Abstract
GPR30 is known as a membrane receptor for picomolar concentrations of estradiol. The GPR30-specific agonist G1 causes a rapid, non-genomic suppression of gonadotropin-releasing hormone (GnRH)-induced luteinizing hormone (LH) secretion from bovine anterior pituitary (AP) cells. A few studies have recently clarified that protein kinase A (PKA) and phosphorylated extracellular signal-regulated kinase (pERK) might be involved in cytoplasmic signaling pathways of GPR30 in other cells. Therefore, we tested the hypothesis that PKA and ERK kinase (MEK) are important cytoplasmic mediators for GPR30-associated non-genomic suppression of GnRH-induced LH secretion from bovine AP cells. Bovine AP cells (n = 8) were cultured for 3 days under steroid-free conditions. The AP cells were previously treated for 30 min with one of the following: 5000 nM of PKA inhibitor (H89), 1000 nM of MEK inhibitor (U0126), or a combination of H89 and U0126. Next, the AP cells were treated with 0.01 nM estradiol for 5 min before GnRH stimulation. Estradiol treatment without inhibitor pretreatment significantly suppressed GnRH-induced LH secretion (P < 0.01). In contrast, estradiol treatment after pretreatment with H89, U0126 or their combination had no suppressive effect on GnRH-induced LH secretion. The inhibitors also inhibited the G1 suppression of GnRH-induced LH secretion. Therefore, these data supported the hypothesis that PKA and MEK (thus, also pERK) are the intracellular mediators downstream of GPR30 that induce the non-genomic suppression of GnRH-induced LH secretion from bovine AP cells by estradiol or G1.
Keywords: Extra-cellular regulated kinases, Gonadotrope, G protein-coupled estrogen receptor-1, Protein kinase A, Ruminant
Estradiol in picomolar concentrations in bovine blood [1, 2] is a powerful negative feedback regulator for the hypothalamus and pituitary to suppress the secretion of gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH), respectively. To induce these important feedback effects from the ovary to the hypothalamus and pituitary, estradiol binds to nuclear-localized estrogen receptors α or β (ERα or ERβ) and alters gene transcription [3,4,5]. However, estradiol has also been shown to bind a plasma membrane receptor in the anterior pituitary (AP), thereby suppressing LH, but not follicle-stimulating hormone (FSH), in a rapid, non-genomic manner [6, 7]. The estradiol receptor G-protein-coupled receptor 30 (GPR30) is present in the plasma membrane of rat AP cells [8, 9] and in bovine gonadotropes, where it contributes to rapid negative estradiol feedback regulation of GnRH-induced LH secretion [10]. Selective agonists of ERα and ERβ at concentrations below 10 nM have no effect on GnRH-induced LH release from ovine AP cells [6]. STX, an agonist for the STX receptor, has no effect on LH secretion from bovine AP cells [11]. We recently demonstrated that estradiol suppresses LH secretion in a rapid, non-genomic manner via GPR30 in cultured bovine AP cells without decreasing gene expression of the LHα and LHβ subunits [12]. Therefore, GPR30 is the only clearly identified membrane estradiol receptor, and its study is important for understanding reproduction in ruminants.
Unlike the cytoplasmic pathways for ERα and ERβ that control gene expression in various cells, little is known about the cytoplasmic signaling pathway of activated GPR30. A few studies have recently revealed that protein kinase A (PKA) might be a pathway for activated GPR30 in mouse trigeminal ganglia [13] and the rat liver [14]. Within 15 min of estradiol treatment, LH secretion from the ovine AP decreases, and phosphorylated extracellular signal-regulated kinase (pERK) increases in the ovine AP, both in vivo and in vitro [7, 15]. A GPR30 antagonist, G36, inhibits ERK phosphorylation by estrogen in SKBr3 cells [16]. Injection of GnRH can induce LH secretion from AP cells within 15 min in cows [17], suggesting that it occurs non-genomically; however, little is known about the signaling pathways associated with GnRH receptor activation in bovine AP cells. Protein kinase A and ERK kinase (MEK) might be the cytoplasmic mediators in pathways for the non-genomic suppression of GnRH-induced LH secretion from AP cells by GPR30 agonists.
