Abstract
Background
Clinical trials have demonstrated the benefit of implantable cardioverter-defibrillators (ICDs) for the primary prevention of sudden cardiac death in selected high-risk individuals. Because of small numbers of women enrolled in these trials, outcomes for women after hospital discharge have not been well described. We compared procedure-related complications and outcomes after hospital discharge between men and women undergoing single- or dual-chamber ICD implantation for primary prevention.
Methods
In patients 65 years or older with Medicare fee-for-service coverage, we identified 38,912 initial implants (25% women) who received single- or dual-chamber ICDs for primary prevention between January 2006 and December 2009 in the NCDR and evaluated gender differences in outcomes.
Results
Women had greater comorbidity and more advanced heart failure (HF) at the time of ICD implantation than did men. Device-related complications, death at 6 months, all-cause readmissions, and HF readmissions at 6 months were significantly more common in women (7.2% vs 4.8%, 6.5% vs 5.6%, 37.2% vs 31.7%, and 14.0% vs 10.0% respectively; P < .001 for all). Women continued to have higher odds of procedural complications (odds ratio [OR] 1.39, 95% CI 1.26–1.53, P < .001), 6-month all-cause readmission (OR 1.22, 95% CI 1.16–1.28, P < .001), and 6-month HF readmission (OR 1.32, 95% CI 1.23–1.42, P < .001), with a trend toward higher 6-month mortality (OR 1.08, 95% CI 0.98–1.20, P = .123), compared with men, after adjusting for differences in baseline characteristics and device type (single vs dual chamber).
Conclusions
Among older patients receiving ICDs for primary prevention in clinical practice, women experience worse outcomes than do men. Reasons for gender differences in outcomes are poorly understood and require further investigation.
Clinical trials have demonstrated the benefit of implantable cardioverter-defibrillator (ICD) therapy for the primary prevention of sudden cardiac death in selected high-risk patients.1–5 With expanding indications for ICDs and the large population of patients receiving this therapy in clinical practice, understanding outcomes beyond clinical trials is important.
In contemporary clinical practice, more than 1 in 4 persons undergoing primary prevention ICD implantation is female.6 However, data on gender differences from clinical trials are limited, as women comprise only 8% to 15% of subjects in primary prevention trials enrolling patients with ischemic heart disease and 23% to 30% of subjects in trials enrolling those with nonischemic heart disease.1–5 Previous studies comparing outcomes between men and women with primary prevention ICDs have produced inconsistent results.7–13 Varying results related to gender differences in complications after ICD implantation have also been described.7,10,14–16 However, prior work in this area is limited by a paucity of outcomes data in clinical practice beyond hospital discharge.
The NCDR ICD Registry provides a unique opportunity to examine outcomes in large numbers of men and women receiving ICDs in “real-world” clinical practice. The aim of this study is to compare intermediate-term outcomes between women and men in a large cohort of patients receiving single- or dual-chamber ICD therapy for primary prevention, including comparison of device-related complications, mortality, and all-cause and heart failure (HF) readmission rates.
Methods
Data source
Analyses in this study are based on data contained in the NCDR ICD Registry, which is a national database developed by the American College of Cardiology and the Heart Rhythm Society. The Centers for Medicare and Medicaid Services (CMS) published the National Coverage Decision in 2005 to expand ICD coverage. Implantable cardioverter-defibrillator implantations for primary prevention indications in Medicare beneficiaries are required to be included in this registry. However, 79% of hospitals submit data on all device recipients, regardless of age or device indication. This has resulted in reporting of data in 90% of all ICDs implanted in the United States at the time of the current study, and therefore, this registry provides the most comprehensive characterization of contemporary practice.17 Detailed demographic, clinical, and device data are collected for each ICD implantation procedure. Data quality procedures for the NCDR, which include audits of registry data compared with clinical records, have been previously described.18 We limited this analysis to patients 65 years or older with Medicare fee-for-service coverage. Longitudinal outcomes after ICD implantation were obtained by linking NCDR data with Medicare inpatient fee-for-service claims using probabilistic matching, which has been previously described.19
Patient population
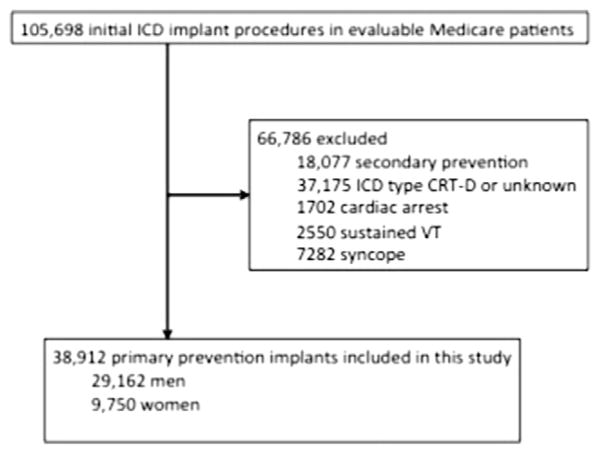
All patients 65 years or older who received an ICD from January 2006 to December 2009 in the NCDR ICD Registry who could be matched to CMS Medicare fee-for-service claims data were identified. A match was performed for the linkage using indirect identifiers of patients’ age, gender, admission date, discharge date, procedure date, and hospital Medicare provider number. The match rate in this study was 71%, which is similar to the match rate in previous NCDR publications in which the clinical records were matched to CMS claims. Previous investigation demonstrated that linked and unlinked CMS patients have similar demographic and clinical features, except for less commercial or health maintenance organization insurance in the linked cohort. 19 Patients were then excluded if they had a previous ICD or pacemaker, received an ICD for secondary prevention, or did not have 3 months of follow-up. Patients who received cardiac resynchronization therapy (CRT) were excluded due to changing adoption rates and changing guidelines related to CRT, as well as previously described gender differences in response to CRT. After excluding patients with CRT-ICD or if device type (single vs dual chamber) was missing, cardiac arrest, sustained ventricular tachycardia (VT), or syncope, 38,912 ICD implantation procedures for primary prevention indications were included in the current analysis (Figure 1).
