Abstract
Poly methyl methacrylate (PMMA) cement produce exothermic reaction during its polymerization process, which damage the surrounding bone tissue during orthopedic surgery. Nanoparticles additives (magnesium oxide, hydroxyapatite, chitosan, barium sulfate and silica) and alternative monomers (glycidyl methacrylate(GMA) tri-methaxysilyl propyl methacrylate (3MPMA)), can be incorporated with the PMMA beads and methyl methacrylate (MMA) monomers, respectively, to reduce the exothermic temperature. A comparative study of the addition of these additives and monomer at different concentration on exothermic temperature of PMMA is not known and significant for designing improved PMMA cement for orthopedic applications. The goal of this study is two folds: (1) to evaluate the effect of the inclusion of the above additives with PMMA on the exothermic temperature of PMMA, (2) to evaluate the effect of the inclusion of the above alternative monomers on the exothermic temperature of PMMA. A commercial bone cement was used in this study as PMMA cement. Two wt% and six wt% of the above nanoparticle were mixed with PMMA beads. Two and six wt% of the above alterative monomers were mixed with MMA monomers. Bead and monomer ratio of 2:1 was maintained to prepare the cement samples. A 4-channel thermocouple was used to determine the temperature changes of the samples in an insulated acrylic mold during the curing period. This study found maximum curing temperature on the 2 wt% Magnesium oxide added PMMA specimen was significantly lower than other samples. Addition of 3MPMA and GMA to MMA decreased the maximum curing temperatures and curing time of specimens compared to other samples.
Keywords: PMMA, Bone cement, Exothermic Temperature, Additives, Nanoparticles, Orthopedics
1. Introduction
The most common bone cement material used clinically today for orthopedic surgeries is poly methyl methacrylate (PMMA) [1]. In general, poly Methyl MethAcrylate (PMMA) beads are added to methyl methacrylate (MMA) monomer with bead and monomer ratio of 2:1 to prepare the PMMA bone cement. Conventional PMMA bone cement has several thermal disadvantages. It is known that polymeric materials have an exothermic reaction during its polymerization process [2]. The problem can cause damage to the surrounding bone cells as well as the tissues [3]. Nanoparticle (NP) additives such as magnesium oxide (MgO), hydroxyapatite (HAp), chitosan (CS), barium sulfate (BaSO4) and silica (SiO2) have been used as additives for the improvement of thermal performances of conventional PMMA bone cement [4]. Glycidyl methacrylate(GMA) and tri-methaxysilyl propyl methacrylate (3MPMA) can be used with MMA to increase the PMMA bone cement thermal properties too. Different types and concentrations of monomers affect the exothermic reaction differently [5]. A comparative study of the effect of the additives and monomers are required for designing improved PMMA cement for orthopedic applications.
It is important to identify if the addition of nanoparticle and alternative monomer with bone cements result in a harmful exothermic process. The exothermic process is a process which the reactants produce heat during the reaction. The process for the PMMA bone cement to solidify is highly exothermic and it can damage the surrounding bone tissue [8]. The more heat it produces during the process, the more harmful it will be to the surrounding bone tissue. Additionally, the residual stresses, caused by the exothermic temperature difference, can influence the fracture energies at the grain boundaries of the nanoparticle-PMMA beads interface [9]. Determining the temperature change of the PMMA cement will provide a good evidence of how much damage the developed cement will cause to the surrounding bone tissue.
The high surface area to volume ratio of nanoparticle additives provides many attractive mechanical, chemical, and thermal qualities regarding bone cement applications. They were previously shown to improve the mechanical performance of acrylic bone cement [10-12]. MgO, HAp, CS, BaSO4 and SiO2 are attractive as additives in bone cement because they can offer the potential to dissipate the heat generated from polymerization reactions throughout the bone cement material, facilitate more uniform cement curing, and minimize the risk of thermal necrosis.
This research will be a good data resource of exothermic measurement of different monomers in bone cement for further studies to develop novel bone cement. Conducting research on materials will also benefit other branches of engineering, such as strength of materials or structural engineering. Veterans who need amputations will have a better chance to avoid the pain from the dying bone tissues. People who were in accidents or disasters will also have a better chance to have better artificial organs. The aging community will benefit from better biomedical devices to increase the length and quality of life.