This study was conducted to test a hypothesis that PKA and MEK are cytoplasmic mediators of the rapid, non-genomic suppression of GnRH-induced LH secretion from the bovine AP by estradiol, or a GPR30-specific agonist, G1 [18].
Materials and Methods
All experiments were performed according to the Guiding Principles for the Care and Use of Experimental Animals in the Field of Physiological Sciences (Physiological Society of Japan) and approved by the Committee on Animal Experiments of the School of Veterinary Medicine, Yamaguchi University.
Effects of H89 and U0126 on LH secretion
This experiment was conducted to verify the effect of H89, U0126 and their combination (in the absence of estradiol) on the GnRH-induced LH secretion from bovine AP cells. Nett et al. [19] reported that LH and GnRH receptor levels in the AP were higher during the luteal phase than during the immediate post-estrus period in heifers. Therefore, APs were obtained from postpubertal Japanese Black heifers in the middle of the luteal phase (n = 8, 26 months of age) from a local slaughterhouse in Yamaguchi Prefecture. The protocol to obtain the AP from the head and the method of transporting the APs to the laboratory were as reported previously [11]. The experiment was repeated 8 times with each of the 8 different pituitary glands, using 4 wells per treatment. Each experiment began with enzymatic dispersal of AP cells by using a method previously described [20, 21], and cell viability of greater than 90% was confirmed by trypan blue exclusion. The total cell yield was 19.9 × 106 ± 0.9 × 106 cells per pituitary gland. The dispersed cells were then suspended in phenol red-free Dulbecco’s Modified Eagle’s Medium (DMEM; 21063-029, Gibco, Grand Island, NY, USA) containing 1% nonessential amino acids (100 ×; Gibco), 100 IU/ml penicillin, 50 µg/ml streptomycin, 10% horse serum (Gibco), and 2.5% fetal bovine serum (FBS; Gibco). The horse serum and FBS had previously been treated with dextran-coated charcoal to remove steroid hormones. After the cells (2.5 × 105 cells/ml, total 0.5 ml) were plated in 24-well culture plates (MS-80240; Sumitomo Bakelite, Tokyo, Japan), they were maintained at 37 C in a humidified atmosphere of 5% CO2 for 82 h. The wells were washed twice with PBS and then incubated with 490 µl of DMEM containing 0.1% BSA for 2 h.
Cells were pretreated with DMEM (5 μl) alone or with DMEM containing 500 µM of a PKA inhibitor, H89 (Lkt Laboratories, St. Paul, MN, USA), and/or 100 µM of a MEK inhibitor, U0126 (Enzo Biochem, New York, USA). After 30 min of incubation, DMEM was added to each culture well. We added DMEM at the same time as adding estradiol or G1 in order to follow the same protocol as that used for estradiol and G1 in the following experiment. Cells were incubated with gentle shaking for 5 min, after which they were incubated for 2 h with 5 µl of 100 nM GnRH (Peptide Institute, Osaka, Japan) to stimulate LH secretion. Fig. 1 summarizes the final concentrations of H89, U0126 and GnRH for each treatment. As previously reported [22], LH secretion was stimulated by increasing the amounts of GnRH, with a peak at 1 nM GnRH, and reducing secretion at GnRH concentrations higher than 1 nM. Therefore, the final concentration of GnRH used in this study was 1 nM in all treatments, except in the “controls,” which were pretreated with 5 µl of DMEM for 30 min, treated with 5 µl of DMEM for 5 min and then treated with 5 µl of DMEM without GnRH for 2 h. The 30-min treatment time and the concentrations of H89 and U0126 used in the present study are commonly used in such studies [23] and are identical to those used in an investigation of signaling pathways in lactotroph cells [24]. After 2 h of incubation, the medium was collected for immunoassay of LH.
