Abstract
Cation-coupled HCO3− transport was initially identified in the mid-1970s when pioneering studies showed that acid extrusion from cells is stimulated by CO2/HCO3− and associated with Na+ and Cl− movement. The first Na+-coupled bicarbonate transporter (NCBT) was expression-cloned in the late 1990s. There are currently five mammalian NCBTs in the SLC4-family: the electrogenic Na,HCO3-cotransporters NBCe1 and NBCe2 (SLC4A4 and SLC4A5 gene products); the electroneutral Na,HCO3-cotransporter NBCn1 (SLC4A7 gene product); the Na+-driven Cl,HCO3-exchanger NDCBE (SLC4A8 gene product); and NBCn2/NCBE (SLC4A10 gene product), which has been characterized as an electroneutral Na,HCO3-cotransporter or a Na+-driven Cl,HCO3-exchanger. Despite the similarity in amino acid sequence and predicted structure among the NCBTs of the SLC4-family, they exhibit distinct differences in ion dependency, transport function, pharmacological properties, and interactions with other proteins. In epithelia, NCBTs are involved in transcellular movement of acid-base equivalents and intracellular pH control. In nonepithelial tissues, NCBTs contribute to intracellular pH regulation; and hence, they are crucial for diverse tissue functions including neuronal discharge, sensory neuron development, performance of the heart, and vascular tone regulation. The function and expression levels of the NCBTs are generally sensitive to intracellular and systemic pH. Animal models have revealed pathophysiological roles of the transporters in disease states including metabolic acidosis, hypertension, visual defects, and epileptic seizures. Studies are being conducted to understand the physiological consequences of genetic polymorphisms in the SLC4-members, which are associated with cancer, hypertension, and drug addiction. Here, we describe the current knowledge regarding the function, structure, and regulation of the mammalian cation-coupled HCO3− transporters of the SLC4-family.
Introduction
CO2 and HCO3− constitute the most important buffer system in the body with a number of unique features: CO2 reacts with H2O to form carbonic acid (H2CO3), which is a weak acid that partially dissociates into H+ and the conjugate base HCO3−. The plasma concentration of H2CO3 is very low (~3 μmol/L), but any H2CO3 consumed is replenished from existing CO2. The partial pressure of arterial CO2 is normally maintained at 40 mmHg (equivalent to 1.2 mmol/L CO2) and is regulated by the respiratory system. Given that the respiratory system has a normal function, the CO2/HCO3− buffer system is “open,” and adding H+ to the body or removing H+ from the body will have minimal effects on the partial pressure of CO2 but affect the concentration of HCO3− ([HCO3−]): adding H+ reduces [HCO3−], whereas removing H+ raises [HCO3−]. Thus, the mechanism of pH regulation in the body becomes the mechanism of maintaining [HCO3−] in the body.
The regulation of intracellular pH (pHi) and extracellular pH (pHo) is biologically very important since the structure and function of virtually all proteins are influenced by pH. A change in pH may for instance affect activities of enzymes and the structure and function of membrane proteins and signaling molecules, resulting in abnormal cell functions (268). Cells are equipped with several acid-base transport pathways that enable them to exert control of pHi. These pathways include primary active transporters such as the H-ATPase (28) and the H,K-ATPase (258); secondary active transporters such as the Na,H-exchangers (39, 227, 329), proton-coupled carboxylate, nitrate, and oligopeptide transporters and the Na+-dependent and -independent HCO3 transporters (255, 295) and channels with conductance for H+ or HCO3− (87, 303). This article focuses on Na+-coupled bicarbonate transporters (NCBTs) that comprise electrogenic Na,HCO3-cotransporters, electroneutral Na,HCO3-cotransporters, and the Na+-driven Cl,HCO3-exchanger (Fig. 1). NCBTs move HCO3− across cell membranes and this movement is equivalent to the movement of H+ in the opposite direction because of the ubiquitous carbonic anhydrase (CA) reaction. NCBTs belong to the SLC4 gene products that also include Cl,HCO3-exchangers (11, 77) and a distantly related Na,borate-transporter (233).
Figure 1.
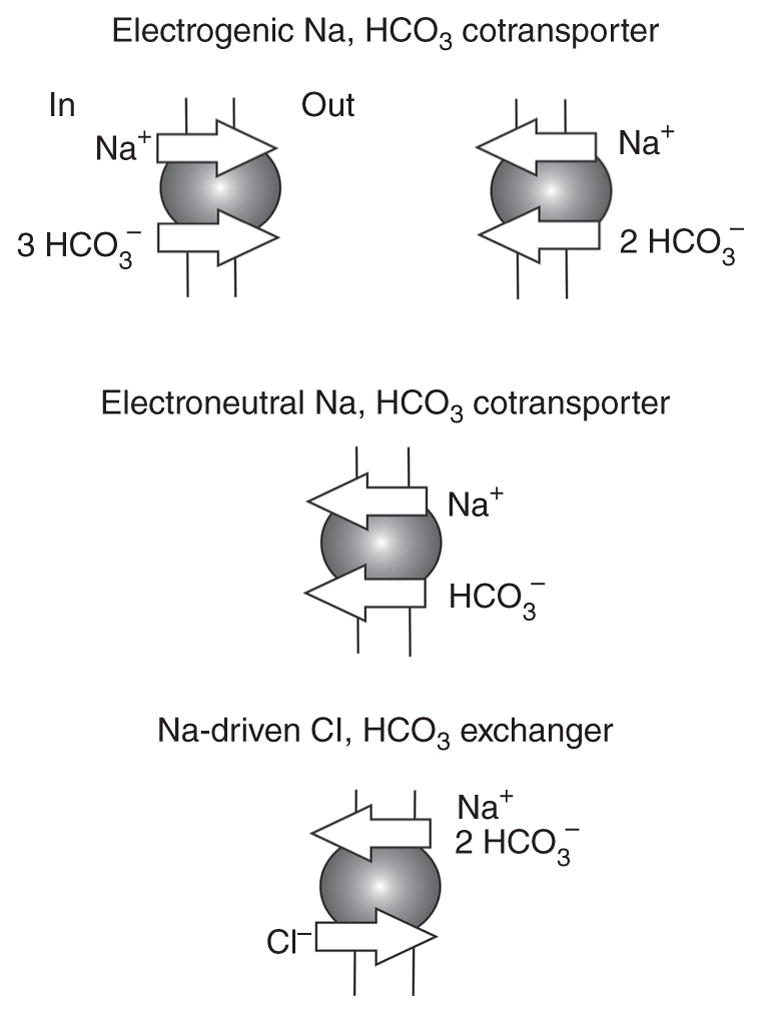
Na+-coupled bicarbonate transporters NCBTs. Electrogenic Na,HCO3 cotransporters mediate HCO3− efflux or HCO3− influx in a tissue-specific manner. Electroneutral Na,HCO3 cotransporters mediate net HCO3− influx. Na+-driven Cl,HCO3 exchangers electroneutrally mediate Cl− influx in exchange for Cl− efflux.
Since the first cloning of the Na,HCO3-cotransporter NBCe1 (266), our understanding of the molecular and cellular physiology of the NCBTs in the body has been significantly advanced. In vitro and in vivo studies have demonstrated the physiological importance of the NCBTs and also the pathological implications of disturbed transporter function are gradually being appreciated. NCBTs are widely expressed in the body and serve many functions in addition to the housekeeping role of maintaining the intracellular H+ concentration at a fairly constant level. In epithelial tissues, such as the kidney, intestine, pancreatic duct, and choroid plexus, NCBTs contribute to transcellular movement of HCO3− and thus to the secretion and excretion of acid or base. In nonepithelial tissues such as the brain, the heart, vascular smooth muscle, and the endothelium, NCBTs primarily regulate pHi. This has distinct physiological consequences depending upon which tissue is affected. Knockout mice with targeted disruption of slc4 genes exhibit abnormalities in pH-related physiology ultimately leading to defects in neuronal activity, sensory function, or the regulation of blood pressure or cerebrospinal fluid pressure and volume. In humans, genome-wide association studies have revealed a significant association between genetic polymorphisms in SLC4 genes and susceptibility to pathological conditions or diseases such as hypertension, cancer, and drug addiction. Furthermore, mutations in NBCe1 have been identified in patients with renal tubular acidosis, ocular defects, and aberrant dentition. Studies have also provided information on biochemical and biophysical properties of the transporters. Each of the known NCBTs exhibits distinct properties of ion dependence, electrogenicity, stoichiometry, and interaction with other proteins although NCBT proteins have considerable sequence homology (>30%) and possibly similarities in protein structures (40). The underlying molecular mechanism of HCO3− transport is likely at least partially conserved among the NCBTs.
This article provides an outline of our current understanding of the mammalian NCBTs. The first section describes studies of Na,HCO3-transport physiology in the precloning era. The second section focuses on postcloning studies of NCBT proteins and describes current progresses in HCO3-transporter physiology, ion transport, structure, and disease.
The Precloning Era
The first Na+-coupled HCO3− described
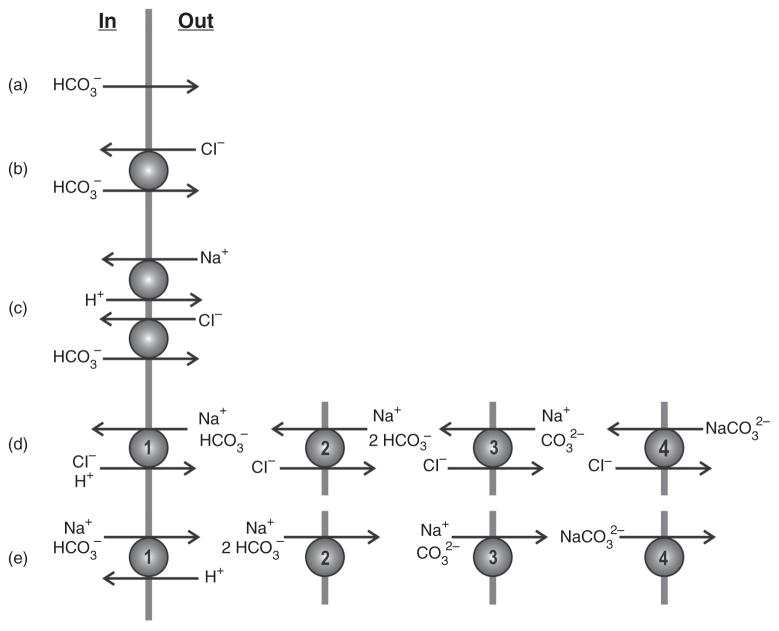
The idea that cation-coupled HCO3− transport is important for regulation of pHi was developed in the mid-1970s when Boron and Thomas first realized that the energetically uphill extrusion of acid, or influx of base, during recovery from an intracellular acid load in squid and snail neurons was stimulated by CO2/HCO3− (41, 314). The pHi-recovery was furthermore [HCO3−]-dependent (42, 315) and was inhibited by the anion transport inhibitors DIDS and SITS (275, 313). They next realized that during recovery from the acid load, intracellular Cl− was required and the recovery of pHi was associated with Cl− efflux (275). It was further demonstrated that the intracellular Cl− concentration ([Cl−]i) fell while the intracellular Na+ concentration ([Na+]i) increased (315). The latter was consistent with the pHi recovery from an acid load in snail neurons being completely blocked under conditions where extracellular Na+ was removed (315). This observation was substantiated in squid giant axons (276) and further elaborated by the demonstration that HCO3−-dependent pHi recovery from intracellular acidosis in the barnacle muscle fiber was dependent on the extracellular [Na+] with a Km for Na+ higher than 50 mmol/L (42). In this latter study, it was concluded, based on measurements of the kinetics of the transport, that likely transport modes were cotransport of Na+ and CO3 2− or Na+ and 2 HCO3− in exchange for Cl−, while the data seemed to exclude that the ion pair NaCO3− was the transported species. This is interesting in relation to a later study in squid axons, where transport of the ion pair NaCO3− could not be ruled out (37). As further discussed below, the exact mode of transport and the ionic species being translocated (Fig. 2) are still matters of debate.
Figure 2.
Five different modes for effective HCO3− extrusion over the basolateral membrane of the proximal tubule [adapted, with permission, from (39)]. The authors ruled out the first four modes because the transport was known to be Na+ dependent and electrogenic. Although this leaves option 5 (e) as the correct transport mode, it is still not decided which of the four different transport modes of (e), that is, which ion species, are actually transported by the transporter. This is not only the case for the Na+-dependent transport of HCO3− or a related species over the basolateral membrane of the proximal tubule, but indeed for all Na+-dependent transporters discussed in this article.
In the 1980s, several groups reported the presence of Na+-driven Cl/HCO3-exchange in crayfish neurons (217, 218) and skeletal muscle (103), among others. It was also demonstrated that the Na+-dependent HCO3− influx operated in parallel with the Na,H-exchanger to protect against intracellular acidification in leech neurons (282) and in a hamster lung fibroblast line (168) where the transport was shown to be important for DNA synthesis and cell growth. The Na+-driven Cl/HCO3-exchange was also found in several other cell lines derived from, for example, the kidney and tumors although not all cell lines exhibited the activity (172, 260). In some cells, for example, Vero cells, it seems that the transport was the dominant acid extrusion pathway in the near-physiological pHi range (318, 319). It should be appreciated that these initial discoveries, which have proven very important for our understanding of mammalian physiology, were made in nonmammalian cells.
The second Na+-coupled HCO3− described
The demonstration
In 1983, Boron and Boulpaep (39) concluded based on a series of elegant measurements of pHi, [Na+]i, [Cl−]i, and membrane potential in salamander proximal tubules that a basolateral, electrogenic Na,HCO3-cotransporter was present and responsible for the reabsorption of filtered HCO3− across the basolateral membrane of the proximal tubule. This transport amounts to 3.5 to 4 moles of HCO3− in the human proximal tubule every day and is crucial for normal acid-base homeostasis in the body. Importantly, the transport was reported to be independent of Cl− and thus different from the Na+-dependent Cl,HCO3-exchange, which was already known (see above). In a figure from this important paper (39), the authors depicted how different substrates in different stoichiometries can provide a net transport of HCO3− which is the result of the transporter activity (Fig. 2). The novel mechanism of HCO3-cotransport was confirmed in vivo by Frömter’s group through demonstration of an electrogenic HCO3− transport over the basolateral membrane of rat proximal tubules (48, 352) and in 1985, it was demonstrated that the transport was coupled to Na+ efflux (353). In the latter study, it was further argued based on thermodynamic arguments that the stoichiometry of the transport to result in net efflux of HCO3− would have to be 1 Na+ to 3 HCO3−. Using pH-sensitive fluorophores (13) and electrophysiological measurements (29), the presence of electrogenic Na,HCO3-cotransport was also demonstrated in rat and rabbit proximal tubules. In a cell line derived from monkey kidney epithelium, the kinetics of the Na,HCO3-cotransporter was investigated (146). Based on these findings, it was suggested that the apparent Km for HCO3− was about 10 mmol/L at 151 mmol/L Na+, but 35 mmol/L at 20 mmol/L Na+ while the apparent Km for Na+ was 19 and 28 mmol/L in the presence of 56 mmol/L and 17 mmol/L HCO3−, respectively. These findings indicate that the apparent Km for Na+ may be in the range of 20 to 25 mmol/L at physiological [HCO3−] (147). In vesicles from basolateral membranes of rabbit proximal tubules, the apparent Km for Na+ was only 10 mmol/L at 21 mmol/L HCO3− (10).
Electrogenic Na,HCO3-cotransport has subsequently been demonstrated in other tissues: in 1984, electrogenic Na,HCO3-cotransport was reported in corneal epithelial cells (145, 148) and later in many other tissues including leech glia (89, 281). In the basolateral membranes of gastric parietal cells (78), hepatocytes (100), and pancreatic ducts (141, 323, 357), Na,HCO3-cotransport was also reported, although the electrogenicity of the transport was not tested in all of these studies. Electrogenic Na,HCO3-cotransport has also been reported in the heart: Vaughan-Jones and his colleagues using guinea pig hearts suggested that the transport might be electroneutral but could not rule out a 1 Na+ to 2 HCO3− stoichiometry (173, 185), while the electrogenic nature of the transport was more apparent in cat and rat hearts (9,53). Electrogenic Na,HCO3-cotransport was also characterized in rat astrocytes (24, 27). The transport in astrocytes had a stoichiometry of 1 Na+ to 2 HCO3− and induced intracellular alkalinization in gliotic hippocampal slices. The Na,HCO3-cotransport was stimulated by membrane depolarization, which occurs due to increased extracellular K+ during neuronal firing (115, 116). This activity, called depolarization-induced alkalinization, helps compensate pHo changes caused by neuronal firing (269).
CO32− or HCO3−?
Already in the first paper (39) describing the electrogenic Na,HCO3-cotransport, Boron and Boulpaep addressed the possibility that the transported anion could be either CO32− or HCO3− (Fig. 2 and Fig. 3). They also pointed out that, if CO3 2− was the transported anion, it might be in the form of the ion pair NaCO3−. Clearly, CO3 2− is present in much lower concentrations than HCO3− (although considerably higher than the concentration of free H+, which is transported by the Na,H-exchangers). It is certainly possible that the affinity for CO3 2− could be much higher than the affinity for HCO3−. This issue was further discussed by Jentsch and co-workers (150), who argued, based on modeling considerations, that the ion pair NaCO3− was an attractive substrate for the electrogenic Na,HCO3-cotransporter, although separate transport of Na+ and CO32− or of HCO3− could not be ruled out. Based on the ability of SO3 2− to support the transport (although this was not confirmed in the study of Jentsch (147)) and the poor ability of Li+ to support the transport, Soleimani and Aronson concluded that the transport likely involved 1 Na+, 1 CO3 2−, and 1 HCO3− (297). Frömter and co-workers (292) suggested a very elegant approach to the question which is illustrated in Figure 3. The basis for this approach is that no matter whether HCO3− or CO3 2− is the transported ion pH near the membrane is expected to decrease when the transport is activated. However, if HCO3− is the transported species, then inhibition of the CA will reduce the activity induced acidification while if CO3 2− is the transported species the acidification will be enhanced (see Fig. 3). It is, therefore, possible to distinguish between CO3 2− and HCO3− transport via the transporter by measurements of pH near the membrane in the absence and presence of a CA inhibitor. Frömter and co-workers applied this idea to their previous data (292) and also exploited the idea in later papers (222). Based on these findings, they suggested that in situations with a stoichiometry of 1 Na+ to 3 HCO3−, 1 CO3 2− is transported with 1 HCO3−, while in situations with a stoichiometry of 1 Na+ to 2 HCO3− it is likely that 2 HCO3− are transported (221).
Figure 3.
Schematic diagram showing the rationale for determination of whether HCO3− or CO3 2− is the transported ion. It has been suggested (38, 292) that it is possible to distinguish between HCO3− and CO3 2− transport by measuring pH in the extracellular space near the membrane following a sudden change in transport activity, before and after inhibition of the carbonic anhydrase. If CO3− is the transported species an exaggerated decrease in pH would develop when the carbonic anhydrase is inhibited, while if HCO3− is the transported species a blunted decrease in pH would be the result. From (38).
The question of the kinetic model of the electrogenic Na,HCO3-cotransporter was investigated in a detailed study by Gross and Hopfer (124). Based on a comparison of experimental data and a model for ordered binding of Na+ and HCO3−, it was concluded that an ordered model is possible and that the major voltage dependence of the transporter is associated with the binding and translocation of HCO3−, whereas the binding of Na+ is voltage independent.
Stoichiometry
To determine the stoichiometry of the electrogenic Na,HCO3-cotransporter, a number of elegant experiments have been performed. In their original observation (39), Boron and Boulpaep depicted several possible modes of ion movement for the HCO3-transporters (Fig. 2) and suggested a stoichiometry of 1 Na+ to 2 HCO3−. As pointed out above, Frömter’s group reported a stoichiometry of 1 Na+ to 3 HCO3− in the rat kidney in situ (353) and also demonstrated that a 1 Na+ to 3 HCO3− stoichiometry was necessary to mediate net efflux of HCO3− through that pathway (353). The same stoichiometry was found in vesicles from the basolateral membranes of rabbit proximal tubules (298). However, in the isolated rabbit proximal tubule, the stoichiometry of the Na,HCO3-cotransporter was found to be 1 Na+ to 2 HCO3− (291), which might suffice to cause net efflux of HCO3− (291) given the intracellular [Na+] and [HCO3−] in this preparation. The observation was provocative as the same group found that in the rat proximal tubules in situ the stoichiometry was 1 Na+ to 3 HCO3− (353) as discussed above. It was concluded that this was not due to species differences but rather reflected that the stoichiometry of the Na,HCO3-cotransporter could vary (291, 292). This statement was strongly supported by another observation from the same year (245) that isohydric hypercapnia of the bath solution in addition to changing the net direction of HCO3− transport from efflux to influx in isolated salamander proximal tubules also changed the stoichiometry of the Na,HCO3-cotransport from 1:3 to 1:2. Frömter’s group also demonstrated that “improved” incubation conditions (i.e., cell culture medium and norepinephrine instead of physiological salt solutions) led to a shift in stoichiometry from 1:2 to 1:3 in isolated rabbit proximal tubules (220) and an increased rate of HCO3− transport, which was approaching the in vivo values under these conditions (165). Further progress was made when the electrogenic Na,HCO3-cotransporter NBCe1 was expressed in Xenopus laevis oocytes. Here, the stoichiometry was found to be 1 Na+ to 2 HCO3− (129) and unaffected by the ATP concentration (129) and the temperature (95). However, it was demonstrated that the stoichiometry was changed from 1:2 to 1:3 when the Ca2+ concentration in the oocytes was increased from <100 to 500 nmol/L (221). This strongly suggests that intracellular Ca2+ can modify the transport function. It was also suggested by the authors that this stoichiometry change was most likely occurring via a Ca2+-dependent protein kinase. In this respect, it may be of interest that an increased intracellular Ca2+ concentration via calmodulin inhibits the HCO3−-dependent uptake of 22Na+ into vesicles of basolateral membranes from rabbit proximal tubules (272).
