Abstract
Background/Aims
To determine if there is a relationship between patient symptoms and functional improvement on inpatient rehabilitation.
Methods
Retrospective review of medical records at an American tertiary referral-based cancer center of all patients admitted to an inpatient rehabilitation unit between 3/1/2013-5/20/2013. Main outcome measures included the Edmonton Symptom and Assessment Scale (ESAS) and Functional Independence Measure (FIM).
Findings
The medical records for 71 unique cancer rehabilitation inpatients were analyzed. Statistical analysis of total admission ESAS on total FIM change found no significant relationships. The symptom burden of the patients was mild. Patients demonstrated statistically significant improvements in function and symptoms during inpatient rehabilitation. The mean change in total FIM and total ESAS were an increase of 19.20 and decrease of 7.41 respectively. Statistically significant changes occurred in fatigue, sleep, pain, and anxiety.
Conclusion
Both symptom and functional scores improved significantly during inpatient rehabilitation. However, no significant relationships were found between symptoms at admission and improvement in FIM.
Keywords: Cancer, Inpatient, Rehabilitation, Symptoms, Function
Introduction
Many cancer patients suffer from a variety of symptoms due to the disease and its treatments.1 Multiple studies have shown that higher functioning cancer patients tend to have less symptoms.2,3 Lin et al. found a strong relationship with pain, fatigue, disturbed sleep, distress and the Karnofsky Performance Scale in cancer patients.4 Hung et al. found a relationship between function and fatigue in non-small cell lung cancer survivors using the Karnofsky Self Report Performance Scale and the Brief Fatigue Inventory.5
This relationship has led to studies on physical activity’s impact on symptoms. Increasing physical activity through exercise programs has been shown in multiple studies to reduce cancer symptoms such as fatigue6-11, cachexia12, and pain.13-21 Studies have demonstrated improvement in symptoms and or quality of life during inpatient rehabilitation of several populations including patients with multiple sclerosis,22,23 chronic obstructive pulmonary disease,24 lung transplant patients,25 cystic fibrosis,26 stroke,27 general rehabilitation28 and cancer.29-31 These prior studies utilized a variety of instruments to assess symptoms and function.
While there has been published research regarding associations between symptoms and function/activity, research on the relationship of symptom severity and improvement/change in function has not been performed in cancer patients. One could postulate that symptoms such as fatigue, depression, and well-being could have an effect on rehabilitation participation and effectiveness. Could cancer symptom severity be predictive of functional improvement on inpatient rehabilitation? There have been prior analyses regarding the relationship of symptoms and functional improvement in stroke rehabilitation inpatients with no clear relationships found.32, 33
The primary objective of this study is to determine if symptom burden at admission to inpatient rehabilitation is predictive of functional improvement in cancer rehabilitation inpatients. If such a relationship existed, it could help consulting physiatrists determine inpatient rehabilitation potential.
METHODS
Subjects
This retrospective study included all patients admitted to inpatient rehabilitation at an American tertiary referral based cancer center from 3/1/2013 through 5/20/2013. If a patient was admitted more than once, only one randomly selected admission would be included. Patients who had incomplete symptom or functional records were excluded.
Procedure
Our rehabilitation inpatients underwent the standard American acute rehabilitation facility therapy of 3 hours/weekday of a combination of physical therapy (PT), occupational therapy (OT), speech therapy (when appropriate) and/or group therapy. Every patient received at least one hour of PT and one hour of OT every weekday. The third weekday hour was either more individual PT/OT, group therapy or speech therapy, depending on the patient’s needs. Therapy on Saturdays consisted of approximately 1 hour of individual (PT or OT) or group therapy. There was no therapy given on Sundays.
