Abstract
Background
There are few sex-specific outcome data in heart failure with preserved ejection fraction.
Methods and Results
We assessed sex differences in baseline characteristics and outcomes among 4128 patients with heart failure with preserved ejection fraction in the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Women (n=2491) with heart failure with preserved ejection fraction were ≈1 year older (72±7 years versus 71±7 years) and more likely to be obese (46% versus 35%) and have chronic kidney disease (34% versus 26%) and hypertension (91% versus 85%) than men but less likely to have an ischemic cause (19% versus 34%), atrial fibrillation (27% versus 33%), or chronic obstructive pulmonary disease (8% versus 13%) (all P<0.001). During a mean of 49.5 months, there were 881 deaths (447 in women, 434 in men; risk ratio, 0.64; 95% CI, 0.56–0.74) and 5776 hospitalizations (3239 in women, 2537 in men; risk ratio, 0.80; 95% CI, 0.76–0.84). Women had lower risk of all-cause events (deaths and hospitalizations), even after adjusting for baseline characteristics (adjusted hazards ratio, 0.81; 95% CI, 0.73–0.89). However, the sex-related difference in risk of all-cause events was modified in the presence or absence of atrial fibrillation, renal dysfunction, stable angina pectoris, or advanced New York Heart Association class symptoms.
Conclusions
In patients with typical heart failure with preserved ejection fraction, there were prominent sex differences in baseline characteristics and outcomes. Women had better overall prognosis, although the presence of 4 common baseline characteristics seemed to moderate this finding.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT000095238.
Keywords: aging, heart failure, sex, prognosis, preserved left ventricular function
Epidemiological studies have revealed striking sex-related differences in clinical presentation,1–5 risk factors,6,7 and prognosis8–10 of heart failure (HF). One of the most notable sex-related differences in HF is that most women have HF with preserved ejection fraction (HFPEF), an important disorder that is incompletely understood.11
Previous studies have been limited by retrospective design,12 underrepresentation of women, or exclusion of HFPEF.13 Three previous trials (DIG-PEF [Digitalis Intervention Group-Preserved Ejection Fraction],14 CHARM-Preserved [Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity: Preserved Ejection fraction],15 Pep-CHF [Perindopril in Elderly People with Chronic Heart Failure]16) have included sizeable numbers of patients with HFPEF but have been criticized for selecting patients who were not necessarily representative of patients with HFPEF seen in population-based studies.17 The Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial18 is the largest HFPEF trial to date and included patients (60% women) who closely resembled those with typical HFPEF as described in population-based epidemiological studies.1,2
Elucidating the effect of sex and risk factors on outcomes in HFPEF may advance fundamental understanding of the HFPEF syndrome and guide the design of tailored intervention strategies. Recognizing that a substantial proportion of events during follow-up in the I-PRESERVE trial were noncardiovascular events,19 in sharp contrast to the profile of events observed in HF studies of predominantly younger men, we hypothesized that women with HFPEF would be older and would have more noncardiovascular comorbidities at baseline compared with men, which could predispose to sex-related differences in outcomes in HFPEF. Accordingly, we aimed to test this hypothesis by examining the differences in clinical characteristics and prognosis in women and men with HFPEF from the large, prospective I-PRESERVE trial.
Methods
Sample
The study sample consisted of the 4128 patients enrolled in the I-PRESERVE trial.18 Inclusion criteria included age ≥60 years old, clinical signs and symptoms of HF, and a left ventricular ejection fraction ≥45%. The intervention was irbesartan 300 mg/day versus placebo. Although the primary trial results,18 mode of death,19 and HF-related quality of life20 have been previously published, where some results were stratified by sex and heterogeneity in the treatment effect by sex was not found, the detailed sex-specific analyses and hospitalization events presented here have not been previously reported.
Outcomes
The primary outcome of I-PRESERVE was a composite of all-cause death or the first hospitalization for a protocol-specified adjudicated cardiovascular hospitalization (defined as worsening HF, unstable angina, myocardial infarction, ventricular arrhythmia, atrial arrhythmia, or stroke). Secondary outcomes included cardiovascular death, all-cause mortality, HF mortality or hospitalization, and change in quality of life related to HF, New York Heart Association (NYHA) functional class, and N-terminal pro-B-type natriuretic peptide level in blood (Roche Elecsys assay). Outcomes (deaths and hospitalizations) were adjudicated by an independent Clinical Endpoint Committee using prespecified criteria that have been previously published.19 HF mortality was defined as death because of worsening or intractable HF, whereas HF hospitalization was defined as one with a primary diagnosis of worsening HF, where patients with worsening HF displayed symptoms and signs of HF, as well as diagnostic evidence, such as a significant increase in natriuretic peptides, radiographic congestion, or prerenal azotemia.
Statistical Analysis
Baseline variables were compared between men and women using 2-sided Student t tests or Wilcoxon rank-sum tests for continuous variables and Fisher exact tests or χ2 tests for categorical variables. For each outcome of interest, time to first event was recorded, whereas other events were censored. Cox proportional hazards analysis was used to model the effect of sex, baseline covariates (age, obesity, NHYA status, HF cause, HF hospitalization 6 months before baseline, history of hypertension, stable angina pectoris, myocardial infarction, percutaneous coronary intervention/coronary artery bypass surgery, atrial fibrillation, diabetes mellitus, stroke/transient ischemic attack, chronic obstructive pulmonary disease/asthma, valve disease, smoking, ejection fraction, heart rate, systolic blood pressure, hemoglobin, N-terminal pro-B-type natriuretic peptide, neutrophil count, glomerular filtration rate, and medications), as well as the interactions between sex and each baseline covariate. The analyses of interactions with sex focused on all-cause events because the results for cardiovascular and noncardiovascular events were similar, there were more all-cause events, and competing risks are incorporated into the end point. To help account for multiple comparisons, the threshold for statistical significance was P<0.01. Estimated P values are presented for readers who would like to make further adjustments, considering the number of tests done to compare women and men.
