Abstract
SLC4A gene family proteins include bicarbonate transporters that move HCO3− across the plasma membrane and regulate intracellular pH and transepithelial movement of acid–base equivalents. These transporters are Cl/HCO3 exchangers, electrogenic Na/HCO3 cotransporters, electroneutral Na/HCO3 cotransporters, and Na+-driven Cl/HCO3 exchanger. Studies of the bicarbonate transporters in vitro and in vivo have demonstrated their physiological importance for acid–base homeostasis at the cellular and systemic levels. Recent advances in structure/function analysis have also provided valuable information on domains or motifs critical for regulation, ion translocation, and protein topology. This chapter focuses on the molecular mechanisms of ion transport along with associated structural aspects from mutagenesis of particular residues and from chimeric constructs. Structure/function studies have helped to understand the mechanism by which ion substrates are moved via the transporters. This chapter also describes some insights into the structure of SLC4A1 (AE1) and SLC4A4 (NBCe1) transporters. Finally, as some SLC4A transporters exist in concert with other proteins in the cells, the structural features associated with protein–protein interactions are briefly discussed.
1. INTRODUCTION
In most cells, the internal ionic environment is regulated by membrane proteins that move ions across the cell membranes. The SLC4A bicarbonate transporters are a subset of these proteins that are specialized to move HCO3−. HCO3− buffers H+, and therefore, HCO3− transport is equivalent to H+ transport in the opposite direction from a pH standpoint. The primary function of the bicarbonate transporters is to regulate intracellular H+. Some bicarbonate transporters move HCO3− into the cytosol and raise intracellular pH (acid extruders), whereas others remove HCO3− from the cytosol and lower intracellular pH (acid loaders). The activity of acid extruders and loaders affects steady-state pH in the cells. Cells also utilize these pH regulators to transfer large amounts of Na+ and HCO3− from one body compartment to another to maintain extracellular and total body acid–base balance. The bicarbonate transporters are also widely distributed in many epithelial tissues where they play a role in transepithelial absorption and secretion of HCO3−. This transepithelial HCO3− movement contributes to tissue-specific physiological processes such as reabsorbing HCO3− or excreting acid into urine (kidney), neutralizing intestinal gastric acid (pancreatic ducts), or maintaining the cerebrospinal fluid pH (choroid plexus).
Many studies have been done to determine the functional and physiological significance of the SLC4A bicarbonate transporters in the body and to understand their pathological involvement in acid–base disorders. Cellular signals responsible for transport regulation have also been examined. In addition, the members of SLC4A transporters exhibit high homology in amino acid sequence, and this homology has enabled researchers to investigate domains or residues important for distinct molecular and functional properties of ion movement. Such efforts have produced valuable results to advance our knowledge of the underlying molecular mechanisms of ion transport. Furthermore, similar structural features among bicarbonate transporters have facilitated our understanding of transporter structure and the macromolecular complexes with other proteins. This chapter provides some insight into ion transport properties, structure, and protein–protein interactions of SLC4A bicarbonate transporters. This chapter focuses on anion exchangers AE1–3 and Na/HCO3 cotransporters NBCe1 and NBCn1 because less has been done with other Na+-coupled transporters.
2. SLC4A TRANSPORTERS
The SLC4A transporters (Table 1) are Cl/HCO3 exchangers, Na/HCO3 cotransporters, Na+-driven Cl/HCO3 exchanger, and Na/borate transporter. These are distinct from the SLC26A anion exchangers that transport a variety of monovalent and divalent anions (Sindic, Chang, Mount, & Romero, 2007). Some SLC26A proteins transport HCO3− in exchange for Cl− or other anions.
Table 1.
Human SLC4A transporters
| Human gene | Protein | Function | pHi regulation | Human chromosome |
|---|---|---|---|---|
| SLC4A1 | AE1 Band 3 | Cl−/HCO3− exchange | Acid loader | 17q21–q22 |
| SLC4A2 | AE2 | Cl−/HCO3− exchange | Acid loader | 7q35–36 |
| SLC4A3 | AE3 | Cl−/HCO3− exchange | Acid loader | 2q36 |
| SLC4A4 | NBCe1 NBC1 | Na+/HCO3− cotransport (electrogenic) | Acid extruder (except NBCe1-A) | 4q21 |
| SLC4A5 | NBCe2 NBC4 | Na+/HCO3− cotransport (electrogenic) | Acid extruder | 2p13 |
| SLC4A6 | – | – | ||
| SLC4A7 | NBCn1 NBC3 | Na+/HCO3− cotransport | Acid extruder | 3p22 |
| SLC4A8 | NDCBE | Na+/Cl−/2HCO3− cotransport/exchange* | Acid extruder | 12q13 |
| SLC4A9 | AE4 | Cl−/HCO3− exchange | Acid loader | 5q31 |
| SLC4A10 | NBCn2 NBCE | Na+/HCO3− cotransport | Acid extruder | 2q23–q24 |
| SLC4A11 | NaBC BTR1 | Na+/B(OH)4−, OH− cotransport | Acid extruder for OH− transport | 20p12 |
NDCBE cotransports external 1Na+ and 2HCO3− in exchange for internal Cl−.
2.1. Cl/HCO3 Exchangers (AEs)
2.1.1. AE1 (SLC4A1), AE2 (SLC4A2), AE3 (SLC4A3)
The three Cl/HCO3 exchangers (AE1–3) primarily move external HCO3− in exchange for internal Cl− (Alper, 2009; Bonar & Casey, 2008). AE1 is the first cloned HCO3− transporter (Kopito & Lodish, 1985). The human AE1 contains 911 amino acids with the predicted structure of 13 transmembrane (TM) (The TM topology of AE1 is defined according to the 13-TM model (Bonar & Casey, 2008), in which 13 TMs and one unusual membrane-spanning segment (called extended structure) between TM11 and TM12 are present. The topology of NBCe1 is defined according to the 14-TM model (Zhu et al., 2010), in which 14 TMs are present.) segments and cytoplasmic N- and C-terminal domains (Zhu, Lee, & Casey, 2003). The large N-terminal domain (~400 amino acids) plays a regulatory role, while the transmembrane domain is responsible for anion exchange activity. The N-terminal domain interacts with other proteins such as ankyrin, glycolytic enzymes, and hemoglobin, whereas the C-terminal domain interacts with carbonic anhydrase II (CA II) (Vince & Reithmeier, 1998). AE1 exists as homodimers (Wang, Sarabia, Reithmeier, & Kuhlbrandt, 1994). AE1 is the most abundant membrane protein in red blood cells and plays a key role in CO2 delivery from tissues to the lungs. AE1 is also localized to the basolateral membrane of type-A intercalated cells in the kidney collecting ducts (van Adelsberg, Edwards, & Al Awqati, 1993). The renal kAE1 lacks the first 65 amino acids because of an alternate promoter site. AE1 also transports other anions such as SO42− at a very low rate (Milanick & Gunn, 1982).
AE2 was originally isolated from the kidney (Alper, Kopito, Libresco, & Lodish, 1988). AE2 is ~300 amino acids larger than AE1 and exists as multiple variants because of alternative promoter sites. Similar to AE1, the N- and C-terminal domains of AE2 play regulatory roles in its pH dependence, as well as response to NH4+ and hypertonicity (Chernova et al., 2003; Stewart, Chernova, Shmukler, Wilhelm, & Alper, 2002), while the transmembrane domains are responsible for exchanger function (Stewart, Kurschat, Vaughan-Jones, Shmukler, & Alper, 2007). AE2 is widely distributed in the body, particularly in epithelial cells including the thick ascending limb and cortical collecting duct intercalated cells in the kidney (Brosius et al., 1995), and contributes to transepithelial movement of HCO3− and salt.
Human AE3 comprised the cardiac variant (cAE3) and the brain variant (bAE3) with different N-terminal domains (Kopito et al., 1989). Both variants are found in many different tissues in addition to the heart and brain. AE3 has a lower exchange activity than AE1 and AE2 because its membrane expression is weak (Fujinaga, Loiselle, & Casey, 2003).
2.1.2. AE4 (SLC4A9)
Human AE4 contains 983 amino acids and has three alternative splice variants according to the NCBI database. AE4 is more closely related to Na/ HCO3 transporters than AEs in amino acid sequence. AE4 is functionally characterized as a Cl/HCO3 exchanger (Tsuganezawa et al., 2001; Ko, Luo et al., 2002), but it is also suggested to be an electroneutral NBC without Cl/HCO3 exchange activity (Parker et al., 2002). AE4 is localized to renal collecting duct type-A intercalated cells, but its membrane polarity appears to be species-specific. It is localized to the basolateral membrane of the intercalated cells in rats and mice, but to the apical and lateral membranes in rabbits (Ko, Luo et al., 2002).
2.2. Na+-Coupled Bicarbonate Transporters (NCBTs)
2.2.1. NBCe1 (SLC4A4), NBCe2 (SLC4A5)
Romero and Boron cloned the electrogenic NBCe1 from the salamander kidney (Romero, Hediger, Boulpaep, & Boron, 1997). On the basis of predicted topology and similarity to AE1, NBCe1 (1035 amino acids) was proposed to have a cytoplasmic N-terminal domain, 12–14 TM segments, and a cytoplasmic C-terminal domain. The most widely distributed variant is NBCe1-B containing the unique N-terminal 85 amino acids, which replace the first 41 amino acids of the renal variant NBCe1-A (Abuladze et al., 1998; Choi, Romero, Khandoudi, Bril, & Boron, 1999). The brain-specific NBCe1-C is identical to NBCe1-B except for the C-terminal 61 amino acids (Bevensee, Schmitt, Choi, Romero, & Boron, 2000). NBCe1-A heterologously expressed in Xenopus oocytes produces an electrogenic HCO3− current with a 1Na+ and 2HCO3− stoichiometry (Ducoudret, Diakov, Muller-Berger, Romero, & Frömter, 2001; Grichtchenko et al., 2001; Sciortino & Romero, 1999), mediating net Ducoudret, Diakov, Muller-Berger, Romero, & Frömter, 2001 influx. In contrast, the transporter in the proximal tubules moves 1Na+ and 2HCO3− across the basolateral membrane, mediating net HCO3− efflux. Studies show that the same transport molecule presumably changes its stoichiometry depending on the cellular environment and parameters such as intracellular Ca2+ levels (Muller-Berger, Ducoudret, Diakov, & Frömter, 2001) or phosphorylation activated by cAMP (Gross et al., 2003). Similar to AEs, NBCe1 forms a homodimer composed of two individually functional subunits (Gill & Boron, 2006a; Kao et al., 2008).
