Abstract
Lung transplantation is a therapeutic option for patients with end-stage pulmonary disorders. Unfortunately, chronic lung allograft dysfunction (CLAD), most commonly manifest as bronchiolitis obliterans syndrome (BOS), continues to be highly prevalent and is the major limitation to long-term survival. The pathogenesis of BOS is complex and involves alloimmune and nonalloimmune pathways. Clinically, BOS manifests as airway obstruction and dyspnea that are classically progressive and ultimately fatal; however, the course is highly variable, and distinguishable phenotypes may exist. There are few controlled studies assessing treatment efficacy, but only a minority of patients respond to current treatment modalities. Ultimately, preventive strategies may prove more effective at prolonging survival after lung transplantation, but their remains considerable debate and little data regarding the best strategies to prevent BOS. A better understanding of the risk factors and their relationship to the pathological mechanisms of chronic lung allograft rejection should lead to better pharmacological targets to prevent or treat this syndrome.
Keywords: lung transplantation, chronic rejection, bronchiolitis obliterans syndrome
Lung transplantation (LT) is a treatment option for select patients with end-stage pulmonary or pulmonary-vascular disease. For the majority of recipients, the procedure is intended to alleviate symptoms, improve quality of life, and improve survival as compared with expectations without LT. Unfortunately, graft failure and mortality rates after LT exceed most other solid organ transplants. According to the most recent report from the International Society for Heart and Lung Transplantation (ISHLT) registry, the median survival after LT is now 5.9 years, improved from 5.3 years among those transplanted between 1996 and 2003 and from 3.9 years among those transplanted before 1996.1 The improvement in survival is mainly because of better operative and perioperative outcomes, whereas mortality rates after the first year posttransplant remain essentially un- changed. The main limitation to better long-term survival after LT remains bronchiolitis obliterans syndrome (BOS), which is the most common form of chronic lung allograft dysfunction (CLAD). Other less common manifestations of CLAD include restrictive allograft syndrome (RAS) and neutrophilic reversible allograft dysfunction (NRAD). These phenotypes are discussed in detail elsewhere in this issue. BOS is the leading cause of death after the first year posttransplant, and, according to the latest ISHLT report, 48% and 76% of patients develop BOS by 5 and 10 years after transplantation, respectively.1 In addition to its impact on long-term survival, BOS causes significant morbidity,2 impairs quality of life,3 and increases costs.4 This article provides an overview of the diagnosis, pathogenesis, and treatment of BOS.
Diagnosis of BOS
The diagnosis of BOS is defined by a sustained (≥ 3 weeks) decline in the forced expiratory volume in the first second of expiration (FEV1) provided alternative causes of pulmonary dysfunction (e.g., anastomotic stricture/complications, infection, acute rejection, and recurrent or progressive native disease) have been excluded.5 The baseline FEV1 is defined as the average of the two highest posttransplant measurements, without the use of a bronchodilator, at least 3 weeks apart.5 A decline in FEV1 from baseline of 20% or more is defined as BOS. Progressive stages of BOS (stages 1 through 3) reflect worsening degrees of airflow obstruction (Table 1).5 In the 2001 updated definition and classification of BOS, stage BOS 0-p (potential BOS) was added to detect early change in lung function and was defined as an FEV1 81 to 90% of baseline and/or forced expiratory flow (FEF) 25 to 75% (measurement of midexpiratory flow rates) less than or equal to 75% of baseline.5 Studies have examined the validity of BOS 0-p as a predictor of future BOS in bilateral and single-lung transplant recipients.6,7 Each study reported similar performance characteristics with the FEF 25 to 75% criterion for BOS 0-p performing poorly, whereas the FEV1 criterion was a modest predictor of BOS. Still, the positive predictive value of BOS 0-p (by FEV1) for progression to BOS within 1 year was less than 60%.6,7
Table 1.
Bronchiolitis obliterans syndrome classification system
| 1993 Classification | 2001 Classification | ||
|---|---|---|---|
| BOS 0 | FEV1 > 80% of baseline | BOS 0 | FEV1 > 90% of baseline and FEF25–75 > 75% of baseline |
| BOS 0-p | FEV1 81-90% of baseline and/or FEF25–75≤ 75% of baseline | ||
| BOS 1 | FEV1 66–80% of baseline | BOS 1 | FEV1 66–80% of baseline |
| BOS 2 | FEV1 51–65% of baseline | BOS 2 | FEV1 51–65% of baseline |
| BOS 3 | FEV1 < 50% of baseline | BOS 3 | FEV1 < 50% of baseline |
Source: Modified from International Society for Heart and Lung Transplantation diagnostic criteria.5
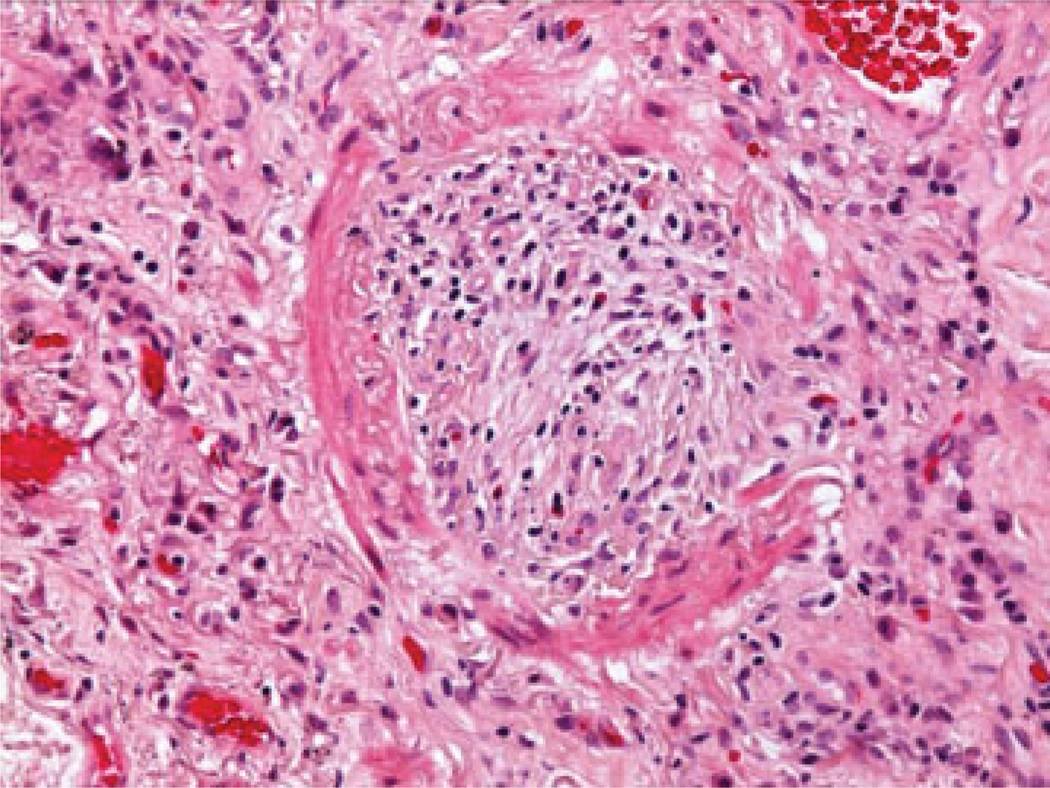
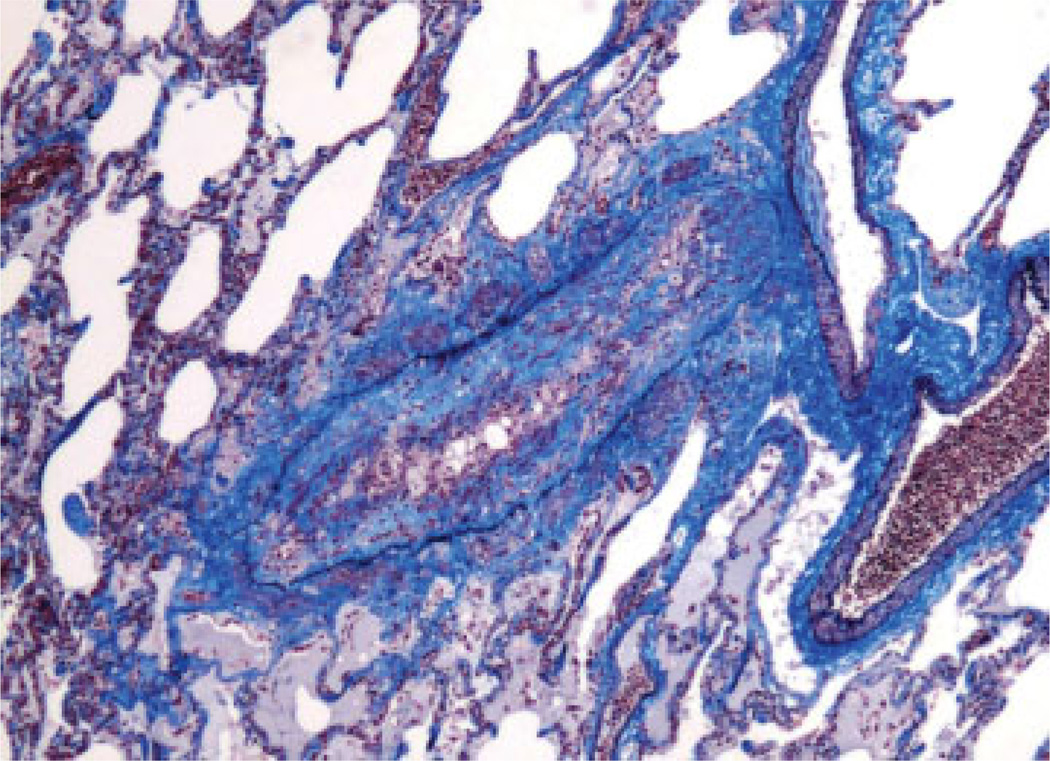
The histological hallmark of BOS is obliterative bronchiolistis (OB) (Fig. 1). OB is an inflammatory/fibrotic process affecting the small noncartilagenous airways (membranous and respiratory bronchioles) characterized by subepithelial fibrosis causing partial or complete luminal occlusion.8,9 The fibro-obliteration may be concentric or eccentric and is often associated with atrophy of the smooth muscle and destruction of elastica of the airway wall.8 The presence of lymphocytic bronchiolitis or intraluminal granulation tissue is not sufficient to diagnose OB.5 Distinctions between subtotal and total obliteration and between active versus inactive lesions (presence or absence of inflammation) have been abandoned in the recent revisions of the nomenclature.8,9 Trichrome and elastic tissue stains may facilitate identification of damaged or obliterated airways5 (Fig. 2).
Fig. 1.
Complete fibrous obliteration of small bronchiole with residual elastic layer and atrophied smooth muscle (hematoxylin and eosin stain; original magnification ×400).
Fig. 2.
Partial obliteration of bronchiole with mononuclear cell infiltration in subepithelial fibrosis (combined Masson trichrome and elastic van Gieson stain; original magnification ×40).
Due to its patchy nature, transbronchial biopsy (TBBx) is an insensitive method for detecting OB, and the clinical use of BOS with its functional grading (to be described) is the preferred means for diagnosis and monitoring.8 Mucostasis and/or foamy histiocytes in the distal air spaces are commonly associated with OB and may be seen on TBBx. However, the pathological term obliterative bronchiolitis should be reserved for histological specimens showing dense fibrosis within the small airways.5 Fibrointimal thickening and mononuclear inflammation of pulmonary arteries and veins, similar to what is seen in chronic allograft vasculopathy of transplanted hearts, may also be present with chronic rejection, but appreciation of this pathology generally requires open biopsy or autopsy and is not generally amenable to TBBx.8,10
Natural History
BOS is not usually diagnosed before 6 months and is most common between ~ 1.5 and 4 years posttransplant.11 Like the time to onset, the subsequent clinical course of BOS is highly variable.5,12–16 The course may be insidious, with a gradual decline in lung function over months to years, or abrupt, with severe decline in lung function over a few weeks.2,15,16 In one study of 111 lung transplant recipients with BOS, the steepest decline in FEV1 occurred in the first 6 months after BOS onset, followed by progressively less steep declines over the next 18 months.16 The time to onset of BOS and rapidity of fall in FEV1 were related to outcome.16 For example, early-onset BOS (within 2 years of transplant) was associated with lower FEV1 than late-onset BOS (after 2 years). Similarly, rapid-onset BOS (FEV1 decline > 20% in the 6 months preceding BOS) was associated with greater dysfunction of the lung allograft (i.e., a lower FEV1% predicted at BOS onset; a steeper decline in the first 6 months after onset of BOS, and a lower FEV1% predicted at 2 years after onset of BOS).16 In another study, the median survival after BOS diagnosis was 2.5 years with only 26% surviving 5 years.11 Not surprisingly, early-onset BOS and high-grade-onset BOS (grade 2 or 3) predicted worse survival following the diagnosis of BOS.11
Mechanisms of BOS Pathogenesis
The pathogenesis of BOS is complex and is driven by both alloimmune and nonalloimmune mechanisms that may act alone or in combination. Histological evaluation of allograft airways suggests that the pathogenesis first involves lymphocytic infiltrates of the submucosa (i.e., lymphocytic bronchiolitis), followed by epithelial cell injury, necrosis, and ulcerations of the mucosa. The associated inflammatory reaction in the airway lumen results in recruitment/proliferation of fibroblasts/myofibroblasts.5,8,17 Epithelial mesenchymal transition (EMT) may play a role in the fibroproliferative process, but this remains controversial.18,19 Ultimately, intraluminal polypoid granulation tissue leads to subtotal or total obliteration of airway lumens.8
Cytokines, Growth Factors, and Chemokines
Critical to airway wound repair is a delicate balance between type 1, 2, 17, and regulatory T (Treg) immune responses. Disruption of this balance may lead to fibro-obliteration of allograft airways and BOS. The type 1 immune response is mainly associated with a cytotoxic T lymphocyte (CTL) and delayed type hypersensitivity (DTH) response. The type 1 immune response is characterized by the production of interleukin (IL)-2, IL-12, γ-interferon (IFN-γ), and lymphotoxin. Classically, type 1 cytokines have been associated with acute cellular rejection, as well as BOS in some but not all studies.20–26 The type 2 immune response is characterized by the production of IL-4, IL-5, and IL-13, which promote mucosal, allergic, and humoral immunity. Although a type 2 immune profile has favored the acquisition of tolerance in some animal models, there is increasing evidence implicating a role for type 2 responses in rejection, especially chronic rejection.27–29
A twist to the classic type 1/2 immune response paradigm was seen in a study using a cardiac allograft rejection model: rodents lacking a type 1 immune response skewed toward a type 17 response.30 A type 17 immune response is characterized by the production of IL-17 and IL-23 and is associated with autoimmunity. Interestingly, recent studies suggest that allograft dysfunction can be associated with immunity against self-antigens (e.g,, col [V] and K-α1 tubulin) that may become unveiled at the time of organ harvest, implantation, ischemia-reperfusion, acute rejection, and infections.31–37 Thus increasing attention is being paid to the role of type 17 immune response in the pathogenesis of allo/autoimmunity in LT. Autoimmunity mediated by col (V)-specific T(H)17 cells predisposes patients to the development of BOS.37 Furthermore, type 17 skewing cytokines— transforming growth factor-β (TGF-β), IL-1 β, IL-6, and IL-23— and the type 17 effector cytokine IL-17 were elevated in the bronchoalveolar lavage fluid (BALF) of patients with BOS.38 Moreover, in a murine model of transplant obliterative airway disease, neutralization of IL-6 led to a reduction in T(H)-17 cells, which was associated with a dramatic reduction in allograft airway obliteration.39 Importantly, the role of IL-17 during lung allograft injury has been confirmed in multiple models of lung alloreactivity.36,40 Thus an understanding of the complex interactions between cytokine networks will be critical for designing therapeutic strategies that can abrogate allograft rejection and induce donor-specific tolerance.