Figure 1.
Study sample. There were 105,698 evaluable Medicare patients in the NCDR who underwent initial ICD implantation from 2006 to 2009 after matching with CMS claims data in the corresponding period. After excluding patients with secondary prevention indications, CRT, cardiac arrest, sustained VT, and syncope, 38,912 implant procedures for primary prevention indications were included in the current analysis.
Outcomes
Outcomes were identified using CMS inpatient claims, which were linked into the NCDR data and included device-related complications, all-cause readmission, HF readmission, and death. Readmission for HF was based on a primary discharge diagnosis of HF. For the outcomes of readmission and death, the 6-month period after implantation was examined.
Device-related complications were evaluated using the definition of the clinical performance measure developed for CMS in partnership with the American College of Cardiology and endorsed by the National Quality Forum (NQF). Device-related complications were included if they occurred within 30 or 90 days of implantation, with the period defined by type of complication, based on specifications used in the hospital-based performance measure previously approved by the NQF.20 Because the measure was developed for public reporting purposes, only the most serious complications were included. Based on input from a technical panel of experts convened during metric development, the time frames used for the assessment of each complication varied based on the extent to which the panel deemed it likely to be attributable to the ICD implantation procedure. Specifically, complications within 30 days include (a) pneumothorax requiring chest tube, (b) hematoma requiring blood transfusion or evacuation, (c) cardiac tamponade, or (d) death. Complications within 90 days include (a) mechanical complications requiring reoperation for system, generator, or lead revision; (b) device-related infection; or (c) recurrent ICD implant at 90 days (defined as any International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis code for subsequent ICD implant procedure within 90 days of the index procedure). The technical expert panel and developers of the CMS measure agreed that subsequent implantation of an ICD within 90 days of the initial implantation procedure would represent an unplanned event.
Statistical analysis
Baseline characteristics and outcomes were compared between men and women using the χ2 test for categorical variables and t test for continuous variables. Percentages and means (±SD) as well as the P value for comparisons are reported.
Multivariable hierarchical logistic regression models were used to examine the independent relationship between gender and outcomes, adjusting for possible confounders including relevant demographics, clinical factors, device type (single vs dual chamber), and patient clustering among hospitals. Factors included in the models were age, admission reason, family history of sudden death, HF history, New York Heart Association (NYHA) functional class, ischemic disease, prior percutaneous coronary intervention (PCI), prior coronary artery bypass graft surgery (CABG), prior myocardial infarction (MI), nonischemic cardiomyopathy, primary valvular disease, atrial fibrillation/flutter, nonsustained VT, cerebrovascular disease, chronic lung disease, dialysis, hypertension, diabetes mellitus, left ventricular ejection fraction (LVEF), QRS duration, device type (single vs dual chamber), and discharge medications. In the models, we imputed the missing values to the most common category for the categorical variables if the corresponding missing rate was less than 1%. Otherwise, we added missing values as a category for the categorical variable in the model. For a continuous variable with missing rate less than 1%, we imputed the missing value to its median; otherwise, we imputed the missing value to its median, created a dummy variable indicating the missing value, and added it into the model.
All analyses were performed using SAS 9.2 (Cary, NC). The Yale Human Investigation Committee approved the analysis and determined that informed consent was not applicable to the data collected by the Registry.
