Abstract
Objective
Methotrexate (MTX) taken as monotherapy is recommended as the initial disease-modifying antirheumatic drug for rheumatoid arthritis (RA). The purpose of this study was to examine outcomes of a blinded trial of initial MTX monotherapy with the option to step-up to combination therapy as compared to immediate combination therapy in patients with early, poor-prognosis RA.
Methods
In the Treatment of Early Rheumatoid Arthritis (TEAR) trial, 755 participants with early, poor-prognosis RA were randomized to receive MTX monotherapy or combination therapy (MTX + etanercept or MTX + sulfasalazine + hydroxychloroquine). Participants randomized to receive MTX monotherapy stepped up to combination therapy at 24 weeks if the Disease Activity Score in 28 joints using the erythrocyte sedimentation rate (DAS28-ESR) was ≥ 3.2.
Results
Attrition at 24 weeks was similar in the MTX monotherapy and combination groups. Of the 370 evaluable participants in the initial MTX group, 28% achieved low levels of disease activity and did not step-up to combination therapy (MTX monotherapy group). The mean ± SD DAS28-ESR in participants continuing to take MTX monotherapy at week 102 was 2.7 ± 1.2, which is similar to that in participants who were randomized to immediate combination therapy (2.9 ± 1.2). Participants who received MTX monotherapy had less radiographic progression at week 102 as compared to those who received immediate combination therapy (mean ± SD change in modified Sharp score 0.2 ± 1.1 versus 1.1 ± 6.4. Participants assigned to initial MTX who required step-up to combination therapy at 24 weeks (72%) demonstrated similar DAS28-ESR values (3.5 ± 1.3 vs 3.2 ± 1.3 at week 48) and radiographic progression (change in modified Sharp score 1.2 ± 4.1 vs 1.1 ± 6.4 at week 102) as those assigned to immediate combination therapy. The results for either of the immediate combination approaches, whether triple therapy or MTX + etanercept, were similar.
Conclusion
These results in patients with early, poor prognosis RA validate the strategy of starting with MTX monotherapy. This study is the first to demonstrate in a blinded trial that initial MTX monotherapy with the option to step-up to combination therapy results in similar outcomes to immediate combination therapy. Approximately 30% of patients will not need combination therapy, and the 70% who will need it are clinically and radiographically indistinguishable from those who were randomized to receive immediate combination therapy.
Methotrexate (MTX) is the cornerstone of successful therapy for rheumatoid arthritis (RA) [1-3]. Not only is it the first disease-modifying antirheumatic drug (DMARD) prescribed by most clinicians, it is the one that is continued the longest and is the foundation for the vast majority of successful DMARD combinations whether conventional (4-12) or biologic (12-19). Guidelines from both the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) recommend MTX as a first line agent for the initial treatment of RA (1,2). However, many recent trials have demonstrated that patients with early RA treated with combination therapy fare better than those given MTX monotherapy, at least initially (5,14,16,20-21). Moreover, some have suggested that there is a “window of opportunity” for therapy early in the disease, implying that if this window closes, therapy will be less effective, highlighting the need to initiate the most effective therapy quickly. Further guidelines (1) suggest that initial therapy with biologic agents may be appropriate in patients with poor-prognosis RA.
Despite these data and beliefs, most RA patients in clinical practice fail to reach the target of low disease activity or remission. Additionally, more intensive therapeutic strategies may come at a cost, both in monetary terms as well as in terms of an increased risk of treatment related adverse events. Therefore, a critically important question is whether starting combination therapy only in those who need it leads to long-term results that are inferior to those that could be achieved with initial combination therapy for all. To date no blinded trials have addressed this fundamentally important question in early RA. To this end the Treatment of Early Rheumatoid Arthritis (TEAR) clinical trial allows for comparison between patients receiving initial MTX monotherapy and those receiving initial combination therapy. Half of the participants were randomized to one of two MTX monotherapy arms: MTX alone for the duration of the trial or MTX with an option to step-up to combination therapy (MTX + etanercept or MTX + sulfasalazine [SSZ] + hydroxychloroquine [HCQ]), at week 24 if the Disease Activity Score in 28 joints using the erythrocyte sedimentation rate (DAS28-ESR) was not < 3.2. Thus, the MTX monotherapy group could be compared to the two immediate combination therapy groups, allowing us to address this important question.
Patients and Methods
Research Design and Methods
Detailed methods on the TEAR trial have been published elsewhere (22). Briefly, TEAR used a randomized double-blind 2×2 factorial design trial with 4 arms: immediate treatment with 1) MTX +ETN; or 2) MTX+SSZ+HCQ (triple therapy); or 3) initial MTX, with step-up treatment if DAS28-ESR was ≥ 3.2 at week 24 to MTX + ETN or 4) MTX+SSZ+HCQ.