The regulation by pH
The regulation of the electrogenic Na,HCO3-cotransporter by pH, [CO2], and [HCO3−] has been investigated by several investigators and turns out to be complex. Based on 22Na+ fluxes in vesicles from the basolateral membrane of rabbit proximal tubules, it was suggested that pHi through an allosteric effect modulates the activity of the electrogenic Na,HCO3-cotransporter (299). This concept was further developed in a more physiological condition (125), and it was concluded that the binding constant for the substrates of the transporter decreased with increasing pHi. An inherent problem in these experiments was that change in pH to a new steady state is associated with changes in [CO2] and/or [HCO3−]. To overcome this problem, Boron’s group developed a technique by which the independent effects of H+, HCO3−, and CO2 can be assessed using out-of-equilibrium solutions (358). Using this technique on rabbit proximal tubules, it was somewhat surprisingly shown that an increase in basolateral [HCO3−] inhibited the HCO3− transport and an increase of [CO2] accelerated the HCO3− transport (Fig. 4), while H+ apparently did not affect transport activity (359, 363). In further studies, it was demonstrated that the signaling pathway for the stimulating effect of CO2 on HCO3− transport in the proximal tubules involves the local renin angiotensin system acting on the angiotensin 1A receptor presumably in an autocrine manner (360, 362) as well as tyrosine kinases presumably in the ErbB family of receptor tyrosine kinases (361). These findings are consistent with a well-known stimulatory effect of angiotensin II on HCO3− reabsorption in the proximal tubules (106). But also nonreceptor tyrosine kinases of the src family and MAP kinases have been suggested to be important for the stimulatory response to both angiotensin II and hypercapnic acidosis (264, 273).
Figure 4.
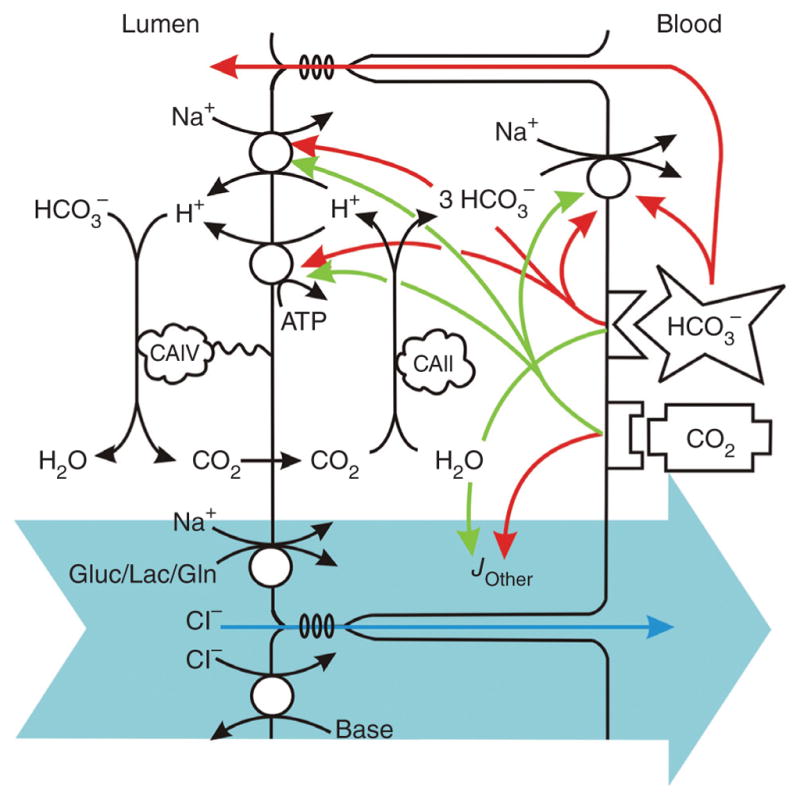
Model of reabsorption of HCO3− in the proximal tubules. The figure underlines the importance of basolateral HCO3− and CO2 for the regulation of HCO3− reabsorption suggested by Zhou et al. (363) and recently documented by Fukuda et al. (102) and others. From (363).
The third Na+-coupled HCO3− described
Na+-coupled HCO3− influx in smooth muscle cells was demonstrated in small arteries (1) where it was DIDS-sensitive, and in the guinea pig ureter (8) where it was DIDS-insensitive and associated with hyperpolarization. The presence of Na+-coupled HCO3− influx was also confirmed in a smooth muscle cell line (257). Here, it was suggested that, in addition to an electroneutral Na+-coupled Cl,HCO3-exchange activity, cells exposed to Cl− free conditions demonstrated Na+-dependent HCO3− uptake. The uptake rate was insensitive to presumed depolarization by increasing extracellular [K+] and it was suggested that this could reflect an electroneutral Na,HCO3-cotransport. As pointed out by Gross and Hopfer (124), the rate of an electrogenic transport is not necessarily strongly dependent on the membrane potential because the effect of the membrane potential depends on how the transport molecule operates, i.e. whether the rate limiting step of the translocation is voltage sensitive. It was, therefore, important to obtain measurements of the membrane potential during activation of the transport and to test the possible involvement of Cl−. This was achieved in small arteries (2) where activation of the Na,HCO3-cotransport had no effect on membrane potential and was not associated with any change in 36Cl− efflux. Based on these findings, it was suggested that an electroneutral Na,HCO3-cotransporter was present in smooth muscle cells. The presence of an electroneutral Na,HCO3-cotransport was also strongly suggested from work in guinea pig heart muscle (174), although the authors did not exclude the possibility that the transport was in fact electrogenic. In smooth muscle, the electroneutral Na,HCO3-cotransport was shown to be activated by norepinephrine and suggested (3) to be important for extrusion of the acid load associated with smooth muscle contraction, that is, a housekeeping role. In the heart the Na,HCO3-cotransport activity was shown to be affected by epinephrine and ATP (174), although for the reasons outlined above it is difficult to know whether this reflects effects on the electroneutral Na,HCO3-cotransporter. Remarkably, little work was done on electroneutral Na,HCO3-cotransport until the transport molecule was cloned.
K+-coupled bicarbonate transport
The K+-coupled HCO3−-transport involves movement of K+ and HCO3− out of cells and thus mediates cellular acid loading. The transport was found in squid axons where it has been suggested to serve a major component of base efflux following intracellular alkali loads (131). Measurements of axonal pHi revealed that the K+-dependent base efflux from the axons is unrelated to those observed with Li+, Na+, or Cs+. This base efflux is not inhibited by DIDS or by inhibitors of the H,K-ATPase or the NHEs but by quaternary ammonium ions (86). The K+-coupled HCO3− transport also appears to exist in mammalian tissues. Electroneutral K,HCO3-cotransport has been observed in the medullary thick ascending limb of the kidney (186). The cotransport is DIDS-sensitive and can be inhibited either by raising extracellular [K+] or by depleting intracellular K+. The mechanism may be mediated either by K+-coupled HCO3− transport or by K,Cl,HCO3 cotransport (43). In rat hippocampal neurons, the pHi recovery from an alkaline load is neither Cl−-dependent nor DIDS sensitive (Bevensee and Boron, personal communication). The protein responsible for K+-coupled HCO3− transport is presently not identified.
The Cloning Era
With the descriptions of the Na,HCO3-transport first in non-mammalian cells and later mammalian cells it was obvious that the genes encoding the proteins mediating the transport should be cloned. Several unsuccessful attempts were done to clone the relevant genes. The first successful cloning was the cloning from the salamander kidney of the gene encoding the electrogenic Na,HCO3-cotransporter NBCe1 in 1997 (266). Mammalian NBCe1 orthologs were then cloned by many researchers, followed by successful cloning and characterization of other mammalian NCBTs.
Also NCBT genes from invertebrates were cloned (243, 267, 325). The Drosophila gene encodes the transporter responsible for Na+-driven Cl/HCO3 exchange (267). The two genes cloned from squid giant axons encode an electrogenic Na,HCO3-cotransporter (243) and a Na+-driven Cl,HCO3 exchanger (325). Apart from providing important information on base transport proteins in vertebrates, these papers also paid tribute to the invaluable information obtained from invertebrate model systems when sodium coupled bicarbonate transport was first described in the cloning era.
Currently, there are five mammalian NCBT genes which exhibit >50% amino acid sequence homology with one another. They are proposed to share a similar topology with 14 transmembrane segments (Fig. 5). One additional gene Slc4a9 codes for a related protein, which has been suggested to be an anion exchanger (159, 320, 341). Interestingly the Slc4a9 gene product has also been suggested to be an electroneutral Na,HCO3-cotransporter (234). This suggestion has recently been supported by the finding that a sodium-dependent bicarbonate transport in the basolateral membrane of β-intercalated cells in mouse collecting ducts disappears in Slc4a9 knockout mice (56). Therefore, the Slc4a9 gene product AE4 could have been discussed here but is probably equally well discussed together with the other anion-exchangers. The current article describes the cloning and functional characterization of the five well-characterized NCBTs. Human canonical proteins, to which all positional information and amino acid variation refer according to the UniProtKB/Swiss-Prot, are summarized in Table 1.
Figure 5.

Structure of NCBT proteins. (A) A generic structure of NCBTs is predicted to have an extended N-terminus, a transmembrane domain containing 14 transmembrane segments, and a relatively short C-terminal domain. The extracellular loop between segments 5 and 6 contains two N-glycosylation sites. (B) Alignment of protein sequence comprising human NCBTs. The alignment was performed using the UniProt (www.uniprot.org) with each canonical protein sequence for NBCe1 (Uniprot ID: Q9Y6R1), NBCe2 (Q9BY07), NBCn1 (Q9Y6M7), NBCn2 (Q2Y0W8), and NDCBE (Q6U841). Sequences highly conserved among NCBTs are shown in brown bars, while sequences moderately conserved are in open bars. Sequences with negligible homology are shown as a horizontal line. Internal splice cassettes are in different colors.
Table 1.
Human Na+-Coupled HCO3− Transporters
| Protein | Gene | Amino acids* | Variants | UniProt ID |
|---|---|---|---|---|
| NBCe1 | SLC4A4 | 1079 | 5 | Q9Y6R1 |
| NBCe2 | SLC4A5 | 1137 | 8 | Q9BY07 |
| NBCn1 | SLC4A7 | 1214 | 5 | Q9Y6M7 |
| NDCBE | SLC4A8 | 1093 | 7 | Q2Y0W8 |
| NBCn2/ NCBE | SLC4A10 | 1118 | 2 | Q6U841 |
Amino acids in canonical proteins assigned by the UniProt.
SLC4A4 (NBCe1)
NBCe1 was isolated by an expression cloning method using the salamander kidney cDNA library (266). NBCe1 contains 1,035 amino acids and has a high sequence homology with previously cloned Cl,HCO3-exchangers (AE1-3). The predicted structure of NBCe1 contains a cytoplasmic N-terminal domain, a transmembrane (TM) domain with 12–14 membrane-spanning segments, and a cytoplasmic C-terminal domain. The mammalian NBCe1 orthologs with similar amino acid sequences (later named NBCe1-A) were subsequently isolated (50, 265), and their variants NBCe1-B and -C were identified (5, 70). NBCe1-A and -B differ in their N-terminal domains in which the first 41 amino acids in NBCe1-A are replaced with 85 alternative amino acids in NBCe1-B. Human NBCe1-B (1079 amino acids) is the canonical protein. NBCe1-C is identical to NBCe1-B except for the C-terminus in which the last 61 amino acids are replaced with 46 alternative amino acids (26). In addition, two additional N-terminal variations were reported by Liu et al. (197). The extracellular loop between TM5 and TM6 has two sites for glycosylation that produces negligible effect on transport function in Xenopus oocytes (69). Similar to the AEs, NBCe1 forms a homodimer comprised of two individually functional subunits (109, 153).
NBCe1-A is predominantly expressed in the renal proximal tubules (283), where it is localized to the basolateral membrane of the tubule cells and mediates HCO3− efflux to the peritubular capillaries. In contrast, NBCe1-B is widely distributed in the body (5, 70). In general, NBCe1-B is localized to the basolateral membrane of epithelial cells and mediates cellular HCO3− uptake (5, 208, 312), but it is also expressed in the apical membrane of pancreatic ducts (270). NBCe1-B is also expressed in many nonepithelial cells such as cardiomyocytes (70, 155), skeletal muscle (162), corneal endothelia (194), oligodendrocytes (263), neurons, and astrocytes (207, 261). NBCe1-C is exclusively expressed in the nervous system, particularly in astrocytes (207).
SLC4A5 (NBCe2)
NBCe2 is an electrogenic Na,HCO3-cotransporter first cloned and characterized by Pushkin et al. (253) and later by Virkki et al. (326) and the human canonical NBCe2 has 1137 amino acids with 55% amino acid sequence homology with NBCe1. Currently, eight different variants are reported in the human protein database due to alternative splicing events, but only NBCe2-C appears to be functionally capable of electrogenic Na,HCO3-cotransport (326). NBCe2 is strongly expressed in the liver, where it is localized to hepatocytes and intrahepatic cholangiocytes in bile ducts (6). The hepatic expression of NBCe2 is consistent with the electrophysiological observation (99, 100) that HCO3−-dependent acid extrusion in hepatocytes is governed by electrogenic Na,HCO3-cotransport. Other cells that express NBCe2 include uroepithelial cells and renal collecting duct intercalated cells (6, 81), choroid plexus epithelial cells (46, 81). In skeletal muscle cells, NBCe2 expression has been described in the sarcolemma including the T-tubular membranes (162).
SLC4A7 (NBCn1)
Mammalian cDNAs encoding the electroneutral Na,HCO3-cotransporter NBCn1 were first cloned from rat aorta (68) and human skeletal muscle (252). NBCn1 has 57% amino acid sequence homology with NBCe1, and its predicted structure is also similar to that of NBCe1. NBCn1 exists as multiple variants due to alternative promoter sites and cytoplasmic N- and C-terminal splicing events. The splicing events result in at least three splice cassettes, cassette I containing 13 amino acids, cassette II containing 123 amino acids (124 in humans), and cassette III containing 36 amino acids. Cassette II is predominantly found in smooth, cardiac, and skeletal muscle tissues (74) and likely reduces protein expression in membranes (349). The human canonical NBCn1 contains 1214 amino acids. NBCn1 is found widely in the body. In most epithelial tissues, the transporter is localized to the basolateral membranes of the cells (113, 230, 248, 249, 327). The transporter is also found in the apical membranes of some epithelial cells such as in pancreatic ducts (205), intercalated cells (256), and salivary glands (113).
NBCn1 moves Na+ and HCO3− into the cells by operating with a stoichiometry of 1 Na+ to 1 HCO3−. Thus, the Na,HCO3-cotransport by NBCn1 occurs with no direct changes in the membrane potential. In most cell types— although not in all (33)—NBCn1 is only weakly inhibited by DIDS (68), a hallmark that distinguishes NBCn1 from other bicarbonate transporters of the SLC4-family. In addition, NBCn1 has Na+-channel-like activity that is uncoupled from the Na,HCO3-cotransport activity and stimulated by DIDS (68). NBCn1 is widely distributed in many epithelial and nonepithelial cells (34), and plays a role in pHi regulation and probably transcellular HCO3− movement.
SLC4A8 (NDCBE)
NDCBE is a Na+-driven Cl,HCO3-exchanger that moves external Na+ and HCO3− into cells in exchange for internal Cl− which was first cloned from Drosophila by Romero et al. (267) and from human brain by Grichtchenko et al. (117) and Virkki et al. (235). NDCBE is different from the Cl/HCO3-exchangers (AEs; SLC4A1-3), which move external Cl− into the cells in exchange for internal HCO3−. Thus, NDCBE serves as an acid extruder, whereas the AEs serve as acid loaders. The human canonical NDCBE contains 1093 amino acids and has 50% amino acid sequence homology with NBCe1 and 70% with NBCn1. Similar to other SLC4 transporters, NDCBE exists as multiple variants due to alternative promoter sites and splicing events.
NDCBE transcripts are strongly expressed in the brain and testes, and weakly in the kidney and ovaries (81, 117). In the brain, the NDCBE protein is localized to cell body membranes, cytosol, and processes of neurons (60, 179), thereby supporting the previous observations that the Na+-driven Cl,HCO3 exchanger primarily governs HCO3−-dependent acid extrusion in neurons (22, 287). NDCBE is found in presynaptic nerve terminals, at the electron microscopic level (296), mostly in glutamatergic terminals and some GABAergic terminals (49). The exchanger likely regulates presynaptic pH that affects neurotransmission. Mice with a targeted disruption of slc4a8 display abolished thiazide-sensitive and amiloride-insensitive Na+-reabsorption in the cortical collecting ducts of the kidney (187).
SLC4A10 (NBCn2/NCBE)
NBCn2/NCBE was originally cloned from a mouse insulinoma cell line (330). The human canonical NBCn2/NCBE contains 1118 amino acids and its amino acid sequence is 71% and 65% identical to NDCBE and NBCn1, respectively. The human clone exists as two variants depending upon the presence of an N-terminal cassette of 30 amino acids according to the Uniprot database. The rat orthologs vary at the C-terminal end, lacking the last 21 amino acids (107). NBCn2/NCBE is found in the brain with strong expression in neurons particularly in postsynaptic membranes (143), and choroid plexus epithelia (59, 81, 107, 135). The transporter is also found in other tissues such as the stomach and duodenum (81).
NBCn2/NCBE was first reported as a Na+-dependent Cl,HCO3-exchanger with a somewhat unusual Cl− dependency (330). In Xenopus oocytes expressing NBCn2/NCBE, the inhibition of 36Cl− efflux by DIDS is far less substantial than inhibition by HCO3− removal. Furthermore, Na+ influx increases linearly with higher external [Cl−], which would otherwise decrease if the transporter mediated Na+-driven Cl/HCO3 exchange. Later, Parker et al. (236) observed that the abnormal Cl− flux by NBCn2/NCBE is due to Cl− self-exchange uncoupled from the transport of Na+ and HCO3−. On the other hand, Damkier et al. (79) observed 36Cl efflux that is DIDS-sensitive and dependent on Na+ and HCO3−. The issue of NBCn2 versus NCBE will be described in detail in the next section.
The Postcloning Era
Following the cloning of the five NCBTs, research has been focused on understanding the cellular, physiological, and pathological roles of NCBT-mediated pH regulation in the body. The results demonstrate that NCBTs play essential roles in HCO3− movement across cell membranes to regulate and maintain acid-base homeostasis in many tissues. The physiological importance of NCBTs has been further supported by studies of knockout mice which exhibit abnormalities in distinct physiological processes in tissue-specific manners (Table 2). Studies have also advanced our understanding of biochemical and biophysical properties of the NCBT proteins. The mechanisms for ion translocation, stoichiometry/electrogenicity, and interactions with other proteins were studied by expressing transporters in heterologous expression systems. This section describes postcloning studies of NCBT physiology, ion transport, structure, and related disease.
Table 2.
Slc4 Knockout and Knockin Mice
| Gene | Site | Method* | Major phenotypes | Reference |
|---|---|---|---|---|
| Slc4a4 | Exon 9 | H | Growth retardation and death before weaning Metabolic acidosis Abnormal dentition and intestinal obstruction Low plasma Na+, hyperaldosteronism, splenomegaly No depolarization-induced alkalinization in neurons |
(105, 170, 310) |
| W516X (exon 11) | H | Growth retardation and death before weaning Proximal renal tubular acidosis Ocular abnormalities Anemia, volume depletion, prerenal azotemia |
(199) | |
| Slc4a5 | Exon 15 | R | Reduced intracerebral volume and pressure Remodeling choroid plexus epithelia |
(152) |
| Exon 7 | H | Hypertension Metabolic acidosis Hyporeninemic hypoadosteronism |
(119) | |
| Slc4a7 | Exon 5 | H | Blindness and auditory impairment | (36, 202) |
| Exon 1 | R | Inhibition of NO-mediated vasorelaxation Resistant to hypertension development |
(35) | |
| Slc4a8 | Exon 12 | H | No thiazide-sensitive Na,Cl transport in collecting ducts Reduced spontaneous glutamate release in CA1 pyramidal layer Increased seizure threshold |
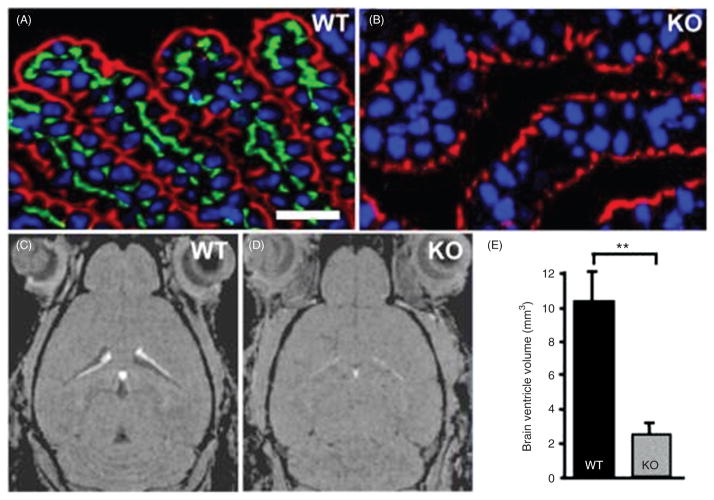
(187, 296) |
| Slc4a10 | Exon 12 | H | Reduced ventricular volume and neuronal excitability Altered expression of other transporters in choroid plexus |
(82, 143) |
H: homologous recombination, R: retroviral integration.