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
Results
Baseline characteristics
Women comprised 25% (n = 9,750) of the study cohort. Differences in baseline characteristics are summarized in Table I. Women were more likely to have a history of HF, more severe HF, lower LVEF, and nonischemic etiology for the cardiomyopathy than men (P < .001 for all). In addition, women were less likely to be admitted for the implantation procedure itself and were more likely to be hospitalized for a cardiac reason. Women also had a shorter mean QRS duration but were more likely to have a left bundle-branch block (LBBB) than men. They were also less likely to receive a dual-chamber device (as compared with single-chamber ICD) than men. Women were also less likely to be taking an angiotensin-converting enzyme inhibitor, aspirin, or statin and more likely to be taking an angiotensin receptor blocker or diuretic than men.
Table I.
Baseline characteristics, device type (single vs dual chamber), and discharge medications
| Men | Women | P | |
|---|---|---|---|
| n | 29,162 | 9750 | |
| Admission characteristics | |||
| Age (y), mean (SD) | 74.0 (6.0) | 74.1 (6.0) | .025 |
| Hospitalization reason | <.001 | ||
| Admitted for this procedure (%) | 70.2 | 65.6 | |
| Hospitalized—cardiac (%) | 11.2 | 15.2 | |
| History and risk factors | |||
| HF history (%) | 76.9 | 83.4 | <.001 |
| NYHA class III–IV (%) | 35.9 | 44.5 | <.001 |
| Class III (%) | 33.9 | 41.8 | |
| Class IV (%) | 2.0 | 2.6 | |
| Atrial fibrillation/flutter (%) | 34.9 | 30.2 | <.001 |
| Nonischemic DCM (%) | 19.5 | 34.4 | <.001 |
| Ischemic heart disease (%) | 80.9 | 64.8 | <.001 |
| Prior MI (%) | 65.9 | 52.2 | <.001 |
| Previous CABG (%) | 47.5 | 28.6 | <.001 |
| Previous PCI (%) | 38.6 | 33.0 | <.001 |
| Previous valvular surgery (%) | 6.1 | 7.3 | <.001 |
| Cerebrovascular disease (%) | 17.1 | 15.6 | <.001 |
| Chronic lung disease (%) | 23.9 | 24.4 | .239 |
| Diabetes (%) | 37.2 | 39.2 | <.001 |
| Hypertension (%) | 79.2 | 80.4 | .012 |
| Dialysis (%) | 3.9 | 3.8 | .502 |
| Diagnostics | |||
| LVEF <30% (%) | 54.4 | 58.2 | <.001 |
| QRS duration, mean (SD) | 114 (27) | 110 (27) | <.001 |
| QRS duration >140 ms (%) | 15.8 | 15.0 | .042 |
| LBBB (%) | 13.2 | 18.0 | <.001 |
| RBBB + fascicular block (%) | 10.1 | 5.4 | <.001 |
| Creatinine (mg/dL), mean (SD) | 1.4 (0.9) | 1.3 (1.0) | <.001 |
| Creatinine >2 mg/dL (%) | 8.8 | 7.2 | <.001 |
| BUN (mg/dL), mean (SD) | 25.1 (13.1) | 25.4 (14.3) | .031 |
| BUN >30 mg/dL (%) | 22.4 | 24.3 | <.001 |
| Sodium <135 (%) | 8.4 | 10.7 | <.001 |
| Systolic BP (mm Hg), mean (SD) | 133.3 (22.2) | 134.4 (23.6) | <.001 |
| Systolic BP <100 mm Hg (%) | 3.9 | 4.5 | .012 |
| ICD type | <.001 | ||
| Single chamber (%) | 36.0 | 37.9 | |
| Dual chamber (%) | 64.0 | 62.1 | |
| Discharge medications | |||
| β-Blocker (%) | 86.7 | 87.3 | .111 |
| ACE inhibitor (%) | 65.3 | 60.1 | <.001 |
| ARB (%) | 16.2 | 20.5 | <.001 |
| Diuretic (%) | 59.7 | 70.4 | <.001 |
| Any antiarrhythmic (%) | 11.8 | 11.3 | .238 |
| Aspirin (%) | 73.2 | 68.2 | <.001 |
| Statin (%) | 72.1 | 64.5 | <.001 |
Abbreviations: DCM, Dilated cardiomyopathy; RBBB, right bundle-branch block; BUN, blood urea nitrogen; BP, blood pressure; ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Device-related complications
Any device-related complication (as specified in the NQF-endorsed performance measure) was more common in women compared with men (7.2% vs 4.8%, P < .001) (Table II). After exclusion of 30-day mortality, device-related complications remained higher in women (5.9% vs 3.9%, P < .001) (Table II). In particular, women had a significantly higher rate of 30-day pneumothorax requiring intervention, hematoma requiring evacuation, cardiac tamponade, and 90-day mechanical complications requiring revision when compared with men. There was no gender difference in occurrence of 90-day device-related infection or recurrent ICD implant. After adjusting for baseline differences between men and women (including differences in demographics, medical history and risk factors, diagnostics, ICD type, and discharge medications), device-related complications remained higher in women (adjusted odds ratio [OR] 1.39 [95% CI 1.26–1.53, P < .001] and unadjusted OR 1.53 [95% CI 1.40–1.68, P < .001]) (Table III).