NBCe2 is the second type of the electrogenic Na/HCO3 cotransporter (Pushkin et al., 2000; Virkki, Wilson, Vaughan-Jones, & Boron, 2002). NBCe2 is strongly expressed in hepatocytes and bile duct cholangiocytes in the liver and renal pelvis uroepithelial cells in the kidney (Abuladze et al., 2004). It is also found in the renal collecting ducts, choroid plexus epithelia (Bouzinova et al., 2005; Damkier, Nielsen, & Praetorius, 2007), and sarcolemmal membranes in skeletal muscles (Kristensen, Kristensen, & Juel, 2004). NBCe2 produces a HCO3−-induced current with the 1Na+:2HCO3− stoichiometry when expressed in Xenopus oocytes (Virkki et al., 2002). However, it operates with the 1Na+:3HCO3− stoichiometry when expressed in the renal proximal tubule cell line (Sassani et al., 2002) and in intact cells prepared from choroid plexus epithelia (Millar & Brown, 2008).
2.2.2. NBCn1 (SLC4A7), NBCn2 (SLC4A10)
The electroneutral NBCn1 was isolated from rat vascular smooth muscle (Choi, Aalkjaer, Boulpaep, & Boron, 2000) and human skeletal muscle (Pushkin et al., 1999). Human NBCn1 contains 1214 amino acids with a predicted structure similar to that of NBCe1. NBCn1 exists as multiple variants because of N- and C-terminal splice events. The transporter is widely distributed in the body (Boedtkjer, Praetorius, Fuchtbauer, & Aalkjaer, 2008). NBCn1 is expressed in vascular smooth muscle cells and endothelial cells and modulates artery tone and blood pressure control (Boedtkjer et al., 2011). The variant containing the N-terminal cassette of 123 amino acids (cassette II) is predominantly found in the cardiovascular system (Cooper et al., 2006). Among SLC4A bicarbonate transporters, NBCn1 is least sensitive to the anion channel/transporter blocker 4,4′-diisothiocyanatostilbene disulfonate (DIDS) (Choi et al., 2000). In addition, NBCn1 has HCO3−-independent, Na+ channel-like activity (Cooper et al., 2005).
NBCn2 is the second cloned electroneutral Na/HCO3 cotransporter (Wang, Yano, Nagashima, & Seino, 2000). This transporter is predominantly expressed in neurons and choroid plexus epithelia (Damkier et al., 2007). NBCn2 operates with the 1Na+:1HCO3− stoichiometry with Cl− self-exchange activity (Parker, Musa-Aziz et al., 2008). Other studies show that NBCn2 directly transports Cl− in exchange for Na/HCO3 cotransport and functions as a Na+-driven Cl/HCO3 exchanger NCBE (Damkier, Aalkjaer, & Praetorius, 2010; Giffard, Lee, Ouyang, Murphy, & Monyer, 2003).
2.2.3. NDCBE1 (SLC4A8)
NDCBE1 is the Na+-driven Cl/HCO3 exchanger (Grichtchenko et al., 2001; Parker, Bouyer, Daly, & Boron, 2008). This transporter is predominantly expressed in neurons (Chen, Haddad, & Boron, 2008; Sinning et al., 2011), where Na+-driven Cl/HCO3 exchange has been known to primarily govern HCO3−-dependent acid extrusion (Schwiening & Boron, 1994). NDCBE moves external 1Na+ and 2HCO3− in exchange for internal Cl−.
2.3. Sodium Borate (NaBC) Transporter
NaBC1 (SLC4A11) transports Na+ and borate (Park, Li, Shcheynikov, Zeng, & Muallem, 2004). This transporter has substantial sequence homology to SLC4A bicarbonate transporters and was assigned as SLC4A11 before its function was characterized. NaBC1 operates with the stoichiometry of 1Na+ and at least 2B(OH)4− (Park et al., 2004) The transporter moves Na+ and OH− (H+) in the absence of borate.
3. ION TRANSPORT PROPERTIES
3.1. Ion Dependence and Selectivity
3.1.1. AEs
AEs are permissive to many anions in addition to HCO3− and Cl−. For example, AE1 has the capacity to move several monovalent and divalent anions in either direction or via anion self exchange (Knauf & Pal, 2003). AE2 also mediates the exchange of anions including NO3−, Br−, and I− with low affinity when expressed in Xenopus oocytes (Humphreys, Jiang, Chernova, & Alper, 1994). The amino acid residue best characterized for anion selectivity is Glu681 (TM8) in erythrocyte AE1 and the corresponding Glu site in AE2. Chemical modification of Glu681 to an alcohol leads to electrogenic SO42−/Cl− exchange and electroneutral SO42−/SO42− self exchange (Jennings, 1995; Jennings & Al Rhaiyel, 1988). Similar changes are also observed from the site-directed mutagenesis of the corresponding Glu in cloned AE1 and AE2 (Chernova et al., 1997; Sekler, Lo, & Kopito, 1995), indicating that the role of Glu681 is conserved among AEs. The underlying mechanism involves an interaction of sulfate with H+ that is donated by the Glu residue lining the pore. Therefore, Glu681 contributes to anion translocation, as discussed below. Other residues in AE are identified to serve as the anion selectivity sites in addition to Glu681 of TM8. By scanning cysteine accessibility mutagenesis (SCAM) of AE1, Casey and his colleagues (Zhu & Casey, 2004) found that the region of Ser852–Leu857 in the extracellular loop between the last two TMs serves as an anion selectivity filter. Whether the residues Ser852–Leu857 are mechanistically associated with Glu681 for function is unclear.
While it is generally accepted that the transmembrane domain plays an essential role in anion selectivity, the cytoplasmic domains may also contribute to maintaining HCO3− selectivity. Deletion and missense mutants without the C-terminal putative CAII-binding site lose Cl−/HCO3− exchange while retaining Cl−/Cl− self-exchange (Dahl et al., 2003).
3.1.2. NCBTs
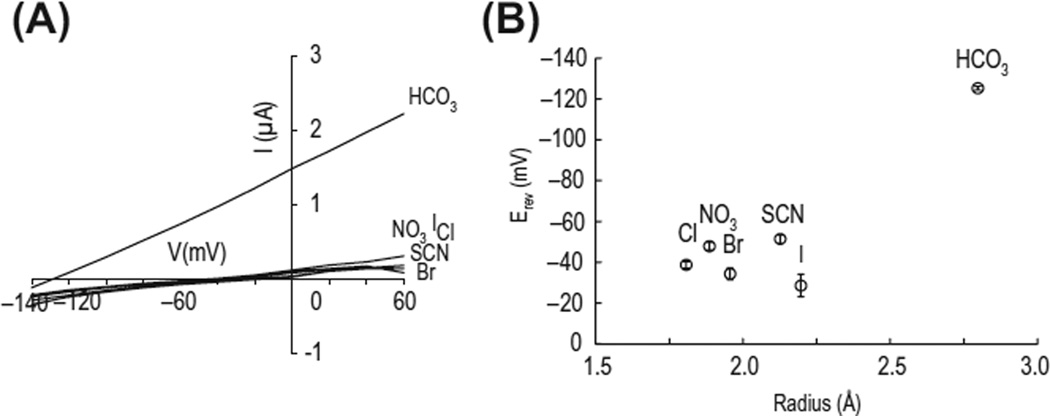
All NCBTs are dependent upon Na+ (Amlal, Wang, Burnham, & Soleimani, 1998; Sciortino & Romero, 1999). The current–voltage relationship for electrogenic HCO3− transport shows a positive and linear slope with outward currents at positive voltages. The current is highly selective to HCO3− and is not elicited by other anions (Fig. 1). The charge-conserved substitution of Glu555 with an Asp (i.e. D555E) produces dose-dependent Cl− currents in the absence of HCO3− (Yang, Kim et al., 2009). Nonetheless, D555E produces a HCO3− current when both Cl− and HCO3− are present. Anion selectivity experiments reveal that D555E is broadly permissive to other anions including NO3−, SCN−, I−, and Br−, whereas wild-type NBCe1 shows a high selectivity to HCO3−. D555 may act to distinguish HCO3− from other trigonal planar polyanions such as NO3−, as well as provide steric hindrance for small anions and SCN−.The mechanism appears to involve a salt bridge between Asp555 and adjacent charge residues in TM5 (unpublished observation by Soojung Lee). NBCe1 may also transport OH− at high extracellular pH (Amlal et al., 1998).
Figure 1.
Anion selectivity of NBCe1. A) NBCe1 expressed in Xenopus oocytes produces electrogenic outward currents in response to HCO3−, but not other anions. Recording was done while oocytes were exposed to different anions (25 mM for each). B) Zero current voltage (Erev) versus anion radius. Note that HCO3− has a large effective radius, but produces a current. The molecular or effective radius (in Å) is 1.81 Cl−, 1.89 NO3−, 1.96 Br−, 2.13 SCN−, 2.20 I− and 2.8 HCO3−. n = 6 for each.
The apparent affinity of NBCe1-A for Na+ is about 30 mM, and independent of different voltages and external [HCO3−] (Sciortino & Romero, 1999). Similar affinity values were also observed for all three NBCe1 variants, -A, -B, and -C (apparent Km of 21–36 mM) (McAlear & Bevensee, 2006). The apparent Km of NBCe1 for HCO3− is 4–6.5 mM (Grichtchenko, Romero, & Boron, 2000; McAlear & Bevensee, 2006).
3.2. Ion Translocation
3.2.1. AEs
In addition to anion dependence and selectivity, Glu681 plays a role in ion translocation. Chemical modification or site-directed mutagenesis of this residue alters anion exchange kinetics (Chernova et al., 1997; Sekler et al., 1995), indicating that it is located in the anion translocation pathway. An SCAM study on TM8 (Tang, Kovacs, Sterling, & Casey, 1999) also identified residues that are predicted to line the translocation pore adjacent to Glu681. These are Ala666, Ser667, Leu669, Leu673, Leu677, and Leu680 located in one face of α-helical TM8. Furthermore, cysteine mutants at Ile684 and Ile688 in the intracellular loop are also inhibited by sulfhydryl reagents and thus may be parts of the pore. Other TMs have been implicated as part of anion translocation. Lys539 in TM5 is the site where the anion channel/transporter blocker 4,4′-diisothiocyanostilbene-2,2′-disulfonate (DIDS) binds (Bartel, Hans, & Passow, 1989). Mutations of His703, Arg731, His735, His816, and His834 located in the region of TM10–13 result in inhibition of Cl− flux (Muller-Berger, Karbach, Konig et al., 1995). The His residues, particularly His735, are proposed to interact with Glu681 and form an access channel (Muller-Berger, Karbach, Kang et al., 1995). Several other TMs from 9 to 13 are required for transport function or contribute to ion translocation. Cysteine substitutions G714C, S725C, S731C, S762C, G790C, and F806C all reduced transporter activity to <40% of wild-type AE1 (Fujinaga, Tang, & Casey, 1999). The authors also found that the sulfhydryl reagent decreases the activity of AE constructs S852C (TM12) and A858C (TM13).