With regard to allo/autoimmunity, the balance between effector type immune responses (type 1,2,17) and regulatory (Treg) type immune responses may dictate outcomes of allograft accommodation or rejection. Treg cells are CD4+ T helper cells characterized by constitutively high expression of the transcription factor FoxP3 and have the ability to “tone-down” effector responses. Interestingly, some but not all studies demonstrate that Tregs are a biomarker of clinical outcomes. In renal transplant recipients there was no significant correlation between intragraft FoxP3+ cells with severity of graft rejection or renal function at 1 or 2 years.41 Similarly, another study found that a transient increase in FoxP3+ Tregs within the graft does occur during rejection but does not correlate with clinical outcomes42 at 3 or 12 months. Conversely, an analysis on peripheral blood FoxP-3 mRNA expression by qPCR (polymerase chain reaction) demonstrated that low expression was associated with more chronic renal allograft rejection.43 In LT, flow cytometry studies performed on BALF cells demonstrated that CD4 + FoxP3+ “Tregs” could distinguish stable lung transplant recipients from those that go on to develop BOS.44 In another study with similar methodologies, phenotypically distinct Tregs (i.e., CD3 + CD4 + CD25hiFoxP3 + CCR7 + ) were key in determining which patients would have long-term graft stability.45 Collectively, these studies suggest that successful prevention of BOS post-LT may depend on the downregulation of all three effector immune responses (type 1,2, and 17) while preserving or augmenting the regulatory immune response.
The proliferation of epithelial cells, myofibroblasts/fibroblasts, smooth muscle, and mesenchymal precursor cells from both the donor and the recipient may all contribute to the development of BOS.16,46,47 Platelet-derived growth factor (PDGF) is elevated in the BALF of patients with BOS, and its inhibition in animal models reduced myofibroblast proliferation, smooth muscle proliferation, and obliterative changes.48–50 TGF-β, hepatocyte growth factor (HGF), and insulin-like growth factor-1 (IGF-1) can contribute to fibroproliferation and have each been implicated in the development of BOS.51–53
Chemokines and their interaction with specific cell receptors are essential components of inflammatory and immune responses via recruitment of specific leukocyte subpopulations. In the lung allograft, regulated on activation, normal T cell expressed and secreted (RANTES)/CCL5 and its interaction with CCR1 and CCR5, as well as CXCR3-CXCL9 biology, proved to be important in acute lung allograft rejection.54,55 Similarly, CXCR3-ligand, CCR2-CCL2, and CXCR2-ligand interactions have all been shown to be important in chronic lung allograft rejection; albeit, through different nonredundant mechanisms.56–58 Recently, IL-8/CXCL8, a CXCR2 ligand, has received a great deal of attention in the lung transplant literature. CXCL8 is elevated in the BALF of BOS patients, and this elevation may precede the development of BOS.56,59,60 Furthermore, CXCL8 is elevated during pseudomonal infections and gastroesophageal reflux (GER) and may be a part of the mechanistic link between these events and BOS.61 CXCL8 is a potent attractor of neutrophils, and BALF neutrophilia has been proposed as a marker for the development of BOS as well.59,62 However, the mechanistic role of neutrophils in the pathogenesis of BOS is unclear, and the role of CXCL8 in promoting airway microvascular remodeling was independent of neutrophils.56 Importantly, CXCR2-ligand biology has also been linked to hypoxia-inducible factor 1a mediated angiogenesis during airway ischemia, which has been shown to be an important pathway during airway rejection.63
Alloimmune Reactivity
The immune response to allogeneic tissues is mediated by major histocompatibility complex (MHC) molecules. In humans, class I MHC molecules are known as human leukocyte antigens (HLA) A, B, and C and are constitutively expressed on most nucleated cells. Class II molecules are known as HLA DR, DP, and DQ and are constitutively expressed only by bone marrow–derived antigen-presenting cells. These molecules play a critical role in the immune system via the presentation of peptides in a form that can be recognized by T cells. Allograft rejection is achieved through cytotoxicity caused by CD4+ or CD8+ T cells that are recognizing donor MHC molecules through the direct or indirect pathway, memory T-cell-mediated delayed-type hypersensitivity, and via complement activation or antibody-dependent cytotoxicity of allograft cells opsonized by allogeneic antibodies.64
Classically, BOS is considered to be the end-stage consequence of alloimmune-mediated injury to the lung allograft. Observations that support this assertion include the following: an increasing number of HLA mismatches between donor and recipient is associated with increased risk of BOS,65,66 T cells from lung transplant recipients with BOS are sensitized to donor antigens presented via the indirect route,67,68 and patients with BOS have an oligoclonal CD4+ T-cell expansion not present in patients without BOS.69
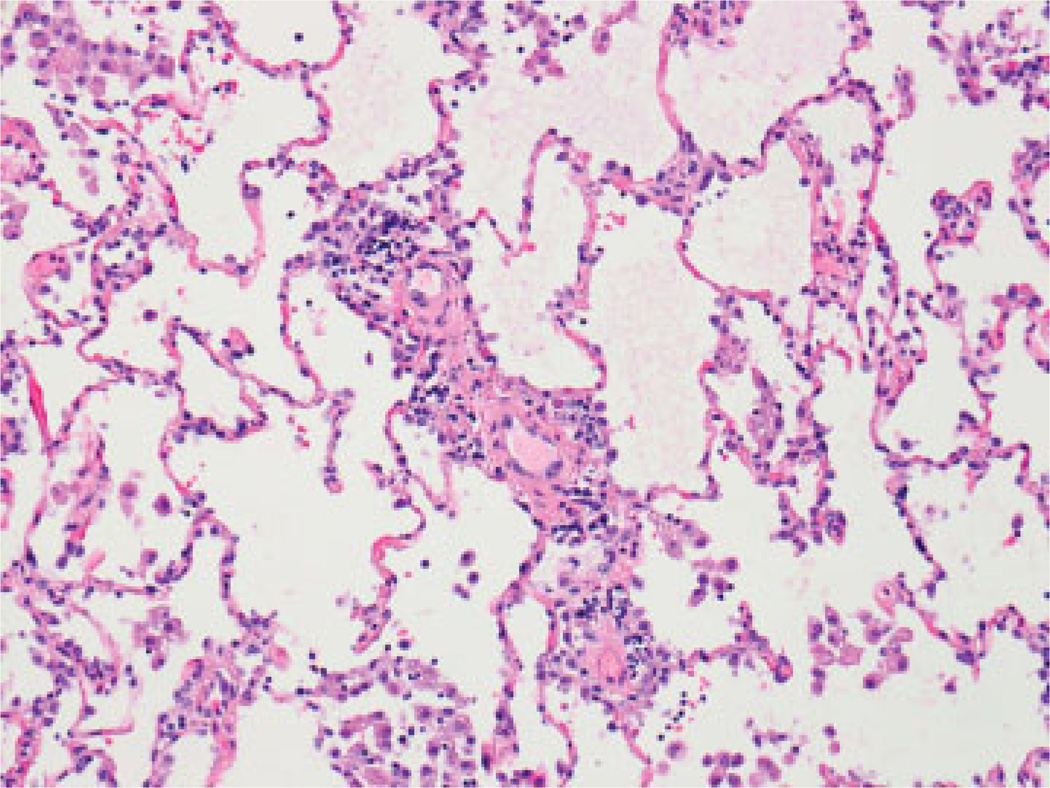
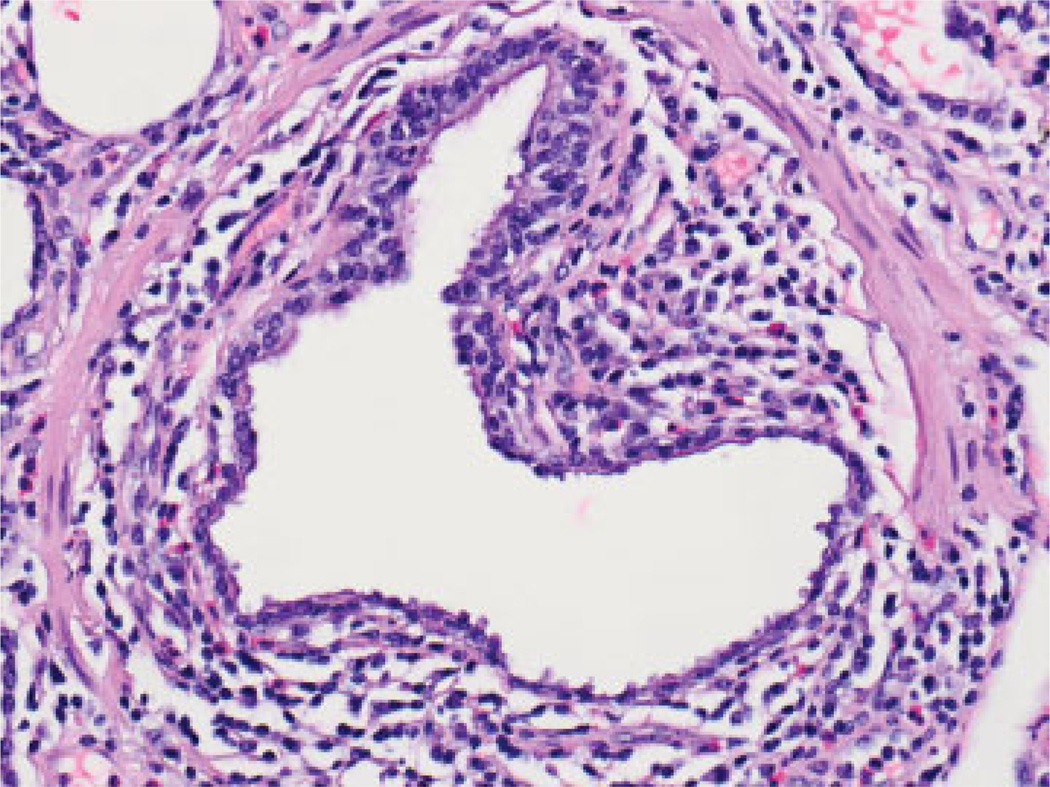
Not surprisingly, numerous studies have also implicated acute cellular rejection (ACR) as the most important risk factor for BOS.5,70–73 The nomenclature for ACR in the lung, adopted by the ISHLT in 1990, modified in 1996 and again in 2007, is based upon TBBx and provides separate A and B grades. A-grade ACR describes the presence and extent of perivascular inflammatory cell infiltrates. With increasing severity, these infiltrates extend into the interstitium and alveolar spaces (Fig. 3). A-grades range from A0 (no rejection) to A4 (severe rejection).8 Multiple episodes,74 high grade,71 or late-onset A-grade ACR71,75,76 predicts a greater risk of BOS. Importantly, even a single episode of any A-grade ACR increases the risk for BOS.74,75,77,78 Because A-grade ACR is characterized by a perivascular lymphocytic infiltrate, the mechanism linking it to small airways obliteration is not well understood. However, B-grade ACR, also known as lympho-cytic bronchiolitis (LB), describes the presence and extent of peri-airway lymphocyte infiltration.79 The ISHLT has recommended comment on the presence and severity of LB for grading ACR since 19969 (Fig. 4). Multiple studies have linked LB with the development of BOS,13,71,80,81 and the severity of LB was the most significant predictor of BOS in one large study.82 Interestingly, A-grade ACR was a risk factor for BOS in univariate analysis, confirming many other studies, but was not an independent risk factor for BOS in multivariable analyses adjusting for LB.82 Importantly, ISHLT guidelines call for rigorous exclusion of infection before ascribing the features of LB to rejection,8 a difficulty that often hampers the interpretation of LB.
Fig. 3.
Perivascular lymphoid infiltrate with rare eosinophils, consistent with mild acute cellular rejection (hematoxylin and eosin stain; original magnification ×200).
Fig. 4.
Circumferential lymphoid infiltration around small bronchiole with frequent eosinophils, consistent with high-grade small airway inflammation (grade B2R) (hematoxylin and eosin stain; original magnification ×400).
Humoral Immunity
Antibody-mediated rejection (AMR) is a recognized clinical entity in renal and heart transplantation and may be a major cause of late graft loss, especially in renal transplantation.83 However, conclusive evidence for the existence of AMR after LT and its role in the pathogenesis of BOS is lacking. In the latest revision (2007) of the ISHLT Lung Rejection Study Group iteration of the nomenclature of lung rejection, there was no consensus reached on the histological hallmarks of AMR in the lung.8 The group therefore urged caution in the diagnosis of AMR until there is further evidence.
The ability of B cells to recognize alloantigen is not controversial. B cells recognize antigen via their B-cell receptor, internalize and process the antigen to peptide epitopes, and then present it in the context of self-MHC to T cells (i.e., indirect pathway). T cells then stimulate B-cell differentiation and antibody class switching. Stimulated B cells become either plasmablasts (i.e., secrete low-affinity antibodies) or activated B cells. Activated B cells, with the help of other mononuclear cells, further proliferate, hypermutate, and undergo affinity maturation resulting in their becoming either plasma cells (PCs) or memory B cells. PCs secrete high-affinity antibodies, whereas memory B cells undergo secondary stimulation, proliferation, and differentiation into PCs when reexposed to alloantigen or other stimuli (e.g., infections). Therefore, the presence of anti-HLA antibodies is undoubtedly a marker of indirect allorecognition. However, the controversy lies in whether or not a specific pathology in the lung allograft is directly attributable to donor-specific alloantibodies (DSA).
The strongest evidence for the concept of AMR is hyperacute rejection, clinically manifested as primary graft failure occurring very early after transplantation in the setting of preformed antibodies to donor HLA antigens or endothelial cells.83 Although rare, hyperacute rejection is well described after lung transplantation.84–88 Features of hyperacute rejection include fibrin thrombi in alveolar septa, fibrinoid necrosis of alveolar septal walls, and hemorrhage.8 These histopathologic features likely represent the severest form of AMR in the lung, but histopathological criteria for AMR outside of the hyperacute rejection clinical scenario remain to be determined.
Among the criteria for a humoral response proposed by the National Conference to Assess AMR in Solid Organ Transplantation, foremost is the detection of circulating DSA. Several early studies demonstrated that an increasing pretransplant panel reactive antibody (PRA) test is associated with increasing mortality, especially in the first 30 days posttransplant (HR 2.6).89,90 Considered together with the hyperacute rejection scenario, these studies suggest that alloantibodies might compound the allograft injury initiated by ischemia reperfusion. Other studies have investigated the impact of incident humoral responses after LT. Infiltration of B cells in the human lung allograft during ACR was associated with refractoriness to augmented immunosuppression,91 and several other studies have also correlated the development of anti-HLA antibodies with steroid-refractory ACR.92,93 Likewise, the development of antibodies specific to HLA predicts the development of BOS.66,94,95 Interestingly, the administration of alloantibody is capable of causing airway obliteration in a murine model, demonstrating that an alloantibody can induce airway injury.96 Collectively, these studies suggest a possible role of alloantibodies in the pathogenesis of acute and chronic allograft rejection.