Table II.
Procedure-related complications by gender
| Men | Women | P | |
|---|---|---|---|
| n | 29,612 | 9750 | |
| Pneumothorax requiring chest tube, 30 d (%) | 0.37 | 0.89 | <.001 |
| Hematoma requiring transfusion or evacuation, 30 d (%) | 0.25 | 0.37 | .047 |
| Cardiac tamponade, 30 d (%) | 0.45 | 1.39 | <.001 |
| Mortality, 30 d (%) | 1.05 | 1.45 | .002 |
| Mechanical complications requiring revision, 90 d (%) | 1.71 | 2.40 | <.001 |
| Device-related infection, 90 d (%) | 0.66 | 0.68 | .874 |
| Post-index ICD, 90 d (%) | 0.81 | 0.86 | .621 |
| Complication, any of the above (%) | 4.84 | 7.20 | <.001 |
Table III.
Relationship of female gender to complications, mortality, and readmission
| Complications, 30 or 90 d
|
Mortality, 6 mo
|
All-cause readmission, 6 mo
|
HF readmission, 6 mo
|
|||||
|---|---|---|---|---|---|---|---|---|
| Description | OR | P | OR | P | OR | P | OR | P |
| Unadjusted | 1.53 (1.40–1.68) | <.001 | 1.18 (1.07–1.29) | .001 | 1.27 (1.21–1.33) | <.001 | 1.45 (1.35–1.55) | <.001 |
| Adjusted* | 1.39 (1.26–1.53) | <.001 | 1.08 (0.98–1.20) | .123 | 1.22 (1.16–1.28) | <.001 | 1.32 (1.23–1.42) | <.001 |
Adjusted for demographics (age and admission reason), medical history, and risk factors (family history of sudden death, chronic heart failure, NYHA class—current status, atrial fibrillation/atrial flutter, nonsustained VT, Nonischemic dilated cardiomyopathy, ischemic heart disease, previous MI, previous CABG, previous percutaneous coronary intervention, previous valvular surgery, cerebrovascular disease, chronic lung disease, diabetes, hypertension, renal failure-dialysis), diagnostics (LVEF, QRS, blood urea nitrogen), ICD type, and discharge medications.
All-cause mortality
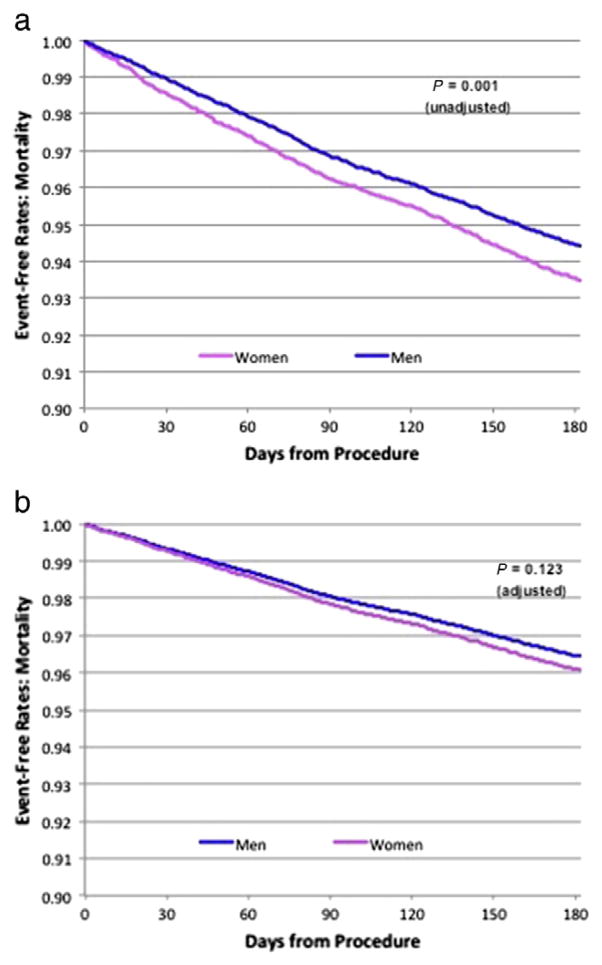
Women had higher 6-month mortality rates than did men (6.5% vs 5.6%, P < .001). The Kaplan-Meier curves illustrate the unadjusted and adjusted differences in mortality between men and women within 6 months (Figure 2). After adjusting for baseline differences between men and women (including differences in demographics, medical history and risk factors, diagnostics, ICD type, and discharge medications), there was a trend toward higher 6-month mortality in women (adjusted OR 1.08 [95% CI 0.98–1.20, P = .123] compared with unadjusted OR 1.18 [95% CI 1.07–1.29, P = .001]) (Table III).
