Abstract
The purpose of this study was to determine whether dithiothreitol (DTT) treatment of sperm and ethanol activation improve embryo production by intracytoplasmic sperm injection (ICSI). Further, we compared ICSI with standard in vitro fertilization (IVF) in oocytes obtained from cattle. We demonstrated that DTT reduced the disulfide bond in the bovine sperm head. Using oocytes obtained from a slaughterhouse, ICSI-DTT treatment without ethanol showed the highest rate of blastocyst formation. We applied these results to fertilization using ovum pick-up (OPU). Eleven Japanese black cattle served as donors for OPU plus standard IVF (OPU-IVF). Of them, four donors with low embryo development rates were selected to determine whether embryo development was enhanced by OPU plus ICSI (OPU-ICSI). We assessed effects on embryo development following IVF and ICSI in oocytes obtained using OPU. Blastocyst rates were significantly higher for OPU-ICSI than for OPU-IVF. Our results suggest that OPU-ICSI improves the blastocyst development rate in donors with low embryo production compared with the standard OPU-IVF.
Keywords: Dithiothreitol treatment, Intracytoplasmic sperm injection, Japanese black cattle, Low embryo development, Ovum pick-up
A combination of ovum pick-up and in vitro fertilization (OPU-IVF) is expected to serve as an alternative technology for inducing superovulation. Moreover, OPU-IVF facilitates the collection of oocytes from the ovary of donor cows with low gonadotropin response, and it is practiced in livestock breeding. However, the rate of embryo development is not always improved using OPU-IVF, and the low success rate is a concern.
The specificity of the individual donor influences the success rate of embryo development following OPU-IVF [1, 2]. Differences in efficiency of embryo production among bulls have also been reported, and it is necessary to determine optimal in vitro fertilization (IVF) conditions by experimentation. Moreover, there are problems associated with sorting semen with weak motility.
The purpose of our study was to evaluate methods for intracytoplasmic sperm injection (ICSI) to generate embryos. ICSI is a microfertilization technique involving the direct injection of a spermatozoon into the ooplasm. In cattle, the advantages of ICSI include effective utilization of spermatozoa for livestock improvement and multiplication of superior animals. Further, ICSI can be used to obtain embryos with fewer spermatozoa. We have reported that spermatozoa immobilization just before injection using the combination of a piezo-micromanipulator and ethanol activation 4 h after ICSI is useful for producing blastocysts [3]. Furthermore, offspring are constantly being produced by embryo transfer using ICSI [3, 4].
A previous study reported that Piezo-ICSI using tail-cut motile spermatozoa is effective for cleavage and subsequent development without exogenous oocyte activation and that it resulted in the birth of five calves [5]. Bovine ICSI without additional activation treatment is important for achieving high rates of production of healthy calves [4]. Therefore, as a future objective to produce healthy calves, we also examined activation treatment after ICSI.
In contrast to conventional IVF, ICSI injects a spermatozoon directly into the ooplasm without an acrosome reaction. Generally, when the sperm enters into the ooplasm, disulfide bonds in the sperm head nucleus, which form disulfide bridges, begin to be reduced by glutathione (GSH) [6, 7]. After fertilization, sperm head nuclei show a reduction of disulfide bounds in protamines and the replacement of protamines with histones [8]. Subsequently, the sperm membrane disappears, and the male pronucleus forms and fuses with the female pronucleus. Compared with sperms of several other species (e.g., the mouse, human, and hamster), bull sperm is more stable and cannot easily decondense in the bovine oocyte [6]. Various chemicals such as heparin [5, 9, 10], caffeine [5, 9,10,11,12] and Ca-ionophore [5, 10] are able to increase sperm membrane permeabilization, the acrosome reaction and sperm head decondensation; for this reason, they are routinely used in bovine IVF. Conversely, the compound dithiothreitol (DTT) has been shown to induce reduction of protamine disulfides bound to the sperm head [13,14,15]. Furthermore, pretreatment of spermatozoa with the abovementioned chemicals may aid embryo development of sperm-injected oocytes [5, 16,17,18].
The present study sought to evaluate the improvement in embryo production using a combination of ovum pick-up and ICSI (OPU-ICSI) after DTT pretreatment to target donors with low embryo production in OPU-IVF.
Materials and Methods
Slaughterhouse oocyte collection
Bovine ovaries from Japanese black cows or heifers were obtained from a local slaughterhouse and transported to the laboratory within 2 h. Cumulus oocyte complexes (COCs) were aspirated from antral follicles 2–8 mm in diameter through a 21-gauge, 1.58-cm (5/8 inches) needle attached to a 10 ml syringe. Oocytes were matured in vitro, as previously described [3, 4].
