Abstract
Spinal cord regenerative ability is lost with development, but the mechanisms underlying this loss are still poorly understood. In chick embryos, effective regeneration does not occur after E13, when spinal cord injury induces extensive apoptotic response and tissue damage. As initial experiments showed that treatment with a calcium chelator after spinal cord injury reduced apoptosis and cavitation, we hypothesized that developmentally regulated mediators of calcium-dependent processes in secondary injury response may contribute to loss of regenerative ability. To this purpose we screened for such changes in chick spinal cords at stages of development permissive (E11) and non-permissive (E15) for regeneration. Among the developmentally regulated calcium-dependent proteins identified was PAD3, a member of the peptidylarginine deiminase (PAD) enzyme family that converts protein arginine residues to citrulline, a process known as deimination or citrullination. This post-translational modification has not been previously associated with response to injury. Following injury, PAD3 up-regulation was greater in spinal cords injured at E15 than at E11. Consistent with these differences in gene expression, deimination was more extensive at the non-regenerating stage, E15, both in the gray and white matter. As deimination paralleled the extent of apoptosis, we investigated the effect of blocking PAD activity on cell death and deiminated-histone 3, one of the PAD targets we identified by mass-spectrometry analysis of spinal cord deiminated proteins. Treatment with the PAD inhibitor, Cl-amidine, reduced the abundance of deiminated-histone 3, consistent with inhibition of PAD activity, and significantly reduced apoptosis and tissue loss following injury at E15. Altogether, our findings identify PADs and deimination as developmentally regulated modulators of secondary injury response, and suggest that PADs might be valuable therapeutic targets for spinal cord injury.
Keywords: apoptosis, deimination/citrullination, development, peptidyl arginine deiminase, regeneration, spinal cord
Introduction
In amniotes, such as birds and mammals, the ability to significantly regenerate the central nervous system (CNS) is lost with development. Comparative analysis of injury responses at permissive and non-permissive stages of regeneration can therefore shed light on the cellular and molecular basis underlying such loss. The chick spinal cord provides an excellent model for these comparative studies, given its accessibility in ovo and the fact that at non-regenerating stages of development it appears to respond to traumatic injury in a similar fashion to the human spinal cord, by forming a large fluid-filled cavity. In contrast, the injury site in the mouse spinal cord is filled in with cells and connective tissue (Inman et al., 2002; Zhang et al., 1996).
The chick spinal cord displays remarkable regenerative ability, even at rather advanced stages of development, but this is eventually lost around embryonic day 13 (E13) (Ferretti and Whalley, 2008; Hasan et al., 1991; Shimizu et al., 1990). The ability to regenerate the spinal cord, as well as the loss of it with development, is most likely due to a combination of several factors, including changes in early response to injury, progression of myelination and possibly intrinsic properties of neurons and of neural stem/progenitor cells (Blackmore and Letourneau, 2006; Keirstead et al., 1992; Whalley et al., 2009; Whalley et al., 2006).
Significantly, early response to injury in the chick spinal cord is strikingly different at regeneration permissive and non-permissive stages of development, with the latter showing much more extensive apoptosis and cavitation by 24 hours after spinal cord injury (McBride et al., 2003; Whalley et al., 2006). Changes in calcium homeostasis following neural trauma play a critical role in the progression of secondary injury in mammals, including triggering an apoptotic response (Norberg et al., 2008; Orrenius et al., 2003; Velardo et al., 2000; Wang et al., 1999). Therefore, we wished to assess whether developmentally regulated calcium-dependent processes might also play a role in the changes in secondary injury response, and consequently in the regenerative ability of the chick spinal cord, and used a microarray-based analysis to identify such genes.
Among differentially regulated calcium-dependent proteins, we identified a member of the rather neglected calcium-dependent protein peptidylarginine deiminase (PAD) family. Enzymes of the PAD family, of which 5 members (PAD1–4 and PAD6) are known in mammals and 3 (PAD 1–3) in chick, convert protein arginine residues to citrulline (deimination/citrullination) (Balandraud et al., 2005; Chavanas et al., 2004). It has been suggested that deimination plays a role in a number of diseases including skin diseases, rheumatoid arthritis and allergic encephalomyelitis (Cao et al., 1998; Doyle and Mamula, 2005; Gyorgy et al., 2006; Mastronardi et al., 2006; Nicholas et al., 2005; Raijmakers et al., 2006; Vossenaar et al., 2003; Wood et al., 2008; Ying et al., 2009). Deimination is a post-translational modification that leads to a charge loss that can alter protein conformation and consequently their structure, function and interaction with other proteins. We show here that PAD3 is developmentally regulated in response to injury and that PAD inhibition results in a reduction in apoptosis and cavitation, supporting a role for PAD and deimination in modulating secondary injury response with development following spinal cord damage. This study also points at PADs as potential therapeutic targets.
Materials and Methods
Animals, surgery and pharmacological treatments
Fertilized Brown Leghorn eggs (Needle Farm, Cambridge, UK) were incubated at 37 °C in a humidified forced flow incubator. All procedures were approved under the Animals Scientific Procedures Act 1986. Surgery and tissue collection was carried out as previously reported (Shimizu et al., 1990; Whalley et al., 2006).
Different concentrations of the Ca++ chelator BAPTA-AM solubilized in DMSO or of the PAD inhibitor, Cl-amidine (Knuckley et al., 2008; Luo et al., 2006), solubilized in phosphate buffer saline (PBS), or of the carrier alone were applied in ovo immediately after spinal cord injury at E15 and their effect on survival assessed at 24 hours (Whalley et al., 2006). Selected concentrations were tested for their effect on apoptosis and deimination. The extent of cavity formation was assessed in hematoxylin and eosin stained longitudinal spinal cord sections and apoptosis detected by TUNEL as previously described (Whalley et al., 2006). Briefly, montages of images of longitudinal sections of spinal cord stained with H&E or TUNEL were created at a level close to the center of the injury and the size of visible cavitation. Tissue damage extending cranially and caudally from the site of injury was measured at 24 hours using ImageJ; apoptosis was assessed by scoring the density of apoptotic cells on a scale of 1–4 using the criteria exemplified in Fig. 4F at different distances from the injury site (1, 2, 3, 4 mm). Statistical significance was evaluated by ANOVA and Student’s t-test and p<0.05 was taken to be significant.
Figure 4.