2. Materials and Methods
2.1. Sample Preparation
Cobalt™ HV bone cement was used in this study as PMMA cement. Two wt% and six wt% of MgO, HAp, CS, BaSO4 and SiO2 were mixed with PMMA beads. The mixer was dissolved in MMA monomer using 2:1 solid: liquid ratio to prepare PMMA-MgO, PMMA-HAp, PMMA -CS, PMMA -BaSO4 and PMMA -SiO2 specimens, respectively. Similarly, two and six wt% of GMA and 3MPMA monomers were mixed with MMA monomers. The solution was dissolved with 2 wt% and 6 wt% of PMMA-MgO, PMMA-HAp, PMMA -CS, PMMA -BaSO4 and PMMA -SiO2 mixtures using 2:1 solid: liquid ratio to prepare the corresponding GMA and 3MPMA included PMMA-MgO, PMMA-HAp, PMMA -CS, PMMA -BaSO4 and PMMA -SiO2 specimens.
2.2. Design and manufacture of the experimental setup
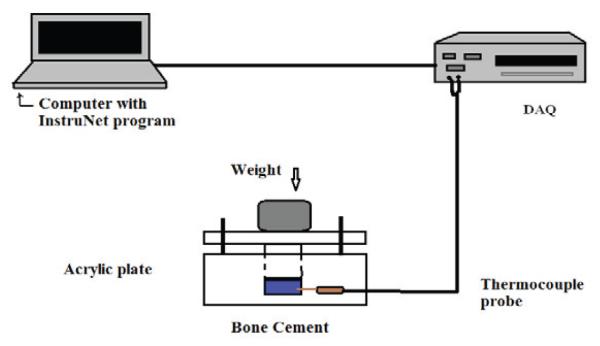
A custom made temperature measurement system was used to determine the temperature changes of the different PMMA cements in an insulated acrylic mold. The 4-channel DI-1000 thermocouple (DATAQ Instruments) was used to measure the temperature changes of the bone cements. The thermocouple was connected to a data acquisition device which was connected to a computer. The computer utilized the InstruNet software for collecting the experimental data. The schematic figure of the setup is shown in the Fig. 1. The experimental setup is shown in the Fig. 2.
Fig. 1.
Schematic diagram for experimental setup
Fig. 2.
Fabricated experimental setup.
2.3. Measurement of exothermic temperature
The changes in temperature of PMMA samples during solidification were measured by using a 4-channel DI-1000thermocouple (DATAQ Instruments). The PMMA sample was placed on an acrylic plate. Set of weights (1633 grams) pressing the sample from above. The temperature change was recorded over various periods of time until the PMMA sample was completely solidified (by using a steel needle to poke the sample in order to identify the curing time). The temperature of pure PMMA cement was used as the reference value. For different types of PMMA samples, the temperatures were measured by thermocouple every 25 seconds.
3. Results and Discussion
Figures 3 to 5 show the variation of curing temperature with respect to time for different PMMA samples. All samples showed the similar characteristic of temperature increase to a peak temperature, Tc, and temperature decrease after Tc. It is also evident from the graphs that nanoparticle additives and alternative monomers influenced the time to reach Tc. The time to reach Tc was lowest for PMMA samples without additives and compared to PMMA samples including additives. MgO-included PMMA cement showed the slowest time to reach maximum temperature. Since it is known that thermal stress is proportional to temperature rise, the thermal stress created in PMMA samples must be higher compared to additive-included PMMA samples.
Fig. 3.
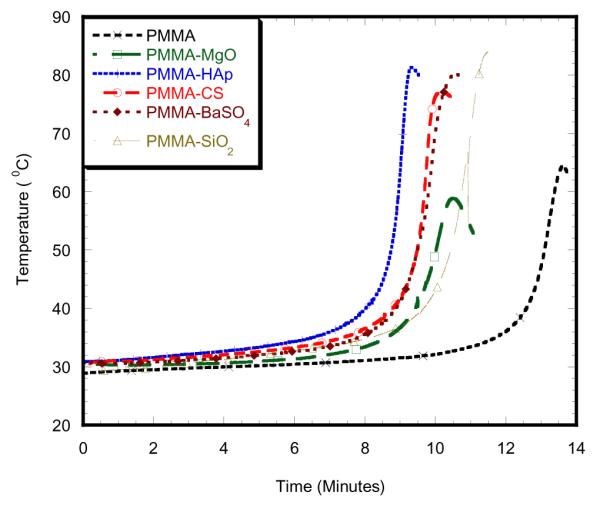
2wt% nanoparticle additives with MMA monomer
Fig. 5.