Results
Baseline Characteristics
Among 4128 elderly patients with HFPEF, 2491 (60%) were women, who were, on average, 1 year older than men (Table 1). Compared with men, women were more likely to be obese and have a history of hypertension; were less likely to have an ischemic cause, stable angina pectoris, previous myocardial infarction, atrial fibrillation, chronic obstructive pulmonary disease, or smoking; and had a similar prevalence of diabetes mellitus. At baseline, heart rate was higher and peripheral edema was more prevalent in women compared with men. HF-specific quality of life was worse in women compared with men. Women were more likely than men to have chronic kidney disease but less likely to be anemic (using differential hemoglobin cut points) and had lower N-terminal pro-B-type natriuretic peptide levels.
Table 1.
Baseline Clinical Characteristics by Sex
| Characteristic | Women (n=2491) | Men (n=1637) | P Value |
|---|---|---|---|
| Clinical | |||
| Age, y | 72±7 | 71±7 | <0.001 |
| Body mass index, kg/m2 | 30±6 | 29±5 | <0.001 |
| Obesity*, % | 46 | 35 | <0.001 |
| Heart failure cause, % ischemic | 19 | 34 | <0.001 |
| Stable angina pectoris, % | 38 | 43 | <0.001 |
| Myocardial infarction, % | 17 | 33 | <0.001 |
| PCI/CABG, % | 9 | 20 | <0.001 |
| Hypertension, % | 91 | 85 | <0.001 |
| Atrial fibrillation, % | 27 | 33 | <0.001 |
| Diabetes mellitus, % | 28 | 27 | 0.74 |
| Chronic obstructive pulmonary disease, % | 8 | 13 | <0.001 |
| Smoking, % | 9 | 32 | <0.001 |
| NYHA class II/III/IV, % | 20/77/2 | 22/75/3 | 0.006 |
| Hospitalization in the last 6 mo, % | 44 | 45 | 0.49 |
| Ejection fraction, % | 61±9 | 58±9 | <0.001 |
| Physical examination | |||
| Heart rate, bpm | 72±11 | 71±10 | 0.003 |
| Systolic blood pressure, mm Hg | 137±15 | 136±15 | 0.14 |
| Diastolic blood pressure, mm Hg | 79±9 | 79±9 | 0.80 |
| S3 gallop, number, % | 8 | 9 | 0.10 |
| Jugular venous distension, % | 7 | 10 | 0.008 |
| Hepatomegaly, % | 17 | 20 | 0.16 |
| Edema, % | 25 | 21 | 0.002 |
| Rales, % | 25 | 24 | 0.43 |
| Quality of life | |||
| Minnesota living with heart failure score | 45±21 | 39±21 | <0.001 |
| Investigations | |||
| Radiological pulmonary congestion, % | 42 | 40 | 0.001 |
| Median (Q1–Q3) NT-pro-BNP, pg/mL | 301 (126–897) | 413 (155–1051) | <0.001 |
| Hemoglobin, g/dL | 13.5±1.8 | 14.5±1.9 | <0.001 |
| Anemia†, % | 11 | 16 | <0.001 |
| Estimated glomerular filtration rate, mL/min per 1.73 m2 | 70.8±22.1 | 75.2±22.9 | <0.001 |
| Chronic kidney disease‡, % | 34 | 26 | <0.001 |
| Potassium, μmol/L | 4.4±0.5 | 4.5±0.5 | 0.10 |
| Medications | |||
| Loop diuretic, % | 51 | 53 | 0.08 |
| Thiazide diuretic, % | 41 | 34 | <0.001 |
| Spironolactone, % | 15 | 17 | 0.08 |
| Angiotensin-converting enzyme inhibitor, % | 23 | 29 | <0.001 |
| Digoxin, % | 12 | 16 | 0.006 |
| β-Blocker, % | 59 | 59 | 0.93 |
| Antiarrythmic, % | 8 | 11 | 0.003 |
| Calcium channel blocker, % | 42 | 37 | <0.001 |
| Nitrate, % | 25 | 30 | <0.001 |
| Oral anticoagulant, % | 55 | 64 | <0.001 |
| Aspirin, % | 52 | 59 | <0.001 |
| Lipid lowering, % | 28 | 35 | <0.001 |
PCI/CABG indicates percutaneous coronary intervention/coronary artery bypass surgery; NYHA, New York Heart Association; bpm, beats per minute; NT-pro-BNP, N-terminal pro-B-type natriuretic peptide.
Data are mean±SD.
Obesity defined as body mass index ≥30 kg/m2.
Anemia defined as hemoglobin ≤13 g/dL in men and ≤12 g/dL in women.
Chronic kidney disease defined as estimated glomerular filtration rate <60 mL/min per 1.73 m2.