SLC4A4 (NBCe1)
Physiology of NBCe1
(i) Renal acid-base regulation
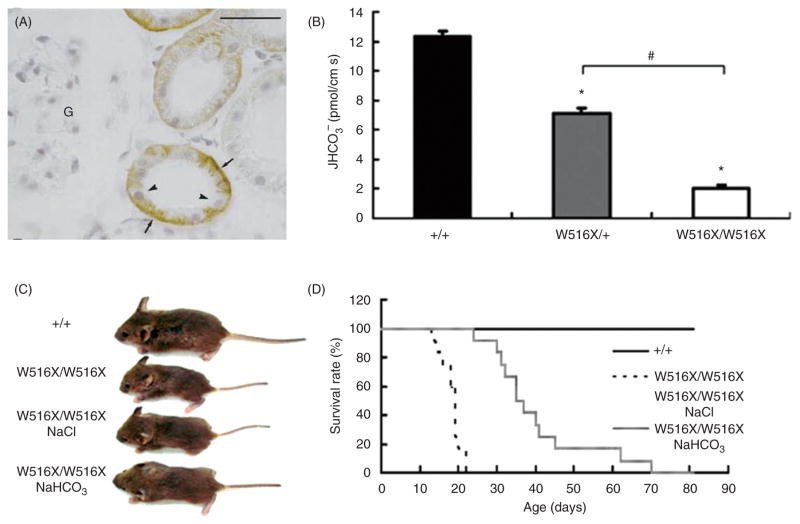
A major task of the kidney is to maintain whole body acid-base homeostasis by reclaiming filtered HCO3− and producing new HCO3− to neutralize acidic by-products of metabolism (39). The proximal tubule plays a central role in these processes, reabsorbing >80% of filtered HCO3−. NBCe1-A is electrogenic (Fig. 6A) have a stoichiometry of 1 Na+ to 3 HCO3− in the basolateral membrane of the proximal tubule (Fig. 7A) and play a major role in reclaiming HCO3− (354). Consistent with this functional knockout of NBCe1 (199) leads to reduced uptake of HCO3− in the proximal tubules (Fig. 7B) and these mice are growth retarded (Fig. 7C) and die early (Fig. 7D). These phenotypes can be partly rescued by feeding the mice HCO3− (Fig. 7C and 7D), which might be expected for a HCO3− loosing condition. NBCe1-A is mostly restricted to the S1 and S2 segments of the proximal tubule (209). The kidney makes adaptive changes in HCO3− reabsorption and NH4+ excretion in response to acid-base disturbances (10, 14, 164, 250), and such adaptive changes involve downregulation or upregulation of NBCe1 in the proximal tubule. NBCe1 in rat proximal tubules is downregulated by metabolic alkalosis (16, 47). This downregulation is expected to blunt HCO3− reabsorption and help prevent metabolic alkalosis. In contrast, the effect of metabolic acidosis on NBCe1 appears to vary depending upon experimental procedures. NBCe1 is upregulated by lithium-treated metabolic acidosis (157) and bilateral ureteral obstruction-induced metabolic acidosis (332), consistent with the previous reports that HCO3− absorptive capacity in the proximal tubules increases with chronic metabolic acidosis (10,250,279) or respiratory acidosis (73,160). However, Kwon et al. (166) and Brandes et al. (47) observed negligible changes in NBCe1 expression during chronic metabolic acidosis. Wang et al. (333) found that the NBCe1 response to acidosis is time/age-dependent in neonatal unilateral ureter obstruction.
Figure 6.
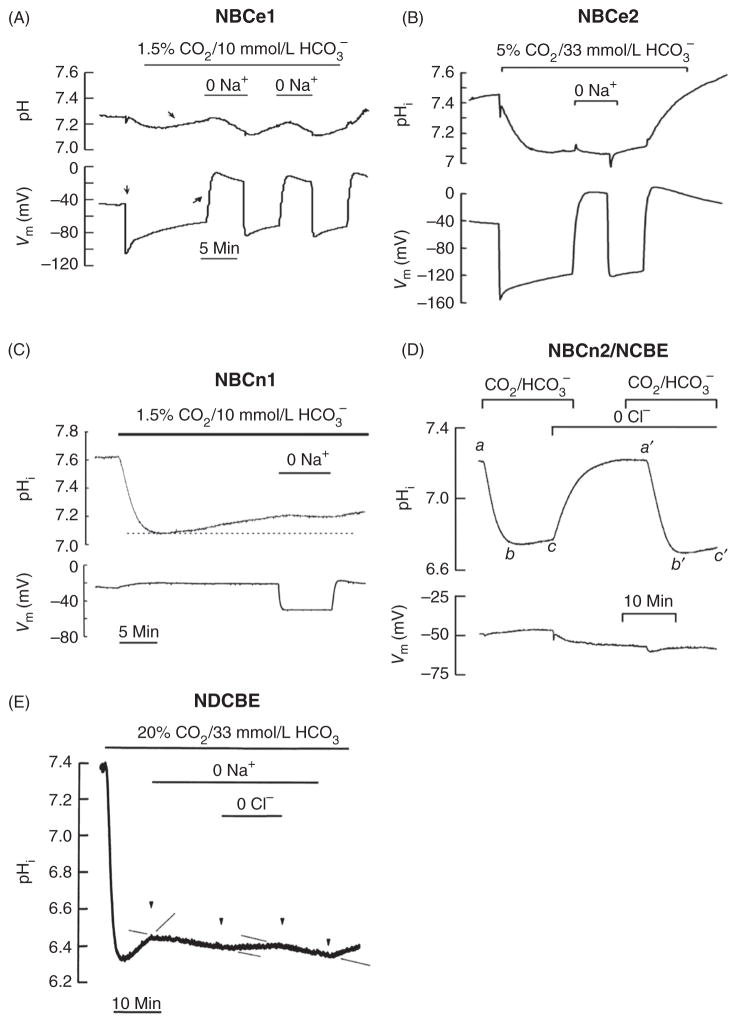
Functional characterization of NBCs expressed in Xenopus oocytes demonstrating Na+-dependent pHi recovery from a CO2/HCO3−-induced acidification. The electrogenic transporters NBCe1 (A) [adapted, with permission, from 70] and NBCe2 (B) [adapted, with permission, from 326] produce a large hyperpolarization due to net negative charge movement into oocytes. Activation of the electroneutral transporters NBCn1 (C) [adapted, with permission, from 75], NBCn2/NCBE (D) [adapted, with permission, from 236], and NDCBE (E) [adapted, with permission, from 117] is not associated with hyperpolarization.
Figure 7.
NBCe1 (A) NBCe1 is highly expressed in the basolateral membranes of the cortical collecting ducts (arrows) but not in glomeruli (G) (209). [(B)–(D)] Functional knockout of NBCe1 in mice (199). (B) Rates of HCO3− absorption from isolated renal proximal tubules. Mice with functional knockout of NBCe1 (W516/W516×) had severely reduced reabsorption of HCO3−, while heterozygous mice had a mildly reduced reabsorption. (C) Mice with functional knockout of NBCe1 were growth retarded compared to wild-type mice (+/+). NaCl in the drinking water had no effect on the growth retardation while NaHCO3-treated mice had attenuated growth retardation. (D) Mice with functional knockout of NBCe1 had severely reduced survival rates with a sharp increase in mortality starting around 17 days, but NaHCO3 treatment of these mice prolonged the survival time up to 81 days of age.
In addition to systemic alkalosis/acidosis, plasma Na+ affects NBCe1 expression in the kidney. NBCe1 is downregulated in proximal tubules of rats exposed to 280 mmol/L NaCl in the drinking water for 5 days (16). Proximal tubule NBC activity is possibly altered in hypertension: NBCe1 is more abundant in the renal cortex of spontaneously hypertensive rats (301) compared to control rats; however, NBCe1 expression and function was lower in a proximal tubule cell line from spontaneously hypertensive rats than in control cell lines (239). Finally, NBCe1 is recognized to be one of the Na+ transporters that are upregulated by chronic exposure to the sympathetic neurotransmitter norepinephrine promoting Na+ and water retention (300).
(ii) HCO3− secretion in exocrine cells
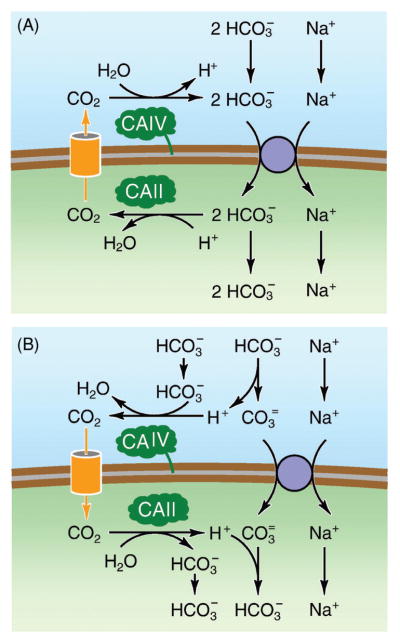
Fluid and HCO3− secretion are vital functions of secretory glands, particularly pancreas and salivary glands. The molecular mechanisms of fluid and HCO3− secretion by these exocrine cells have recently been described by Lee et al. (180). In the pancreatic ducts, NBCe1-B is localized to the basolateral membrane, mediates transport with a stoichiometry of 1 Na+ to 2 HCO3 −, and mediates HCO3− influx (208). Intracellular HCO3− becomes a source for luminal HCO3− secretion mediated by the SLC26 transporters and CFTR (219). In addition to this basolateral HCO3− influx mediated by NBCe1, HCO3− is also produced from hydration of CO2 catalyzed by CA II. A basolateral Na/H-exchanger (NHE1) contributes to the accumulation of intracellular HCO3− by extruding the proton produced by hydration of CO2. Recent studies suggest that basolateral NBCe1 and luminal CFTR are coordinately regulated (347,348). NBCe1 and CFTR interact with IRBIT (IP3 receptor binding protein released with IP3), a signaling protein that is released from IP3 receptors and regulates downstream target molecules (294). The interaction stimulates fluid and HCO3− secretion, which can result in a luminal fluid containing up to 150 mmol/L NaHCO3.
NBCe1 is also important for the exocrine function of the salivary glands. Secretion of HCO3− to the saliva is essential for oral function and health, as HCO3− in saliva buffers acids produced by oral bacteria. HCO3−-dependent Na+ influx has been functionally detected in mammalian parotid acinar cells (246, 304). Immunohistochemistry of rat and human parotid glands shows an abundant expression of NBCe1 in the basolateral membranes of acinar and ducts cells (231, 271) and submandibular duct glands (271). By patch clamp recordings of acutely dissociated bovine parotid acinar cells, Yamaguchi et al. (343) have identified a Na+-dependent and DIDS-sensitive HCO3− current that has an apparent coupling ratio of 1 Na+ and 2 HCO3−. RT-PCR analyses recognizes NBCe1-B, but not NBCe1-A, in these cells. It has also been shown that HCO3− secretion involves NBCe1 endocytosis and is regulated by acetylcholine, which is responsible for production of fluid and electrolyte secretion in the salivary glands (343). Perry et al. (242) observed in parotid ParC5 cells that NBCe1 is internalized from the basolateral membranes by carbachol and phorbol-12-myristate-13-acetate (PMA) to appear in early endosomes. The internalization of NBCe1 is prevented by PKC inhibitors (240).
(iii) HCO3− secretion in the intestine
In the intestine, NBCe1 is abundantly localized to the basolateral membranes of colonic crypts, thereby contributing to intestinal anion secretion (290). cAMP stimulates NBCe1-mediated HCO3− transport in crypts (19). Forskolin and carbachol stimulate NBCe1 membrane expression by vesicle trafficking and exocytosis (355). NBCe1 knockout mice exhibit a significant reduction in cAMP-mediated HCO3− secretion in the proximal colon (105). NBCe1 is also expressed in the duodenum, although the role here is unclear (64).
(iv) pH regulation during amelogenesis
NBCe1 is essential for the development of normal enamel. The physiological importance of NBCe1 for enamel development was revealed based on patients with NBCe1 mutations, who have abnormal dentition (91, 140). In addition, the enamel of NBCe1 knockout mice was structurally defective and low in mineral content (170). The incisors of these mice are chalky-white and prone to enamel fracture. Immunohistochemistry shows NBCe1 being localized to the basolateral membrane of ameloblasts and RT-PCR analyses detects NBCe1-B transcripts in ameloblast-like LS8 cells (229). Transcellular HCO3− secretion probably regulates extracellular pH to support proton buffering during the growth of hydroxyapatite crystals (169). Many proteins capable of HCO3− transport, including NBCe1, are upregulated at the onset of the maturation stage of amelogenesis (171).
(v) Intracellular pH regulation in astrocytes and neurons
Astrocytes represent the most studied nonepithelial cells where NBCe1 contributes to pH regulation (67). NBCe1-B and -C expression has been detected (26,207,261), and electrogenic Na,HCO3-cotransport has been functionally characterized (24) in astrocytes. The cotransport has a stoichiometry of 1 Na+ to 2 HCO3− and induces alkalinization in gliotic hippocampal slices. Electrogenic Na,HCO3-cotransport activity is stimulated by membrane depolarization that occurs due to increased extracellular K+ during neuronal firing (116) and the cotransport activity helps compensate extracellular pH changes under these conditions (269). Interestingly, NBCe1-A is found in some neurons, where electroneutral NCBTs have been known to govern HCO3−-dependent acid extrusion. The expression of NBCe1-A is supported by recent reports of NBCe1 activity in cultured mammalian neurons (206, 310). Assessed by in situ hybridization (108) and immunoblotting (93), NBCe1 mRNA and protein expression begin at the time of birth and persist throughout adulthood.
Early studies have investigated potential pathological implications of disturbed NBCe1 function. Giffard et al. (108) tested whether NBCe1 is associated with vulnerability to acidic injury in ischemia and found that blocking the transporter with DIDS inhibits acidic injury in primary cultures of astrocytes. Jung et al. (149) observed a significant upregulation of Na+ transporters including NBCe1 and down-regulation of the Na,K-ATPase in ischemic penumbra in rats with permanent middle cerebral artery occlusion. The authors proposed that NBCe1 upregulation contributes to cell damage through intracellular Na+ accumulation that leads to cell swelling and Ca2+ overload. NBCe1 is also upregulated in the left ventricle following myocardial infarction (278). This regulation seemed to be dependent on the function of the angiotensin system. Taken together, the increased expression or activity of NBCe1 is likely deleterious to cells. Douglas et al. (94) examined the effect of chronic intermittent hypoxia on NBCe1 protein expression and found downregulation of the transporter. The level of downregulation varies between NBCe1 variants and in different brain regions. Consistent with the downregulation of NBCe1, acute hypoxia inhibits electrogenic Na,HCO3-cotransport activity in hippocampal astrocytes (25). In addition, recent studies show that NBCe1 activity enhances glutamine efflux via the glutamine transporter SLC38A3 (335) and fast glycolysis in response to excitatory synaptic transmission (274).
SLC4A4-related diseases in humans
Missense and nonsense mutations in human SLC4A4 have been identified in patients with proximal renal tubular acidosis (pRTA), visual and hearing defects, and abnormal dentition (90, 91, 133, 137–140, 199, 293, 307, 308, 322). These phenotypes are recapitulated in knockout mice with targeted disruption of the slc4a4 gene (105,170), or knockin mice with a point mutation (199), although both knockout and knockin mice exhibit additional abnormalities. Studies by many different researchers show that some of these mutations result in loss of NBCe1 activity due to impaired membrane trafficking. For example, by cysteine mutagenesis of these pRTA-associated residues, Zhu et al. (366) found that most of the mutants are not accessible to the methylsulfonate reagents. These pRTA-associated residues are buried in the membrane field and are important for the protein structure. Surface biotinylation or immunoblot experiments by other researchers show comparable impairment of protein expression in membranes (90, 91, 133, 137–140, 199, 293, 307, 308, 322). Some pRTA-associated mutations result in a partial loss of function. For example, G486R and T485S mutants have reduced Na,HCO3-cotransport activity despite normal membrane expression in heterologous expression systems (307). Other mutations result in gain of a new function: A799V gains an unusual HCO3−-independent conductance that might be associated with hypokalemic paralysis (237).
Signaling molecules affecting NBCe1 activity
Little is known about the effect of signaling molecules on NBCe1 activity. It has been suggested that phosphorylation activated by cAMP or cytosolic [Ca2+] change the stoichiometry of the transporter in heterologous expression systems. In addition, ATP can stimulate NBCe1 activity (129). On the other hand, NBCe1 is negatively affected by protein kinase C. Perry et al. (241) observed that PKC activated by phorbol-12-myristate-13-acetate (PMA) inhibits NBCe1 in Xenopus oocytes. Angiotensin II affects NBCe1 endocytosis in a biphasic manner: at 10−11 to 10−10 M, angiotensin II stimulates NBCe1 currents; but at 10−6 M, angiotensin II reduces NBCe1 currents and surface expression (241). This biphasic effect is similar to that in the proximal tubules. Wu et al. (340) reported that NBCe1 is stimulated by phosphatidylinositol 4,5-bisphosphate (PIP2). DIDS-sensitive NBCe1 currents exhibit run-down in oocytes; and by patch-clamp recordings, the authors found that cytosolic PIP2 reduces the rate of rundown and stimulates NBCe1 current. PIP2 is known to bind to a stretch of positively charged amino acids in membrane-associated proteins or signaling molecules near the membrane and thereby regulate their activity (130). It is thus proposed that PIP2 interacts with NBCe1 and stimulates the Na,HCO3-cotransport activity. Hong et al. (132) recently reported that residues 37 to 65 in NBCe1-B are required for the interaction with and regulation by IRBIT as well as PIP2. Phosphorylation of Ser65 mediates NBCe1-B regulation by SPAK, while phosphorylation of Thr49 is required for regulation by IRBIT and SPAK.
Mechanism of ion transport
(i) Ion dependence
In Xenopus oocytes, NBCe1 produces currents in response to Na+ and HCO3− (Fig. 6A) (118,210,288). The currents are outwardly directed at the resting membrane potential and get progressively larger at more positive voltages. The current-voltage relationship exhibits a positive and linear slope with a reversal potential (Erev) ranging between −120 and −140 mV. The NBCe1 current is dependent on Na+ and the slope of the I/V-curve becomes progressively larger at higher [Na+]. The apparent affinity Km for Na+ is 19 to 36 mmol/L for three NBCe1 variants (288). NBCe1 does not produce currents to K+, NMDG+, or choline, although it appears to generate a very small current to Li+. The strict dependence on Na+ is confirmed by pHi experiments in which pHi recovery from intracellular acidification requires external Na+ (17, 210).
The current-voltage relationship for NBCe1 has a positive, almost linear slope and displays a strong dependence on HCO3− (118, 210, 288). Determined by peak currents at −60 mV under voltage-clamp conditions, Grichtchenko et al. (118) proposed a kinetic model for a single Michaelis-Menten process (with an apparent Km for HCO3− of 6.5 mmol/L) and a linear component. NBCe1 does not produce current in response to other anions such as Cl−, Br−, I−, or NO3− (350). The strict HCO3− dependence of NBCe1 is distinct from AEs that can move several monovalent and divalent anions with low affinity in either direction or via anion self exchange (158).
(ii) HCO3− versus CO3 2− transport
Since equilibrated solutions containing HCO3− will also contain CO3 2−, it has been a challenging question to determine which ion is in fact being transported. To address this question, Boron and his colleagues (114, 184) co-expressed NBCe1 and GPI-linked CA IV in Xenopus oocytes and measured surface pH (pHs) while voltage-clamping oocytes. The idea is that if NBCe1 transports HCO3−, CA IV would replenish the lost HCO3− at the oocyte surface and then pHs would decrease. Inhibiting CA IV would consequently blunt the decrease in pHs. However, if NBCe1 transports CO3 2−, then a large increase in [H+] would occur because HCO3− is deprotonated to produce CO3 2−. CA IV would then consume H+ by catalyzing the generation of OH− by conversion of HCO3− to CO2 + OH− as discussed above. In this case, inhibiting CA IV would enhance the pHs decrease (Fig. 3). Boron and his colleagues found that stimulating NBCe1 (by positively shifting the holding potential) decreased pHs, whereas slowing NBCe1 (by negatively shifting the holding potential) increased pHs. The CA inhibitor acetazolamide augmented all pHS changes. Thus, NBCe1 appears to transport CO3 2−, not HCO3−. These results are comparable with the observation by Grichtchenko and Chesler (115) that the CA inhibitor benzolamide augments a pHo decrease mediated by NBCe1 in hippocampal glial cultures. It is unclear whether HCO3− and CO3 2− can be transported simultaneously (see discussion above). In this case, NBCe1 in the renal proximal tubules would have a stoichiometry of 1 Na+ to 1 CO3 2− and 1 HCO3− (221,292,297).
(iii) Ion translocation
The NBCe1 amino acid sequence has facilitated structure-function studies to determine the molecular mechanism of ion transport and the structural requirements for function. Abduladze et al. (4) performed site-directed mutagenesis of charged and polar residues and identified many mutations affecting transport function. They proposed that charged and polar residues serve as ion binding sites or interact with other charged residues constituting structural components of the transporter. Other researchers focused on amino acid residues in single TMs or cytoplasmic N-and C-terminal domains. Dinour et al. (91) investigated the function of S427L (located in TM1), which is one of the human mutations exhibiting visual defects and renal tubular acidosis. The mutation was found to cause the protein to lose voltage-sensing capacity or a Na+ binding site and additionally reduced membrane expression of the protein. The reversal potential for the S427L-mediated current was extremely negative (< −160 mV) indicating no HCO3− efflux under normal conditions in native cells. Li et al. (192) report that S427L is incorrectly targeted to the apical membrane instead of the basolateral membrane. Using cysteine mutagenesis of all amino acids in TM1(Gln424–Gly448), Zhu et al. (364) found that Ala428, Ala435, and Thr442 affect cotransport function. The access of these mutants to methylsulfonate reagents significantly inhibits pHi recovery, indicating that these residues are located on the same face of the TM 1 α-helix lining the pore.