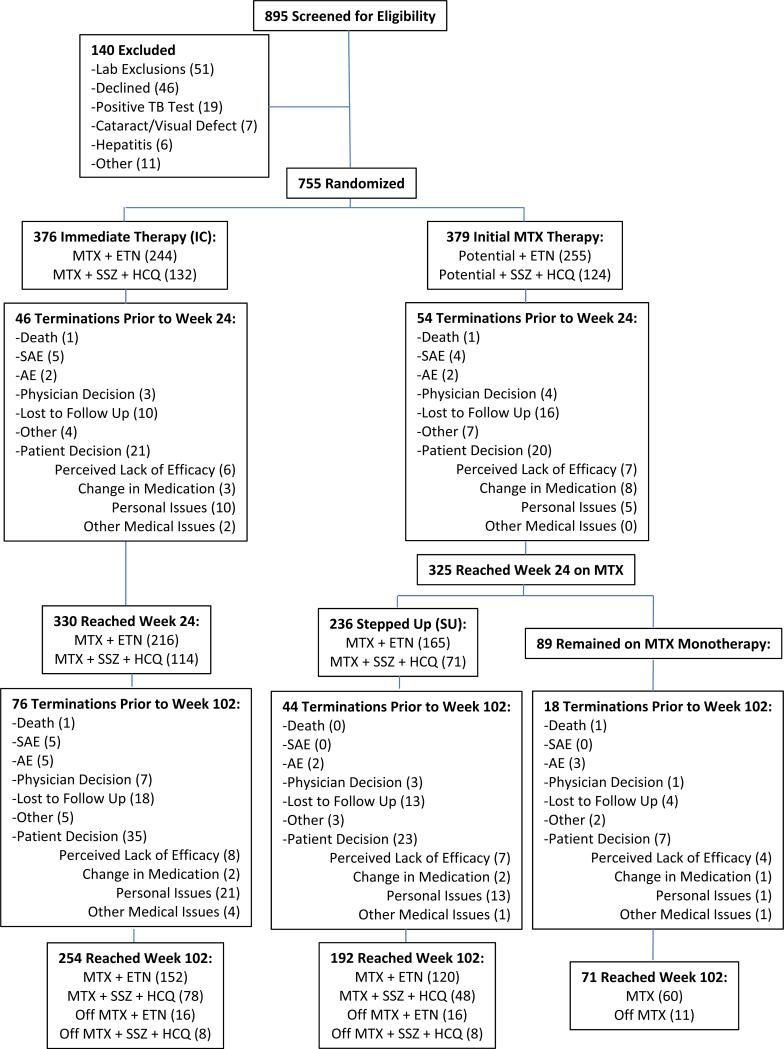
For the purpose of this analysis, two additional groups were retrospectively created to compare the effectiveness of continued MTX monotherapy among participants whose DAS28-ESR at week 24 was < 3.2 and therefore did not receive any step-up therapy. The 2 comparator groups consisted of patients who were randomized to receive MTX monotherapy but who required a step-up to additional active therapy at week 24 (step-up therapy group) and patients who were randomized to receive combination therapy (immediate combination therapy group) (see Figure 1). Treatment arms 1 (initial MTX + etanercept) and 2 (initial triple therapy) were combined to create an immediate treatment arm. Those initially treated with MTX who stepped up therapy at 24 weeks (DAS28-ESR ≥ 3.2) in arm 3 (to MTX + etanercept) & arm 4 (to triple therapy) were combined into a single step-up therapy group. Because participants had to reach week 24 to be evaluated for step-up to additional active medications (arms 3 & 4), only participants that were taking TEAR medications per protocol at week 24 were included in this analysis. Thus, this analysis compared outcome among patients in the MTX monotherapy group who did not step up (MTX monotherapy) versus those who did (step-up therapy) versus those in the immediate combination arms.
Figure 1.
Flow diagram showing the distribution of the study subjects from initial assessment to analysis of the study data. Details are given according to the Consolidated Standards of Reporting Trials (CONSORT) statement for reporting randomized controlled trials. Although 89 participants had a Disease Activity Score in 28 joints using the erythrocyte sedimentation rate of 3.2 at week 24, only 81 continued in the study past week 24. Off medication means the subject was off any/all active study drugs. TB, tuberculosis; MTX, methotrexate; ETN, etanercept; SSZ, sulfasalazine; HCQ, hydroxychloroquine; SAE, serious adverse event; AE, adverse event; MM, MTX monotherapy.
All participants and site personnel, including the treating rheumatologists, were blinded with regard to treatment assignment and step-up to additional active medications at week 24.
Study Organization
The Clinical Coordinating Center and Statistical and Data Management Center for the study were located at the University of Alabama at Birmingham (UAB). The protocol was developed in 2003; recruitment began in May 2004 and concluded in January 2007. The TEAR trial was registered with clinicaltrials.gov (NCT00259610) and was approved by central and local institutional review board committees. All patients participating in the study provided written informed consent.
Participants
Entry criteria for the TEAR Trial included disease duration < 3 years from the time of diagnosis; age >18 years; RA diagnosis by the 1987 ACR criteria (23); active disease (at least 4 swollen and 4 tender joints, using the 28-joint count); positive for rheumatoid factor (RF) or anti-cyclic citrullinated peptide (CCP) antibodies, or if autoantibody-negative, at least 2 erosions by radiographs of hands, wrists, or feet. Stable doses of ≤ 10 mg/day of prednisone and nonsteroidal anti-inflammatory drugs were allowed. Exclusion criteria were contraindications to study medications; prior use of biologic therapy; corticosteroid injections during the 4 weeks prior; or diagnosis of serious infection, including positive skin test for TB.
Study Protocol
Treatment was allocated via a computerized data entry system in a 2:1 ratio for ETN versus triple therapy in a standard permuted block approach, by study site, with block sizes of 6 or 12. Investigators and participants remained masked to treatment assignment until the end of year 2. Matching placebos were utilized throughout the trial, including the step-up period. Joint assessment occurred every 6 weeks during the first 48 weeks and every 12 weeks thereafter. Radiographs of hands, wrists, and feet were acquired at baseline, and weeks 48 and 102.
Study Medications: MTX
All participants received oral MTX, which was escalated to a dosage of 20 mg/wk or was maintained at a lower dose if by week 12, the patient had no active tender/painful or swollen joints.
SSZ + HCQ
For those assigned to receive triple therapy, either immediately or as part of step-up, SSZ was initiated at a dose of 500 mg twice a day, and if tolerated, escalated to 1000 mg twice a day after 6 weeks; HCQ was administered at a dose of 200 mg twice a day.
Etanercept
For those randomized to receive etanercept, either immediately or a part of step-up therapy, the drug was administered at a dosage of 50 mg/wk subcutaneously.