Figure 2.
Mortality rate. The Kaplan-Meier curves illustrate the difference in mortality event-free rates, unadjusted (A) and adjusted (B), in men and women within 6 months. Women had higher unadjusted 6-month mortality rates than did men, with a trend toward higher mortality rates after adjusting for baseline differences.
All-cause and HF readmissions
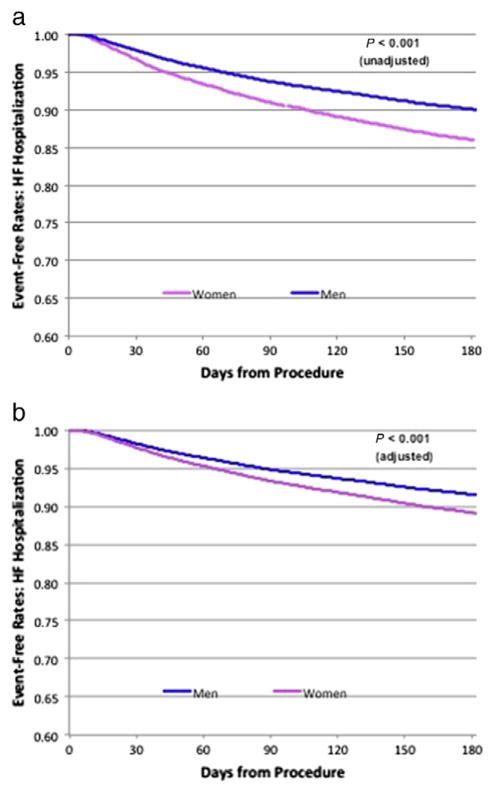
Heart failure readmission within 6 months occurred in 14.0% of women compared with 10.0% of men (P < .001) (Figure 3). After adjusting for baseline differences between men and women (including differences in demographics, medical history and risk factors, diagnostics, ICD type, and discharge medications), HF readmission rates at 6 months remained higher in women (adjusted OR 1.32 [95% CI 1.23–1.42, P < .001] and unadjusted OR 1.45 [95% CI 1.35–1.55, P < .001]) (Table III).
Figure 3.
Heart failure rehospitalization rate. The Kaplan-Meier curves illustrate the difference in unadjusted HF hospitalization event-free rates, unadjusted (A) and adjusted (B), in men and women within 6 months. HF rehospitalization rates at 6 months were higher in women than in men.
All-cause readmission within 6 months was also higher in women (37.2%) than in men (31.7% P < .001). These differences persisted after adjusting for baseline differences between men and women (adjusted OR 1.22 [95% CI 1.16–1.28, P < .001] and unadjusted OR 1.27 [95% CI 1.21–1.33, P < .001]) (Table III).
Although class III and class IV HF were significantly more frequent in women (Table I), it should be noted that differences in HF and all-cause readmissions persisted after controlling for HF severity.
Discussion
This study demonstrates gender differences in intermediate-term outcomes in older patients after initial ICD implantation for primary prevention indications. Women have (1) higher device-related complication rates, (2) higher 6-month all-cause and HF readmission rates, and (3) higher 6-month mortality rates compared with men. These gender differences in complication rates and readmission rates persisted after adjusting for differences in baseline characteristics and the type of ICD used.
Although randomized clinical trials reported outcomes of men and women receiving ICDs in a limited number of subjects meeting specific enrollment criteria, the current study focuses on real-world outcomes in 38,912 patients receiving ICDs in clinical practice. Importantly, the study cohort includes the largest number of women undergoing primary prevention ICD implantation to date. Finally, although prior studies have focused on in-hospital outcomes after ICD implantation procedures, the current article contributes to our understanding about gender differences in outcomes beyond initial hospitalization.
Procedure-related complications
In the current study, women had higher procedure-related complication rates within 90 days than did men. Several prior studies suggested no apparent gender differences in complication rates in men and women undergoing ICD implantation for secondary or primary prevention indications.7,14,15 In contrast, more recent data from real-world clinical practice in an ICD Registry from the United States revealed higher in-hospital complication rates in women; however, this earlier report from the NCDR was limited to in-hospital outcomes, whereas the current article provides information on outcomes beyond hospital discharge, including 6-month all-cause readmission, HF readmission, and mortality.16 Our findings are similar to a Canadian health payer-mandated prospective study of patients referred for ICD implantation, where women were significantly more likely than men to experience major complications up to 1 year after implantation.10 However, in contrast to the Canadian study, the current study demonstrates a higher risk of cardiac perforation or pneumothorax in women.
