Our published report in this issue of Cell Metabolism (Oh et al., 2015) documents a role for the exocytosis protein Syntaxin 4 (Syn4) in extending longevity and health-span in mice. The study was initially conducted at Indiana University School of Medicine (IU), and given the significant longevity phenotype of the IU cohort (+33%), an independent Syn4 transgenic (Tg) mouse colony was established at the University of Michigan (UM), a site with ample experience in conducting mouse lifespan studies, to assess the reproducibility of the longevity phenotype.
To our surprise, the lifespan table showed only a small trend for the UM Syn4 Tg mice to live slightly longer than control mice at the UM site, especially at the end of the lifespan in males (Figures 1A–1C), but the difference did not reach statistical significance. In an attempt to understand the discrepancy in results between the UM and IU cohorts, we quantified transgene expression (Figure 1D). IU Tg males at 5 or 18 months of age exhibited larger increases in Syn4 abundance in skeletal muscle compared with IU controls (5 months, 3.11-fold, n = 3; 18 months, 3.30-fold, n = 4), while UM Tg males showed only modest increases relative to UM controls (5 months, 1.30-fold, n = 3; 18 months, 1.50-fold, n = 4). Notably, DNA genotyping of the mice from which muscle lysates were taken for protein analyses clearly showed the presence of the Syn4 transgene in both IU and UM colonies (Figure 1D, lanes 3 and 4, and lanes 7 and 8). IU- and UM-derived control mice showed similar endogenous Syn4 levels, suggesting that the difference in Syn4 abundance observed between Tg mice is linked to the low level of transgene expression in the UM cohort.
Figure 1. Lifespan and Syn4 Abundance in an Independent Colony of Syn4 Transgenic Mice.
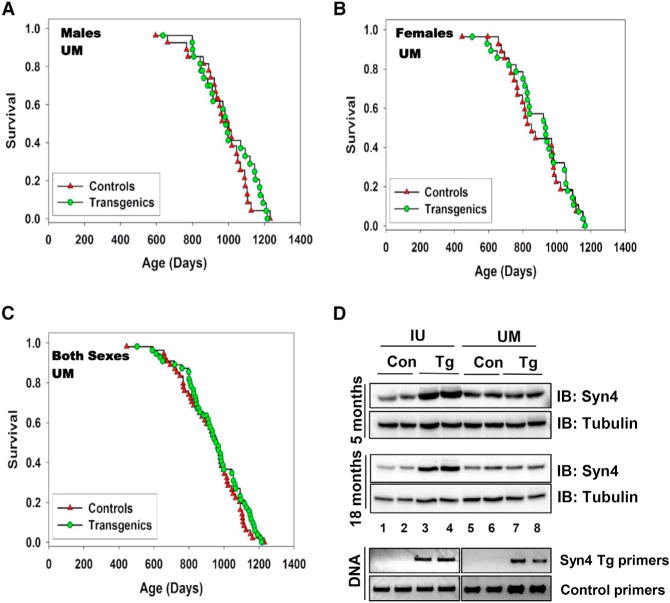
Survival curves for mice in the Michigan (UM) cohort for (A) males (27 Tg and 27 Control; p = 0.46) and (B) females (27 Con and 28 Tg; p = 0.63) versus (C) both sexes pooled (55 Con and 56 Tg; p = 0.34). Statistical analyses were conducted using ANOVA. (D) Levels of transgene expression in male mice from IU versus UM cohorts in skeletal hindlimb muscle lysates (representative of n = 3/group for 5-month-old mice, n = 4/group for 18-month-old mice). Lysate proteins were resolved using 10% SDS-PAGE followed by transfer and immunoblot (IB) detection, and DNA genotyping was performed using Syn4 Tg primers (Oh et al., 2014). Tubulin served as the protein gel loading control. Equal quantities of PCR product were loaded per lane in the DNA gels, with control primers included to assess DNA quality and suitability for PCR analysis.