Step-up Therapy
At week 24, participants in the 2 step-up arms who had a DAS28-ESR of ≥ 3.2 continued with the same dosage of MTX and added either etanercept or SSZ+HCQ (triple therapy). Participants in the 2 step-up arms who had a DAS28-ESR < 3.2 at week 24 continued with the same dosage of MTX and placebos.
Folic Acid
All participants were prescribed folic acid 1 mg per day orally.
Study Outcomes
The primary end point for the parent trial was an observed-group analysis of the DAS28-ESR between weeks 48 and 102 (24,25), with secondary endpoints defined by the ACR criteria for 20% improvement (ACR 20), 50% improvement (ACR50), and 70% improvement (ACR70), physical disability [as determined by the modified Health Assessment Questionnaire (m-HAQ)], and joint damage (as determined by radiographic assessments) (26). Weeks 48 to 102 time points were used to allow sufficient time for those receiving step-up medication at week 24 to achieve maximal efficacy.
Radiographic progression was evaluated in the hands, wrists and feet. The radiographs were scored according to the modified Sharp/van der Heijde method by two independent readers (27). The readers scored all the films grouped by patient but blinded with regard to time sequence and treatment arm. The mean score of the two readers was used (Intraclass Correlation Coefficient 0.96).
Statistical Analysis
Analysis was performed using a modified intent-to-treat approach, including those participants in the groups to which they were randomized who reached week 24 while receiving allocated TEAR medications, regardless of compliance with the drug regimen. Baseline demographic features were compared between groups by analysis of variance (ANOVA) with Tukeys honestly significant difference for multiple comparisons, Welch ANOVA, or Wilcoxon-Kruskal-Wallis test, as applicable. The DAS28-ESR values between the postbaseline visits (weeks 12-102) were examined using a two-way, repeated-measures, mixed-model analysis, adjusting for the DAS28-ESR at baseline (25). Secondary outcomes included assessing treatment differences in radiographic scores and radiographic disease progression between baseline and week 102 (24), and differences in the numbers achieving ACR20, ACR50, and ACR70 responses between weeks 24 and 102.
Radiographic changes were analyzed using an ANOVA approach, where the differences in mean progression scores between treatment groups at two years were assessed after adjustment for the baseline radiographic scores. Chi-square tests were used to assess differences in the proportion of participants achieving radiographic remission or progression at week 102 and those achieving ACR20, AR50 and ACR79 at weeks 24, 48 and 102 (with dropouts considered treatment failures [i.e. non-responder imputation]). Remission was defined as change in the total Sharp score of ≤0.5 from baseline.
Results are presented as all observed data, without imputation for missing values, or as a nonresponders analysis as indicated. Data are reported as the mean ± SD or as the number (%). P values less than 0.5 were considered significant. Tukeys honestly significant difference was used to adjust for multiple comparisons (where indicated). SAS v9 or JMP v8 software was used.
Results
Demographic characteristics and early termination of study participants
Table 1 details the baseline demographic features of the study participants who reached week 24 and had been randomized to immediate combination, initial MTX but requiring subsequent step-up therapy, and initial MTX but not requiring step-up therapy (MTX, monotherapy). Participants receiving MTX monotherapy had a statistically lower DAS28-ESR at baseline compared to the other groups (P = 0.0027). Further, although the differences were not statistically significant, patients receiving MTX alone had numerically lower radiographic damage at baseline and were more likely to be male, Caucasian, and younger and to have a lower body mass index (BMI). Importantly, factors that were positively associated with better outcomes in the parent TEAR trial (22) included male sex, lower BMI and DAS28-ESR values, shorter disease duration and Caucasian race. There were no differences in baseline characteristics between participants who reached week 24 and those who did not, with the exception that patients who did not reach week 24 had a higher number of total painful joints (data not shown).
Table 1.
Baseline Demographic and Disease characteristics in patients who reached week 24, by treatment group*
| Characteristic | Immediate Combination Therapy (n=315) | Step-Up to Combination Therapy (n=234) | MTX Monotherapy (n=81) |
|---|---|---|---|
| Female: Number (%) | 236 (74.9) | 172 (73.5) | 52 (64.2) |
| Caucasian: Number (%) | 250 (79.4) | 187 (79.9) | 71 (87.7) |
| Age (years): mean (SD) | 49.9 (12.9) | 49.6 (13.0) | 48.2 (11.7) |
| BMI (mg/kg2): mean (SD) | 29.4 (7.4) | 30.0 (7.2) | 28.7 (5.5) |
| Disease Duration (months): | |||
| mean (SD) | 3.6 (6.5) | 3.4 (6.2) | 3.2 (6.3) |
| Median (range) | 1.0 (0-35.8) | 1.0 (0-32.7) | 1.2 (0-33.1) |
| Prednisone Use: Number (%) | 136 (43.2) | 87 (37.2) | 39 (48.2) |
| Total Tender Joints: mean (SD) | 14.0 (6.6) | 14.5 (7.2) | 12.3 (5.5)† |
| Total Swollen Joints: mean (SD) | 12.5 (5.8) | 13.5 (6.2) | 11.4 (5.1)‡ |
| ESR (mm/hr): median (range) | 27 (1-150) | 27 (1-126) | 21 (2-173) |
| DAS28-ESR: mean (SD) | 5.8 (1.1) | 5.9 (1.1) | 5.5 (1.0)§ |
| No. of Erosions: mean (SD) | 3.3 (6.7) | 3.2 (5.5) | 1.9 (2.3) |
| median (range) | 1.5 (0-74.5) | 1.5 (0-42) | 1 (0-10) |
| Modified Sharp Score: | |||
| mean (SD) | 6.9 (15.0) | 6.2 (13.4) | 2.9 (3.6) |
| median (range) | 2 (0-175.5) | 2 (0-117.5) | 1.5 (0-19) |
Patients were randomly assigned to 1 of the following 3 regimens: immediate combination therapy with either methotrexate (MTX) plus etanercept or MTX plus sulfasalazine plus hydroxychloroquine, initial MTX with step-up to combination therapy at 24 weeks if the Disease Activity Score in 28 joints using the erythrocyte sedimentation rate (DAS28-ESR) was ≥ 3.2, or MTX monotherapy. P values across treatment groups were significant for the number of tender joints (P = 0.0148 by Welch analysis of variance [ANOVA]), the number of swollen joints (P = 0.0106 by Welch ANOVA), the median ESR (P = 0.0468 by Wilcoxon-Kruskal-Wallis test), and the DAS28-ESR (P = 0.0027 by ANOVA with Tukey's honestly significant difference used to adjust for multiple comparisons). BMI = body mass index.