Reasons for the higher rate of complications in women are unclear. Vascular access may be more difficult in women due to smaller body size and smaller vessels, perhaps placing them at greater risk for pneumothorax. Women may also be at higher risk for perforation due to a thinner walled right ventricle. Although most complications are related to mechanical adverse events, delayed presentation in women or greater severity of illness may also place them at increased risk for anesthesia or implant-related complications.
HF readmission
In the current study, women had higher HF readmission rates than did men. Women appeared to be sicker at the time of initial ICD implantation than did men, with more severe HF (classes III–IV) and worse left ventricular function, at least partially explaining differences in outcome. Although adjusting for comorbidities and severity of HF did result in a slight reduction in the OR for HF readmission (adjusted OR 1.32 [95% CI 1.23–1.42, P < .001] and unadjusted OR 1.45 [95% CI 1.35–1.55, P < .001]), readmission rates at 6 months remained higher in women (Table III).
Prior studies have shown that nonischemic cardiomyopathy is more frequent in women than in men, also noted in the current study. However, controlling for cardiomyopathy etiology did not eliminate gender differences. Women were more likely to have LBBB, which has been associated with increased morbidity and mortality in HF.21 It is possible that CRT may have been underused in women, potentially contributing to worse outcome. In fact, a recent meta-analysis revealed that women actually derived greater benefit from CRT than did men.22 Additional study is needed to determine if there are gender differences in referral patterns or timing of device therapy, or if other unmeasured confounders (such as frailty) might contribute to these gender differences in outcomes.
Mortality
Gender differences in mortality after ICD implantation were noted in the current study, with higher 6-month mortality rates in women compared with men, and a trend persisted after adjusting for baseline differences and ICD type. Prior post hoc analyses of multicenter randomized trials have demonstrated varying results related to gender differences in mortality after ICD implantation.2,4,5,7–9,11,12 However, only a small number of women received ICDs in these clinical trials, ranging from <20 each in the Multicenter Unsustained Tachycardia Trial (MUSTT) and Multicenter Automatic Defibrillator Implantation Trial (MADIT) to 185 in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT).1,3,5
In contrast to the current study, other cohort studies have also demonstrated varying results related to mortality in men and women after ICD implantation. One study demonstrated a lower all-cause mortality in women than in men,23 whereas another study demonstrated no gender differences in mortality.10 Differences in baseline characteristics and study methodology may account for differences in outcomes between other cohort studies and the current NCDR study.
Previous investigation examining Medicare patients revealed a much lower mortality rate in patients who underwent same-day ICD implantation (25%) compared with those who were already hospitalized for more than 1 week prior to implantation (53%).24 In the current study, women were more likely to be admitted to the hospital for reasons other than device implantation, also suggesting greater severity or acuity of illness, perhaps contributing to gender differences in outcome.
Gender differences in mortality might be explained by differences in mode of death between men and women. Previous studies have shown that the incidence of sudden death is lower in women than in men,25,26 and women who present with out-of-hospital cardiac arrest demonstrate a lower incidence of VT/ventricular fibrillation compared with men.27–29 As the benefit of ICD therapy is likely related to reduced sudden death risk and effective treatment of VT/ventricular fibrillation, it is certainly feasible that women who receive an ICD may be more likely to die of nonarrhythmic or noncardiac causes compared with men who receive an ICD. Future studies should compare cardiac arrhythmic, cardiac nonarrhythmic, and noncardiac mortality among women and men who receive ICDs to help address this question.
Limitations
This study included only fee-for-service Medicare patients, so the results may not apply to younger patients. Although the current study examines gender differences in outcomes, we cannot examine potential gender differences in relative ICD benefit due the lack of a control group without an ICD. There are many differences between characteristics of men and women in the NCDR, suggesting more severe illness at the time of initial ICD implantation in women. Although we accounted for a wide range of baseline clinical variables, the possibility of residual unmeasured confounding cannot be excluded. It is also unclear why women seem to receive ICDs at a more advanced stage of illness, as data related to access to HF or ICD therapy and patient refusal are not available in this registry. More recent data illustrate the impact of device programming and unnecessary shocks on outcomes; however, information related to device programming and arrhythmic events during follow-up is not available in the current study.
In prior analysis of the NCDR ICD population, men were slightly more likely to receive non–evidence-based ICDs than women.30 In the current study, only individuals with LVEF ≤35% were included, and non–evidence-based ICD implantation was not examined in order to capture real-world experience, regardless of adherence to clinical guidelines. Although the possibility that gender-differences in nonindicated devices may have influenced the results cannot be excluded, prior investigation demonstrated that these differences were minimal (as 75.4% vs 74.5% of patients who received non–evidence-based vs evidence--based devices were men). Furthermore, the multivariable models should further minimize any potential impact of gender differences in non–evidence-based ICD usage.