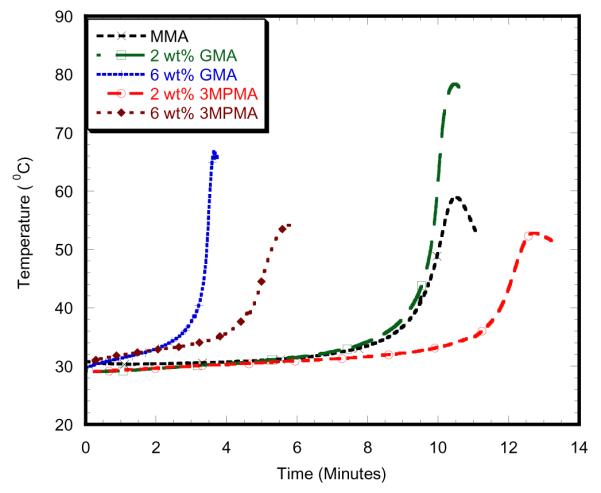
2wt% MgO with alternative monomers
Table 1 and Table 2 report the curing temperatures and times of different samples at different concentrations. This study found that maximum curing temperature on the 2wt% PMMA-MgO with MMA monomer specimen was 58.91 °C, which was significantly lower than PMMA specimen as shown in Figure 3. The addition of 2wt%3MPMA to MMA monomer decreased maximum curing temperature for 2wt% MgO as shown in Figure 5. The maximum curing temperature on the 6wt% PMMA-SiO2 with MMA monomer specimen was 77.01 ° C, which is significantly lower than other 6wt% specimens, but higher than PMMA specimen as shown in Figure 4. The addition of 2wt%3MPMA to MMA monomer decreased maximum curing temperature for 6wt% SiO2as shown in Figure 6. 2wt% HAp with 2wt% 3MPMA specimen has the lower curing temperature of 49.74 ° C by comparing to PMMA-MMA specimens as shown in Table 1. However, the specimens mixed with higher wt% of GMA monomer had shorter time of curing by comparing to PMMA-MMA specimens as shown in Table 2.
Table 1.
Maximum curing temperatures of different specimens
| Control (Unit: °C) | NP | MgO | |||||
|---|---|---|---|---|---|---|---|
| PMMA | only MMA | Monomer | only MMA | 2 wt% GMA | 6 wt% GMA | ||
| 64.42059 | 2 wt% | 58.9129 | 78.34302 | 67.06444 | |||
| 6 wt% | 80.2923 | 72.35516 | 70.43481 | ||||
| Monomer | 2 wt% 3MPMA | 6 wt% 3MPMA | |||||
| 2 wt% | 52.84161 | 54.1815 | |||||
| 6 wt% | 53.57946 | 60.6967 | |||||
| NP | Chitosan | BaSO4 | |||||
| Monomer | only MMA | 2 wt% GMA | 6 wt% GMA | only MMA | 2 wt% GMA | 6 wt% GMA | |
| 2 wt% | 78.08844 | 81.3611 | 73.59322 | 80.2 | 75.18993 | 70.51448 | |
| 6 wt% | 82.10461 | 76.89606 | 74.64117 | 79.39277 | 58.59829 | 70.75648 | |
| Monomer | 2 wt% 3MPMA | wt% 3MPM | 2 wt% 3MPMA | 6 wt% 3MPMA | |||
| 2 wt% | 58.3 | 69.08008 | 62.5 | 75.14077 | |||
| 6 wt% | 57 | 64.3562 | 54.93084 | 65.32405 | |||
| NP | HA | SiO2 | |||||
| Monomer | only MMA | 2 wt% GMA | 6 wt% GMA | only MMA | 2 wt% GMA | 6 wt% GMA | |
| 2 wt% | 81.32119 | 77.01936 | 55.00045 | 84.12207 | 83.56549 | 75.09467 | |
| 6 wt% | 85.15068 | 57.73536 | 70.26519 | 77.00518 | 65.9138 | 66.55793 | |
| Monomer | 2 wt% 3MPMA | wt% 3MPM | 2 wt% 3MPMA | 6 wt% 3MPMA | |||
| 2 wt% | 49.74053 | 71.98364 | 64.03219 | 73.8733 | |||
| 6 wt% | 55.39149 | 66.80482 | 58.72564 | 71.31774 | |||
Table 2.