P = 0.0286 versus step-up therapy
P = 0.0161 versus step-up therapy
P = 0.0063 versus step-up therapy, and P = 0.0258 versus Immediate Combination Therapy
The overall early termination rate in the parent trial was 32%, with no differential dropout by treatment arm prior to week 102. There was no difference in the proportion of participants who terminated treatment prior to week 24 in the immediate combination versus initial MTX groups (16.2% vs 15.6%, P=0.84) nor for those who completed week 102 among the remaining patients who reached week 24 (immediate combination 73.3% versus step-up 75.2% versus MTX alone 75.3%, P=0.86). The last observed DAS28-ESR for earlier terminators in the immediate combination therapy group was not different from that for earlier terminators in the step-up therapy group (mean ± SD 5.1±1.5 versus 5.3±1.5, P = 0.48), further indicating that during the first 24 weeks, there was no differential reason for early termination between those receiving immediate combination treatment and those receiving MTX alone. Those terminating prior to week 24 began the trial with a higher number of tender joints than those reaching week 24 (mean ± SD 16.0±6.9 versus 14.0±6.7, P=0.0023); however, the number of tender joints was not significantly different between the two treatment groups (immediate combination versus step-up) for those who terminated prior to week 24 (P=0.68), again indicating that the treatment arm itself was not associated with early termination. Importantly, the TEAR trial (22) showed no difference in adverse events across treatment arms.
Changes in the DAS28-ESR from week 48 to week 102
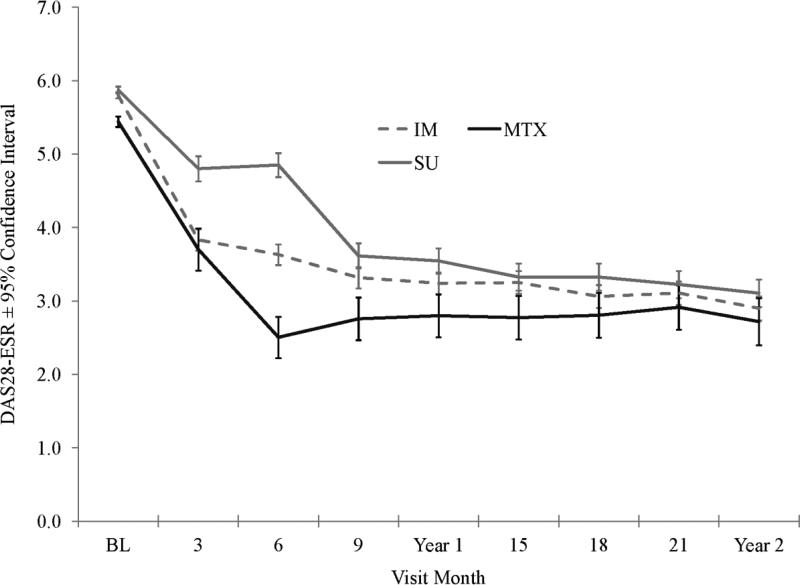
The primary endpoint of the parent TEAR trial was the mean DAS28-ESR from week 48 to weeks 102 (Figure 2 and Table 2). Patients responding well to initial MTX demonstrated similar-to-lower DAS28-ESR scores compared to the immediate combination and step-up therapy groups prior to week 24. However, after allowing 24 weeks for step-up treatment to achieve maximum efficacy, the only statistically significant difference in DAS28-ESR that remained after week 24 was at week 48 in those who stepped up therapy as compared to those who did not (3.5±1.3 versus 2.8±1.3, P=0.005); however, this difference was no longer present by week 60 (Month 15, P=0.50) (Figure 2).
Figure 2.
Disease Activity Scores in 28 joints using the erythrocyte sedimentation rate (DAS28-ESR) throughout the study period, by treatment group. Patients received immediate (IM) combination therapy (broken line), methotrexate (MTX) monotherapy (solid black line), or initial MTX therapy with step-up (SU) to combination therapy (solid gray line) (see Patients and Methods for details). Values are the mean ± 95% confidence interval.
Table 2.
DAS28-ESR values at baseline and weeks 24, 48, and 102, by treatment group*
| Visit Week | ||||
|---|---|---|---|---|
| Assessment Mean ± SD (N) | Baseline | Week 24 | Week 48 | Week 102 |
| IM (n = 315) | 5.8±1.1 | 3.6±1.3‡ | 3.2±1.3 | 2.9±1.2 |
| SU (n = 234) | 5.9±1.1 | 4.9±1.3 | 3.5±1.3 | 3.1±1.3 |
| MTX (n = 81) | 5.4±1.0† | 2.5±1.3§ | 2.8±1.3¶ | 2.7±1.2 |
Data at week 48 are from 288 patients in the immediate combination therapy group, 209 in the step-up therapy group, and 75 in the methotrexate (MTX) monotherapy group; data at week 102 are from 231, 176, and 61 patients in the respective groups. Values are the mean ± SD. P values were determined as follows: baseline data were compared by one-way analysis of variance; weeks 24, 48, and 102 data were compared by repeated-measures mixed-model analysis, adjusting for screening Disease Activity Score in 28 joints using the erythrocyte sedimentation rate (DAS28-ESR), with Tukey's honestly significant difference used to adjust for multiple comparisons.