The prevalence of a history of HF in this study was 77% to 83%, which appears somewhat low in a cohort of patients receiving ICDs for primary prevention, although this is similar to prior study from the NCDR.16 Although the American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines still consider ICD implantation in asymptomatic patients with left ventricular systolic dysfunction reasonable with a class IIb recommendation for NYHA class I patients,31 it is certainly possible that documentation of HF class may be limited in some medical records, and abstractors might have difficulty identifying functional class based on symptoms in the medical record. NCDR Registry audits demonstrated that participants had an average raw accuracy of data abstraction of 91.2% for the ICD Registry. Nonetheless, it is certainly possible that variability in documentation of different variables may exist, representing a limitation of registry studies.
Conclusions
Women receiving an ICD for primary prevention indications have higher device-related complication rates, higher 6-month all-cause and HF readmission rates, and a trend toward higher 6-month mortality rates compared with men. Although some of these differences may be due to women receiving ICDs at later stages of disease, further investigation to explore reasons for gender differences in outcomes is needed. Ongoing surveillance of ICD-related outcomes in clinical practice, focusing on gender differences in arrhythmic events and mode of death, or analyses of databases including patients with and without ICDs are recommended.
Footnotes
Disclosures
This research was supported by the American College of Cardiology Foundation’s NCDR. No additional extramural funding was used to support this work. The views expressed in this manuscript represent those of the author(s) and do not necessarily represent the official views of the NCDR or its associated professional societies identified at www.ncdr.com.
ICD Registry is an initiative of the American College of Cardiology Foundation and the Heart Rhythm Society.
References
- 1.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–40. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Zareba W, Hall J, et al. Prophylactic Implantation of a Defibrillator in Patients With Myocardial Infarction and Reduced Ejection Fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 3.Buxton AE, Lee KL, Fisher JD, et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med. 1999;341:1882–90. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 4.Kadish A, Dyer A, Daubert JP, et al. for the Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–8. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 5.Bardy GH, Lee KL, Mark DB, et al. for the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 6.Kremers MS, Hammill SC, Berul CI, et al. The National ICD Registry Report: version 2. 1 including leads and pediatrics for years 2010 and 2011. Heart Rhythm. 2013;10:e59–65. doi: 10.1016/j.hrthm.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 7.Russo AM, Poole JE, Mark DB, et al. Primary prevention with defibrillator therapy in women: results from the Sudden Cardiac Death in Heart Failure Trial. J Cardiovasc Electrophysiol. 2008;19:720–4. doi: 10.1111/j.1540-8167.2008.01129.x. [DOI] [PubMed] [Google Scholar]
- 8.Albert CM, Quigg R, Saba S, et al. Sex differences in outcome after implantable cardioverter defibrillator implantation in nonischemic cardiomyopathy. Am Heart J. 2008;156:367–72. doi: 10.1016/j.ahj.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Santangeli P, Pelargonio G, Dello A, et al. Gender differences in clinical outcome and primary prevention defibrillator benefit in patients with severe left ventricular dysfunction: a systemic review and meta-analysis. Heart Rhythm. 2010;7:876–82. doi: 10.1016/j.hrthm.2010.03.042. [DOI] [PubMed] [Google Scholar]
- 10.MacFadden DR, Crystal E, Krahn AD, et al. Sex differences in implantable cardioverter-defibrillator outcomes: findings from a prospective defibrillator database. Ann Intern Med. 2012;156:195–203. doi: 10.7326/0003-4819-156-3-201202070-00007. [DOI] [PubMed] [Google Scholar]
- 11.Russo AM, Stamato NJ, Lehmann MH, et al. Influence of gender on arrhythmia characteristics in the multicenter unsustained tachycardia trial. J Cardiovasc Electrophysiol. 2004;5:993–8. doi: 10.1046/j.1540-8167.2004.04050.x. [DOI] [PubMed] [Google Scholar]
- 12.Zareba W, Moss AJ, Jackson Hall W, et al. Clinical course and implantable cardioverter defibrillator therapy in postinfarction women with severe left ventricular dysfunction. J Cardiovasc Electrophysiol. 2005;16:1265–70. doi: 10.1111/j.1540-8167.2005.00224.x. [DOI] [PubMed] [Google Scholar]