Ovum pick-up
Ovarian follicles were aspirated from eleven Japanese black cows at 7-day intervals using a transvaginal ultrasound-guided device (Aloka, SSD-1200, Overseas Monitor, Richmond, BC, Canada) equipped with a 7.5-MHz convex probe and a stainless steel needle guide. A 17-gauge × 60-cm echogenic needle (Misawa Medical Industry, Ibaraki, Japan) was used to penetrate the vaginal wall and ovarian tissue for follicle aspiration. Follicle contents were aspirated into one-way tubing using a constant aspiration pressure of 100 mmHg. Follicular aspiration fluids were collected in 50-ml conical centrifuge tubes (Nunc, Roskilde, Denmark) warmed to 30 C using a tube warmer (Model FV5, FHK, Tokyo, Japan). The aspiration medium was modified phosphate-buffered saline supplemented with 10 IU/ml of sodium heparin (NOVO Heparin, Novo Nordisk A/S, Bagsvaerd, Denmark) and 0.05 mg/ml of gentamicin (Sigma-Aldrich, St. Louis, MO, USA). Only follicles of > 2 mm in diameter were aspirated. After follicle aspiration, an Em-Con filter (Immuno Systems, Spring Valley, WI, USA) was used to separate any blood components and to collect the oocytes from the follicular fluid aspirate.
Oocyte maturation
The isolated COCs were washed three times. Groups of 10 COCs were matured in 100 μl TCM199 (Gibco BRL, Grand Island, NY, USA) supplemented with 4 mg/ml of bovine serum albumin (BSA; Sigma-Aldrich), 0.1 IU/ml of FSH (Antrin; Kyoritsu Seiyaku, Tokyo, Japan) and 50 ng/ml of epidermal growth factor (EGF; Upstate Biotechnology, Lake Placid, NY, USA) under mineral oil (Nacalai Tesque, Kyoto, Japan) at 38.5 C in a 5% CO2 atmosphere for 22 h.
Sperm preparation
Straws containing frozen spermatozoa from a Japanese black bull were thawed at 37 C for 30 sec in a water bath, washed with BSA-free mTALP [19], supplemented with 10 mM caffeine (Wako Pure Chemical Industries, Osaka, Japan) and centrifuged at 500 × g for 5 min [20]. Washed spermatozoa were then incubated with BSA-free mTALP supplemented with 5 mM DTT (Wako Pure Chemical Industries) for 10 min at 38.5 C [16,17,18] and washed again. DTT was not added to the control group. Pelleted spermatozoa were resuspended in the same medium. To prepare spermatozoa for ICSI, a 10-μl sperm suspension was mixed with 30-μl 12% PVP K90 (MP Biomedicals, solon, OH, USA).
Labeling of bull spermatozoa with monobromobimane (mBBr)
Monobromobimane (Calbiochem, La Jolla, CA, USA) was added to a 1.0-ml sperm suspension (4–5 × 106 sperm/ml) in mTALP at a final reagent concentration of 0.1 mM in a 1.5-ml microtube. Sperm samples were covered with aluminum foil during the labeling reaction to minimize bimane photolysis. After a 5-min incubation in the dark at 37 C, the sperm suspension was mounted on glass slides, covered with coverslips and examined using fluorescence microscopy [21, 22]. Fluorescence emissions of sperm were recorded by digital camera using a stabilized mercury lamp and fluorescence filters (excitation at 480 nm and emission at 510 nm) to determine emission values using ImageJ 1.48 (National Institutes of Health, Bethesda, MD, USA).
Intracytoplasmic sperm injection
After maturation, cumulus cells were thoroughly dispersed with 0.1% bovine testicular hyaluronidase (Sigma-Aldrich) and removed by gentle pipetting. After the removal of cumulus cells, only oocytes with a visible first polar body were selected, and they were washed three times in TCM199 supplemented with 5% calf serum (CS; Gibco BRL) and kept in the same medium until further treatment at 38.5 C in a 5% CO2 atmosphere.
During ICSI, oocytes were suspended in M2 medium supplemented with 5% CS (Gibco BRL). ICSI was performed using a piezo-driven micromanipulator (PMM-150; Prime Tech, Ibaraki, Japan) according to published methods [3, 4]. Immediately before sperm injection, a motile spermatozoon was immobilized by breaking its tail with the tip of an injection needle. An oocyte was secured using a pipette, and the polar body was vertically positioned. The immobilized spermatozoon was then transported tail-first into the injection pipette. The zona pellucida was penetrated by applying several piezo pulses. The spermatozoon was pushed forward until its head approached the center of the oocyte. The oolemma was punctured using a single piezo pulse, and the spermatozoon was injected into the ooplasm. Injected oocytes were transferred into 50 μl TCM199 supplemented with 5% CS under paraffin oil and stored at 38.5 C in a 5% CO2 atmosphere. Thereafter, we selected 2PB oocytes at 4 h following ICSI and excluded 1PB oocytes [3, 4].
In vitro fertilization
IVF was performed as described previously using one straw of frozen sperm [23]. Frozen and thawed spermatozoa were washed twice with mTALP medium supplemented with 10 mM caffeine (Wako Pure Chemical Industries) and centrifuged at 500 × g for 5 min. The final sperm pellet was resuspended in the same medium at a final concentration of 1.0–2.0 × 107 sperm/ml. An equal volume of mTALP medium supplemented with 3 mg/ml BSA and 10 IU/ml heparin (NOVO Heparin, Novo Nordisk A/S) was added to the sperm suspension. Heparin-treated spermatozoa were placed under paraffin oil and incubated for 15 min at 38.5 C in an atmosphere containing 5% CO2. Groups of COCs (≤ 50) matured in vitro were introduced into 100-μl microdrops of sperm suspension in a 35-mm culture dish. Six hours after the initiation of insemination, the cumulus cells were removed with a pipette.