Institutional Review Board (IRB) approval was obtained. A waiver of informed consent in compliance with federal and institutional guideline was granted by the IRB. Patients during this time period were analyzed because Edmonton Symptom and Assessment Scores (ESAS) were obtained during this time period. Physiatrists reviewed the medical records and collected the data. Collected data included the 12-item Edmonton Symptom Assessment Scores – Financial/Spiritual (ESAS-FS), functional information, demographic information, medical information, and laboratory values. Functional information included Functional Independence Measure (FIM) scores, reason for admission to rehabilitation, and length of stay on rehabilitation. Demographic information included age, race, sex, marital status, insurance type, and discharge disposition. Medical information included primary cancer type, length of stay prior to inpatient rehabilitation transfer, and if the patient returned to the primary acute care service. Laboratory values included white blood cell count, platelet count, prealbumin, albumin, and serum creatinine at the time of inpatient rehabilitation transfer.
ESAS subscores included the ESAS-Psychologic (sum of anxiety and depression scores) and ESAS-Physical (sum of pain, dyspnea, appetite, nausea, fatigue, and drowsiness) were calculated. FIM subscores included FIM-Activities of Daily Living (sum of eating, grooming, bathing, upper extremity dressing, lower extremity dressing and toileting), FIM-Mobility (sum of bed/chair transfers, toilet transfers, tub/shower transfers, mobility and stairs) and FIM-Cognition (sum of comprehension, expression, social interaction, problem solving and memory) were calculated.
Dependent-samples t-tests were conducted to compare the admission subscales and total ESAS score means paired to their discharge scores at a 95% confidence level. The same procedure was conducted on the FIM subscales and total scores to their discharge scores. In both analyses a specific, directional difference was hypothesized that due to patient improvement, symptoms would decrease and function would increase.
The third analysis directly tested the hypothesis that functional improvement could be predicted from admission symptoms. The hypothesized relationship between symptom severity and functional improvement were tested by examining the bivariate fit parameters of the total change in FIM by ESAS-psychological, ESAS-physical, and ESAS-total at the level of 95% confidence.
RESULTS
95 inpatient rehabilitation admissions occurred during the study time period (3/1/2013-5/20/2013). 23 patients with incomplete functional and/or symptom data were excluded. One patient was admitted twice to inpatient rehabilitation, therefore, only 1 of this patient’s admissions was analyzed. In total, 71 unique patient admissions were tested for this study.
Table 1 displays demographic information of the study group. The median age was 63 years (Standard Deviation (SD)=13.3). The primary cancer types, along with their frequencies (and percentages) are listed in Table 2.
Table 1. Categorical Demographic Variables.
| Category | Frequency (Percentage) |
|---|---|
|
| |
| Age | |
| Below 65 | 43 (60.5) |
| Above 65 | 28 (39.4) |
|
| |
| Race | |
| White | 49 (69.0) |
| Black | 10 (14.0) |
| Hispanic | 8 (11.2) |
| Asian | 4 (5.6) |
|
| |
| Sex | |
| Female | 39 (54.9) |
| Male | 32 (45.0) |
|
| |
| Marital status | |
| Married | 52 (73.2) |
| Divorced | 8 (11.2) |
| Single | 7 (9.85) |
| Widowed | 4 (5.63) |
|
| |
| Payer source | |
| Private ins. | 35 (49.2) |
| Medicare | 35 (49.2) |
| Self-pay | 1 (1.4) |
|
| |
| Discharge Destination | |
| Home | 60 (84.5) |
| Acute | 7 (9.8) |
| Skilled Nursing Facility | 4 (5.6) |
Table 2. Primary Cancer Types.
| Cancer Type | Frequency (Percentage) |
|---|---|
| Primary CNS Lesions | 24 (33.8) |
| Glioblastoma Multiforme | 13 |
| Anaplastic Astrocytoma | 3 |
| Medulloblastoma | 2 |
| Oligodendroglioma | 1 |
| Rheumatologic Brain Lesion | 1 |
| Pineal Tumor | 1 |
| Cervical Ependymoma | 1 |
| Cavernoma | 1 |
| Neurofibromatosis | 1 |
| Liquid Tumors | 13 (18.3) |
| Multiple Myeloma | 3 |
| Leukemia | 5 |
| Lymphoma | 5 |
| Sarcomas | 10 (14.0) |
| Chondrosarcoma | 4 |
| Liposarcoma | 2 |
| Fibrous Histiocytoma | 1 |
| Ewing’s Sarcoma | 1 |
| Pigmented Villonodular Synovitis | 1 |
| Hemangiopericytoma | 1 |
| Dermatologic | 4 (5.6) |
| Melanoma | 2 |
| Dermatologic SCC | 2 |
| Head & Neck | 2 (2.8) |
| Sinus SCC | 1 |
| Oral SCC | 1 |
| Genitourinary | 4 (5.6) |
| Renal Cell Carcinoma | 2 |
| Prostate Cancer | 2 |
| Other Cancers | 14 (19.7) |
| Rectal Cancer | 1 |
| Pheochromocytoma | 1 |
| Breast Cancer | 6 |
| Lung Cancer | 6 |
CNS, Central Nervous System; SCC, Squamous Cell Carcinoma
Median white blood cell, platelet, and creatinine levels at rehabilitation admission were within normal limits. Median prealbumin and albumin were below clinically accepted limits at 18.1 and 3.0 respectively.