According to the National Conference to Assess AMR in Solid Organ Transplantation, any degree of humoral reaction greater than a latent humoral response requires the demonstration of C4d deposition in the allograft. But there may be problems extending this proposed criterion to LT because positive C4d staining in a lung allograft may lack the specificity seen in other solid-organ allografts. For instance, in a cohort of 33 lung transplant recipients, C3d (positive in 20) and C4d (positive in 11) staining was associated with primary graft dysfunction (PGD) and airway infection, but not with ACR or chronic rejection, or with presumed morphological features of AMR (necrotizing septal capillary injury or the presence of intra-capillary macrophages).97 Another study demonstrated variable nonspecific C4d staining without any consistent pattern among lung transplant cases grouped according to the presence of acute and/or chronic rejection.98 Interestingly, half of nontransplant constrictive bronchiolitis and diffuse alveolar damage (DAD) controls also had positive C4d staining.98 Recently, Yousem and Zeevi examined characteristics of 17 biopsies from patients with ACR and DSA compared with 26 biopsies from patients with ACR and no anti-HLA antibodies. In this study, C4d staining was more common in the group with DSA, but it did not reliably separate the two groups.99 Likewise, we have recently shown that C3d and C4d staining showed no correlation with each other, the presence of DSA, or histopathologic findings.100 Collectively, these studies demonstrate that C4d and C3d are not specific enough to distinguish AMR from other lung pathologies.
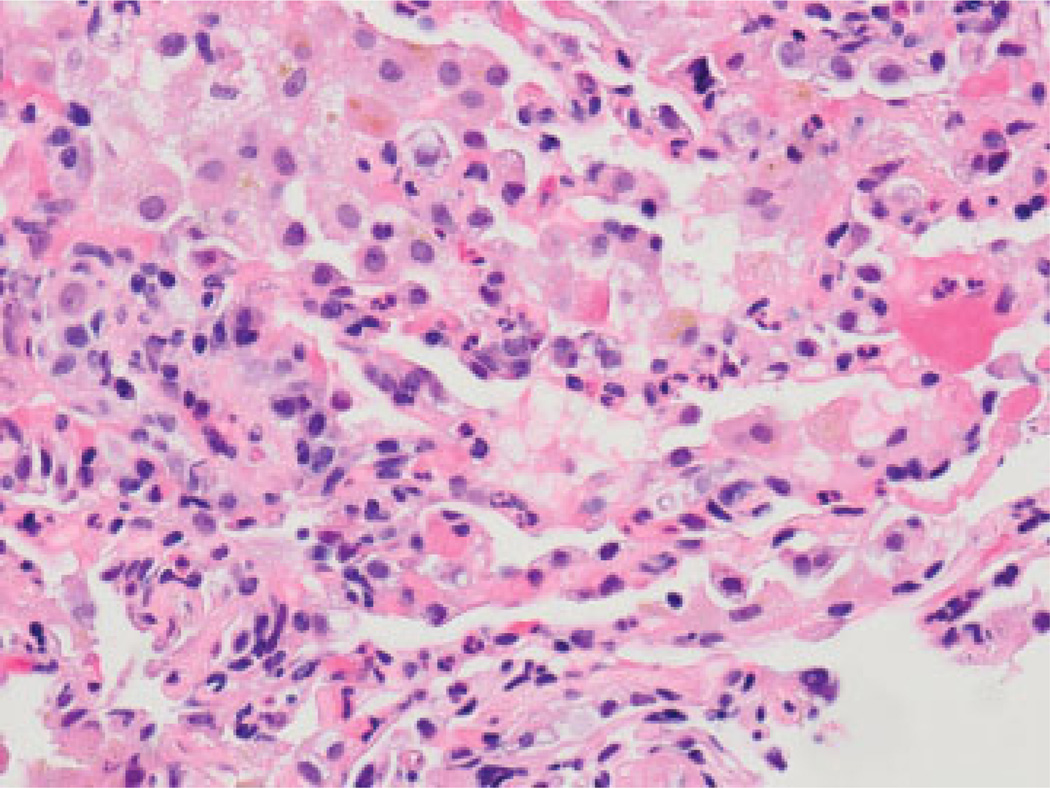
Possibly, the combination of DSA with a characteristic histopathology and the appropriate clinical scenario will improve our ability to detect AMR in the lung allograft. More work is required to define AMR histopathology in the lung, but capillary inflammation and injury are likely to be key. In the recent study by Yousem and Zeevi,99 capillaritis was the only histopathologic feature that separated groups with and without anti-HLA antibodies, although it was seen in only a minority of cases. Along these lines, we have also shown that capillary inflammation, defined as capillary neutrophilic infiltration with at least two back-to-back intracapillary neutrophils (Fig. 5), or DAD, in the absence of infection, was significantly associated with DSA (69 vs. 24%).100 Although more work is required, these studies suggest that the finding of capillary inflammation, in combination with DSA and the right clinical scenario, may be a useful tool for identifying AMR in the lung.
Fig. 5.
Diffuse and back-to-back capillary neutrophils (hematoxylin and eosin stain; original magnification ×600).
Autoimmunity
Immunologic response to cryptic self-antigens and/or their determinants has recently been shown to possibly contribute to the pathogenesis of chronic rejection in many solid organ transplants. Thus any injurious process to the donor organ may lead to the unveiling of intercalated self-antigens and/or their determinant initiating an immune response that has been coined “autoimmunity” posttransplantation. This response is due to a combination of cellular and antibody-mediated injuries and has been described in cardiac transplantation (e.g., autoimmune response to myosin and vimentin),101,102 renal transplantation.(e.g., autoimmune response to antiangiotensin type I receptor antibodies, col (IV), and MHC class 1 chain-related peptide A)103–105 and LT (e.g., autoimmune response to col (V) and K-α1 tubulin).31–37 Human LT studies have demonstrated an association between responses to self-antigens and BOS.33,37,106,107 Mechanistic studies involving humans and animals suggest that any inflammatory change in the lung allograft (e.g., PGD, ACR, and/or infection) allows cryptic self-antigens to be exposed to the immune system causing a sensitization that can then lead to immune-mediated allograft injury and eventual lung allograft dysfunction.35–37,40,107
Col (V) is ubiquitously expressed in perivascular/bronchial connective tissues where it is incorporated within collagen I fibrils that protect it from immunological responses.108 Following LT col (V) fibrils can be detected in BALF in a rat model system.34 Additionally, animals can develop a T-cell immune response to col (V) characterized by IFN-γ expression that is associated with rejection. Furthermore, the transfer of col (V)–specific T cells to rats with lung isografts develops perivascular/ bronchial mononuclear cell infiltration (e.g., areas full of exposed col [V] due to ischemia-reperfusion) mimicking the pathology of ACR and LB.34,109 This suggests that a lymphocyte-specific response to self-antigens can cause rejection. Moreover, oral tolerance to col (V) in a non–fully mismatched rat transplant model was protective of lung allograft rejection.109 Translational human studies have also shown that human lung transplant recipients with detectable col (V) specific immune responses in patients before (likely from there underlying lung disease) or after transplantation are possibly at risk for the development of PGD and BOS.33,37,110
Studies have also found that their may be a pathogenic role for an autoimmune response to K-α1 tubulin in the development of chronic rejection.106 K-α1 tubulin is one of six isotypes of α-tubulin, is expressed in the gap junction of airway epithelial and endothelial cells, and is a component of cellular microtubules,111–113 making it important in microtubule formation, GTP binding, and cellular movement.114,115 Antibodies to K-α1 tubulin correlate with the development of chronic rejection.106,107 Mechanistically, these antibodies can bind to epithelial cells and stimulate profibrotic growth-factor signals.106 Other studies demonstrate that the responses to col (V) and K-α1 tubulin are both important in allograft injury, and these recipient responses can lead to a skewed immune response (e.g., high type 1/17 and low type 2) favoring the development of BOS.36,107,110
Both allo- and autoimmunity occur together and may have the potential to drive one another in the immunopathogenesis of BOS. Mice treated with anti-MHC class 1 antibodies developed de novo antibodies to self-antigens col (V) and K-α1 tubulin.36 Likewise, in human lung transplant recipients, there is a strong correlation between the development of donor-specific anti-HLA antibodies and antibodies to self-antigens col (V) and K-α1 tubulin, with the development of DSA preceding antibodies to self-antigens.107 Conversely, pretransplant antibodies to self-antigens were associated with the posttrans-plant development of DSA, as well as with increased risk of PGD and BOS.116 Importantly, recipients with both DSA and antibodies to self-antigens who cleared both antibody types after treatment (rituximab and/or intravenous immunoglobulin) were at lower risk of developing BOS than those who cleared only the DSA but had persistent antibodies to self-antigens.33 Collectively these studies demonstrate cross-talk between auto- and alloimmune responses that may perpetuate lung allograft injury, ultimately leading to BOS.
Innate Immunity
A growing body of literature supports an association between BOS and ostensibly nonalloimmune responses to local injury and foreign antigens unrelated to donor-specific MHC. Theoretically, any insult, including infection, aspiration, and ischemia-reperfusion injury can lead to the propagation of “danger signals” that activate professional antigen-presenting cells (e. g., dendritic cells) via Toll-like receptors (TLRs), leading to optimized antigen presentation to alloreactive T cells.117 In animal models, TLR engagement has been found to hinder the induction of transplant tolerance.118,119 Local innate immune activation through lipopolysaccharide has been shown to induce alloimmune lung injury via TLR4 activation.120 Polymorphisms in TLR2, TLR4, TLR9,121,122 the lipopolysaccharide receptor CD14,123 as well as altered levels of mannose-binding lectin in transplant recipients124–126 have all been associated with BOS. This supports the hypothesis that innate immunity appears to be an important cofactor linking alloimmune-independent mechanisms of lung injury to accelerated alloimmune responses and BOS.
Primary graft dysfunction (PGD) is a form of acute lung injury that arises within the first 72 hours of transplantation, resulting from multiple pathological mechanisms inherent to the process of transplantation, including physiological changes in the donor following brain death, ex-plantation, cold ischemia, and reperfusion within the recipient.127 The innate immune response fundamental to PGD and other forms of ischemia-reperfusion injury involves activation of TLR signaling,128–130 increased expression of proinflammatory cytokines,131–133 and recruitment of recipient lymphocytes and antigen-presenting cells,134,135 and has been associated with enhanced expression of major histocompatibility class (MHC) II antigens.136,137 Although PGD is a well-described major factor in early mortality following lung transplantation, there is increasing evidence that PGD also contributes to late morbidity and mortality.138,139 In two single-center studies, PGD was found to be an independent risk factor for the subsequent development of BOS.140,141 Polymorphisms in pentraxin-3, a key mediator of innate immunity, were associated with PGD, suggesting that variations in recipient innate immunity may affect the incidence of PGD and the subsequent risk of BOS.142,143
Following transplantation, recipients may be at increased risk of developing GER and/or aspiration of gastric fluids due to delayed gastric emptying, lung denervation, impaired cough reflex, and abnormal mucociliary clearance.144,145 In a rat model of LT, histological findings consistent with OB have been reproduced by instillation of gastric fluid into allografts.146 Two centers have found that the presence of bile acid in BALF from transplant recipients was associated with BOS, whereas a more recent study found that the presence of bile acid in lavage specimens from patients who already have BOS is associated with a more rapid decline in lung function and an increased rate of mortality.147–149 GER confirmed by pH probe testing has also been associated with an increased rate of acute rejection, as well as reduced plateaus in lung function following LT.150–152 High levels of bile acids within the allografts of patients with GER have been associated with lower surfactant collectin proteins and surfactant phospholipids, all components of innate immunity.153 Treatment of GER is recommended posttransplant; however, proton pump inhibitors may not affect non–acid reflux, and in the previously noted rat model, pH neutralization of the instilled gastric fluid had no impact on the subsequent induction of OB.148,154 In transplant recipients with known reflux, retrospective studies have found that more aggressive treatment with early gastric fundoplication may be associated with greater freedom from BOS, and improved lung function.152,155
Multiple infectious processes have been linked to the development of BOS. The best evidence for this is in patients who develop cytomegalovirus (CMV) pneumonitis.14,76,95,156–161 CMV infection increases epithelial expression of donor HLA in transplant recipients95 and upregulates proinflammatory cytokine expression.162 The virus has also been found to share nucleic acid sequence homology with specific HLA antigens.159 Two nonrandomized trials have found that pharmacological prophylaxis against CMV leads to both a reduced rate of CMV as well as BOS.163,164 Similarly, associations between BOS and community-acquired respiratory viruses (CARVs),165–168 human herpesvirus-6,169 and Chlamydophila pneumonia170 infection have been described in retrospective single-center series. Our group recently found that CXCR3 chemokines are upregulated during CARV infection, and elevated expression of these chemokines among infected patients is associated with chronic allograft dysfunction, suggesting a potential mechanistic link between nonalloimmune responses to these acute infections and the subsequent development of alloreactivity.171
Low-grade chronic infections may also be important risk factors for BOS. Lung transplant recipients, especially those transplanted for cystic fibrosis, commonly develop lower airway colonization with Pseudomonas aeruginosa and/or Aspergillus species. In two retrospective studies, pseudomonal colonization was associated with increased risk and higher stage of BOS,172–174 and levels of antipseudomonal antibodies in BAL among colonized transplant recipients have been associated with local innate immune responses.175 Similar findings were reported with Aspergillus colonization. In a time-dependent analysis, Aspergillus colonization was a risk factor for the development of BOS, independent of ACR.176 Furthermore, those with new or persistent Aspergillus colonization after the development of BOS had a greater risk of progression to severe BOS (stage 3) or death.176
Treatment of BOS
Treatment options for BOS generally remain disappointing. Historically, uncontrolled studies have cited treatment responses with diverse strategies, but interpretation is often clouded by small sample sizes and lack of suitable controls. Frequently in these studies, favorable responses were defined as “stabilization” or reduction in the rate of decline of FEV1; improvement was rarely documented. Importantly, “stabilization” may reflect the natural history of the disease.16 Anecdotal improvements in FEV1 are also reported. However, it is interesting that lung biopsies from patients with BOS typically have varying degrees of ACR.177 Thus responses to therapy may be due to resolving ACR rather than BOS.
Changes in Maintenance Immunosuppression
Uncontrolled studies have cited slower rates of FEV1 decline after conversion to mycophenolate mofetil (MMF) from azathioprine (AZA),178,179 or to tacrolimus from cyclosporine.180–182 Controlled data confirming benefit are not available, and the positive findings in these studies probably reflect the natural history of BOS. We believe that conversion from AZA to MMF or cyclosporine to tacrolimus is unlikely to benefit most patients with BOS.
Cytolytic Therapy
Antilymphocyte and antithymocyte preparations deplete T cells and can have prolonged effects on T-cell function through nondepletive mechanisms (e.g., effects on antigen-presenting cells and B cells). Salvage treatment for BOS with cytolytic therapies has been reported to slow the decline of FEV1.183,184 Alemtuzumab (Campath 1H, Genzyme, Cambridge, MA) is a humanized CD52 directed cytolytic antibody that results in a rapid and sustained (6 months or longer) depletion of lymphocytes. In a small cohort (n = 10) of patients with BOS, FEV1 improved in four patients and remained stable in an additional three patients, but overall the FEV1 for the group was unchanged 6 months after treatment.185 Importantly, infectious complications following alemtuzumab treatment were common (73%), limiting our enthusiasm for its use in BOS. No controlled data exist for alemtuzumab or other cytolytics for the treatment of BOS at this time.
