Abstract
Linear polyacrylamide (PAAm) is modified with dopamine or nitrodopamine (PAAm-D and PAAm-ND, respectively) to evaluate the effect of nitro-group modification on the interfacial binding properties of polymer-bound catechol. Nanocomposite hydrogels are prepared by mixing PAAm-based polymers with Laponite and the viscoelastic properties of these materials are determined using oscillatory rheometry. The incorporation of a small amount of catechol (≈0.1 wt% in swollen hydrogel) drastically increases the shear moduli by 1–2 orders of magnitude over those of the catechol-free control. Additionally, PAAm-ND exhibits higher shear moduli values than PAAm-D across the whole pH range tested (pH 3.0–9.0). Based on the calculated effective crosslinking density, effective functionality, and molecular weight between crosslinks, nitro-group functionalization of dopamine results in a polymer network with increased crosslinking density and crosslinking points with higher functionality. Nitro-functionalization enhances the interfacial binding property of dopamine and increases its resistant to oxidation, which results in nanocomposite hydrogels with enhanced stiffness and a viscous dissipation property.
Keywords: catechol, hydrogels, interfacial binding, nanocomposites, nitro-group functionalization
1. Introduction
The mixing of polymer with nanoparticles is an efficient strategy to fabricate nanocomposite systems with high performance and multiple functionalities.[1–6] Nanocomposite materials rely on weak physical interactions (e.g., hydrogen bonding, ionic interactions, etc.) between the polymer matrix and the inorganic nanoparticles to achieve elevated mechanical properties and extensibility, as well as the ability to recover after large, repeated deformation. Strong interfacial binding is necessary to acheive effective stress transfer between the nanoparticles and the polymer matrix.[7–9] A strong interfacial bond is especially critical for materials that experience cyclic loading to prevent rapid breakdown at the interface.[10] Although the use of covalent bonds to bridge the polymer matrix with incorporated nanoparticles has been described,[11–13] covalently crosslinked composite materials exhibit a significant increase in mechanical properties but a reduction in flexibility and recovery efficiency when the irreversible covalent bonds are broken during deformation.[14] A strong, reversible bond found in marine adhesive chemistries is exploited herein to investigate the effect of interfacial binding strength on the viscoelasticity of nanocomposite hydrogels.
Marine mussel secretes protenaceous adhesives to enable its attachment to various substrate surfaces under wet, saline conditions.[15,16] The excellent bonding ability of these adhesive proteins is attributed to the presence of a unique amino acid, 3,4-dihydroxyphenylalanine (DOPA).[17] The catechol side chain of DOPA is capable of forming strong reversible bonds with metal oxide with a measured adhesion strength averaging around 800 pN, roughly 40% that of covalent bonds.[18] The use of network-bound catechol (e.g., DOPA, dopamine) to increase the interfacial binding strength between polymer networks and encapsulated nanoparticles in creating nanocomposite materials (e.g., hydrogels,[19] films,[20] nanofibers,[21] rubber,[22,23] and tissue adhesive[24] with drastically improved materials properties has been described. However, the effect of pH on the material properties of these nanocomposites has not yet been reported. pH plays an important role in the oxidation state of catechol and its ability to bind to metal oxide and ions.[25–27] When the pH approaches and exceeds the first dissociation constant of the catechol–OH group (pKa1 = 9.0), DOPA auto-oxidizes to its quinone form with reduced adhesive trength.[18,25,28]
DOPA is also found in adhesive proteins utilized by sandcastle worms to cement sand fragments into tube-shaped dwellings.[29] The catechol side chain in these adhesive proteins is further modified with an electron withdrawing chloro-functional group at the para position (2-chloro-4,5-dihydroxyphenylalanine), which was proposed as a natural adaptation to increase interfacial binding strength.[30] Substituting −H with an electron withdrawing group (EWG) lowers the dissociation constant (pKa) of the catechol hydroxyl groups and their redox potential, thus making the oxidation of the catechol group more difficult.[31] The oxidized quinone forms weak bonds with the substrate surface when compared to its reduced catechol counterpart.[25,28,32] Similarly, nitro-substituted catechols have been found to form complexes with metal oxides that are more stable than unsubstituted catechol.[33–35] Nanoparticles modified with nitro-DOPA- or nitrodopamine-functionalized polymers were found to have significantly higher colloidal stability when compared to other catechol groups (e.g., DOPA, dopamine, etc.) and could be repeatedly heated to 90 °C without noticeable agglomeration.[34] An electron paramagnetic resonance study indicated that enhanced electron delocalization for nitrocatechols may have contributed to its remarkable binding strength to metal oxide surfaces.[33] Most recently, the nitro-functionalization of dopamine has been found to drastically alter the rate of crosslinking and the degradation rate of nitrodopamine-modified bioadhesives.[36,37]
The present work seeks to enhance the interfacial binding strength of the catechol moiety with EWG modification and evaluate its effect on the viscoelastic properties of nanocomposite hydrogels. To this end, a linear and water soluble polyacrylamide (PAAm) polymer was functionalized with catechol with and without EWG modification (e.g., nitrodopamine and dopamine, respectively). These polymers were mixed with nanoparticles to yield a homogenous nanocomposite hydrogel using a biocompatible nanosilicate, Laponite (Na0.7+(Mg5.5Li0.3Si8) O20(OH)4)0.7−). Laponite was chosen for this study as its reversible interaction between dopamine has been previously established.[19,24] Additionally, pH was used to further modulate the interfacial binding strength of the polymer-bound catechol moieties and to determine its effect on the viscoelastic properties of the nanocomposite hydrogels.