Association Between Sex and Outcomes
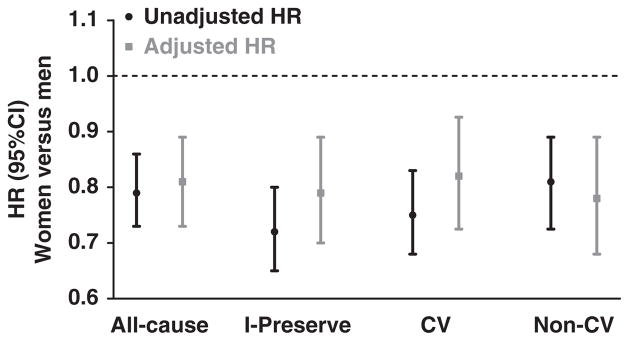
In time-to-first event (death or hospitalization) analyses, there were 2430 all-cause events during a mean follow-up of 49.5 months, of which 1754 were of cardiovascular causes (Table 2; Figure 1). Women had a 21% lower unadjusted risk of all-cause events than men, which persisted after adjusting for the differences in baseline characteristics. As with all-cause events, the unadjusted risk of the primary outcome of I-PRESERVE (all-cause death or hospitalization for protocol-specified cardiovascular causes) was 28% lower in women than men, remaining significant (albeit attenuated) after adjusting for baseline covariates. Similarly, in women compared with men, the unadjusted risk of cardiovascular events alone was 25% lower, and the unadjusted risk of noncardiovascular events was 19% lower, with the difference remaining significant even after adjusting for baseline covariates. There was a trend for lower risk of HF-specific events in women than men who did not reach statistical significance. For all-cause mortality, there were a total of 881 deaths (447 [17.9%] in women, 434 [26.5%] in men; unadjusted hazards ratio, 0.64; 95% CI, 0.56–0.73).
Table 2.
Association Between Sex and Time to First Outcomes
| Outcome | No. of Events
|
Event Rate Per 100 Patient-Years
|
Univariable Analysis
|
Multivariable Analysis*
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| All Patients | Women | Men | Women | Men | HR (95% CI), Women vs Men | P Value | HR (95% CI), Women vs Men | P Value | |
| All-cause death | 881 | 447 | 434 | 4.32 | 6.72 | 0.64 (0.56–0.73) | <0.001 | 0.70 (0.59–0.83) | <0.001 |
| All-cause hospitalization or death | 2430 | 1382 | 1049 | 19.42 | 25.05 | 0.79 (0.73–0.86) | <0.001 | 0.80 (0.72–0.89) | <0.001 |
| Cardiovascular hospitalization or death | 1754 | 970 | 784 | 11.76 | 15.97 | 0.75 (0.68–0.83) | <0.001 | 0.81 (0.72–0.92) | 0.001 |
| Noncardiovascular hospitalization or death | 1483 | 846 | 638 | 9.89 | 12.40 | 0.81 (0.72–0.89) | <0.001 | 0.78 (0.69–0.90) | <0.001 |
| Heart failure hospitalization or death | 716 | 420 | 296 | 4.43 | 5.02 | 0.89 (0.77–1.04) | 0.140 | 0.94 (0.77–1.14) | 0.51 |
| First all-cause hospitalization | 2278 | 1314 | 964 | 18.43 | 23.14 | 0.82 (0.75–0.88) | <0.001 | 0.77 (0.66–0.89) | <0.001 |
HR indicates hazards ratio; HF, heart failure; PCI/CABG, percutaneous coronary intervention/coronary artery bypass surgery; TIA, transient ischemic attack; COPD, chronic obstructive pulmonary disease; NT-pro-BNP, N-terminal pro-B-type natriuretic peptide.
Adjusted for age, obesity, New York Heart Association status, HF cause, HF hospitalization within 6 mo, comorbidities/risk factors (history of hypertension, stable angina, myocardial infarction, PCI/CABG, atrial fibrillation, diabetes, stroke/TIA, COPD/asthma, valve disease, smoking), ejection fraction capped at 60%, heart rate, systolic blood pressure, hemoglobin, ln-NT-pro-BNP, natural log-neutrophil count, glomerular filtration rate capped at 90 mL/min per 1.73 m2, and all medications.
Death from any cause or hospitalization for protocol-specified cardiovascular cause (HF, myocardial infarction, arrhythmia, or stroke).
Figure 1.
Association between sex and time to first event. Hazard ratios (HRs) for women versus men for first events, where HR <1 indicates lower risk in women. Event categories include the following: all cause (all-cause death or hospitalization); I-PRESERVE (the primary outcome of the Irbesartan in Heart Failure with Preserved Ejection Fraction trial, which was all-cause death or hospitalization for protocol-specified cardiovascular cause, including heart failure, myocardial infarction, unstable angina, arrhythmia, or stroke); CV (cardiovascular events); and non-CV (noncardiovascular events). Black circles and lines represent unadjusted HR and 95% CI. Gray squares and lines represent HR and 95% CI adjusted for age, obesity, New York Heart Association status, heart failure (HF) cause, HF hospitalization within 6 months, comorbidities/risk factors (history of hypertension, stable angina pectoris, myocardial infarction, percutaneous coronary intervention/coronary artery bypass surgery, atrial fibrillation, diabetes mellitus, stroke/transient ischemic attack, chronic obstructive lung disease, valve disease, smoking), ejection fraction, heart rate, systolic blood pressure, hemoglobin, N-terminal pro-B-type natriuretic peptide, neutrophil count, glomerular filtration rate, and medications.
Table 3 shows the total events in women and men. In both women and men with HFPEF, there were more deaths from cardiovascular than noncardiovascular causes, with the most common cardiovascular cause of death being sudden death. Noncardiovascular causes comprised 29.1% of all deaths in women and 31.8% of all deaths in men. There were a total of 5776 all-cause hospitalizations (including repeat admissions; 3239 in women and 2537 in men). In both women and men, there were more hospitalizations for cardiovascular than noncardiovascular causes, with the most common cardiovascular cause of hospitalization being worsening HF. Noncardiovascular causes comprised 45.1% of hospitalizations in women and 44.5% of hospitalizations in men. The risk ratio for overall hospitalizations in women versus men was 0.80 (95% CI, 0.76–0.84). For cardiovascular and noncardiovascular hospitalizations, risk was similarly lower in women, with risk ratios of 0.81 (0.75–0.87) and 0.78 (0.73–0.86), respectively. Virtually, all hospitalization subcategories were also lower in women than men.