Azithromycin
Gerhardt et al first reported the results of a small pilot study using add-on azithromycin (250 mg three times a week) for BOS in 2003.186 In this study, five of six patients had a significant improvement in lung function over a short follow-up period. This sparked intense interest in azithromycin as an immune-modulating agent with relatively few side effects. Most, but not all, subsequent studies have also suggested that a subset of patients with BOS do respond to treatment with azithromycin.73,187–190 Response may be predicted by pretreatment BALF neutrophilia.187 Some have proposed that this group of patients represents a distinct phenotype of CLAD, termed neutrophilic reversible allograft dysfunction (NRAD),191 although this diagnosis has not yet been formally recognized. Recently, the results of a small, single-center, randomized trial of azithromycin for the treatment of BOS were published in abstract form.192 In this study, azithromycin treatment was associated with improved FEV1 at 12 weeks, relative to placebo. At the press time for this review, the final peer-reviewed publication is not yet available. However, as the drug is relatively inexpensive, has few side effects, and there is little else to offer, a trial of therapy seems indicated for any patient who develops BOS.
Extracorporeal Photopheresis
Extracorporeal photopheresis (ECP) involves the removal of a fraction of the patient’s blood and the isolation of leukocytes, which are then exposed to ultraviolet light in the presence of 8-methoxypsoralen. This forms covalent bonds to DNA pyrimidine bases, cell-surface molecules, and cytoplasmic components in exposed cells. ECP is a safe and effective treatment of cutaneous T-cell lymphoma.193 It also has been used successfully to treat graft versus host disease (GVHD) in hematopoietic stem cell transplant recipients194 and for the prevention and treatment of acute cellular rejection in heart transplant recipients.195,196 ECP therapy involves multiple treatment cycles (ECP on 2 consecutive days) at regular intervals for a total of 3 to 12 months.197–199 The mechanisms of action are not fully understood. However, studies suggest that ECP results in leukocyte apoptosis and induction of regulatory T cells.200
There are no controlled studies of ECP in lung transplant recipients. Several observational studies have shown that the rate of decline in FEV1 is reduced after initiation of ECP for the majority of patients, whereas a minority experience improved lung function.197–199 In the largest published series including 56 lung transplant recipients with BOS, 25% had an increase in their FEV1.199 In another recent study including 51 patients, 30% had at least an initial improvement in lung function, and 18% had a sustained improvement in FEV1 12 months after starting ECP.198 Early BOS, defined as onset within 3 years of transplant, was associated with a greater likelihood of response in one study198 but not in the other, where early BOS was defined as onset within 2 years of transplant.199 ECP is generally well tolerated without an appreciable increased risk of infections,197–199 but it is relatively expensive.199 Unfortunately, the evidence for benefit with ECP for BOS is insufficient to support a recommendation at this time. However, appropriately controlled studies examining this question are welcomed.
Fundoplication
Given the relationship between GER and BOS, there may be a role for antireflux surgery in patients with GER who develop BOS. In a series of 43 lung transplant recipients who underwent fundoplication after transplantation, FEV1 improved by an average of 24% by 6 months after antireflux surgery.201 Fifty percent of the 26 patients with BOS at the time of the antireflux surgery no longer met criteria for BOS after fun-doplication.201 The potential benefit of fundoplication in patients with GER and BOS needs to be weighed against the risks of surgery in patients with obstructive lung disease.
Retransplantation
Retransplantation has been performed for lung transplant recipients with BOS, with lower survival rates than with initial transplants.202,203 However, survival after retransplantation for BOS is better than survival after retransplant for early (within 30 days of transplant) causes of graft failure.203 The incidence of BOS after retransplant is higher than after initial transplant (HR 2.0 [1.4–3.0]).203 In light of limited availability of donor lungs, the role of retransplantation for BOS remains controversial.
Strategies for the Prevention of BOS
By the time BOS is diagnosed, it may be too late for treatments to reverse the airways pathology for the majority of patients. Therefore, strategies aimed at the prevention of BOS are most likely to favorably impact long-term morbidity and mortality outcomes after LT.
Induction Therapy
According to the latest report of the ISHLT registry, the overall percentage of lung transplant recipients treated with induction therapy in 2010 declined to 51%, down from more than 60% in the 3 years prior.1 However, over the past decade there has been an overall increase in the use of IL-2 receptor antagonists (e.g., daclizumab or basiliximab) and alemtuzumab.1 Interestingly, the use of polyclonal antilymphocyte globulin/antithymocyte globulin (ALG/ATG) induction has been falling over this same time period. In this same report, any induction therapy was associated with a significantly better overall survival.1 However, these analyses were not adjusted for the propensity to receive induction regimens and thus may be confounded by center, diagnosis, and other recipient variables. Furthermore, the large sample size in this comparison permits statistical significance for a small difference that may not be clinically meaningful.
There is a limited clinical trial experience examining the effectiveness of different induction therapies. In one small randomized trial, induction with rabbit ATG yielded a significant reduction in ACR ≥ 2 compared with no induction.204 However, in a study by the same group that included longer-term follow-up, there was no difference in freedom of ACR ≥ 1, and there was no difference in freedom of BOS, infection, malignancy, or survival.205 In other studies of lung transplant recipients, the incidence of ACR was lower with daclizumab than with ATG in some,206–208 but not all,75,209 studies. The incidence of BOS was lower with daclizumab in one study206 and similar to ATG in two studies.208,209 In a nonrandomized trial, induction therapy with alemtuzumab was associated with greater freedom from rejection compared with ATG or daclizumab.210 Recently, the same group has published their longer-term follow-up and the findings continue to appear favorable: alemtuzumab was associated with improved survival as well as a greater 5-year freedom from BOS.211 There was no difference in the incidence of PTLD, and although rates of infection were not described, alemtuzumab-treated patients were not more likely to die of infection than patients treated with other or no induction.211 However, in the absence of randomized, controlled trial data, concerns remain about the risks of infection and malignancy after alemtuzumab induction. The most important message may be that prospective, multicenter studies are needed to determine if and which induction therapy is beneficial in LT.
Maintenance Immunosuppression
ISHLT registry data suggest that rates of ACR are lower with immunosuppressive regimens employing mycophenolate mofetil (MMF) compared with azathioprine (AZA).1 However, a randomized, open-label trial involving 22 sites found similar rates of ACR, BOS, and survival at 3 years with these agents.212 ACR rates are also lower with immunosuppressive regimens employing tacrolimus as compared with cyclosporine in the most recent report of the ISHLT registry,1 and prospective trials appear to confirm the slight advantage for tacrolimus. In one randomized trial, the incidence of ACR trended lower (p = 0.07), and the incidence of BOS was lower (p = 0.025) in the tacrolimus group.213 A second trial found fewer ACR episodes in the tacrolimus cohort.214 A third trial also reported a lower burden of ACR (both A and B grades), as well as a trend to a greater freedom from BOS (p = 0.09) in the tacrolimus group.215 In the most recent and largest randomized trial, tacrolimus was associated with a lower cumulative incidence of BOS despite no difference in the rates of ACR.216 Importantly, in each of these trials cyclosporine dosing relied on blood trough concentrations (C0) for some or all patients receiving cyclosporine. Studies have demonstrated that 2-hour postdrug concentrations (C2) are a more accurate measure of drug exposure than C0 levels.217 Therefore, it is possible that C2 optimized cyclosporine dosing would perform better and be more comparable to tacrolimus for the prevention of ACR and BOS in lung transplant recipients.
Sirolimus and Everolimus
Sirolimus (rapamycin) and related compounds (e.g., everolimus) bind to the same intracellular target as tacrolimus, FK binding protein.13,218 However, thereafter their activity involves modulation of the activity of the mammalian target of rapamycin (mTOR), which in turn inhibits IL-2-mediated signal transduction, thus blocking the activation and proliferation of T and B cells. In a multicenter, randomized, double-blind trial, 223 lung transplant recipients who were free of BOS received maintenance immunosuppression consisting of cyclosporine and corticosteroids together with either everolimus or AZA.218 Efficacy failure (i.e., drop in FEV1 > 15%, graft loss, death, or loss to follow-up) at 12 months was less frequent (22%) among patients receiving everolimus compared with AZA (34%). However, by 24 months, freedom from BOS was similar between groups. Interestingly, the incidence of treated ACR was significantly reduced at both 12 and 24 months in the everolimus cohort. Although everolimus is a promising therapy, a potential serious concern raised in this study was the increased rate of adverse reactions in the everolimus-treated patients, including bacterial infections, fungal infections, and elevated serum creatinine.218 Theoretically these antiproliferative agents could have deleterious effects on healing of the bronchial anastomoses following LT and should probably be avoided in the early posttransplant period.219
Surveillance Bronchoscopy with Transbronchial Biopsy
Given the relationship between the severity and recurrence of ACR with the development of BOS, a surveillance protocol aimed at the early diagnosis and treatment of ACR has been advocated by some as a strategy for the prevention of BOS. TBBx is the principal diagnostic modality for the assessment of ACR in the lung allograft, but the sensitivity of this procedure is dependent upon the number of samples taken. In one study, 18 samples per bronchoscopy were required to have a 95% confidence of finding rejection.220 Most programs report practices of obtaining far fewer biopsies.221 The specificity of ACR histopathology is also of some concern: the reported interobserver agreement of ACR grading is moderate at best, even between experienced pathologists.222,223
Irrespective of these concerns, studies have demonstrated that surveillance bronchoscopy protocols can detect asymptomatic acute rejection. In one study of 1,235 TBBx in 230 lung transplant recipients, 836 (67.7%) were performed for sur-veillance.224 ACR was diagnosed in 18.9% of surveillance procedures, and 86.4% of clinically indicated TBBx. However, the yield of surveillance TBBx to diagnose ACR between 4 and 12 months decreased to 6.1%. Therefore, the utility of surveillance TBBx beyond 4 to 6 months is a matter of debate.
Still others advocate for no routine surveillance after LT. In fact, there is no current evidence that demonstrates surveillance protocols including TBBx have any impact on BOS or survival after LT. Valentine et al reported a small multicenter trial comparing surveillance with clinically indicated TBBx and BAL225 The clinically indicated group (n = 23) underwent fewer TBBx/BAL than the surveillance group (n = 24) (84 vs 156, respectively). In the surveillance group, 54 TBBx/ BAL procedures were defined as true surveillance procedures, and no episode of ACR was diagnosed by a true surveillance procedure. In this study, there were no differences in freedom from BOS or survival between groups receiving either clinically indicated or surveillance bronchoscopy, but the small size of the study was vastly underpowered to answer this question conclusively.
There remains no consensus on the best practice, but most programs report some version of a surveillance bronchoscopy protocol after LT. In a 2004 survey of lung transplant centers, 69% of responding programs performed surveillance TBBx,226 which was nearly identical to the 68% reported in a separate survey in 1997.221
Hematopoietic Cell Transplant
Full or mixed-chimerism has long been recognized for the potential to facilitate allograft tolerance. Patients who have undergone myeloablative conditioning and human lymphocyte antigen (HLA)-matched bone marrow transplantation for a hematologic-oncology disorder, who later receive a renal transplant from the same donor, have not required immuno-suppression, confirming that chimerism can lead to tolerance.227 Similarly, a recipient of simultaneous renal and hematopoietic-cell transplant treated with a conditioning regimen of total lymphoid irradiation and ATG developed a persistent mixed chimerism with no rejection or GVHD following discontinuation of immunosuppressive drugs.228 Likewise, a pretransplant nonmyeloablative conditioning regimen (anti-CD2 antibody, cyclophosphamide, thymic irradiation, and ± rituximab) resulted in four of five patients with stable immunosuppression-free long-term kidney graft survival.229 Another recent small series also demonstrated successful graft acceptance using a different conditioning strategy (e.g., total body irradiation, 200 Gy, fludarabine, cyclophosphamide, and administration of a special population of bone marrow-derived cells termed facilitator cells along with donor hematopoietic stem cells) in non-HLA matched kidney transplant pairs.230 This regimen resulted in a durable mixed chimerism for five of eight recipients without GVHD and allowed weaning from all immunosuppression by 1 year after transplant.
Unfortunately, even if effective, the sporadic and unpredictable timing of cadaveric donors for LT make the implementation of pretransplant conditioning logistically impossible at this time. Furthermore, the risks of infections with conditioning regimens required to induce chimerism may outweigh the benefits for LT. Lung transplant recipients are uniquely susceptible to posttransplant infections for a variety of reasons, including chronic pretransplant immunosuppression, pretransplant colonization, PGD, prolonged mechanical ventilation, and disrupted cough reflex. Even nonmyeloablative conditioning regimens can lead to prolonged neutropenia/lymphopenia and thus a high risk of serious infections.
Protocols for the simple infusion of donor bone marrow (BM), without conditioning, simultaneous or prior to transplant, would be preferable in LT. In a human study, 26 lung transplant recipients receiving infusion of donor BM (without conditioning) in combination with LT were compared with 13 patients receiving LT alone.231 Chimerism was detectable in more than half of the recipients 1 year posttransplant and in none of the control recipients tested. Among patients surviving > 4 months, OB developed in 1 of 22 BM and 4 of 12 control patients (p = 0.04). Patient survival and freedom from ACR were similar between groups. This technique has promise, but additional studies are required to determine the efficacy, safety, and role of this procedure in humans.
Conclusion
BOS is the dominant factor as to why long-term outcomes after LT remain disappointing. Although alloimmune pathways have a clear role in the pathogenesis of chronic rejection, nonspecific causes of airway injury also appear to promote the development of BOS. Airway injury can accelerate alloimmune responses via innate immune pathways and/or may expose antigens that activate autoimmune responses that lead to BOS independent of alloimmunity. Although nonspecific immunosuppression seems to allow lung allograft accommodation for some, most lung transplant recipients eventually experience late allograft dysfunction in the form of BOS. Unfortunately, there is currently no proven therapy for the prevention or treatment of BOS. Advances in our knowledge of risk factors and pathogenesis should lead to novel strategies for the prevention/treatment of BOS and improvements in long-term outcomes after LT.