Embryo culture
The culture medium was modified synthetic oviduct fluid (mSOF) [24, 25] supplemented with 20 μl/ml essential amino acid solution (50 ×, Gibco BRL), 10 μl/ml nonessential amino acid solution (100 ×, Gibco BRL), 1 mM glycine, 2 mM taurine, ITS supplement (final concentrations of 5 μg/ml insulin, 5 μg/ml transferrin and 5 ng/ml selenium; Sigma-Aldrich) and 6 mg/ml fatty acid-free BSA (Sigma-Aldrich). ICSI and IVF oocytes were cultured in groups of 10–15 in 50-μl drops of mSOF medium at 38.5 C in an atmosphere containing 5% CO2, 5% O2 and 90% N2. Cleavage rates and blastocyst formation rates were assessed at 72 h and 192 h, respectively, after both ICSI and IVF.
Differential staining of inner cell mass and trophectoderm cells
The cell allocation of blastocysts (196 h post insemination) was assessed by differential staining of inner cell mass (ICM) and trophectoderm (TE) cells, as described previously [26]. Briefly, blastocyst TE cells were stained for 40 sec with 100 μg/ml propidium iodide (Sigma-Aldrich) in a permeabilizing solution of 0.2% (v/v) Triton X-100 (Sigma-Aldrich). Blastocysts were then counterstained and simultaneously fixed for 5 min with 25 μg/ml Hoechst 33342 (Calbiochem, La Jolla, CA, USA) in 99.5% ethanol. Fixed and stained whole blastocysts were mounted, and the number of ICM cells and TE cells was assessed using fluorescence microscopy. ICM and TE nuclei were identified by blue and pink to red staining, respectively.
Analysis of pronuclear formation
For observation of pronuclei, at 18 h post IVF and ICSI, oocytes were fixed for 48 h in acetic acid-ethanol (3:1), stained with 1% acetic acid-orcein and examined under a phase-contrast microscope.
Experimental design
In Experiment 1, the effect of DTT on disulfide bonds of sperm was examined using mBBr staining. The control received no DTT treatment. In Experiment 2, we examined the embryo production rate following DTT-ICSI and ICSI without DTT using oocytes obtained from a slaughterhouse. We then observed embryo development. Additionally, cell allocation in blastocysts was assessed by the differential staining of ICM and TE cells. In Experiment 3, we examined improvements in the embryo production rate following DTT-ICSI using oocytes obtained by OPU from four donors with low embryo production. These oocytes were subjected to IVF and DTT-ICSI. To evaluate fertilization in OPU-IVF and OPU-ICSI, we examined formation of the male pronucleus (MPN) at 18 h post IVF and ICSI. In the present study, we used frozen spermatozoa from a single sire.
Statistical analyses
Data for the labeling of bull spermatozoa with mBBr staining and differential staining of ICM and TE cells were analyzed by one-way ANOVA. Means were compared among the different groups by Tukey’s multiple comparison tests. Percentage data for cleavage and blastocyst formation in the 11 Japanese black cattle subjected to OPU were arcsine-transformed prior to one-way ANOVA. Means were compared among the different groups by Tukey’s multiple comparison tests. Other percentage data were analyzed using the chi-square test and Fisher’s exact probability test. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R (version 2.13.0, The R Foundation for Statistical Computing). More precisely, it is a modified version of R Commander (version 1.6.3) that includes statistical functions frequently used in biostatistics. P values of < 0.05 were considered statistically significant.
Results
Experiment 1
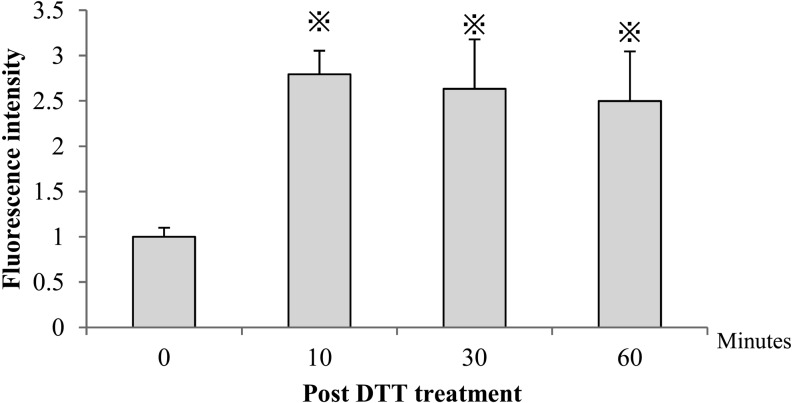
We analyzed sperm numbers after DTT treatment (0 min, 37; 10 min, 46; 30 min, 49; 60 min, 50). Bovine spermatozoa pretreated with DTT showed higher (P < 0.05) fluorescein emission values (10 min, 2.7 ± 0.3; 30 min, 2.6 ± 0.5; 60 min, 2.5 ± 0.5) compared with those shown by the control group (1.0; Fig. 1).
Fig. 1.