Patients did demonstrate statistically significant improvements in their ESAS scores. The median total ESAS score was 30 (M = 29.25, SD = 15.59) at admission and 19 (M = 21.84, SD = 15.16) at discharge. Total admission ESAS also was aligned with a significant proportion of variance in discharge scores, R2 = .1289, F(1. 62) = 9.175, p < .0036. Table 3 lists ESAS scores at admission and discharge. Individual patients exhibited severe scores on occasional subscales but no patients exhibited a severe total ESAS score either at admission or discharge.
Table 3. Admission and Discharge ESAS Scores.
| Admission |
Severe | Discharge |
Severe | |||||
|---|---|---|---|---|---|---|---|---|
| ESAS Score Item | M | SD | f | Freq | SD | f | df | t |
| Pain | 3.25 | 2.77 | 8 | 2.26 | 2.56 | 2 | 64 | −3.24** |
| Fatigue | 4.24 | 2.55 | 8 | 2.66 | 2.59 | 3 | 63 | −5.13** |
| Nausea | 0.57 | 1.83 | 2 | 0.75 | 1.75 | 1 | 64 | 0.53 |
| Depression | 1.27 | 2.29 | 2 | 0.87 | 1.89 | 1 | 64 | −1.38 |
| Anxiety | 1.64 | 2.34 | 3 | 0.81 | 1.59 | 0 | 64 | −2.65* |
| Drowsiness | 1.97 | 2.54 | 3 | 1.61 | 2.37 | 2 | 64 | −1.36 |
| Appetite | 3.83 | 2.99 | 9 | 3.36 | 2.61 | 6 | 64 | −1.52 |
| Wellbeing | 3.60 | 2.43 | 5 | 3.10 | 2.48 | 4 | 64 | −1.56 |
| Shortness of Breath | 1.25 | 1.91 | 0 | 1.27 | 2.03 | 1 | 50 | −0.44 |
| Sleep | 4.08 | 3.11 | 14 | 2.87 | 2.61 | 3 | 50 | −2.36* |
| Financial | 2.02 | 2.79 | 5 | 1.46 | 2.32 | 2 | 50 | −1.20 |
| Spiritual | 1.19 | 2.14 | 2 | 0.76 | 1.62 | 1 | 50 | 0.06 |
| Total | 29.25 | 15.59 | 0 | 21.84 | 15.16 | 0 | 63 | −3.75** |
p < .05.
p < .001.
M=mean; SD=standard deviation; f = frequency; df=degrees of freedom; t=t-value Severe individual ESAS scores are considered to be scores of 7-10. A severe total ESAS score is considered to be 70-100.
The median length of stay before transfer to the inpatient rehabilitation unit was 10 days (SD=12.7). The median length of stay on inpatient rehabilitation was 11 days (SD=5.2). Patients with a longer length of stay also had a larger change in FIM (p=0.0003). Patients with a higher admission total FIM score tended to have a higher total discharge FIM score (b=0.355, t(62) = 3.03, p < .0036). Additionally, admission FIM scores significantly predicted discharge scores. Table 4 demonstrates changes in FIM from admission to discharge. Statistically significant changes were found in Total FIM scores and FIM subscales.