Table 3.
Total Mortality and Hospitalizations and Causes in Women and Men
| Events | Numbers (%) of Events*
|
Event Rate Per 100 Patient-Years of Follow-Up†
|
||||
|---|---|---|---|---|---|---|
| Women (n=2491) | Men (n=1637) | Women | Men | Risk Ratio (95% CI) | P Value | |
| Deaths | ||||||
| All-cause deaths | 447 | 434 | 4.32 | 6.72 | 0.64 (0.56–0.74) | <0.0001 |
| Noncardiovascular deaths | 130 (29.1) | 138 (31.8) | 1.26 | 2.14 | 0.59 (0.46–0.75) | <0.0001 |
| Cardiovascular deaths | 260 (58.2) | 272 (62.7) | 2.51 | 4.21 | 0.60 (0.50–0.71) | <0.0001 |
| Sudden death | 104 (23.3) | 127 (29.3) | 1.01 | 1.97 | 0.51 (0.39–0.67) | <0.0001 |
| Pump failure | 62 (13.9) | 63 (14.5) | 0.6 | 0.98 | 0.61 (0.43–0.89) | 0.007 |
| Stroke | 45 (10.1) | 31 (7.1) | 0.44 | 0.48 | 0.91 (0.56–1.48) | 0.67 |
| Myocardial infarction | 23 (5.1) | 22 (5.1) | 0.22 | 0.34 | 0.65 (0.34–1.23) | 0.16 |
| Other vascular death | 17 (3.8) | 15 (3.5) | 0.16 | 0.23 | 0.70 (0.33–1.52) | 0.33 |
| Other cardiac death | 9 (2.0) | 14 (3.2) | 0.09 | 0.22 | 0.40 (0.15–0.99) | 0.033 |
| Unknown/unclassified deaths | 57 (12.8) | 24 (5.5) | 0.55 | 0.37 | 1.48 (0.91–2.50) | 0.1 |
| Hospitalizations | ||||||
| All-cause hospitalizations | 3239 | 2537 | 31.33 | 39.29 | 0.80 (0.76–0.84) | <0.0001 |
| Noncardiovascular hospitalizations | 1462 (45.1) | 1130 (44.5) | 14.1 | 17.5 | 0.81 (0.75–0.87) | <0.0001 |
| Cardiovascular hospitalizations | 1761 (54.4) | 1407 (55.5) | 17.0 | 21.8 | 0.78 (0.73–0.86) | <0.0001 |
| Worsening heart failure | 736 (22.7) | 506 (19.9) | 7.1 | 7.8 | 0.91 (0.81–1.02) | 0.098 |
| Cardiovascular procedure | 147 (4.5) | 182 (7.2) | 1.42 | 2.81 | 0.50 (0.40–0.63) | <0.0001 |
| Unstable angina/MI | 196 (6.1) | 162 (6.4) | 1.90 | 2.50 | 0.76 (0.61–0.94) | 0.009 |
| Arrhythmia | 166 (5.1) | 117 (4.6) | 1.61 | 1.81 | 0.89 (0.70–1.13) | 0.32 |
| Chest pain | 131 (4.0) | 122 (4.8) | 1.27 | 1.89 | 0.67 (0.52–0.87) | 0.002 |
| Stroke/transient ischemic attack | 126 (3.9) | 105 (4.1) | 1.22 | 1.63 | 0.75 (0.57–0.98) | 0.03 |
| Other cardiac | 79 (2.4) | 53 (2.1) | 0.76 | 0.82 | 0.93 (0.65–1.34) | 0.68 |
| Peripheral vascular disease | 59 (1.8) | 57 (2.2) | 0.57 | 0.88 | 0.65 (0.44–0.95) | 0.02 |
| Syncope | 42 (1.3) | 61 (2.4) | 0.41 | 0.94 | 0.43 (0.28–0.65) | <0.0001 |
| Hypertension | 58 (1.8) | 16 (0.6) | 0.56 | 0.25 | 2.26 (1.28–4.22) | 0.002 |
| Hypotension | 19 (0.6) | 19 (0.7) | 0.18 | 0.29 | 0.62 (0.31–1.25) | 0.15 |
| Sudden death | 2 (0.1) | 1 (<0.1) | 0.02 | 0.015 | 1.25 (0.07–73.7) | 0.9 |
MI indicates myocardial infarction.
Numbers represent absolute numbers of events (% of total events in women and men), including multiple hospitalizations per patient.
Numbers represent rates of death and first admission to hospital for specified causes per 100 patient-years of follow-up.
Sex Differences in the Predictors of All-Cause Events
In sex-stratified multivariable analyses, including all baseline covariates, variables associated with all-cause events in both men and women included higher age, HF hospitalization within 6 months, history of chronic obstructive pulmonary disease, lower hemoglobin, and higher N-terminal pro-B-type natriuretic peptide (Table 4). In women, obesity, diabetes mellitus, lower glomerular filtration rate, and anti-arrythmic medications were also significantly associated with higher risk of all-cause events. In men, higher NYHA status (class III/IV versus I/II), a history of coronary revascularization, higher neutrophil count, and smoking were also significantly associated with higher risk of all-cause events.