Integrity of disulfide bonds in bovine sperm treated with DTT. The level of disulfide bonding is expressed as the relative fluorescein intensity of monobromobimane (mBBr). Each column shows the mean ± SEM. P < 0.05; one-way ANOVA and Tukey’s multiple comparison test.
Experiment 2
Results were obtained from three replicates, with a total of 347 oocytes injected with spermatozoa either pretreated with DTT (ICSI-DTT) or untreated (ICSI-Sp) and the oocytes either activated using 7% ethanol or not activated (four conditions total). Table 1 shows results of in vitro development of bovine oocytes fertilized by ICSI. ICSI-Sp with ethanol treatment (88.8%) showed a significantly higher cleavage rate than ICSI-DTT with ethanol (76.4%), ICSI-DTT without ethanol (77.1%) and ICSI-Sp without ethanol (70.7%) (P < 0.05). ICSI-DTT without ethanol produced the highest rate of blastocyst formation (P < 0.05; 33.3% vs. 18.0%, 18.8% and 7.3% for ICSI-DTT with ethanol, ICSI-Sp with ethanol and ICSI-Sp without ethanol, respectively).
Table 1. Effect of different sperm pretreatments on the in vitro development of bovine oocytes generated by intracytoplasmic sperm injection.
| Treatment | EtOH | No. of oocytes | No. (%) of cleavages | No. (%) of blastocysts | ||
| ICSI-DTT | + | 89 | 67 | (76.4) a | 16 | (18.0) a |
| ICSI-DTT | – | 96 | 74 | (77.1) a | 32 | (33.3) b |
| ICSI-Sp | + | 80 | 71 | (88.8) b | 15 | (18.8) a |
| ICSI-Sp | – | 82 | 58 | (70.7) a | 6 | (7.3) c |
Values with superscript letters are statistically significant (P < 0.05). The number of replicates was three. Cleavage and blastocyst rates were evaluated 72 h and 192 h, respectively. Activation treatment (7% EtOH, 5 min). ICSI-Sp, treatment only with mTALP before intracytoplasmic sperm injection (ICSI); ICSI-DTT, treatment with 5 mM dithiothreitol.
Embryo quality was assessed on the basis of total number of cells, TE cells and ICM cells. Table 2 shows the blastocyst cell allocation, as assessed by differential staining of ICM and TE cells. In the mean numbers of total nuclei in blastocysts, ICSI-DTT without ethanol treatment (165.6 ± 8.5) was significantly higher than both ICSI-Sp with ethanol and without ethanol (124 ± 9.8 and 109 ± 10.3, respectively, P < 0.05).
Table 2. Effect of sperm pretreatments prior to intracytoplasmic sperm injection on the total number of cells and numbers of inner cell mass and trophectoderm cells.
| Treatment | EtOH | No. of oocytes | No. of cells |
||||||||
| TE | ICM | Total | |||||||||
| ICSI-DTT | + | 16 | 104.1 | ± | 7.9 ab | 47.3 | ± | 5.8 | 151.4 | ± | 10.8 ab |
| ICSI-DTT | – | 32 | 116.3 | ± | 8.2 a | 49.3 | ± | 2.9 | 165.6 | ± | 8.5 a |
| ICSI-Sp | + | 15 | 86.3 | ± | 7.6 ab | 38.2 | ± | 3.9 | 124.5 | ± | 9.8 b |
| ICSI-Sp | – | 6 | 69.2 | ± | 8.7 b | 40.3 | ± | 6.4 | 109.5 | ± | 10.3 b |
Values with superscript letters are statistically significant (P < 0.05). The number of replicates was three. Activation treatment (7% EtOH, 5 min). ICSI-Sp, treatment only with mTALP before intracytoplasmic sperm injection (ICSI); ICSI-DTT, treatment with 5 mM dithiothreitol.
Experiment 3
Blastocyst rates among the 11 donors ranged from 2.7% to 50.0%. Donor A had significantly lower embryo development rates than donors G, H, I, J and K. In the present study, the spermatozoa were from a single sire, indicating that differences in embryo development rates could be attributed to the different oocyte donors used (Table 3). The rates of embryo development among donors A, B, C and D differed by ≤ 20%.
Table 3. Effect of different donors on in vitro development of bovine embryos generated by OPU-IVF.
| Group | Donor | Age | Parity | No. of oocytes | No. (%) of cleaved embryos | No. (%) of blastocysts | ||
| Low embryo production | A | 16 | 9 | 74 | 11 | (16.2 ± 7.6) a | 2 | (2.7 ± 2.4) a |
| B | 10 | 2 | 73 | 19 | (31.5 ± 10.2) ab | 4 | (5.5 ± 2.7) ab | |
| C | 10 | 2 | 83 | 28 | (38.6 ± 3.7) ab | 6 | (7.2 ± 0.6) ab | |
| D | 9 | 1 | 96 | 32 | (33.3 ± 6.4) ab | 13 | (13.5 ± 2.1) ab | |
| Conventional | E | 13 | 2 | 52 | 18 | (34.6 ± 10.7) ab | 13 | (25.0 ± 9.4) ab |
| F | 13 | 1 | 49 | 24 | (49.0 ± 7.4) ab | 14 | (28.6 ± 6.5) ab | |
| G | 13 | 3 | 45 | 36 | (80.0 ± 5.0) b | 16 | (35.6 ± 9.8) b | |
| H | 11 | 4 | 126 | 93 | (73.8 ± 16.1) b | 45 | (35.7 ± 7.9) b | |
| I | 14 | 8 | 76 | 50 | (65.8 ± 5.6) b | 33 | (43.4 ± 9.2) b | |
| J | 12 | 3 | 70 | 46 | (65.7 ± 8.7) ab | 32 | (45.7 ± 4.4) b | |
| K | 15 | 6 | 64 | 49 | (76.6 ± 18.0) b | 32 | (50.0 ± 12.7) b | |
Values with superscript letters are statistically significant (P < 0.05). The number of replicates was five. Cleavage and blastocyst rates were evaluated at 72 h and 192 h. Low embryo production differed by ≤ 20% in terms of blastocyst rate.