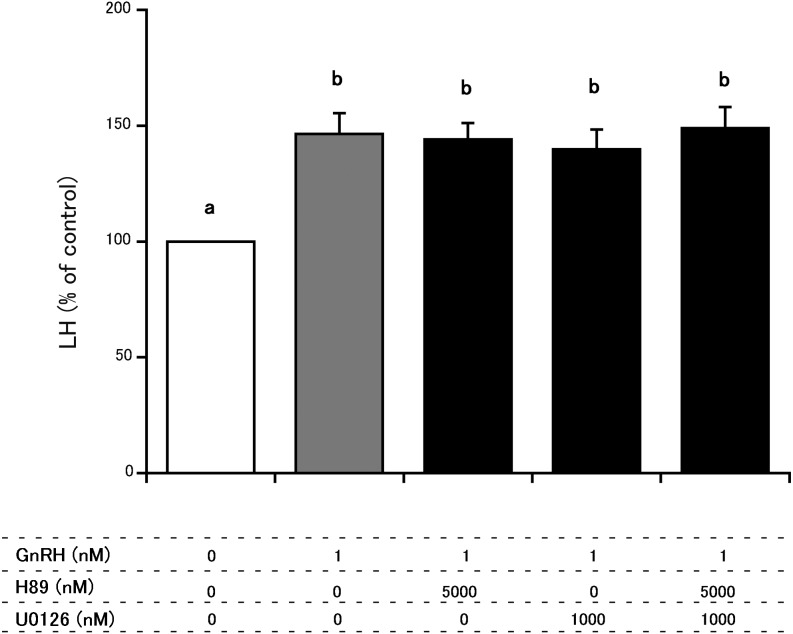
Fig. 1.
Comparison of the effects H89 and U0126 on GnRH-induced LH secretion from cultured bovine AP cells. The final concentrations of H89, U01226 and GnRH were 5000 nM, 1000 nM and 1 nM, respectively. LH concentrations in control cells (cultured in medium alone) were averaged, and the mean LH concentrations of treated groups were expressed as percentages of the average control value. a vs. b: significant difference (P < 0.05)
Effects of H89 and U0126 on the suppression of GnRH-induced LH secretion by estradiol and G1, respectively
Three separate experiments were conducted to evaluate the effect of H89, U0126 and their combination on the suppression of GnRH-induced LH secretion from bovine AP cells by estradiol and G1, respectively. Anterior pituitaries were obtained from postpubertal Japanese Black heifers in the middle of the luteal phase (n = 8, 26 months of age).
Each experiment was repeated 8 times with each of the 8 different pituitary glands, using 4 wells per treatment. After enzymatic dispersal of AP cells, the cells were cultured in the medium described in the previous section for 82 h. The wells were washed twice with PBS and then incubated with 485 µl of DMEM containing 0.1% BSA for 2 h. Cells were pretreated with 5 µl of DMEM alone or with 5 µl of DMEM containing 500 µM of H89 (final concentration, 5000 nM) and/or 100 µM of U0126 (final concentration, 1000 nM). For the estradiol experiment, after 30 min of incubation, either 5 µl of DMEM alone or 5 µl of DMEM containing 1 nM estradiol (final concentration, 0.01 nM; Wako Pure Chemical Industries, Osaka, Japan) was added to each culture well. For the G1 experiment, G1 (final concentration, 0.01 nM; Azano Biotech, Albuquerque, NM, USA) was used instead of estradiol.
The cells were incubated with gentle shaking for 5 min, after which they were incubated for 2 h with 5 µl of 100 nM GnRH (final concentration, 1 nM) dissolved in DMEM to stimulate LH secretion. There were 6 treatment conditions each for estradiol (Fig. 2) and G1 (Fig. 3). According to previous studies [10, 11], the concentrations of the 2 agents used can significantly suppress GnRH-induced LH secretion from bovine AP cells. In all 2 experiments, after 2 h of incubation, the medium was collected for LH immunoassay.