Based on sequence comparisons among bicarbonate transporters, Yang et al. (350) identified Asp555 as a residue responsible for anion selectivity. Mutation of this residue to a Glu (i.e., D555E) causes NBCe1 to be permissive to other anions. All electroneutral Na,HCO3-transporters possess a Glu in this position, suggesting that the anion selectivity would be associated with electrogenicity although the mechanism of how the two properties are related is unclear. Yamazaki et al. (346) found that the single nucleotide polymorphism K558R results in reduced transport activity without changing protein trafficking or the apparent Km for Na+. McAlear and Bevensee (210) performed cysteine-scanning mutagenesis of amino acid residues in TM8 (Ala739-Thr758) and found Leu750 as a residue involved in ion translocation. The L750C mutant mediates HCO3− currents that are strongly inhibited by sulfhydryl reagents. Chen and Boron (63) proposed TM6 and TM12 as part of a functional unit.
In addition to the TM domains, the cytoplasmic N-terminal domain is proposed to contribute to ion translocation. Chang et al. (58) demonstrated that the low Na,HCO3-cotransport activity of the human mutant R298S can be rescued by substituting Glu91 with an Arg. The authors propose that an electrostatic interaction or hydrogen bond between Arg298 and Glu91 forms a permeation tunnel, through which HCO3− is translocated. Whether Arg298 lines the pore remains unknown.
Taken together, several residues in TMs and the cytoplasmic N-terminus have been identified to be critical for ion translocation (Table 3). Not all of these residues are charged or polar, and they may be lining the pore or serve as structural residues for protein conformation.
Table 3.
Amino Acid Residues in NBCe1 Identified as Sites for Ion Transport
| Domain | Residue | Potential role | Reference |
|---|---|---|---|
| Nt | Arg298, Glu91 | HCO3− permeation tunnel | (58) |
| TM1 | Ser427 | Na+ binding or voltage sensor | (91) |
| Ala428, Ala435, Thr442 | Ion translocation in the pore | (364) | |
| TM5 | Asp555 | Anion selectivity | (350) |
| Lys558 | Pore entry or DIDS-binding site | (346) | |
| Lys559 | DIDS-binding site | (203) | |
| EL4 | not specified | Electrogenicity | (62) |
| TM6 | not specified | Functional unit | (63) |
| TM8 | L750 | Ion translocation in the pore | (210) |
| TM12 | not specified | Functional unit | (63) |
Electrogenicity and stoichiometry
To understand the molecular basis of electrogenicity, Choi et al. (71) performed experiments in which a series of chimeric transporters from NBCe1 and NBCn1 were constructed and their electrogenicity tested. They found that the transmembrane domain of NBCe1 is essential for electrogenicity, while the cytoplasmic N- and C-terminal domains and the large third extracellular loop (EL3; located between TM5 and TM6) have negligible effect on transport function despite the fact that these regions are unique to NBCe1. Furthermore, electrogenicity of NBCe1 requires an interaction between the two regions of TM1-5 and TM6-14. The interaction might be essential for Na+ and HCO3− binding or a part of the ion-binding vestibule. The interaction might not be limited to TMs, but could involve the loops between TMs. Chen et al. (62) identified the loop EL4 between TM7 and TM8 as the region critical for electrogenicity. EL4, which contains 32 amino acids, may coordinate with residues in TM1-5 to produce Na,HCO3-current.
The issue of Na+ to HCO3− stoichiometry is related to the electrogenicity of NBCe1. In Xenopus oocytes, the stoichiometry of NBCe1 was found to be 1 Na+ to 2 HCO3− (129) and unaffected by the ATP concentration (129) and the temperature (95). However, when the [Ca2+] in the oocytes was increased from 100 nmol/L or less to 500 nmol/L the 1 Na+ to 2 HCO3− stoichiometry was shifted to a 1 Na+ to 3HCO3− stoichiometry (221). It was suggested by the authors that this was most likely occurring via a Ca2+-dependent protein kinase. In this respect, it may be of interest that an increase of [Ca2+] via calmodulin was shown to inhibit the HCO3−-dependent uptake of 22Na+ into vesicles of basolateral membranes from rabbit proximal tubules (272).
Also, Gross and colleagues have addressed the question of stoichiometry. In immortalized rat proximal tubule cells grown in a monolayer and placed in Ussing chambers, the apical membrane was permeabilized with amphotericin B. The transport through the basolateral Na,HCO3-cotransporter was assessed based on the reversal potential and conductance of the Na+- and HCO3−-dependent, dinitro-stilbene disulfonate sensitive current and the stoichiometry was found to be 1 Na+ to 3 HCO3− (123). After cloning of the NCBTs, Gross and colleagues expressed the pancreatic variant of NBCe1 in a mouse pancreatic cell line and found the stoichiometry to be 1 Na+ to 2 HCO3− under those conditions (120). These differences in stoichiometry are consistent with the transporter mediating influx of HCO3− over the basolateral membrane of pancreatic ducts but efflux of HCO3− over the basolateral membrane of the proximal tubule. The same year, Gross and colleagues expressed both the pancreatic and the renal variant of NBCe1 in a mouse proximal tubule cell line as well as in a mouse collecting duct cell line (121). Both NBCe1 variants showed a 1 Na+ to 3 HCO3− stoichiometry in the proximal tubule cell line but a 1 Na+ to 2 Na+ stoichiometry in the collecting duct cell line. These findings provide strong further arguments that the same transport molecule can mediate transport with different stoichiometries depending on the cellular environment. To provide insight into which signals in the cells could be important for this shift in stoichiometry, the role of a cAMP-dependent phosphorylation of the transporter was investigated (122). Addition of a membrane permeable variant of cAMP (8-Br-cAMP) resulted in a shift from a 1 Na+ to 3 HCO3− stoichiometry to a 1 Na+ to 2 HCO3− stoichiometry and was associated with phosphorylation of NBCe1. The phosphorylation and shift in stoichiometry was abolished when Ser982 in the C-terminus was mutated to an alanine. It was further demonstrated that the catalytic subunit of PKA was able to phosphorylate the protein. These findings are consistent with cAMP-dependent signaling modifying the stoichiometry and could explain how cAMP reduces the reabsorption of HCO3− in the proximal tubules through a change in transport stoichiometry.
Pharmacological inhibition
A number of pharmacological agents have been shown to inhibit NBCe1 with reasonable potency (31) but development of more specific compounds applicable under both in vitro and in vivo experimental conditions would be a substantial improvement.
The stilbene derivatives such as DIDS and SITS are potent inhibitors of Cl,HCO3-exchangers (12, 51) and also of NBCe1. Studies of erythrocyte AE1 reveal that the DIDS inhibition occurs in two steps (52): a rapid ionic interaction, which can be reversed by scavenging DIDS with albumin, and a slower covalent reaction. In either case, DIDS blocks transport. In AE1, the covalent binding site is Lys539 in TM5 as determined by site-directed mutagenesis (21) and biochemical analysis (225). At high pH, H2DIDS also binds to Lys851. The DIDS binding motif in AEs was recognized as KLXK (X = I and Y), where the first K binds to DIDS.
Based on the fact that NBCe1 has the similar DIDS motif (KMIK) in TM5, Lu et al. (203) performed mutagenesis of the KMIK motif and found that Lys559 (first K) produces the most substantial effect on DIDS sensitivity. The inhibitory constant for DIDS increases in the order of Asp > Glu > Gln > Lys at this position, indicating that a charge environment near the site is important for DIDS binding. DIDS is known to block anion channels/transporters by binding to the pore entry of the protein and occluding the pore (317). Thus, Lys559 is expected to be located near the pore entry. The NBCe1 mutated at Lys559 has similar HCO3− conductance compared to NBCe1 mutated at other residues in the KMIK motif (203). Thus, it is unclear whether Lys559 serves as the site for HCO3− binding. The inhibition constant Ki of NBCe1 for DIDS is 36 μmol/L (196), which is higher than those of the Cl,HCO3-exchangers AE1-3.
In addition to DIDS and SITS, there are other blockers that have inhibitory effects on NBCe1, and probably other NCBTs. Fluorescent oxonol dyes such as diBA(3)C4 and diBA(5)C4 inhibit NBCe1 (196) and are more potent than DIDS, although DIDS and oxonol dyes partially share the same binding site and compete with each other. Other anion transport blockers, including niflumic acid, benzamil, and tenidap, also inhibit NBCe1 (95,203). The inhibition by a broad spectrum of anion blockers implies that the pore of NBCe1 may contain a relatively large inner vestibule.
The N-cyanosulphonamide compound S0859 has been described as a more specific and reversible inhibitor of Na,HCO3-cotransport (57, 286, 345). The Ki for S0859 is 1.7 μM in cultured rat ventricular myocytes (57). At 30 μmol/L, which gives full inhibition of Na,HCO3-cotransport, S0859 does not affect Cl−/HCO3−-, Cl−/OH−-, or Na+/H+-exchange activity (57). Thus, its effect is specific to NCBTs, at least in ventricular cardiomyocytes. More recent studies also show that S0859 inhibits NBCn1 activity in the human breast cancer cell line MCF-7 (175) whereas surprisingly S0859 had no effect on Na,HCO3-cotransport activity in isolated arteries or slices of murine breast tumors (175). NBCn1 is responsible for Na,HCO3-cotransport activity in both of these tissues and differences in transporter isoform, therefore, does not appear to explain the difference. Instead, the authors suggested that a very strong binding to extracellular proteins (studied for plasma samples) may explain the effect of S0859 in isolated cells but not in tissues (175). Therefore, despite S0859 being a useful generic Na,HCO3-cotransport inhibitor and a definite improvement compared to the stilbene derivatives, it does not appear to distinguish between different NCBT isoforms and delivery of the compound in tissues and in vivo may be an issue.
Also functionally active antibodies have been used to modify NBC activity. An antibody was raised against NBCe1 (155) and shown to inhibit 50% to 66% of the recovery from acidosis in rat cardiomyocytes and HEK cells transfected with NBCe1. Interestingly, the antibody reduced the adverse effects of ischemia on performance of the isolated perfused rat heart in that study (155). Also De Giusti et al. (88) raised antibodies against NBCe1 and reported on two antibodies with concentration dependent activating and inhibitory effects, respectively on Na,HCO3-cotransport activity in isolated cat ventricular myocytes. It would be of substantial interest if this area was further developed.
Autostimulatory versus autoinhibitory domains
In Xenopus oocytes, all three variants NBCe1-A, -B, -C move 1 Na+ and at least 2 HCO3− into the cells, thus inducing HCO3−-dependent acid extrusion and electrogenic currents. Nonetheless, the different N- and C-terminal amino acids in the variants contribute to modulating cotransport activity, making them functionally distinct. McAlear and Bevensee (211) found that deleting the N-terminal 41 amino acids uniquely present in NBCe1-A decreases the current, whereas deleting the N-terminal 85 amino acids in NBCe1-B/C increases the current. The authors proposed that the N-terminal region in NBCe1-A serves as an autostimulatory domain, while the N-terminal region in NBCe1-B/C serves as an autoinhibitory domain. The autoinhibitory domain appears to be found in other NCBTs, according to the sequence comparison among the transporters (40). IRBIT binds to the N-terminal 85 (294) amino acids of NBCe1-B, and increases HCO3− transport activity (348). Deletion of residues 2 to 16 in the N-terminus abolishes the IRBIT-induced increase in transport activity without disrupting autoinhibition (183). These findings indicate that the structural determinant for autoinhibition is different from that for IRBIT binding.
Interaction with carbonic anhydrases
Some HCO3− transporters have been proposed to interact with CAs to form a functionally relevant protein complex (213). Most importantly, AE1 has been proposed to interact with CAII (324) through an acidic cluster DADD in the cytoplasmic C-terminus of AE1, which appears to serve as a consensus CAII binding site. The critical residue is the first Asp (D877) to which the basic amino acids in the N-terminal region of CAII bind by electrostatic interactions involving His and/or Lys residues. The AE1/CAII interaction has been reported to increase the rate of HCO3− transport (302). Cl−/HCO3− exchange via AE1 in erythrocytes is a rate-limiting step for CO2 delivery from tissues to the lungs (338), and maximizing the HCO3− transport rate helps utilize the full CO2 transport capacity of the blood. Other AEs and different CA isoforms are also reported to interact with each other (55).
Similarly, NBCe1 is reported to interact with CAII. NBCe1 contains D986NDD in the C-terminal domain (251), and—based on measurements of membrane conductance and intracellular [Na+]—the interaction has been proposed to help maximize the HCO3− gradient local to NBCe1 and enhance the cotransport rate (23, 251). NBCe1 also interacts with CAI and CAIII (285), CA IV (15), and CA IX (226, 309). Mutational studies has suggested that Gly767 in the fourth extracellular loop of the transporter is a site important for binding in a GST pull-down assay and for the stimulatory effect of CA IV on HCO3− transport (15). Missense mutations in CA IV associated with autosomal dominant rod-cone dystrophy disrupt NBCe1-mediated HCO3− transport in the eye, thus indicating that the interaction is essential for function (351).
Nonetheless, Boron and his colleagues found that neither CAII nor CAII-NBCe1 fusion affect NBCe1 currents or the pHi recovery rate mediated by NBCe1 in Xenopus oocytes (204). They propose a model where CAII in the immediate vicinity of NBCe1 has a minimal effect on local [HCO3−] and is thus unlikely to enhance NBCe1 activity (204). Furthermore, a recent report shows that a deletion mutant lacking the putative CAII-binding domain has normal transport activity (342). The role of CA binding to NBCe1 is thus controversial.
Membrane expression
A series of studies by Soleimani and colleagues suggest that the cytoplasmic C-terminal domain of NBCe1 is important for its targeting to the basolateral membrane. Deletion of the residues QQPFLS (position 1010–1015) causes the mutant protein to be misplaced to the apical membrane in the kidney epithelial cell line MDCK (193). In particular, Phe1013 and Leu1014 are essential. Substitution of Phe1013 or Leu1014 with Ala causes the transporter to be misplaced to the apical membrane, whereas substitution of Phe1013 with a Leu has no effect and substitution of Leu1014 with a Phe results in intracellular retention of the transporter (191). Shifting the Phe1013-Leu1014 pair by one amino acid position results in either misplacement or intracellular retention (189). These data indicate that the exact orientation and location of the two residues are critical for basolateral expression of NBCe1.
Several studies have been performed to determine structural elements important for intracellular trafficking. Espiritu et al. (97) demonstrated that deletion mutants lacking the cytoplasmic N- or C-terminal domains showed reduced basolateral membrane expression in opossum kidney OK cells. A recent report by Soleimani’s group shows that Asp405 and Asp416 in the N-terminal domain of NBCe1 are critical for protein expression in plasma membranes (190).
Protein structure
Similar to AEs (331, 356), NBCe1 forms a homodimer comprised of two individually functional subunits (109,153). The dimerization is mediated by the N-terminal domain, and also involves an intramolecular interaction between Cys630 and Cys642 in the EL3, although this stable linkage is unnecessary for function (153). Despite this progress, our knowledge on the NBCe1 protein structure is very limited. The topology is currently based on the hydropathy plot of the amino acid residues and biochemical analyses such as glycosylation, cysteine-scanning mutagenesis, and molecular modeling. It is generally accepted that the N-terminal and C-terminal domains of the SLC4-proteins are intracellular, but it has been debated how many TMs are present in the NCBTs. In particular, the second part of the TM domain (between TM6 and the last TM) has been debated. Zhu et al. (365) addressed this issue by cysteine-scanning mutagenesis of Ala800-Lys967 covering the second part of the TM domain, and identified the presence of 6 TMs in the region. The current NBCe1 topology model has 14 TMs. NBCe1 has a large extracellular loop between TM5 and TM6, whereas AEs have a large extracellular loop between TM7 and TM8. Recent work has further shown that in NBCe1 the important TM1 is longer compared to TM1 in AE1 and the TM1 in NBCe1 is predicted to take up a tilted position in the membrane (367).
SLC4A5 (NBCe2)
Physiology of NBCe2
Despite the cloning of NBCe2 in the early 2000s, its physiological importance in tissues is poorly understood. NBCe2 moves 1 Na+ and at least 2 HCO3− across the plasma membrane and thus is electrogenic (Fig. 6B). The transporter operates with a 1 Na+ to 2 HCO3− stoichiometry in Xenopus oocytes (326). In the renal proximal tubule cell line mPCT (280) and in intact cells isolated from choroid plexus epithelia (214), however, NBCe2 operates with a 1 Na+ to 3 HCO3− stoichiometry and is expected to mediate HCO3− efflux from the cells. NBCe2 is expressed in the apical membrane of choroid plexus epithelia (Fig. 8A) and thus transports Na+ and HCO3− to the cerebrospinal fluid (46). Very interestingly it has recently been shown that the NBCe2 expressed in the apical membrane of the choroid is a novel shorter variant of the known NBCe2, and is under control of an alternative promoter (102). Similarly, NBCe2 in the apical membrane of the renal collecting duct intercalated cells (81) is expected to contribute to HCO3− secretion.
Figure 8.
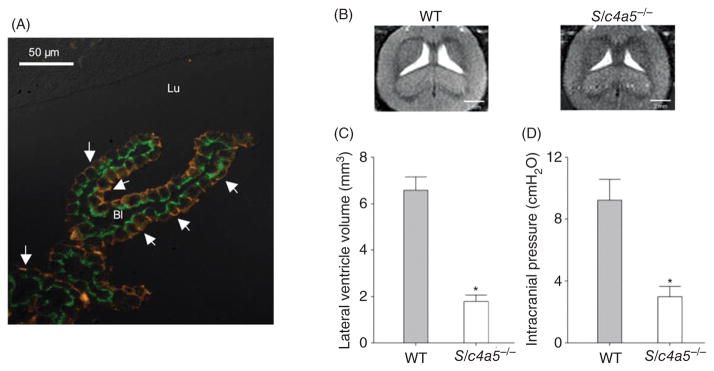
(A) Double-labeling immunofluorescence microscopic analysis of NBCe2 (red) and NCBE/NBCn2 (green) localization in rat choroid plexus (46). The fluorescence image was overlaid a differential interference contrast image and shows apical localization of NBCe2 (arrows) and basolateral localization of NCBE/NBCn2. Panels B and C show ventricular volume and (D) intracranial pressure in wild-type (WT) and NBCe2 knockout (Slc4a5−/−) mice (152). Panel B shows MRI imaging (horizontal plane) of the lateral ventricles in WT and Slc4a5−/− mice. The ventricular volume and intracranial pressure are significantly reduced in Slc4a5−/− mice.
Knockout mice with targeted disruption of the slc4a5 gene have profoundly decreased intracranial volume and pressure (Fig. 8B, 8C, and 8D) due to an abnormal cerebrospinal fluid volume (152). Mitochondria, Na+ transporting proteins, and cytoskeletal components are misplaced in choroid plexus epithelia from NBCe2 knockout mice, implying that NBCe2 affects the cellular structure of choroid plexus epithelia. These knockout mice have normal livers and kidneys, as well as normal plasma electrolytes. Another line of NBCe2 knockout mice develops arterial hypertension and metabolic acidosis (119). These mice also develop hyporeninemic hypoaldosteronism, elevated fluid intake and urine excretion, and increased glomerular filtration rate. Based on these findings, Groger et al. (119) suggested that lack of renal NBCe2 results in a compensatory increase in HCO3− absorption via other Na,HCO3-transporters such as NBCn1, which could cause an increase in Na+ uptake due to the difference in transport stoichiometry. While this could contribute to hypertension development, there is currently no strong evidence for luminal expression of NBCn1 in the relevant nephron segments at least in wild type mice.
Genetic polymorphisms
Genome-wide association analyses show significant levels of association between single nucleotide polymorphisms (SNPs) in SLC4 genes and pathological conditions (Table 4). In particular, several SNPs in SLC4A5 are associated with hypertension (20). SLC4A5 is one gene on chromosome 2 that is known to influence blood pressure (134, 163). A significant association of rs10177833 (Celera database: hcv1137534) with blood pressure reactivity, and systolic and diastolic blood pressures was observed in the Utah pedigree samples (136). SNP rs10177833 is also identified as one of the two SNPs (the other is rs7571842) that are associated with salt sensitivity of blood pressure in Caucasian Americans (salt sensitivity is defined as a ≥7 mmHg increase in mean arterial pressure during a transition between low and high [Na+] diet) (54). Other studies show that African American women with rs8179526 who consumed high sodium levels have lower systolic blood pressure than controls (311). The results support the idea of SLC4A5 serving as a hypertension susceptibility gene. In addition, certain SNPs are also associated with atherosclerotic peripheral arterial disease (154). Currently there is no understanding of how a modification in SLC4A5 influences development of atherosclerosis. Most SNPs in SLC4A5 are located in introns or nontranslated regions of the gene, and therefore, the observed associations of SLC4A5 polymorphisms with the cardiovascular phenotypes are unlikely due to direct changes in transport function. Instead, these SNPs may be in disequilibrium with functionally important SNPs in the coding region or may influence the expression levels of the transporters. The promoter activity of the SLC4A5 gene is not affected by polymorphisms (305).
Table 4.