P = 0.0063 versus step-up therapy.
P = 0.0001 versus step-up therapy and versus MTX monotherapy.
P = 0.0001 versus step-up therapy.
P = 0.0052 versus step-up therapy.
In participants who responded well initially to MTX alone, the DAS28-ESR scores remained significantly lower at 36 weeks and nominally lower for the duration of the trial through 102 weeks. Overall, 28% of participants receiving MTX alone initially had DAS28-ESR scores < 3.2 at 24 weeks (89 of 317 participants) and therefore did not need to step-up to combination therapy. These patients continued to take MTX alone for the duration of the 102-week trial (Figure1). Importantly, within 12 weeks of stepping up to combination therapy, this group of patients achieved DAS28-ESR scores equivalent to those in patients who received combinations from the outset (Figure 2). This indicated that in terms of the primary outcome (mean DAS28-ESR between weeks 48 and 102 in the parent trial), there was no penalty for delaying combination therapy until it was clinically indicated and that if participants were responding well to MTX therapy at week 24, they would continue to respond well through 102 weeks of the same therapy.
Radiographic progression
Progression of the modified Sharp score was an important secondary outcome of the study. The cumulative probability plot for the three treatment strategies that emerged from the parent TEAR trial (immediate combination therapy, step-up therapy, and MTX monotherapy) were completely superimposable (data plot not shown). Table 3 shows the modified Sharp score. While there were statistically significant differences between treatment arms at both weeks 48 and 102 in favor of the MTX monotherapy group, the maximum difference in the median modified sharp scores was only 1.5 points (immediate combination therapy versus MTX monotherapy) at both weeks 48 and 102 (Table 3). In addition, there were no group differences in the proportion of patients with erosive disease or mean erosion scores at any of the visits (data not shown).
Table 3.
Modified Sharp Scores at baseline, week 48, and week 102, as well as changes in modified Sharp scores from baseline to week 102, by treatment group*
| Assessment | Change in modified Sharp Score | |||||||
|---|---|---|---|---|---|---|---|---|
| Group (N) | Baseline | 48 weeks | 102 weeks | |||||
| Total | Median (range) | Mean ± SD | Median (range) | Mean ± SD | Median (range) | Mean ± SD | Median (range) | Mean ± SD |
| Immediate Combination Therapy (n = 330) | 2 (0-175.5) | 7 ± 14.9 | 3 (0-174.5) | 7.9 ± 15.9 | 3.0 (0-175.5) | 7.5 ± 15.7 | 0 (−13-78.5) | 1.1 6.4 |
| Step Up to Combination Therapy (n = 236) | 2 (0-117.5) | 6.2 ± 13.4 | 2.5 (0-115.5) | 7.3 ± 14.6 | 2.8 (0-52) | 6.2 ± 8.7 | 0 (−4-32) | 1.2 ± 4.1 |
| MTX Monotherapy (n = 84) | 1.5 (0-19) | 2.9 ± 3.5 | 1.5 (0-19.5) | 2.9 ± 3.6 | 1.3 (0-14) | 2.6 ± 2.9 | 0 (−1.5-5) | 0.2 ± 1.1 |
P values across treatment groups were significant at week 48 (P = 0.0321) and week 102 (P = 0.0074), as was the change in modified Sharp score at week 102 (P = 0.0467). MTX = methotrexate.
In terms of radiographic progression and remission, a higher proportion of participants in the step-up therapy group experienced progression as compared to the immediate combination therapy and MTX monotherapy groups (49.3% versus 40.0% versus 24.4%, P = 0.010), with a higher proportion of nonprogressors at week 102 in the MTX monotherapy group as compared to the immediate combination and step-up groups (87.8% versus 73.0% versus 66.7%, P=0.017). Importantly, if looking at the strategy of starting MTX therapy as compared to starting combination therapy, patients who were started on MTX alone fared as well as those who were started on combination therapy.
Time course of response to MTX therapy
Figure 2 shows the time course of response to therapy as determined by the DAS28-ESR. The MTX monotherapy group had a significant response by week 12 of therapy and, as a group, did not improve further following week 24, as has been previously reported by other investigators (28). MTX was started at a dosage of 10 mg/week, escalated to 15 mg/week at week 6 and then to 20 mg/week at week 12. Importantly, the MTX dosage escalation occurred unless the patient had zero tender joints and zero swollen joints at that visit. This time course of response is important, and these data would suggest that the clinical decision to add therapy (in this case, either etanercept or SSX+HCQ) should be made at 24 weeks if MTX is escalated, as was done in the trial. The mean MTX dosage was similar across groups: 19.2 mg/week in the immediate combination therapy group, 19.5 mg/week in the step-up therapy group, and 19.6 mg/week in the MTX monotherapy group. The median MTX dosage was 20 mg/week for all treatment groups.