Table 4. Admission and Discharge FIM Scores.
| Admission | Discharge | Δ | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| FIM Subscale | M | SD | M | SD | M | SD | t (68) |
| ADL | 33.08 | 6.36 | 42.20 | 6.40 | 9.15 | 5.25 | 14.48** |
| Mobility | 17.36 | 4.57 | 24.42 | 4.50 | 7.02 | 3.97 | 14.54** |
| Cognition | 30.97 | 5.25 | 31.57 | 4.35 | 0.65 | 1.96 | 2.60* |
| Total | 88.54 | 14.34 | 107.79 | 14.90 | 19.2 | 9.93 | 16.06** |
p < .05.
p < .001.
M=mean; SD=standard deviation; t = t-value
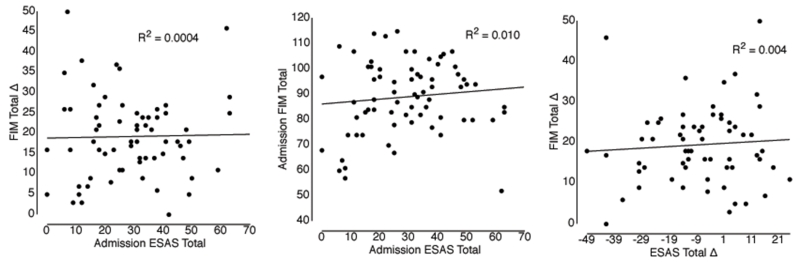
While significant improvements in cancer symptoms and functional improvement were found, further analysis of the relationship between ESAS and FIM found no significant results. Figure 1 demonstrates the correlation analysis of 1) Admission Total ESAS and Admission Total FIM, 2) Admission Total ESAS and Change in Total FIM, and 3) Absolute Change in Total ESAS and Change in Total FIM.
Figure 1. Regression Analysis of ESAS and FIM Scores.
DISCUSSION
The primary objective of our study was to analyze if a relationship existed between cancer inpatient rehabilitation admission symptom severity and functional improvement This is the first published study to do so in cancer patients specifically (a population that often suffers from a variety of severe symptoms) and the third to analyze this relationship in a rehabilitation setting on any patient population to our knowledge. Our study with a n=71 was unable to identify a statistically significant relationship.
Symptom improvements from admission to discharge were statistically significant. Multiple studies of cancer rehabilitation inpatients have shown similar results.34 Heim et al. demonstrated improvement in global quality of life, physical well-being, and functionality from beginning to end of inpatient rehabilitation. Instruments used included the Functional Assessment of Chronic Illness Therapy Measurement System for Anemia/Fatigue, Hospital Anxiety and Depression Scale, and Multidimensional Fatigue Inventory.35 Guo et al. obtained ESAS scores from 63 cancer rehabilitation inpatients and demonstrated a statistically significant improvement in anxiety, constipation, fatigue, pain, appetite, sense of well-being, and insomnia.29 Functionally, patients improved significantly on inpatient rehabilitation which has also been demonstrated in prior studies with the American standard of 3 hours of therapy/day.36,37 A relationship between a longer inpatient rehabilitation length of stay and increased change in total FIM has also been found.38 The longer one receives intense rehabilitation the more improvement is to be expected. Our findings of longer stay is associated with increased functional improvement raises the possibility that there might be a “sweet spot” for rehabilitation as compared to cost. Portions of inpatient rehabilitation might be performed as an outpatient with the same functional improvement. More research is necessary to identify the length of stay to achieve optimal improvement.
With respect to our primary objective, Madden et al. studied health related quality of life and FIM scores of 116 Canadian post-stroke rehabilitation inpatients. Subscores and total score of the FIM along with Short Form-36 (SF-36) patient survey results were evaluated. They analyzed if there was a correlation between changes in SF-36 individual symptom scores and total symptom score with changes in FIM scores using Pearson correlation tests. Similar to our study, both outcomes improved significantly, but there was no correlation between the two.32 The SF-36 measures some symptoms including pain and vitality but also function. The ESAS measures symptoms only. In an inpatient and outpatient study of 223 Dutch post-stroke rehabilitation patients, fatigue versus functional scores were assessed at 6 months, 12 months, and 36 months post-stroke. Fatigue, basic ADL function, and advanced ADL function were measured using the Fatigue Severity Scale, Barthel Index, and Frenchay Activities Index respectively. The authors concluded that lower fatigue severity scale scores were not associated with longitudinal improvement in basic or higher activity of daily living scores.33 The results of Madden et al., van de Port et al., and our study suggest that symptom severity does not affect functional improvement in rehabilitation.