SLC4 Genetic Polymorphisms Associated with Diseases
| Gene | Chrom | Total SNPs | Syn | Nonsyn | Disease-associated SNP | Disease | Reference |
|---|---|---|---|---|---|---|---|
| SLC4A4 | 4q21 | 7163 | 56 | 57 | Rs72650362 A/G (exon) | reduced activity* | (346) |
| SLC4A5 | 2p13 | 9750 | 68 | 93 | rs10177833 A/C (intron) | hypertension | (136) |
| SLC4A7 | 3p22 | 2357 | 51 | 73 | rs7571842 A/G (intron) | hypertension | (54) |
| SLC4A8 | 12q13 | 2312 | 46 | 47 | rs4973768 C/T (3′ UTR) | breast cancer | (7, 65, 127, 195, 201, 306) |
| SLC4A10 | 2q24 | 6234 | 46 | 43 | rs3278 A/G (intron) | drug addiction | (142, 188, 321) |
| rs13082711 C/T (5′ untream) | hypertension | (96) |
Association with disease is not known.
SLC4A7 (NBCn1)
Physiology of NBCn1
Among the NCBTs, NBCn1 is the second most studied transporter and transport in an electroneutral manner (Fig. 6C). Molecular and cellular studies have demonstrated the physiological importance of NBCn1 for acid-base regulation in a variety of tissues and expanded our understanding of how acid-base disturbances interfere with normal physiological functions.
(i) Renal acid-base regulation
NBCn1 is found in the renal thick ascending limb epithelium and in intercalated cells of the collecting ducts (167, 248, 327). In the medullary thick ascending limb, where 10% to 15% of filtered HCO3− is reabsorbed (112, 228), NBCn1 is localized to the basolateral side of the epithelium. Furthermore, DIDS-insensitive Na+-dependent HCO3− influx has been described in this segment. Considering the direction of transport, the role of NBCn1 in this nephron segment is unlikely associated with HCO3− reabsorption but instead is proposed to compensate for intracellular H+ liberated during transcellular transport of NH4+/NH3 across the thick ascending limb (166, 224). Importantly this function of NBCn1 could—together with NHE4 (44)—assist in transepithelial transport of NH4+ in the thick ascending limb. NH4+ transport and subsequent dissociation of intracellular NH4+ to NH3 and H+ are significantly enhanced during metabolic acidosis due to increased ammoniagenesis in the proximal tubules and luminal NH4+ delivery to the thick ascending limb. Consistent with this idea, NBCn1 expression and function are significantly upregulated in rats fed with a low-K+ diet (144) or treated with NH4+ in the drinking water (166), both of which increases NH4+ delivery to the thick ascending limb. NBCn1 in a medullary thick ascending limb cell line is also upregulated under an acidic incubation and enhances NH4+ transport (181). NBCn1 is sensitive to cellular and systemic pH changes, and its expression and activity are affected by acidosis and alkalosis. NBCn1 is downregulated by hypercalcemia-induced chronic metabolic alkalosis (334). Conversely, NBCn1 is upregulated by chronic metabolic acidosis induced by NH4Cl loading (166). The transporter is also upregulated after long-term lithium treatment that induces hyperchloremic metabolic acidosis (157). The upregulation of NBCn1 under these conditions may account for adaptive changes in HCO3− and NH4+ absorption in the thick ascending limb during chronic metabolic acidosis (111). Interestingly, acid/base transporters such as NBCn1, NBCe1, and NHE3 are downregulated in rat kidneys following ureter obstruction (332) or calcineurin inhibition (216), both of which cause metabolic acidosis. The downregulation of these transporters might be responsible for the development of metabolic acidosis.
(ii) HCO3− secretion in exocrine cells
A variety of secretory epithelial cells express NBCn1, but the physiological contribution of this transporter to HCO3− secretion has not been extensively investigated. In most epithelial tissues, NBCn1 is localized to the basolateral membranes (113, 230, 248, 249, 327). Thus, similar to NBCe1 in many secretory cells, NBCn1 raises intracellular HCO3− levels and stimulates apical HCO3− secretion mediated by other HCO3−-releasing proteins. On the other hand, some secretory cells such as pancreatic ducts and salivary glands also express NBCn1 in the apical membranes (113). Park et al. (232) demonstrated that NBCn1 interacts with the chloride channel CFTR in the apical membranes of pancreatic ducts and submandibular glands. At rest, NBCn1 salvages HCO3− in the lumen to minimize HCO3− secretion, but this salvage mechanism is unnecessary and inhibited upon stimulation of CFTR that regulates the activity of all luminal HCO3− secretion. In the duodenum NBCn1 is present in the basolateral membrane (64, 247), where it is shown to play a major role in basal and forskolin-induced secretion of HCO3− from the duodenum (64).
(iii) Arterial tone regulation
Both pHi (Fig. 8) and pHo (328) affect arterial tone regulation. Many proteins in the vasculature are sensitive to pH, and severe pH changes influence both vasoconstriction and vasodilation in resistance arteries (30). AEs and NHEs have been known to govern pHi regulation in vascular smooth muscle cells (150, 151), but more recently, it has become clear that HCO3−-dependent acid extrusion also plays a role for pHi regulation in vascular smoothmuscle cells (1,2). NBCn1 is found in vascular smooth muscle and endothelial cells determined by RT-PCR analyses, immunohistochemistry, and transgenic reporter mice (33, 34, 80). The transporter is found in intact mouse mesenteric, coronary, and cerebral small arteries. The molecular detection of NBCn1 (33) is consistent with the functional observation of Na+-dependent HCO3− transport in vascular smooth muscles from all of these vascular beds. Furthermore, knocking down NBCn1 in mouse mesenteric small arteries using small interference RNA decreases the steady-state pHi by ~0.2 pH unit and the net amiloride-insensitive, Na+-dependent base influx by ~70% (33), reflecting a significant contribution of NBCn1 to the net acid extrusion. Knockout mice with targeted disruption of slc4a7 show completely abolished Na,HCO3-cotransport activity and exhibit abnormalities in artery function (Fig. 9) and blood pressure regulation (35). These mice have a lower steady-state pHi in arterial endothelial cells (Fig. 9A) and smooth muscle cells (Fig. 9C), and also develop reduced nitric oxide-mediated vasorelaxation (Fig. 9B) and smooth muscle Rho-kinase dependent Ca2+ sensitivity with consequent reduced contraction to vasoconstrictors like norepinephrine (Fig. 9D). The NBCn1 knockout mice are mildly hypertensive, but resistant to developing hypertension to angiotensin II and nitric oxide synthase inhibition. Together with the recent observation that SNPs in SLC4A7 are associated with hypertension in humans (96), the knockout mouse study provides very strong arguments for NBCn1’s importance in the pathogenesis of hypertension and as a potential target in this condition.
Figure 9.
Effect of intracellular pH on vascular tone (35). In the NBCn1 (Slc4a7) knockout mouse (red) vascular endothelial cell (A) and smooth muscle cell (C) pH is reduced (vertical bars show SEM, n = 5 and 6, respectively). This is associated with a reduced endothelial cell mediated smooth muscle cell relaxation to acetylcholine (ACh) of norepinephrine (NE) activated mesenteric small arteries (B) and reduced tension development to NE in the presence of 100 μmol/L nitric oxide synthase inhibitor N(G)-nitro-L-arginine methyl ester (L-NAME) E), (vertical bars show SEM, n = 10). In the presence of 10 μmol/L Rho-kinase inhibitor fasudil, the tension development to NE is similar in arteries from wild type and knockout mice (D) (n = 5), consistent with the Rho-kinase being pH sensitive allowing for an effect of intracellular pH on smooth muscle cell tone.
(iv) Development of sensory neurons
Knockout mice with targeted disruption of the slc4a7 gene slowly develop blindness and auditory impairment due to degeneration of retinal photoreceptors and inner ear hair cells (36,202). In the inner ear, a progressive loss of inner and outer hair cells and spiral ganglia neurons was shown in knockout mice beginning at postnatal day 21. Type II and IV fibrocytes in the spiral ligament were also progressively lost. The pathological mechanism appears to involve a disruption of protein-protein interactions in the macromolecular network in the synaptic terminals of inner ear hair cells and retinal photoreceptors (259). The slc4a7 knockout mice have been proposed as a model to study Usher syndrome type IIB, but the clinical and genetic analyses of Usher patients show no mutations in NBCn1 (178).
(iv) Modulation of neuronal activity
Neuronal activity is affected by local pH at synapses and by systemic pH (67). Na+-driven Cl/HCO3-exchange has been functionally characterized as a major HCO3−-dependent acid extrusion pathway in neurons (22, 287). However, Cooper et al. (75) found NBCn1 protein expression in primary cultures of rat hippocampal neurons. NBCn1 in adult neurons lacks the 123-amino acid long cassette II and is localized to somatodendrites and synapses. It is found in both GABAergic and non-GABAergic neurons determined by single-cell RT-PCR analyses. NBCn1 is markedly upregulated following incubation in acidic or Mg2+-free media, probably to compensate intracellular acidification during acid loads or neuronal activity (76). NBCn1 knockdown attenuates excitotoxicity mediated by NMDA receptors in Mg2+ depletion, which is consistent with previous data that pharmacological inhibition of Na,H-exchange or Na,HCO3-transport protects neurons against damage or injury in cerebral ischemia (67). NBCn1 in the brain is upregulated in chronic metabolic acidosis (230), and this upregulation could be the basis for acidic injury of neurons during cerebral ischemia or chronic metabolic acidosis. Chen et al. (59) examined NBCn1 and NBCn2/NCBE expression in mouse brains subjected to chronic continuous hypoxia (11% O2 for 14 or 28 d) and found a decreased expression of the transporters in many different brain regions. The authors proposed that the decreased expression of NBCn1-B and NBCn2/NCBE reflect decreased energy consumption in the brain.
(v) Other roles
In osteoclasts, NBCn1 is involved in bone resorption (262). Knocking down NBCn1 inhibits bone resorption and increases intracellular acidification in osteoclasts. NBCn1 is also involved in osteoclast survival (45). In addition, NBCn1 is found in matrix vesicles released from osteoblasts during bone development (316). Electroneutral Na,HCO3-cotransport has been reported in ventricular cardiomyocytes and Purkinje fibers (85, 173). The cotransport affects excitation-contraction coupling and subsequent contractility. The mRNA levels and activities of both NBCn1 and NBCe1 are upregulated following ventricular hypertrophy and are induced by suprarenal abdominal aortic constriction (344). Enhanced NBC activity during hypertrophic development is expected to cause intracellular Na+ overload, which would be detrimental to myocytes during ischemic reperfusion due to Ca2+ overload via Na,Ca-exchangers. Immunohistochemical analysis also shows NBCn1 being localized to the capillaries of the heart ventricles (80).
Association with cancer development
The challenges to pH homeostasis in tumors are different from those in normal tissues. The pHi of tumor cells is near neutral or slightly alkaline, while the extracellular pH is very acidic usually between pH 6.5 and 6.8 (110). This reversed pH gradient is largely due to abnormal metabolism and affects tumor growth and progression (104). Tumor cells can thrive in this acidic pH environment and expand into the area of the dying normal tissues. NHE1 is one of the proteins responsible for extruding acids from tumor cells (212), but recent studies suggest that NBCn1 also plays a role (176, 238). Boedtkjer et al. (32) demonstrated that NBCn1 expression in membranes of human primary breast carcinomas and metastases is 20% to 30% higher than that in matched normal breast tissue (10). Functionally, cellular acid extrusion in the human primary breast carcinomas is mainly governed by Na,HCO3-cotransport (Fig. 10D) that is modestly sensitive to DIDS, a hallmark for NBCn1. NBCn1 is upregulated in tumors obtained after subcutaneous injection of breast cancer cells to mice and is one of the novel target proteins for tyrosine kinase phosphorylation in these tumors (66). Furthermore, expression of NBCn1, but not NHE1, is upregulated by the N-terminally truncated tyrosine kinase ErbB2/HER-2 (i.e., ΔNErbB2 or p95HER-2) (176), a constitutively active kinase involved in increased metastasis in breast cancers (72) and a protein conveying poor prognosis. This upregulation induced by ΔNErbB2 is comparable with an increased rate of pHi recovery after acid load. However, whereas NHE1 knockdown or inhibition causes ΔNErbB2-expressing cells to undergo programmed cell death and enhance cancer cell motility, NBCn1 knockdown or pharmacological inhibition shows no effects on these parameters (176,177). Thus, NBCn1 and NHE1 appear to have different effects on cancer progression although they share similar acid extrusion functions (31).
Figure 10.
NBCn1 expression in cancer cells. Micrographs of normal human breast (A) and breast cancer (B) immunostained (brown) for NBCn1. (C) Average (with SEM, n = 5) membrane density of NBCn1 immunostaining in normal breast, primary breast carcinomas, and metastases as indicated. (D) Na+-dependent ethylispropylamilroide (EIPA) insensitive recovery of intracellular pH in a biopsy of human breast cancer following washout of NH4Cl (vertical bars show SEM, n = 5). The recovery of intracellular pH was Na+ and HCO3− dependent and thus demonstrates a NCBT transport activity in breast cancer. The authors also showed that the NCBT activity was modestly DIDS-sensitive (34±9%), consistent with NBCn1 being responsible. Scale bars = 20 μm. Adapted, with permission, from (32).
Genetic polymorphisms
Genome-wide association studies have identified the SNP rs4973768 in SLC4A7 as one of the new breast cancer susceptibility loci (7, 127, 201, 215, 306). The SNP rs4973768 is tightly linked to increased breast cancer risk for BRCA2 carriers (18,223) and is particularly associated with the Luminal A subtype (127). This SNP is located in the 3′ untranslated region of the SLC4A7 gene, implying that the observed association with breast cancer phenotypes does not involve altered function of the transporter, but instead could be due to a change in NBCn1 mRNA stability.
SLC4A7 gene polymorphisms are also linked to other pathological or behavioral states. The marker rs3278 is identified as one of the addiction phenotypic marker loci (321). The minor allele frequency of rs3278 is prevalent in drug abusers who are strongly dependent on at least one illegal substance of abuse (142). The marker rs3278 is located in intron 7 of the gene. Another SNP rs13082711 is associated with hypertension (96), reflecting the complex blood pressure phenotype in slc4a7 knockout mice (35). In addition, the SLC4A7 gene locus shows a strong linkage to the levels of environmental toxic elements in erythrocytes (336, 337).
Channel-like activity
NBCn1 has intrinsic channel-like activity (68, 75). This channel-like activity is mediated by Na+ and raises intracellular Na+ levels in HEK 293 cells and Xenopus oocytes. It is not coupled to Na,HCO3-cotransport and occurs in the absence of HCO3−. Chimeric transporters with NBCe1 and NBCn1 show that the region of TM6–14 of NBCn1 is responsible for channel-like activity (71). Replacing the TM6-14 region of NBCn1 with the corresponding region of NBCe1 abolishes steady-state Na+ conductance, whereas the opposite replacement retains the conductance. The channel-like activity is not just an oddity of NBCn1 because other HCO3− transporters exhibit similar activities. Erythrocyte AE1 has an anion conductance that progressively increases as the membranes are hyperpolarized (101). Trout AE1 is known to produce a robust Cl− current and amino acids responsible for the currents have been examined (98). Other HCO3− transporters such as SLC26A7 and SLC26A9 have the capability to produce anion currents in addition to being exchangers (92, 156). The channel-like activity affects membrane potential and ion movement across the membrane at least in heterologous expression systems. Whether a similar effect occurs in the cells in vivo is unclear.
Interaction with other proteins
NBCn1 contains the PDZ binding motif in the C-terminal. NBCn1 can interact with many different PDZ proteins in cell/tissue-specific manners. In the kidney collecting ducts (254, 256), NBCn1 interacts with the 56 kDa subunit of H-ATPase. The interaction may help coordinate the two acid-base proteins NBCn1 and H-ATPase in the same side of the membrane. In the pancreatic ducts and salivary glands, NBCn1 interacts with ezrin-binding protein 50 (EBP50) (232), which is a binding protein of CFTR. NBCn1/EBP50 interaction allows NBCn1 to cluster with CFTR so that NBCn1 and CFTR are coordinately regulated by ATP. In the ear, NBCn1 interacts with harmonin to form a protein complex (259). In the nervous system, NBCn1 interacts with the postsynaptic density protein PSD-95 at synapses (230). Interestingly, the interaction does not affect Na,HCO3-cotransport activity, but stimulates channel-like activity (182).
NBCn1 has also been shown to interact physically and functionally with calcineurin Aβ (84). This interaction involves a calcineurin binding motif PTVVIH in cassette II in NBCn1. Calcineurin is required for Ca2+-induced activation of NBCn1 and inhibition of calcineurin augments intracellular acidification during rat mesenteric artery contraction (84). Since cassette II is predominantly expressed in smooth, cardiac and skeletal muscle tissues (74) this observation is an important step towards understanding the functional implication of different splice variants and a mechanism of tissue specific regulation of NBCn1. The role of this regulation for vascular function is still not clear.
NBCn1 expressed as GST-fusion protein has also been suggested to interact with CAII (200). The interaction purportedly involves the consensus CAII binding sites D1135D1136 in the C-terminal domain of NBCn1. The dominant-negative CAII mutant V143Y was furthermore found to reduce the rate of pHi recovery mediated by NBCn1 in HEK 293 cells, indicating that the interaction is required for optimal NBCn1 function. It should be noted, however, that binding of CAII to SLC4-termini (AE1, NBCe1, and NDCBE) was subsequently questioned by another group (244) who suggested that the original signs of binding could be explained by the ability of GST alone to bind CAII.
PKA reduces NBCn1 transport activity by a mechanism that neither involves CAII/NBCn1 interaction nor phosphorylation of the C-terminus.
SLC4A8 (NDCBE)
Paradoxically, the first Na+-coupled HCO3− transport to be described (i.e., Na+-dependent Cl,HCO3-exchange) has probably been the least studied in the postcloning period. When expressing the Slc4a9 gene product an electroneutral, sodium-dependent transport is apparent (Fig. 6E). Antibodies against NDCBE have been developed though and used to describe the distribution of NDCBE in the brain of humans (81), rats (49, 81, 179), and mice (61, 296). In all species immunohistochemistry suggested the presence of NDCBE in pyramidal neurons in hippocampus (Fig. 11A) and in Purkinje cells in cerebellum. In both mice and rats, where a more extensive study of the brain distribution of NDCBE was made, the immunostaining showed a widespread distribution in neurons of the cerebral cortex, in several places in the cerebellum as well as in substantia nigra and in medulla. A detailed analysis revealed that NDCBE is predominantly expressed in presynaptic glutamatergic terminals (49, 296). These morphological results were strongly supported by data from Slc4a8 knockout mice (296). Cultured hippocampal neurons from these mice had a low steady-state pHi and reduced acid extrusion, thus strongly supporting that NDCBE is an important acid extruder in hippocampal neurons. Functionally, it was shown that spontaneous glutamate release from the CA1 pyramidal layer of slc4a8 knockout mice was reduced compared to the release from the same site in wild-type mice, and more importantly this reduction could be rescued by increasing pHi. These data provide very strong arguments for NDCBE serving an important role in regulating pHi in glutamatergic neurons with consequences for glutamate release. This conclusion was corroborated by the finding that the knockout mice have an increased seizure threshold (296).
Figure 11.
Localization of NDCBE to the hippocampus of the human brain (adapted, with permission, from 81). (B) Effects of knockout of NDCBE (Slc4a8) on Na+-dependent pHi changes in intercalated cells in mice cortical collecting ducts (adapted, with permission, from 187). Traces are the average of pHi changes recorded when luminal Na+ was removed and readded, in the presence of extracellular HCO3−. The data provides functional evidence for the presence of NDCBE in the apical membrane, which is important for reabsorption of Na+ by these cells.
The antibody developed by Chen et al. (61) was also used to determine the effect of chronic hypoxia on the expression of NDCBE in the brain (60). In this study, neonatal (postnatal age of 2 days) and adult mice were exposed to normobaric hypoxia (11%) for 14 or 28 days. Based on Western blot analyses, it was found that NDCBE expression was reduced in the brain of adult mice. In the neonatal mice, the pattern was more complex because reduced expression was only seen in the hippocampus and subcortical areas. Also, the effect of metabolic acidosis on NDCBE expression was assessed (179). As expected, metabolic acidosis was associated with upregulation of NDCBE. Interestingly, the upregulation was not general but confined to specific areas in the brain, and the NDCBE downregulation was even seen in some areas such as the olfactory bulb.
In addition, a novel role of NDCBE for sodium reabsorption in mouse cortical collecting ducts has been suggested (187). In intercalated cells in the cortical collecting ducts of Na+-deprived mice a Na+-dependent regulation of intracellular pH occurs, which disappears in NDCBE knockout mice (Fig. 11B). In this segment an amiloride-insensitive and thiazide-sensitive sodium absorption is also present. This Na+ absorption is not explained by transport via the thiazide-sensitive Na,Cl-cotransporter, which is normally present in the distal tubules, but not present in this segment. This conclusion was further strengthened by the observation that the amiloride-insensitive and thiazide-sensitive sodium absorption was also present in knockout mice for the Na,Cl-cotransporter (187). Based on a series of experiments, it was suggested that the uptake was mediated by the combined activity of the Na+-independent Cl,HCO3-exchanger (pendrin or slc26a4) and NDCBE. Part of the evidence for this mechanism was the observation that in NDCBE knockout mice the thiazide-sensitive Na+ absorption is lost in the cortical collecting ducts. It now remains to be seen whether NDCBE is important during the development of salt-dependent hypertension.