2. Experimental Section
2.1. Materials
Dopamine hydrochloride (99%) and ammonium persulfate (APS, 98.0%) were purchased from Acros (New Jersey, USA). Sulfuric acid (H SO , ACS PLUS), hydrochloric acid (HCl, ACS grade), acrylamide (AAm, ≥ 99%), acrylic acid N-hydroxysuccinimide ester (AA-NHS, 90%), and N,N,N′,N′-tetramethylethylenediamine (TEMED, ≈99%) were purchased from Sigma-Aldrich (USA). Laponite XLG was donated by Southern Clay Products Inc. (Texas, USA). All reagents were used as received. Deionized water (D.I. H2O) was used to prepare all aqueous solutions. 0.1 M NaCl solutions with a pH range from 3.0 to 9.0 were prepared as buffer solutions for hydrogel soaking. pH values of 3.0 and 5.0 were adjusted with 0.1 m HCl, and pH values of 7.4 and 9.0 were adjusted using 0.1 m Na2HPO4 and 0.1 m HCl solutions at appropriate ratios. All reagents were used as received. Nitrodopamine hemisulfate was synthesized according to a published protocol.[38]
2.2. Synthesis of Catechol-Functionalized PAAm
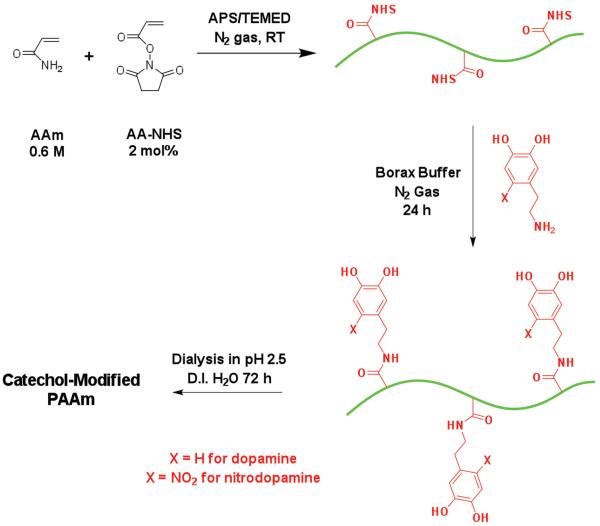
Dopamine- and nitrodopamine-functionalized polyacrylamide (PAAm-D and PAAm-ND, respectively) were prepared in two steps (Scheme 1). In the first step, a precursor polyacrylamide (PAAm) copolymer containing reactive −NHS ester groups (PAAm-co-AA-NHS) was prepared by free radical polymerization. 50 mL of D.I. H2O containing 0.6 m of AAm and 2 mol% of AA-NHS (relative to AAm) was purged with nitrogen for 30 min before APS and TEMED (APS: 0.2 mol% relative to AAm, TEMED:APS = 2.3:1 mol:mol) were added to trigger the polymerization at room temperature under a nitrogen atmosphere for 3 h. The as-made precursor polymer was precipitated in chilled acetone to remove the redox initiator and unreacted monomers to yield the PAAm-co-AA-NHS precursor polymer.
Scheme 1.
General synthesis procedure for catechol-functionalized PAAm.
In the second step, PAAm-co-AA-NHS was redissolved in 50 mL of D.I. H2O and mixed with 40 mL of borax buffer (0.025 m Na2B4O7·10H2O adjusted to pH 8.0) containing either dopamine hydrochloride or nitrodopamine hemisulfate. The molar ratio between the catechol amine and AA–NHS was fixed at 1.5:1. The reaction mixture was stirred under a nitrogen atmosphere for 24 h. The polymer was dialyzed in dialysis tubing with a molecular weight cut off of 3500 Da for 72 h in D.I. H2O acidified to pH 2.5 by adding 0.1 m HCl and collected by precipitating in chilled acetone to obtain the catechol-functionalized polymers, and dried. The PAAm control was also prepared using same free radical polymerization process without the addition of AA-NHS. 1H NMR (D2O:DMSO-d6, 4:1 v:v, δ): PAAm-D 7.7–7.4 (m, 2H; −C(=O)−NHH), 6.9 (m, H; −C(=O) −NHH), 6.8–6.5 (m, 3H; C6H3), 3.2 (s, 2H; −NH−CH2−CH2−), 2.8 (s, 2H; −NH−CH2−CH2−), 2.1 (m, 1H; −CH−CH2−), 1.5 (m, 2H; −CH−CH2−) (Figure S1, Supporting Information); PAAm-ND 8.0 (s, 1H; C6HH), 7.7–7.4 (m, 2H; −C(=O)−NHH), 6.9 (m, H; −C(=O)=NHH), 6.7 (s, 3H; C6HH), 3.2 (s, 4H; −NH−CH2−CH2−), 2.1 (m, 1H; −CH−CH2−), 1.5 (m, 2H; −CH−CH2−) (Figure S2, Supporting Information).
2.3. Characterization of Catechol-Functionalized PAAm Polymers
The molecular weight of the synthesized polymers was analyzed using a gel-permeation chromatograph (GPC, Agilent 1260) equipped with an Agilent G1362A 1260 refractive index detector. Three Waters Ultrahydrogel columns were used, aligned in series, and an aqueous mobile phase (0.1 m sodium nitrate, 0.02 wt% sodium azide; flow rate = 1 mL min−1). Shodex Standard P-82 pullulan was used as the standard. Polymers were dissolved in D.I. H2O at a concentration of 2 mg mL−1, stirred for 5 h, and injected with a 0.45 μm filter. The catechol content in PAAm-D and PAAm-ND was quantitatively determined by UV-vis spectrometry (Lambda 35, Perkin Elmer Inc., USA).[39,40] Briefly, a known amount of the PAAm-D or PAAn-ND was dissolved in D.I. H2O acidified to pH 2.5 and its absorbance was determined at 279 nm and 310 nm, respectively (Figure S3, Supporting Information). Standard curves for dopamine (279 nm) and nitrodopamine (310 nm) were used to determine the amount of catechol coupled to each polymer.
2.4. Preparation of Nanocomposite Hydrogels
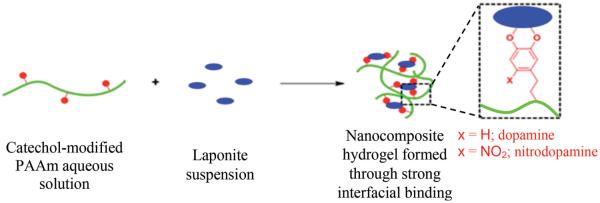
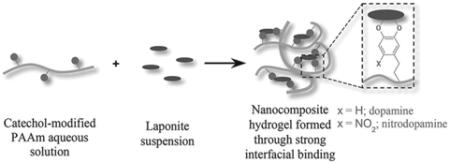
PAAm polymers with or without dopamine or nitrodopamine were homogenously mixed with Laponite nanoparticles to form nanocomposite hydrogels (Scheme 2). Unless otherwise specified, hydrogels were prepared with a ratio of 60:40 (wt%:wt%) polymer:Laponite (i.e., starting polymer and Laponite contents were 60 and 40 wt%, respectively). The polymer (0.100 g) was dissolved in pH 4.4 D.I. H2O (20.0 mL) with vigorous magnetic stirring overnight to give a clear solution (5.0 mg mL−1). Separately, Laponite nanoparticles (0.067 g) were dispersed in D.I. H2O (26.8 mL) with vigorous magnetic stirring for 20 min, and then 0.15 mL of 0.1 m NaCl solution was added with sonication for 5 min to give 2.5 mg mL−1 of clear Laponite suspension containing 0.56 × 10−3 M NaCl. The polymer solution was added dropwise to the Laponite suspension with vigorous magnetic stirring and the stirring was continued for an additional 4 h to obtain a homogenous mixture. The mixture was then transferred into a pre-weighed petri dish and evaporated in the fume hood to give a condensed nanocomposite mixture (≈2.0 wt%) and further added to a circular mold with a diameter of 20 mm and a depth of 2 mm (Figure S4, Supporting Information) and freeze dried. To reconstitute the hydrogels, the freeze dried nanocomposite discs were soaked in excess 0.1 m NaCl buffer solution at a desired pH (3.0, 5.0, 7.4, and 9.0) for 48 h.