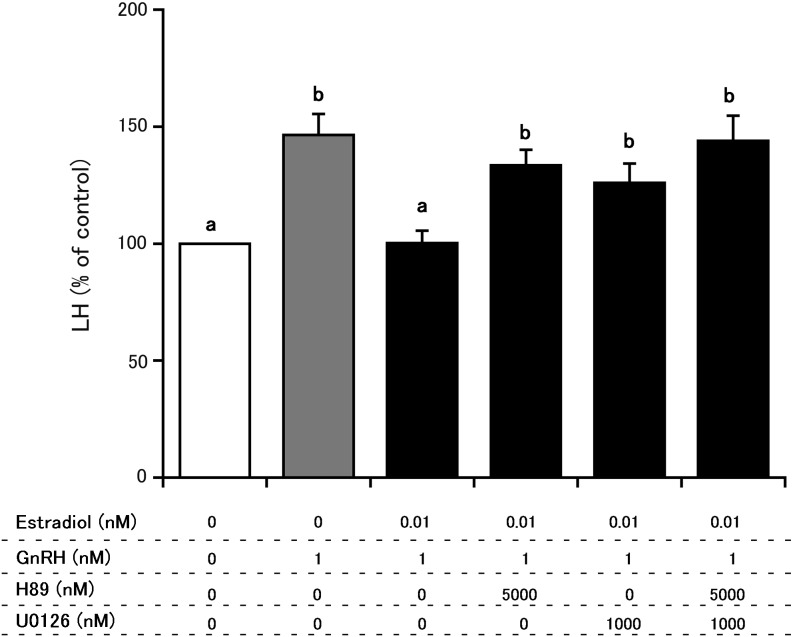
Fig. 2.
Comparison of the effects of H89 and U0126 on estradiol (0.01 nM) suppression of GnRH-induced LH secretion from cultured bovine AP cells. The final concentrations of H89, U01226 and GnRH were 5000 nM, 1000 nM and 1 nM, respectively. LH concentrations in control cells (cultured in medium alone) were averaged, and the mean LH concentrations of treated groups were expressed as percentages of the average control value. a vs. b: significant difference (P < 0.05).
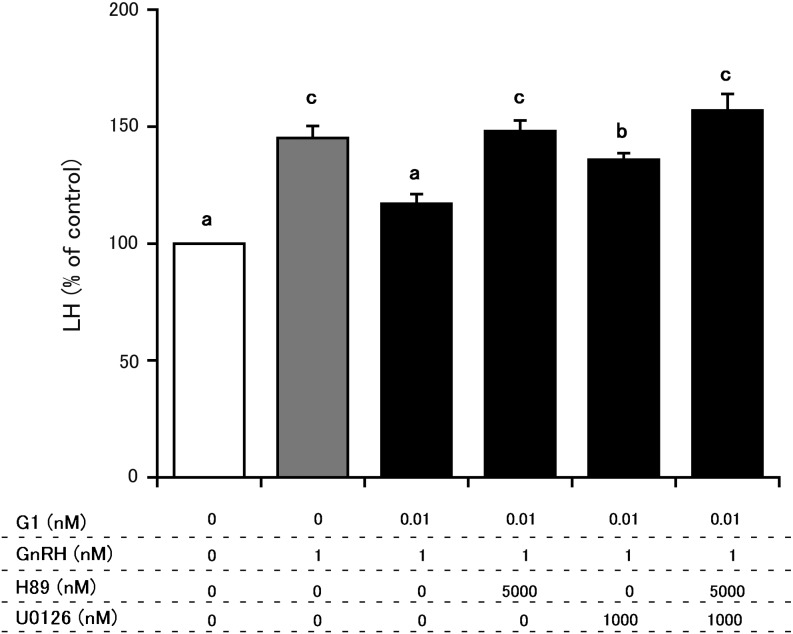
Fig. 3.
Comparison of the effects of H89 and U0126 on G1 (0.01 nM) suppression of GnRH-induced LH secretion from cultured bovine AP cells. The final concentrations of H89, U01226 and GnRH were 5000 nM, 1000 nM and 1 nM, respectively. LH concentrations in control cells (cultured in medium alone) were averaged, and the mean LH concentrations of treated groups were expressed as percentages of the average control value. a vs. b vs. c: significant differences (P < 0.05).