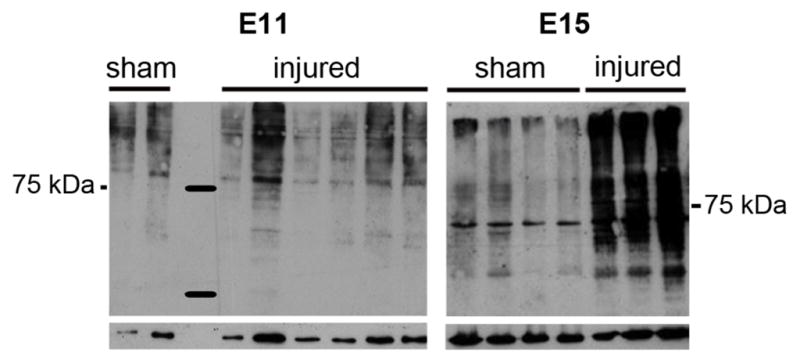
Western blot of proteins from individual E11 and E15 sham-operated and injured spinal cords 2 hours after surgery. Deiminated proteins detected by F95 are shown in the upper panel and the loading protein control in the lower panel.
RNA analysis
Total mRNA was extracted from the cervical peri-injury region or the equivalent region from sham-operated embryos of five individual spinal cords for each treatment group (E11 and E15 24 hours post-surgery) following the protocol of ARK genomics (www.ark-genomics.org/protocols). mRNA quality was checked using Agilent 2100 Bioanalyzer and mRNA expression assessed using chick GeneChip according to manufacturer’s protocol (Affymetrix). RNA (1μg) from individual spinal cords was used to provide independent replicates for each array. GeneSpring software (Agilent Technologies) was used for expression analysis and filtering was performed by Volcano plot (1.5 fold changes, p<0.05) and verified using functional annotation clustering in David 6.7.
PAD3 and Gapdh gene expression was determined by quantitative real time PCR (7500 Fast Real Time PCR System, Applied Biosystems) using the SYBR GreenER reagent kit (Invitrogen)(Gögel et al., 2010). Each sample was run in duplicate. Primers were designed to amplify specific regions to the chick PAD3 flanking region 987–1123: PAD3 chick forward: 5′ CGTGAAGGACAATGAGGACT 3′; PAD3 chick reverse: 5′GCACATAGCCAAACTCCACT3′. All results were normalized with respect to Gapdh expression; GAPDH chick forward 5′CCAGGTTGTCTCCTGTGACT3′; GAPDH chick reverse 5′CACAACACGGTTGCTGTATC3′.
Protein analysis
Immunohistochemical staining and Western blotting were carried out as previously described (Whalley et al., 2009). Sections were imaged either under a Zeiss Axioplan or by confocal laser scanning microscopy (LSM 710, Zeiss, Germany). Complete “z” series optical sections were collected and projected onto a single plane using Zeiss software and ImageJ. Nuclear and cytoplasmic protein fractions were obtained by homogenizing pools of three cervical spinal cords in ice-cold hypotonic buffer (10 mM Hepes, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT) supplemented with complete protease inhibitor cocktail (Sigma). Lysis was achieved using QIAshredder spin columns (QIAGEN) at 500g. Cytoplasmic fractions were stored for further analysis. Nuclear pellets resuspended in 0.5 ml of ice-cold high salt buffer (20 mM Hepes pH 7.9, 25% glycerol, 1.5 mM MgCl2, 0.2 mM EDTA, 350 mM NaCl, 0.5 mM DTT) containing protease inhibitors and TritonX100 were kept on ice for 30 minutes. After centrifugation at 13,000g (30 minutes, 4°C), supernatant containing nuclear proteins were collected. Proteins (2–4 μg/lane) resolved by 12% SDS-PAGE were analyzed by Western blotting.
A pool of 5 spinal cords per treatment group was used for immunoprecipitation of deiminated proteins, using the Catch and Release® v2.0 Kit according to the manufacturer’s instructions (Upstate). An identical amount of proteins (5 μg) from each treatment group pool was immunoprecipitated. Immunoprecipitated protein fractions were analysed by 12% SDS-PAGE and Western blotting. Candidate bands were excised from silver stained gels, and analyzed by liquid chromatography electrospray tandem mass spectrometry (LC-ESI-MS/MS) as previously described (Gögel et al., 2010).
Primary antibodies were: F95 monoclonal mouse IgM antibody (Nicholas and Whitaker, 2002) (2 μg for immunoprecipitation; 1/200 for immunohistochemistry; 1/5000 for Western blotting); rabbit anti-PAD3 (Chemicon, 1:200 for immunohistochemistry; 1:1000 for Western blotting), mouse anti-NeuN (1;50, Millipore); rabbit anti-GFAP (1:1000, Sigma); rat anti-MBP, and rabbit anti-deiminated histone 3 (citH3; 1:100 and 1:250, Abcam). Secondary antibodies were: HRP-conjugated goat anti-mouse IgM (Serotec, USA 1:2000), HRP-conjugated goat anti-rabbit (DAKO, 1:2000); Alexa 568-conjugated goat anti-mouse IgM and Alexa 488-conjugated goat anti-rabbit (1:400; Molecular Probes), Cy5-conjugated anti-rat (Invitrogen, 1:50).
Results
To establish whether inhibition of calcium influx at the time of injury reduces injury response in E15 spinal cords, we administered 25 mg/kg of the calcium chelator BAPTA in ovo either in sham-operated or injured embryos; this dose did not to impair embryonic survival, and slightly increased it (Supplementary Table 1). By 24 hours after injury E15 control embryos displayed an extensive apoptotic response, as indicated by TUNEL, and significant tissue loss was already evident (Fig. 1A). In contrast, in BAPTA-treated embryos little tissue damage was observed and apoptotic cells were restricted to the region close to the injury site (Fig. 1B–C).
Figure 1.
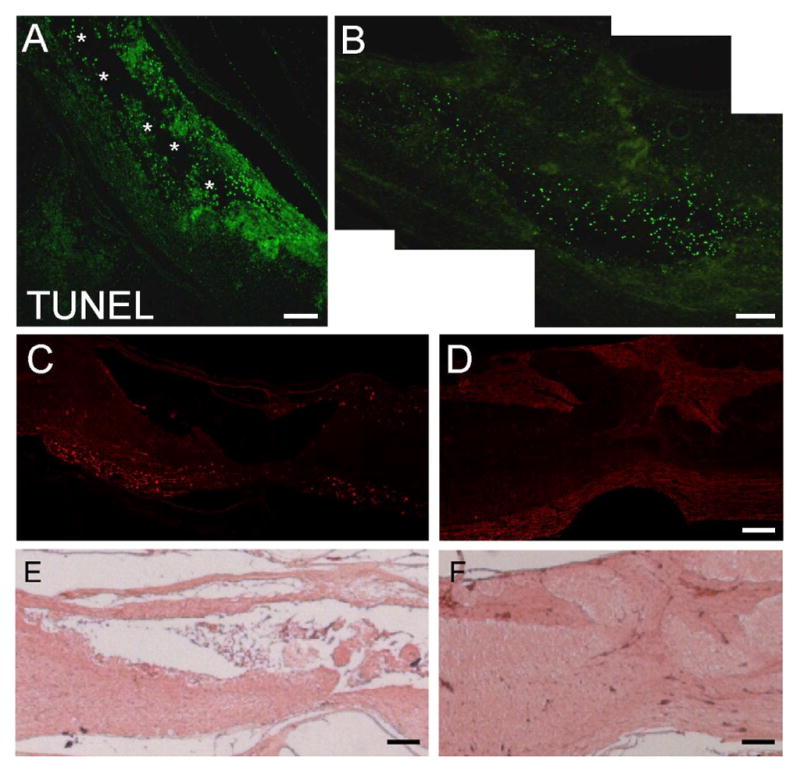
Detection of apoptosis by TUNEL in control and BAPTA-treated E15 spinal cords 24 hours after injury. A) TUNEL staining of control spinal cord; note extensive apoptosis far from the injury site; asterisks indicate the extensive cavity. B) TUNEL staining of spinal cord treated with the Ca++ ionophore BAPTA at the time of injury; note the reduction in apoptosis. C) Phase image of region boxed in (B); note the reduction in cavity formation (asterisks). Scale bars = 130 μm (A), 65 μm (B,C).