Scheme 2.
General process of forming nanocomposite hydrogel containing catechol-modified PAAm and Laponite.
2.5. Characterization of Nanocomposite Networks
The chemical composition of the freeze dried nanocomposite network was determined using Fourier transform infrared (FTIR) spectroscopy (Perkin Elmer Spectrum One). The morphologies of the dried nanocomposite networks were characterized using field emission scanning electronic microscopy (FE-SEM, Hitachi S-4700). A freshly formed cross-section was created by cutting the network in half and coating with Pt/Pd alloy. The equilibrium water content (EWC) of the reconstituted hydrogels was calculated by measuring the mass of the hydrogel before (Ms) and after (Md) drying the samples in vacuum for at least 48 h. EWC was determined using Equation (1).[41]
| (1) |
2.6. Oscillatory Rheometry
Oscillatory rheometry was carried out using an HR-2 rheometer (TA Instruments Inc., USA) to perform frequency sweep experiments (0.01–600 rad s−1 with a strain of 10%) to collect storage (G′) and loss (G″) moduli. The reconstituted nanocomposite hydrogel discs (diameter = 20 mm, replicate n = 3) were tested using parallel plate (20 mm) with a gap that was 85% that of the individual hydrogel thickness, as measured by a digital calliper. Mineral oil was applied around the edge of the hydrogel disc to avoid dehydration during testing.
2.7. Statistical Analysis
Statistical analysis was performed using JPM Pro 9 software (SAS, Cary, NC). The student t-test and one-way analysis of variance (ANOVA) with Tukey-Kramer HSD analysis were performed for comparing means of two and more than two groups, respectively. A p-value less than 0.05 was considered significant.
3. Results and Discussion
3.1. Synthesis of Catechol-Functionalized PAAm Polymers
PAAm modified with either dopamine or nitrodopamine (PAAm-D and PAAm-ND, respectively) was prepared using a two-step sythesis process (Scheme 1). In the first step, free-radical polymerization was carried out to yield a PAAm copolymerized with AA-NHS, which contained pendant activated ester groups. The reactive −NHS groups were further reacted with the primary amine on either dopamine or nitrodopamine to covalently link these adhesive moieties to the PAAm chain. Both 1H NMR (Figure S1 and S2, Supporting Information) and UV–vis (Figure S3, Supporting Information) confirmed the presence of dopamine and nitrodopamine in PAAm-D and PAAm-ND, respectively. On average there were nearly 3 catechol pendant groups coupled to each polymer chain based on UV-vis (Table 1). For both PAAm-D and PAAm-ND, the resulting yield of the coupled catechol accounted for around 0.5 mol% relative to that of AAm repeating units, corresponding to around a 25% yield based on the theoretical starting concentration of AA-NHS (2 mol%) used during the polymerization step. This yield is comparable to published results (13–35%) for polyacrylate-based polymer containing NHS activated esters.[42] PAAm homopolymer had a higher molecular weight compared to the copolymers, indicating that the presence of AA-NHS affected the growth of the polymer chain. The polydispersity index (PDI) was found to be around 2–2.4. This relatively high PDI was attributed to the fast reaction rate of the free radical polymerization, which resulted in a weight-average molecular weight (M̄w) of around 106 Da within 3 h. Additionally, the high viscosity achieved during the polymerization process of this high molecular weight polymer also contributed to the measured PDI. Nevertheless, both PAAm-D and PAAm-ND were prepared with comparable catechol content and molecular weight to investigate the effect of nitro-group functionalization on the polymer-nanoparticle interfacial binding within nanocomposite hydrogel networks.
Table 1.
Reaction parameters, yield, weight average molecular weight (M̄w ), and catechol content of PAAm and catechol-modified PAAm.
| Sample | [AAm]:[AA-NHS]: [APS]: [TEMED] |
Yield [%] |
Catechol a)
[mol%] |
mol catechol/ mol polymer |
M̄w
[kDa]/PDI |
|---|---|---|---|---|---|
| PAAm | 100 : 0 : 0.2 : 0.46 | 94.7 | – | – | 1080/2.41 |
| PAAm-D | 100 : 2 : 0.2 : 0.46 | 79.5 | 0.47 | 2.7 | 852/2.31 |
| PAAm-ND | 100 : 2 : 0.2 : 0.46 | 81.6 | 0.53 | 2.9 | 900/1.92 |
Relative to the repeating AAm monomers.
3.2. Preparation of Nanocomposite Networks
To ensure a homogenous distribution of Laponite within the PAAm-based hydrogel network, both the nanoparticle and polymer were mixed in a dilute concentration (combined concentration of 3.5 mg mL−1) and sequentially condensed and freeze dried (Figure S4, Supporting Information). The freeze dried PAAm-D nanocomposite network was white, indicating that no oxidation of the dopamine side chain had occured during the process of network formation. On the other hand, the mixture of PAAm-ND and Laponite both in solution and in the freeze dried network was yellowish in color. This color indicated the presence of nitrodopamine in the nanocomposite network, as nitrodopamine powder is dark yellowish-brown (not shown) in color. FTIR spectra (Figure 1) of various PAAm nanocomposites revealed the characteristic primary amide (3305, 3178, and 1606 cm−1 for primary −NH2 and 1647 cm−1 for C=O) and aliphatic acrylate backbone (2964 and 1448 cm−1 for −CH2−) of PAAm. Additionally, these composites also exhibited a strong contribution from the Si−O−Si stretching of the nanoparticle (984 cm−1). For both PAAm-D and PAAm-ND, catechol peaks were not readily visible due to the low content of the adhesive moieties in the polymer. SEM images of the freeze-dried nanocomposites confirmed that these materials formed porous networks (Figure 2).
Figure 1.
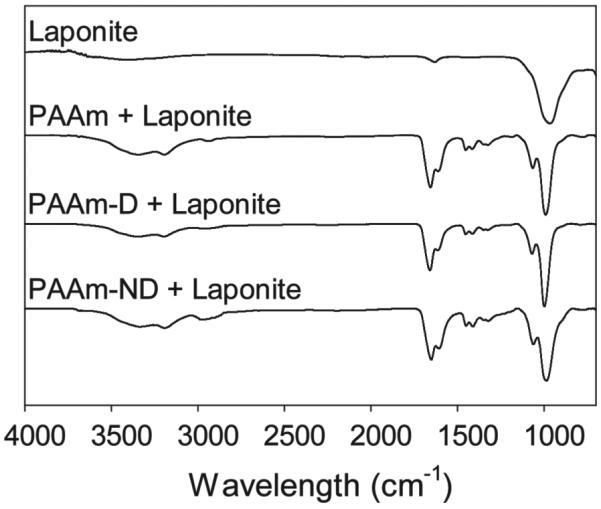
FTIR spectra of freeze dried nanocomposite networks.