SLC4A10 (NBCn2/NCBE)
Following the cloning and characterization of the SLC4A10 gene product as an electroneutral Na+-coupled (Fig. 6D) Cl,HCO3-exchanger NCBE, an important concern over the substrate dependence of the transporter has arisen (Fig. 12). Parker et al. (236) expressed SLC4A10 in oocytes and used electrodes to monitor surface [Cl−] and pHi. They furthermore measured the transmembrane flux of 36Cl− and provided convincing evidence that the SLC4A10 gene product does not provide net transport of Cl− under physiological conditions, although net transport of Cl− was found when extracellular Cl− was removed. Therefore, they suggested that the SLC4A10 gene product mediates electroneutral Na,HCO3-cotransport in parallel to Cl− self-exchange under physiological conditions and should be given the name NBCn2. This name reflects that it is the second electroneutral Na,HCO3-cotransporter. However, Damkier et al. (79) provided equally convincing evidence that net transport of Cl− was associated with activity of the SLC4A10 gene product when expressed in a mammalian fibroblast cell line. In this case, 36Cl− efflux was also examined, while pHi and [Na+]i were measured with fluorescent dyes. The authors found that, although expression of SLC4A10 induced a Na+- and HCO3−-dependent unidirectional efflux of 36Cl−, no matching increase in unidirectional 36Cl influx was seen. Apart from the very different experimental conditions, it is presently difficult to explain the different findings. An interesting possibility is that one of the HCO3− sites (i.e., one of the 2 HCO3− sites in the Na+-driven Cl,HCO3-exchanger of Fig. 12) prefers Cl− under some conditions. If this is the case, the transporter would behave as an NBCn2 with Cl− self-exchange when Cl− is the preferred substrate, and behave as an NCBE when HCO3− is the preferred substrate.
Figure 12.
NBCn2 versus NCBE. In Xenopus oocytes and HEK 293 cells, SLC4A10 encoding NBCn2/NCBE produces Cl− flux. This flux is either due to Cl− self-exchange uncoupled to Na/HCO3 transport (236) or Na,HCO3-dependent Cl− transport (79).
Notwithstanding the ambiguity concerning the substrate dependence, an understanding of the physiological role of NBCn2/NCBE is beginning to emerge. The transporter is predominantly found in the brain with the strongest expression in the cortex, hippocampus and cerebellum (59, 107, 143, 198). The expression appears in neurons rather than glia. With the development of antibodies distinguishing some subtypes, it was possible to demonstrate that four different subtypes have differential distribution profiles in the brain (198). Subtype A is the dominant form in the brain, while subtype D is mainly expressed in the medulla and subcortical regions. There is, however, currently little information on the specific physiological importance of NBCn2/NCBE in the brain. Chen et al. (59) report that chronic hypoxia leads to downregulation of NBCn2/NCBE (and also NBCn1) in several brain regions. This downregulation might be part of a general downregulation of transporters seen in the brain during hypoxia to reduce energy consumption. The relevance of the reduced expression of these transporters for the pathology associated with hypoxia was not studied.
A better defined role for NBCn2/NBCE has been obtained in the choroid plexus (Fig. 13A and 13B). The presence of NBCn2/NCBE in the basolateral membranes of choroid plexus is reported in several papers (46, 107, 249). With the development of slc4a10 knockout mice, the role of NBCn2/NCBE in producing cerebrospinal fluid became apparent (143). The ventricular volume of the knockout mice was substantially reduced (Fig. 13C, 13D, and 13E), consistent with NBCn2/NCBE playing an important role in production of CSF. This is consistent with an old observation demonstrating that the CSF production is dependent on HCO3− (277) and may be of clinically relevant as it has been suggested that CSF production may be important in, for example, Alzheimer’s disease (339). Although basal pHi in neurons from the slc4a10 knockout mice was not different from that of wild type mice, the knockout mice exhibit increased seizure threshold. No other major CNS phenotype was detected (143). The increased seizure threshold in the knockout mice is consistent with the observation that knockout of AE3 lowers the threshold for seizures (128).
Figure 13.
NBCn2/NCBE (Slc4a10) is present in the basolateral membrane of the choroid plexus epithelial cells and Slc4a10 knockout mice have reduced decreased volume of the brain ventricles (adapted, with permission, from 143). (A) Slc4a10 (green) localizes to the basolateral, but not the apical, membrane of the choroid plexus epithelium. The apical membrane is stained for the Na+/K+-ATPase (red) and the blue stain is nuclei (scale bar 30 μm). (B) In Slc4a10 knockout mice no NBCn2/NCBE staining was seen. [(C) and (D)] MRI scans of mice brain show the brain ventricles. (E) The volume of the brain ventricles from knockout mice is reduced to ≈25% of the volume in wild-type mice.
These findings in mice are, however, contrasted with the observation that in a patient with epilepsy, mental retardation, and cognitive impairment, a disruption of the SLC4A10 gene was found as the sole genetic abnormality (126, 161). Interestingly, partial deletion of SLC4A10 has also been associated to autism (289). Association between gene variations in SLC4A10 and open-angle glaucoma (198) (which is dependent on CSF production) and major depression (284) were investigated, but no convincing association was found. Interestingly, in the choroid plexus of slc4a10 knockout mice, NHE1 is abnormally expressed in the basolateral membranes, whereas in the wild type mice, this transporter is located to the apical membrane (83). The expression of the water channel AQP1 and the Na,K-pump in the choroid plexus were also reduced in NHE1 knockout mice (82). The importance of this is currently unknown.
Conclusion
With the molecular characterization of the five NCBTs described in this article, the field of pH regulation has come to a new level. Virtually, every cell in the body has one or more of these transporters. By transporting the base HCO3− into or out of cells, these transporters play a central role in regulation of pHi. With the expression in many epithelial cells, they also contribute substantially to the whole body acid-base homeostasis. For example, HCO3− reabsorption mediated by NBCe1 in the proximal tubules is crucial for normal acid-base homeostasis in the body. Despite the substantial advance in our knowledge regarding the molecular, cellular, and physiological implications of the transporters, much more experimental evidence is required to fully understand the physiological and pathophysiological importance of the transporters. It is unclear how the transporters are precisely regulated, both at the expression and activity levels. Also, since only very limited crystal structure information is available, the knowledge on structure-function relationships is primitive. We also need specific drugs to alter transport activity and help characterize the physiological importance and therapeutic potential of the transporters. Some examples of mutations leading to severe conditions have been described and there are likely many more conditions to be described, where these transporters are dysfunctional. Also, even though disturbed bicarbonate transport clearly is associated with conditions like cancer, cardiovascular disease, and metabolic disturbances, the precise mechanistic role and therapeutic potential of the NCBTs remains to be investigated. In conclusion, we expect very novel and exciting results coming from the studies of bicarbonate transporters in the near future.
Acknowledgments
We would like to thank Min Hyung Kwon, Courtni Andrews, and Akanksha Samal for illustration work. This work was supported by the NIH GM078502 (IC) and The Danish Council for Independent Research (12-126232 to CA) and (10-094816 to EB).
References
- 1.Aalkjaer C, Cragoe EJ., Jr Intracellular pH regulation in resting and contracting segments of rat mesenteric resistance vessels. J Physiol. 1988;402:391–410. doi: 10.1113/jphysiol.1988.sp017211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aalkjaer C, Hughes A. Chloride and bicarbonate transport in rat resistance arteries. J Physiol. 1991;436:57–73. doi: 10.1113/jphysiol.1991.sp018539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aalkjaer C, Mulvany MJ. Steady-state effects of arginine vasopressin on force and pHi of isolated mesenteric resistance arteries from rats. Am J Physiol. 1991;261:C1010–C1017. doi: 10.1152/ajpcell.1991.261.6.C1010. [DOI] [PubMed] [Google Scholar]
- 4.Abuladze N, Azimov R, Newman D, Sassani P, Liu W, Tatishchev S, Pushkin A, Kurtz I. Critical amino acid residues involved in the electrogenic sodium-bicarbonate cotransporter kNBC1-mediated transport. J Physiol. 2005;565:717–730. doi: 10.1113/jphysiol.2005.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem. 1998;273:17689–17695. doi: 10.1074/jbc.273.28.17689. [DOI] [PubMed] [Google Scholar]
- 6.Abuladze N, Pushkin A, Tatishchev S, Newman D, Sassani P, Kurtz I. Expression and localization of rat NBC4c in liver and renal uroepithelium. Am J Physiol Cell Physiol. 2004;287:C781–C789. doi: 10.1152/ajpcell.00590.2003. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed S, Thomas G, Ghoussaini M, Healey CS, Humphreys MK, Platte R, Morrison J, Maranian M, Pooley KA, Luben R, Eccles D, Evans DG, Fletcher O, Johnson N, dos SSI, Peto J, Stratton MR, Rahman N, Jacobs K, Prentice R, Anderson GL, Rajkovic A, Curb JD, Ziegler RG, Berg CD, Buys SS, McCarty CA, Feigelson HS, Calle EE, Thun MJ, Diver WR, Bojesen S, Nordestgaard BG, Flyger H, Dork T, Schurmann P, Hillemanns P, Karstens JH, Bogdanova NV, Antonenkova NN, Zalutsky IV, Bermisheva M, Fedorova S, Khusnutdinova E, Kang D, Yoo KY, Noh DY, Ahn SH, Devilee P, van Asperen CJ, Tollenaar RA, Seynaeve C, Garcia-Closas M, Lissowska J, Brinton L, Peplonska B, Nevanlinna H, Heikkinen T, Aittomaki K, Blomqvist C, Hopper JL, Southey MC, Smith L, Spurdle AB, Schmidt MK, Broeks A, van Hien RR, Cornelissen S, Milne RL, Ribas G, Gonzalez-Neira A, Benitez J, Schmutzler RK, Burwinkel B, Bartram CR, Meindl A, Brauch H, Justenhoven C, Hamann U, Chang-Claude J, Hein R, Wang-Gohrke S, Lindblom A, Margolin S, Mannermaa A, Kosma VM, Kataja V, Olson JE, Wang X, Fredericksen Z, Giles GG, Severi G, Baglietto L, English DR, Hankinson SE, Cox DG, Kraft P, Vatten LJ, Hveem K, Kumle M, Sigurdson A, Doody M, Bhatti P, Alexander BH, Hooning MJ, van den Ouweland AM, Oldenburg RA, Schutte M, Hall P, Czene K, Liu J, Li Y, Cox A, Elliott G, Brock I, Reed MW, Shen CY, Yu JC, Hsu GC, Chen ST, Anton-Culver H, Ziogas A, Andrulis IL, Knight JA, Beesley J, Goode EL, Couch F, Chenevix-Trench G, Hoover RN, Ponder BA, Hunter DJ, Pharoah PD, Dunning AM, Chanock SJ, Easton DF. Newly discovered breast cancer susceptibility loci on 3p24 and 17q23.2. Nat Genet. 2009;41:585–590. doi: 10.1038/ng.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aickin CC. Movement of acid equivalents across the mammalian smooth muscle cell membrane. Ciba Found Symp. 1988;139:3–22. doi: 10.1002/9780470513699.ch2. [DOI] [PubMed] [Google Scholar]
- 9.Aiello EA, Petroff MG, Mattiazzi AR, Cingolani HE. Evidence for an electrogenic Na+-HCO3− symport in rat cardiac myocytes. J Physiol. 1998;512:137–148. doi: 10.1111/j.1469-7793.1998.137bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akiba T, Rocco VK, Warnock DG. Parallel adaptation of the rabbit renal cortical sodium/proton antiporter and sodium/bicarbonate cotransporter in metabolic acidosis and alkalosis. J Clin Invest. 1987;80:308–315. doi: 10.1172/JCI113074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alper SL. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J Exp Biol. 2009;212:1672–1683. doi: 10.1242/jeb.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alper SL, Chernova MN, Williams J, Zasloff M, Law FY, Knauf PA. Differential inhibition of AE1 and AE2 anion exchangers by oxonol dyes and by novel polyaminosterol analogs of the shark antibiotic squalamine. Biochem Cell Biol. 1998;76:799–806. doi: 10.1139/bcb-76-5-799. [DOI] [PubMed] [Google Scholar]
- 13.Alpern RJ. Mechanism of basolateral membrane H+/OH−/HCO3− transport in the rat proximal convoluted tubule. A sodium-coupled electrogenic process. J Gen Physiol. 1985;86:613–636. doi: 10.1085/jgp.86.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alpern RJ. Cell mechanisms of proximal tubule acidification. Physiol Rev. 1990;70:79–114. doi: 10.1152/physrev.1990.70.1.79. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez BV, Loiselle FB, Supuran CT, Schwartz GJ, Casey JR. Direct extracellular interaction between carbonic anhydrase IV and the human NBC1 sodium/bicarbonate co-transporter. Biochemistry. 2003;42:12321–12329. doi: 10.1021/bi0353124. [DOI] [PubMed] [Google Scholar]
- 16.Amlal H, Chen Q, Greeley T, Pavelic L, Soleimani M. Coordinated down-regulation of NBC-1 and NHE-3 in sodium and bicarbonate loading. Kidney Int. 2001;60:1824–1836. doi: 10.1046/j.1523-1755.2001.00995.x. [DOI] [PubMed] [Google Scholar]
- 17.Amlal H, Wang Z, Burnham C, Soleimani M. Functional characterization of a cloned human kidney Na+:HCO3− cotransporter. J Biol Chem. 1998;273:16810–16815. doi: 10.1074/jbc.273.27.16810. [DOI] [PubMed] [Google Scholar]
- 18.Antoniou AC, Beesley J, McGuffog L, Sinilnikova OM, Healey S, Neuhausen SL, Ding YC, Rebbeck TR, Weitzel JN, Lynch HT, Isaacs C, Ganz PA, Tomlinson G, Olopade OI, Couch FJ, Wang X, Lindor NM, Pankratz VS, Radice P, Manoukian S, Peissel B, Zaffaroni D, Barile M, Viel A, Allavena A, Dall’Olio V, Peterlongo P, Szabo CI, Zikan M, Claes K, Poppe B, Foretova L, Mai PL, Greene MH, Rennert G, Lejbkowicz F, Glendon G, Ozcelik H, Andrulis IL, Thomassen M, Gerdes AM, Sunde L, Cruger D, Birk JU, Caligo M, Friedman E, Kaufman B, Laitman Y, Milgrom R, Dubrovsky M, Cohen S, Borg A, Jernstrom H, Lindblom A, Rantala J, Stenmark-Askmalm M, Melin B, Nathanson K, Domchek S, Jakubowska A, Lubinski J, Huzarski T, Osorio A, Lasa A, Duran M, Tejada MI, Godino J, Benitez J, Hamann U, Kriege M, Hoogerbrugge N, van der Luijt RB, van Asperen CJ, Devilee P, Meijers-Heijboer EJ, Blok MJ, Aalfs CM, Hogervorst F, Rookus M, Cook M, Oliver C, Frost D, Conroy D, Evans DG, Lalloo F, Pichert G, Davidson R, Cole T, Cook J, Paterson J, Hodgson S, Morrison PJ, Porteous ME, Walker L, Kennedy MJ, Dorkins H, Peock S, Godwin AK, Stoppa-Lyonnet D, de PA, Mazoyer S, Bonadona V, Lasset C, Dreyfus H, Leroux D, Hardouin A, Berthet P, Faivre L, Loustalot C, Noguchi T, Sobol H, Rouleau E, Nogues C, Frenay M, Venat-Bouvet L, Hopper JL, Daly MB, Terry MB, John EM, Buys SS, Yassin Y, Miron A, Goldgar D, Singer CF, Dressler AC, Gschwantler-Kaulich D, Pfeiler G, Hansen TV, Jonson L, Agnarsson BA, Kirchhoff T, Offit K, Devlin V, Dutra-Clarke A, Piedmonte M, Rodriguez GC, Wakeley K, Boggess JF, Basil J, Schwartz PE, Blank SV, Toland AE, Montagna M, Casella C, Imyanitov E, Tihomirova L, Blanco I, Lazaro C, Ramus SJ, Sucheston L, Karlan BY, Gross J, Schmutzler R, Wappenschmidt B, Engel C, Meindl A, Lochmann M, Arnold N, Heidemann S, Varon-Mateeva R, Niederacher D, Sutter C, Deissler H, Gadzicki D, Preisler-Adams S, Kast K, Schonbuchner I, Caldes T, de la Hoya M, Aittomaki K, Nevanlinna H, Simard J, Spurdle AB, Holland H, Chen X, Platte R, Chenevix-Trench G, Easton DF. Common breast cancer susceptibility alleles and the risk of breast cancer for BRCA1 and BRCA2 mutation carriers: Implications for risk prediction. Cancer Res. 2010;70:9742–9754. doi: 10.1158/0008-5472.CAN-10-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachmann O, Rossmann H, Berger UV, Colledge WH, Ratcliff R, Evans MJ, Gregor M, Seidler U. cAMP-mediated regulation of murine intestinal/pancreatic Na+/HCO3− cotransporter subtype pNBC1. Am J Physiol Gastrointest Liver Physiol. 2003;284:G37–G45. doi: 10.1152/ajpgi.00209.2002. [DOI] [PubMed] [Google Scholar]
- 20.Barkley RA, Chakravarti A, Cooper RS, Ellison RC, Hunt SC, Province MA, Turner ST, Weder AB, Boerwinkle E. Positional identification of hypertension susceptibility genes on chromosome 2. Hypertension. 2004;43:477–482. doi: 10.1161/01.HYP.0000111585.76299.f7. [DOI] [PubMed] [Google Scholar]
- 21.Bartel D, Hans H, Passow H. Identification by site-directed mutagenesis of Lys-558 as the covalent attachment site of H2DIDS in the mouse erythroid band 3 protein. Biochim Biophys Acta. 1989;985:355–358. doi: 10.1016/0005-2736(89)90427-6. [DOI] [PubMed] [Google Scholar]
- 22.Baxter KA, Church J. Characterization of acid extrusion mechanisms in cultured fetal rat hippocampal neurones. J Physiol. 1996;493:457–470. doi: 10.1113/jphysiol.1996.sp021396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker HM, Deitmer JW. Carbonic anhydrase II increases the activity of the human electrogenic Na+/HCO3− cotransporter. J Biol Chem. 2007;282:13508–13521. doi: 10.1074/jbc.M700066200. [DOI] [PubMed] [Google Scholar]
- 24.Bevensee MO, Apkon M, Boron WF. Intracellular pH regulation in cultured astrocytes from rat hippocampus. II. Electrogenic Na+/HCO3− cotransport. J Gen Physiol. 1997;110:467–483. doi: 10.1085/jgp.110.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bevensee MO, Boron WF. Effects of acute hypoxia on intracellular-pH regulation in astrocytes cultured from rat hippocampus. Brain Res. 2008;1193:143–152. doi: 10.1016/j.brainres.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF. An electrogenic Na+-HCO3− cotransporter (NBC) with a novel COOH-terminus, cloned from rat brain. Am J Physiol Cell Physiol. 2000;278:C1200–C1211. doi: 10.1152/ajpcell.2000.278.6.C1200. [DOI] [PubMed] [Google Scholar]
- 27.Bevensee MO, Weed RA, Boron WF. Intracellular pH regulation in cultured astrocytes from rat hippocampus. I. Role Of HCO3−. J Gen Physiol. 1997;110:453–465. doi: 10.1085/jgp.110.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beyenbach KW, Wieczorek H. The V-type H+ ATPase: Molecular structure and function, physiological roles and regulation. J Exp Biol. 2006;209:577–589. doi: 10.1242/jeb.02014. [DOI] [PubMed] [Google Scholar]
- 29.Biagi BA, Sohtell M. Electrophysiology of basolateral bicarbonate transport in the rabbit proximal tubule. Am J Physiol. 1986;250:F267–F272. doi: 10.1152/ajprenal.1986.250.2.F267. [DOI] [PubMed] [Google Scholar]
- 30.Boedtkjer E, Aalkjaer C. Intracellular pH in the resistance vasculature: Regulation and functional implications. J Vasc Res. 2012;49:479–496. doi: 10.1159/000341235. [DOI] [PubMed] [Google Scholar]
- 31.Boedtkjer E, Bunch L, Pedersen SF. Physiology, pharmacology and pathophysiology of the pH regulatory transport proteins NHE1 and NBCn1: Similarities, differences, and implications for cancer therapy. Curr Pharm Des. 2012;18:1345–1371. doi: 10.2174/138161212799504830. [DOI] [PubMed] [Google Scholar]