References
- 1.Christie JD, Edwards LB, Kucheryavaya AY, et al. International Society of Heart and Lung Transplantation The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant. 2012;31(10):1073–1086. doi: 10.1016/j.healun.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Knoop C, Estenne M. Acute and chronic rejection after lung transplantation. Semin Respir Crit Care Med. 2006;27(5):521–533. doi: 10.1055/s-2006-954609. [DOI] [PubMed] [Google Scholar]
- 3.Vermeulen KM, Groen H, van der Bij W, Erasmus ME, Koëter GH, TenVergert EM. The effect of bronchiolitis obliterans syndrome on health related quality of life. Clin Transplant. 2004;18(4):377–383. doi: 10.1111/j.1399-0012.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- 4.van den Berg JW, van Enckevort PJ, TenVergert EM, Postma DS, van der Bij W, Koëter GH. Bronchiolitis obliterans syndrome and additional costs of lung transplantation. Chest. 2000;118(6):1648–1652. doi: 10.1378/chest.118.6.1648. [DOI] [PubMed] [Google Scholar]
- 5.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21(3):297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 6.Hachem RR, Chakinala MM, Yusen RD, et al. The predictive value of bronchiolitis obliterans syndrome stage 0-p. Am J Respir Crit Care Med. 2004;169(4):468–472. doi: 10.1164/rccm.200307-1018OC. [DOI] [PubMed] [Google Scholar]
- 7.Lama VN, Murray S, Mumford JA, et al. Prognostic value of bronchiolitis obliterans syndrome stage 0-p in single-lung transplant recipients. Am J Respir Crit Care Med. 2005;172(3):379–383. doi: 10.1164/rccm.200501-097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26(12):1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Yousem SA, Berry GJ, Cagle PT, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15(1Pt1):1–15. [PubMed] [Google Scholar]
- 10.Saggar R, Ross DJ, Saggar R, et al. Pulmonary hypertension associated with lung transplantation obliterative bronchiolitis and vascular remodeling of the allograft. Am J Transplant. 2008;8(9):1921–1930. doi: 10.1111/j.1600-6143.2008.02338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finlen Copeland CA, Snyder LD, Zaas DW, Turbyfill WJ, Davis WA, Palmer SM. Survival after bronchiolitis obliterans syndrome among bilateral lung transplant recipients. Am J Respir Crit Care Med. 2010;182(6):784–789. doi: 10.1164/rccm.201002-0211OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton CM, Carlsen J, Mortensen J, Andersen CB, Milman N, Iversen M. Long-term survival after lung transplantation depends on development and severity of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2007;26(7):681–686. doi: 10.1016/j.healun.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med. 2002;166(4):440–444. doi: 10.1164/rccm.200201-003pp. [DOI] [PubMed] [Google Scholar]
- 14.Heng D, Sharples LD, McNeil K, Stewart S, Wreghitt T, Wallwork J. Bronchiolitis obliterans syndrome: incidence, natural history, prognosis, and risk factors. J Heart Lung Transplant. 1998;17(12):1255–1263. [PubMed] [Google Scholar]
- 15.Jackson CH, Sharples LD, McNeil K, Stewart S, Wallwork J. Acute and chroniconsetof bronchiolitis obliterans syndrome (BOS): are they different entities? J Heart Lung Transplant. 2002;21(6):658–666. doi: 10.1016/s1053-2498(02)00381-9. [DOI] [PubMed] [Google Scholar]
- 16.Lama VN, Murray S, Lonigro RJ, et al. Course of FEV(1) after onset of bronchiolitis obliterans syndrome in lung transplant recipients. Am J Respir Crit Care Med. 2007;175(11):1192–1198. doi: 10.1164/rccm.200609-1344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verleden G, Dupont L. Lung and Heart–Transplantation Lung Obliterative bronchiolitis. In: Lynch JP III, Ross DJ, editors. Lung Biology in Health and Disease. Vol. 217. New York: Taylor & Francis; 2006. pp. 723–751. [Google Scholar]
- 18.Borthwick LA, McIlroy EI, Gorowiec MR, et al. Inflammation and epithelial to mesenchymal transition in lung transplant recipients: role in dysregulated epithelial wound repair. Am J Transplant. 2010;10(3):498–509. doi: 10.1111/j.1600-6143.2009.02953.x. [DOI] [PubMed] [Google Scholar]
- 19.Borthwick LA, Parker SM, Brougham KA, et al. Epithelial to mesenchymal transition (EMT) and airway remodelling after human lung transplantation. Thorax. 2009;64(9):770–777. doi: 10.1136/thx.2008.104133. [DOI] [PubMed] [Google Scholar]
- 20.Hodge S, Holmes M, Banerjee B, et al. Posttransplant bronchiolitis obliterans syndrome is associated with bronchial epithelial to mesenchymal transition. Am J Transplant. 2009;9(4):727–733. doi: 10.1111/j.1600-6143.2009.02558.x. [DOI] [PubMed] [Google Scholar]
- 21.Iacono A, Dauber J, Keenan R, et al. Interleukin 6 and interferon-gamma gene expression in lung transplant recipients with refractory acute cellular rejection: implications for monitoring and inhibition by treatment with aerosolized cyclosporine. Transplantation. 1997;64(2):263–269. doi: 10.1097/00007890-199707270-00015. [DOI] [PubMed] [Google Scholar]
- 22.Lu KC, Jaramillo A, Lecha RL, et al. Interleukin-6 and interferon-gamma gene polymorphisms in the development of bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2002;74(9):1297–1302. doi: 10.1097/00007890-200211150-00017. [DOI] [PubMed] [Google Scholar]
- 23.Meloni F, Vitulo P, Cascina A, et al. Bronchoalveolar lavage cytokine profile in a cohort of lung transplant recipients: a predictive role of interleukin-12 with respect to onset of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2004;23(9):1053–1060. doi: 10.1016/j.healun.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Moudgil A, Bagga A, Toyoda M, Nicolaidou E, Jordan SC, Ross D. Expression of gamma-IFN mRNA in bronchoalveolar lavage fluid correlates with early acute allograft rejection in lung transplant recipients. Clin Transplant. 1999;13(2):201–207. doi: 10.1034/j.1399-0012.1999.130208.x. [DOI] [PubMed] [Google Scholar]
- 25.Neuringer IP, Walsh SP, Mannon RB, Gabriel S, Aris RM. Enhanced T cell cytokine gene expression in mouse airway obliterative bronchiolitis. Transplantation. 2000;69(3):399–405. doi: 10.1097/00007890-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 26.Räisänen-Sokolowski A, Glysing-Jensen T, Russell ME. Leukocyte-suppressing influences of interleukin (IL)-10 in cardiac allografts: insights from IL-10 knockout mice. Am J Pathol. 1998;153(5):1491–1500. doi: 10.1016/S0002-9440(10)65737-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keane MP, Gomperts BN, Weigt S, et al. IL-13 is pivotal in the fibroobliterative process of bronchiolitis obliterans syndrome. J Immunol. 2007;178(1):511–519. doi: 10.4049/jimmunol.178.1.511. [DOI] [PubMed] [Google Scholar]
- 28.Lama VN, Harada H, Badri LN, et al. Obligatory role for interleu-kin-13 in obstructivelesion development in airway allografts. Am J Pathol. 2006;169(1):47–60. doi: 10.2353/ajpath.2006.050975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhai Y, Ghobrial RM, Busuttil RW, Kupiec-Weglinski JW. Th1 and Th2 cytokines in organ transplantation: paradigm lost? Crit Rev Immunol. 1999;19(2):155–172. [PubMed] [Google Scholar]
- 30.Heidt S, Segundo DS, Chadha R, Wood KJ. The impact of Th17 cells on transplant rejection and the induction of tolerance. Curr Opin Organ Transplant. 2010;15(4):456–461. doi: 10.1097/MOT.0b013e32833b9bfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chanut-Delalande H, Fichard A, Bernocco S, Garrone R, Hulmes DJ, Ruggiero F. Control of heterotypic fibril formation by collagen V is determined by chain stoichiometry. J Biol Chem. 2001;276(26):24352–24359. doi: 10.1074/jbc.m101182200. [DOI] [PubMed] [Google Scholar]
- 32.Iwata T, Chiyo M, Yoshida S, et al. Lung transplant ischemia reperfusion injury: metalloprotease inhibition down-regulates exposure of type V collagen, growth-related oncogene-induced neutrophil chemotaxis, and tumor necrosis factor-alpha expression. Transplantation. 2008;85(3):417–426. doi: 10.1097/TP.0b013e31815e91b6. [DOI] [PubMed] [Google Scholar]
- 33.Hachem RR, Tiriveedhi V, Patterson GA, Aloush A, Trulock EP, Mohanakumar T. Antibodies to K-α 1 tubulin and collagen V are associated with chronic rejection after lung transplantation. Am J Transplant. 2012;12(8):2164–2171. doi: 10.1111/j.1600-6143.2012.04079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haque MA, Mizobuchi T, Yasufuku K, et al. Evidence for immune responses to a self-antigen in lung transplantation: role of type V collagen-specific T cells in the pathogenesis of lung allograft rejection. J Immunol. 2002;169(3):1542–1549. doi: 10.4049/jimmunol.169.3.1542. [DOI] [PubMed] [Google Scholar]
- 35.Bharat A, Fields RC, Trulock EP, Patterson GA, Mohanakumar T. Induction of IL-10 suppressors in lung transplant patients by CD4+25+ regulatory T cells through CTLA-4 signaling. J Immunol. 2006;177(8):5631–5638. doi: 10.4049/jimmunol.177.8.5631. [DOI] [PubMed] [Google Scholar]
- 36.Fukami N, Ramachandran S, Saini D, et al. Antibodies toMHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182(1):309–318. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burlingham WJ, Love RB, Jankowska-Gan E, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliter-ative bronchiolitis in human lung transplants. J Clin Invest. 2007;117(11):3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanaudenaerde BM, De Vleeschauwer SI, Vos R, et al. The role of the IL23/IL17 axis in bronchiolitis obliterans syndrome after lung transplantation. Am J Transplant. 2008;8(9):1911–1920. doi: 10.1111/j.1600-6143.2008.02321.x. [DOI] [PubMed] [Google Scholar]
- 39.Nakagiri T, Inoue M, Morii E, et al. Local IL-17 production and a decrease in peripheral blood regulatory T cells in an animal model of bronchiolitis obliterans. Transplantation. 2010;89(11):1312–1319. doi: 10.1097/TP.0b013e3181d8ea16. [DOI] [PubMed] [Google Scholar]
- 40.Fan L, Benson HL, Vittal R, et al. Neutralizing IL-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation. Am J Transplant. 2011;11(5):911–922. doi: 10.1111/j.1600-6143.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kollins D, Stoelcker B, Hoffmann U, et al. FOXP3+ regulatory T-cells in renal allografts: correlation with long-term graft function and acute rejection. Clin Nephrol. 2011;75(2):91–100. [PubMed] [Google Scholar]
- 42.Batsford S, Dickenmann M, Dürmüller U, Hopfer H, Gudat F, Mihatsch M. Ismonitoring of FOXP3 Treg cells in renal transplants during acute cellular rejection episodes useful? Clin Nephrol. 2011;75(2):101–106. [PubMed] [Google Scholar]
- 43.Iwase H, Kobayashi T, Kodera Y, et al. Clinical significance of regulatory T-cell-related gene expression in peripheral blood after renal transplantation. Transplantation. 2011;91(2):191–198. doi: 10.1097/TP.0b013e3181ffbab4. [DOI] [PubMed] [Google Scholar]
- 44.Bhorade SM, Chen H, Molinero L, et al. Decreased percentage of CD4+FoxP3+ cells in bronchoalveolar lavage from lung transplant recipients correlates with development of bronchiolitis obliterans syndrome. Transplantation. 2010;90(5):540–546. doi: 10.1097/TP.0b013e3181e8dabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gregson AL, Hoji A, Palchevskiy V, et al. Protection against bronchiolitis obliterans syndrome is associated with allograft CCR7+ CD45RA- T regulatory cells. PLoS ONE. 2010;5(6):e11354. doi: 10.1371/journal.pone.0011354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bröcker V, Länger F, Fellous TG, et al. Fibroblasts of recipient origin contribute to bronchiolitis obliterans in human lung transplants. Am J Respir Crit Care Med. 2006;173(11):1276–1282. doi: 10.1164/rccm.200509-1381OC. [DOI] [PubMed] [Google Scholar]
- 47.Kleeberger W, Versmold A, Rothämel T, et al. Increased chime-rism of bronchial and alveolar epithelium in human lung allografts undergoing chronic injury. Am J Pathol. 2003;162(5):1487–1494. doi: 10.1016/S0002-9440(10)64281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hertz MI, Henke CA, Nakhleh RE, et al. Obliterative bronchiolitis after lung transplantation: a fibroproliferative disorder associated with platelet-derived growth factor. Proc Natl Acad Sci U S A. 1992;89(21):10385–10389. doi: 10.1073/pnas.89.21.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kallio EA, Koskinen PK, Aavik E, Buchdunger E, Lemström KB. Role of platelet-derived growth factor in obliterative bronchiolitis (chronic rejection) in the rat. Am J Respir Crit Care Med. 1999;160(4):1324–1332. doi: 10.1164/ajrccm.160.4.9802006. [DOI] [PubMed] [Google Scholar]
- 50.Tikkanen JM, Hollmén M, Nykänen AI, Wood J, Koskinen PK, Lemström KB. Role of platelet-derived growth factor and vascular endothelial growth factor in obliterative airway disease. Am J Respir Crit Care Med. 2006;174(10):1145–1152. doi: 10.1164/rccm.200601-044OC. [DOI] [PubMed] [Google Scholar]
- 51.Aharinejad S, Taghavi S, Klepetko W, Abraham D. Prediction of lung-transplant rejection by hepatocyte growth factor. Lancet. 2004;363(9420):1503–1508. doi: 10.1016/S0140-6736(04)16148-5. [DOI] [PubMed] [Google Scholar]
- 52.Charpin JM, Stern M, Grenet D, Israël-Biet D. Insulinlike growth factor-1 in lung transplants with obliterative bronchiolitis. Am J Respir Crit Care Med. 2000;161(6):1991–1998. doi: 10.1164/ajrccm.161.6.9905049. [DOI] [PubMed] [Google Scholar]
- 53.El-Gamel A, Sim E, Hasleton P, et al. Transforming growth factor beta (TGF-beta) and obliterative bronchiolitis following pulmonary transplantation. J Heart Lung Transplant. 1999;18(9):828–837. doi: 10.1016/s1053-2498(99)00047-9. [DOI] [PubMed] [Google Scholar]
- 54.Belperio JA, Burdick MD, Keane MP, et al. The role of the CC chemokine, RANTES, in acute lung allograft rejection. J Immunol. 2000;165(1):461–472. doi: 10.4049/jimmunol.165.1.461. [DOI] [PubMed] [Google Scholar]
- 55.Belperio JA, Keane MP, Burdick MD, et al. Role of CXCL9/CXCR3 chemokine biology during pathogenesis of acute lung allograft rejection. J Immunol. 2003;171(9):4844–4852. doi: 10.4049/jimmunol.171.9.4844. [DOI] [PubMed] [Google Scholar]
- 56.Belperio JA, Keane MP, Burdick MD, et al. Role of CXCR2/CXCR2 ligands in vascular remodeling during bronchiolitis obliterans syndrome. J Clin Invest. 2005;115(5):1150–1162. doi: 10.1172/JCI24233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belperio JA, Keane MP, Burdick MD, et al. Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J Clin Invest. 2001;108(4):547–556. doi: 10.1172/JCI12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Belperio JA, Keane MP, Burdick MD, et al. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol. 2002;169(2):1037–1049. doi: 10.4049/jimmunol.169.2.1037. [DOI] [PubMed] [Google Scholar]
- 59.DiGiovine B, Lynch JP, III, Martinez FJ, et al. Bronchoalveolar lavage neutrophilia is associated with obliterative bronchiolitis after lung transplantation: role of IL-8. J Immunol. 1996;157(9):4194–4202. [PubMed] [Google Scholar]