Curing times for different specimens
| Unit: mins | 2wt%GMA | 2WT%3MPMA | ||||||||
| MgO | HA | Chitosan | BaSO4 | SiO2 | MgO | HA | Chitosan | BaSO4 | SiO2 | |
| 2wt%nanoparticle | 7:30 | 6:44 | 7:58 | 7:30 | 9:37 | 10:33 | 11:38 | 12:10 | 12:30 | 11:58 |
| 6wt%nanoparticle | 8:37 | 7:50 | 9:01 | 8:45 | 11:07 | 12:06 | 12:48 | 11:30 | 9:30 | 11:01 |
| 6wt%GMA | 6wt%3MPMA | |||||||||
| MgO | HA | Chitosan | BaSO4 | SiO2 | MgO | HA | Chitosan | BaSO4 | SiO2 | |
| 2wt%nanoparticle | 7:20 | 7:22 | 8:09 | 7:45 | 8:50 | 9:11 | 8:57 | 9:33 | 8:06 | 9:02 |
| 6wt%nanoparticle | 7:36 | 7:21 | 7:23 | 6:30 | 6:45 | 8:02 | 9:11 | 8:33 | 9:57 | 9:35 |
| MMA | PMMA with alternative monomers | |||||||||
| MgO | HA | Chitosan | BaSO4 | SiO2 | MMA | 2wt%3MPMA | 2wt%GMA | 6wt%3MPMA | 6wt%GMA | |
| 2wt%nanoparticle | 9:31 | 7:50 | 9:10 | 8:45 | 10:50 | 9:55 | 12:33 | 10:05 | 4:55 | 7:08 |
| 6wt%nanoparticle | 7:05 | 7:35 | 8:15 | 7:57 | 10:15 | |||||
Fig. 4.
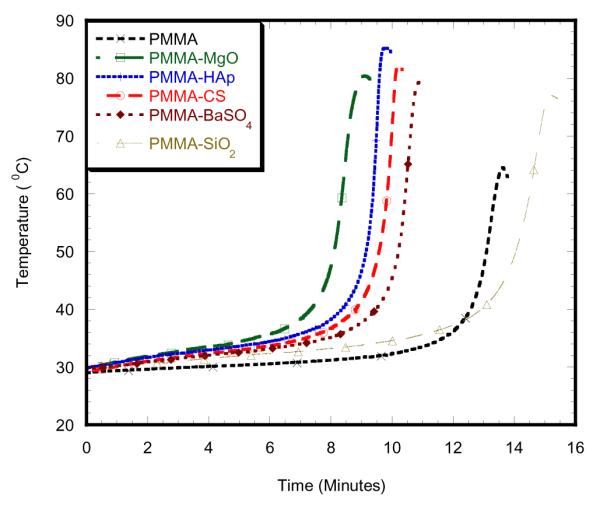
6wt% nanoparticle additives with MMA monomer
Fig. 6.
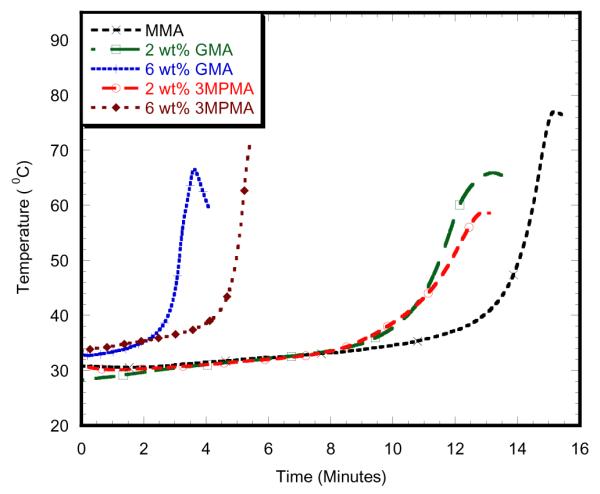
6wt% SiO2 with alternative monomers
The results shows that a specific combination of nanoparticle and alternative monomers can significantly and positively affected the thermal properties of Cobalt HV bone cement. They increased the maximum curing temperature and the time required to completely polymerize the methyl methacrylate monomer, which increases the time required for polymerization. Additional benefits due to nanoparticle and alternative monomer incorporation to bone cement include: minimized risk for thermal necrosis of bone at the bone-cement interfaces that lead to the improved implant longevity in orthopedic and orthodontic applications.
4. Conclusion
The goal for this research was to measure the temperature changes on the different bone cements with different concentrations of nanoparticles (2 wt% and 6 wt% of nanoparticles beads in PMMA beads) and monomers (2 wt% and 6 wt% of alternative monomers in MMA monomers). The maximum curing temperature for 2wt% MgO was significantly lower than the maximum curing temperature for other PMMA specimen having MMA monomer only. However, specimens with higher concentration of nanoparticles had higher maximum curing temperature by comparing to the other PMMA specimen having MMA monomer only. Addition of 3MPMA to monomer decreased the maximum curing temperatures of specimens, but the addition of GMA to monomer decreased the curing time of the specimens.
Acknowledgements
This project was supported by the National Institute of General Medical Sciences of the National Institutes of Health through Grant Number 8P20GM103447 and University of Central Oklahoma CURE-STEM faculty scholar award support from University of Central Oklahoma (UCO). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH or UCO.