Table 4.
Sex Differences in the Multivariable Predictors of All-Cause Events
| Variable | HR (95% CI); P Value
|
P Value Interaction | |
|---|---|---|---|
| Women | Men | ||
| Age | 1.024 (1.014–1.035); <0.001 | 1.025 (1.013–1.037); <0.001 | 0.45 |
| Obesity | 1.210 (1.063–1.377); 0.004 | 1.136 (0.969–1.330); 0.115 | 0.52 |
| NYHA (class III/IV vs I/II) | 1.019 (0.852–1.220); 0.835 | 1.329 (1.076–1.640); 0.008 | 0.006 |
| HF cause (ischemic vs nonischemic) | 1.046 (0.868–1.262); 0.635 | 1.159 (0.956–1.406); 0.134 | 0.054 |
| Hypertension | 0.845 (0.677–1.055); 0.138 | 0.999 (0.813–1.228); 0.990 | 0.41 |
| Stable angina pectoris | 1.020 (0.880–1.182); 0.795 | 1.117 (0.947–1.317); 0.191 | 0.007 |
| Myocardial infarction | 1.035 (0.859–1.247); 0.718 | 1.040 (0.867–1.248); 0.669 | 0.13 |
| PCI/CABG | 1.107 (0.870–1.408); 0.408 | 1.242 (1.011–1.525); 0.039 | 0.96 |
| Atrial fibrillation | 1.115 (0.928–1.341); 0.244 | 0.834 (0.680–1.022); 0.080 | 0.005 |
| Diabetes mellitus | 1.454 (1.128–1.872); 0.004 | 1.178 (0.851–1.631); 0.323 | 0.49 |
| Smoking | 1.117 (0.905–1.378); 0.301 | 1.208 (1.035–1.410); 0.016 | 0.58 |
| Chronic obstructive lung disease | 1.351 (1.081–1.689); 0.008 | 1.301 (1.054–1.606); 0.014 | 0.59 |
| Valve disease | 1.117 (0.925–1.349); 0.248 | 1.140 (0.909–1.431); 0.257 | 0.47 |
| Heart rate (per 1 bpm) | 1.004 (0.998–1.010); 0.247 | 1.004 (0.996–1.011); 0.336 | 0.97 |
| SBP (per 1 mm Hg) | 1.000 (0.996–1.005); 0.889 | 1.000 (0.995–1.005); 0.917 | 0.90 |
| Hemoglobin (per 1 g/dL) | 0.962 (0.929–0.996); 0.029 | 0.946 (0.912–0.982); 0.003 | 0.95 |
| NT-pro-BNP (per 1 log unit) | 1.172 (1.085–1.265); <0.001 | 1.174 (1.077–1.281); ≤0.001 | 0.44 |
| Hospitalization in 6 mo | 1.447 (1.240–1.690); <0.001 | 1.411 (1.171–1.700); <0.001 | 0.049 |
| Neutrophil count (per 1 log unit) | 1.154 (0.966–1.377); 0.114 | 1.634 (1.309–2.041); <0.001 | 0.048 |
| Ejection fraction | 0.985 (0.972–0.999); 0.033 | 0.992 (0.977–1.007); 0.281 | 0.70 |
| eGFR (per 1 mL/min per 1.73 m2) | 0.991 (0.987–0.994); <0.001 | 0.997 (0.993–1.002); 0.211 | 0.01 |
| Antiarrythmic | 1.320 (1.052–1.656); 0.016 | 1.036 (0.806–1.333): 0.781 | 0.004 |
HR indicates hazards ratio; NYHA, New York Heart Association; HF, heart failure; PCI/CABG, percutaneous coronary intervention/coronary artery bypass surgery; bpm, beats per minute; SBP, systolic blood pressure; NT-pro-BNP, N-terminal pro-B-type natriuretic peptide; eGFR, estimated glomerular filtration rate.
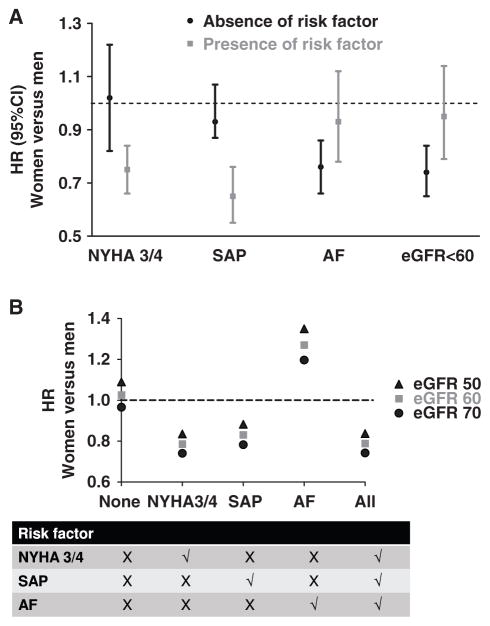
To gain further insights into the hazards ratio for all-cause events in women relative to men, we tested for interactions between sex and baseline variables. There were significant interactions between sex and 4 baseline characteristics: stable angina pectoris, atrial fibrillation, NYHA class, and estimated glomerular filtration rate (Table 4 last column). The effect of each interaction, analyzed one at a time, is shown in Figure 2A. The risk of all-cause events was not lower in women in the presence of atrial fibrillation or renal dysfunction and in the absence of advanced NYHA class III/IV symptoms or stable angina pectoris. To show how the women-to-men all-cause hazards ratios varied with all 4 interacting variables, we entered different values of the interacting variables into the estimated Cox regression model as shown in Figure 2B. There was also a significant interaction between sex and antiarrythmic medications, but numbers were too small for further analyses.