- 60.Reynaud-Gaubert M, Marin V, Thirion X, et al. Upregulation of chemokines in bronchoalveolar lavage fluid as a predictive marker of post-transplant airway obliteration. J Heart Lung Transplant. 2002;21(7):721–730. doi: 10.1016/s1053-2498(02)00392-3. [DOI] [PubMed] [Google Scholar]
- 61.Vos R, Blondeau K, Vanaudenaerde BM, et al. Airway colonization and gastric aspiration after lung transplantation: do birds of a feather flock together? J Heart Lung Transplant. 2008;27(8):843–849. doi: 10.1016/j.healun.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 62.Neurohr C, Huppmann P, Samweber B, et al. Munich Lung Transplant Group Prognostic value of bronchoalveolar lavage neutrophilia in stable lung transplant recipients. J Heart Lung Transplant. 2009;28(5):468–474. doi: 10.1016/j.healun.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 63.Jiang X, Khan MA, Tian W, et al. Adenovirus-mediated HIF-1α gene transfer promotes repair of mouse airway allograft micro-vasculature and attenuates chronic rejection. J Clin Invest. 2011;121(6):2336–2349. doi: 10.1172/JCI46192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Le Moine A, Goldman M, Abramowicz D. Multiple pathways to allograft rejection. Transplantation. 2002;73(9):1373–1381. doi: 10.1097/00007890-200205150-00001. [DOI] [PubMed] [Google Scholar]
- 65.Chalermskulrat W, Neuringer IP, Schmitz JL, et al. Human leukocyte antigen mismatches predispose to the severity of bronchiolitis obliterans syndrome after lung transplantation. Chest. 2003;123(6):1825–1831. doi: 10.1378/chest.123.6.1825. [DOI] [PubMed] [Google Scholar]
- 66.Sundaresan S, Mohanakumar T, Smith MA, et al. HLA-A locus mismatches and development of antibodies to HLA after lung transplantation correlate with the development of bronchiolitis obliterans syndrome. Transplantation. 1998;65(5):648–653. doi: 10.1097/00007890-199803150-00008. [DOI] [PubMed] [Google Scholar]
- 67.SivaSai KS, Smith MA, Poindexter NJ, et al. Indirect recognition of donor HLA class I peptides in lung transplant recipients with bronchiolitis obliterans syndrome. Transplantation. 1999;67(8):1094–1098. doi: 10.1097/00007890-199904270-00002. [DOI] [PubMed] [Google Scholar]
- 68.Stanford RE, Ahmed S, Hodson M, Banner NR, Rose ML. A role for indirect allorecognition in lung transplant recipients with obliterative bronchiolitis. Am J Transplant. 2003;3(6):736–742. doi: 10.1034/j.1600-6143.2003.00142.x. [DOI] [PubMed] [Google Scholar]
- 69.Duncan SR, Leonard C, Theodore J, et al. Oligoclonal CD4(+) T cell expansions in lung transplant recipients with obliterative bronchiolitis. Am J Respir Crit Care Med. 2002;165(10):1439–1444. doi: 10.1164/rccm.2107009. [DOI] [PubMed] [Google Scholar]
- 70.Bando K, Paradis IL, Similo S, et al. Obliterative bronchiolitis after lung and heart-lung transplantation An analysis of risk factors and management. J Thorac Cardiovasc Surg. 1995;110(1):4–13. doi: 10.1016/S0022-5223(05)80003-0. discussion 13–14. [DOI] [PubMed] [Google Scholar]
- 71.Husain AN, Siddiqui MT, Holmes EW, et al. Analysis of risk factors for the development of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 1999;159(3):829–833. doi: 10.1164/ajrccm.159.3.9607099. [DOI] [PubMed] [Google Scholar]
- 72.Scott AI, Sharples LD, Stewart S. Bronchiolitis obliterans syndrome: risk factors and therapeutic strategies. Drugs. 2005;65(6):761–771. doi: 10.2165/00003495-200565060-00004. [DOI] [PubMed] [Google Scholar]
- 73.Verleden GM, Dupont LJ, Van Raemdonck DE. Is it bronchiolitis obliterans syndrome or is it chronic rejection: a reappraisal? Eur Respir J. 2005;25(2):221–224. doi: 10.1183/09031936.05.00057404. [DOI] [PubMed] [Google Scholar]
- 74.Hopkins PM, Aboyoun CL, Chhajed PN, et al. Association of minimal rejection in lung transplant recipients with obliterative bronchiolitis. Am J Respir Crit Care Med. 2004;170(9):1022–1026. doi: 10.1164/rccm.200302-165OC. [DOI] [PubMed] [Google Scholar]
- 75.Burton CM, Iversen M, Scheike T, Carlsen J, Andersen CB. Minimal acute cellular rejection remains prevalent up to 2 years after lung transplantation: a retrospective analysis of 2697 transbronchial biopsies. Transplantation. 2008;85(4):547–553. doi: 10.1097/TP.0b013e3181641df9. [DOI] [PubMed] [Google Scholar]
- 76.Kroshus TJ, Kshettry VR, Savik K, John R, Hertz MI, Bolman RM., III Risk factors for the development of bronchiolitis obliterans syndrome after lung transplantation. J Thorac Cardiovasc Surg. 1997;114(2):195–202. doi: 10.1016/S0022-5223(97)70144-2. [DOI] [PubMed] [Google Scholar]
- 77.Hachem RR, Khalifah AP, Chakinala MM, et al. The significance of a single episode of minimal acute rejection after lung transplantation. Transplantation. 2005;80(10):1406–1413. doi: 10.1097/01.tp.0000181161.60638.fa. [DOI] [PubMed] [Google Scholar]
- 78.Khalifah AP, Hachem RR, Chakinala MM, et al. Minimal acute rejection after lung transplantation: a risk for bronchiolitis obliterans syndrome. Am J Transplant. 2005;5(8):2022–2030. doi: 10.1111/j.1600-6143.2005.00953.x. [DOI] [PubMed] [Google Scholar]
- 79.Tazelaar HD, Yousem SA. The pathology of combined heart-lung transplantation: an autopsy study. Hum Pathol. 1988;19(12):1403–1416. doi: 10.1016/s0046-8177(88)80233-8. [DOI] [PubMed] [Google Scholar]
- 80.Girgis RE, Tu I, Berry GJ, et al. Risk factors for the development of obliterative bronchiolitis after lung transplantation. J Heart Lung Transplant. 1996;15(12):1200–1208. [PubMed] [Google Scholar]
- 81.Sharples LD, McNeil K, Stewart S, Wallwork J. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. J Heart Lung Transplant. 2002;21(2):271–281. doi: 10.1016/s1053-2498(01)00360-6. [DOI] [PubMed] [Google Scholar]
- 82.Glanville AR, Aboyoun CL, Havryk A, Plit M, Rainer S, Malouf MA. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. Am J Respir Crit Care Med. 2008;177(9):1033–1040. doi: 10.1164/rccm.200706-951OC. [DOI] [PubMed] [Google Scholar]
- 83.Takemoto SK, Zeevi A, Feng S, et al. National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant. 2004;4(7):1033–1041. doi: 10.1111/j.1600-6143.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 84.Bittner HB, Dunitz J, Hertz M, Bolman MR, III, Park SJ. Hyperacute rejection in single lung transplantation—case report of successful management by means of plasmapheresis and antithymocyte globulin treatment. Transplantation. 2001;71(5):649–651. doi: 10.1097/00007890-200103150-00012. [DOI] [PubMed] [Google Scholar]
- 85.Choi JK, Kearns J, Palevsky HI, et al. Hyperacute rejection of a pulmonary allograft Immediate clinical and pathologic findings. Am J Respir Crit Care Med. 1999;160(3):1015–1018. doi: 10.1164/ajrccm.160.3.9706115. [DOI] [PubMed] [Google Scholar]
- 86.de Jesus Peixoto Camargo J, Marcantonio Camargo S, Marcelo Schio S, Noguchi Machuca T, Adélia Perin F. Hyperacute rejection after single lung transplantation: a case report. Transplant Proc. 2008;40(3):867–869. doi: 10.1016/j.transproceed.2008.02.052. [DOI] [PubMed] [Google Scholar]
- 87.Frost AE, Jammal CT, Cagle PT. Hyperacute rejection following lung transplantation. Chest. 1996;110(2):559–562. doi: 10.1378/chest.110.2.559. [DOI] [PubMed] [Google Scholar]
- 88.Scornik JC, Zander DS, Baz MA, Donnelly WH, Staples ED. Susceptibility of lung transplants to preformed donor-specific HLA antibodies as detected by flow cytometry. Transplantation. 1999;68(10):1542–1546. doi: 10.1097/00007890-199911270-00018. [DOI] [PubMed] [Google Scholar]
- 89.Hadjiliadis D, Chaparro C, Reinsmoen NL, et al. Pre-transplant panel reactive antibody in lung transplant recipients is associated with significantly worse post-transplant survival in a multicenter study. J Heart Lung Transplant. 2005;24(7, Suppl):S249–S254. doi: 10.1016/j.healun.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 90.Shah AS, Nwakanma L, Simpkins C, Williams J, Chang DC, Conte JV. Pretransplant panel reactive antibodies in human lung transplantation: an analysis of over 10,000 patients. Ann Thorac Surg. 2008;85(6):1919–1924. doi: 10.1016/j.athoracsur.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 91.Yousem SA, Martin T, Paradis IL, Keenan R, Griffith BP. Can immunohistological analysis of transbronchial biopsy specimens predict responder status in early acute rejection of lung allografts? Hum Pathol. 1994;25(5):525–529. doi: 10.1016/0046-8177(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 92.Girnita AL, McCurry KR, Iacono AT, et al. HLA-specific antibodies are associated with high-grade and persistent-recurrent lung allograft acute rejection. J Heart Lung Transplant. 2004;23(10):1135–1141. doi: 10.1016/j.healun.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 93.Girnita AL, Duquesnoy R, Yousem SA, et al. HLA-specific antibodies are risk factors for lymphocytic bronchiolitis and chronic lung allograft dysfunction. Am J Transplant. 2005;5(1):131–138. doi: 10.1111/j.1600-6143.2004.00650.x. [DOI] [PubMed] [Google Scholar]
- 94.Palmer SM, Davis RD, Hadjiliadis D, et al. Development of an antibody specific to major histocompatibility antigens detectable by flow cytometry after lung transplant is associated with bronchiolitis obliterans syndrome. Transplantation. 2002;74(6):799–804. doi: 10.1097/00007890-200209270-00011. [DOI] [PubMed] [Google Scholar]
- 95.Smith MA, Sundaresan S, Mohanakumar T, et al. Effect of development of antibodies to HLA and cytomegalovirus mismatch on lung transplantation survival and development of bronchiolitis obliterans syndrome. J Thorac Cardiovasc Surg. 1998;116(5):812–820. doi: 10.1016/S0022-5223(98)00444-9. [DOI] [PubMed] [Google Scholar]
- 96.Maruyama T, Jaramillo A, Narayanan K, Higuchi T, Mohanakumar T. Induction of obliterative airway disease by anti-HLA class I antibodies. Am J Transplant. 2005;5(9):2126–2134. doi: 10.1111/j.1600-6143.2005.00999.x. [DOI] [PubMed] [Google Scholar]
- 97.Westall GP, Snell GI, McLean C, Kotsimbos T, Williams T, Magro C. C3d and C4d deposition early after lung transplantation. J Heart Lung Transplant. 2008;27(7):722–728. doi: 10.1016/j.healun.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 98.Wallace WD, Reed EF, Ross D, Lassman CR, Fishbein MC. C4d staining of pulmonary allograft biopsies: an immunoperoxidase study. J Heart Lung Transplant. 2005;24(10):1565–1570. doi: 10.1016/j.healun.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 99.Yousem SA, Zeevi A. The histopathology of lung allograft dysfunction associated with the development of donor-specific HLA alloantibodies. Am J Surg Pathol. 2012;36(7):987–992. doi: 10.1097/PAS.0b013e31825197ae. [DOI] [PubMed] [Google Scholar]
- 100.DeNicola MM, Weigt SS, Belperio JA, Reed EF, Ross DJ, Wallace WD. Pathologic findings in lung allografts with anti-HLA antibodies. J Heart Lung Transplant. 2013;32(3):326–332. doi: 10.1016/j.healun.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kalache S, Dinavahi R, Pinney S, Mehrotra A, Cunningham MW, Heeger PS. Anticardiac myosin immunity and chronic allograft vasculopathy in heart transplant recipients. J Immunol. 2011;187(2):1023–1030. doi: 10.4049/jimmunol.1004195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nath DS, Ilias Basha H, Tiriveedhi V, et al. Characterization of immune responses to cardiac self-antigens myosin and vimentin in human cardiac allograft recipients with antibody-mediated rejection and cardiac allograft vasculopathy. J Heart Lung Transplant. 2010;29(11):1277–1285. doi: 10.1016/j.healun.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lemy A, Andrien M, Lionet A, et al. Posttransplant major histocompatibility complex class I chain-related gene A antibodies and long-term graft outcomes in a multicenter cohort of 779 kidney transplant recipients. Transplantation. 2012;93(12):1258–1264. doi: 10.1097/TP.0b013e31824fd8f1. [DOI] [PubMed] [Google Scholar]
- 104.Reinsmoen NL, Lai CH, Heidecke H, et al. Antiangiotensin type 1 receptor antibodies associated with antibody mediated rejection in donor HLA antibody negative patients. Transplantation. 2010;90(12):1473–1477. doi: 10.1097/TP.0b013e3181fd97f1. [DOI] [PubMed] [Google Scholar]
- 105.Utsumi K, Sawada T, Adachi E, et al. Collagen type IV in acute rejection of kidney. Transplant Proc. 2000;32(7):1785. doi: 10.1016/s0041-1345(00)01374-9. [DOI] [PubMed] [Google Scholar]
- 106.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180(7):4487–4494. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saini D, Weber J, Ramachandran S, et al. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant. 2011;30(6):624–631. doi: 10.1016/j.healun.2011.01.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Adachi E, Hopkinson I, Hayashi T. Basement-membrane stromal relationships: interactions between collagen fibrils and the lamina densa. Int Rev Cytol. 1997;173:73–156. doi: 10.1016/s0074-7696(08)62476-6. [DOI] [PubMed] [Google Scholar]
- 109.Yasufuku K, Heidler KM, Woods KA, et al. Prevention of bronchiolitis obliterans in rat lung allografts by type V collagen-induced oral tolerance. Transplantation. 2002;73(4):500–505. doi: 10.1097/00007890-200202270-00002. [DOI] [PubMed] [Google Scholar]
- 110.Tiriveedhi V, Angaswamy N, Brand D, et al. A shift in the collagen Vantigenic epitope leads toT helper phenotype switch and immune response to self-antigen leading to chronic lung allograft rejection. Clin Exp Immunol. 2012;167(1):158–168. doi: 10.1111/j.1365-2249.2011.04486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gull K, Hussey PJ, Sasse R, Schneider A, Seebeck T, Sherwin T. Tubulin isotypes: generation of diversity in cells and microtubular organelles. J Cell Sci Suppl. 1986;5:243–255. doi: 10.1242/jcs.1986.supplement_5.16. [DOI] [PubMed] [Google Scholar]
- 112.Takano T, Hasegawa Y, Miyauchi A, et al. Overexpression of kalpha1 tubulin mRNA in thyroid anaplastic carcinoma. Cancer Lett. 2001;168(1):51–55. doi: 10.1016/s0304-3835(01)00509-2. [DOI] [PubMed] [Google Scholar]
- 113.Villasante A, Wang D, Dobner P, Dolph P, Lewis SA, Cowan NJ. Six mouse alpha-tubulin mRNA sencode five distinct isotypes: testis-specific expression of two sister genes. Mol Cell Biol. 1986;6(7):2409–2419. doi: 10.1128/mcb.6.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Murphy DB. Functions of tubulin isoforms. Curr Opin Cell Biol. 1991;3(1):43–51. doi: 10.1016/0955-0674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 115.Wang N, Yan K, Rasenick MM. Tubulin binds specifically to the signal-transducing proteins, Gs alpha and Gi alpha 1. J Biol Chem. 1990;265(3):1239–1242. [PubMed] [Google Scholar]