There are limitations to our retrospective study. First, we only analyzed 71 patients. A preliminary analysis by a statistician hypothesized that a sample size of 60 should have been adequate to determine a relationship. Because of this, we felt 71 would have been able to reveal a relationship if one existed. Secondly, a proportion of patients had crucial data missing (23/94, 24.5%) that made analysis impossible. Third, this study consisted of a variety of primary cancer types, however, a more focused cancer population could have yielded more specific results. Last, the symptom burden of the majority of our cancer patients was quite mild. A severe individual ESAS symptom score is defined as 7-10. The percentage of patients with such scores never exceeded 25%. That being said, in our population, symptom severity was not a barrier to further functional improvement. Guo et al. also had similar mean ESAS scores ranging from 2.1 to 3.9 for the 7 worst symptoms in a group of cancer rehabilitation inpatients.29 Using thousands of 9 item ESAS assessments (the first 9 items of the 12 item ESAS), a mean total score of 19.9 has been reported in cancer patients 6 months before death.39 Total 9 item ESAS scores for our group were slightly worse at 21.98. Patients with higher symptom burdens have had lower function in prior studies.2-5 Cancer patients who are accepted to inpatient rehabilitation are screened by a consulting physiatrist. Patients have to be able to tolerate 3 hours of therapy per day to qualify for acute inpatient rehabilitation. A relationship has been identified in prior studies between symptom burden and performance status.4,5 The prequalification to tolerate 3 hours of therapy per day may have reduced the numbers of patients with high symptom burdens from the study.
There are a number of possible explanations for the lack of association between inpatient rehabilitation symptom burden and functional improvement. One possibility is that the physiatry team was able to successfully manage distressing symptoms such as pain, nausea, anorexia and fatigue during the rehabilitation admission, thereby allowing patients who had these symptoms to adhere to their intense rehabilitation. Our findings of significant improvement of overall symptoms and particularly pain and fatigue upon rehabilitation discharge are reassuring and suggest that the physiatry team may have successfully controlled these symptoms during the rehabilitation admission. Another possible explanation is that some symptoms/characteristics that are not measured with the ESAS could have impacted adherence to rehabilitation. These might include, for example, resilience or motivation. More research is needed to better understand the association of symptom burden, symptom relief and functional improvement in the cancer rehabilitation population.
CONCLUSIONS
The symptom burden and function of these cancer rehabilitation inpatients improved during the rehabilitation admission. No significant relationship was found between admission total ESAS scores and total FIM change.
Table 5. Bivariate fit of FIM total change by ESAS subscales and total.
| Subscale | M | SD | Median | n | Parameter | SE |
|---|---|---|---|---|---|---|
| Psychological | 2.92 | 4.01 | 1.0 | 71 | −0.0734 | 0.299 |
| Physical | 15.2 | 14.5 | 18.0 | 70 | −0.0472 | 0.145 |
| Total | 26.2 | 13.9 | 27 | 70 | −0.0132 | 0.080 |
Note: None of the bivariate parameters were statistically significant.
M=mean; SD = standard deviation; SE = standard error.
Acknowledgments
Supported in part by the M.D. Anderson Cancer Center support grant CA 016672. Eduardo Bruera is supported in part by National Institutes of Health grants RO1NR010162-01A1, RO1CA122292-01, and RO1CA124481-01. This study was presented as a poster presentation at the 91st American Congress of Rehabilitation Medicine Annual Conference on October 9, 2014.