Figure 2.
SEM images of A) PAAm, B) PAAm-D, and C) PAAm-ND nanocomposite networks. Scale bars = 50 μm.
Dry nanocomposite networks were reconstituted in excess aqueous buffer solutions and equiliated for 48 h to form hydrogels. The equilibrium water content (EWC) of these nanocomoposite hydrogels was between 92 and 97 wt% (Figure 3). EWC is an important property of a hydrogel and is inversely proportional to the crosslinking density and mechanical properties of the network.[27,43,44] At all the pH levels tested, the EWC values of PAAm-D and PAAm-ND hydrogels were significantly lower than those of PAAm. This indicates that nanocomposite hydrogels containing the adhesive catechol moieties were more densely crosslinked, presumably due to strong interfacial binding between polymer-bound catechol and Laponite.[19,24] Although there was no significant difference for EWC values measured for PAAm-D and PAAm-ND at pHs between 3.0 and 7.4, EWC for PAAm-ND measured at pH 9.0 was lower than that of PAAm-D. These results suggested that the nitrodopamine still formed strong bonds with Laponite even at a pH above the proton dissociation constant of the first −OH group (pKa1 = 6.5 and 9 for nitrodopamine and dopamine, respectively.[33,45]
Figure 3.
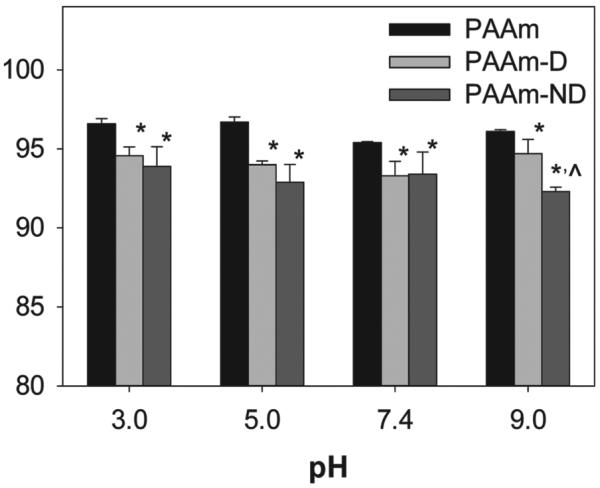
Equilibrium water content (EWC) of nanocomposite hydrogels equilibrated in various pH levels. Hydrogels were formed with an initial polymer:Laponite weight ratio of 60:40 (wt%:wt%). * Statistically different from PAAm (p < 0.05); ^ Statistically different from PAAm-D (p < 0.05).
3.3. Oscillatory Rheometry
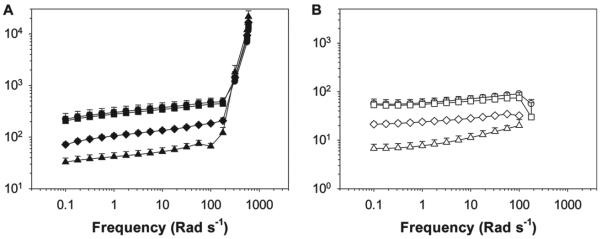
The viscoelasticity properties of PAAm and Laponite nanocomposite hydrogels with or without the incorporation of dopamine and nitrodopamine moieties were determined using oscillatory rheometry (Figure 4,5 and 6). Regardless of the polymer or pH, nanocomposite hydrogels exhibited very similar responses. For all samples, the measured storage modulus (G′) values were greater than those of the loss modulus (G′′) values across all the frequency ranges tested, indicating that these materials behaved as crosslinked hydrogel networks. For PAAm-D and PAAm-ND, crosslinks were formed between polymer-bound catechol and Laponite.[19] The catechol-free PAAm control also formed a hydrogel network but with lower G′ and G″, presumably due to the weak PAAm-Laponite interaction.[46,47] Additionally, both G′ and G″ increased with increasing frequency, similar to other reported physically crosslinked hydrogels.[46–48] At a frequency less than 100 rad s−1, both G′ and G″ exhibited power law behavior in response to the shear frequency (ω), where G′ ~ ωn′, G″ ~ ωn″, and n′ and n″ are the relaxation exponent for G′ and G″, respectively.[49] The relaxation exponent values reported here (Table S1, Supporting Information) are in agreement with physically crosslinked nanocomposite hydrogels composed of PAAm and Laponite (0.1–0.18).[47] n′ for both PAAm-D and PAAm-ND were lower than that of PAAm, indicating that the catechol-containing nanocomposite behaved more elastically.[49] G′ rose sharply while G″ decreased sharply when the frequency increased beyond 100 rad s−1. When the hydrogels were repeatedly deformed at a high frequency, the polymer chains failed to rearrange themselves within the short time scale of the imposed mechanical deformation, which led to stiffening of the networks.[50,51] This result further confirmed the formation of hydrogel networks.
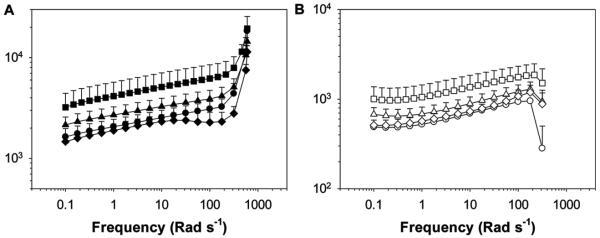
Figure 4.
A) Storage and B) loss moduli of PAAm-ND nanocomposite hydrogels during oscillatory frequency sweep experiment. Hydrogels were equilibrated in pH 3 (●, ○), Z5 (■, □), 7.4 (▲, △), and 9 (◆, ◇) prior to testing. The filled and empty symbols indicate G′ and G″, respectively.
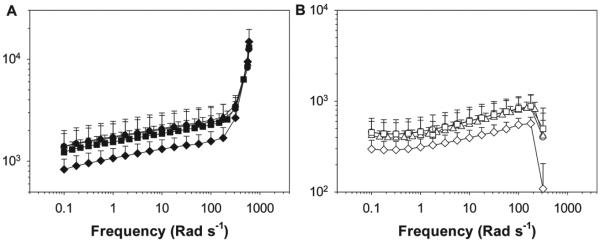
Figure 5.
A) Storage and B) loss moduli PAAm-D nanocomposite hydrogels during the oscillatory frequency sweep experiment. Hydrogels were equilibrated in pH 3 (●, ○), Z5 (■, □), 7.4 (▲, △), and 9 (◆, ◇ prior to testing. The filled and empty symbols indicate G′ and G″, respectively.