These four donors with low embryo development were selected to determine whether embryo development could be improved with OPU-ICSI. For the four donors with low embryo developmental ability in OPU-IVF, under similar conditions as those in experiment 2, we determined whether there was an improvement in the embryo developmental rate. Table 4 shows the results for embryo development following IVF and ICSI for oocytes obtained using OPU. In donors A, B and D, OPU-ICSI produced significantly higher cleavage rates than IVF (42.9% vs. 20.4%, 74.3% vs. 24.5% and 65.3% vs. 21.9%, respectively). Similarly, in donors A, B, C and D, blastocyst rates were significantly higher for ICSI than for IVF (19.0% vs. 1.9%, 31.4% vs. 4.1%, 25.6 vs. 7.0 and 28.6% vs. 10.9%, respectively). In summary, OPU-ICSI produced significantly higher overall cleavage and blastocyst rates than IVF.
Table 4. Comparison of embryo production efficiency between IVF and ICSI in donors.
| Donor | Method | No. of replicates | No. of oocytes | No. (%) of cleavages | No. (%) of blastocysts | ||
| A | ICSI | 3 | 21 | 9 | (42.9) a | 4 | (19.0) a |
| IVF | 3 | 54 | 11 | (20.4) b | 1 | (1.9) b | |
| B | ICSI | 3 | 35 | 26 | (74.3) a | 11 | (31.4) a |
| IVF | 3 | 49 | 12 | (24.5) b | 2 | (4.1) b | |
| C | ICSI | 3 | 39 | 19 | (48.7) ab | 10 | (25.6) a |
| IVF | 3 | 43 | 14 | (32.6) ab | 3 | (7.0) b | |
| D | ICSI | 3 | 49 | 32 | (65.3) a | 14 | (28.6) a |
| IVF | 3 | 64 | 14 | (21.9) b | 7 | (10.9) b | |
| Total | ICSI | 12 | 144 | 86 | (59.7) a | 39 | (27.1) a |
| IVF | 12 | 210 | 51 | (27.9) b | 13 | (6.2) b | |
Values with superscript letters are statistically significant (P < 0.05).
The results for male pronuclear formation at 18 h after OPU-IVF and OPU-ICSI are shown in Table 5. No statistically significant difference was found between IVF and ICSI in donor I. The 1-MPN rates of ICSI were significantly higher than that of IVF in donors C and D (P < 0.05).
Table 5. Results of male pronuclear formation following OPU-IVF and OPU-ICSI.
| Group | Donor | Method | No. of oocytes examined | No. of male pronuclei |
|||
| 1 MPN (%) | 2 or more MPN (%) | ||||||
| Low embryo production | C | IVF | 59 | 26 | (44.1) a | 3 | (5.1) |
| ICSI | 35 | 23 | (65.7) b | - | - | ||
| D | IVF | 60 | 21 | (35.0) a | 2 | (3.3) | |
| ICSI | 30 | 21 | (70.0) b | - | - | ||
| Conventional | I | IVF | 45 | 34 | (75.6) | 1 | (2.2) |
| ICSI | 39 | 33 | (84.6) | - | - | ||
Values with superscript letters within the same donor are statistically significant (P < 0.05) between IVF and ICSI. Oocytes were examined 18 h post IVF and ICSI. MPN: male pronucleus.
Discussion
Bovine sperm nuclei have particularly strong disulfide bonds [6], which can be expected to result in hyperstabilized chromatin, preventing the sperm nucleus from decondensing [27]. The protamine in bull sperm chromatin is a cysteine-rich type 1 protein, resulting in the chromatin configuration being tightly packaged and stable [28]. DTT is an agent that specially reduces disulfide bonds in sperm chromatin. Its use has been suggested for gaining access to the sperm nucleus through the perinuclear theca membrane by reduction of the protamine disulfide bond, resulting in decondensation of chromosomes [7, 16]. We demonstrated the optimal DTT treatment time to be 10 min and observed the reduction of disulfide bonds in the sperm head with DTT treatment. Staining with mBBr may have detected additional changes in proteins other than the sperm-histone proteins, resulting in a net increase in fluorescence.
Moreover, we examined the efficacy of ICSI-DTT for improving OPU-IVF using low embryo production donors. Pretreatment of spermatozoa with DTT has been shown to enhance the overall success rate of ICSI-fertilized embryos in all cows [16, 17]. Thus, we examined the effect of sperm pretreated with DTT on the developmental competence of bovine oocytes fertilized by ICSI. Extending the results of previous reports, we also observed significantly increased rates of blastocyst formation with DTT-ICSI.