- 32.Boedtkjer E, Moreira JM, Mele M, Vahl P, Wielenga VT, Christiansen PM, Jensen VE, Pedersen SF, Aalkjaer C. Contribution of Na+,HCO3−-cotransport to cellular pH control in human breast cancer: A role for the breast cancer susceptibility locus NBCn1 (SLC4A7) Int J Cancer. 2013;132:1288–1299. doi: 10.1002/ijc.27782. [DOI] [PubMed] [Google Scholar]
- 33.Boedtkjer E, Praetorius J, Aalkjaer C. NBCn1 (slc4a7) mediates the Na+-dependent bicarbonate transport important for regulation of intracellular pH in mouse vascular smooth muscle cells. Circ Res. 2006;98:515–523. doi: 10.1161/01.RES.0000204750.04971.76. [DOI] [PubMed] [Google Scholar]
- 34.Boedtkjer E, Praetorius J, Fuchtbauer EM, Aalkjaer C. Antibody-independent localization of the electroneutral Na+-HCO3− cotransporter NBCn1 (slc4a7) in mice. Am J Physiol Cell Physiol. 2008;294:C591–C603. doi: 10.1152/ajpcell.00281.2007. [DOI] [PubMed] [Google Scholar]
- 35.Boedtkjer E, Praetorius J, Matchkov VV, Stankevicius E, Mogensen S, Fuchtbauer AC, Simonsen U, Fuchtbauer EM, Aalkjaer C. Disruption of Na+,HCO3− cotransporter NBCn1 (slc4a7) inhibits NO-mediated vasorelaxation, smooth muscle Ca2+ sensitivity, and hypertension development in mice. Circulation. 2011;124:1819–1829. doi: 10.1161/CIRCULATIONAHA.110.015974. [DOI] [PubMed] [Google Scholar]
- 36.Bok D, Galbraith G, Lopez I, Woodruff M, Nusinowitz S, BeltrandelRio H, Huang W, Zhao S, Geske R, Montgomery C, Van SSI, Friddle C, Platt K, Sparks MJ, Pushkin A, Abuladze N, Ishiyama A, Dukkipati R, Liu W, Kurtz I. Blindness and auditory impairment caused by loss of the sodium bicarbonate cotransporter NBC3. Nat Genet. 2003;34:313–319. doi: 10.1038/ng1176. [DOI] [PubMed] [Google Scholar]
- 37.Boron WF. Intracellular pH-regulating mechanism of the squid axon. Relation between the external Na+ and HCO3− dependences. J Gen Physiol. 1985;85:325–345. doi: 10.1085/jgp.85.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boron WF. Evaluating the role of carbonic anhydrases in the transport of HCO3−-related species. Biochim Biophys Acta. 2010;1804:410–421. doi: 10.1016/j.bbapap.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boron WF, Boulpaep EL. Intracellular pH regulation in the renal proximal tubule of the salamander. Na+-H+ exchange. J Gen Physiol. 1983;81:29–52. doi: 10.1085/jgp.81.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boron WF, Chen L, Parker MD. Modular structure of sodium-coupled bicarbonate transporters. J Exp Biol. 2009;212:1697–1706. doi: 10.1242/jeb.028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boron WF, De Weer P. Active proton transport stimulated by CO2/HCO3−, blocked by cyanide. Nature. 1976;259:240–241. doi: 10.1038/259240a0. [DOI] [PubMed] [Google Scholar]
- 42.Boron WF, McCormick WC, Roos A. pH regulation in barnacle muscle fibers: Dependence on extracellular sodium and bicarbonate. Am J Physiol. 1981;240:C80–C89. doi: 10.1152/ajpcell.1981.240.1.C80. [DOI] [PubMed] [Google Scholar]
- 43.Bourgeois S, Masse S, Paillard M, Houillier P. Basolateral membrane Cl−-, Na+-, and K+-coupled base transport mechanisms in rat MTALH. Am J Physiol Renal Physiol. 2002;282:F655–F668. doi: 10.1152/ajprenal.00220.2000. [DOI] [PubMed] [Google Scholar]
- 44.Bourgeois S, Meer LV, Wootla B, Bloch-Faure M, Chambrey R, Shull GE, Gawenis LR, Houillier P. NHE4 is critical for the renal handling of ammonia in rodents. J Clin Invest. 2010;120:1895–1904. doi: 10.1172/JCI36581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouyer P, Sakai H, Itokawa T, Kawano T, Fulton CM, Boron WF, Insogna KL. Colony-stimulating factor-1 increases osteoclast intracellular pH and promotes survival via the electroneutral Na+/HCO3− cotransporter NBCn1. Endocrinology. 2007;148:831–840. doi: 10.1210/en.2006-0547. [DOI] [PubMed] [Google Scholar]
- 46.Bouzinova EV, Praetorius J, Virkki LV, Nielsen S, Boron WF, Aalkjaer C. Na+-dependent HCO3− uptake into the rat choroid plexus epithelium is partially DIDS sensitive. Am J Physiol Cell Physiol. 2005;289:C1448–C1456. doi: 10.1152/ajpcell.00313.2005. [DOI] [PubMed] [Google Scholar]
- 47.Brandes A, Oehlke O, Schumann A, Heidrich S, Thevenod F, Roussa E. Adaptive redistribution of NBCe1-A and NBCe1-B in rat kidney proximal tubule and striated ducts of salivary glands during acid-base disturbances. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2400–R2411. doi: 10.1152/ajpregu.00208.2007. [DOI] [PubMed] [Google Scholar]
- 48.Burckhardt BC, Sato K, Fromter E. Electrophysiological analysis of bicarbonate permeation across the peritubular cell membrane of rat kidney proximal tubule. I. Basic observations. Pflugers Arch. 1984;401:34–42. doi: 10.1007/BF00581530. [DOI] [PubMed] [Google Scholar]
- 49.Burette AC, Weinberg RJ, Sassani P, Abuladze N, Kao L, Kurtz I. The sodium-driven chloride/bicarbonate exchanger in presynaptic terminals. J Comp Neurol. 2012;520:1481–1492. doi: 10.1002/cne.22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burnham CE, Amlal H, Wang Z, Shull GE, Soleimani M. Cloning and functional expression of a human kidney Na+:HCO3− cotransporter. J Biol Chem. 1997;272:19111–19114. doi: 10.1074/jbc.272.31.19111. [DOI] [PubMed] [Google Scholar]
- 51.Cabantchik ZI, Knauf PA, Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of ‘probes’. Biochim Biophys Acta. 1978;515:239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- 52.Cabantchik ZI, Rothstein A. The nature of the membrane sites controlling anion permeability of human red blood cells as determined by studies with disulfonic stilbene derivatives. J Membr Biol. 1972;10:311–330. doi: 10.1007/BF01867863. [DOI] [PubMed] [Google Scholar]
- 53.Camilion de Hurtado MC, Perez NG, Cingolani HE. An electrogenic sodium-bicarbonate cotransport in the regulation of myocardial intracellular pH. J Mol Cell Cardiol. 1995;27:231–242. [PubMed] [Google Scholar]
- 54.Carey RM, Schoeffel CD, Gildea JJ, Jones JE, McGrath HE, Gordon LN, Park MJ, Sobota RS, Underwood PC, Williams J, Sun B, Raby B, Lasky-Su J, Hopkins PN, Adler GK, Williams SM, Jose PA, Felder RA. Salt sensitivity of blood pressure is associated with polymorphisms in the sodium-bicarbonate cotransporter. Hypertension. 2012;60:1359–1366. doi: 10.1161/HYPERTENSIONAHA.112.196071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Casey JR, Sly WS, Shah GN, Alvarez BV. Bicarbonate homeostasis in excitable tissues: Role of AE3 Cl−/HCO3− exchanger and carbonic anhydrase XIV interaction. Am J Physiol Cell Physiol. 2009;297:C1091–C1102. doi: 10.1152/ajpcell.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chambrey R, Kurth I, Peti-Peterdi J, Houillier P, Purkerson JM, Leviel F, Hentschke M, Zdebik AA, Schwartz GJ, Hubner CA, Eladari D. Renal intercalated cells are rather energized by a proton than a sodium pump. Proc Natl Acad Sci U S A. 2013;110:7928–7933. doi: 10.1073/pnas.1221496110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ch’en FF, Villafuerte FC, Swietach P, Cobden PM, Vaughan-Jones RD. S0859, an N-cyanosulphonamide inhibitor of sodium-bicarbonate cotransport in the heart. Br J Pharmacol. 2008;153:972–982. doi: 10.1038/sj.bjp.0707667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang MH, DiPiero J, Sonnichsen FD, Romero MF. Entry to “HCO3− tunnel” revealed by SLC4A4 human mutation and structural model. J Biol Chem. 2008;283:18402–18410. doi: 10.1074/jbc.M709819200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen LM, Choi I, Haddad GG, Boron WF. Chronic continuous hypoxia decreases the expression of SLC4A7 (NBCn1) and SLC4A10 (NCBE) in mouse brain. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2412–R2420. doi: 10.1152/ajpregu.00497.2007. [DOI] [PubMed] [Google Scholar]
- 60.Chen LM, Haddad GG, Boron WF. Effects of chronic continuous hypoxia on the expression of SLC4A8 (NDCBE) in neonatal versus adult mouse brain. Brain Res. 2008;1238:85–92. doi: 10.1016/j.brainres.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen LM, Kelly ML, Parker MD, Bouyer P, Gill HS, Felie JM, Davis BA, Boron WF. Expression and localization of Na+-driven Cl−-HCO3− exchanger (SLC4A8) in rodent CNS. Neuroscience. 2008;153:162–174. doi: 10.1016/j.neuroscience.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen LM, Liu Y, Boron WF. Role of an extracellular loop in determining the stoichiometry of Na+-HCO3− cotransporters. J Physiol. 2011;589:877–890. doi: 10.1113/jphysiol.2010.198713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen LM, Qin X, Moss FJ, Liu Y, Boron WF. Effect of simultaneously replacing putative TM6 and TM12 of human NBCe1-A with those from NBCn1 on surface abundance in Xenopus oocytes. J Membr Biol. 2012;245:131–140. doi: 10.1007/s00232-012-9421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen M, Praetorius J, Zheng W, Xiao F, Riederer B, Singh AK, Stieger N, Wang J, Shull GE, Aalkjaer C, Seidler U. The electroneutral Na+:HCO3− cotransporter NBCn1 is a major pHi regulator in murine duodenum. J Physiol. 2012;590:3317–3333. doi: 10.1113/jphysiol.2011.226506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen W, Zhong R, Ming J, Zou L, Zhu B, Lu X, Ke J, Zhang Y, Liu L, Miao X, Huang T. The SLC4A7 variant rs4973768 is associated with breast cancer risk: Evidence from a case-control study and a meta-analysis. Breast Cancer Res Treat. 2012;136:847–857. doi: 10.1007/s10549-012-2309-9. [DOI] [PubMed] [Google Scholar]
- 66.Chen Y, Choong LY, Lin Q, Philp R, Wong CH, Ang BK, Tan YL, Loh MC, Hew CL, Shah N, Druker BJ, Chong PK, Lim YP. Differential expression of novel tyrosine kinase substrates during breast cancer development. Mol Cell Proteomics. 2007;6:2072–2087. doi: 10.1074/mcp.M700395-MCP200. [DOI] [PubMed] [Google Scholar]
- 67.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 68.Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature. 2000;405:571–575. doi: 10.1038/35014615. [DOI] [PubMed] [Google Scholar]
- 69.Choi I, Hu L, Rojas JD, Schmitt BM, Boron WF. Role of glycosylation in the renal electrogenic Na+/HCO3− cotransporter (NBCe1) Am J Physiol Renal Physiol. 2003;284:F1199–F1206. doi: 10.1152/ajprenal.00131.2002. [DOI] [PubMed] [Google Scholar]
- 70.Choi I, Romero MF, Khandoudi N, Bril A, Boron WF. Cloning and characterization of a human electrogenic Na+-HCO3− cotransporter isoform (hhNBC) Am J Physiol. 1999;276:C576–C584. doi: 10.1152/ajpcell.1999.276.3.C576. [DOI] [PubMed] [Google Scholar]
- 71.Choi I, Soo YH, Boron WF. The electrogenicity of the rat sodium-bicarbonate cotransporter NBCe1 requires interactions among transmembrane segments of the transporter. J Physiol. 2007;578:131–142. doi: 10.1113/jphysiol.2006.114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Christianson TA, Doherty JK, Lin YJ, Ramsey EE, Holmes R, Keenan EJ, Clinton GM. NH2-terminally truncated HER-2/neu protein: Relationship with shedding of the extracellular domain and with prognostic factors in breast cancer. Cancer Res. 1998;58:5123–5129. [PubMed] [Google Scholar]
- 73.Cogan MG. Chronic hypercapnia stimulates proximal bicarbonate reabsorption in the rat. J Clin Invest. 1984;74:1942–1947. doi: 10.1172/JCI111614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cooper DS, Lee HJ, Yang HS, Kippen J, Yun CC, Choi I. The electroneutral sodium/bicarbonate cotransporter containing an amino terminal 123-amino-acid cassette is expressed predominantly in the heart. J Biomed Sci. 2006;13:593–595. doi: 10.1007/s11373-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cooper DS, Saxena NC, Yang HS, Lee HJ, Moring AG, Lee A, Choi I. Molecular and functional characterization of the electroneutral Na+/HCO3− cotransporter NBCn1 in rat hippocampal neurons. J Biol Chem. 2005;280:17823–17830. doi: 10.1074/jbc.M408646200. [DOI] [PubMed] [Google Scholar]
- 76.Cooper DS, Yang HS, He P, Kim E, Rajbhandari I, Yun CC, Choi I. Sodium/bicarbonate cotransporter NBCn1/slc4a7 increases cytotoxicity in magnesium depletion in primary cultures of hippocampal neurons. Eur J Neurosci. 2009;29:437–446. doi: 10.1111/j.1460-9568.2008.06611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cordat E, Casey JR. Bicarbonate transport in cell physiology and disease. Biochem J. 2009;417:423–439. doi: 10.1042/BJ20081634. [DOI] [PubMed] [Google Scholar]
- 78.Curci S, Debellis L, Fromter E. Evidence for rheogenic sodium bicarbonate cotransport in the basolateral membrane of oxyntic cells of frog gastric fundus. Pflugers Arch. 1987;408:497–504. doi: 10.1007/BF00585075. [DOI] [PubMed] [Google Scholar]
- 79.Damkier HH, Aalkjaer C, Praetorius J. Na+-dependent HCO3− import by the slc4a10 gene product involves Cl− export. J Biol Chem. 2010;285:26998–27007. doi: 10.1074/jbc.M110.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Damkier HH, Nielsen S, Praetorius J. An anti-NH2-terminal antibody localizes NBCn1 to heart endothelia and skeletal and vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;290:H172–H180. doi: 10.1152/ajpheart.00713.2005. [DOI] [PubMed] [Google Scholar]
- 81.Damkier HH, Nielsen S, Praetorius J. Molecular expression of SLC4-derived Na+-dependent anion transporters in selected human tissues. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2136–R2146. doi: 10.1152/ajpregu.00356.2007. [DOI] [PubMed] [Google Scholar]
- 82.Damkier HH, Praetorius J. Genetic ablation of Slc4a10 alters the expression pattern of transporters involved in solute movement in the mouse choroid plexus. Am J Physiol Cell Physiol. 2012;302:C1452–C1459. doi: 10.1152/ajpcell.00285.2011. [DOI] [PubMed] [Google Scholar]
- 83.Damkier HH, Prasad V, Hubner CA, Praetorius J. Nhe1 is a luminal Na+/H+ exchanger in mouse choroid plexus and is targeted to the basolateral membrane in Ncbe/Nbcn2-null mice. Am J Physiol Cell Physiol. 2009;296:C1291–C1300. doi: 10.1152/ajpcell.00062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Danielsen AA, Parker MD, Lee S, Boron WF, Aalkjaer C, Boedtkjer E. Splice cassette II of NBCn1 (slc4a7) interacts with calcineurin A: Implications for transporter activity and intracellular pH control during rat artery contractions. J Biol Chem. 2013;288:8146–8155. doi: 10.1074/jbc.M113.455386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dart C, Vaughan-Jones RD. Na+-HCO3− symport in the sheep cardiac Purkinje fibre. J Physiol. 1992;451:365–385. doi: 10.1113/jphysiol.1992.sp019169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Davis BA, Hogan EM, Cooper GJ, Bashi E, Zhao J, Boron WF. Inhibition of K+/HCO3− cotransport in squid axons by quaternary ammonium ions. J Membr Biol. 2001;183:25–32. doi: 10.1007/s00232-001-0050-0. [DOI] [PubMed] [Google Scholar]
- 87.DeCoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- 88.De Giusti VC, Orlowski A, Villa-Abrille MC, de Cingolani GE, Casey JR, Alvarez BV, Aiello EA. Antibodies against the cardiac sodium/bicarbonate co-transporter (NBCe1) as pharmacological tools. Br J Pharmacol. 2011;164:1976–1989. doi: 10.1111/j.1476-5381.2011.01496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deitmer JW, Schlue WR. The regulation of intracellular pH by identified glial cells and neurones in the central nervous system of the leech. J Physiol. 1987;388:261–283. doi: 10.1113/jphysiol.1987.sp016614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Demirci FY, Chang MH, Mah TS, Romero MF, Gorin MB. Proximal renal tubular acidosis and ocular pathology: A novel missense mutation in the gene (SLC4A4) for sodium bicarbonate cotransporter protein (NBCe1) Mol Vis. 2006;12:324–330. [PubMed] [Google Scholar]
- 91.Dinour D, Chang MH, Satoh J, Smith BL, Angle N, Knecht A, Serban I, Holtzman EJ, Romero MF. A novel missense mutation in the sodium bicarbonate cotransporter (NBCe1/SLC4A4) causes proximal tubular acidosis and glaucoma through ion transport defects. J Biol Chem. 2004;279:52238–52246. doi: 10.1074/jbc.M406591200. [DOI] [PubMed] [Google Scholar]
- 92.Dorwart MR, Shcheynikov N, Wang Y, Stippec S, Muallem S. SLC26A9 is a Cl− channel regulated by the WNK kinases. J Physiol. 2007;584:333–345. doi: 10.1113/jphysiol.2007.135855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Douglas RM, Schmitt BM, Xia Y, Bevensee MO, Biemesderfer D, Boron WF, Haddad GG. Sodium-hydrogen exchangers and sodium-bicarbonate co-transporters: Ontogeny of protein expression in the rat brain. Neuroscience. 2001;102:217–228. doi: 10.1016/s0306-4522(00)00473-5. [DOI] [PubMed] [Google Scholar]
- 94.Douglas RM, Xue J, Chen JY, Haddad CG, Alper SL, Haddad GG. Chronic intermittent hypoxia decreases the expression of Na+/H+ exchangers and HCO3− dependent transporters in mouse CNS. J Appl Physiol. 2003;95:292–299. doi: 10.1152/japplphysiol.01089.2002. [DOI] [PubMed] [Google Scholar]
- 95.Ducoudret O, Diakov A, Muller-Berger S, Romero MF, Fromter E. The renal Na+-HCO3− cotransporter expressed in Xenopus laevis oocytes: Inhibition by tenidap and benzamil and effect of temperature on transport rate and stoichiometry. Pflugers Arch. 2001;442:709–717. doi: 10.1007/s004240100594. [DOI] [PubMed] [Google Scholar]
- 96.Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, Pihur V, Vollenweider P, O’Reilly PF, Amin N, Bragg-Gresham JL, Teumer A, Glazer NL, Launer L, Zhao JH, Aulchenko Y, Heath S, Sober S, Parsa A, Luan J, Arora P, Dehghan A, Zhang F, Lucas G, Hicks AA, Jackson AU, Peden JF, Tanaka T, Wild SH, Rudan I, Igl W, Milaneschi Y, Parker AN, Fava C, Chambers JC, Fox ER, Kumari M, Go MJ, van der Harst P, Kao WH, Sjogren M, Vinay DG, Alexander M, Tabara Y, Shaw-Hawkins S, Whincup PH, Liu Y, Shi G, Kuusisto J, Tayo B, Seielstad M, Sim X, Nguyen KD, Lehtimaki T, Matullo G, Wu Y, Gaunt TR, Onland-Moret NC, Cooper MN, Platou CG, Org E, Hardy R, Dahgam S, Palmen J, Vitart V, Braund PS, Kuznetsova T, Uiterwaal CS, Adeyemo A, Palmas W, Campbell H, Ludwig B, Tomaszewski M, Tzoulaki I, Palmer ND, Aspelund T, Garcia M, Chang YP, O’Connell JR, Steinle NI, Grobbee DE, Arking DE, Kardia SL, Morrison AC, Hernandez D, Najjar S, McArdle WL, Hadley D, Brown MJ, Connell JM, Hingorani AD, Day IN, Lawlor DA, Beilby JP, Lawrence RW, Clarke R, Hopewell JC, Ongen H, Dreisbach AW, Li Y, Young JH, Bis JC, Kahonen M, Viikari J, Adair LS, Lee NR, Chen MH, Olden M, Pattaro C, Bolton JA, Kottgen A, Bergmann S, Mooser V, Chaturvedi N, Frayling TM, Islam M, Jafar TH, Erdmann J, Kulkarni SR, Bornstein SR, Grassler J, Groop L, Voight BF, Kettunen J, Howard P, Taylor A, Guarrera S, Ricceri F, Emilsson V, Plump A, Barroso I, Khaw KT, Weder AB, Hunt SC, Sun YV, Bergman RN, Collins FS, Bonnycastle LL, Scott LJ, Stringham HM, Peltonen L, Perola M, Vartiainen E, Brand SM, Staessen JA, Wang TJ, Burton PR, Soler AM, Dong Y, Snieder H, Wang X, Zhu H, Lohman KK, Rudock ME, Heckbert SR, Smith NL, Wiggins KL, Doumatey A, Shriner D, Veldre G, Viigimaa M, Kinra S, Prabhakaran D, Tripathy V, Langefeld CD, Rosengren A, Thelle DS, Corsi AM, Singleton A, Forrester T, Hilton G, McKenzie CA, Salako T, Iwai N, Kita Y, Ogihara T, Ohkubo T, Okamura T, Ueshima H, Umemura S, Eyheramendy S, Meitinger T, Wichmann HE, Cho YS, Kim HL, Lee JY, Scott J, Sehmi JS, Zhang W, Hedblad B, Nilsson P, Smith GD, Wong A, Narisu N, Stancakova A, Raffel LJ, Yao J, Kathiresan S, O’Donnell CJ, Schwartz SM, Ikram MA, Longstreth WT, Jr, Mosley TH, Seshadri S, Shrine NR, Wain LV, Morken MA, Swift AJ, Laitinen J, Prokopenko I, Zitting P, Cooper JA, Humphries SE, Danesh J, Rasheed A, Goel A, Hamsten A, Watkins H, Bakker SJ, van Gilst WH, Janipalli CS, Mani KR, Yajnik CS, Hofman A, Mattace-Raso FU, Oostra BA, Demirkan A, Isaacs A, Rivadeneira F, Lakatta EG, Orru M, Scuteri A, Ala-Korpela M, Kangas AJ, Lyytikainen LP, Soininen P, Tukiainen T, Wurtz P, Ong RT, Dorr M, Kroemer HK, Volker U, Volzke H, Galan P, Hercberg S, Lathrop M, Zelenika D, Deloukas P, Mangino M, Spector TD, Zhai G. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Espiritu DJ, Bernardo AA, Arruda JA. Role of NH2 and COOH termini in targeting, stability, and activity of sodium bicarbonate cotransporter 1. Am J Physiol Renal Physiol. 2006;291:F588–F596. doi: 10.1152/ajprenal.00361.2005. [DOI] [PubMed] [Google Scholar]