3.2.2. NCBTs
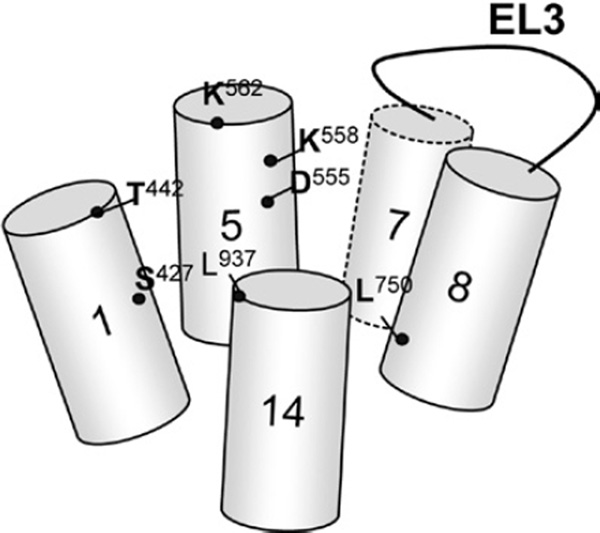
Regarding ion translocation of NBCe1, site-directed mutagenesis of amino acid residues in NBCe1 has been performed to identify the structural requirements for cotransport function (Abuladze et al., 2005). Many charged and polar residues, such as Glu, Asp and Arg, scattered throughout all the TMs were found critical for transport function. Nonetheless, while these residues contribute to charge interaction with ion substrates, it is unclear whether they are responsible for ion translocation or structural components of the protein. Furthermore, noncharged or nonpolar residues may play a more important role in ion translocation. An SCAM analysis of residues in TM8 identified Leu750 among others involved in ion translocation (McAlear & Bevensee, 2006). The sulfhydryl reagent pCMBS accessibility of L750C is strongly influenced by Na+ and HCO3−, DIDS binding, and membrane potential. Kurtz and his colleagues used a similar approach to evaluate amino acid residues in TM1 and found that T442C, A435C, and A428C have substantial sensitivity to the methylsulfonate reagents (Zhu et al., 2009). The α-helical structure of TM1 suggests that these residues would be located on the same face of the TM and serve as binding sites for ion substrates or lining the pore. The functional significance of TM1 is also evident for S427L, which has a defect in either the voltage sensor or Na+-binding site and fails to mediate Na/HCO3 efflux (Dinour et al., 2004). An SCAM analysis of residues in TM10–14 reveals that the C-terminal TMs are tightly folded with limited access to the extracellular aqueous environment (Zhu et al., 2010). D894C (in a loop between TM12 and TM13) and L937C (in TM14) appear to display a severe decrease in NBC function. Figure 2 summarizes amino acid residues that have been identified as residues critical for ion translocation via NBCe1. These residues are located in TM1, TM5, TM7, TM8, and TM14.
Figure 2.
Amino acid residues critical for ion translocation through NBCe1. The residues are located in TM1, TM5, TM7, TM8, and TM14. Identification and characterization of these residues are described in the text.
While structure/function studies continue to investigate the transmembrane domain for ion translocation, the cytoplasmic domain may also play a role. The naturally occurring mutant R298S is located in the cytoplasmic N-terminal domain of NBCe1 and has reduced transport activity. By modeling NBCe1 onto the crystal structure of the AE1 N-terminal domain, Romero and his colleagues hypothesized that Arg298 interacts with Glu91 or Glu295 via hydrogen bonds or charge–charge interactions (Chang, Dipiero, Sonnichsen, & Romero, 2008). The double mutation E91R/R298E rescues transport activity, indicating that the N-terminal domain contributes to HCO3− permeation.
3.3. Mechanisms of Ion Transport
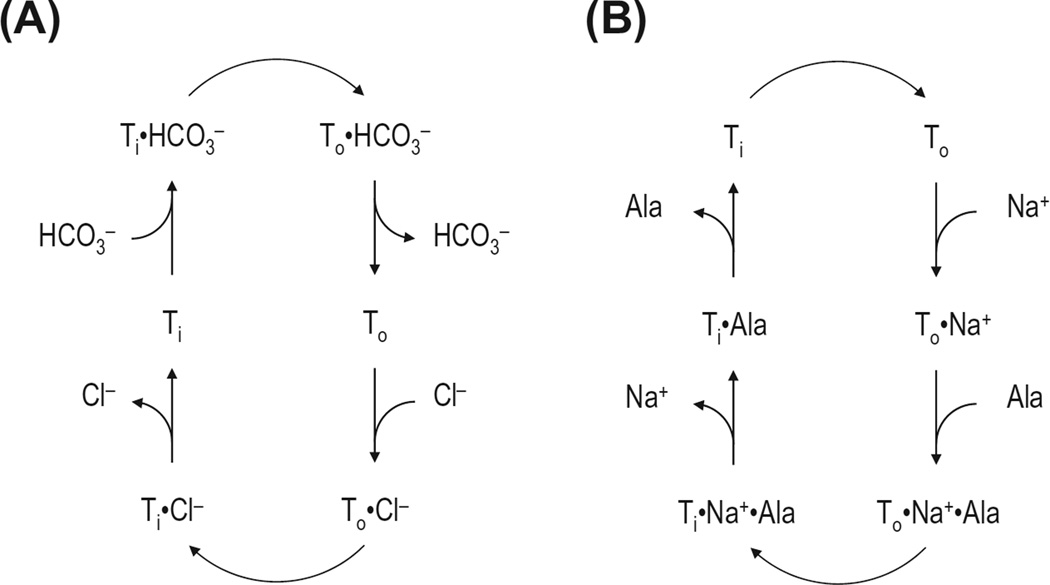
The following two different mechanistic models are considered for SLC4A transporters: a sequential (or ping-pong) mechanism and a simultaneous mechanism (Fig. 3). In the sequential mechanism, one ion substrate binds to the transporter, causes a conformational change, and is released at the trans side of the membrane. Then, a second ion substrate binds to the transporter, regenerates the original conformation, and is released on the original cis side of the membrane. Therefore, the sequential mechanism involves a series of association–dissociation steps and conformational changes. However, the simultaneous mechanism proposes that the transporter forms a ternary complex with substrates before translocation occurs. The first and second ion substrates bind to the transporter and form a tertiary complex. This causes a conformational transition that makes the bound substrates face the trans side of the membrane. The two substrates are subsequently released.
Figure 3.
Sequential (ping pong) vs simultaneous transport mechanisms. A) Cl−/HCO3 exchange via a sequential mechanism. In this mechanism, the transporter (T) accepts only one ion substrate at a time at each membrane surface. Ti and To represent the transporter in the inward- and outward-facing states, respectively. B) Na+/alanine cotransport via a simultaneous mechanism. Both Na+ and alanine (Ala) bind to the transporter before translocation occurs. The tertiary complex T·Na+·Ala undergoes a conformational change. Releasing the two substrates on the opposite side of the membrane causes the transporter to be returned to the original state.
The sequential mechanism has been proposed to account for the exchange kinetics of Cl− and HCO3− by AEs (Frohlich & Gunn, 1986). H2-DIDS can bind preferentially to the outward-facing state (Jennings, Whitlock, & Shinde, 1998). The exchange rates for HCO3− or Cl− are different depending upon the inward facing or outward facing state (Knauf, Law, Leung, Gehret, & Perez, 2002). In addition, for the E699Q mutation of mouse AE1 (corresponding to E681Q in human AE1), elevating intracellular [SO42−] increases the apparent Km for extracellular Cl− and SO42− (Chernova, Stewart, Barry, Jennings, & Alper, 2008). All these data are consistent with the sequential kinetics.
It is unclear if the mechanism used by NCBTs is simultaneous or sequential. The simultaneous mechanism can be distinguished from the sequential mechanism experimentally by studying the relationship of Km and Vmax as shown for the Na/alanine transporter (Jauch & Lauger, 1986). The simultaneous mechanism exhibits a variable Km/Vmax ratio for the variable substrate at different concentrations of the fixed substrate, whereas the sequential mechanism shows a constant Km/Vmax ratio. The apparent Km and Vmax of NBCe1 for Na+ are unaffected at 10 and 33 mM [HCO3−] (Sciortino & Romero, 1999), implying that NBCe1-mediated transport may occur via a sequential mechanism although additional experiments are needed to confirm this. The ability of D555E to produce a Cl− current (Yang, Kim et al., 2009) provides some insights into the binding order. The mutant transporter favorably selects HCO3− when HCO3− and Cl− are present, but this HCO3− selection requires Na+. In the absence of Na+, D555E fails to select HCO3− and instead selects Cl−. This indicates that HCO3− binding requires the precondition of Na+ binding from the perspective of the ion binding order.
3.4. Electrogenicity and Stoichiometry
The electrogenicity of a given ion transporter determines whether it is affected by the membrane voltage in addition to the ion gradient across the cell membrane. Among SLC4A transporters, NBCe1 and NBCe2 are electrogenic despite their sequence similarity with other transporters. The molecular basis of electrogenicity is poorly understood. Studies on chimeric transporters constructed from NBCe1 and NBCn1 reveal that the NBCe1 electrogenicity is conferred by the conserved transmembrane domain, while the cytoplasmic N and C termini and the large third extracellular loop (EL3) between TM5 and TM6 are not critical (Choi, Yang, & Boron, 2007). Furthermore, electrogenicity is mediated via interactions between TM1–5 and TM6–14 because swapping these two regions with NBCe1 and NBCn1 results in electroneutral Na/HCO3 transporters. The EL4 (32 amino acids) between TM7 and TM8 has been found to be critical for electrogenicity (Chen, Liu, & Boron, 2011). Chimeric transporters with NBCe1–EL4 exhibit electrogenic properties, while those with NBCn1– EL4 exhibit electroneutral properties. EL4 may directly constitute a part of the ion-binding vestibule or coordinate with TMs to modulate ion binding.
Related to the issue of electrogenicity is the ion transport stoichiometry. The Na+:HCO3− stoichiometry of NBCe1 is particularly essential for HCO3 reabsorption in the proximal tubules of the kidney, where the basolateral NBCe1-A moves 1Na+ and 3HCO3− into the interstitium (Soleimani, Grassl, & Aronson, 1987). If the stoichiometry were 1Na+ and 2HCO3−, then the transporter would instead move ions in the opposite direction (i.e. into the cell) because the equilibrium potential for 1Na+/2HCO3− transport is estimated to be more negative than the resting potential, typically −70 mV (Boron & Boulpaep, 1983). Indeed, NBCe1 in astrocytes has a 1Na+:2HCO3− stoichiometry and transports ions into the cells (Bevensee, Apkon, & Boron, 1997; Bevensee, Weed, & Boron, 1997). Similarly, NBCe1 in pancreatic ducts has a 1Na+:2HCO3− stoichiometry and transports ions into the cells (Ishiguro, Steward, Lindsay, & Case, 1996; Zhao, Star, & Muallem, 1994). Given the different N-terminal amino acids between NBCe1-A and NBCe1-B/C, it was initially expected that the stoichiometry would be determined by the N-terminal domain of the transporter. However, NBCe1-A expressed in Xenopus oocytes mediates Na/HCO3 influx with a 1Na+:2HCO3− stoichiometry (Ducoudret et al., 2001; Grichtchenko et al., 2001; Sciortino & Romero, 1999). The stoichiometry changes from 1Na+:2HCO3− to 1Na+:3HCO3− when the cytosolic Ca2+ concentration increases (Muller-Berger et al., 2001). Both NBCe1-A and -B show a 1Na+:3HCO3− stoichiometry when expressed in a proximal tubule cell line and a 1Na+:2HCO3− stoichiometry when expressed in a mouse collecting tubule cell line (Gross et al., 2001). These findings provide a strong argument that the same transport molecule can express different stoichiometries, depending on the cellular environment. Gross and his colleagues demonstrate that the stoichiometry can be changed by a cAMP-dependent protein kinase A phosphorylation site (Ser982 in NBCe1-A and Ser1026 in NBCe1-B) in the C-terminal domain (Gross et al., 2003).
