. doi: 10.1523/JNEUROSCI.1970-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spencer KM. The functional consequences of cortical circuit abnormalities on gamma oscillations in schizophrenia: insights from computational modeling. Front Hum Neurosci. 2009;3:33. doi: 10.3389/neuro.09.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gandal MJ, Sisti J, Klook K, et al. GABAB-mediated rescue of altered excitatory–inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction. Transl Psychiatry. 2012;2(7):e142. doi: 10.1038/tp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Del Pino I, Garcia-Frigola C, Dehorter N, et al. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron. 2013;79(6):1152–1168. doi: 10.1016/j.neuron.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 63.Fejgin K, Nielsen J, Birknow MR, et al. A mouse model that recapitulates cardinal features of the 15q13.3microdeletion syndrome including schizophrenia- and epilepsy-related alterations. Biol Psychiatry. 2014;76(2):128–137. doi: 10.1016/j.biopsych.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 64.Andersson RH, Johnston A, Herman PA, et al. Neuregulin and dopamine modulation of hippocampal gamma oscillations is dependent on dopamine D4 receptors. Proc Natl Acad Sci U S A. 2012;109(32):13118–13123. doi: 10.1073/pnas.1201011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kocsis B, Lee P, Deth R. Enhancement of gamma activity after selective activation of dopamine D4 receptors in freely moving rats and in a neurodevelopmental model of schizophrenia. Brain Struct Funct. 2014;219(6):2173–2180. doi: 10.1007/s00429-013-0607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.