Previous studies reported improved results in terms of damage to the sperm head plasma membrane before ICSI, which may contribute to the release of factors involved in sperm head decondensation and oocyte activation [29,30,31]. DTT treatment destroys the sperm plasma membrane in a time-dependent manner in bovine [32]. In the process of fertilization, the sperm triggers a series of intracellular Ca2+ releases [21, 33, 34]. These Ca2+ oscillations activate the egg and determine maturation promoting factor (MPF) inactivation, sperm head decondensation and pronucleus formation, and they are involved in the initiation of embryo development [35, 36]. Soluble sperm factors are involved in the regulation of calcium waves [37]. It is known that cytosolic sperm extracts (CFs) of several species can activate mammalian eggs [38,39,40,41,42]. This suggests that the present sperm injection procedure was sufficient to trigger oocyte activation.
The comparison of effects suggested that the low blastocyst rates of low embryo production cows were caused by low MPN rates in IVF. In OPU-ICSI, sperm was directly injected into the oocyte, and we inferred that DTT pretreatment of sperm promoted male pronuclear formation [43]. Higher rates of MPN were obtained when sperm pretreated with DTT was injected into ooplasm, which suggest that an appropriate chemical pretreatment of sperm would be greatly beneficial for bovine ICSI [5, 16, 44]. Thus, the high rate of zygotes with two pronuclei after ICSI suggests that sperm contains all factors necessary to induce the formation of two pronuclei [45].
Bovine ICSI usually requires oocyte activation after sperm injection. One plausible reason for the low embryo production rate in bovine ICSI is the lower MPF activity of oocytes. Fujinami et al. reported that ethanol treatment after ICSI temporarily inhibited MPF activity at 6 h after ICSI [35] and, thus, promoted subsequent embryonic development. We obtained good results from ethanol treatment for 4 h following ICSI, but these results indicate that there is no need for treatment with ethanol when sperms are pretreated with DTT. This is in agreement with a report of Galli et al. [29]. The precise mechanism of this finding is the subject of a future investigation.
In our organization, blastocyst rates using OPU-IVF range between 2.7% and 50.0%, and they vary among individual animals. Generally, rates of embryo development differ depending on the donor [1], an observation consistent with the present results using four low embryo production donors. The use of ICSI showed that the rates of cleavage and embryo development in low production donors can be improved. Examination of total and TE cell numbers in blastocysts revealed that ICSI-DTT embryos had significantly increased numbers of these cells as compared with those in ICSI-Sp embryos. Thus, we demonstrated the ability of ICSI-DTT to produce high-quality embryos.
In conclusion, we demonstrated that the OPU-ICSI after DTT sperm treatment (OPU-ICSI-DTT) procedure using OPU-IVF improved the rate of blastocyst generation for donors with low embryo production capability. In bovine OPU-ICSI after DTT sperm treatment, sperm was directly injected into the oocyte, and ICSI promoted male pronuclear formation. To the best of our knowledge, the developmental competence observed in the present study was the highest reported in bovine embryo production by OPU-ICSI-DTT in low embryo production cows. The increase in embryo productivity following OPU-ICSI-DTT suggests the utility of this OPU-IVF method for increasing the production of Japanese black cattle. Moreover, our results indicate the potential to produce embryos with higher developmental competence.
References
- 1.Imai K, Tagawa M, Yoshioka H, Matoba S, Narita M, Inaba Y, Aikawa Y, Ohtake M, Kobayashi S. The efficiency of embryo production by ovum pick-up and in vitro fertilization in callte. J Reprod Dev 2006; 52: S19–S29. [Google Scholar]
- 2.Skrzyszowska M, Smorag Z, Katska L, Bochenek M, Gogol P, Kania G, Rynska B. Development of bovine embryos after intracytoplasmic sperm injection (ICSI): effect of gamete donors, sperm chromatin structure and activation treatment. Czech J Anim Sci 2002; 47: 85–91. [Google Scholar]
- 3.Horiuch T, Emuta C, Yamauchi Y, Oikawa T, Numabe T, Yanagimachi R. Birth of normal calves after intracytoplasmic sperm injection of bovine oocytes: a methodological approach. Theriogenology 2002; 57: 1013–1024. [DOI] [PubMed] [Google Scholar]
- 4.Oikawa T, Takada N, Kikuchi T, Numabe T, Takenaka M, Horiuchi T. Evaluation of activation treatments for blastocyst production and birth of viable calves following bovine intracytoplasmic sperm injection. Anim Reprod Sci 2005; 86: 187–194. [DOI] [PubMed] [Google Scholar]