3.5. Pharmacological Inhibition of HCO3− Transport
All SLC4A HCO3− transporters except NBCn1 are inhibited by stilbene derivatives such as DIDS, 4-acetamide-4′-isothiocyanatostilbene-2,2′-disulfonic acid (SITS) and 4,4′-dinitrostilbene-2,2′ disulfonate (DNDS). These derivatives are broad inhibitors of anion transporters/channels (Cabantchik, Knauf, & Rothstein, 1978). DIDS binds to the pore entry of anion channels/transporters and occludes the pore vestibule (Tombola, Del Giudice, Papini, & Zoratti, 2000). The inhibition constant Ki of AE1 in erythrocytes for DIDS is as low as 0.04 µM (Knauf, Law, & Hahn, 1995), while the values of cloned AEs are much larger. For NBCe1, the inhibition constant is 36 µM (Liu, Williams, Sumpter, & Bevensee, 2007). DIDS reversibly and covalently binds to the first lysine (K) in the SKLIK motif in TM 5 of AE1 (Bartel et al., 1989). NBCe1 has the homologous KKMIK motif, and mutation analysis of this motif shows the second K (i.e. K559) with the most profound effect on the reversible DIDS binding (Lu & Boron, 2007). However, in the absence of DIDS, the mutation at K559 produces a HCO3− conductance similar to those of other mutations in the KKMIK motif. Thus, K559 does not appear to serve directly as a HCO3− translocation site. Other NCBTs contain similar DIDS motifs in the corresponding region of TM5, but their role in DIDS sensitivity has not been tested.
In addition to DIDS, fluorescent oxonol dyes such as WW-781, diBA(3) C4 and diBA(5)C4 are potent inhibitors for AEs (Alper et al., 1988). The inhibition potency of these oxonol dyes is higher than that of DIDS and varies among AE isoforms. The oxonol dyes diBA(3)C4 and diBA(5)C4 also inhibit NBCe1 (Liu et al., 2007). The oxonol dyes and DIDS compete with each other, and therefore appear to share at least part of the same external binding site. It is suggested that SLC4A transporters may contain a relatively large inner vestibule, in which a number of large anions bind and block Cl− permeation. Other chemicals that inhibit AEs and/or NBCs include niflumic acid (Cousin & Motais, 1976; Liu et al., 2007), benzamil and tenidap (Ducoudret et al., 2001; Lu & Boron, 2007), and an N-cyanosulphonamide inhibitor S8059 (Ch’en, Villafuerte, Swietach, Cobden, & Vaughan-Jones, 2008).
3.6. HCO3− vs CO3− Transport
The possibility that HCO3 transporters would carry CO32− is based on an unexpected observation by Grichtchenko and Chesler (1994). They monitored extracellular pH in the brain slices and found a pH decrease that was caused by electrogenic Na/HCO3 influx via NBCe1 into the astrocytes. They anticipated that inhibiting extracellular carbonic anhydrase (CA) would reduce the supply of extracellular HCO3− and thus blunt the pH decrease. However, CA inhibition had the opposite effect and stimulated the pH decrease. They hypothesized that NBCe1 transports CO32− instead of HCO3−. At physiological pH (i.e. pH 7.4 in the extracellular space), HCO3− is in the millimolar range, while CO32− is in the micromolar range and H+ is in the nanomolar range. If CO32− is consumed via NBCe1, the reaction HCO3− → CO32− + H+ would produce a large increase in [H+]. However, [HCO3−] would remain relatively unchanged as it is in the millimolar range. From the reaction CO2 + H2O ↔ HCO3− + H+, one can expect that an increase in [H+] makes the reaction run leftward. Therefore, inhibiting extracellular carbonic anhydrase would blunt this leftward reaction and consequently enhance a decrease in extracellular pH. Boron and his colleagues (Grichtchenko & Boron, 2002; Lee, Grichtchenko, & Boron, 2011) tested this model by coexpressing NBCe1 and GPI-linked CAIV in Xenopus oocytes and measuring surface pH (pHs). They found that stimulating NBCe1 caused pHs to change as predicted from CO32− transport and inhibiting CAIV augmented all pHS changes. These results are consistent with CO32− transport rather than HCO3− by NBCe1.
3.7. Channel-Like Activity
Historically, ion transporters have been considered mechanistically and structurally different from ion channels (Hille, 2001). Ion transporters move ions slowly and exhibit distinct substrate coupling (i.e. stoichiometry) and kinetics, whereas ion channels move ions rapidly (>106 ions per second) and exhibit gating and permeation. A typical view is that ion transporters structurally undergo rather marked conformational changes between inward-facing and outward-facing states, whereas ion channels undergo more subtle gating changes that open and close the pore. However, this distinction between transporters and channels became increasingly unclear as some membrane proteins share functional characteristics of both groups. Neurotransmitter transporters, including serotonin transporters, norepinephrine transporters, glutamate transporters and dopamine transporters, have imbedded channel properties (DeFelice & Blakely, 1996; Torres & Amara, 2007). Similarly, NBCn1 has robust Na+ channel-like activity (Na+ ≫ K+ = Cs+ = NMDG+) (Choi et al., 2000; Cooper et al., 2005). This activity can cause a positive shift in the membrane potential, and raise intracellular Na+ when NBCn1 is expressed in HEK 293 cells or Xenopus oocytes. The channel-like activity is not coupled to Na/HCO3 cotransport. The domain responsible for the channel-like activity of NBCn1 is the region between TM6 and TM14 (Choi et al., 2007). NBCn1 is not the only HCO3− transporter exhibiting this activity, other SLC4A proteins also have similar activities. Erythrocyte AE1 mediates an anion flux that is stimulated by cell hyperpolarization, implicating a conductive pathway (Freedman & Novak, 1997). Trout AE1 expressed in Xenopus oocytes induces Cl− currents (Fievet, Gabillat, Borgese, & Motais, 1995). The electroneutral NDAE1 in Drosophila has a small inward current (Romero et al., 2000). NBCe1 variants display a Na+-independent HCO3− current (McAlear, Liu, Williams, McNicholas-Bevensee, & Bevensee, 2006). Furthermore, the Cl/HCO3 exchangers Slc26a3 and Slc26a6 produce large NO3− and SCN− currents (Ko, Shcheynikov et al., 2002; Shcheynikov et al., 2008).
The channel-like activity is more than just an oddity of the transporter because it affects cellular membrane potential and controls entry of ions at least in heterologous expression systems. For example, the Na+ channel activity of NBCn1 lowers an electrochemical Na+ gradient across the cell membrane. The lowered Na+ gradient may alter activities of other Na+-conducting ion channels if such channels and NBCn1 are present together. The significance of this effect has yet to be explored.
4. STRUCTURE OF SLC4A TRANSPORTERS
AE1 is predominantly composed of dimers although both monomers and tetramers are known to exist (Reithmeier, Chan, & Popov, 1996). A structural analysis by electron microscopy and three-dimensional image reconstruction at 20 Å resolution reveal the dimeric membrane domain in the U-shaped structure (Dolder, Walz, Hefti, & Engel, 1993; Wang et al., 1994). The two monomers are in contact at the lower part (called the basal domain) embedded in the membrane, while forming a pair of protrusions at the upper part that faces the cytosol. The protrusions form the sides of a canyon, which surrounds a wide space and then converges into a depression in the basal domain. This depression may represent the pore entry or the opening to a transporter vestibule located at the dimer interface.
In support of the above model, the three-dimensional structure of the AE1 membrane domain shows V-shaped densities near the center of the dimer at 7.5 Å resolution (Hirai, Hamasaki, Yamaguchi, & Ikeda, 2011; Yamaguchi et al., 2010). Interestingly, similar V-shaped densities exist in the previously reported structure of a prokaryotic chloride channel (ClC) protein. The ClC protein contains 14 TMs with two internal repeats of 7 TMs inserted into the membrane in the transverse orientation (Dutzler, Campbell, Cadene, Chait, & MacKinnon, 2002). A V-shaped pair of helical repeats (TM1 +2 and TM8 +9) in AE1 are similar to corresponding repeats in the ClC. This similarity in the density and projection maps coincides with a sequence identity of 20%. Nonetheless, there are difficulties with this model. All the TMs cannot be clearly accounted for at 7.5 Å. The beginning of TM1 predicted from NMR studies (Chambers, Bloomberg, Ring, & Tanner, 1999) does not align with the beginning of that for ClC.
The cytoplasmic N-terminal domain of NBCe1-A has been crystallized at 3 Å (Gill & Boron, 2006b). The domain forms two monomers related by a 2-fold axis. The dimer architecture is similar to that observed for the N-terminal crystals of AE1 (Zhang, Kiyatkin, Bolin, & Low, 2000).
5. INTERACTION WITH OTHER PROTEINS
Similar to many other membrane proteins, some SLC4A transporters exist in concert with other proteins and form macromolecular complexes. It would be worth discussing the structural features associated with protein– protein interactions, which mainly occur in the cytoplasmic N- and C-terminal domains of the transporters.
5.1. AEs
The N-terminal domain in AE1 has an ankyrin-binding motif in residues 175–185 (Chang & Low, 2003). The AE1/ankyrin interaction tethers the cytoskeletal protein spectrin to the microdomains near the plasma membrane. Tyrosine phosphorylation promotes dissociation of AE1 from the spectrin-actin skeleton and may enable erythrocytes to undergo adaptive changes in response to stimuli such as malaria parasite invasion, cell shrinkage, and aging (Ferru et al., 2011). AE1 also binds to glycolytic enzymes, hemoglobin and protein 4.1 and 4.2.
The cytoplasmic C-terminal domain of AE1 interacts with CAII (McMurtrie et al., 2004; Vince & Reithmeier, 2000). The consensus sequence for the CAII binding site is DADD, where the first Asp makes an electrostatic interaction with His or Lys residues in the N-terminal region of CAII. Other AEs and different CA isoforms also interact with each other (Casey, Sly, Shah, & Alvarez, 2009). The AE1/CAII interaction significantly increases the rate of HCO3− transport (Sterling, Reithmeier, & Casey, 2001). Cl−/HCO3− exchange via AE1 in erythrocytes is a rate-limiting step for CO2 delivery from tissues to the lungs (Wieth, Andersen, Brahm, Bjerrum, & Borders, 1982). Maximizing the HCO3− transport rate helps fully utilize the CO2-transport capacity of the blood.