Absolute responses to MTX monotherapy
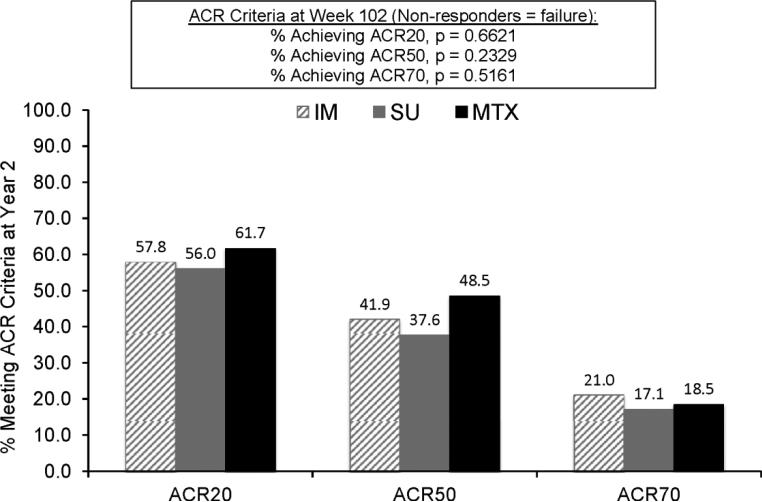
While the target of therapy in this trial was a reduction of DAS28-ESR to < 3.2, different targets may be appropriate for different patients. The ACR responses in the three treatment groups are shown in Figure 3. The percentages of participants who met other possible targets were as follows: for a clinically meaningful response (reduction in DAS28-ESR of > 1.2 units), 81.0% in the immediate combination therapy group, 73.9% in the step-up therapy group, and 85.2% in the MTX monotherapy group; for low disease activity (DAS28-ESR < 3.2), 58.1% in the immediate combination therapy group, 49.6% in the step-up therapy group, and 67.9% in the MTX monotherapy group; and for minimal disease activity (DAS28-ESR < 2.6), 39.7% in the immediate combination therapy group, 34.2% in the step-up therapy group, and 50.6% in the MTX monotherapy group. Unfortunately, we were unable to calculate ACR/EULAR remission rates, since C-reactive protein levels were not determined as part of this study.
Figure 3.
Percentages of patients achieving a response according to the American College of Rheumatology criteria for 20% improvement (ACR20), 50% improvement (ACR50), and 70% improvement (ACR70) at year 2. Patients received immediate combination therapy (hatched bars), initial methotrexate (MTX) therapy with step-up to combination therapy (shaded bars), or methotrexate (MTX) monotherapy (solid bars) (see Patients and Methods for details). Numbers across the top of the bars are the values represented by the bars themselves. Comparisons across groups for the numbers of patients achieving ACR20, ACR50, and ACR70 improvement were not significant (P = 0.66, 0.23, and 0.51, respectively).
Discussion
This post hoc analysis of data from the TEAR trial is the first to validate in a randomized double-blind trial the most commonly recommended initial approach to the treatment of RA. Clinical (DAS28-ESR, m-HAQs, and scores on the patient's global assessment of disease activity and patient's assessment of pain) and radiographic outcomes were indistinguishable from week 48 to week 102 for those participants who were started on MTX monotherapy and then stepped-up to combination therapy only when clinically indicated as compared to those who were started on combination therapy initially. Although the results of this trial have shown that in this population of RA patients, the results with triple therapy were equivalent to the results with MTX plus etanercept (22), some still hold the belief that the MTX plus tumor necrosis factor inhibitor strategy is superior. To this point, if data only from those patients who stepped-up to MTX plus etanercept or only those who randomized to MTX plus etanercept are analyzed, the results are the same – at the end of the trial, there was no clinical or radiographic advantage to the initial combination of MTX plus etanercept over the addition of etanercept in only those patients who did not meet the treatment goal (DAS28-ESR <3.2).
It is critical to point out that TEAR was a study of participants with early, poor-prognosis RA. All participants in the trial were either rheumatoid factor-positive or anti-CCP antibody-positive or had at least two erosions noted on baseline radiographs. Further, the mean DAS28-ESR at entry for the parent trial was 5.8 (consistent with high levels of disease activity) and 76% of the participants had erosive disease at baseline. Finally, TEAR was a trial of early disease, with a mean RA duration of 3.6 months. Therefore, the findings of this analysis apply to the early treatment of poor-prognosis RA. It is also well known that not all RA patients in clinical practice are treated to target and that early treatment yields better results. Therefore, our excellent results when patients with inadequate responses to MTX are stepped-up to combinations after 6 months may not be generalizable to patients in practice who have been taking MTX for years and still have active disease.
Recently, the concept of measuring an absolute level of disease activity in all patients and treating to target has been widely embraced. The ACR and EULAR have collaborated on a better way to define remission in clinical trials (29), and hopefully, this can be applied to clinical practice (30). The ACR has recently published recommendations on acceptable ways to document disease activity in the clinic (31). However, despite all these recommendations, there is no uniform agreement on what the target should be in all patients (1-3,32). Some suggest that remission should be targeted in all patients, while others believe that low levels of disease activity may be sufficient. In this trial, we defined the target in two different ways. With regard to escalation of the MTX dosage through week 12, we did so unless the patient had no tender or swollen joints (essentially remission). With regard to stepping up to combination therapy, we used low disease activity (DAS28 < 3.2) as the target. Figure 2 details the level of response that was seen in the MTX monotherapy group at 24 weeks.
Many patients and some clinicians would be satisfied with responses that are clinically significant but fall short of the “target” of remission or low levels of disease activity. Just how far to push therapy in the individual patient remains an opportunity for rheumatologists to continue to practice the art of medicine. Clearly there are those patients in whom adding a biologic agent for one or two tender joints is not practical. As endocrinologists have taught us, tighter control is not always better (33). The TEAR trial design reflects the views of the investigators, in that escalation of MTX dosage was done unless remission had occurred (clinically defined in this trial as no tender or swollen joints), but escalation to combination therapy, either biologic or conventional, was done only if there was failure to attain a low disease state (DAS28-ESR >3.2).
The vast majority of RA clinical trials, including TEAR, have a clinical end point as their primary outcome, typically defined as an ACR20 response or DAS28-ESR response. This has lead at times to the confusing result that clinical outcomes, like ACR20 or DAS28-ESR responses, are not always completely concordant with radiographic outcomes, with perhaps the PREMIER trial being the best example (16). Those in the TEAR trial who continued to take MTX alone, by definition, did very well clinically; all achieved a DAS28-ESR of <3.2 by 24 weeks. As a group, they also did extremely well radiographically (mean ± SD change in modified Sharp score at 2 years 0.2 ± 1.1). However, several participants receiving MTX alone did experience radiographic progression, with 3 of these participants accounting for the majority of the damage in those who remained on MTX monotherapy. Identifying participants who do very well clinically but progress radiographically is important, in part because it is rare (< 1% of those randomized to MTX monotherapy in the TEAR trial), but this goal remains elusive.