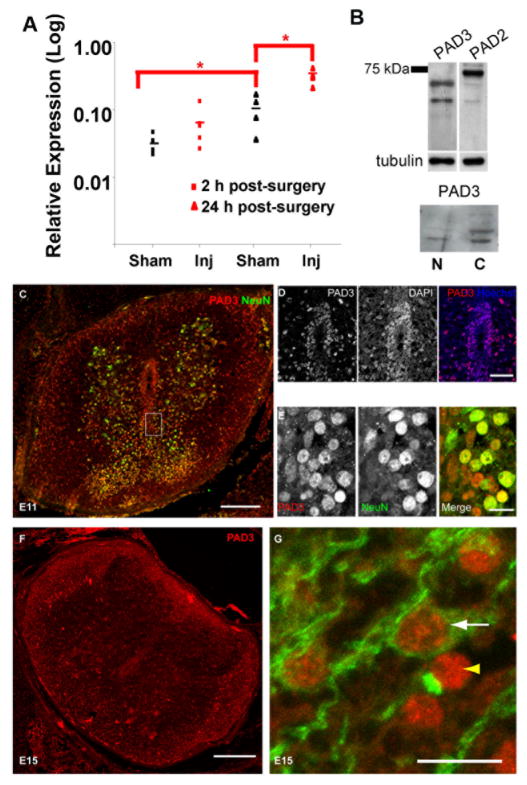
To identify possible modulators of this response, we carried out gene profiling experiments aimed at discovering molecules with calcium-dependent activity differentially regulated in the chick spinal cord in response to injury at permissive (E11) and non-permissive (E15) stages for regeneration (Table 1). Among calcium-dependent molecules, PAD3 was expressed at higher levels at E15 than E11 and also differently up-regulated in response to injury at these developmental stages. Expression of PAD3 transcript in the spinal cord increased six-fold after spinal cord injury at E15, but less than two-fold after injury at E11 as compared to sham-operated controls (Table 1). To confirm that the chick PAD identified in our screen was homologous to mammalian PAD3, we designed primers specific for chick PAD3 and assessed immunoreactivity using an anti-PAD3 antibody. RT-qPCR showed that PAD3 mRNA was indeed expressed in the chick spinal cord, increased significantly from E15 to E16 and was greatly up-regulated after injury at E15 (Fig 2A). As observed in the microarray study, only a small PAD3 mRNA up-regulation was observed in response to injury at E11 by qRT-PCR (not shown). Expression of PAD3 protein was also assessed by Western blotting and immunohistochemistry. The anti-PAD3 antibody reacted with a chick protein of molecular weight consistent with that of human PAD3 (Nachat et al., 2005) and reactivity was detected both in the nucleus and in the cytoplasm, with a main band of approximately 70 kDa being detected (Fig. 2B). PAD3. Consistent with both nuclear and cytoplasmic localization of PAD3 was the identity of proteins immunoprecipitated by the PAD3 antibody identified by mass spectrometry, which included histones and tubulin. Given the high sequence similarity between PADs and the fact that both PAD2 and PAD3 are expressed in the spinal cord, we cannot fully rule out that the antibody we used might to some extent react also with other PADs. However, the difference in staining pattern observed with antibodies to PAD3 and PAD2 (Fig. 2B), with the latter detecting a higher molecular weight band, suggests specificity of the PAD3 staining observed in the chick spinal cord. In spinal cord sections (Fig. 2C–G), PAD3 reactivity in cells surrounding the central canal was mainly nuclear (Fig. 2C–D) and appeared to be stronger at E12 (Fig. 2C) than at E15 (Fig. 2F). At E11, PAD3 was also detected in a subset of neurons, as indicated by co-labelling with the neuronal marker NeuN, and in the white matter (Fig. 2C, E). At E15 PAD staining was still observed in the gray matter and had increased in the white matter (Fig. 2F). Double-staining for MBP and PAD3 showed PAD3 expression in developing oligodendrocytes, but some MBP-negative/PAD-positive cells, presumably astrocytes, were also observed in the white matter (Fig. 2G).
Table 1.
Calcium-dependent molecules differentially regulated following spinal cord injury with development
| mRNA Fold changes | |||
|---|---|---|---|
|
| |||
| Gene (GenBank) | E11 inj/sh | E15 inj/sh | E15/E11 sh |
| Peptidyl arginine deiminase, type III (NM_205043) | 1.8 | 6.0 | 2.1 |
| S100 calcium binding protein A6 (calcyclin) (NM_204148) | 4.4 | 22 | nc |
| S100 calcium binding protein A9 (calgranulin B) (X61200) | nc | 4.1 | 2.4 |
| S100 calcium binding protein A11 (calgizzarin) (NM_205166) | nc | 2.5 | nc |
| Cellular ligand of annexin 2(p11) (NM_205506) | 1.7 | 4.3 | nc |
| Annexin A1 (NM_206906) | 14.7 | 3.5 | nc |
| Annexin A11 (CR390633) | nc | 1.7 | nc |
| Myosin light chain 2 (LC2f) (M11030) | nc | 3.7 | 3 |
| Myosin IF (MYO1F; CBBMIB) (NM_205254) | 1.7 | 3.1 | 1.8 |
| Myosin Ie (Myosin heavy chain myr 3) (ENSGALT00000006599) | nc | 2.1 | nc |
| Myosin, light polypeptide kinase (MYLK) (M96655) | nc | 1.5 | nc |
| Phospholipase A2, group IVA (cytosolic, calcium-dependent) (NM_205423) | nc | 1.9 | nc |
| Janus kinase 1 (JAK1) (NM_204870) | nc | 1.6 | nc |
| Plastin 3 (T isoform) (PLS3; fimbrin) (AJ720945) | nc | 2.1 | nc |
| Similar to calcium-regulated heat-stable protein (24kD); calcineurin substrate CRHSP-24 (ENSGALT00000011834) | nc | 2.2 | nc |
| Calbindin 1, 28kDa (NM_205513) | nc | 0.4 | nc |
| Kv channel interacting protein 4 (KCNIP4) (NM_204555) | nc | 0.6 | 0.7 |
| Follistatin-like 4 (FSTL4) (NM_204502) | nc | 0.6 | nc |
Figure 2.