5.2. NCBTs
IP3 receptor binding protein released with IP3 (IRBIT) is a signaling protein that is released from IP3 receptors and regulates downstream target molecules. IRBIT binds to the N terminus of NBCe1-B, but not NBCe1-A, and increases HCO3− transport activity (Shirakabe et al., 2006). IRBIT-mediated NBCe1-B stimulation has been demonstrated in pancreatic ducts (Yang, Shcheynikov et al., 2009a). The N-terminal 87 amino acids of NBCe1-B is an autoinhibitory domain that, when deleted, stimulates transport activity (McAlear et al., 2006). Deleting the N-terminal residues 2–16 of NBCe1-B abolishes the IRBIT-stimulated effect while maintaining autoinhibition (Lee, Boron, & Parker, 2012). Thus, autoinhibitory and IRBIT-binding determinants are not identical.
Some SLC4A transporters contain a motif in their C terminus to which PDZ proteins bind (PDZ stands for Post synaptic density protein PSD95, Drosophila disc large tumor suppressor Dlg1, and Zonula occludens-1 protein zo-1). PDZ proteins are proteins that interact with a variety of membrane and cytosolic proteins, modulate activities of their binding partners, associate the partners with cytoskeletal protein complexes near the membrane, and recruit signaling proteins (Kim & Sheng, 2004). NBCn1 contains the PDZ-binding motif (amino acid sequence ETSL) and interacts with the 56 kDa subunit of H-ATPase (Pushkin et al., 2003). The two proteins colocalize to the collecting duct intercalated cells of the kidney. NBCn1 also interacts with ezrin-binding protein 50 (EBP50; alias Na/H exchanger regulatory factor 1 NHERF-1) in the apical membrane of the pancreatic ducts and salivary glands (Park et al., 2002). The NBCn1/EBP50 interaction helps scavenge luminal HCO3− at rest but facilitates Cl−-mediated HCO3− release during stimulation. NBCn1 also interacts with the postsynaptic density protein PSD-95 at synapses in the hippocampus (Park et al., 2010). The NBCn1/PSD-95 interaction appears to increase channel-like activity of the transporter. NBCn1 also interacts with harmonin to form a protein complex in the ear (Reiners et al., 2005). Other SLC4A bicarbonate transporters capable of interacting with PDZ proteins include NBCn2-C, which contains ETCL at its C terminus and interacts with the cytoskeleton and EBP5 (Lee, Ouyang, & Giffard, 2006).
Similar to AEs, NCBTs are also reported to interact with CAII isoforms. For example, the cytoplasmic C-terminal domain of NBCe1 contains D986NDD for the CA II binding and the interaction enhances transport function (Becker & Deitmer, 2007; Pushkin et al., 2004). In contrast, other studies reveal no interaction between NBCe1 and CA II (Lu et al., 2006) or normal transport activity of a mutant lacking the CAII-binding domain (Yamada, Horita, Suzuki, Fujita, & Seki, 2011).
6. CONCLUSION
SLC4A transporters regulate intracellular pH in a variety of cells. Almost all cells in the body possess at least one of these transporters, and changes in pH influence activities of other physiologically important proteins. For example, pH alters ion permeation, sensitivity, and response to agonists of ion channel receptors in the nervous system. Slc4a-knockout mice exhibit aberrant neuronal activities that are caused by acid–base disturbance. In addition, the bicarbonate transporters are essential for transepithelial movement of acid–base equivalents in epithelial tissues such as the kidney where the transporters contribute to regulating plasma pH. Studying the molecular mechanism of how ions are selected and carried via the transporters helps better understand acid–base homeostasis at the cellular and whole-body levels. Studying the transporter structure is valuable for drug designs for transporter-related pathophysiology. Future molecular studies of SLC4A bicarbonate transporters will certainly advance our understanding of transporter physiology.
Acknowledgments
I thank Drs Mark Bevensee and Christian Aalkjaer for their valuable input. This work was supported by the NIH GM078502.
REFERENCES
- Abuladze N, Azimov R, Newman D, Liu W, Tatishchev S, Pushkin A, et al. Critical amino acid residues involved in the electrogenic sodium bicarbonate cotransporter kNBC1-mediated transport. Journal of Physiology. 2005;565(15):717–730. doi: 10.1113/jphysiol.2005.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, et al. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. Journal of Biological Chemistry. 1998;273(28):17689–17695. doi: 10.1074/jbc.273.28.17689. [DOI] [PubMed] [Google Scholar]
- Abuladze N, Pushkin A, Tatishchev S, Newman D, Sassani P, Kurtz I. Expression and localization of rat NBC4c in liver and renal uroepithelium. American Journal of Physiology Cell Physiology. 2004;287(3):C781–C789. doi: 10.1152/ajpcell.00590.2003. [DOI] [PubMed] [Google Scholar]
- Alper SL. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. Journal of Experimental Biology. 2009;212(11):1672–1683. doi: 10.1242/jeb.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper SL, Chernova MN, Williams J, Zasloff M, Law FY, Knauf PA. Differential inhibition of AE1 and AE2 anion exchangers by oxonol dyes and by novel polyaminosterol analogs of the shark antibiotic, squalamine. Biochemistry and Cell Biology. 1988;76(5):799–806. doi: 10.1139/bcb-76-5-799. [DOI] [PubMed] [Google Scholar]
- Alper SL, Kopito RR, Libresco SM, Lodish HF. Cloning and characterization of a murine band 3-related cDNA from kidney and from a lymphoid cell line. Journal of Biological Chemistry. 1988;263(32):17092–17099. [PubMed] [Google Scholar]
- Amlal H, Wang Z, Burnham C, Soleimani M. Functional characterization of a cloned human kidney Na+:HCO3 − cotransporter. Journal of Biological Chemistry. 1998;273(27):16810–16815. doi: 10.1074/jbc.273.27.16810. [DOI] [PubMed] [Google Scholar]
- Bartel D, Hans H, Passow H. Identification by site directed mutagenesis of Lys-558 as the covalent attachment site of dihydro DIDS in the mouse erythroid band 3 protein. Biochimica et Biophysica Acta. 1989;985(3):355–358. doi: 10.1016/0005-2736(89)90427-6. [DOI] [PubMed] [Google Scholar]
- Becker HM, Deitmer JW. Carbonic anhydrase II increases the activity of the human electrogenic Na+/HCO3 − cotransporter. Journal of Biological Chemistry. 2007;282(18):13508–13521. doi: 10.1074/jbc.M700066200. [DOI] [PubMed] [Google Scholar]
- Bevensee MO, Apkon M, Boron WF. Intracellular pH regulation in cultured astrocytes from rat hippocampus. II. Electrogenic Na/HCO3 cotransport. Journal of General Physiology. 1997;110(4):467–483. doi: 10.1085/jgp.110.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF. An electrogenic Na/HCO3 cotransporter (NBC) with a novel C terminus, cloned from rat brain. American Journal of Physiology Cell Physiology. 2000;278(6):C1200–C1211. doi: 10.1152/ajpcell.2000.278.6.C1200. [DOI] [PubMed] [Google Scholar]
- Bevensee MO, Weed RA, Boron WF. Intracellular pH regulation in cultured astrocytes from rat hippocampus. I. Role of HCO3 − . Journal of General Physiology. 1997;110(4):453–465. doi: 10.1085/jgp.110.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedtkjer E, Praetorius J, Fuchtbauer EM, Aalkjaer C. Antibody-independent localization of the electroneutral Na+-HCO3 cotransporter NBCn1 (Slc4a7) in mice. American Journal of Physiology Cell Physiology. 2008;294(2):C591–C603. doi: 10.1152/ajpcell.00281.2007. [DOI] [PubMed] [Google Scholar]
- Boedtkjer E, Praetorius J, Matchkov VV, Stankevicius E, Mogensen S, Fuchtbauer AC, et al. Disruption of Na+, HCO3 − cotransporter NBCn1 (slc4a7) inhibits NO-mediated vasorelaxation, smooth muscle Ca2+ sensitivity, and hypertension development in mice. Circulation. 2011;124(17):1819–1829. doi: 10.1161/CIRCULATIONAHA.110.015974. [DOI] [PubMed] [Google Scholar]
- Bonar PT, Casey JR. Plasma membrane Cl−/HCO3 − exchangers: structure, mechanism and physiology. Channels (Austin) 2008;2(5):337–345. doi: 10.4161/chan.2.5.6899. [DOI] [PubMed] [Google Scholar]
- Boron WF, Boulpaep EL. Intracellular pH regulation in the renal proximal tubule of the salamander: basolateral HCO3 − transport. Journal of General Physiology. 1983;81(1):53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzinova EV, Praetorius J, Virkki LV, Nielsen S, Boron WF, Aalkjaer C. Na+-dependent HCO3 − uptake into the rat choroid plexus epithelium is partially DIDS sensitive. American Journal of Physiology Cell Physiology. 2005;289(6):C1448–C1456. doi: 10.1152/ajpcell.00313.2005. [DOI] [PubMed] [Google Scholar]
- Brosius FC, Nguyen K, Stuart-Tilley AK, Haller C, Briggs JP, Alper SL. Regional and segmental localization of AE2 anion exchanger mRNA and protein in rat kidney. American Journal of Physiology. 1995;269(4 Pt 2):F461–F468. doi: 10.1152/ajprenal.1995.269.4.F461. [DOI] [PubMed] [Google Scholar]
- Cabantchik ZI, Knauf PA, Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of ‘probes’. Biochimica et Biophysica Acta. 1978;515(3):239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Casey JR, Sly WS, Shah GN, Alvarez BV. Bicarbonate homeostasis in excitable tissues: role of AE3 Cl−/HCO3 − exchanger and carbonic anhydrase XIV interaction. American Journal of Physiology Cell Physiology. 2009;297(5):C1091–C1102. doi: 10.1152/ajpcell.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch’en FF, Villafuerte FC, Swietach P, Cobden PM, Vaughan-Jones RD. S0859, an N-cyanosulphonamide inhibitor of sodium-bicarbonate cotransport in the heart. British Journal of Pharmacology. 2008;153(5):972–982. doi: 10.1038/sj.bjp.0707667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers EJ, Bloomberg GB, Ring SM, Tanner MJ. Structural studies on the effects of the deletion in the red cell anion exchanger (band 3, AE1) associated with South East Asian ovalocytosis. Journal of Molecular Biology. 1999;285(3):1289–1307. doi: 10.1006/jmbi.1998.2392. [DOI] [PubMed] [Google Scholar]
- Chang MH, Dipiero J, Sonnichsen FD, Romero MF. Entry to “Formula Tunnel” revealed by SLC4A4 human mutation and structural model. Journal of Biological Chemistry. 2008;283(26):18402–18410. doi: 10.1074/jbc.M709819200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SH, Low PS. Identification of a critical ankyrin-binding loop on the cytoplasmic domain of erythrocyte membrane band 3 by crystal structure analysis and site-directed mutagenesis. Journal of Biological Chemistry. 2003;278(9):6879–6884. doi: 10.1074/jbc.M211137200. [DOI] [PubMed] [Google Scholar]