- 13.Bhavnani SP, Pavuluri V, Coleman CI, et al. The gender-paradox among patients with implantable cardioverter-defibrillators: a propensity-matched study. Pacing Clin Electrophysiol. 2013;36:878–84. doi: 10.1111/pace.12141. [DOI] [PubMed] [Google Scholar]
- 14.Pires LA, Sethuraman B, Guduguntla VD, et al. Outcome of women versus men with ventricular tachyarrhythmias treated with the implantable cardioverter defibrillator. J Cardiovasc Electrophysiol. 2002:563–568. doi: 10.1046/j.1540-8167.2002.00563.x. [DOI] [PubMed] [Google Scholar]
- 15.Kudenchuk PJ, Bardy GH, Poole JE, et al. Malignant sustained ventricular tachyarrhythmias in women: characteristics and outcome of treatment with an implantable cardioverter defibrillator. J Cardiovasc Electrophysiol. 1997;8:2–10. doi: 10.1111/j.1540-8167.1997.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 16.Peterson PN, Daugherty SL, Wang Y, et al. Gender differences in procedure-related adverse events in patients receiving implantable cardioverter-defibrillator therapy. Circulation. 2009;119:1078–84. doi: 10.1161/CIRCULATIONAHA.108.793463. [DOI] [PubMed] [Google Scholar]
- 17.Hammill SC, Kremers MS, Stevenson LW, et al. Review of the Registry’s fourth year, incorporating lead data and pediatric ICD procedures, and use as a national performance measure. Heart Rhythm. 2010;7:1340–5. doi: 10.1016/j.hrthm.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Messenger JC, Ho KK, Young CH, et al. NCDR Science and Quality Oversight Committee Data Quality Workgroup: The National Cardiovascular Data Registry (NCDR) Data Quality Brief: the NCDR Data Quality Program in 2012. J Am Coll Cardiol. 2012;60:1484–8. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Brennan JM, Peterson ED, Messenger JC, et al. Linking the National Cardiovascular Data Registry CathPCI Registry with Medicare claims data: validation of a longitudinal cohort of elderly patients undergoing cardiac catheterization. Circ Cardiovasc Qual Outcomes. 2012;5:134–40. doi: 10.1161/CIRCOUTCOMES.111.963280. [DOI] [PubMed] [Google Scholar]
- 20.Hospital Risk-Standardized Complication Rate following Implantation of Implantable Cardioverter-Defibrillator (ICD), Measure #0694, steward= Centers for Medicare and Medicaid Services; http://www.qualityforum.org/QPS/QPSTool.aspx. [Google Scholar]
- 21.Reil JC, Robertson M, Ford I, et al. Impact of left bundle branch block on heart rate and its relationship to treatment with ivabradine in chronic heart failure. Eur J Heart Fail. 2013;15:1044–52. doi: 10.1093/eurjhf/hft072. [DOI] [PubMed] [Google Scholar]
- 22.Zusterzeel R, Selzman KA, Sanders WE, et al. Cardiac resynchronization therapy in women: US Food and Drug Administration meta-analysis of patient-level data. JAMA Intern Med. 2014;174:1340–8. doi: 10.1001/jamainternmed.2014.2717. [DOI] [PubMed] [Google Scholar]
- 23.van der Heijden AC, Thijssen J, Borleffs CJ, et al. Gender-specific differences in clinical outcome of primary prevention implantable cardioverter defibrillator recipients. Heart. 2013;99:1244–9. doi: 10.1136/heartjnl-2013-304013. [DOI] [PubMed] [Google Scholar]
- 24.Chen CY, Stevenson LW, Stewart GC, et al. Impact of baseline heart failure burden on post-implantable cardioverter-defibrillator mortality among medicare beneficiaries. J Am Coll Cardiol. 2013;61:2142–50. doi: 10.1016/j.jacc.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kannel WB, Schatzkin A. Sudden death: lessons from subsets in population studies. J Am Coll Cardiol. 1985;5:141B–9B. doi: 10.1016/s0735-1097(85)80545-3. [DOI] [PubMed] [Google Scholar]
- 26.Rho RW, Patton KK, Poole JE, et al. Important differences in mode of death between men and women with heart failure who would qualify for a primary prevention implantable cardioverter-defibrillator. Circulation. 2012;126:2402–7. doi: 10.1161/CIRCULATIONAHA.111.069245. [DOI] [PubMed] [Google Scholar]
- 27.Kim C, Fahrenbruch CE, Cobb LA, et al. Out-of-hospital cardiac arrest in men and women. Circulation. 2001;104:2699–703. doi: 10.1161/hc4701.099784. [DOI] [PubMed] [Google Scholar]
- 28.Wigginton JG, Pepe PE, Bedolla JP, et al. Sex-related differences in the presentation and outcome of out-of-hospital cardiopulmonary arrest: a multiyear, prospective, population-based study. Crit Care Med. 2002;30:S131–6. doi: 10.1097/00003246-200204001-00002. [DOI] [PubMed] [Google Scholar]
- 29.Herlitz J, Rundqvist S, Bång A, et al. Is there a difference between women and men in characteristics and outcome after in hospital cardiac arrest? Resuscitation. 2001;49:15–23. doi: 10.1016/s0300-9572(00)00342-7. [DOI] [PubMed] [Google Scholar]
- 30.Al-Khatib SM, Hellkamp A, Curtis J, et al. Non–evidence-based ICD implantations in the United States. JAMA. 2011;305:43–9. doi: 10.1001/jama.2010.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51:e1–62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]