- 116.Bharat A, Saini D, Steward N, et al. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann Thorac Surg. 2010;90(4):1094–1101. doi: 10.1016/j.athoracsur.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Andrade CF, Waddell TK, Keshavjee S, Liu M. Innate immunity and organ transplantation: the potential role of toll-like receptors. Am J Transplant. 2005;5(5):969–975. doi: 10.1111/j.1600-6143.2005.00829.x. [DOI] [PubMed] [Google Scholar]
- 118.Chen L, Wang T, Zhou P, et al. TLR engagement prevents transplantation tolerance. Am J Transplant. 2006;6(10):2282–2291. doi: 10.1111/j.1600-6143.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 119.Porrett PM, Yuan X, LaRosa DF, et al. Mechanisms underlying blockade of allograft acceptance by TLR ligands. J Immunol. 2008;181(3):1692–1699. doi: 10.4049/jimmunol.181.3.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Garantziotis S, Palmer SM, Snyder LD, et al. Alloimmune lung injury induced by local innate immune activation through inhaled lipopolysaccharide. Transplantation. 2007;84(8):1012–1019. doi: 10.1097/01.tp.0000286040.85007.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Palmer SM, Burch LH, Trindade AJ, et al. Innate immunity influences long-term outcomes after human lung transplant. Am J Respir Crit Care Med. 2005;171(7):780–785. doi: 10.1164/rccm.200408-1129OC. [DOI] [PubMed] [Google Scholar]
- 122.Kastelijn EA, van Moorsel CH, Rijkers GT, et al. Polymorphisms in innate immunity genes associated with development of bronchi-olitis obliterans after lung transplantation. J Heart Lung Transplant. 2010;29(6):665–671. doi: 10.1016/j.healun.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 123.Palmer SM, Klimecki W, Yu L, et al. Genetic regulation of rejection and survival following human lung transplantation by the innate immune receptor CD14. Am J Transplant. 2007;7(3):693–699. doi: 10.1111/j.1600-6143.2007.01669.x. [DOI] [PubMed] [Google Scholar]
- 124.Carroll KE, Dean MM, Heatley SL, et al. High levels of mannose-binding lectin are associated with poor outcomes after lung transplantation. Transplantation. 2011;91(9):1044–1049. doi: 10.1097/TP.0b013e318212c7d6. [DOI] [PubMed] [Google Scholar]
- 125.Hodge S, Dean M, Hodge G, Holmes M, Reynolds PN. Decreased efferocytosis and mannose binding lectin in the airway in bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2011;30(5):589–595. doi: 10.1016/j.healun.2011.01.710. [DOI] [PubMed] [Google Scholar]
- 126.Budd SJ, Aris RM, Medaiyese AA, Tilley SL, Neuringer IP. Increased plasma mannose binding lectin levels are associated with bronchiolitis obliterans after lung transplantation. Respir Res. 2012;13:56. doi: 10.1186/1465-9921-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. ISHLTWorking Group on Primary Lung Graft Dysfunction. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24(10):1454–1459. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 128.Andrade CF, Kaneda H, Der S, et al. Toll-like receptor and cytokine gene expression in the early phase of human lung transplantation. J Heart Lung Transplant. 2006;25(11):1317–1323. doi: 10.1016/j.healun.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 129.Sugimoto S, Lin X, Okazaki M, et al. Monocyte differentiation is controlled by MyD88 after mouse orthotopic lung transplantation. Transplant Proc. 2009;41(1):388–390. doi: 10.1016/j.transproceed.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 130.Kaczorowski DJ, Tsung A, Billiar TR. Innate immune mechanisms in ischemia/reperfusion. Front Biosci (Elite Ed. 2009;1:91–98. doi: 10.2741/E10. [DOI] [PubMed] [Google Scholar]
- 131.Moreno I, Vicente R, Ramos F, Vicente JL, Barberá M. Determination of interleukin-6 in lung transplantation: association with primary graft dysfunction. Transplant Proc. 2007;39(7):2425–2426. doi: 10.1016/j.transproceed.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 132.Serrick C, Adoumie R, Giaid A, Shennib H. The early release of interleukin-2, tumor necrosis factor-alpha and interferon-gamma after ischemia reperfusion injury in the lung allograft. Transplantation. 1994;58(11):1158–1162. [PubMed] [Google Scholar]
- 133.Karakurum M, Shreeniwas R, Chen J, et al. Hypoxic induction of interleukin-8 gene expression in human endothelial cells. J Clin Invest. 1994;93(4):1564–1570. doi: 10.1172/JCI117135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Belperio JA, Keane MP, Burdick MD, et al. CXCR2/CXCR2 ligand biology during lung transplant ischemia-reperfusion injury. J Immunol. 2005;175(10):6931–6939. doi: 10.4049/jimmunol.175.10.6931. [DOI] [PubMed] [Google Scholar]
- 135.Shah RJ, Diamond JM, Lederer DJ, et al. Plasma monocyte chemo-tactic protein-1 levels at 24 hours are a biomarker of primary graft dysfunction after lung transplantation. Transl Res. 2012;160(6):435–442. doi: 10.1016/j.trsl.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Waddell TK, Gorczynski RM, DeCampos KN, Patterson GA, Slutsky AS. Major histocompatibility complex expression and lung ischemia-reperfusion in rats. Ann Thorac Surg. 1996;62(3):866–872. doi: 10.1016/s0003-4975(96)00509-7. [DOI] [PubMed] [Google Scholar]
- 137.Bharat A, Narayanan K, Street T, et al. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83(2):150–158. doi: 10.1097/01.tp.0000250579.08042.b6. [DOI] [PubMed] [Google Scholar]
- 138.Prekker ME, Nath DS, Walker AR, et al. Validation of the proposed International Society for Heart and Lung Transplantation grading system for primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2006;25(4):371–378. doi: 10.1016/j.healun.2005.11.436. [DOI] [PubMed] [Google Scholar]
- 139.Christie JD, Kotloff RM, Ahya VN, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005;171(11):1312–1316. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175(5):507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 141.Whitson BA, Prekker ME, Herrington CS, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant. 2007;26(10):1004–1011. doi: 10.1016/j.healun.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 142.Diamond JM, Lederer DJ, Kawut SM, et al. Lung Transplant Outcomes Group Elevated plasma long pentraxin-3 levels and primary graft dysfunction after lung transplantation for idiopathic pulmonary fibrosis. Am J Transplant. 2011;11(11):2517–2522. doi: 10.1111/j.1600-6143.2011.03702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Diamond JM, Meyer NJ, Feng R, et al. Lung Transplant Outcomes Group Variation in PTX3 is associated with primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2012;186(6):546–552. doi: 10.1164/rccm.201204-0692OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Young LR, Hadjiliadis D, Davis RD, Palmer SM. Lung transplantation exacerbates gastroesophageal reflux disease. Chest. 2003;124(5):1689–1693. doi: 10.1378/chest.124.5.1689. [DOI] [PubMed] [Google Scholar]
- 145.Basseri B, Conklin JL, Pimentel M, et al. Esophageal motor dysfunction and gastroesophageal reflux are prevalent in lung transplant candidates. Ann Thorac Surg. 2010;90(5):1630–1636. doi: 10.1016/j.athoracsur.2010.06.104. [DOI] [PubMed] [Google Scholar]
- 146.Li B, Hartwig MG, Appel JZ, et al. Chronic aspiration of gastric fluid induces the development of obliterative bronchiolitis in rat lung transplants. Am J Transplant. 2008;8(8):1614–1621. doi: 10.1111/j.1600-6143.2008.02298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.D’Ovidio F, Mura M, Tsang M, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. J Thorac Cardiovasc Surg. 2005;129(5):1144–1152. doi: 10.1016/j.jtcvs.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 148.Blondeau K, Mertens V, Vanaudenaerde BA, et al. Gastro-oesophageal reflux and gastric aspiration in lung transplant patients with or without chronic rejection. Eur Respir J. 2008;31(4):707–713. doi: 10.1183/09031936.00064807. [DOI] [PubMed] [Google Scholar]
- 149.Mertens V, Blondeau K, Van Oudenhove L, et al. Bile acids aspiration reduces survival in lung transplant recipients with BOS despite azithromycin. Am J Transplant. 2011;11(2):329–335. doi: 10.1111/j.1600-6143.2010.03380.x. [DOI] [PubMed] [Google Scholar]
- 150.Shah N, Force SD, Mitchell PO, et al. Gastroesophageal reflux disease is associated with an increased rate of acute rejection in lung transplant allografts. Transplant Proc. 2010;42(7):2702–2706. doi: 10.1016/j.transproceed.2010.05.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Murthy SC, Nowicki ER, Mason DP, et al. Pretransplant gastro-esophageal reflux compromises early outcomes after lung transplantation. J Thorac Cardiovasc Surg. 2011;142(1):47–52. doi: 10.1016/j.jtcvs.2011.04.028. e3. [DOI] [PubMed] [Google Scholar]
- 152.Hartwig MG, Anderson DJ, Onaitis MW, et al. Fundoplication after lung transplantation prevents the allograft dysfunction associated with reflux. Ann Thorac Surg. 2011;92(2):462–468. doi: 10.1016/j.athoracsur.2011.04.035. 468–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.D’Ovidio F, Mura M, Ridsdale R, et al. The effect of reflux and bile acid aspiration on the lung allograft and its surfactant and innate immunity molecules SP-A and SP-D. Am J Transplant. 2006;6(8):1930–1938. doi: 10.1111/j.1600-6143.2006.01357.x. [DOI] [PubMed] [Google Scholar]
- 154.Tang T, Chang JC, Xie A, Davis RD, Parker W, Lin SS. Aspiration of gastric fluid in pulmonary allografts: Effect of pH. J Surg Res. 2013;181(1):e31–e38. doi: 10.1016/j.jss.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 155.Hoppo T, Jarido V, Pennathur A, et al. Antireflux surgery preserves lung function in patients with gastroesophageal reflux disease and end-stage lung disease before and after lung transplantation. Arch Surg. 2011;146(9):1041–1047. doi: 10.1001/archsurg.2011.216. [DOI] [PubMed] [Google Scholar]
- 156.Keenan RJ, Lega ME, Dummer JS, et al. Cytomegalovirus serologic status and postoperative infection correlated with risk of developing chronic rejection after pulmonary transplantation. Transplantation. 1991;51(2):433–438. doi: 10.1097/00007890-199102000-00032. [DOI] [PubMed] [Google Scholar]
- 157.Cerrina J, Le Roy Ladurie F, Herve PH, et al. Role of CMV pneumonia in the development of obliterative bronchiolitis in heart-lung and double-lung transplant recipients. Transpl Int. 1992;5(Suppl 1):S242–S245. doi: 10.1007/978-3-642-77423-2_77. [DOI] [PubMed] [Google Scholar]
- 158.Paraskeva M, Bailey M, Levvey BJ, et al. Cytomegalovirus replication within the lung allograft is associated with bronchiolitis obliterans syndrome. Am J Transplant. 2011;11(10):2190–2196. doi: 10.1111/j.1600-6143.2011.03663.x. [DOI] [PubMed] [Google Scholar]
- 159.Luckraz H, Sharples L, McNeil K, Wreghitt T, Wallwork J. Cytomegalovirus antibody status of donor/recipient does not influence the incidence of bronchiolitis obliterans syndrome in lung transplantation. J Heart Lung Transplant. 2003;22(3):287–291. doi: 10.1016/s1053-2498(02)00471-0. [DOI] [PubMed] [Google Scholar]
- 160.Keller CA, Cagle PT, Brown RW, Noon G, Frost AE. Bronchiolitis obliterans in recipients of single, double, and heart-lung transplantation. Chest. 1995;107(4):973–980. doi: 10.1378/chest.107.4.973. [DOI] [PubMed] [Google Scholar]
- 161.Reichenspurner H, Girgis RE, Robbins RC, et al. Stanford experience with obliterative bronchiolitis after lung and heart-lung transplantation. Ann Thorac Surg. 1996;62(5):1467–1472. doi: 10.1016/0003-4975(96)00776-X. discussion 1472–1473. [DOI] [PubMed] [Google Scholar]
- 162.Weigt SS, Elashoff RM, Keane MP, et al. Altered levels of CC chemokines during pulmonary CMV predict BOS and mortality post-lung transplantation. Am J Transplant. 2008;8(7):1512–1522. doi: 10.1111/j.1600-6143.2008.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ruttmann E, Geltner C, Bucher B, et al. Combined CMV prophylaxis improves outcome and reduces the risk for bronchiolitis obliterans syndrome (BOS) after lung transplantation. Transplantation. 2006;81(10):1415–1420. doi: 10.1097/01.tp.0000209439.27719.ed. [DOI] [PubMed] [Google Scholar]
- 164.Chmiel C, Speich R, Hofer M, et al. Ganciclovir/valganciclovir prophylaxis decreases cytomegalovirus-related events and bronchiolitis obliterans syndrome after lung transplantation. Clin Infect Dis. 2008;46(6):831–839. doi: 10.1086/528689. [DOI] [PubMed] [Google Scholar]
- 165.Khalifah AP, Hachem RR, Chakinala MM, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170(2):181–187. doi: 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]
- 166.Kumar D, Erdman D, Keshavjee S, et al. Clinical impact of community-acquired respiratory viruses on bronchiolitis obliterans after lung transplant. Am J Transplant. 2005;5(8):2031–2036. doi: 10.1111/j.1600-6143.2005.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kumar D, Husain S, Chen MH, et al. A prospective molecular surveillance study evaluating the clinical impact of community-acquired respiratory viruses in lung transplant recipients. Transplantation. 2010;89(8):1028–1033. doi: 10.1097/TP.0b013e3181d05a71. [DOI] [PubMed] [Google Scholar]
- 168.Gottlieb J, Schulz TF, Welte T, et al. Community-acquired respiratory viral infections in lung transplant recipients: a single season cohort study. Transplantation. 2009;87(10):1530–1537. doi: 10.1097/TP.0b013e3181a4857d. [DOI] [PubMed] [Google Scholar]
- 169.Neurohr C, Huppmann P, Leuchte H, et al. Munich Lung Transplant Group Human herpesvirus 6 in bronchalveolar lavage fluid after lung transplantation: a risk factor for bronchiolitis obliterans syndrome? Am J Transplant. 2005;5(12):2982–2991. doi: 10.1111/j.1600-6143.2005.01103.x. [DOI] [PubMed] [Google Scholar]
- 170.Kotsimbos TC, Snell GI, Levvey B, et al. Chlamydia pneumoniae serology in donors and recipients and the risk of bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2005;79(3):269–275. doi: 10.1097/01.tp.0000149839.87843.64. [DOI] [PubMed] [Google Scholar]
- 171.Weigt SS, Derhovanessian A, Liao E, et al. CXCR3 chemokine ligands during respiratory viral infections predict lung allograft dysfunction. Am J Transplant. 2012;12(2):477–484. doi: 10.1111/j.1600-6143.2011.03859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Botha P, Archer L, Anderson RL, et al. Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation. 2008;85(5):771–774. doi: 10.1097/TP.0b013e31816651de. [DOI] [PubMed] [Google Scholar]
- 173.Vos R, Vanaudenaerde BM, Geudens N, Dupont LJ, Van Raemdonck DE, Verleden GM. Pseudomonal airway colonisation: risk factor for bronchiolitis obliterans syndrome after lung transplantation? Eur Respir J. 2008;31(5):1037–1045. doi: 10.1183/09031936.00128607. [DOI] [PubMed] [Google Scholar]