- Chen LM, Haddad GG, Boron WF. Effects of chronic continuous hypoxia on the expression of SLC4A8 (NDCBE) in neonatal versus adult mouse brain. Brain Research. 2008;1238:85–92. doi: 10.1016/j.brainres.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LM, Liu Y, Boron WF. Role of an extracellular loop in determining the stoichiometry of Na+-HCO3 − cotransporters. Journal of Physiology. 2011;589(Pt 4):877–890. doi: 10.1113/jphysiol.2010.198713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernova MN, Jiang L, Crest M, Hand M, Vandorpe DH, Strange K, et al. Electrogenic sulfate/chloride exchange in Xenopus oocytes mediated by murine AE1 E699Q. Journal of General Physiology. 1997;109(3):345–360. doi: 10.1085/jgp.109.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernova MN, Stewart AK, Barry PN, Jennings ML, Alper SL. Mouse Ae1 E699Q mediates SO42−i/aniono exchange with [SO4 2−]i-dependent reversal of wild-type pHo sensitivity. American Journal of Physiology Cell Physiology. 2008;295(2):C302–C312. doi: 10.1152/ajpcell.00109.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernova MN, Stewart AK, Jiang L, Friedman DJ, Kunes YZ, Alper SL. Structure-function relationships of AE2 regulation by Cai 2+-sensitive stimulators NH4 + and hypertonicity. American Journal of Physiology. Cell Physiology. 2003;284(5):C1235–C1246. doi: 10.1152/ajpcell.00522.2002. [DOI] [PubMed] [Google Scholar]
- Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature. 2000;405(6786):571–575. doi: 10.1038/35014615. [DOI] [PubMed] [Google Scholar]
- Choi I, Romero MF, Khandoudi N, Bril A, Boron WF. Cloning and characterization of a human electrogenic Na+-HCO3 − cotransporter isoform (hhNBC) American Journal of Physiology. 1999;276(3 Pt 1):C576–C584. doi: 10.1152/ajpcell.1999.276.3.C576. [DOI] [PubMed] [Google Scholar]
- Choi I, Yang HS, Boron WF. The electrogenicity of the sodium/bicarbonate cotransporter NBCe1 requires interactions among transmembrane segments of the transporter. Journal of Physiology (London) 2007;578(Pt 1):131–142. doi: 10.1113/jphysiol.2006.114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DS, Lee HJ, Yang HS, Kippen J, Yun CC, Choi I. The electroneutral sodium/bicarbonate cotransporter containing an amino terminal 123-amino-acid cassette is expressed predominantly in the heart. Journal of Biomedical Science. 2006;13(4):593–595. doi: 10.1007/s11373-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DS, Saxena NC, Yang HS, Lee HJ, Moring AG, Lee A, et al. Molecular and functional characterization of the electroneutral Na/HCO3 cotransporter NBCn1 in rat hippocampal neurons. Journal of Biological Chemistry. 2005;280(18):17823–17830. doi: 10.1074/jbc.M408646200. [DOI] [PubMed] [Google Scholar]
- Cousin JL, Motais R. The role of carbonic anhydrase inhibitors on anion permeability into ox red blood cells. Journal of Physiology. 1976;256(1):61–80. doi: 10.1113/jphysiol.1976.sp011311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl NK, Jiang L, Chernova MN, Stuart-Tilley AK, Shmukler BE, Alper SL. Deficient HCO3 − transport in an AE1 mutant with normal Cl− transport can be rescued by carbonic anhydrase II presented on an adjacent AE1 protomer. Journal of Biological Chemistry. 2003;278(45):44949–44958. doi: 10.1074/jbc.M308660200. [DOI] [PubMed] [Google Scholar]
- Damkier HH, Aalkjaer C, Praetorius J. Na+-dependent HCO3 − import by the slc4a10 gene product involves Cl− export. Journal of Biological Chemistry. 2010;285(35):26998–27007. doi: 10.1074/jbc.M110.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damkier HH, Nielsen S, Praetorius J. Molecular expression of SLC4-derived Na+-dependent anion transporters in selected human tissues. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2007;293(5):R2136–R2146. doi: 10.1152/ajpregu.00356.2007. [DOI] [PubMed] [Google Scholar]
- DeFelice LJ, Blakely RD. Pore models for transporters? Biophysical Journal. 1996;70(2):579–580. doi: 10.1016/S0006-3495(96)79604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinour D, Chang MH, Satoh J, Smith BL, Angle N, Knecht A, et al. A novel missense mutation in the sodium bicarbonate cotransporter (NBCe1/SLC4A4) causes proximal tubular acidosis and glaucoma through ion transport defects. Journal of Biological Chemistry. 2004;279(50):52238–52246. doi: 10.1074/jbc.M406591200. [DOI] [PubMed] [Google Scholar]
- Dolder M, Walz T, Hefti A, Engel A. Human erythrocyte band 3. Solubilization and reconstitution into two-dimensional crystals. Journal of Molecular Biology. 1993;231(1):119–132. doi: 10.1006/jmbi.1993.1261. [DOI] [PubMed] [Google Scholar]
- Ducoudret O, Diakov A, Muller-Berger S, Romero MF, Frömter E. The renal Na-HCO3 −cotransporter expressed in Xenopus laevis oocytes: inhibition by tenidap and benzamil and effect of temperature on transport rate and stoichiometry. Pflügers Archiv European Journal of Physiology. 2001;442(5):709–717. doi: 10.1007/s004240100594. [DOI] [PubMed] [Google Scholar]
- Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 A° reveals the molecular basis of anion selectivity. Nature. 2002;415(6869):287–294. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- Ferru E, Giger K, Pantaleo A, Campanella E, Grey J, Ritchie K, et al. Regulation of membrane-cytoskeletal interactions by tyrosine phosphorylation of erythrocyte band 3. Blood. 2011;117(22):5998–6006. doi: 10.1182/blood-2010-11-317024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fievet B, Gabillat N, Borgese F, Motais R. Expression of band 3 anion exchanger induces chloride current and taurine transport: structure-function analysis. EMBO Journal. 1995;14(21):5158–5169. doi: 10.1002/j.1460-2075.1995.tb00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman JC, Novak TS. Electrodiffusion, barrier, and gating analysis of DIDS-insensitive chloride conductance in human red blood cells treated with valinomycin or gramicidin. Journal of General Physiology. 1997;109(2):201–216. doi: 10.1085/jgp.109.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich O, Gunn RB. Erythrocyte anion transport: the kinetics of a single-site obligatory system. Biochimica et Biophysica Acta. 1986;864(2):169–194. doi: 10.1016/0304-4157(86)90010-9. [DOI] [PubMed] [Google Scholar]
- Fujinaga J, Loiselle FB, Casey JR. Transport activity of chimaeric AE2–AE3 chloride/bicarbonate anion exchange proteins. Biochemical Journal. 2003;371(Pt 3):687–696. doi: 10.1042/BJ20030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga J, Tang XB, Casey JR. Topology of the membrane domain of human erythrocyte anion exchange protein, AE1. Journal of Biological Chemistry. 1999;274(10):6626–6633. doi: 10.1074/jbc.274.10.6626. [DOI] [PubMed] [Google Scholar]
- Giffard RG, Lee YS, Ouyang YB, Murphy SL, Monyer H. Two variants of the rat brain sodium-driven chloride bicarbonate exchanger (NCBE): developmental expression and addition of a PDZ motif. European Journal of Neuroscience. 2003;18(11):2935–2945. doi: 10.1046/j.1460-9568.2003.03053.x. [DOI] [PubMed] [Google Scholar]
- Gill HS, Boron WF. Expression and purification of the cytoplasmic N-terminal domain of the Na/HCO3 cotransporter NBCe1-A: structural insights from a generalized approach. Protein Expression and Purification. 2006a;49(2):228–234. doi: 10.1016/j.pep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Gill HS, Boron WF. Preliminary X-ray diffraction analysis of the cytoplasmic N-terminal domain of the Na/HCO3 cotransporter NBCe1-A. Acta Crystallographica Section F: Structural Biology and Crystallization Communications. 2006b;62(Pt 6):534–537. doi: 10.1107/S1744309106015181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichtchenko, Boron Evidence for CO3 − transport by NBCe1, based on surface-pH measurements in voltage-clamped Xenopus oocytes co-expressing NBCe1 and CAIV: evidence for CO3 − transport. FASEB Journal. 2002;16:A795. [Google Scholar]
- Grichtchenko II, Choi I, Zhong X, Bray-Ward P, Russell JM, Boron WF. Cloning, characterization, and chromosomal mapping of a human electroneutral Na+-driven Cl-HCO3 exchanger. Journal of Biological Chemistry. 2001;276(11):8358–8363. doi: 10.1074/jbc.C000716200. [DOI] [PubMed] [Google Scholar]
- Grichtchenko II, Romero MF, Boron WF. Extracellular HCO3 − dependence of electrogenic Na/HCO3 cotransporters cloned from salamander and rat kidney. Journal of General Physiology. 2000;115(5):533–545. doi: 10.1085/jgp.115.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E, Hawkins K, Abuladze N, Pushkin A, Cotton CU, Hopfer U, Kurtz I. The stoichiometry of the electrogenic sodium bicarbonate cotransporter NBC1 is cell-type dependent. Journal of Physiology. 2001;531(Pt 3):597–603. doi: 10.1111/j.1469-7793.2001.0597h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E, Fedotoff O, Pushkin A, Abuladze N, Newman D, Kurtz I. Phosphorylation-induced modulation of pNBC1 function: distinct roles for the amino- and carboxy-termini. Journal of Physiology. 2003;549(Pt 3):673–682. doi: 10.1113/jphysiol.2003.042226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion channels of Exitable Membranes (chapter 11: Elementary properties of pores) Massachusetts: Sinauer Associates, Inc.; 2001. [Google Scholar]
- Hirai T, Hamasaki N, Yamaguchi T, Ikeda Y. Topology models of anion exchanger 1 that incorporate the anti-parallel V-shaped motifs found in the EM structure. Biochemistry and Cell Biology. 2011;89(2):148–156. doi: 10.1139/o10-160. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Jiang L, Chernova MN, Alper SL. Functional characterization and regulation by pH of murine AE2 anion exchanger expressed in Xenopus oocytes. American Journal of Physiology. 1994;267(5 Pt 1):C1295–C1307. doi: 10.1152/ajpcell.1994.267.5.C1295. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Steward MC, Lindsay ARG, Case RM. Accumulation of intracellular HCO3 − by Na+-HCO3 − cotransport in interlobular ducts from the guinea-pig pancreas. Journal of Physiology. 1996;495(Pt 1):169–178. doi: 10.1113/jphysiol.1996.sp021582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauch P, Lauger P. Electrogenic properties of the sodium-alanine cotransporter in pancreatic acinar cells: II. Comparison with transport models. Journal of Membrane Biology. 1986;94(2):117–127. doi: 10.1007/BF01871192. [DOI] [PubMed] [Google Scholar]