Figure 2.
Effect of interactions on association between sex and all-cause events. The y axis indicates hazards ratios (HRs) for women versus men for all-cause events where HR <1 indicates lower risk in women. A, Results of univariable analyses showing the HRs in the absence (black) or presence (gray) of specific risk factors, including New York Heart Association (NYHA) class 3 or 4, stable angina pectoris (SAP), atrial fibrillation (AF), and reduced estimated glomerular filtration rate (eGFR). B, Results of multivariable analyses accounting for all 4 significant interactions and adjusting for age, obesity, NYHA status, heart failure (HF) cause, HF hospitalization within 6 months, comorbidities/ risk factors (history of hypertension, SAP, myocardial infarction, percutaneous coronary intervention/ coronary artery bypass surgery, AF, diabetes mellitus, stroke/transient ischemic attack, chronic obstructive lung disease, valve disease, smoking), ejection fraction, heart rate, systolic blood pressure, hemoglobin, N-terminal pro-B-type natriuretic peptide, neutrophil count, glomerular filtration rate, and medications. The table (bottom) indicates situations where specific risk factors are present (cross) or absent (tick) at levels of estimated eGFR of 70 mL/min per 1.73 m2 (black circles), 60 mL/min per 1.73 m2 (gray squares), and 50 mL/min per 1.73 m2 (black triangles).
Discussion
In this large sample of women and men with HFPEF, there were notable sex-related differences in baseline risk factors. Women were more likely to be obese and have a history of hypertension or renal impairment and were only ≈1 year older than men. Men were more likely to have an ischemic cause for HFPEF, atrial fibrillation, chronic obstructive pulmonary disease, and anemia. Even accounting for these baseline differences, women with HFPEF were ≈20% less likely than men to experience death or hospitalization of any cause during follow-up. A lower risk was observed in women for both cardiovascular and noncardiovascular events. This lower relative risk for women could not be explained by adjustment for differences in baseline characteristics. However, the sex-related difference in risk of all-cause events was modified by atrial fibrillation, stable angina pectoris, NYHA class, and renal function.
Prior reports from HF trials that examined sex differences were largely limited to HF with reduced ejection fraction and included relatively small numbers of women.13 I-PRESERVE is the largest prospective trial of HFPEF to date and the first in which the majority of patients were women, reflecting its prevalence in the population. The number of women in I-PRESERVE (n=2491) was more than twice that of CHARM-Preserved, the largest previously reported trial of HFPEF. Furthermore, the baseline characteristics of the I-PRESERVE patients closely resembled those with HFPEF described in population-based studies.1,2 In contrast, in CHARM-Preserved there was a dominance of men, coronary artery disease, and relatively low mean ejection fraction in patients who were, on average, younger than those seen in population and community studies. This was also the case for the report from the earlier DIG [Digitalis Intervention Group] trial.14
These are important considerations because age, sex, comorbidities, and outcomes are closely related in HF: women with HF tend to be older than men with HF, leading to the speculation that women with HFPEF, being older, would have more noncardiovascular comorbidities, thus predisposing them to a higher rate of noncardiovascular events than men.21 Our findings are contrary to this hypothesis. In this sample, the age distributions were similar between women and men with HFPEF, and the risk of noncardiovascular events was lower in women, even when adjustments were made for several significant sex differences in the baseline characteristics. It is notable that noncardiovascular events were common (although cardiovascular events predominated), with ≈30% of all deaths and 45% of hospitalizations because of noncardiovascular causes. These rates of noncardiovascular outcomes are similar to those reported from population-based studies and Medicare claims data of HFPEF21–26 and far exceed the rates of noncardiovascular events typically seen in patients with HF with reduced ejection fraction where men predominate.27
Our analyses of I-PRESERVE data indicate that among elderly patients with HFPEF, women, in general, had a lower risk of adverse clinical events than men. These results are consistent with previous reports, including the recently published Meta-Analysis Global Group In Chronic Heart Failure (MAGGIC) meta-analysis,28 where women with HF were shown to have lower all-cause mortality over 3 years than men, irrespective of ejection fraction and even after accounting for baseline differences in risk factors. Similarly, in the overall CHARM program, first cardiovascular events were lower in women than men, regardless of ejection fraction. However, our results differ from CHARM because all-cause and noncardiovascular hospitalization risks for women were also lower than in men in our cohort. Sex-specific data in HFPEF alone from CHARM-Preserved were displayed but were not analyzed in depth,29 and the estimated sex-specific event rates did not seem to be lower in women. Of note, unlike in CHARM-Preserved, we used the composite end point of death or hospitalization. We, therefore, extend the previously published data by showing that lower risk with typical HFPEF persisted in women than men using composite outcomes for mortality, as well as hospitalizations, and was observed not only for first events but also for multiple all-cause, cardiovascular, and noncardiovascular events experienced throughout the entire 49-month period of follow-up.
We further examined whether the relative risk in women versus men depended on the values of specific baseline characteristics. Atrial fibrillation and renal dysfunction seemed to carry greater risk in women than men. Previous studies have shown that women are more likely than men to experience symptomatic episodes of atrial fibrillation, higher heart rates during episodes, and a higher frequency of recurrences.30 Although further research is needed to understand these sex differences in risk, potential reasons may include the lower rate of treatment with statins31 or the higher rate of obesity among women compared with men in our cohort, both of which may predispose women to more severe arrhythmic episodes (though not necessarily greater prevalence of disease). The coexistence of renal dysfunction in HFPEF is common but poorly understood and often under-diagnosed.32 Despite recognition of renal dysfunction as an important predictor of outcomes in HFPEF among women,33 the sex difference in the extent of risk imparted by similar degrees of renal dysfunction has not been widely appreciated previously. The differential impact of symptoms of angina and HF on women versus men may relate to known difficulties of interpreting symptoms of ischemic heart disease in women, which are more often atypical in women than men, or differences in perception of symptoms between women and men. Although these data extend previous reports, we agree with other commentators28 that there remains uncertainty regarding the interaction between HF cause and sex-related outcomes, as well as the need for further studies to understand this relationship.