Glucocorticoid therapy is clearly an effective adjunct to DMARD therapy in selected RA patients and has been shown to yield additional benefits to MTX therapy in patients with early disease (34). The TEAR trial, like most recent trials, allowed patients to be taking low-dose prednisone at study entry, but the use of steroids in RA was not examined in this trial, and we therefore cannot comment on this question. There was no difference in the response to treatment of those taking versus those not taking steroids.
The ‘holy grail’ of treatment in many diseases, and certainly in RA, is being able to predict at the outset and in a differential manner which patients will benefit most from a given intervention. To date, analyses of our data have not elucidated factors that are present at disease onset that would have allowed us to predict at the level of the individual patient who would respond best to MTX monotherapy and who needed combinations as initial therapy. We have published studies on factors that may be useful for predicting response at the 12 week time point, which could accelerate combination therapy in those who will eventually need it (35). Table 1 does show that the MTX responders were more likely to be male, have a lower BMI, and have a lower level of disease activity and less radiographic damage at baseline. All of these factors in addition to disease duration were associated with positive outcomes in the TEAR trial. However, none of these factors by themselves or in aggregate analyses preformed so far has proven powerful enough to be clinically useful predictors of response for the individual patient. In this trial, serial serum samples were collected and DNA was banked, and further analyses of these data are in progress.
Finally, the huge economic implications of our findings should not be overlooked. In this trial, 28% of participants who were randomized to receive MTX monotherapy had a very good outcome and did not need other potentially costly or toxic therapies. This, of course, means that many participants who initially received costly biologic agents did not need them to achieve a DAS28-ESR of <3.2. This proportion of participants with an excellent initial response to MTX is remarkably similar to that found in the open-label Swedish Pharmacotherapy (SWEFOT) (36) Behandelstrategieën voor Reumatoide Artritis (BeSt) (9,10) trials, where 30% of participants were found to do well with MTX monotherapy. In light of our findings that patients who ultimately need to step-up to combination therapy had equivalent outcomes to those who were randomized to combination therapy, the less expensive strategy of starting with MTX alone is clearly the most practical.
In summary, the TEAR trial has demonstrated that the strategy of initiating MTX monotherapy in patients with early, poor-prognosis RA results in clinical and radiographic outcomes that are similar to those seen when combinations of drugs are started at the beginning. This finding is in contrast to the current recommendations of the ACR (1) that call for patients with poor-prognosis RA to initially receive combination therapy and will need to be considered when these recommendations are updated. The subset of patients (≈30%) who maintain an excellent response to initial MTX monotherapy continue to do well clinically and radiographically after two years of treatment. Critical unanswered questions include: what should the ultimate treatment target be in early RA? and how do we, in a differential fashion, predict which patients will respond to which therapy so that the best therapy can be initiated from the outset in all patients?
Acknowledgments
Role of the Study Sponsor
The investigator-initiated TEAR trial used a planning grant from National Institute of Arthritis and Musculoskeletal and Skin Diseases. After completion of the planning process, Amgen Inc. provided funding for the TEAR trial itself. Amgen had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. The University of Alabama at Birmingham and the study investigators are solely responsible for all data collection, data management, statistical analysis, the decision to publish these results and manuscript development. Publication of this article was not contingent upon approval by Amgen.
References
- 1.Singh JA, Furst DE, Bharat A, et al. 2012 Update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care and Research. 2012;64:625–39. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69(6):964–75. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McInnes IB, O'Dell JR. Rheumatoid arthritis. Ann Rheum Dis. 2010;69:1898–906. doi: 10.1136/ard.2010.134684. [DOI] [PubMed] [Google Scholar]
- 4.O'Dell JR, Haire CE, Erikson N, et al. Treatment of rheumatoid arthritis with methotrexate alone, sulfasalazine and hydroxychloroquine, or a combination of all three medications. N Engl J Med. 1996;334(20):1287–91. doi: 10.1056/NEJM199605163342002. [DOI] [PubMed] [Google Scholar]
- 5.Mottonen T, Hannonen P, Leirisalo-Repo M, et al. Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. FIN-RACo trial group. Lancet. 1999;353(9164):1568–73. doi: 10.1016/s0140-6736(98)08513-4. [DOI] [PubMed] [Google Scholar]
- 6.Kremer J, et al. The combination of Leflunomide and Methotrexate in patients with active rheumatoid arthritis who are failing on methotrexate alone: A Double-blind placebo controlled study. Ann Int Med. 2002;137:726–33. doi: 10.7326/0003-4819-137-9-200211050-00007. [DOI] [PubMed] [Google Scholar]
- 7.O'Dell J, Leff R, Paulsen G, Haire C, Mallek J, Eckhoff PJ, Fernandez A, Blakely K, Wees S, Stoner J, Hadley S, Felt J, Palmer W, Waytz P, Churchill M, Klassen L, Moore G. Treatment of rheumatoid arthritis with methotrexate and hydroxychloroquine, methotrexate and sulfasalazine, or a combination of all three. Arthritis Rheum. 2002;46:1164–1170. doi: 10.1002/art.10228. [DOI] [PubMed] [Google Scholar]
- 8.Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364(9430):263–9. doi: 10.1016/S0140-6736(04)16676-2. [DOI] [PubMed] [Google Scholar]