- Jennings ML. Rapid electrogenic sulfate-chloride exchange mediated by chemically modified band 3 in human erythrocytes. Journal of General Physiology. 1995;105(1):21–47. doi: 10.1085/jgp.105.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings ML, Al Rhaiyel S. Modification of a Carboxyl Group that appears to cross the permeability barrier in the red blood cell anion transporter. Journal of General Physiology. 1988;92(2):161–178. doi: 10.1085/jgp.92.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings ML, Whitlock J, Shinde A. Pre-steady state transport by erythrocyte band 3 protein: uphill countertransport induced by the impermeant inhibitor H2DIDS. Biochemistry and Cell Biology. 1998;76(5):807–813. doi: 10.1139/bcb-76-5-807. [DOI] [PubMed] [Google Scholar]
- Kao L, Sassani P, Azimov R, Pushkin A, Abuladze N, Peti-Peterdi J, et al. Oligomeric structure and minimal functional unit of the electrogenic sodium bicarbonate cotransporter NBCe1-A. Journal of Biological Chemistry. 2008;283(39):26782–26794. doi: 10.1074/jbc.M804006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nature Reviews Neuroscience. 2004;5(10):771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Knauf PA, Law FY, Hahn K. An oxonol dye is the most potent known inhibitor of band 3-mediated anion exchange. American Journal of Physiology. 1995;269(4 Pt 1):C1073–C1077. doi: 10.1152/ajpcell.1995.269.4.C1073. [DOI] [PubMed] [Google Scholar]
- Knauf PA, Law FY, Leung TW, Gehret AU, Perez ML. Substrate-dependent reversal of anion transport site orientation in the human red blood cell anion-exchange protein, AE1. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(16):10861–10864. doi: 10.1073/pnas.162402399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf PA, Pal P. Band 3-mediated transport in red cell membrane transport in Health and Disease. Berlin: Springer; 2003. [Google Scholar]
- Ko SB, Luo X, Hager H, Rojek A, Choi JY, Licht C, et al. AE4 is a DIDS-sensitive Cl−/HCO3 − exchanger in the basolateral membrane of the renal CCD and the SMG duct. American Journal of Physiology Cell Physiology. 2002;283(4):C1206–C1218. doi: 10.1152/ajpcell.00512.2001. [DOI] [PubMed] [Google Scholar]
- Kopito RR, Lee BS, Simmons DM, Lindsey AE, Morgans CW, Schneider K. Regulation of intracellular pH by a neuronal homolog of the erythrocyte anion exchanger. Cell. 1989;59(5):927–937. doi: 10.1016/0092-8674(89)90615-6. [DOI] [PubMed] [Google Scholar]
- Kopito RR, Lodish HF. Primary structure and transmembrane orientation of the murine anion exchange protein. Nature. 1985;316(6025):234–238. doi: 10.1038/316234a0. [DOI] [PubMed] [Google Scholar]
- Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, et al. A molecular mechanism for aberrant CFTR-dependent HCO3 − transport in cystic fibrosis. EMBO Journal. 2002;21(21):5662–5672. doi: 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen JM, Kristensen M, Juel C. Expression of Na+/HCO3 − co-transporter proteins (NBCs) in rat and human skeletal muscle. Acta Physiologica Scandinavica. 2004;182(1):69–76. doi: 10.1111/j.1365-201X.2004.01297.x. [DOI] [PubMed] [Google Scholar]
- Lee SK, Boron WF, Parker MD. Relief of autoinhibition of the electrogenic Na-HCO3 cotransporter NBCe1-B: role of IRBIT vs. amino-terminal truncation. A American Journal of Physiology Cell Physiology. 2012;302(3):C518–C526. doi: 10.1152/ajpcell.00352.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Grichtchenko II, Boron WF. Distinguishing HCO3 − from CO3 2− transport by NBCe1-A. FASEB Journal. 2011;25:656.9. [Google Scholar]
- Lee YS, Ouyang YB, Giffard RG. Regulation of the rat brain Na+ -driven Cl−/HCO3 − exchanger involves protein kinase A and a multiprotein signaling complex. FEBS Letters. 2006;580(20):4865–4871. doi: 10.1016/j.febslet.2006.07.075. [DOI] [PubMed] [Google Scholar]
- Liu X, Williams JB, Sumpter BR, Bevensee MO. Inhibition of the Na/bicarbonate cotransporter NBCe1-A by diBAC oxonol dyes relative to niflumic acid and a stilbene. Journal of Membrane Biology. 2007;215(2–3):195–204. doi: 10.1007/s00232-007-9018-z. [DOI] [PubMed] [Google Scholar]
- Lu J, Boron WF. Reversible and irreversible interactions of DIDS with the human electrogenic Na/HCO3 cotransporter NBCe1-A: role of lysines in the KKMIK motif of TM5. American Journal of Physiology Cell Physiology. 2007;292(5):C1787–C1798. doi: 10.1152/ajpcell.00267.2006. [DOI] [PubMed] [Google Scholar]
- Lu J, Daly CM, Parker MD, Gill HS, Piermarini PM, Pelletier MF, et al. Effect of human carbonic anhydrase II on the activity of the human electrogenic Na/ HCO3 cotransporter NBCe1-A in Xenopus oocytes. Journal of Biological Chemistry. 2006;281(28):19241–19250. doi: 10.1074/jbc.M602181200. [DOI] [PubMed] [Google Scholar]
- McAlear SD, Bevensee MO. A cysteine-scanning mutagenesis study of transmembrane domain 8 of the electrogenic Sodium/Bicarbonate cotransporter NBCe1. Journal of Biological Chemistry. 2006;281(43):32417–32427. doi: 10.1074/jbc.M607253200. [DOI] [PubMed] [Google Scholar]
- McAlear SD, Liu X, Williams JB, McNicholas-Bevensee CM, Bevensee MO. Electrogenic Na/HCO3 cotransporter (NBCe1) variants expressed in Xenopus oocytes: functional comparison and roles of the amino and carboxy termini. Journal of General Physiology. 2006;127(6):639–658. doi: 10.1085/jgp.200609520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurtrie HL, Cleary HJ, Alvarez BV, Loiselle FB, Sterling D, Morgan PE, et al. The bicarbonate transport metabolon. Journal of Enzyme Inhibition and Medicinal Chemistry. 2004;19(3):231–236. doi: 10.1080/14756360410001704443. [DOI] [PubMed] [Google Scholar]
- Milanick MA, Gunn RB. Proton-sulfate co-transport: mechanism of H+ and sulfate addition to the chloride transporter of human red blood cells. Journal of General Physiology. 1982;79(1):87–113. doi: 10.1085/jgp.79.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar ID, Brown PD. NBCe2 exhibits a 3 HCO3 −:1 Na+ stoichiometry in mouse choroid plexus epithelial cells. Biochemical and Biophysical Research. 2008;373(4):550–554. doi: 10.1016/j.bbrc.2008.06.053. [DOI] [PubMed] [Google Scholar]
- Muller-Berger S, Ducoudret O, Diakov A, Frömter E. The renal Na-HCO3 cotransporter expressed in Xenopus laevis oocytes: change in stoichiometry in response to elevation of cytosolic Ca2+ concentration. Pflügers Archiv European Journal of Physiology. 2001;442(5):718–728. doi: 10.1007/s004240100592. [DOI] [PubMed] [Google Scholar]
- Muller-Berger S, Karbach D, Kang D, Aranibar N, Wood PG, Ruterjans H, et al. Roles of histidine 752 and glutamate 699 in the pH dependence of mouse band 3 protein-mediated anion transport. Biochemistry. 1995;34(29):9325–9332. doi: 10.1021/bi00029a007. [DOI] [PubMed] [Google Scholar]
- Muller-Berger S, Karbach D, Konig J, Lepke S, Wood PG, Appelhans H, et al. Inhibition of mouse erythroid band 3-mediated chloride transport by site-directed mutagenesis of histidine residues and its reversal by second site mutation of Lys 558, the locus of covalent H2DIDS binding. Biochemistry. 1995;34(29):9315–9324. doi: 10.1021/bi00029a006. [DOI] [PubMed] [Google Scholar]
- Parker MD, Bouyer P, Daly CM, Boron WF. Cloning and characterization of novel human SLC4A8 gene products encoding Na+-driven Cl−/HCO3 − exchanger variants NDCBE-A, -C, and -D. Physiological Genomics. 2008;34(3):265–276. doi: 10.1152/physiolgenomics.90259.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MD, Musa-Aziz R, Rojas jd, Choi I, Daly CM, Boron WF. Characterization of human SLC4A10 as an electroneutral Na/HCO3 cotransporter (NBCn2) with Cl− self-exchange activity. Journal of Biological Chemistry. 2008;283(19):12777–12788. doi: 10.1074/jbc.M707829200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Ko SBH, Davidson N, Muallem G, Thomas PJ, Pushkin A, et al. The cystic fibrosis transmembrane conductance regulator interacts with and regulates the activity of the HCO salvage transporter human Na+-HCO3 cotransporter isoform 3. Journal of Biological Chemistry. 2002;277(52):50503–50509. doi: 10.1074/jbc.M201862200. [DOI] [PubMed] [Google Scholar]
- Park M, Li Q, Shcheynikov N, Zeng W, Muallem S. NaBC1 is a ubiquitous electrogenic Na+ -coupled borate transporter essential for cellular boron homeostasis and cell growth and proliferation. Molecular Cell. 2004;16(3):331–341. doi: 10.1016/j.molcel.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Park HJ, Rajbhandari I, Yang HS, Lee S, Cucoranu D, Cooper DS, et al. Neuronal expression of sodium/bicarbonate cotransporter NBCn1 (SLC4A7) and its response to chronic metabolic acidosis. American Journal of Physiology Cell Physiology. 2010;298(5):C1018–C1028. doi: 10.1152/ajpcell.00492.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushkin A, Abuladze N, Gross E, Newman D, Tatishchev S, Lee I, et al. Molecular mechanism of kNBC1-carbonic anhydrase II interaction in proximal tubule cells. Journal of Physiology. 2004;559(Pt 1):55–65. doi: 10.1113/jphysiol.2004.065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I. Cloning, tissue distribution, genomic organization, and functional characterization of NBC3, a new member of the sodium bicarbonate cotransporter family. Journal of Biological Chemistry. 1999;274(23):16569–16575. doi: 10.1074/jbc.274.23.16569. [DOI] [PubMed] [Google Scholar]
- Pushkin A, Abuladze N, Newman D, Lee I, Xu G, Kurtz I. Cloning, characterization and chromosomal assignment of NBC4, a new member of the sodium bicarbonate cotransporter family. Biochimica et Biophysica Acta. 2000;1493(1–2):215–218. doi: 10.1016/s0167-4781(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Pushkin A, Abuladze N, Newman D, Muronets V, Sassani P, Tatishchev S, et al. The COOH termini of NBC3 and the 56-kDa H+-ATPase subunit are PDZ motifs involved in their interaction. American Journal of Physiology Cell Physiology. 2003;284(3):C667–C673. doi: 10.1152/ajpcell.00225.2002. [DOI] [PubMed] [Google Scholar]