Footnotes
Disclosures:
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
REFERENCES
- 1.Mao JJ, Armstrong K, Bowman MA, Xie SX, Kadakia R, Farrar JT. Symptom burden among cancer survivors: impact of age and comorbidity. J Am Board Fam Med. 2007 Sep-Oct;20(5):434–43. doi: 10.3122/jabfm.2007.05.060225. [DOI] [PubMed] [Google Scholar]
- 2.Lewis C, Xun P, He K. Physical activity in relation to quality of life in newly diagnosed colon cancer patients: a 24-month follow-up. Qual Life Res. 2014 Oct;23(8):2235–46. doi: 10.1007/s11136-014-0679-7. [DOI] [PubMed] [Google Scholar]
- 3.Dimeo F, Stieglitz RD, Novelli-Fischer U, Fetscher S, Mertelsmann R, Keul J. Correlation between physical performance and fatigue in cancer patients. Ann Oncol. 1997 Dec;8(12):1251–5. doi: 10.1023/a:1008234310474. [DOI] [PubMed] [Google Scholar]
- 4.Lin S, Chen Y, Yang L, Zhou J. Pain, fatigue, disturbed sleep and distress comprised a symptom cluster that related to quality of life and functional status of lung cancer surgery patients. J Clin Nurs. 2013 May;22(9-10):1281–90. doi: 10.1111/jocn.12228. [DOI] [PubMed] [Google Scholar]
- 5.Hung R, Krebs P, Coups EJ, Feinstein MB, Park BJ, Burkhalter J, Ostroff JS. Fatigue and functional impairment in early-stage non-small cell lung cancer survivors. J Pain Symptom Manage. 2011 Feb;41(2):426–35. doi: 10.1016/j.jpainsymman.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimeo FC, Stieglitz RD, Novelli-Fischer U, Fetscher S, Keul J. Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer. 1999 May 15;85(10):2273–7. [PubMed] [Google Scholar]
- 7.Vardar Yağlı N, Şener G, Arıkan H, Sağlam M, İnal İnce D, Savcı S, Çalık Kutukcu E, Altundağ K, Kaya EB, Kutluk T, Özışık Y. Do Yoga and Aerobic Exercise Training Have Impact on Functional Capacity, Fatigue, Peripheral Muscle Strength, and Quality of Life in Breast Cancer Survivors? Integr Cancer Ther. 2015 Jan 6; doi: 10.1177/1534735414565699. pii: 1534735414565699. [Epub ahead of print]) [DOI] [PubMed] [Google Scholar]
- 8.Eyigor S, Akdeniz S. Is exercise ignored in palliative cancer patients? World J Clin Oncol. 2014 Aug 10;5(3):554–9. doi: 10.5306/wjco.v5.i3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gracey JH, Watson M, Payne C, Rankin J, Dunwoody L. Translation research: ‘Back on Track’, a multiprofessional rehabilitation service for cancer-related fatigue. BMJ Support Palliat Care. 2014 Dec 19; doi: 10.1136/bmjspcare-2014-000692. pii: bmjspcare-2014-000692. doi: 10.1136/bmjspcare-2014-000692. [Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell SA, Hoffman AJ, Clark JC, DeGennaro RM, Poirier P, Robinson CB, Weisbrod BL. Putting evidence into practice: an update of evidence-based interventions for cancer-related fatigue during and following treatment. Clin J Oncol Nurs. 2014 Dec;18(Suppl):38–58. doi: 10.1188/14.CJON.S3.38-58. [DOI] [PubMed] [Google Scholar]
- 11.Bergenthal N, Will A, Streckmann F, Wolkewitz KD, Monsef I, Engert A, Elter T, Skoetz N. Aerobic physical exercise for adult patients with haematological malignancies. Cochrane Database Syst Rev. 2014 Nov 11;11:CD009075. doi: 10.1002/14651858.CD009075.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Lira FS, Neto JC, Seelaender M. Exercise training as treatment in cancer cachexia. Appl Physiol Nutr Metab. 2014 Jun;39(6):679–86. doi: 10.1139/apnm-2013-0554. [DOI] [PubMed] [Google Scholar]
- 13.Eickmeyer SM, Gamble GL, Shahpar S, Do KD. The role and efficacy of exercise in persons with cancer. PM&R. 2012 Nov;4(11):874–81. doi: 10.1016/j.pmrj.2012.09.588. [DOI] [PubMed] [Google Scholar]