PAD3 is up-regulated with development and following injury at E15. A) PAD mRNA detected by qPCR shows almost 10 fold increase 24 hours after injury at E15; note also increased PAD3 in sham-operated spinal cords between E15 and E16. *p<0.05. B) Western blot with an anti-PAD3 antibody of total proteins from E11 spinal cords and reactivity of anti-PAD3 and anti-PAD2 antibodies in nuclear (N) and cytoplasmic (C) fractions. C) E12 spinal cord double-labelled for PAD3 (red) and NeuN (green). D) Central canal region in an E12 spinal cord stained for PAD3 (red) and counterstained with the nuclear stain Hoechst dye (blue); note the largely nuclear localization of PAD3. E) High magnification of the gray matter (region boxed in C) showing some neurones double-labelled for PAD3 (red) and NeuN (green). F) PAD3 expression in E15 spinal cord; note PAD3 expression both in gray and white matter. G) Cells double-labelled for the oligodendrocyte marker MBP (green) and PAD3 (red) are present in the white matter of E16 spinal cords (white arrow); PAD3-positive/MBP-negative cells are also observed (yellow arrowhead). Scale bars = 200 μm (C,F); 40 μm (D); 10 μm (E, G).
To determine whether elevated PAD abundance resulted in increased deimination activity, we used an antibody, F95, that specifically recognizes the citrulline in proteins (Nicholas et al., 2003). No F95 reactivity was detectable in sham-operated spinal cords either at E11 or E15 (Supplementary Fig. 1A–B). Following injury at E11 some F95 reactivity was detectable in the peri-injury region by 24 hours (Fig. 3A–B). In contrast, extensive deimination was observed as early as 2 hours after injury at E15 (Fig. 3C, D, D′). Consistent with immunohistochemical staining, Western blotting showed that protein deimination was greatly increased in protein extracts from individual sham-operated and injured E15 spinal cords, but not following injury at E11 (Fig. 4).
Figure 3.
Detection of deiminated proteins with F95 antibody in injured E11 and E15 spinal cords. F95 is red and nuclear counterstain is blue. A) Hematoxylin and eosin (H&E) staining of E11 spinal cord 24 hours after injury; arrow: site of injury; arrowheads: regions of noticeable haemorrhage. B) F95 staining (section from the same spinal cord shown in (A; region boxed)). Note limited deimination; some autofluorescence is observed in regions of haemorrhage (*). C) H&E staining of E15 spinal cord two hours after injury. D) F95 staining (section from the same spinal cord shown in (C); extensive deimination is already apparent at 2 hours close to the injury site as more easily observed at high magnification (D′). Scale bars = 50 μm (A), 260 μm (B,C,D); 65 μm (D′).
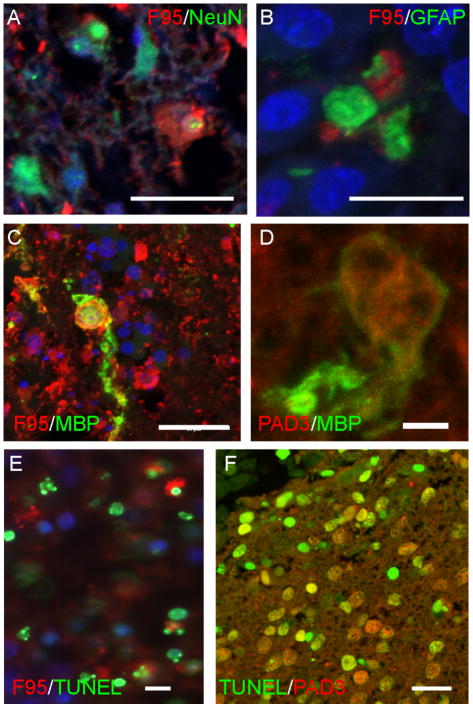
Deimination occurred both in the injured grey and white matter 24 hours after injury, as shown by double-staining with F95 and neural markers (Fig. 5A–C). Notwithstanding the extensive tissue damage and the rapid loss of NeuN reactivity observed following injury (not shown) that could make precise co-localization difficult to assess, some cells double-stained for F95 and neuronal (NeuN) or glia (GFAP, MBP) markers were detected, suggesting that deimination was activated in both lineages, consistent with PAD3 expression (Fig. 2C, E, G, 5D).
Figure 5.
Detection of deiminated proteins and PAD3 in injured E11 and E15 spinal cords. F95 is red and nuclear counterstain is blue A–C) Double-labelling for F95 (red) and NeuN (A), GFAP (B) and MBP (C) (all green) in E15 spinal cords 24 hours after injury. D) Double-labelling for PAD3 (red) and MBP (green) in E15 spinal cords 24 hours after injury. E) Double-labelling for F95 (red) and TUNEL (green) in E11 spinal cord 24 hors post-injury. F) Double-labelling for PAD3 (red) and TUNEL (green) in E15 24 hours post-injury. Scale bars = 20 μm (A, C, E,F); 10 μm (D).
The more extensive deimination observed after injury at E15 than at E11 paralleled the previously reported differences in apoptosis at these developmental stages. Therefore, injured spinal cord sections were double-labeled for deimination and TUNEL at different times after injury. Some double-labeled cells were indeed observed in injured spinal cords at 8 hours (Supplementary Fig. 2A) and at 24 hours after surgery both at E11 and E15 (Fig. 5E, Fig. 6). By 4 days after spinal cord injury at E15, both apoptosis and F95 reactivity was greatly reduced, but some hot spots of F95 reactivity were still observed in clumps of tissue debris sometimes found within cavities, and some double labeled-cells were still observed (Supplementary Fig. 2B, C–C″). Consistent with a role in apoptosis, PAD3 expression could be detected in several TUNEL-positive cells 24 hours after injury, though it was undetectable in very intensely labeled apoptotic cells, probably due to its degradation at the late apoptotic stages (Fig. 5F).
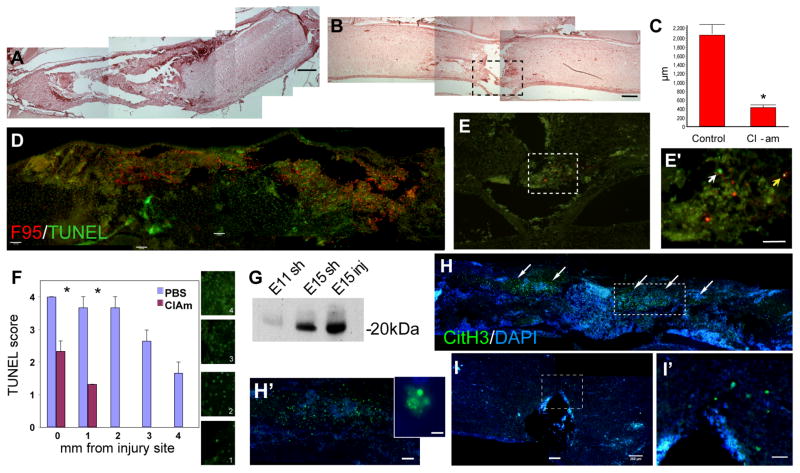
Figure 6.