- 9.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52(11):3381–90. doi: 10.1002/art.21405. [DOI] [PubMed] [Google Scholar]
- 10.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Comparison of treatment strategies in early rheumatoid arthritis: a randomized trial. Ann Intern Med. 2007;146(6):406. doi: 10.7326/0003-4819-146-6-200703200-00005. [DOI] [PubMed] [Google Scholar]
- 11.Tugwell P, Bombardier C, Gent M, et al. Low-dose cyclosporine versus placebo in patients with rheumatoid arthritis. Lancet. 1990;335:1051. doi: 10.1016/0140-6736(90)92630-z. [DOI] [PubMed] [Google Scholar]
- 12.O'Dell JR, Elliott JR, Mallek JA, et al. Treatment of early seropositive rheumatoid arthritis: doxycycline plus methotrexate versus methotrexate alone. Arthritis Rheum. 2006;54(2):621–7. doi: 10.1002/art.21620. [DOI] [PubMed] [Google Scholar]
- 13.Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675–81. doi: 10.1016/S0140-6736(04)15640-7. [DOI] [PubMed] [Google Scholar]
- 14.Emery P, Breedveld FC, Hall S, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet. 2008;372(9636):375–82. doi: 10.1016/S0140-6736(08)61000-4. [DOI] [PubMed] [Google Scholar]
- 15.Lipsky P, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. N. Engl J Med. 2000;343(22):1594–1602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- 16.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54(1):26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 17.Emery P, Fleischmann R, Filipowicz-Sosnowska A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 2006;54:1390–400. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]
- 18.Kremer JM, Russell AS, Emery P, et al. Long-term safety, efficacy and inhibition of radiographic progression with abatacept treatment in patients with rheumatoid arthritis and an inadequate response to methotrexate: 3-year results from the AIM trial. Ann Rheum Dis. 2011;70(10):1826–30. doi: 10.1136/ard.2010.139345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genovese MC, McKay JD, Nasonov EL, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968–80. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- 20.Boers M, Verhoeven AC, Markusse HM, et al. Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet. 1997;350(9074):309–18. doi: 10.1016/S0140-6736(97)01300-7. [DOI] [PubMed] [Google Scholar]
- 21.Landewe RB, Boers M, Verhoeven AC, et al. COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheum. 2002;46(2):347–56. doi: 10.1002/art.10083. [DOI] [PubMed] [Google Scholar]
- 22.Moreland L, O'Dell J, Paulus H, et al. A Randomized Comparative Effectiveness Study of Oral Triple Therapy versus Etanercept plus Methotrexate in Early, Aggressive Rheumatoid Arthritis: The TEAR Trial. Arthritis Rheum. 2012;64:2836–46. doi: 10.1002/art.34498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis & Rheumatism. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 24.van der Heijde D, Klareskog L, Boers M, Landewe R, Codreanu C, Bolosiu HD, et al. Comparison of different definitions to classify remission and sustained remission: 1 year TEMPO results. Ann Rheum Dis. 2005;64(11):1582–7. doi: 10.1136/ard.2004.034371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aletaha D, Landewe R, Karonitsch T, Bathon J, Boers M, Bombardier C, et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Arthritis Rheum. 2008;59(10):1371–7. doi: 10.1002/art.24123. [DOI] [PubMed] [Google Scholar]
- 26.van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 1999;26(3):743–5. [PubMed] [Google Scholar]
- 27.van der Heijde D, Klareskog L, Landewe R, Bruyn GA, Cantagrel A, Durez P, et al. Disease remission and sustained halting of radiographic progression with combination etanercept and methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 2007;56(12):3928–39. doi: 10.1002/art.23141. [DOI] [PubMed] [Google Scholar]
- 28.Kremer JM, Lee JK. The safety and efficacy of the use of methotrexate in long-term therapy for rheumatoid arthritis. Arthritis Rheum. 1986;29:822–31. doi: 10.1002/art.1780290702. [DOI] [PubMed] [Google Scholar]
- 29.Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum. 2011;63(3):573–86. doi: 10.1002/art.30129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Dell JR, Mikuls TR. To improve outcomes we must define and measure them: toward defining remission in rheumatoid arthritis. Arthritis Rheum. 2011;63(3):587–9. doi: 10.1002/art.30199. [DOI] [PubMed] [Google Scholar]
- 31.Anderson J, Caplan L, Yazdany J, Robbins ML, Neogi T, Michaud K, Saag KG, O'Dell JR, Kazi S. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care and Research. 2012;64:640–7. doi: 10.1002/acr.21649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American College of Rheumatology Rheumatoid Arthritis Clinical Trial Investigators Ad Hoc Task Force Arthritis Rheum; American College of Rheumatology Clinical Trial Priorities and Design Conference; July 22–23, 2010; 2011. pp. 2151–6. [DOI] [PubMed] [Google Scholar]
- 33.ACCORD Study Group. Gerstein HC, Miller ME, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818–28. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bakker MF, Jacobs JW, Welsing PM, Verstappen SM, Tekstra J, Ton E, et al. on behalf of the Utrecht Rheumatoid Arthritis Cohort Study Group Low-dose prednisone inclusion in a methotrexate-based, tight control strategy for early rheumatoid arthritis: a randomized trial. Ann Intern Med. 2012;156:329–39. doi: 10.7326/0003-4819-156-5-201203060-00004. [DOI] [PubMed] [Google Scholar]
- 35.Curtis JR, McVie T, Mikuls TR, O'Dell JR, Bridges SL, Jr, Moreland LW, Cofield S. Clinical response within 12 weeks as a predictor of future low disease activity in early RA patients: results from the TEAR trial. Arthritis Rheum. 2011;63(10 Suppl):S155. doi: 10.3899/jrheum.120715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Vollenhoven RF, Ernestam S, Geborek P, et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet. 2009;374(9688):459–66. doi: 10.1016/S0140-6736(09)60944-2. [DOI] [PubMed] [Google Scholar]