Limitations
Our study was performed in the context of a clinical trial, which may limit the generalizability of our findings. However, the I-PRESERVE cohort at baseline closely resembled the patients with typical HFPEF described in population-based epidemiological studies.1,2 Furthermore, I-PRESERVE had the largest group of women with HFPEF in a prospective study of HFPEF and included detailed characterization, systematic long-term follow-up, and adjudication of outcomes. As in all studies, we cannot definitively exclude that some of the outcomes that seemed to be sex related were instead because of other unmeasured factors or chance, given the number of comparisons. Thus, these results should be interpreted with caution and confirmed in future studies.
Conclusion
There were several sex differences in elderly patients with typical HFPEF in I-PRESERVE. Women had better overall prognosis than men and were at lower risk of both cardiovascular and noncardiovascular events, although this effect was modified by the presence or absence of atrial fibrillation, renal dysfunction, stable angina pectoris, or advanced NYHA class symptoms. Further research is needed to understand the complex sex-related differences in risk among patients with HF. A better understanding of sex-specific risk factors may help inform strategies aimed at improving outcomes in this important disorder.
CLINICAL PERSPECTIVE.
Epidemiological studies have revealed sex-related differences in clinical presentation, risk factors, and prognosis of heart failure (HF). One of the most notable sex-related differences in HF is that most women have HF with preserved ejection fraction (HFPEF). However, there are few sex-specific outcome data for HFPEF. We assessed sex differences in baseline characteristics and outcomes among 4128 patients with HFPEF in the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. There were notable sex-related differences in baseline risk factors. Women were more likely to be obese and have a history of hypertension or renal impairment and were only ≈1 year older than men. Men were more likely to have an ischemic cause for HFPEF, atrial fibrillation, chronic obstructive pulmonary disease, and anemia. Even accounting for these baseline differences, women with HFPEF were ≈20% less likely than men to experience death or hospitalization for any cause during follow-up. A lower risk was observed in women for both cardiovascular and noncardiovascular events. This lower relative risk for women could not be explained by adjustment for differences in baseline characteristics. However, the sex-related difference in risk of all-cause events was modified by atrial fibrillation, stable angina pectoris, New York Heart Association functional class, and renal function. A better understanding of sex-specific risk factors may help inform strategies aimed at improving outcomes in this important disorder.
Acknowledgments
Sources of Funding
The I-PRESERVE trial was funded by Bristol-Myers Squibb and Sanofi-Aventis, as was the present subanalysis. Also, it was supported, in part, by National Institutes of Health grants 37AG18915 and P30AG21222 (Dalane W. Kitzman).
Footnotes
Disclosures
The following authors served as consultants and received honoraria from Bristol-Myers Squibb for their roles in conducting the I-PRESERVE trial, including serving on the Coordinating Committee, Executive Committee, and Endpoint Committee: Dalane W. Kitzman, Michael R. Zile, Barry Massie, and Peter E Carson, Inder S. Anand, John J. McMurray, Robert S. McKelvie, and Michel Komajda. The other authors have no conflicts to report.
References
- 1.Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL Cardiovascular Health Study Research Group. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 2.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 3.Devereux RB, Roman MJ, Liu JE, Welty TK, Lee ET, Rodeheffer R, Fabsitz RR, Howard BV. Congestive heart failure despite normal left ventricular systolic function in a population-based sample: the Strong Heart Study. Am J Cardiol. 2000;86:1090–1096. doi: 10.1016/s0002-9149(00)01165-6. [DOI] [PubMed] [Google Scholar]
- 4.Kupari M, Lindroos M, Iivanainen AM, Heikkilä J, Tilvis R. Congestive heart failure in old age: prevalence, mechanisms and 4-year prognosis in the Helsinki Ageing Study. J Intern Med. 1997;241:387–394. doi: 10.1046/j.1365-2796.1997.129150000.x. [DOI] [PubMed] [Google Scholar]
- 5.Masoudi FA, Havranek EP, Smith G, Fish RH, Steiner JF, Ordin DL, Krumholz HM. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41:217–223. doi: 10.1016/s0735-1097(02)02696-7. [DOI] [PubMed] [Google Scholar]
- 6.Galvao M, Kalman J, DeMarco T, Fonarow GC, Galvin C, Ghali JK, Moskowitz RM. Gender differences in in-hospital management and outcomes in patients with decompensated heart failure: analysis from the Acute Decompensated Heart Failure National Registry (ADHERE) J Card Fail. 2006;12:100–107. doi: 10.1016/j.cardfail.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Nieminen MS, Harjola VP, Hochadel M, Drexler H, Komajda M, Brutsaert D, Dickstein K, Ponikowski P, Tavazzi L, Follath F, Lopez-Sendon JL. Gender related differences in patients presenting with acute heart failure. Results from EuroHeart Failure Survey II. Eur J Heart Fail. 2008;10:140–148. doi: 10.1016/j.ejheart.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Deswal A, Bozkurt B. Comparison of morbidity in women versus men with heart failure and preserved ejection fraction. Am J Cardiol. 2006;97:1228–1231. doi: 10.1016/j.amjcard.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 9.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 10.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 11.Kitzman DW. Diastolic dysfunction: one piece of the heart failure with normal ejection fraction puzzle. Circulation. 2008;117:2044–2046. doi: 10.1161/CIRCULATIONAHA.108.770602. [DOI] [PubMed] [Google Scholar]