- 14.Knobf MT, Thompson AS, Fennie K, Erdos D. The Effect of a Community-Based Exercise Intervention on Symptoms and Quality of Life. Cancer Nurs. 2013 Mar 20; doi: 10.1097/NCC.0b013e318288d40e. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012 Nov 14;11:CD006145. doi: 10.1002/14651858.CD006145.pub3. doi: 10.1002/14651858.CD006145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra SI, Scherer RW, Snyder C, Geigle P, Gotay C. The effectiveness of exercise interventions for improving health-related quality of life from diagnosis through active cancer treatment. Oncol Nurs Forum. 2015 Jan 1;42(1):E33–53. doi: 10.1188/15.ONF.E33-E53. [DOI] [PubMed] [Google Scholar]
- 17.Dimeo FC, Tilmann MH, Bertz H, Kanz L, Mertelsmann R, Keul J. Aerobic exercise in the rehabilitation of cancer patients after high dose chemotherapy and autologous peripheral stem cell transplantation. Cancer. 1997 May 1;79(9):1717–22. [PubMed] [Google Scholar]
- 18.Zeng Y, Huang M, Cheng AS, Zhou Y, So WK. Meta-analysis of the effects of exercise intervention on quality of life in breast cancer survivors. Breast Cancer. 2014 May;21(3):262–74. doi: 10.1007/s12282-014-0521-7. [DOI] [PubMed] [Google Scholar]
- 19.Chen HM, Tsai CM, Wu YC, Lin KC, Lin CC. Randomised controlled trial on the effectiveness of home-based walking exercise on anxiety, depression and cancer-related symptoms in patients with lung cancer. Br J Cancer. 2014 Dec 9; doi: 10.1038/bjc.2014.612. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eyigor S, Kanyilmaz S. Exercise in patients coping with breast cancer: An overview. World J Clin Oncol. 2014 Aug 10;5(3):406–11. doi: 10.5306/wjco.v5.i3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kummer F, Catuogno S, Perseus JM, Bloch W, Baumann FT. Relationship between cancer-related fatigue and physical activity in inpatient cancer rehabilitation. Anticancer Res. 2013 Aug;33(8):3415–22. [PubMed] [Google Scholar]
- 22.Judica E, Martinelli Boneschi F, Ungaro D, Comola M, Gatti R, Comi G, Rossi P. Impact of fatigue on the efficacy of rehabilitation in multiple sclerosis. J Neurol. 2011 May;258(5):835–9. doi: 10.1007/s00415-010-5851-6. [DOI] [PubMed] [Google Scholar]
- 23.Drulovic J, Bursac LO, Milojkovic D, Tepavcevic DK, Gazibara T, Pekmezovic T. MSQoL-54 predicts change in fatigue after inpatient rehabilitation for people with multiple sclerosis. Disabil Rehabil. 2013 Mar;35(5):362–6. doi: 10.3109/09638288.2012.704122. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs-Climent D, Le Gallais D, Varray A, Desplan J, Cadopi M, Préfaut C. Quality of life and exercise tolerance in chronic obstructive pulmonary disease: effects of a short and intensive inpatient rehabilitation program. Am J Phys Med Rehabil. 1999 Jul-Aug;78(4):330–5. doi: 10.1097/00002060-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Ihle F, Neurohr C, Huppmann P, Zimmermann G, Leuchte H, Baumgartner R, Kenn K, Sczepanski B, Hatz R, Czerner S, Frey L, Ueberfuhr P, Bittmann I, Behr J, Munich Lung Transplant Group Effect of inpatient rehabilitation on quality of life and exercise capacity in long-term lung transplant survivors: a prospective, randomized study. J Heart Lung Transplant. 2011 Aug;30(8):912–9. doi: 10.1016/j.healun.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz TG, Goldbeck L. The effect of inpatient rehabilitation programmes on quality of life in patients with cystic fibrosis: a multi-center study. Health Qual Life Outcomes. 2006 Feb 3;4:8. doi: 10.1186/1477-7525-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hopman WM, Verner J. Quality of life during and after inpatient stroke rehabilitation. Stroke. 2003 Mar;34(3):801–5. doi: 10.1161/01.STR.0000057978.15397.6F. [DOI] [PubMed] [Google Scholar]