Figure 6.
A) Storage and B) loss moduli PAAm nanocomposite hydrogels during the oscillatory frequency sweep experiment. Hydrogels were equilibrated in pH 3 (●, ○), Z5 (■, □), 7.4 (▲, △), and 9 (◆, ◇) prior to testing. The filled and empty symbols indicate G′ and G″, respectively.
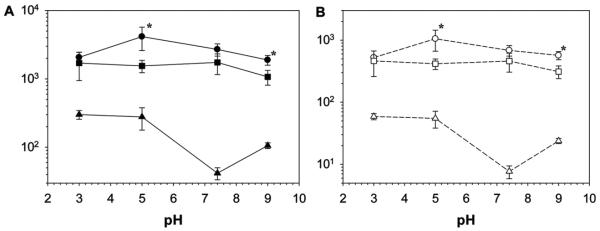
Nanocomposite hydrogels containing catechol adheisve moieties exhibited G′ and G″ values that were 1–2 orders of magnitude higher than those of the catechol-free hydrogel (Figure 7). This drastic difference in the measured moduli values was attributed to the strong wet adhesive properties of the catechol group. Increased measured G′ values for catechol-containing materials indicated an elevated stiffness as a result of increased effective crosslinking density due to the formation of strong catechol-Laponite bonds. Increased crosslinking density in the catechol-containing network was corroborated with reduced EWC (Figure 3). Elevated G″ values indicated strong viscous dissipation properties resulting from the breaking of reversible physical bonds between catechol and Laponite.[19,24] The measured increase in materials properties as a result of catechol-functionalization was remakable, considering the extremely low content of catechol moiety in the nanocomposite hydrogel (≈0.1 wt% in the swollen network).
Figure 7.
Summary of oscillatory rheology data from frequency sweep experiment for PAAm-ND (●, ○), PAAm-D (■, □), and PAAm (▲, △). The filled and empty symbols represent G′ and G″, respectively. Values were taken from a frequency of 1 rad s−1 and a strain of 0.1. * Denotes significant different from PAAm-D hydrogel for a given pH (p < 0.05).
Both PAAm-D and PAAm-ND nanocomposite hydrogels exhibited higher G′ and G″ values under acidic conditions (Figure 7). For PAAm-ND, maximum G′ and G″ values (4200 and 1000 Pa, respectively) were observed at pH 5.0 and both moduli decreased in value with a further increase in pH. On the other hand, G′ and G″ was maintained at around 1700 and 500 Pa, respectively, for PAAm-D tested at pH values between 3.0 and 7.4, before these values decreased to 1000 and 300 Pa, respectively, when the pH was raised to 9.0. When comparing the rheological response of PAAm-D and PAAm-ND nanocomposite hydrogels, PAAm-ND demonstrated equivalent or higher G′ and G″ values when compared to those of PAAm-D. Measured shear moduli values for PAAm-ND were statistically higher at pH 5.0 and 9.0 when compared to those of PAAm-D.
Rheological data further confirmed that the interfacial binding properties of the catechol side chain were responsible for the elevated materials properties. For example, 4-nitrocatechol demonstrated maximum absorption to inorganic substrates at a pH of around 6,[52] which corresponded well with the pH dependent behavior of the shear moduli measured for PAAm-ND. Additionally, the point of zero charge (PZC) for silica oxide (SiO2) occurs at around a pH of 5,[53] and under a more acidic or basic condition, proton or hydroxide ions can compete with catechol for binding onto the charged oxide surface.[54,55] Furthermore, the reduced form of catechol is responsible for the strong moisture-resistant adhesion to inorganic substrates and its binding strength decreases with oxidation.[25,28,32] When both nitrodopamine and dopamine exist predominantly in their reduced forms at pH 5.0, PAAm-ND significantly out performed PAAm-D, indicating that EWG modification greatly enhanced the affinity of catechol towards oxide surfaces. When the pH is raised above the pKa1 of nitrodopamine (pKa1 = 6.5) but below that of dopamine (pKa1 = 9) at pH = 7.4, the shear moduli of PAAm-ND decreased and there was no statistical difference between PAAm-ND and PAAm-D samples. Further increasing the pH to around the pKa1 of dopamine (pH = 9.0) resulted in a further decrease in the measured shear moduli values. However, PAAm-ND exhibited higher shear moduli than PAAm-D, suggesting that the presence of an electronegative −NO2 group rendered the nitrodopamine less prone to oxidation.[56–58]
3.4. Effective Crosslinking Density and Molecular Weight between Crosslinks
The rheological and EWC data were used to calculate the effective crosslink density (veff) of the nanocomposite hydrogels using the Equation (2) while assuming an affine phantom network:[59,60]
| (2) |
where G is the shear modulus, feff is the effective functionality of the crosslinks, R is the gas constant, T is the temperature in Kelvin, and φ2 is the volume fraction of the polymer in the hydrogel. G′ recorded at a frequency of 1 rad s−1 was used as G.[61] In the nanocomposite hydrogels used here, Laponite acts as the site for forming multifunctional crosslinking points and feff was calculated based on Equation (3):[62]
| (3) |
where Mw,L is the molecular weight of Laponite (2.5 × 106 g mol−1)[61] and CL is the mass concentration of Laponite in the hydrogel. φ2 was calculated based on the EWC data using the density of water (1 g cm−3), PAAm (1.42 g cm−3),[63] and Laponite (2.53 g cm−3),[64] while assuming the mass ratio of PAAm and Laponite remain the same as the starting mixture at a 60:40 wt%:wt% ratio.
For a given pH, veff values for PAAm were significantly lower than those of PAAm-D and PAAm-ND (Table 2), indicating that the presence of catechol drastically increased crosslinking density. Similarly, PAAm-ND were more densely crosslinked than PAAm-D. This increase in the crosslinking density was associated with the formation of crosslinking points with higher functionality (i.e., a number of elastically effective network chains extending from Laponite) as measured by feff. These results are in agreement with published observations, where increased interfacial binding strength between polymer matrix and encapsultated nanoparticles increased both the crosslinking density and functionality of the network.[65] Specifically, PAAm-ND exhibited equal or higher veff and feff values when compared to those of PAAm-D. Additionally, PAAm-ND equillibriated at pH 5.0 exhibited the highest veff and feff values reported for all the formulations tested. At pH 9.0, both veff and feff values for PAAm-ND and PAAm-D were equivalent. Given that PAAm-ND exhibited significantly higher moduli values than PAAm-D (Figure 7), our results further confirmed that nitrodopamine formed stronger bonds with Laponite even at a basic pH. Both veff and feff values reported here are of the same order of magnitude as other physically crosslinked nanocomposite hydrogels.[61,62,65]
Table 2.