- Reiners J, van Wijk E, Marker T, Zimmermann U, Jurgens K, te BH, et al. Scaffold protein harmonin (USH1C) provides molecular links between Usher syndrome type 1 and type 2. Human Molecular Genetics. 2005;14(24):3933–3943. doi: 10.1093/hmg/ddi417. [DOI] [PubMed] [Google Scholar]
- Reithmeier RA, Chan SL, Popov M. Structure of the erythrocyte Band 3 anion exchanger. In: Konings WN, Kaback HR, Lolkema JS, editors. Handbook of biological physics. Amsterdam: Elsevier Science; 1996. pp. 281–309. [Google Scholar]
- Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/HCO3 − cotransporter. Nature. 1997;387(6631):409–413. doi: 10.1038/387409a0. [DOI] [PubMed] [Google Scholar]
- Romero MF, Henry D, Nelson S, Harte PJ, Dillon AK, Sciortino CM. Cloning and characterization of a Na+-driven anion exchanger (NDAE1). A new bicarbonate transporter. Journal of Biological Chemistry. 2000;275(32):24552–24559. doi: 10.1074/jbc.M003476200. [DOI] [PubMed] [Google Scholar]
- Sassani P, Pushkin A, Gross E, Gomer A, Abuladze N, Dukkipati R, et al. Functional characterization of NBC4: a new electrogenic sodium- bicarbonate cotransporter. American Journal of Physiology Cell Physiology. 2002;282(2):C408–C416. doi: 10.1152/ajpcell.00409.2001. [DOI] [PubMed] [Google Scholar]
- Schwiening CJ, Boron WF. Regulation of intracellular pH in pyramidal neurons from the rat hippocampus by Na+-dependent Cl−-HCO3 − exchange. Journal of Physiology. 1994;475(1):59–67. doi: 10.1113/jphysiol.1994.sp020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciortino CM, Romero MF. Cation and voltage dependence of rat kidney electrogenic Na+-HCO3 − cotransporter, rkNBC, expressed in oocytes. American Journal of Physiology. 1999;277(4 Pt 2):F611–F623. doi: 10.1152/ajprenal.1999.277.4.F611. [DOI] [PubMed] [Google Scholar]
- Sekler I, Lo RS, Kopito RR. A conserved glutamate is responsible for ion selectivity and pH dependence of the mammalian anion exchangers AE1 and AE2. Journal of Biological Chemistry. 1995;270(48):28751–28758. doi: 10.1074/jbc.270.48.28751. [DOI] [PubMed] [Google Scholar]
- Shcheynikov N, Yang D, Wang Y, Zeng W, Karniski LP, So I, et al. The Slc26a4 transporter functions as an electroneutral Cl−/I−/HCO3 − exchanger: role of Slc26a4 and Slc26a6 in I− and HCO3 − secretion and in regulation of CFTR in the parotid duct. Journal of Physiology. 2008;586(16):3813–3824. doi: 10.1113/jphysiol.2008.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, et al. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3 − cotransporter 1 (pNBC1) Proceedings of the National Academy of Sciences of the United States of America. 2006;103(25):9542–9547. doi: 10.1073/pnas.0602250103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindic A, Chang MH, Mount DB, Romero MF. Renal physiology of SLC26 anion exchangers. Current Opinion in Nephrology and Hypertension. 2007;16(5):484–490. doi: 10.1097/MNH.0b013e3282e7d7d0. [DOI] [PubMed] [Google Scholar]
- Sinning A, Liebmann L, Kougioumtzes A, Westermann M, Bruehl C, Hubner CA. Synaptic glutamate release is modulated by the Na+ -driven Cl−/HCO3 − exchanger Slc4a8. Journal of Neuroscience. 2011;31(20):7300–7311. doi: 10.1523/JNEUROSCI.0269-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani M, Grassl SM, Aronson PS. Stoichiometry of Na+-HCO3 − cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. Journal of Clinical Investigation. 1987;79:1276–1280. doi: 10.1172/JCI112948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling D, Reithmeier RA, Casey JR. A transport metabolon: functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. Journal of Biological Chemistry. 2001;276(51):47886–47894. doi: 10.1074/jbc.M105959200. [DOI] [PubMed] [Google Scholar]
- Stewart AK, Chernova MN, Shmukler BE, Wilhelm S, Alper SL. Regulation of AE2-mediated Cl− transport by intracellular or by extracellular pH requires highly conserved amino acid residues of the AE2 NH2-terminal cytoplasmic domain. Journal of General Physiology. 2002;120(5):707–722. doi: 10.1085/jgp.20028641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AK, Kurschat CE, Vaughan-Jones RD, Shmukler BE, Alper SL. Acute regulation of mouse AE2 anion exchanger requires isoform-specific amino acid residues from most of the transmembrane domain. Journal of Physiology. 2007;584(Pt 1):59–73. doi: 10.1113/jphysiol.2007.136119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XB, Kovacs M, Sterling D, Casey JR. Identification of residues lining the translocation pore of human AE1, plasma membrane anion exchange protein. Journal of Biological Chemistry. 1999;274(6):3557–3564. doi: 10.1074/jbc.274.6.3557. [DOI] [PubMed] [Google Scholar]
- Tombola F, Del Giudice G, Papini E, Zoratti M. Blockers of VacA provide insights into the structure of the pore. Biophysical Journal. 2000;79(2):863–873. doi: 10.1016/S0006-3495(00)76342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres GE, Amara SG. Glutamate and monoamine transporters: new visions of form and function. Current Opinion In Neurobiology. 2007;17(3):304–312. doi: 10.1016/j.conb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Tsuganezawa H, Kobayashi K, Iyori M, Araki T, Koizumi A, Watanabe SI, et al. A new member of the HCO3 − transporter superfamily is an apical anion exchanger of beta-intercalated cells in the kidney. Journal of Biological Chemistry. 2001;276(16):8180–8189. doi: 10.1074/jbc.M004513200. [DOI] [PubMed] [Google Scholar]
- van Adelsberg JS, Edwards JC, Al Awqati Q. The apical Cl/HCO3 exchanger of β intercalated cells. Journal of Biological Chemistry. 1993;268(15):11283–11289. [PubMed] [Google Scholar]
- Vince JW, Reithmeier RA. Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte Cl−/HCO3 − exchanger. Journal of Biological Chemistry. 1998;273(43):28430–28437. doi: 10.1074/jbc.273.43.28430. [DOI] [PubMed] [Google Scholar]
- Vince JW, Reithmeier RA. Identification of the carbonic anhydrase II binding site in the Cl−/HCO3 − anion exchanger AE1. Biochemistry. 2000;39(18):5527–5533. doi: 10.1021/bi992564p. [DOI] [PubMed] [Google Scholar]
- Virkki LV, Wilson DA, Vaughan-Jones RD, Boron WF. Functional characterization of human NBC4 as an electrogenic Na+-HCO3 − cotransporter (NBCe2) American Journal of Physiology Cell Physiology. 2002;282(6):C1278–C1289. doi: 10.1152/ajpcell.00589.2001. [DOI] [PubMed] [Google Scholar]
- Wang DN, Sarabia VE, Reithmeier RA, Kuhlbrandt W. Three-dimensional map of the dimeric membrane domain of the human erythrocyte anion exchanger, Band 3. EMBO Journal. 1994;13(14):3230–3235. doi: 10.1002/j.1460-2075.1994.tb06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Yano H, Nagashima K, Seino S. The Na+ -driven Cl−/HCO3 − exchanger: cloning, tissue distribution, and functional characterization. Journal of Biological Chemistry. 2000;275(45):35486–35490. doi: 10.1074/jbc.C000456200. [DOI] [PubMed] [Google Scholar]
- Wieth JO, Andersen OS, Brahm J, Bjerrum PJ, Borders CL., Jr Chloride–bicarbonate exchange in red blood cells: physiology of transport and chemical modification of binding sites. Philosophical Transactions of the Royal Society of London B Biological Sciences. 1982;299(1097):383–399. doi: 10.1098/rstb.1982.0139. [DOI] [PubMed] [Google Scholar]
- Yamada H, Horita S, Suzuki M, Fujita T, Seki G. Functional role of a putative carbonic anhydrase II-binding domain in the electrogenic Na+-HCO3 − cotransporter NBCe1 expressed in Xenopus oocytes. Channels (Austin) 2011;5(2):106–109. doi: 10.4161/chan.5.2.14341. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Ikeda Y, Abe Y, Kuma H, Kang D, Hamasaki N, et al. Structure of the membrane domain of human erythrocyte anion exchanger 1 revealed by electron crystallography. Journal of Molecular Biology. 2010;397(1):179–189. doi: 10.1016/j.jmb.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Yang D, Shcheynikov N, Zeng W, Ohana E, So I, Ando H, et al. IRBIT coordinates epithelial fluid and HCO3 − secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. Journal of Clinical Investigation. 2009;119(1):193–202. doi: 10.1172/JCI36983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HS, Kim E, Lee S, Park HJ, Cooper DS, Rajbhandari I, et al. Mutation of aspartate 555 of the sodium/bicarbonate transporter SLC4A4/NBCe1 induces chloride transport. Journal of Biological Chemistry. 2009;284(23):15970–15979. doi: 10.1074/jbc.M109.001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Kiyatkin A, Bolin JT, Low PS. Crystallographic structure and functional interpretation of the cytoplasmic domain of erythrocyte membrane band 3. Blood. 2000;96(6):2925–2933. [PubMed] [Google Scholar]
- Zhao H, Star RA, Muallem S. Membrane localization of H+ and HCO3 − transporters in the rat pancreatic duct. Journal of General Physiology. 1994;104(1):57–85. doi: 10.1085/jgp.104.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Azimov R, Kao L, Newman D, Liu W, Abuladze N, et al. NBCe1-A transmembrane segment 1 lines the ion translocation pathway. Journal of Biological Chemistry. 2009;284(13):8918–8929. doi: 10.1074/jbc.M806674200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Casey JR. The substrate anion selectivity filter in the human erythrocyte Cl−/HCO3 − exchange protein, AE1. Journal of Biological Chemistry. 2004;279(22):23565–23573. doi: 10.1074/jbc.M401380200. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Kao L, Azimov R, Abuladze N, Newman D, Pushkin A, et al. Structural and functional characterization of the C-terminal transmembrane region of NBCe1-A. Journal of Biological Chemistry. 2010;285(48):37178–37187. doi: 10.1074/jbc.M110.169201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Lee DW, Casey JR. Novel topology in C-terminal region of the human plasma membrane anion exchanger, AE1. Journal of Biological Chemistry. 2003;278(5):3112–3120. doi: 10.1074/jbc.M207797200. [DOI] [PubMed] [Google Scholar]