Radioimmunoassay to measure LH concentration in culture media
LH concentrations in the culture media were assayed in duplicate by double-antibody radioimmunoassay using 125I-labeled bLH and anti-oLH-antiserum [AFP11743B and AFP192279, National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Bethesda, MD, USA]. This assay has been previously described in detail [12] and has been used to measure changes in blood LH concentration in Holstein heifers before and after GnRH treatment [25]. The detection limit was 0.40 ng/ml. At 2.04 ng/ml, the intra- and inter-assay coefficients of variation were 3.6% and 6.2%, respectively.
Statistical analysis
LH concentrations in the control samples for each pituitary were averaged, and the mean value was set as 100%. LH concentrations in the treated samples for each pituitary were averaged, and the mean LH values were expressed as a percentage of the control value. Data were analyzed using StatView version 5.0 for Windows (SAS Institute, Cary, NC, USA). The statistical significance of differences in LH concentration were analyzed by one-factor analysis of variance (ANOVA) followed by post hoc comparisons using Fisher’s protected least significant difference test. The level of significance was set at P < 0.05. Data are expressed as the mean ± standard error of the mean (SEM).
Results
Effects of H89 and U0126 on GnRH-induced LH secretion in AP cells
Figure 1 depicts the effects of H89 alone, U0126 alone and the combination of both inhibitors on GnRH-induced LH secretion from cultured AP cells in the absence of estradiol. The LH concentration in the medium of GnRH wells was higher than in the control wells. None of the treatments had an effect on GnRH-induced LH secretion.
Effects of H89 and U0126 on estradiol-mediated suppression of GnRH-induced LH secretion in AP cells
Figure 2 depicts the effects of H89 and U0126 on estradiol-mediated suppression of GnRH-induced LH secretion from cultured AP cells. Treatment with 0.01 nM estradiol in the absence of inhibitors suppressed GnRH-induced LH secretion. In contrast, pretreatment with H89 alone, U0126 alone or their combination inhibited estradiol suppression of GnRH-induced LH secretion. The effects among treatments with H89 alone, U0126 alone or their combination did not differ significantly.
Effects of H89 and U0126 on G1-mediated suppression of GnRH-induced LH secretion in AP cells
Figure 3 depicts the effects of H89 and U0126 on the G1-mediated suppression of GnRH-induced LH secretion from cultured AP cells. Treatment with 0.01 nM G1 in the absence of inhibitors suppressed GnRH-induced LH secretion. In contrast, pretreatments with H89 alone, U0126 alone or the combination of H89 and U0126 inhibited the G1-mediated suppression of GnRH-induced LH secretion. The effect of U0126 alone was weaker (P < 0.01) than that of H89 alone and the combination treatment.
Discussion
In the present study, pretreatment with PKA or MEK inhibitors inhibited the estradiol- or G1-induced suppression of GnRH-induced LH secretion. The inhibitors had no significant effect on nonactivated GPR30. Therefore, we addressed each inhibitor separately and the possible association between PKA and MEK signaling as the cytoplasmic pathways for GPR30 activated by estradiol and G1.
A few studies have recently indicated that PKA is a potential intracellular downstream mediator of the GPR30 pathway in non-gonadotroph cells, mouse trigeminal ganglia [13] and the rat liver [14], although the role of PKA is not linked to controlling LH secretion. G1, as well as estradiol, increases the current amplitude of voltage-gated Na+ channels in human breast cancer cells, and a PKA inhibitor can abolish such an effect [26]. Therefore, the present data suggested that PKA might be an intracellular mediator downstream of GPR30 that induces estradiol-mediated suppression of LH secretion from AP cells in a non-genomic manner. Further studies are required to clarify the contribution of Na+ channels to estradiol’s non-genomic suppression of LH secretion.
Estradiol increases pERK in the ovine AP both in vivo and in vitro within 15 min of treatment [7, 15]. The GPR30 antagonist G36 inhibits ERK phosphorylation by estrogen in SKBr3 cells [16]. Estrogen activates ERK even in ER-negative SKBr3 cells [27]. Therefore, the present findings suggest that MEK and pERK could be other intracellular mediators downstream of GPR30 that induce estradiol-mediated suppression of LH secretion from AP cells via a non-genomic mechanism.