Effect of Cl-amidine treatment on E15 chick spinal cords 24 hours after injury assessed by hematoxylin and eosin staining (A–B), co-labelling for deiminated proteins with the F95 antibody and for apoptosis by TUNEL (D–E), and staining for deiminated H3 (H–I′). A) Control spinal cord injury; note large cavity. B) Injured spinal cord treated with 80 mg/Kg Cl-amidine; note the reduction in cavity size. C) Quantitative analysis of cavity size (n=6; m±sd); note significant reduction (ANOVA, p≤0.001) following Cl-amidine treatment. D) F95 (red) and TUNEL (green) labelling of injured control spinal cord; note extensive deimination and apoptosis. E) F95 (red) and TUNEL (green) labelling of injured Cl-amidine-treated spinal cord. E′) Higher magnification of the dotted box in (E); note the reduction in both deimination and apoptosis in the treated spinal cord; the white arrowhead points to an apoptotic cell and the yellow arrow to a cell double-labelled for TUNEL and F95. F) Density of TUNEL-positive cells in control and Cl-amidine-treated animals scored at different distances from the injury (n=4; m±sd). The boxes indicate the scoring criteria used to evaluate the extent of apoptosis from very low/negative (1) to high (4). Note significant reduction (* p≤0.04; Student’s t-test) following Cl-amidine treatment close to the injury side and absence of TUNEL-positive cells from 2 mm from the injury site in Cl-amidine-treated spinal cords. G) Western blot detecting histone 3 (H3) in immunoprecipitated deiminated protein fractions from 1) E11 sham-operated, 2) E15 sham-operated and E15 injured spinal cords. H) Control injured spinal cord; deiminated H3 (CitH3, green) is detected in the injured region in E15 spinal cords (arrows) and commonly associated with nuclei of apoptotic morphology (see insert). I–I′) Cl-amidine-treated injured spinal cord; note greatly decreased expression of CitH3 around the injury site (box enlarged in I′) compared to (H–H′). Scale bars = 260 μm (A,B,D,H,I); 130 μm (E); 65 μm (E′, I′), 20 μm (insert in H).
In E15 injured spinal cords, increased deimination could already be detected at 2 hours after injury, before apoptotic cells were detectable by TUNEL, and was hardly visible in BAPTA-treated embryos (not shown), consistent with deimination being a calcium-dependent process. Therefore, we tested the hypothesis that PAD may play a role in early injury response by treating E15 spinal cords with the PAD inhibitor, Cl-amidine (80mg/kg; Supplementary Table 1). Treatment was performed immediately after injury and changes at the morphological level and in apoptosis were assessed 24 hours later (Fig. 6). All embryos treated with Cl-amidine displayed a significant reduction in cavity size at 24 hours after injury (Fig 6A–C). Apoptosis was also significantly reduced in Cl-amidine treated embryos (Fig. 6D–F), as observed following treatment with BAPTA (Fig. 1).
We also examined the effect of Cl-amidine treatment on abundance of deiminated histone 3 (CitH3), that was identified as a PAD target by mass-spectrometry analysis of deiminated proteins immunoprecipitated by F95 (Fig. 6G) and also by the anti-PAD3 antibody (not shown). Immunostaining for CitH3, was greatly reduced in Cl-amidine-treated spinal cords as compared to untreated controls (Fig. 6H–I).
Discussion
This study has identified PAD3 as a developmentally-regulated enzyme expressed both in progenitors and mature neural cells that appears to be an important player in the early spinal cord injury response and in modulating its severity.
Secondary injury in non-regenerating chick spinal cord is reduced by decreasing intracellular Ca++
Reduction in the secondary injury response is a first crucial step towards improving repair within a damaged central nervous system. It is well established that initial necrotic cell death induced by mechanical injury is followed by more extensive tissue loss due to a secondary injury response involving apoptotic neural cell death, both in mammals and birds, and that its extent is related to functional recovery (Basso et al., 1996; Beattie et al., 2000; Liu et al., 1997; McBride et al., 2003; Whalley et al., 2006). The elevation in intracellular free calcium levels following neural injury is known to play a key role in this response in non-regenerating mammalian models. We have now shown this to also be the case in the E15 injured chick spinal cord, as indicated by the ability of the Ca++ chelator BAPTA to decrease apoptotic response and cavity size to an extent comparable to that observed at stages of development permissive for regeneration.
Ca++-dependent molecules are differently up-regulated following neural injury at permissive and non-permissive stages for regeneration
Ca++ influx can affect several apoptosis associated molecules. With the exception of calbidin, that was down-regulated following injury at E15, the Ca++-dependent transcripts differently expressed in response to injury were up-regulated. Among them, only annexin 1 displayed greater up-regulation after injury at E11 than at E15. The precise role of annexins in neural injury remains to be elucidated (Liu et al., 2004), but given suggestions that annexin 1 plays a neuroprotective role following cerebral ischaemia (Gavins et al., 2007; Solito et al., 2008), its up-regulation in injured E11 spinal cord may contribute to the high regenerative ability at this stage of development. Significant up-regulation of members of the S100 family was observed in E15 injured spinal cords. The greater up-regulation at E15 of calcyclin (S100A6), that has been associated with astrogliosis (Lesniak et al., 2009; Yachnis et al., 1993; Yamashita et al., 1999), may be due to the increase in astrocyte number with development and consequently greater astrogliotic response following injury. Altogether, the changes in the expression of Ca++-dependent molecules revealed by our screen reflect the changes in apoptosis and the degree of spinal cord damage occurring at the two developmental stages studied, strengthening the view that early response to injury is developmentally regulated and plays a crucial role in neural repair.