Footnotes
Peer-review under responsibility of organizing committee of the 6th BSME International Conference on Thermal Engineering (ICTE 2014)
References
- [1].Van der Geer J, Hanraads JAJ, Lupton RA. The art of writing a scientific article. J. Sci. Commun. 2000;163:51–59. [Google Scholar]
- [2].Strunk W, Jr., White EB. The Elements of Style. third ed Macmillan; New York: 1979. [Google Scholar]
- [3].Mettam GR, Adams LB. How to prepare an electronic version of your article. In: Jones BS, Smith RZ, editors. Introduction to the Electronic Age. E-Publishing Inc.; New York: 1999. pp. 281–304. [Google Scholar]
- [1].Ricker A, Liu-Snyder P, Webster TJ. The influence of nano MgO and BaSO4 particle size additives on properties of PMMA bone cement. International Journal of Nanomedicine. 2008:125–132. [PMC free article] [PubMed] [Google Scholar]
- [2].Serbetci K, Korkusuz F, Hasirci N. Thermal and mechanical properties of hydroxypaptite impregnated acrylic bone cements. Polymer Testing. 2004;23-2:145–155. [Google Scholar]
- [3].Dunne NJ, Orr JF. Thermal characteristics of curing acrylic bone cement. Journal of Materials Science: Materials in Medicine. 2001;22-2:88–97. doi: 10.1023/a:1013670132001. [DOI] [PubMed] [Google Scholar]
- [4].Khandaker M, Vaughan M, Morris T, White J, Meng Z. Effect of additives particles on mechanical, thermal and cell functions properties of poly (methyl methacrylate) cement. International Journal Nanomedicine. 2014;9-1:2699–2712. doi: 10.2147/IJN.S61964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pascual B, Vazquez B, Gurrachaga M, Goni I, Ginebra MP, Gil FJ, Planell JA, Levenfield B, San Roman J. New aspects of the effect of size and size distribution on the setting parameters and mechanical properties of acrylic bone cements. Biomaterials. 1996;17-5:509–516. doi: 10.1016/0142-9612(96)82725-6. [DOI] [PubMed] [Google Scholar]
- [6].Kim SB, Kim YJ, Yoon TL, Park SA, Cho IH, Kim EJ, Kim IA, Shin J-W. The characteristics of a hydroxyapatite-chitosan-PMMA bone cement. Biomaterials. 2004;25-26:5715–5723. doi: 10.1016/j.biomaterials.2004.01.022. [DOI] [PubMed] [Google Scholar]
- [7].Liacouras PC, Owen JR, Jiranek WA, Wayne JS. Effect of pigmentation on the mechanical and polymerization characteristics of bone cement. The Journal of Arthroplasty. 2006;21-4:606–611. doi: 10.1016/j.arth.2005.07.006. [DOI] [PubMed] [Google Scholar]
- [8].Alt V, Bechert T, Steinrucke P, Wagener M, Seidel P, Dingeldein E, Domann E, Schnettler R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials. 2004;25-18:4383–4391. doi: 10.1016/j.biomaterials.2003.10.078. [DOI] [PubMed] [Google Scholar]
- [9].Catharina SJ, van H-C, Govaert LE, Spoelstra AB, Bulstra SK, Wetzels GMR, Koole LH. Mechanical behaviour of a new acrylic radiopaque iodine-containing bone cement. Biomaterials. 2004;25-13:2657–2667. doi: 10.1016/j.biomaterials.2003.09.038. [DOI] [PubMed] [Google Scholar]
- [10].Rao M, Su Q, Liu Z, et al. Preparation and characterization of a poly(methyl methacrylate) based composite bone cement containing poly(acrylate-co-silane) modified hydroxyapatite nanoparticles. Journal of Applied Polymer Science. 2014;131(15) [Google Scholar]
- [11].Gutierrez-Mejia A, Herrera-Kao W, Duarte-Aranda S, et al. Synthesis and characterization of core-shell nanoparticles and their influence on the mechanical behavior of acrylic bone cements. Materials Science and Engineering C. 2013;33(3):1737–1743. doi: 10.1016/j.msec.2012.12.087. [DOI] [PubMed] [Google Scholar]
- [12].Fang C, Hou R, Zhou K, et al. Surface functionalized barium sulfate nanoparticles: Controlled in situ synthesis and application in bone cement. Journal of Materials Chemistry B. 2014;2(9):1264–1274. doi: 10.1039/c3tb21544j. [DOI] [PubMed] [Google Scholar]