Additionally, we observed that the control population of C57BL/6 mice at UM exhibited a median survival of 851 days for females and 963 days for males, while corresponding values for the IU cohort were considerably smaller, at 807 days for females and 758 days for males. This overall lifespan of the IU control mice is somewhat shorter than expected for this strain of mice, which has been reported to be as high as 900 (Pugh et al., 1999) or 935 days for males in some colonies (Ikeno et al., 2005), or as low as 790 in others (Forster et al., 2003). Moreover, the mice in the UM cohort died between the ages of 700 and 1,100 days, contrasting with a surprisingly steep survival curve in the IU cohort, where nearly all the deaths occurred in mice between the ages of 750 and 850 days. It is common to see site-to-site variation in mouse survival curves, presumably reflecting poorly understood variations in water impurities, odors, bedding, feed sources, noise levels, treatments for benign nonpathogens (furmites, pinworms), and gut microbial flora. Environmental factors have been shown to impact survival: for example, site effects were noted in genetically heterogeneous mice studied by the NIA Intervention Testing Program (Miller et al., 2011; Strong et al., 2008). In each of five subsequent cohorts, UM control males lived longer than control males at the other two sites, the Jackson Laboratories (TJL) and the University of Texas Health Science Center at San Antonio (UT), although there were no site-specific differences in longevity of female mice. For this reason, drugs with dramatic effects on male survival at UT and TJL have sometimes shown less dramatic effects at the UM site (Harrison et al., 2014; Strong et al., 2008). The longer lifespan of C57BL/6 control mice at UM may have contributed to the smaller effect of the Syn4 transgene at this site.
Reports of diminished Syn4 abundance under certain environmental conditions and in distinct strains of mice (Keller et al., 2008; Oh et al., 2014; Yechoor et al., 2002) raise the unresolved question as to whether the transgene expression difference was the result of a site effect and/or related to the derivation of a new Syn4 Tg colony at UM. Importantly, however, the differences in Syn4 transgene expression between the IU and UM colonies ultimately afforded a “Syn4 dosage” comparison of the IU and UM mouse lifespan cohorts within this single study, providing corroborating evidence for the initial observation of an association between Syn4 protein abundance in tissues responsible for maintaining glucose homeostasis and lifespan. The lower level of Syn4 transgene expression at UM, together with the diminished lifespan effect, is consistent with the idea that Syn4 expression levels may contribute to lifespan outcome, a point that would need to be tested more carefully using stocks with graded doses of transgene expression, preferably in multiple tissues. It is possible that the lifespan effect may depend on exceeding a threshold for Syn4 level.
In sum, our report of this UM cohort as a follow-up to the IU cohort study provides a cautionary note that effects of transgenes on health outcomes, including lifespan, may be conditional on site-specific factors that modulate transgene expression, survival patterns of control stocks, or both. We hope that bringing issues such as site effects and transgene expression discrepancies to the discussion table will be helpful to the field, as these and other issues may contribute significantly to lack of reproducibility in this area of research.
References
- Forster MJ, Morris P, Sohal RS. FASEB J. 2003;17:690–692. doi: 10.1096/fj.02-0533fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, Fernandez E, Flurkey K, Javors MA, Nadon NL, et al. Aging Cell. 2014;13:273–282. doi: 10.1111/acel.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno Y, Hubbard GB, Lee S, Richardson A, Strong R, Diaz V, Nelson JF. J Gerontol A Biol Sci Med Sci. 2005;60:1510–1517. doi: 10.1093/gerona/60.12.1510. [DOI] [PubMed] [Google Scholar]
- Keller MP, Choi Y, Wang P, Davis DB, Rabaglia ME, Oler AT, Stapleton DS, Argmann C, Schueler KL, Edwards S, et al. Genome Res. 2008;18:706–716. doi: 10.1101/gr.074914.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, et al. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Stull ND, Mirmira RG, Thurmond DC. J Clin Endocrinol Metab. 2014;99:E866–E870. doi: 10.1210/jc.2013-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Miller RA, Thurmond DC. Cell Metab. 2015;22 doi: 10.1016/j.cmet.2015.07.023. this issue, ■■■–■■■. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh TD, Oberley TD, Weindruch R. Cancer Res. 1999;59:1642–1648. [PubMed] [Google Scholar]
- Strong R, Miller RA, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, et al. Aging Cell. 2008;7:641–650. doi: 10.1111/j.1474-9726.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yechoor VK, Patti ME, Saccone R, Kahn CR. Proc Natl Acad Sci USA. 2002;99:10587–10592. doi: 10.1073/pnas.142301999. [DOI] [PMC free article] [PubMed] [Google Scholar]