- 5.Wei H, Fukui Y. Effects of bull, sperm type and sperm pretreatment on male pronuclear formation after intracytoplasmic sperm injection in cattle. Reprod Fertil Dev 1999; 11: 59–65. [DOI] [PubMed] [Google Scholar]
- 6.Perreault SD, Barbee RR, Slott VL. Importance of glutathione in the acquisition and maintenance of sperm nuclear decondensing activity in maturing hamster oocytes. Dev Biol 1988; 125: 181–186. [DOI] [PubMed] [Google Scholar]
- 7.Sutovsky P, Schatten G. Depletion of glutathione during bovine oocyte maturation reversibly blocks the decondensation of the male pronucleus and pronuclear apposition during fertilization. Biol Reprod 1997; 56: 1503–1512. [DOI] [PubMed] [Google Scholar]
- 8.Zirkin BR, Perreault SD, Naish SJ. Formation and function of the male pronucleus during mammalian fertilization. In: The Molecular Biology of fertilization. San Diego CA: Academic Press; 1989: 91–114. [Google Scholar]
- 9.Keefer CL, Younis AI, Brackett BG. Cleavage development of bovine oocytes fertilized by sperm injection. Mol Reprod Dev 1990; 25: 281–285. [DOI] [PubMed] [Google Scholar]
- 10.Chen SH, Seidel GE. Effect of oocytes activation and treatment of spermatozoa on embryonic development following intracytoplasmic sperm injection in cattle. Theriogenology 1997; 48: 1265–1273. [Google Scholar]
- 11.Goto K, Kinoshita A, Takuma Y, Ogawa K. Fertilisation of bovine oocytes by the injection of immobilised, killed spermatozoa. Vet Rec 1990; 127: 517–520. [PubMed] [Google Scholar]
- 12.Iwasaki S, Li X. Experimental production of triploid bovine embryos by microinjection of two sperm and their development. J Reprod Dev 1994; 40: 317–322. [Google Scholar]
- 13.Calvin HI, Bedford JM. Formation of disulphide bonds in the nucleus and accessory structures of mammalian spermatozoa during maturation in the epididymis. J Reprod Fertil Suppl 1971; 13: 65–75. [PubMed] [Google Scholar]
- 14.Bedford JM, Calvin HI. The occurrence and possible functional significance of -S-S- crosslinks in sperm heads, with particular reference to eutherian mammals. J Exp Zool 1974; 188: 137–155. [DOI] [PubMed] [Google Scholar]
- 15.Tateno H, Kamiguchi Y. Dithiothreitol induces sperm nuclear decondensation and protects against chromosome damage during male pronuclear formation in hybrid zygotes between Chinese hamster spermatozoa and Syrian hamster oocytes. Zygote 1999; 7: 321–327. [DOI] [PubMed] [Google Scholar]
- 16.Rho GJ, Kawarsky S, Johnson WH, Kochhar K, Betteridge KJ. Sperm and oocyte treatments to improve the formation of male and female pronuclei and subsequent development following intracytoplasmic sperm injection into bovine oocytes. Biol Reprod 1998; 59: 918–924. [DOI] [PubMed] [Google Scholar]
- 17.Suttner R, Zakhartchenko V, Stojkovic P, Müller S, Alberio R, Medjugorac I, Brem G, Wolf E, Stojkovic M. Intracytoplasmic sperm injection in bovine: effects of oocyte activation, sperm pretreatment and injection technique. Theriogenology 2000; 54: 935–948. [DOI] [PubMed] [Google Scholar]
- 18.Qian XQ, Inagaki H, Sasada H, Sugawara S. Decondensation and pronuclear formation in bovine oocytes after microinjection of bovine sperm pre-treated by with disulfide bond reducing agents. J Mamm Ova Res 1996; 13: 118–121. [Google Scholar]
- 19.Parrish JJ, Susko-Parrish J, Winer MA, First NL. Capacitation of bovine sperm by heparin. Biol Reprod 1988; 38: 1171–1180. [DOI] [PubMed] [Google Scholar]
- 20.Numabe T, Oikawa T, Kikuchi T, Horuchi T. Pentoxifylline improves in vitro fertilization and subsequent development of bovine oocytes. Theriogenology 2001; 56: 225–233. [DOI] [PubMed] [Google Scholar]
- 21.Huang TTF, Kosower NS, Yanagimachi R. Localization of thiol and disulfide groups in guinea pig spermatozoa during maturation and capacitation using bimane fluorescent labels. Biol Reprod 1984; 31: 797–809. [DOI] [PubMed] [Google Scholar]
- 22.Kosower NS, Katayose H, Yanagimachi R. Thiol-disulfide status and acridine orange fluorescence of mammalian sperm nuclei. J Androl 1992; 13: 342–348. [PubMed] [Google Scholar]
- 23.Takada N, Ohisa N, Numabe T, Ishikawa Y. Production of twin calves by transfer of embryos produced in vitro. Vet Rec 1991; 128: 307. [DOI] [PubMed] [Google Scholar]
- 24.Holm P, Booth PJ, Schmidt MH, Greve T, Callesen H. High bovine blastocyst development in a static in vitro production system using SOFaa medium supplemented with sodium citrate and myo-inositol with or without serum-proteins. Theriogenology 1999; 52: 683–700. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi Y, First NL. In vitro development of bovine one-cell embryos: Influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogenology 1992; 37: 963–978. [DOI] [PubMed] [Google Scholar]