Considering the inhibitory effect of estradiol mediated by GPR30 on pain perception, PKA might be an upstream mediator of MEK [28]. In this study, there was no synergistic effect between U0126 and H89 on the estradiol-mediated suppression of GnRH-induced LH secretion. Therefore, PKA could be a downstream mediator of GPR30 and an upstream mediator of MEK. However, the effect of pretreatment with U0126 alone was weaker against G1-mediated suppression than pretreatment with H89 alone or with the combination of H89 and U0126. Therefore, there could be another mediator downstream of PKA that mediates GPR30 activation. Terasawa and Kenealy [29] reported that estradiol affects various pathways in GnRH neurons and induces cross-talk between cell surface receptors, GPR30 and the nuclear receptors ERα and ERβ. Treatment with ERβ-specific ligand (diarylpropionitrile) as well as estradiol and G1 was reported to rapidly increase pERK in inflammatory breast cancer cell lines [30], suggesting that a combination of ERβ and GPR30 is involved in promoting invasion through the activation of MEK in the non-genomic signaling pathway. When viewed in the context of this recent study, data from the present study suggest that GPR30 plays an important role in the suppression of LH secretion but that ERα and ERβ could also be involved in inducing the rapid suppression of LH secretion.
LH is secreted from the AP into circulating blood in a pulsatile manner during most of the estrous cycle [31, 32]. The pulsatile secretion of GnRH from the hypothalamus into hypophyseal portal blood is the most important factor that controls the parameters of pulsatile LH secretion, particularly the LH pulse frequency [33]. However, changes in the PKA and MEK pathways in AP cells might contribute to other parameters of pulsatile LH secretion, namely, the LH amplitude and mean LH concentration [34, 35].
In conclusion, our study supported the hypothesis that PKA and MEK are intracellular mediators downstream of GPR30 that induce non-genomic suppression of GnRH-induced LH secretion from bovine AP cells by estradiol and G1.
Acknowledgments
This research was partly supported by a Grant-in Aid for Scientific Research (JSPS Kakenhi Grant Number 15K07693) from the Japan Society for the Promotion of Science (Tokyo, Japan) to HK. Faidiban O Rudolf was supported by the Ministry of National Education, Republic of Indonesia, through a scholarship.
References
- 1.Endo N, Nagai K, Tanaka T, Kamomae H. Comparison between lactating and non-lactating dairy cows on follicular growth and corpus luteum development, and endocrine patterns of ovarian steroids and luteinizing hormone in the estrous cycles. Anim Reprod Sci 2012; 134: 112–118. [DOI] [PubMed] [Google Scholar]
- 2.Spicer LJ, Echternkamp SE. Ovarian follicular growth, function and turnover in cattle: a review. J Anim Sci 1986; 62: 428–451. [DOI] [PubMed] [Google Scholar]
- 3.Gieske MC, Kim HJ, Legan SJ, Koo Y, Krust A, Chambon P, Ko C. Pituitary gonadotroph estrogen receptor-alpha is necessary for fertility in females. Endocrinology 2008; 149: 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hewitt SC, Korach KS. Oestrogen receptor knockout mice: roles for oestrogen receptors alpha and beta in reproductive tissues. Reproduction 2003; 125: 143–149. [DOI] [PubMed] [Google Scholar]
- 5.Sánchez-Criado JE, Trudgen K, Millán Y, Blanco A, Monterde J, Garrido-Gracia JC, Gordon A, Aguilar R, de Las Mulas JM, Ko C. Estrogen receptor (ESR) 2 partially offsets the absence of ESR1 in gonadotropes of pituitary-specific Esr1 knockout female mice. Reproduction 2012; 143: 549–558. [DOI] [PubMed] [Google Scholar]
- 6.Arreguin-Arevalo JA, Nett TM. A nongenomic action of 17beta-estradiol as the mechanism underlying the acute suppression of secretion of luteinizing hormone. Biol Reprod 2005; 73: 115–122. [DOI] [PubMed] [Google Scholar]