- 28.Ploypetch T, Dajpratham P. Change in quality of life of disabled patients after intensive inpatient rehabilitation at Siriraj Hospital. J Med Assoc Thai. 2011 Oct;94(10):1245–51. [PubMed] [Google Scholar]
- 29.Guo Y, Young BL, Hainley S, Palmer JL, Bruera E. Evaluation and pharmacologic management of symptoms in cancer patients undergoing acute rehabilitation in a comprehensive cancer center. Arch Phys Med Rehabil. 2007 Jul;88(7):891–5. doi: 10.1016/j.apmr.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 30.Riesenberg H, Lübbe AS. In-patient rehabilitation of lung cancer patients--a prospective study. Support Care Cancer. 2010 Jul;18(7):877–82. doi: 10.1007/s00520-009-0727-y. [DOI] [PubMed] [Google Scholar]
- 31.Spruit MA, Janssen PP, Willemsen SC, Hochstenbag MM, Wouters EF. Exercise capacity before and after an 8-week multidisciplinary inpatient rehabilitation program in lung cancer patients: a pilot study. Lung Cancer. 2006 May;52(2):257–60. doi: 10.1016/j.lungcan.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Madden S, Hopman WM, Bagg S, Verner J, O’Callaghan CJ. Functional status and health-related quality of life during inpatient stroke rehabilitation. Am J Phys Med Rehabil. 2006 Oct;85(10):831–8. doi: 10.1097/01.phm.0000240666.24142.f7. quiz 839-41, 857. [DOI] [PubMed] [Google Scholar]
- 33.van de Port IG, Kwakkel G, Schepers VP, Heinemans CT, Lindeman E. Is fatigue an independent factor associated with activities of daily living, instrumental activities of daily living and health-related quality of life in chronic stroke? Cerebrovasc Dis. 2007;23(1):40–5. doi: 10.1159/000095757. [DOI] [PubMed] [Google Scholar]
- 34.Bertheussen GF, Kaasa S, Hokstad A, Sandmæl JA, Helbostad JL, Salvesen Ø , Oldervoll LM. Feasibility and changes in symptoms and functioning following inpatient cancer rehabilitation. Acta Oncol. 2012 Nov;51(8):1070–80. doi: 10.3109/0284186X.2012.699684. [DOI] [PubMed] [Google Scholar]
- 35.Heim ME, v d Malsburg ML, Niklas A. Randomized controlled trial of a structured training program in breast cancer patients with tumor-related chronic fatigue. Onkologie. 2007 Sep;30(8-9):429–34. doi: 10.1159/000104097. [DOI] [PubMed] [Google Scholar]
- 36.Hunter EG, Baltisberger J. Functional Outcomes by Age for Inpatient Cancer Rehabilitation: A Retrospective Chart Review. J Appl Gerontol. 2013 Jun 1;32(4):443–456. doi: 10.1177/0733464811432632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang ME, Sliwa JA. Inpatient rehabilitation of patients with cancer: efficacy and treatment considerations. PM R. 2011 Aug;3(8):746–57. doi: 10.1016/j.pmrj.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 38.Fu JB, Parsons HA, Shin KY, Guo Y, Konzen BS, Yadav RR, Smith DW. Comparison of functional outcomes in low- and high-grade astrocytoma rehabilitation inpatients. Am J Phys Med Rehabil. 2010 Mar;89(3):205–12. doi: 10.1097/PHM.0b013e3181ca2306. [DOI] [PubMed] [Google Scholar]
- 39.Seow H, Barbera L, Sutradhar R, Howell D, Dudgeon D, Atzema C, Liu Y, Husain A, Sussman J, Earle C. Trajectory of performance status and symptom scores for patients with cancer during the last six months of life. J Clin Oncol. 2011 Mar 20;29(9):1151–8. doi: 10.1200/JCO.2010.30.7173. [DOI] [PubMed] [Google Scholar]