Estimated effective crosslinking density (veff), effective functionality of crosslinks (feff), and molecular weight between crosslinks (M̄c) for nanocomposite hydrogels.
| pH |
v eff
[mol m −3] |
f eff
|
M̄c
[kDa] |
||||||
|---|---|---|---|---|---|---|---|---|---|
| PAAm | PAAm-D | PAAm-ND | PAAm | PAAm-D | PAAm-ND | PAAm | PAAm-D | PAAm-ND | |
| 3.0 | 8.6 ± 1.2 | 30 ± 11 | 32 ± 5.8 | 45 ± 6.4 | 160 ± 56 | 170 ± 31 | 170 ± 24 | 55 ± 28 | 46 ± 7.7 |
| 5.0 | 8.0 ± 2.6 | 24 ± 4.9 | 55 ± 20a) | 42 ± 14 | 130 ± 26 | 290 ± 100* |
190 ± 69 | 60 ± 13 | 30 ± 14a) |
| 7.4 | 1.2 ± 0.17 | 24 ± 8.0 | 38 ± 7.7a) | 6.5 ± 0.88 | 130 ± 43 | 200 ± 41* | 1170 ± 160 |
64 ± 26 | 38 ± 8.4 |
| 9.0 | 2.9 ± 0.25 | 19 ± 4.6 | 23 ± 3.7 | 16 ± 1.3 | 100 ± 24 | 120 ± 19 | 490 ± 40 | 77 ± 17 | 62 ± 9.1 |
Denotes significance different from PAAm-D hydrogel for a given pH ( p < 0.05).
The calculated effective crosslinking density was further used to determine the molecular weight between crosslinks (M̄c ) using Equation (4):[66]
| (4) |
where ρpolymer is the density of the PAAm polymer chain. Calculated M̄c values revealed that the amine side chain of PAAm was involved in interfacial binding. Both the catechol-functionalized PAAm contained approximately 3 catechols per polymer chain (Table 1), which would yield M̄c values of around 300 kDa (i.e., M̄w of polymer divided by the number of catechols) if only these adhesive moieties were involved forming crosslinking points with Laponite. Given that the calculated M̄c values were 5–10 times lower for PAAm-D and PAAm-ND, the strong interfacial binding properties of catechol likely faciliated PAAm-Laponite interactions, potentially by enhancing the proximity between the polymer network and the nanoparticles. Finally, data reported here further confirmed that nitro-functionalization greatly enhanced the interfacial binding strength of the catechol. Equation (4) assumes an ideal network consisting only of effective network chains. As such, the reported M̄c values are likely to be overestimated.[62]
3.5. Effect of Laponite Concentration on Viscoelastic Properties
Up to this point, testing was performed using nanocomposite formulated with a starting polymer:Laponite wt%:wt% ratio of 60:40 (i.e., 40 wt% Laponite). The effect of increasing the starting Laponite content on the viscoelastic properties of these nanocomposite hydrogels was also investigated (Figure S5, S6 and S7, Supporting Information). Both G′ and G″ values for PAAm-ND and PAAm-D showed little or no increase when the starting Laponite content increased from 40 wt% to 60 wt% (Figure 8). Shear moduli values did not decrease, even though there was a reduction in the catechol content with increasing nanoparticle wt%. On the other hand, there was a drastic increase in the measured moduli values for PAAm with increasing Laponite content (43 and 55 times increase for G′ and G″, respectively). Both shear moduli values for PAAm nanocomposite approached those of PAAm-ND and PAAm-D at the highest Laponite content tested. These results indicated that, while catechol was responsible for strong wet adhesion with the nanoparticles, the primary amine side chain of PAAm also contributed to the interaction with Laponite.[46,47]
Figure 8.
Summary of oscillatory rheology data from frequency sweep experiment for PAAm-ND (●, ○), PAAm-D (■, □), and PAAm (▲, △) equilibrated at pH 7.4. The starting Laponite wt% is the mass of Laponite in relation to the combined mass of the polymer and Laponite in the initial mixture of the nanocomposite. The filled and empty symbols represent G′ and G″, respectively. Values were taken from a frequency of 1 rad s−1 and a strain of 0.1. Within the same Laponite concentration, data points not linked by the same letters (a–c) are statistically different (p < 0.05).
Taken together, this report demonstrates that the viscoelasticity property of a nanocomposite hydrogel is highly dependent on the strength of the interaction between the polymer matrix and the nanoparticles. When no catechol moieties were immobilized onto PAAm chains, the interaction between the polymer matrix and nanoparticles was dominated by weak physical bonds (i.e., hydrogen bonding, electrostatic interactions etc.). Although these weak interactions supported network formation, the weakly associated networks demonstrated reduced material properties, especially when the nanoparticle content was low. When a small amount of catechol was introduced, nanocomposite hydrogels exibited a significant increase in measured shear moduli.
Laponite was chosen in the current study as its strong, reversible interaction with network-bound dopamine has previously been reported.[19,24] Additionally, Laponite is known to be biocompatible and support cellular attachment and proliferation,[67,68] which will aid in the future design of mechanically strong biomaterials. The exact molecular interaction between catechol and Laponite is still unknown. Catechol can potentially form hydrogen bonds with silica oxide.[69] However, hydrogen bonding alone cannot account for the drastic increase in the measured storage and loss moduli (an increase of 1–2 orders of magnitude) when an extremely small amount of the catechol group (≈0.1 wt%) was introduced into the polymer network, especially given the fact that acrylamide side chains are capable of hydrogen bond formation. From density functional theory analysis, the binding energy of catechol with a silica oxide surface was estimated to be 33 kcal mol−1, which is significantly higher than that of hydrogen bonds (binding energy = 14 kcal mol−1).[70,71] The theoretical catechol–SiO2 binding energy value approaches the experimental and theoretical values of catechol bound to a titanium oxide surface.[18,72] As such, the combination of hydrogen bonding and the dispersion interaction of the phenylene ring are likely to be involved in the interfacial binding of catechol and Laponite.[71] A more comprehensive study will be required to verify the specific nature of this interaction.
Both EWG modification and pH changes were performed to further modulate the interfacial binding strength between the network-bound catechol and the nanoparticles. The results confirmed that −NO2 group modification greatly enhanced the binding strength of catechol towards Laponite, which resulted in the formation of a more densely crosslinked network with enhanced mechanical properties. The presence of nitrodopamine contributed to forming multifunctional crosslinking points on Laponite, likely through facilitating nanoparticle interactions with PAAm chains. Finally, the material properties of both dopamine- and nitrodopamine-functionalized nanocomposites were highly dependent on the pH and the oxidation state of these catechol moieties. Although measured shear moduli decreased with increasing pH as a result of catechol oxidation, these materials still exhibited moduli values that were 1–2 orders of magnitude higher than catechol-free control. Nitrodopamine was also less prone to oxidation and exhibited stronger binding to Laponite when compared to dopamine when tested above the dissociation constants of these adhesive moieties. Recently, polymer-bound nitrodopamine was demonstrated to be susceptible to light-mediated degradation,[73] which may represent an additional opportunity to tune the material properties of these nanocomposite materials.