- 26.Thouas GA, Korfiatis NA, French AJ, Jones GM, Trounson AO. Simplified technique for differential staining of inner cell mass and trophectoderm cells of mouse and bovine blastocysts. Reprod Biomed Online 2001; 3: 25–29. [DOI] [PubMed] [Google Scholar]
- 27.Huret JL. Nuclear chromatin decondensation of human sperm: a review. Arch Androl 1986; 16: 97–109. [DOI] [PubMed] [Google Scholar]
- 28.Bedford JM. The bearing of epididymal function in strategies for in vitro fertilization and gamete intrafallopian transfer. Ann N Y Acad Sci 1988; 541: 284–291. [DOI] [PubMed] [Google Scholar]
- 29.Galli C, Vassiliev I, Lagutina I, Galli A, Lazzari G. Bovine embryo development following ICSI: effect of activation, sperm capacitation and pre-treatment with dithiothreitol. Theriogenology 2003; 60: 1467–1480. [DOI] [PubMed] [Google Scholar]
- 30.Kolbe T, Holtz W. Birth of a piglet derived from an oocyte fertilized by intracytoplasmic sperm injection (ICSI). Anim Reprod Sci 2000; 64: 97–101. [DOI] [PubMed] [Google Scholar]
- 31.Probst S, Rath D. Production of piglets using intracytoplasmic sperm injection (ICSI) with flowcytometrically sorted boar semen and artificially activated oocytes. Theriogenology 2003; 59: 961–973. [DOI] [PubMed] [Google Scholar]
- 32.Ock SA, Bhak JS, Balasubramanian S, Lee HJ, Choe SY, Rho GJ. Different activation treatments for successful development of bovine oocytes following intracytoplasmic sperm injection. Zygote 2003; 11: 69–76. [DOI] [PubMed] [Google Scholar]
- 33.Cuthbertson KSR, Whittingham DG, Cobbold PH. Free Ca2+ increases in exponential phases during mouse oocyte activation. Nature 1981; 294: 754–757. [DOI] [PubMed] [Google Scholar]
- 34.Sun FZ, Bradshaw JP, Galli C, Moor RM. Changes in intracellular calcium concentration in bovine oocytes following penetration by spermatozoa. J Reprod Fertil 1994; 101: 713–719. [DOI] [PubMed] [Google Scholar]
- 35.Fujinami N, Hosoi Y, Kato H, Matsumoto K, Saeki K, Iritani A. Activation with ethanol improves embryo development of ICSI-derived oocytes by regulation of kinetics of MPF activity. J Reprod Dev 2004; 50: 171–178. [DOI] [PubMed] [Google Scholar]
- 36.Cheng WM, An L, Wu ZH, Zhu YB, Liu JH, Gao HM, Li XH, Zheng SJ, Chen DB, Tian JH. Effects of disulfide bond reducing agents on sperm chromatin structural integrity and developmental competence of in vitro matured oocytes after intracytoplasmic sperm injection in pigs. Reproduction 2009; 137: 633–643. [DOI] [PubMed] [Google Scholar]
- 37.Palermo GD, Avrech OM, Colombero LT, Wu H, Wolny YM, Fissore RA, Rosenwaks Z. Human sperm cytosolic factor triggers Ca2+ oscillations and overcomes activation failure of mammalian oocytes. Mol Hum Reprod 1997; 3: 367–374. [DOI] [PubMed] [Google Scholar]
- 38.Stice SL, Robl JM. Activation of mammalian oocytes by a factor obtained from rabbit sperm. Mol Reprod Dev 1990; 25: 272–280. [DOI] [PubMed] [Google Scholar]
- 39.Wu H, He CL, Fissore RA. Injection of a porcine sperm factor induces activation of mouse eggs. Mol Reprod Dev 1998; 49: 37–47. [DOI] [PubMed] [Google Scholar]
- 40.Macháty Z, Bonk AJ, Kühholzer B, Prather RS. Porcine oocyte activation induced by a cytosolic sperm factor. Mol Reprod Dev 2000; 57: 290–295. [DOI] [PubMed] [Google Scholar]
- 41.Tang T-S, Dong J-B, Huang X-Y, Sun F-Z. Ca(2+) oscillations induced by a cytosolic sperm protein factor are mediated by a maternal machinery that functions only once in mammalian eggs. Development 2000; 127: 1141–1150. [DOI] [PubMed] [Google Scholar]
- 42.Okitsu O, Yamano S, Aono T. Activation of bovine oocytes matured in vitro by injection of bovine and human spermatozoa or their cytosolic fractions. Zygote 2001; 9: 89–95. [DOI] [PubMed] [Google Scholar]
- 43.Li X, Hamano K, Qian XQ, Funauchi K, Furudate M, Minato Y. Oocyte activation and parthenogenetic development of bovine oocytes following intracytoplasmic sperm injection. Zygote 1999; 7: 233–237. [DOI] [PubMed] [Google Scholar]
- 44.Chankitisakul V, Am-In N, Tharasanit T, Somfai T, Nagai T, Techakumphu M. Sperm pretreatment with dithiothreitol increases male pronucleus formation rates after intracytoplasmic sperm injection (ICSI) in swamp buffalo oocytes. J Reprod Dev 2013; 59: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galli C, Drotti G, Notari C, Lazzari G. High rate of activation and fertilization following intracytoplasmic sperm injection (ICSI) in cattle. Theriogenology 1999; 51: 355 (abstract). [Google Scholar]