- 174.Gottlieb J, Mattner F, Weissbrodt H, et al. Impact of graft colonization with gram-negative bacteria after lung transplantation on the development of bronchiolitis obliterans syndrome in recipients with cystic fibrosis. Respir Med. 2009;103(5):743–749. doi: 10.1016/j.rmed.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 175.Vos R, Vanaudenaerde BM, Verleden SE, et al. Diagnostic value of antibodies against Pseudomonas aeruginosa in bronchoalveolar lavage fluid after lung transplantation. Transplant Proc. 2010;42(10):4415–4420. doi: 10.1016/j.transproceed.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 176.Weigt SS, Elashoff RM, Huang C, et al. Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. Am J Transplant. 2009;9(8):1903–1911. doi: 10.1111/j.1600-6143.2009.02635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Martinu T, Howell DN, Davis RD, Steele MP, Palmer SM. Pathologic correlates of bronchiolitis obliterans syndrome in pulmonary retransplant recipients. Chest. 2006;129(4):1016–1023. doi: 10.1378/chest.129.4.1016. [DOI] [PubMed] [Google Scholar]
- 178.Speich R, Boehler A, Thurnheer R, Weder W. Salvage therapy with mycophenolate mofetil for lung transplant bronchiolitis obliterans: importance of dosage. Transplantation. 1997;64(3):533–535. doi: 10.1097/00007890-199708150-00027. [DOI] [PubMed] [Google Scholar]
- 179.Whyte RI, Rossi SJ, Mulligan MS, et al. Mycophenolate mofetil for obliterative bronchiolitis syndrome after lung transplantation. Ann Thorac Surg. 1997;64(4):945–948. doi: 10.1016/s0003-4975(97)00845-x. [DOI] [PubMed] [Google Scholar]
- 180.Borro JM, Bravo C, Solé A, et al. Conversion from cyclosporine to tacrolimus stabilizes the course of lung function in lung transplant recipients with bronchiolitis obliterans syndrome. Transplant Proc. 2007;39(7):2416–2419. doi: 10.1016/j.transproceed.2007.06.071. [DOI] [PubMed] [Google Scholar]
- 181.Cairn J, Yek T, Banner NR, Khaghani A, Hodson ME, Yacoub M. Time-related changes in pulmonary function after conversion to tacrolimus in bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2003;22(1):50–57. doi: 10.1016/s1053-2498(02)00548-x. [DOI] [PubMed] [Google Scholar]
- 182.Sarahrudi K, Carretta A, Wisser W, et al. The value of switching from cyclosporine to tacrolimus in the treatment of refractory acute rejection and obliterative bronchiolitis after lung transplantation. Transpl Int. 2002;15(1):24–28. doi: 10.1007/s00147-001-0370-0. [DOI] [PubMed] [Google Scholar]
- 183.Snell GI, Esmore DS, Williams TJ. Cytolytic therapy for the bronchiolitis obliterans syndrome complicating lung transplantation. Chest. 1996;109(4):874–878. doi: 10.1378/chest.109.4.874. [DOI] [PubMed] [Google Scholar]
- 184.Kesten S, Rajagopalan N, Maurer J. Cytolytic therapy for the treatment of bronchiolitis obliterans syndrome following lung transplantation. Transplantation. 1996;61(3):427–430. doi: 10.1097/00007890-199602150-00019. [DOI] [PubMed] [Google Scholar]
- 185.Reams BD, Musselwhite LW, Zaas DW, et al. Alemtuzumab in the treatment of refractory acute rejection and bronchiolitis obliterans syndrome after human lung transplantation. Am J Transplant. 2007;7(12):2802–2808. doi: 10.1111/j.1600-6143.2007.02000.x. [DOI] [PubMed] [Google Scholar]
- 186.Gerhardt SG, McDyer JF, Girgis RE, Conte JV, Yang SC, Orens JB. Maintenance azithromycin therapy for bronchiolitis obliterans syndrome: results of a pilot study. Am J Respir Crit Care Med. 2003;168(1):121–125. doi: 10.1164/rccm.200212-1424BC. [DOI] [PubMed] [Google Scholar]
- 187.Gottlieb J, Szangolies J, Koehnlein T, Golpon H, Simon A, Welte T. Long-term azithromycin for bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2008;85(1):36–41. doi: 10.1097/01.tp.0000295981.84633.bc. [DOI] [PubMed] [Google Scholar]
- 188.Porhownik NR, Batobara W, Kepron W, Unruh HW, Bshouty Z. Effect of maintenance azithromycin on established bronchiolitis obliterans syndrome in lung transplant patients. Can Respir J. 2008;15(4):199–202. doi: 10.1155/2008/158681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shitrit D, Bendayan D, Gidon S, Saute M, Bakal I, Kramer MR. Long-term azithromycin use for treatment of bronchiolitis obliterans syndrome in lung transplant recipients. J Heart Lung Transplant. 2005;24(9):1440–1443. doi: 10.1016/j.healun.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 190.Yates B, Murphy DM, Forrest IA, et al. Azithromycin reverses airflow obstruction in established bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2005;172(6):772–775. doi: 10.1164/rccm.200411-1537OC. [DOI] [PubMed] [Google Scholar]
- 191.Vanaudenaerde BM, Meyts I, Vos R, et al. A dichotomy in bronchiolitis obliterans syndrome after lung transplantation revealed by azithromycin therapy. Eur Respir J. 2008;32(4):832–843. doi: 10.1183/09031936.00134307. [DOI] [PubMed] [Google Scholar]
- 192.Corris P, Small T, Ryan V, et al. A Randomised Controlled Trial Of Azithromycin Therapy In Bronchiolitis Obliterans Syndrome (BOS) Post Lung Transplantation [abstract] Am J Respir Crit Care Med. 2012;185:A5324. doi: 10.1136/thoraxjnl-2014-205998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Knobler R. Extracorporeal photochemotherapy—present and future. Vox Sang. 2000;78(Suppl 2):197–201. [PubMed] [Google Scholar]
- 194.Rook AH, Cohen JH, Lessin SR, Vowels BR. Therapeutic applications of photopheresis. Dermatol Clin. 1993;11(2):339–347. [PubMed] [Google Scholar]
- 195.Barr ML, Meiser BM, Eisen HJ, et al. Photopheresis Transplantation Study Group Photopheresis for the prevention of rejection in cardiac transplantation. N Engl J Med. 1998;339(24):1744–1751. doi: 10.1056/NEJM199812103392404. [DOI] [PubMed] [Google Scholar]
- 196.Costanzo-Nordin MR, Hubbell EA, O’Sullivan EJ, et al. Successful treatment of heart transplant rejection with photopheresis. Transplantation. 1992;53(4):808–815. doi: 10.1097/00007890-199204000-00021. [DOI] [PubMed] [Google Scholar]
- 197.Benden C, Speich R, Hofbauer GF, et al. Extracorporeal photopheresis after lung transplantation: a 10-year single-center experience. Transplantation. 2008;86(11):1625–1627. doi: 10.1097/TP.0b013e31818bc024. [DOI] [PubMed] [Google Scholar]
- 198.Jaksch P, Scheed A, Keplinger M, et al. A prospective interventional study on the use of extracorporeal photopheresis in patients with bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2012;31(9):950–957. doi: 10.1016/j.healun.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 199.Morrell MR, Despotis GJ, Lublin DM, Patterson GA, Trulock EP, Hachem RR. The efficacy of photopheresis for bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2010;29(4):424–431. doi: 10.1016/j.healun.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 200.George JF, Gooden CW, Guo L, Kirklin JK. Role for CD4(+) CD25(+) T cells in inhibition of graft rejection by extracorporeal photopheresis. J Heart Lung Transplant. 2008;27(6):616–622. doi: 10.1016/j.healun.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 201.Davis RD, Jr, Lau CL, Eubanks S, et al. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. J Thorac Cardiovasc Surg. 2003;125(3):533–542. doi: 10.1067/mtc.2003.166. [DOI] [PubMed] [Google Scholar]
- 202.Brugière O, Thabut G, Castier Y, et al. Lung retransplantation for bronchiolitis obliterans syndrome: long-term follow-up in a series of 15 recipients. Chest. 2003;123(6):1832–1837. doi: 10.1378/chest.123.6.1832. [DOI] [PubMed] [Google Scholar]
- 203.Kawut SM, Lederer DJ, Keshavjee S, et al. Outcomes after lung retransplantation in the modern era. Am J Respir Crit Care Med. 2008;177(1):114–120. doi: 10.1164/rccm.200707-1132OC. [DOI] [PubMed] [Google Scholar]
- 204.Palmer SM, Miralles AP, Lawrence CM, Gaynor JW, Davis RD, Tapson VF. Rabbit antithymocyte globulin decreases acute rejection after lung transplantation: results of a randomized, prospective study. Chest. 1999;116(1):127–133. doi: 10.1378/chest.116.1.127. [DOI] [PubMed] [Google Scholar]
- 205.Hartwig MG, Snyder LD, Appel JZ, III, et al. Rabbit anti-thymocyte globulin induction therapy does not prolong survival after lung transplantation. J Heart Lung Transplant. 2008;27(5):547–553. doi: 10.1016/j.healun.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 206.Ailawadi G, Smith PW, Oka T, et al. Effects of induction immuno-suppression regimen on acute rejection, bronchiolitis obliterans, and survival after lung transplantation. J Thorac Cardiovasc Surg. 2008;135(3):594–602. doi: 10.1016/j.jtcvs.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 207.Garrity ER, Jr, Villanueva J, Bhorade SM, Husain AN, Vigneswaran WT. Low rate of acute lung allograft rejection after the use of daclizumab, an interleukin 2 receptor antibody. Transplantation. 2001;71(6):773–777. doi: 10.1097/00007890-200103270-00015. [DOI] [PubMed] [Google Scholar]
- 208.Lischke R, Simonek J, Davidová R, et al. Induction therapy in lung transplantation: initial single-center experience comparing daclizumab and antithymocyte globulin. Transplant Proc. 2007;39(1):205–212. doi: 10.1016/j.transproceed.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 209.Brock MV, Borja MC, Ferber L, et al. Induction therapy in lung transplantation: a prospective, controlled clinical trial comparing OKT3, anti-thymocyte globulin, and daclizumab. J Heart Lung Transplant. 2001;20(12):1282–1290. doi: 10.1016/s1053-2498(01)00356-4. [DOI] [PubMed] [Google Scholar]
- 210.McCurry KR, Iacono A, Zeevi A, et al. Early outcomes in human lung transplantation with Thymoglobulin or Campath-1H for recipient pretreatment followed by posttransplant tacrolimus near-mono-therapy. J Thorac Cardiovasc Surg. 2005;130(2):528–537. doi: 10.1016/j.jtcvs.2004.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shyu S, Dew MA, Pilewski JM, et al. Five-year outcomes with alemtuzumab induction after lung transplantation. J Heart Lung Transplant. 2011;30(7):743–754. doi: 10.1016/j.healun.2011.01.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.McNeil K, Glanville AR, Wahlers T, et al. Comparison of mycophenolate mofetil and azathioprine for prevention of bronchiolitis obliterans syndrome in de novo lung transplant recipients. Transplantation. 2006;81(7):998–1003. doi: 10.1097/01.tp.0000202755.33883.61. [DOI] [PubMed] [Google Scholar]
- 213.Keenan RJ, Konishi H, Kawai A, et al. Clinical trial of tacrolimus versus cyclosporine in lung transplantation. Ann Thorac Surg. 1995;60(3):580–584. doi: 10.1016/0003-4975(95)00407-C. discussion 584–585. [DOI] [PubMed] [Google Scholar]
- 214.Treede H, Klepetko W, Reichenspurner H, et al. Munich Vienna Lung Transplant Group Tacrolimus versus cyclosporine after lung transplantation: a prospective, open, randomized two-center trial comparing two different immunosuppressive protocols. J Heart Lung Transplant. 2001;20(5):511–517. doi: 10.1016/s1053-2498(01)00244-3. [DOI] [PubMed] [Google Scholar]
- 215.Hachem RR, Yusen RD, Chakinala MM, et al. A randomized controlled trial of tacrolimus versus cyclosporine after lung transplantation. J Heart Lung Transplant. 2007;26(10):1012–1018. doi: 10.1016/j.healun.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 216.Treede H, Glanville AR, Klepetko W, et al. European Australian Investigators in Lung Transplantation Tacrolimus and cyclosporine have differential effects on the risk of development of bronchiolitis obliterans syndrome: results of a prospective, randomized international trial in lung transplantation. J Heart Lung Transplant. 2012;31(8):797–804. doi: 10.1016/j.healun.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 217.Levy G, Thervet E, Lake J, Uchida K. Consensus on Neoral C(2): Expert Review in Transplantation (CONCERT) Group. Patient management by Neoral C(2) monitoring: an international consensus statement. Transplantation. 2002;73(9, Suppl):S12–S18. doi: 10.1097/00007890-200205151-00003. [DOI] [PubMed] [Google Scholar]
- 218.Snell GI, Valentine VG, Vitulo P, et al. RAD B159 Study Group Everolimus versus azathioprine in maintenance lung transplant recipients: an international, randomized, double-blind clinical trial. Am J Transplant. 2006;6(1):169–177. doi: 10.1111/j.1600-6143.2005.01134.x. [DOI] [PubMed] [Google Scholar]
- 219.Dutly AE, Gaspert A, Inci I, Schneiter D, Korom S, Weder W. The influence of the rapamycin-derivate SDZ RAD on the healing of airway anastomoses. Eur J Cardiothorac Surg. 2003;24(1):154–158. doi: 10.1016/s1010-7940(03)00182-9. discussion 158. [DOI] [PubMed] [Google Scholar]
- 220.Scott JP, Smyth RL, Higenbottam T, Mullins P, Solis E, Wallwork J. Transbronchial biopsy after lung transplantation. J Thorac Cardiovasc Surg. 1991;101(5):935–937. [PubMed] [Google Scholar]
- 221.Kukafka DS, O’Brien GM, Furukawa S, Criner GJ. Surveillance bronchoscopy in lung transplant recipients. Chest. 1997;111(2):377–381. doi: 10.1378/chest.111.2.377. [DOI] [PubMed] [Google Scholar]
- 222.Arcasoy SM, Berry G, Marboe CC, et al. Pathologic interpretation of transbronchial biopsy for acute rejection of lung allograft is highly variable. Am J Transplant. 2011;11(2):320–328. doi: 10.1111/j.1600-6143.2010.03382.x. [DOI] [PubMed] [Google Scholar]
- 223.Stephenson A, Flint J, English J, et al. Interpretation of trans-bronchial lung biopsies from lung transplant recipients: inter-and intraobserver agreement. Can Respir J. 2005;12(2):75–77. doi: 10.1155/2005/483172. [DOI] [PubMed] [Google Scholar]
- 224.Hopkins PM, Aboyoun CL, Chhajed PN, et al. Prospective analysis of 1,235 transbronchial lung biopsies in lung transplant recipients. J Heart Lung Transplant. 2002;21(10):1062–1067. doi: 10.1016/s1053-2498(02)00442-4. [DOI] [PubMed] [Google Scholar]
- 225.Valentine VG, Gupta MR, Weill D, et al. Single-institution study evaluating the utility of surveillance bronchoscopy after lung transplantation. J Heart Lung Transplant. 2009;28(1):14–20. doi: 10.1016/j.healun.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 226.Levine SM. Transplant/Immunology Network of the American College of Chest Physicians A survey of clinical practice of lung transplantation in North America. Chest. 2004;125(4):1224–1238. doi: 10.1378/chest.125.4.1224. [DOI] [PubMed] [Google Scholar]
- 227.Sayegh MH, Fine NA, Smith JL, Rennke HG, Milford EL, Tilney NL. Immunologic tolerance to renal allografts after bone marrow transplants from the same donors. Ann Intern Med. 1991;114(11):954–955. doi: 10.7326/0003-4819-114-11-954. [DOI] [PubMed] [Google Scholar]
- 228.Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358(4):362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 229.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358(4):353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Leventhal J, Abecassis M, Miller J, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4(124):24ra28. doi: 10.1126/scitranslmed.3003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Pham SM, Rao AS, Zeevi A, et al. Effects of donor bone marrow infusion in clinical lung transplantation. Ann Thorac Surg. 2000;69(2):345–350. doi: 10.1016/s0003-4975(99)01471-x. [DOI] [PMC free article] [PubMed] [Google Scholar]