PAD and deimination modulate secondary injury response
PAD activity, resulting in protein deimination, has been recently suggested to play a role in a number of autoimmune diseases, including multiple sclerosis, indicating a role for members of this family in the central nervous system (Chavanas et al., 2004; Doyle and Mamula, 2005; Gyorgy et al., 2006; Suzuki et al., 2007). All members of the PAD family are expressed to some extent in the mammalian nervous system, with the exception of PAD6 (also known as ePAD), though PAD2 is considered to be the main brain PAD (Esposito et al., 2007; Keilhoff et al., 2008; Shimada et al., 2009; Vossenaar et al., 2003). PAD4, like PAD2, has been detected in myelin, and it has also been shown to translocate to the nucleus where it can deiminate histones, while PAD3 expression was found in Schwann cells and nuclei of some cultured cerebellar neurons (Keilhoff et al., 2008; Mastronardi and Moscarello, 2005; Mastronardi et al., 2006; Wood et al., 2008). Only 3 PAD genes, the homologues of mammalian PAD1, 2 and 3, appear to be present in the chick (Balandraud et al., 2005; Chavanas et al., 2004; Vossenaar et al., 2003). Chick PAD3 protein shares a similar degree of amino acid identity with human PAD3 and PAD4, 57% and 55%, respectively. We have shown here that chick PAD3 is expressed in the spinal cord, where it can be found both in both nucleus and cytoplasm, and its expression increases between the regeneration-permissive and non-permissive stages of development examined. In addition, like its mammalian counterpart (Chavanas et al., 2006), PAD3 is strongly expressed in the skin (not shown). Nuclear localization of PAD3 suggests similarities with PAD4, which has been shown to deiminate histones (Cuthbert et al., 2004; Wang et al., 2009; Wang et al., 2004). Therefore, chick PAD3 expression appears to encompass some features of both mammalian PAD3 and PAD4.
In the context of spinal cord repair in the chick, PAD3 mRNA is up-regulated to a greater extent in response to injury at the non-permissive stage for regeneration, and this is mirrored by increased enzyme activity, as indicated by the extensive deimination observed after injury at E15. Altogether, the regenerative ability of the spinal cord appears to be associated with low levels and a later onset of deimination, since in E11 injured cords, unlike in E15, deimination is not detectable at two hours and is relatively low at 24 hours. Additional evidence for an early role of protein deimination in response to injury comes from analysis of deimination in relation to the apoptotic response previously reported in non-regenerating spinal cords (McBride et al., 2003; Whalley et al., 2006). Apoptotic cells identified by TUNEL are not yet detectable six hours after injury at E15, but the injured region contains many TUNEL–positive cells by eight hours (Whalley et al., 2006), while cells labeled for deimination are detected several hours earlier. Although TUNEL is a relatively late marker of apoptosis, these results suggest that up-regulation of deimination represent an early response to spinal cord damage. This is also consistent with in vitro experiments in which increase in intracellular Ca++ following thapsigargin treatment of cultured neural cells was found to induce a rapid increase in protein deimination (not shown). Conversely, when intracellular Ca++ is decreased by BAPTA in injured E15 spinal cords, neural damage is reduced, as reported in other injury models (Tymianski et al., 1993). Significantly, the reduction in secondary injury response in spinal cords treated with a PAD inhibitor is comparable to that observed following BAPTA treatment. Impairment of Ca++ homeostasis following injury is known to affect several intracellular processes, including release of free radicals from mitochondria, leading to activation of a number of independent death programmes. It is therefore remarkable that PAD inhibition is able to counteract much of this response. Altogether, this suggests that PAD3 is an important mediator of Ca++-dependent damage both in neurons and oligodendrocytes, where PAD3 is detected. The large population of mature oligodendrocytes and myelination present at E15 but not at E11 is likely to contribute to the extensive tissue loss observed in E15 injured spinal cords (McBride et al., 2003). This together with the fact that myelin-associated proteins are known PAD targets (Harauz and Musse, 2007) suggests that oligodendrocyte sparing following PAD inhibition may greatly contribute to reduced cavity size. The possible involvement of deimination in astrocytic response is currently not clear, as poor staining in injured spinal cord sections due to the extensive tissue damage and the lack of suitable antibodies to double-stain for GFAP and PAD3 did not allow extensive analysis of this cell population.
We cannot rule out at this stage that other PADs may also play a role in the calcium-dependent secondary injury response, as the inhibitor used is not highly selective for PAD3. However, the differential up-regulation of PAD3 in regenerating and non-regenerating spinal cords is consistent with a crucial role for this PAD in the early response to injury at least in the chick. While PADs have been implicated in a number of neurodegenerative diseases, this to our knowledge is the first evidence that they can be key mediators of secondary injury response.
Given the limited repair ability of the CNS, the prospect of administering pharmacologic agents shortly after injury to reduce cellular and functional loss is very attractive. Several cellular processes are finely tuned by Ca++ levels, and disruption of Ca++ homeostasis occurs within minutes after injury contributing to secondary injury response, for example by activating apoptotic pathways and impairing mitochondrial function. In a clinical context, modulation of Ca++-dependent effectors of neural damage that display prolonged activity following spinal cord injury can represent a more viable and effective alternative than modulation of Ca++ levels, that has not proven very successful in clinical trials (Horn and Limburg, 2001; Szydlowska and Tymianski, 2010). Indeed, both PAD mRNA levels and PAD activity are still much higher in injured E15 spinal cords than in normal tissue several hours after injury, when apoptosis is still occurring. This suggests that there may be a window for intervention for targeting PAD following spinal cord injury and this will require in depth evaluation.
Analysis of PAD targets can both provide valuable information on mechanisms underlying changes in injury response with development and help to identify additional potential targets for intervention. Histone modifications are important modulators of several key processes including cell death and survival. Significantly, PADs have been recently proposed as regulators of other histone modifications (Cuthbert et al., 2004; Denis et al., 2009; Li et al., 2010; Wang et al., 2004). The fact that H3 is deiminated in response to spinal cord injury and that reduction in H3 deimination following Cl-amidine treatment parallels reduction in apoptosis suggests a possible epigenetic role for this histone post-translational modification in early injury response that will deserve further investigation.
Conclusions
Developmentally regulated mediators of calcium-dependent processes in secondary injury response may contribute to loss of regenerative ability. In particular, this study has identified PAD3 as one of these mediators in the developing spinal cord and highlighted the importance of deimination in spinal cord injury response. Indeed, post-translational modifications are emerging as key players in the regulation of many cellular processes and we propose a role for deimination in modulating the extent of injury-induced neural tissue damage with development. Identification of this novel role for PAD activity in spinal cord injury, together with the possibility of modulating it pharmacologically with a compound that appears to display little toxicity in vivo (unlike calcium chelators), may open new avenues to reducing tissue damage in injured spinal cords.
Supplementary Material
Acknowledgments
This work was supported by the BBSRC.