- 7.Iqbal J, Latchoumanin O, Clarke IJ. Rapid in vivo effects of estradiol-17beta in ovine pituitary gonadotropes are displayed by phosphorylation of extracellularly regulated kinase, serine/threonine kinase, and 3′,5′-cyclic adenosine 5′-monophosphate-responsive element-binding protein. Endocrinology 2007; 148: 5794–5802. [DOI] [PubMed] [Google Scholar]
- 8.Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol 2007; 193: 311–321. [DOI] [PubMed] [Google Scholar]
- 9.Hazell GG, Yao ST, Roper JA, Prossnitz ER, O’Carroll AM, Lolait SJ. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol 2009; 202: 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudolf FO, Kadokawa H. Expression of estradiol receptor, GPR30, in bovine anterior pituitary and effects of GPR30 agonist on GnRH-induced LH secretion. Anim Reprod Sci 2013; 139: 9–17. [DOI] [PubMed] [Google Scholar]
- 11.Rudolf FO, Kadokawa H. Effects of STX, a novel estrogen membrane receptor agonist, on GnRH-induced luteinizing hormone secretion from cultured bovine anterior pituitary cells. J Vet Med Sci 2014; 76: 1623–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura U, Rudolf FO, Pandey K, Kadokawa H. The non-steroidal mycoestrogen zeranol suppresses luteinizing hormone secretion from the anterior pituitary of cattle via the estradiol receptor GPR30 in a rapid, non-genomic manner. Anim Reprod Sci 2015; 156: 118–127. [DOI] [PubMed] [Google Scholar]
- 13.Yue J, Zhang Y, Li X, Gong S, Tao J, Jiang X. Activation of G-protein-coupled receptor 30 increases T-type calcium currents in trigeminal ganglion neurons via the cholera toxin-sensitive protein kinase A pathway. Pharmazie 2014; 69: 804–808. [PubMed] [Google Scholar]
- 14.Zucchetti AE, Barosso IR, Boaglio AC, Basiglio CL, Miszczuk G, Larocca MC, Ruiz ML, Davio CA, Roma MG, Crocenzi FA, Pozzi EJ. G-protein-coupled receptor 30/adenylyl cyclase/protein kinase A pathway is involved in estradiol 17ß-D-glucuronide-induced cholestasis. Hepatology 2014; 59: 1016–1029. [DOI] [PubMed] [Google Scholar]
- 15.Iqbal J, Latchoumanin O, Sari IP, Lang RJ, Coleman HA, Parkington HC, Clarke IJ. Estradiol-17beta inhibits gonadotropin-releasing hormone-induced Ca2+ in gonadotropes to regulate negative feedback on luteinizing hormone release. Endocrinology 2009; 150: 4213–4220. [DOI] [PubMed] [Google Scholar]
- 16.Dennis MK, Field AS, Burai R, Ramesh C, Petrie WK, Bologa CG, Oprea TI, Yamaguchi Y, Hayashi S, Sklar LA, Hathaway HJ, Arterburn JB, Prossnitz ER. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. J Steroid Biochem Mol Biol 2011; 127: 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadokawa H, Takusari N, Takahashi H, Yamada Y, Kariya T. GnRH inducing LH release, nutrition and plasma cortisol in high producing dairy cows postpartum. J Reprod Dev 1998; 44: 197–203. [Google Scholar]
- 18.Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol 2006; 2: 207–212. [DOI] [PubMed] [Google Scholar]
- 19.Nett TM, Cermak D, Braden T, Manns J, Niswender G. Pituitary receptors for GnRH and estradiol, and pituitary content of gonadotropins in beef cows. I. Changes during the estrous cycle. Domest Anim Endocrinol 1987; 4: 123–132. [DOI] [PubMed] [Google Scholar]
- 20.Hashizume T, Soliman EB, Kanematsu S. Effects of pituitary adenylate cyclase-activating polypeptide (PACAP), prostaglandin E2 (PGE2) and growth hormone releasing factor (GRF) on the release of growth hormone from cultured bovine anterior pituitary cells in vitro. Domest Anim Endocrinol 1994; 11: 331–337. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki S, Kadokawa H, Hashizume T. Direct kisspeptin-10 stimulation on luteinizing hormone secretion from bovine and porcine anterior pituitary cells. Anim Reprod Sci 2008; 103: 360–365. [DOI] [PubMed] [Google Scholar]
- 22.Kadokawa H, Pandey K, Nahar A, Nakamura U, Rudolf FO. Gonadotropin-releasing hormone (GnRH) receptors of cattle aggregate on the surface of gonadotrophs and are increased by elevated GnRH concentrations. Anim Reprod Sci 2014; 150: 84–95. [DOI] [PubMed] [Google Scholar]
- 23.Akiyama T, Kohu K. 2006. U0126 and H89. In: Akiyama T, and Kohu K (eds.), Handbook of Inhibitors. Tokyo: Yodosha; 2006: 28–95 (in Japanese).