4. Conclusion
PAAm nanocomposite hydrogels were formulated with Laponite nanosilicate and network bound mussel-mimetic catechol adhesive. The catechol exhibited a strong affinity toward Laponite, as a small amount of catechol incorporation (ca. 0.1 wt% in the swollen hydrogel) resulted in a drastic enhancement in material properties and the crosslinking density of the nanocomposite hydrogels. The catechol group was further modified with an electron withdrawing nitro-functional group in the form of nitrodopamine, which further enhanced the measured shear moduli across the whole pH range tested. Although, the moduli decreased under basic conditions as a result of catechol oxidation, PAAm-ND still exhibited significantly higher moduli values when compared to those of PAAm-D, potentially due to the increased resistant to oxidation of the nitrodopamine. This paper confirms that strong interfacial binding between the polymer matrix and encapsulated nanoparticles is critical to the fabrication of nanocomposite hydrogels with improved material properties.
Supplementary Material
Acknowledgements
This project was supported by the National Institutes of Health (GM104846). The HR-2 rheometer was supported in part by the Biotechnology Research Center and Research Excellence Fund (Michigan Technological University). GPC characterization was performed at the Materials Research Science and Engineering Center at University of Massachusetts Amherst, which is supported by the Materials Research Facilities Network of the National Science Foundation (DMR-0820506).
References
- [1].Haraguchi K. Polym. J. 2011;43:223. [Google Scholar]
- [2].Sarkar S, Guibal E, Quignard F, SenGupta A. Journal of Nanoparticle Research. 2012;14:1. [Google Scholar]
- [3].Meng H, Gazella J, Lee BP. In: Bio- and Bioinspired Nanomaterials. Ruiz-Molina D, Novio F, Roscini C, editors. Wiley-VCH; Weinheim, Germany: 2014. p. 309. [Google Scholar]
- [4].Kumar SK, Krishnamoorti R. Annual Review of Chemical and Biomolecular Engineering. 2010;1:37. doi: 10.1146/annurev-chembioeng-073009-100856. [DOI] [PubMed] [Google Scholar]
- [5].Das D, Kar T, Das PK. Soft Matter. 2012;8:2348. [Google Scholar]
- [6].Amoli BM, Marzbanrad E, Hu A, Zhou YN, Zhao B. Macromol. Mater. Eng. 2014;299:739. [Google Scholar]
- [7].Fu S-Y, Feng X-Q, Lauke B, Mai Y-W. Composites Part B. 2008;39:933. [Google Scholar]
- [8].Rusli R, Eichhorn SJ. Appl. Phys. Lett. 2008;93:033111. [Google Scholar]
- [9].McIntosh D, Khabashesku VN, Barrera EV. Chem. Mater. 2006;18:4561. [Google Scholar]
- [10].Rusli R, Eichhorn SJ. Nanotechnology. 2011;22:325706. doi: 10.1088/0957-4484/22/32/325706. [DOI] [PubMed] [Google Scholar]
- [11].Ding X, Heiden PA. Macromol. Mater. Eng. 2014;299:268. [Google Scholar]
- [12].Sangermano M, Vitale A, Dietliker K. Polymer. 2014;55:1628. [Google Scholar]
- [13].Kim D, Lee H, Sohn D. J. Nanosci. Nanotechnol. 2014;14:6427. doi: 10.1166/jnn.2014.8792. [DOI] [PubMed] [Google Scholar]
- [14].Jiang FZ, Huang T, He CC, Brown HR, Wang HL. J. Phys. Chem. B. 2013;117:13679. doi: 10.1021/jp4069587. [DOI] [PubMed] [Google Scholar]
- [15].Waite JH. Int. J. Adhes. Adhes. 1987;7:9. [Google Scholar]
- [16].Yamamoto H. Biotechnol. Genet. Eng. Rev. 1996;13:133. doi: 10.1080/02648725.1996.10647926. [DOI] [PubMed] [Google Scholar]
- [17].Lee BP, Messersmith PB, Israelachvili JN, Waite JH. Annu. Rev. Mater. Res. 2011;41:99. doi: 10.1146/annurev-matsci-062910-100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee H, Scherer NF, Messersmith PB. Proc. Natl. Acad. Sci. USA. 2006;103:12999. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Skelton S, Bostwick M, O’Connor K, Konst S, Casey S, Lee BP. Soft Matter. 2013;9:3825. [Google Scholar]
- [20].Podsiadlo P, Liu Z, Paterson D, Messersmith PB, Kotov NA. Adv. Mater. 2007;19:949. [Google Scholar]
- [21].Ryu S, Lee Y, Hwang JW, Hong S, Kim C, Park TG, Lee H, Hong SH. Adv. Mater. 2011;23:1971. doi: 10.1002/adma.201004228. [DOI] [PubMed] [Google Scholar]
- [22].Pan X-D, Qina Z, Yana Y-Y, Sadhukhana P. Polymer. 2010;51:3453. [Google Scholar]
- [23].Pan X-D, Yan Y-Y, Qin Z, Brumbaugh DR, Sadhukhan P. Rubber Chem. Technol. 2012;85:526. [Google Scholar]
- [24].Liu Y, Meng H, Konst S, Sarmiento R, Rajachar R, Lee BP. ACS Appl. Mater. Interfaces. 2014;6:16982. doi: 10.1021/am504566v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yu J, Wei W, Menyo MS, Masic A, Waite JH, Israelachvili JN. Biomacromol. 2013;14:1072. doi: 10.1021/bm301908y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lee BP, Konst S. Adv. Mater. 2014;26:3415. doi: 10.1002/adma.201306137. [DOI] [PubMed] [Google Scholar]
- [27].Lee BP, Lin M-H, Narkar A, Konst S, Wilharm R. Sens. Actuators B. 2015;206:456. [Google Scholar]
- [28].Lee BP, Chao C-Y, Nunalee FN, Motan E, Shull KR, Messersmith PB. Macromolecules. 2006;39:1740. [Google Scholar]
- [29].Wang C, Svendsen K, Stewart R. In: Biological Adhesive Systems. Byern J, Grunwald I, editors. Springer; Vienna: 2010. p. 169. [Google Scholar]