References
- Balandraud N, Gouret P, Danchin EG, Blanc M, Zinn D, Roudier J, Pontarotti P. A rigorous method for multigenic families’ functional annotation: the peptidyl arginine deiminase (PADs) proteins family example. BMC Genomics. 2005;6:153. doi: 10.1186/1471-2164-6-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–56. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Farooqui AA, Bresnahan JC. Review of current evidence for apoptosis after spinal cord injury. J Neurotrauma. 2000;17:915–25. doi: 10.1089/neu.2000.17.915. [DOI] [PubMed] [Google Scholar]
- Blackmore M, Letourneau PC. Changes within maturing neurons limit axonal regeneration in the developing spinal cord. J Neurobiol. 2006;66:348–60. doi: 10.1002/neu.20224. [DOI] [PubMed] [Google Scholar]
- Cao L, Sun D, Whitaker JN. Citrullinated myelin basic protein induces experimental autoimmune encephalomyelitis in Lewis rats through a diverse T cell repertoire. J Neuroimmunol. 1998;88:21–9. doi: 10.1016/s0165-5728(98)00063-0. [DOI] [PubMed] [Google Scholar]
- Chavanas S, Mechin MC, Nachat R, Adoue V, Coudane F, Serre G, Simon M. Peptidylarginine deiminases and deimination in biology and pathology: relevance to skin homeostasis. J Dermatol Sci. 2006;44:63–72. doi: 10.1016/j.jdermsci.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Chavanas S, Mechin MC, Takahara H, Kawada A, Nachat R, Serre G, Simon M. Comparative analysis of the mouse and human peptidylarginine deiminase gene clusters reveals highly conserved non-coding segments and a new human gene, PADI6. Gene. 2004;330:19–27. doi: 10.1016/j.gene.2003.12.038. [DOI] [PubMed] [Google Scholar]
- Cuthbert GL, Daujat S, Snowden AW, Erdjument-Bromage H, Hagiwara T, Yamada M, Schneider R, Gregory PD, Tempst P, Bannister AJ, Kouzarides T. Histone deimination antagonizes arginine methylation. Cell. 2004;118:545–53. doi: 10.1016/j.cell.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Denis H, Deplus R, Putmans P, Yamada M, Metivier R, Fuks F. Functional connection between deimination and deacetylation of histones. Mol Cell Biol. 2009;29:4982–93. doi: 10.1128/MCB.00285-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle HA, Mamula MJ. Posttranslational modifications of self-antigens. Ann N Y Acad Sci. 2005;1050:1–9. doi: 10.1196/annals.1313.001. [DOI] [PubMed] [Google Scholar]
- Esposito G, Vitale AM, Leijten FP, Strik AM, Koonen-Reemst AM, Yurttas P, Robben TJ, Coonrod S, Gossen JA. Peptidylarginine deiminase (PAD) 6 is essential for oocyte cytoskeletal sheet formation and female fertility. Mol Cell Endocrinol. 2007;273:25–31. doi: 10.1016/j.mce.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Ferretti P, Whalley K. Molecular and Cellular Basis of Regeneration and Tissue Repair: Successful neural regeneration in amniotes: the developing chick spinal cord. Cell Mol Life Sci. 2008;65:45–53. doi: 10.1007/s00018-007-7430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavins FN, Dalli J, Flower RJ, Granger DN, Perretti M. Activation of the annexin 1 counter-regulatory circuit affords protection in the mouse brain microcirculation. Faseb J. 2007;21:1751–8. doi: 10.1096/fj.06-7842com. [DOI] [PubMed] [Google Scholar]
- Gögel S, Lange S, Leung KY, Greene NDE, Ferretti P. Post-translational regulation of Crmp in developing and regenerating chick spinal cord. Dev Neurobiol. 2010 doi: 10.1002/dneu.20789. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Gyorgy B, Toth E, Tarcsa E, Falus A, Buzas EI. Citrullination: a posttranslational modification in health and disease. Int J Biochem Cell Biol. 2006;38:1662–77. doi: 10.1016/j.biocel.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Harauz G, Musse AA. A tale of two citrullines--structural and functional aspects of myelin basic protein deimination in health and disease. Neurochem Res. 2007;32:137–58. doi: 10.1007/s11064-006-9108-9. [DOI] [PubMed] [Google Scholar]
- Hasan SJ, Nelson BH, Valenzuela JI, Keirstead HS, Shull SE, Ethell DW, Steeves JD. Functional repair of transected spinal cord in embryonic chick. Restorative Neurology And Neuroscience. 1991;2:137–154. doi: 10.3233/RNN-1991-2303. [DOI] [PubMed] [Google Scholar]
- Horn J, Limburg M. Calcium antagonists for ischemic stroke: a systematic review. Stroke. 2001;32:570–6. doi: 10.1161/01.str.32.2.570. [DOI] [PubMed] [Google Scholar]
- Inman D, Guth L, Steward O. Genetic influences on secondary degeneration and wound healing following spinal cord injury in various strains of mice. J Comp Neurol. 2002;451:225–35. doi: 10.1002/cne.10340. [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Prell T, Langnaese K, Mawrin C, Simon M, Fansa H, Nicholas AP. Expression pattern of peptidylarginine deiminase in rat and human Schwann cells. Dev Neurobiol. 2008;68:101–14. doi: 10.1002/dneu.20578. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Hasan SJ, Muir GD, Steeves JD. Suppression of the onset of myelination extends the permissive period for the functional repair of embryonic spinal cord. Proceedings Of The National Academy Of Sciences Of The United States Of America. 1992;89:11664–11668. doi: 10.1073/pnas.89.24.11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuckley B, Luo Y, Thompson PR. Profiling Protein Arginine Deiminase 4 (PAD4): a novel screen to identify PAD4 inhibitors. Bioorg Med Chem. 2008;16:739–45. doi: 10.1016/j.bmc.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniak W, Slomnicki LP, Filipek A. S100A6 - new facts and features. Biochem Biophys Res Commun. 2009;390:1087–92. doi: 10.1016/j.bbrc.2009.10.150. [DOI] [PubMed] [Google Scholar]
- Li P, Wang D, Yao H, Doret P, Hao G, Shen Q, Qiu H, Zhang X, Wang Y, Chen G, Wang Y. Coordination of PAD4 and HDAC2 in the regulation of p53-target gene expression. Oncogene. 2010;29:3153–62. doi: 10.1038/onc.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Han S, Lu PH, Xu XM. Upregulation of annexins I, II, and V after traumatic spinal cord injury in adult rats. J Neurosci Res. 2004;77:391–401. doi: 10.1002/jnr.20167. [DOI] [PubMed] [Google Scholar]
- Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, Hsu CY, Choi DW. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17:5395–406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Knuckley B, Lee YH, Stallcup MR, Thompson PR. A fluoroacetamidine-based inactivator of protein arginine deiminase 4: design, synthesis, and in vitro and in vivo evaluation. J Am Chem Soc. 2006;128:1092–3. doi: 10.1021/ja0576233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronardi FG, Moscarello MA. Molecules affecting myelin stability: a novel hypothesis regarding the pathogenesis of multiple sclerosis. J Neurosci Res. 2005;80:301–8. doi: 10.1002/jnr.20420. [DOI] [PubMed] [Google Scholar]