- 12.Jessup M, Piña IL. Is it important to examine gender differences in the epidemiology and outcome of severe heart failure? J Thorac Cardiovasc Surg. 2004;127:1247–1252. doi: 10.1016/j.jtcvs.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 13.Petrie MC, Dawson NF, Murdoch DR, Davie AP, McMurray JJ. Failure of women’s hearts. Circulation. 1999;99:2334–2341. doi: 10.1161/01.cir.99.17.2334. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, Love TE, Aronow WS, Adams KF, Jr, Gheorghiade M. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 16.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J PEP-CHF Investigators. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 17.Tomoda H. Irbesartan for heart failure with preserved ejection fraction. N Engl J Med. 2009;360:1257–8. author reply 1258. [PubMed] [Google Scholar]
- 18.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 19.Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, Lopez-Sendon J, Teerlink JR, White M, McMurray JJ, Komajda M, McKelvie R, Ptaszynska A, Hetzel SJ, Massie BM, Carson PE I-Preserve Investigators. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-Preserve) trial. Circulation. 2010;121:1393–1405. doi: 10.1161/CIRCULATIONAHA.109.909614. [DOI] [PubMed] [Google Scholar]
- 20.Rector TS, Carson PE, Anand IS, McMurray JJ, Zile MR, McKelvie RS, Komajda M, Kuskowski M, Massie BM I-PRESERVE Trial Investigators. Assessment of long-term effects of irbesartan on heart failure with preserved ejection fraction as measured by the minnesota living with heart failure questionnaire in the irbesartan in heart failure with preserved systolic function (I-PRESERVE) trial. Circ Heart Fail. 2012;5:217–225. doi: 10.1161/CIRCHEARTFAILURE.111.964221. [DOI] [PubMed] [Google Scholar]
- 21.Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail. 2008;1:91–97. doi: 10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krumholz HM, Parent EM, Tu N, Vaccarino V, Wang Y, Radford MJ, Hennen J. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157:99–104. [PubMed] [Google Scholar]
- 24.Grigorian-Shamagian L, Otero Raviña F, Abu Assi E, Vidal Perez R, Teijeira-Fernandez E, Varela Roman A, Moreira Sayagues L, Gonzalez-Juanatey JR. Why and when do patients with heart failure and normal left ventricular ejection fraction die? Analysis of >600 deaths in a community long-term study. Am Heart J. 2008;156:1184–1190. doi: 10.1016/j.ahj.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, Portnay EL, Marshalko SJ, Radford MJ, Krumholz HM. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol. 2003;42:736–742. doi: 10.1016/s0735-1097(03)00789-7. [DOI] [PubMed] [Google Scholar]
- 26.Muzzarelli S, Leibundgut G, Maeder MT, Rickli H, Handschin R, Gutmann M, Jeker U, Buser P, Pfisterer M, Brunner-La Rocca HP TIME-CHF Investigators. Predictors of early readmission or death in elderly patients with heart failure. Am Heart J. 2010;160:308–314. doi: 10.1016/j.ahj.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The S investigators. New Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 28.Martínez-Sellés M, Doughty RN, Poppe K, Whalley GA, Earle N, Tribouilloy C, McMurray JJ, Swedberg K, Køber L, Berry C, Squire I Meta-Analysis Global Group In Chronic Heart Failure (MAGGIC) Gender and survival in patients with heart failure: interactions with diabetes and aetiology. Results from the MAGGIC individual patient meta-analysis. Eur J Heart Fail. 2012;14:473–479. doi: 10.1093/eurjhf/hfs026. [DOI] [PubMed] [Google Scholar]
- 29.O’Meara E, Clayton T, McEntegart MB, McMurray JJ, Piña IL, Granger CB, Ostergren J, Michelson EL, Solomon SD, Pocock S, Yusuf S, Swedberg K, Pfeffer MA CHARM Investigators. Sex differences in clinical characteristics and prognosis in a broad spectrum of patients with heart failure: results of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2007;115:3111–3120. doi: 10.1161/CIRCULATIONAHA.106.673442. [DOI] [PubMed] [Google Scholar]
- 30.Volgman AS, Manankil MF, Mookherjee D, Trohman RG. Women with atrial fibrillation: Greater risk, less attention. Gend Med. 2009;6:419–432. doi: 10.1016/j.genm.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Amar D, Zhang H, Heerdt PM, Park B, Fleisher M, Thaler HT. Statin use is associated with a reduction in atrial fibrillation after noncardiac thoracic surgery independent of C-reactive protein. Chest. 2005;128:3421–3427. doi: 10.1378/chest.128.5.3421. [DOI] [PubMed] [Google Scholar]
- 32.Lazzeri C, Valente S, Tarquini R, Gensini GF. Cardiorenal syndrome caused by heart failure with preserved ejection fraction. Int J Nephrol. 2011;2011:634903. doi: 10.4061/2011/634903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bibbins-Domingo K, Lin F, Vittinghoff E, Barrett-Connor E, Grady D, Shlipak MG. Renal insufficiency as an independent predictor of mortality among women with heart failure. J Am Coll Cardiol. 2004;44:1593–1600. doi: 10.1016/j.jacc.2004.07.040. [DOI] [PubMed] [Google Scholar]