- [30].Sun CJ, Srivastava A, Reifert JR, Waite JH. J. Adh. 2009;85:126. doi: 10.1080/00218460902782188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Proudfoot GM, Ritchie IM. Aust. J. Chem. 1983;36:885. [Google Scholar]
- [32].Yu M, Hwang J, Deming TJ. J. Am. Chem. Soc. 1999;121:5825. [Google Scholar]
- [33].Amstad E, Gehring AU, Fischer H, Nagaiyanallur VV, Hahner G, Textor M, Reimhult E. J. Phys. Chem. C. 2011;115:683. [Google Scholar]
- [34].Amstad E, Gillich T, Bilecka I, Textor M, Reimhult E. Nano Lett. 2009;9:4042. doi: 10.1021/nl902212q. [DOI] [PubMed] [Google Scholar]
- [35].Malisova B, Tosatti S, Textor M, Gademann K, Zürcher S. Langmuir. 2010;26:4018. doi: 10.1021/la903486z. [DOI] [PubMed] [Google Scholar]
- [36].Cencer M, Murley M, Liu Y, Lee BP. Biomacromol. 2015;16:404. doi: 10.1021/bm5016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Anderson J, Lin M-H, Privette C, Flowers M, Murley M, Lee BP, Ong KG. ScienceJet. 2015;4:80. [PMC free article] [PubMed] [Google Scholar]
- [38].Napolitano A, Dischia M, Costantini C, Prota G. Tetrahedron. 1992;48:8515. [Google Scholar]
- [39].Lee BP, Dalsin JL, Messersmith PB. Biomacromol. 2002;3:1038. doi: 10.1021/bm025546n. [DOI] [PubMed] [Google Scholar]
- [40].Murphy JL, Vollenweider L, Xu F, Lee BP. Biomacromol. 2010;11:2976. doi: 10.1021/bm1007794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cencer MM, Liu Y, Winter A, Murley M, Meng H, Lee BP. Biomacromol. 2014;15:2861. doi: 10.1021/bm500701u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Theato P, Kim J-U, Lee J-C. Macromolecules. 2004;37:5475. [Google Scholar]
- [43].Anseth KS, Bowman CN, Brannon-Peppas L. Biomaterials. 1996;17:1647. doi: 10.1016/0142-9612(96)87644-7. [DOI] [PubMed] [Google Scholar]
- [44].Peppas NA, Bures P, Leobandung W, Ichikawa H. Eur. J. Pharm. Biopharm. 2000;50:27. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- [45].Nurchi VM, Pivetta T, Lachowicz JI, Crisponi G. J. Inorg. Biochem. 2009;103:227. doi: 10.1016/j.jinorgbio.2008.10.011. [DOI] [PubMed] [Google Scholar]
- [46].Xiong L, Hu X, Liu X, Tong Z. Polymer. 2008;49:5064. [Google Scholar]
- [47].Xiong L, Zhu M, Hu X, Liu X, Tong Z. Macromolecules. 2009;42:3811. [Google Scholar]
- [48].Abdurrahmanoglu S, Okay O. J. Appl. Polym. Sci. 2010;116:2328. [Google Scholar]
- [49].Madbouly SA, Otaigbe JU. Macromolecules. 2005;38:10178. [Google Scholar]
- [50].Moura MJ, Figueiredo MM, Gil MH. Biomacromol. 2007;8:3823. doi: 10.1021/bm700762w. [DOI] [PubMed] [Google Scholar]
- [51].Liu M, Li W, Rong J, Zhou C. Colloid Polym. Sci. 2012;290:895. [Google Scholar]
- [52].Vasudevan D, Stone AT. J. Colloid Interface Sci. 1998;202:1. [Google Scholar]
- [53].Tombácz E, Szekeres M. Appl. Clay Sci. 2004;27:75. [Google Scholar]
- [54].Kummert R, Stumm W. J. Colloid Interface Sci. 1980;75:373. [Google Scholar]
- [55].Stone AT, Torrents A, Smolen J, Vasudevan D, Hadley J. Environ. Sci. Technol. 1993;27:895. [Google Scholar]
- [56].Kawabata T, Schepkin V, Haramaki N, Phadke RS, Packer L. Biochemical Pharmacology. 1996;51:1569. doi: 10.1016/0006-2952(96)00101-3. [DOI] [PubMed] [Google Scholar]
- [57].Girerd JJ, Boillot ML, Blain G, Riviere E. Inorg. Chim. Acta. 2008;361:4012. [Google Scholar]
- [58].Higuchi M, Hitomi Y, Minami H, Tanaka T, Funabiki T. Inorg. Chem. 2005;44:8810. doi: 10.1021/ic051173y. [DOI] [PubMed] [Google Scholar]
- [59].Flory PJ. Principles of Polymer Chemistry. Cornell University Press; Ithaca, NY, USA: 1953. [Google Scholar]
- [60].Treloar LRG. The Physics of Rubber Elasticity. 2nd Clarendon Press; Oxford, UK: 1958. p. 342. [Google Scholar]
- [61].Okay O, Oppermann W. Macromolecules. 2007;40:3378. [Google Scholar]
- [62].Nie J, Du B, Oppermann W. Macromolecules. 2005;38:5729. [Google Scholar]
- [63].Hecht AM, Duplessix R, Geissler E. Macromolecules. 1985;18:2167. [Google Scholar]
- [64].Cummins HZ. J. Non-Cryst. Solids. 2007;353:3891. [Google Scholar]
- [65].Abdurrahmanoglu S, Can V, Okay O. J. Appl. Polym. Sci. 2008;109:3714. [Google Scholar]
- [66].Barcellos IO, Pires ATN, Katime I. Polym. Int. 2000;49:825. [Google Scholar]
- [67].Gaharwar AK, Schexnailder PJ, Kline BP, Schmidt G. Acta Biomater. 2011;7:568. doi: 10.1016/j.actbio.2010.09.015. [DOI] [PubMed] [Google Scholar]
- [68].Gaharwar AK, Rivera CP, Wu CJ, Schmidt G. Acta Biomater. 2011;7:4139. doi: 10.1016/j.actbio.2011.07.023. [DOI] [PubMed] [Google Scholar]
- [69].Lu Q, Danner E, Waite JH, Israelachvili JN, Zeng H, Hwang DS. Journal of The Royal Society Interface. 2013:10. doi: 10.1098/rsif.2012.0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Mian SA, Gao X, Nagase S, Jang J. Theoretical Chemistry Accounts. 2011;130:333. [Google Scholar]
- [71].Mian SA, Saha LC, Jang J, Wang L, Gao X, Nagase S. J. Phys. Chem. C. 2010;114:20793. [Google Scholar]
- [72].Vega-Arroyo M, LeBreton PR, Rajh T, Zapol P, Curtiss LA. Chem. Phys. Lett. 2005;406:306. [Google Scholar]
- [73].Shafiq Z, Cui J, Pastor-Pérez L, San Miguel V, Gropeanu RA, Serrano C, del Campo A. Angew. Chem., Int. Ed. 2012;51:4332. doi: 10.1002/anie.201108629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.