- Mastronardi FG, Wood DD, Mei J, Raijmakers R, Tseveleki V, Dosch HM, Probert L, Casaccia-Bonnefil P, Moscarello MA. Increased citrullination of histone H3 in multiple sclerosis brain and animal models of demyelination: a role for tumor necrosis factor-induced peptidylarginine deiminase 4 translocation. J Neurosci. 2006;26:11387–96. doi: 10.1523/JNEUROSCI.3349-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride CB, McPhail LT, Vanderluit JL, Tetzlaff W, Steeves JD. Caspase inhibition attenuates transection-induced oligodendrocyte apoptosis in the developing chick spinal cord. Mol Cell Neurosci. 2003;23:383–97. doi: 10.1016/s1044-7431(03)00063-0. [DOI] [PubMed] [Google Scholar]
- Nachat R, Mechin MC, Takahara H, Chavanas S, Charveron M, Serre G, Simon M. Peptidylarginine deiminase isoforms 1–3 are expressed in the epidermis and involved in the deimination of K1 and filaggrin. J Invest Dermatol. 2005;124:384–93. doi: 10.1111/j.0022-202X.2004.23568.x. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, King JL, Sambandam T, Echols JD, Gupta KB, McInnis C, Whitaker JN. Immunohistochemical localization of citrullinated proteins in adult rat brain. J Comp Neurol. 2003;459:251–66. doi: 10.1002/cne.10607. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Sambandam T, Echols JD, Barnum SR. Expression of citrullinated proteins in murine experimental autoimmune encephalomyelitis. J Comp Neurol. 2005;486:254–66. doi: 10.1002/cne.20527. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Whitaker JN. Preparation of a monoclonal antibody to citrullinated epitopes: its characterization and some applications to immunohistochemistry in human brain. Glia. 2002;37:328–36. [PubMed] [Google Scholar]
- Norberg E, Gogvadze V, Ott M, Horn M, Uhlen P, Orrenius S, Zhivotovsky B. An increase in intracellular Ca2+ is required for the activation of mitochondrial calpain to release AIF during cell death. Cell Death Differ. 2008;15:1857–64. doi: 10.1038/cdd.2008.123. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–65. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- Raijmakers R, Vogelzangs J, Raats J, Panzenbeck M, Corby M, Jiang H, Thibodeau M, Haynes N, van Venrooij WJ, Pruijn GJ, Werneburg B. Experimental autoimmune encephalomyelitis induction in peptidylarginine deiminase 2 knockout mice. J Comp Neurol. 2006;498:217–26. doi: 10.1002/cne.21055. [DOI] [PubMed] [Google Scholar]
- Shimada N, Handa S, Uchida Y, Fukuda M, Maruyama N, Asaga H, Choi EK, Lee J, Ishigami A. Developmental and age-related changes of peptidylarginine deiminase 2 in the mouse brain. J Neurosci Res. 2009 doi: 10.1002/jnr.22255. [DOI] [PubMed] [Google Scholar]
- Shimizu I, Oppenheim RW, Obrien M, Shneiderman A. Anatomical and functional recovery following spinal cord transection in the chick embryo. Journal of Neurobiology. 1990;21:918–937. doi: 10.1002/neu.480210609. [DOI] [PubMed] [Google Scholar]
- Solito E, McArthur S, Christian H, Gavins F, Buckingham JC, Gillies GE. Annexin A1 in the brain--undiscovered roles? Trends Pharmacol Sci. 2008;29:135–42. doi: 10.1016/j.tips.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Yamada R, Yamamoto K. Citrullination by peptidylarginine deiminase in rheumatoid arthritis. Ann N Y Acad Sci. 2007;1108:323–39. doi: 10.1196/annals.1422.034. [DOI] [PubMed] [Google Scholar]
- Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47:122–9. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Tymianski M, Wallace MC, Spigelman I, Uno M, Carlen PL, Tator CH, Charlton MP. Cell-permeant Ca2+ chelators reduce early excitotoxic and ischemic neuronal injury in vitro and in vivo. Neuron. 1993;11:221–35. doi: 10.1016/0896-6273(93)90180-y. [DOI] [PubMed] [Google Scholar]
- Velardo MJ, Reier PJ, Anderson DK. Spinal cord injury. In: Crockard A, et al., editors. Neurosurgery. The scientific basis of clinical practice. Vol. 1. Blackwell Scientific; Oxford: 2000. pp. 499–515. [Google Scholar]
- Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays. 2003;25:1106–18. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–43. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, Allis CD, Coonrod SA. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–13. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wysocka J, Sayegh J, Lee YH, Perlin JR, Leonelli L, Sonbuchner LS, McDonald CH, Cook RG, Dou Y, Roeder RG, Clarke S, Stallcup MR, Allis CD, Coonrod SA. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004;306:279–83. doi: 10.1126/science.1101400. [DOI] [PubMed] [Google Scholar]
- Whalley K, Gogel S, Lange S, Ferretti P. Changes in progenitor populations and ongoing neurogenesis in the regenerating chick spinal cord. Dev Biol. 2009;332:234–245. doi: 10.1016/j.ydbio.2009.05.569. [DOI] [PubMed] [Google Scholar]
- Whalley K, O’Neill P, Ferretti P. Changes in response to spinal cord injury with development: vascularization, haemorrhage and apoptosis. Neuroscience. 2006;137:821–832. doi: 10.1016/j.neuroscience.2005.07.064. [DOI] [PubMed] [Google Scholar]
- Wood DD, Ackerley CA, Brand B, Zhang L, Raijmakers R, Mastronardi FG, Moscarello MA. Myelin localization of peptidylarginine deiminases 2 and 4: comparison of PAD2 and PAD4 activities. Lab Invest. 2008;88:354–64. doi: 10.1038/labinvest.3700748. [DOI] [PubMed] [Google Scholar]
- Yachnis AT, Rorke LB, Lee VM, Trojanowski JQ. Expression of neuronal and glial polypeptides during histogenesis of the human cerebellar cortex including observations on the dentate nucleus. J Comp Neurol. 1993;334:356–69. doi: 10.1002/cne.903340303. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Ilg EC, Schafer BW, Heizmann CW, Kosaka T. Distribution of a specific calcium-binding protein of the S100 protein family, S100A6 (calcyclin), in subpopulations of neurons and glial cells of the adult rat nervous system. J Comp Neurol. 1999;404:235–57. [PubMed] [Google Scholar]
- Ying S, Dong S, Kawada A, Kojima T, Chavanas S, Mechin MC, Adoue V, Serre G, Simon M, Takahara H. Transcriptional regulation of peptidylarginine deiminase expression in human keratinocytes. J Dermatol Sci. 2009;53:2–9. doi: 10.1016/j.jdermsci.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Fujiki M, Guth L, Steward O. Genetic influences on cellular reactions to spinal cord injury: a wound-healing response present in normal mice is impaired in mice carrying a mutation (WldS) that causes delayed Wallerian degeneration. J Comp Neurol. 1996;371:485–95. doi: 10.1002/(SICI)1096-9861(19960729)371:3<485::AID-CNE10>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.