- 24.Ishida M, Mitsui T, Izawa M, Arita J. Absence of ligand-independent transcriptional activation of the estrogen receptor via the estrogen response element in pituitary lactotrophs in primary culture. J Steroid Biochem Mol Biol 2010; 118: 93–101. [DOI] [PubMed] [Google Scholar]
- 25.Kadokawa H. Seasonal differences in the parameters of luteinizing hormone release to exogenous gonadotropin releasing hormone in prepubertal Holstein heifers in Sapporo. J Reprod Dev 2007; 53: 121–125. [DOI] [PubMed] [Google Scholar]
- 26.Fraser SP, Ozerlat-Gunduz I, Onkal R, Diss JK, Latchman DS, Djamgoz MB. Estrogen and non-genomic upregulation of voltage-gated Na+ channel activity in MDA-MB-231 human breast cancer cells: role in adhesion. J Cell Physiol 2010; 224: 527–539. [DOI] [PubMed] [Google Scholar]
- 27.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 2000; 14: 1649–1660. [DOI] [PubMed] [Google Scholar]
- 28.Lu Y, Jiang Q, Yu L, Lu ZY, Meng SP, Su D, Burnstock G, Ma B. 17β-estradiol rapidly attenuates P2X3 receptor-mediated peripheral pain signal transduction via ERα and GPR30. Endocrinology 2013; 154: 2421–2433. [DOI] [PubMed] [Google Scholar]
- 29.Terasawa E, Kenealy BP. Neuroestrogen, rapid action of estradiol, and GnRH neurons. Front Neuroendocrinol 2012; 33: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohshiro K, Schwartz AM, Levine PH, Kumar R. Alternate estrogen receptors promote invasion of inflammatory breast cancer cells via non-genomic signaling. PLoS ONE 2012; 7: e30725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawken PA, Beard AP, Esmaili T, Kadokawa H, Evans AC, Blache D, Martin GB. The introduction of rams induces an increase in pulsatile LH secretion in cyclic ewes during the breeding season. Theriogenology 2007; 68: 56–66. [DOI] [PubMed] [Google Scholar]
- 32.Kadokawa H, Yamada Y. Enhancing effect of acute fasting on ethanol suppression of pulsatile luteinizing hormone release via an estrogen-dependent mechanism in Holstein heifers. Theriogenology 1999; 51: 673–680. [DOI] [PubMed] [Google Scholar]
- 33.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 2010; 30: 3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadokawa H, Yamada Y. Effect of a long-lasting opioid receptor antagonist (naltrexone) on pulsatile LH release in early postpartum Holstein dairy cows. Theriogenology 2000; 54: 75–81. [DOI] [PubMed] [Google Scholar]
- 35.Kadokawa H, Blache D, Martin GB. Plasma leptin concentrations correlate with luteinizing hormone secretion in early postpartum Holstein cows. J Dairy Sci 2006; 89: 3020–3027. [DOI] [PubMed] [Google Scholar]