- 98.Fievet B, Gabillat N, Borgese F, Motais R. Expression of band 3 anion exchanger induces chloride current and taurine transport: Structure-function analysis. EMBO J. 1995;14:5158–5169. doi: 10.1002/j.1460-2075.1995.tb00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fitz JG, Lidofsky SD, Xie MH, Scharschmidt BF. Transmembrane electrical potential difference regulates Na+/HCO3− cotransport and intracellular pH in hepatocytes. Proc Natl Acad Sci U S A. 1992;89:4197–4201. doi: 10.1073/pnas.89.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fitz JG, Persico M, Scharschmidt BF. Electrophysiological evidence for Na+-coupled bicarbonate transport in cultured rat hepatocytes. Am J Physiol. 1989;256:G491–G500. doi: 10.1152/ajpgi.1989.256.3.G491. [DOI] [PubMed] [Google Scholar]
- 101.Freedman JC, Novak TS. Electrodiffusion, barrier, and gating analysis of DIDS-insensitive chloride conductance in human red blood cells treated with valinomycin or gramicidin. J Gen Physiol. 1997;109:201–216. doi: 10.1085/jgp.109.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fukuda H, Hirata T, Nakamura N, Kato A, Kawahara K, Wakabayashi S, Chang MH, Romero MF, Hirose S. Identification and properties of a novel variant of NBC4 (Na+/HCO3− co-transporter 4) that is predominantly expressed in the choroid plexus. Biochem J. 2013;450:179–187. doi: 10.1042/BJ20121515. [DOI] [PubMed] [Google Scholar]
- 103.Galler S, Moser H. The ionic mechanism of intracellular pH regulation in crayfish muscle fibres. J Physiol. 1986;374:137–151. doi: 10.1113/jphysiol.1986.sp016071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nat Rev Cancer. 2008;8:56–61. doi: 10.1038/nrc2255. [DOI] [PubMed] [Google Scholar]
- 105.Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, Clarke LL, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/HCO3− cotransporter. J Biol Chem. 2007;282:9042–9052. doi: 10.1074/jbc.M607041200. [DOI] [PubMed] [Google Scholar]
- 106.Geibel J, Giebisch G, Boron WF. Angiotensin II stimulates both Na+-H+ exchange and Na+/HCO3− cotransport in the rabbit proximal tubule. Proc Natl Acad Sci U S A. 1990;87:7917–7920. doi: 10.1073/pnas.87.20.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Giffard RG, Lee YS, Ouyang YB, Murphy SL, Monyer H. Two variants of the rat brain sodium-driven chloride bicarbonate exchanger (NCBE): developmental expression and addition of a PDZ motif. Eur J Neurosci. 2003;18:2935–2945. doi: 10.1046/j.1460-9568.2003.03053.x. [DOI] [PubMed] [Google Scholar]
- 108.Giffard RG, Papadopoulos MC, van Hooft JA, Xu L, Giuffrida R, Monyer H. The electrogenic sodium bicarbonate cotransporter: Developmental expression in rat brain and possible role in acid vulnerability. J Neurosci. 2000;20:1001–1008. doi: 10.1523/JNEUROSCI.20-03-01001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gill HS, Boron WF. Expression and purification of the cytoplasmic N-terminal domain of the Na+/HCO3− cotransporter NBCe1-A: Structural insights from a generalized approach. Protein Expr Purif. 2006;49:228–234. doi: 10.1016/j.pep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 110.Gillies RJ, Martinez-Zaguilan R, Martinez GM, Serrano R, Perona R. Tumorigenic 3T3 cells maintain an alkaline intracellular pH under physiological conditions. Proc Natl Acad Sci U S A. 1990;87:7414–7418. doi: 10.1073/pnas.87.19.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Good DW. Adaptation of HCO3− and NH4+ transport in rat MTAL: Effects of chronic metabolic acidosis and Na+ intake. Am J Physiol. 1990;258:F1345–F1353. doi: 10.1152/ajprenal.1990.258.5.F1345. [DOI] [PubMed] [Google Scholar]
- 112.Good DW. Ammonium transport by the thick ascending limb of Henle’s loop. Annu Rev Physiol. 1994;56:623–647. doi: 10.1146/annurev.ph.56.030194.003203. [DOI] [PubMed] [Google Scholar]
- 113.Gresz V, Kwon TH, Vorum H, Zelles T, Kurtz I, Steward MC, Aalkjaer C, Nielsen S. Immunolocalization of electroneutral Na+-HCO3− cotransporters in human and rat salivary glands. Am J Physiol Gastrointest Liver Physiol. 2002;283:G473–G480. doi: 10.1152/ajpgi.00421.2001. [DOI] [PubMed] [Google Scholar]
- 114.Grichtchenko II, Boron WF. Evidence for CO3= transport by NBCe1, based on surface-pH measurements in voltage-clamped Xenopus oocytes coexpressing NBCe1 and CAIV: Evidence for CO3= transport. FASEB J. 2002;16:A795. [Google Scholar]
- 115.Grichtchenko II, Chesler M. Depolarization-induced acid secretion in gliotic hippocampal slices. Neuroscience. 1994a;62:1057–1070. doi: 10.1016/0306-4522(94)90343-3. [DOI] [PubMed] [Google Scholar]
- 116.Grichtchenko II, Chesler M. Depolarization-induced alkalinization of astrocytes in gliotic hippocampal slices. Neuroscience. 1994b;62:1071–1078. doi: 10.1016/0306-4522(94)90344-1. [DOI] [PubMed] [Google Scholar]
- 117.Grichtchenko II, Choi I, Zhong X, Bray-Ward P, Russell JM, Boron WF. Cloning, characterization, and chromosomal mapping of a human electroneutral Na+-driven Cl−-HCO3− exchanger. J Biol Chem. 2001;276:8358–8363. doi: 10.1074/jbc.C000716200. [DOI] [PubMed] [Google Scholar]
- 118.Grichtchenko II, Romero MF, Boron WF. Extracellular HCO3− dependence of electrogenic Na+/HCO3− cotransporters cloned from salamander and rat kidney. J Gen Physiol. 2000;115:533–546. doi: 10.1085/jgp.115.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Groger N, Vitzthum H, Frohlich H, Kruger M, Ehmke H, Braun T, Boettger T. Targeted mutation of SLC4A5 induces arterial hypertension and renal metabolic acidosis. Hum Mol Genet. 2012;21:1025–1036. doi: 10.1093/hmg/ddr533. [DOI] [PubMed] [Google Scholar]
- 120.Gross E, Abuladze N, Pushkin A, Kurtz I, Cotton CU. The stoichiometry of the electrogenic sodium bicarbonate cotransporter pNBC1 in mouse pancreatic duct cells is 2 HCO3−:1 Na+ J Physiol. 2001;531:375–382. doi: 10.1111/j.1469-7793.2001.0375i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gross E, Hawkins K, Abuladze N, Pushkin A, Cotton CU, Hopfer U, Kurtz I. The stoichiometry of the electrogenic sodium bicarbonate cotransporter NBC1 is cell-type dependent. J Physiol. 2001;531:597–603. doi: 10.1111/j.1469-7793.2001.0597h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gross E, Hawkins K, Pushkin A, Sassani P, Dukkipati R, Abuladze N, Hopfer U, Kurtz I. Phosphorylation of Ser982 in the sodium bicarbonate cotransporter kNBC1 shifts the HCO3− : Na+ stoichiometry from 3 : 1 to 2 : 1 in murine proximal tubule cells. J Physiol. 2001;537:659–665. doi: 10.1111/j.1469-7793.2001.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gross E, Hopfer U. Activity and stoichiometry of Na+:HCO3− cotransport in immortalized renal proximal tubule cells. J Membr Biol. 1996;152:245–252. doi: 10.1007/s002329900102. [DOI] [PubMed] [Google Scholar]
- 124.Gross E, Hopfer U. Voltage and cosubstrate dependence of the Na+-HCO3− cotransporter kinetics in renal proximal tubule cells. Biophys J. 1998;75:810–824. doi: 10.1016/S0006-3495(98)77570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gross E, Hopfer U. Effects of pH on kinetic parameters of the Na+-HCO3− cotransporter in renal proximal tubule. Biophys J. 1999;76:3066–3075. doi: 10.1016/S0006-3495(99)77459-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gurnett CA, Veile R, Zempel J, Blackburn L, Lovett M, Bowcock A. Disruption of sodium bicarbonate transporter SLC4A10 in a patient with complex partial epilepsy and mental retardation. Arch Neurol. 2008;65:550–553. doi: 10.1001/archneur.65.4.550. [DOI] [PubMed] [Google Scholar]
- 127.Han W, Woo JH, Yu JH, Lee MJ, Moon HG, Kang D, Noh DY. Common genetic variants associated with breast cancer in Korean women and differential susceptibility according to intrinsic subtype. Cancer Epidemiol Biomarkers Prev. 2011;20:793–798. doi: 10.1158/1055-9965.EPI-10-1282. [DOI] [PubMed] [Google Scholar]
- 128.Hentschke M, Wiemann M, Hentschke S, Kurth I, Hermans-Borgmeyer I, Seidenbecher T, Jentsch TJ, Gal A, Hubner CA. Mice with a targeted disruption of the Cl−/HCO3− exchanger AE3 display a reduced seizure threshold. Mol Cell Biol. 2006;26:182–191. doi: 10.1128/MCB.26.1.182-191.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Heyer M, Muller-Berger S, Romero MF, Boron WF, Fromter E. Stoichiometry of the rat kidney Na+-HCO3− cotransport expressed in Xenopus laevis oocytes. Pflugers Arch. 1999;438:322–329. doi: 10.1007/s004240050916. [DOI] [PubMed] [Google Scholar]
- 130.Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE. 2001:re19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- 131.Hogan EM, Cohen MA, Boron WF. K+- and HCO3− -dependent acid-base transport in squid giant axons II. Base influx. J Gen Physiol. 1995;106:845–862. doi: 10.1085/jgp.106.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hong JH, Yang D, Shcheynikov N, Ohana E, Shin DM, Muallem S. Convergence of IRBIT, phosphatidylinositol (4,5) bisphosphate, and WNK/SPAK kinases in regulation of the Na+-HCO3− cotransporters family. Proc Natl Acad Sci U S A. 2013;110:4105–4110. doi: 10.1073/pnas.1221410110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Horita S, Yamada H, Inatomi J, Moriyama N, Sekine T, Igarashi T, Endo Y, Dasouki M, Ekim M, Al-Gazali L, Shimadzu M, Seki G, Fujita T. Functional analysis of NBC1 mutants associated with proximal renal tubular acidosis and ocular abnormalities. J Am Soc Nephrol. 2005;16:2270–2278. doi: 10.1681/ASN.2004080667. [DOI] [PubMed] [Google Scholar]
- 134.Hsueh WC, Mitchell BD, Schneider JL, Wagner MJ, Bell CJ, Nanthakumar E, Shuldiner AR. QTL influencing blood pressure maps to the region of PPH1 on chromosome 2q31–34 in Old Order Amish. Circulation. 2000;101:2810–2816. doi: 10.1161/01.cir.101.24.2810. [DOI] [PubMed] [Google Scholar]
- 135.Hubner CA, Hentschke M, Jacobs S, Hermans-Borgmeyer I. Expression of the sodium-driven chloride bicarbonate exchanger NCBE during prenatal mouse development. Gene Expr Patterns. 2004;5:219–223. doi: 10.1016/j.modgep.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 136.Hunt SC, Xin Y, Wu LL, Cawthon RM, Coon H, Hasstedt SJ, Hopkins PN. Sodium bicarbonate cotransporter polymorphisms are associated with baseline and 10-year follow-up blood pressures. Hypertension. 2006;47:532–536. doi: 10.1161/01.HYP.0000196949.26088.3c. [DOI] [PubMed] [Google Scholar]
- 137.Igarashi T, Inatomi J, Sekine T, Cha SH, Kanai Y, Kunimi M, Tsukamoto K, Satoh H, Shimadzu M, Tozawa F, Mori T, Shiobara M, Seki G, Endou H. Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat Genet. 1999;23:264–266. doi: 10.1038/15440. [DOI] [PubMed] [Google Scholar]
- 138.Igarashi T, Inatomi J, Sekine T, Seki G, Shimadzu M, Tozawa F, Takeshima Y, Takumi T, Takahashi T, Yoshikawa N, Nakamura H, Endou H. Novel nonsense mutation in the Na+/HCO3− cotransporter gene (SLC4A4) in a patient with permanent isolated proximal renal tubular acidosis and bilateral glaucoma. J Am Soc Nephrol. 2001;12:713–718. doi: 10.1681/ASN.V124713. [DOI] [PubMed] [Google Scholar]
- 139.Igarashi T, Sekine T, Inatomi J, Seki G. Unraveling the molecular pathogenesis of isolated proximal renal tubular acidosis. J Am Soc Nephrol. 2002;13:2171–2177. doi: 10.1097/01.asn.0000025281.70901.30. [DOI] [PubMed] [Google Scholar]
- 140.Inatomi J, Horita S, Braverman N, Sekine T, Yamada H, Suzuki Y, Kawahara K, Moriyama N, Kudo A, Kawakami H, Shimadzu M, Endou H, Fujita T, Seki G, Igarashi T. Mutational and functional analysis of SLC4A4 in a patient with proximal renal tubular acidosis. Pflugers Arch. 2004;448:438–444. doi: 10.1007/s00424-004-1278-1. [DOI] [PubMed] [Google Scholar]
- 141.Ishiguro H, Steward MC, Lindsay AR, Case RM. Accumulation of intracellular HCO3− by Na+-HCO3− cotransport in interlobular ducts from the guinea-pig pancreas. J Physiol. 1996;495:169–178. doi: 10.1113/jphysiol.1996.sp021582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ishiguro H, Walther D, Arinami T, Uhl GR. Variation in a bicarbonate co-transporter gene family member SLC4A7 is associated with propensity to addictions: A study using fine-mapping and three samples. Addiction. 2007;102:1320–1325. doi: 10.1111/j.1360-0443.2007.01877.x. [DOI] [PubMed] [Google Scholar]
- 143.Jacobs S, Ruusuvuori E, Sipila ST, Haapanen A, Damkier HH, Kurth I, Hentschke M, Schweizer M, Rudhard Y, Laatikainen LM, Tyynela J, Praetorius J, Voipio J, Hubner CA. Mice with targeted Slc4a10 gene disruption have small brain ventricles and show reduced neuronal excitability. Proc Natl Acad Sci U S A. 2008;105:311–316. doi: 10.1073/pnas.0705487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jakobsen JK, Odgaard E, Wang W, Elkjaer ML, Nielsen S, Aalkjaer C, Leipziger J. Functional up-regulation of basolateral Na+-dependent. Pflugers Arch. 2004;448:571–578. doi: 10.1007/s00424-004-1303-4. [DOI] [PubMed] [Google Scholar]
- 145.Jentsch TJ, Keller SK, Koch M, Wiederholt M. Evidence for coupled transport of bicarbonate and sodium in cultured bovine corneal endothelial cells. J Membr Biol. 1984;81:189–204. doi: 10.1007/BF01868713. [DOI] [PubMed] [Google Scholar]
- 146.Jentsch TJ, Schill BS, Schwartz P, Matthes H, Keller SK, Wiederholt M. Kidney epithelial cells of monkey origin (BSC-1) express a sodium bicarbonate cotransport. Characterization by 22Na+ flux measurements. J Biol Chem. 1985;260:15554–15560. [PubMed] [Google Scholar]
- 147.Jentsch TJ, Schwartz P, Schill BS, Langner B, Lepple AP, Keller SK, Wiederholt M. Kinetic properties of the sodium bicarbonate (carbonate) symport in monkey kidney epithelial cells (BSC-1). Interactions between Na+, HCO3−, and pH. J Biol Chem. 1986;261:10673–10679. [PubMed] [Google Scholar]
- 148.Jentsch TJ, Stahlknecht TR, Hollwede H, Fischer DG, Keller SK, Wiederholt M. A bicarbonate-dependent process inhibitable by disulfonic stilbenes and a Na+/H+ exchange mediate 22Na+ uptake into cultured bovine corneal endothelium. J Biol Chem. 1985;260:795–801. [PubMed] [Google Scholar]
- 149.Jung YW, Choi IJ, Kwon TH. Altered expression of sodium transporters in ischemic penumbra after focal cerebral ischemia in rats. Neurosci Res. 2007;59:152–159. doi: 10.1016/j.neures.2007.06.1470. [DOI] [PubMed] [Google Scholar]
- 150.Kahn AM, Cragoe EJ, Jr, Allen JC, Halligan RD, Shelat H. Na+-H+ and Na+-dependent Cl−-HCO3− exchange control pHi in vascular smooth muscle. Am J Physiol. 1990;259:C134–C143. doi: 10.1152/ajpcell.1990.259.1.C134. [DOI] [PubMed] [Google Scholar]
- 151.Kahn AM, Cragoe EJ, Jr, Allen JC, Seidel CL, Shelat H. Effects of pHi on Na+-H+, Na+-dependent, and Na+-independent Cl−-HCO3− exchangers in vascular smooth muscle. Am J Physiol. 1991;261:C837–C844. doi: 10.1152/ajpcell.1991.261.5.C837. [DOI] [PubMed] [Google Scholar]
- 152.Kao L, Kurtz LM, Shao X, Papadopoulos MC, Liu L, Bok D, Nusinowitz S, Chen B, Stella SL, Andre M, Weinreb J, Luong SS, Piri N, Kwong JM, Newman D, Kurtz I. Severe neurologic impairment in mice with targeted disruption of the electrogenic sodium bicarbonate cotransporter NBCe2 (Slc4a5 gene) J Biol Chem. 2011;286:32563–32574. doi: 10.1074/jbc.M111.249961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kao L, Sassani P, Azimov R, Pushkin A, Abuladze N, Peti-Peterdi J, Liu W, Newman D, Kurtz I. Oligomeric structure and minimal functional unit of the electrogenic sodium bicarbonate cotransporter NBCe1-A. J Biol Chem. 2008;283:26782–26794. doi: 10.1074/jbc.M804006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kardia SL, Greene MT, Boerwinkle E, Turner ST, Kullo IJ. Investigating the complex genetic architecture of ankle-brachial index, a measure of peripheral arterial disease, in non-Hispanic whites. BMC Med Genomics. 2008;1:16. doi: 10.1186/1755-8794-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Khandoudi N, Albadine J, Robert P, Krief S, Berrebi-Bertrand I, Martin X, Bevensee MO, Boron WF, Bril A. Inhibition of the cardiac electrogenic sodium bicarbonate cotransporter reduces ischemic injury. Cardiovasc Res. 2001;52:387–396. doi: 10.1016/s0008-6363(01)00430-8. [DOI] [PubMed] [Google Scholar]
- 156.Kim KH, Shcheynikov N, Wang Y, Muallem S. SLC26A7 is a Cl− channel regulated by intracellular pH. J Biol Chem. 2005;280:6463–6470. doi: 10.1074/jbc.M409162200. [DOI] [PubMed] [Google Scholar]
- 157.Kim YH, Kwon TH, Christensen BM, Nielsen J, Wall SM, Madsen KM, Frokiaer J, Nielsen S. Altered expression of renal acid-base transporters in rats with lithium-induced NDI. Am J Physiol Renal Physiol. 2003;285:F1244–F1257. doi: 10.1152/ajprenal.00176.2003. [DOI] [PubMed] [Google Scholar]
- 158.Knauf PA, Pal P. Red Cell Membrane Transport in Health and Disease. Berlin: Springer; 2003. Band 3-mediated transport; pp. 253–301. [Google Scholar]
- 159.Ko SB, Luo X, Hager H, Rojek A, Choi JY, Licht C, Suzuki M, Muallem S, Nielsen S, Ishibashi K. AE4 is a DIDS-sensitive Cl−/HCO3− exchanger in the basolateral membrane of the renal CCD and the SMG duct. Am J Physiol Cell Physiol. 2002;283:C1206–C1218. doi: 10.1152/ajpcell.00512.2001. [DOI] [PubMed] [Google Scholar]
- 160.Krapf R. Mechanisms of adaptation to chronic respiratory acidosis in the rabbit proximal tubule. J Clin Invest. 1989;83:890–896. doi: 10.1172/JCI113973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Krepischi AC, Knijnenburg J, Bertola DR, Kim CA, Pearson PL, Bijlsma E, Szuhai K, Kok F, Vianna-Morgante AM, Rosenberg C. Two distinct regions in 2q24.2-q24.3 associated with idiopathic epilepsy. Epilepsia. 2010;51:2457–2460. doi: 10.1111/j.1528-1167.2010.02742.x. [DOI] [PubMed] [Google Scholar]
- 162.Kristensen JM, Kristensen M, Juel C. Expression of Na+/HCO3− cotransporter protein (NBCs) in rat and human skeletal muscle. Acta Physiol Scand. 2004;182:69–76. doi: 10.1111/j.1365-201X.2004.01297.x. [DOI] [PubMed] [Google Scholar]
- 163.Krushkal J, Ferrell R, Mockrin SC, Turner ST, Sing CF, Boerwinkle E. Genome-wide linkage analyses of systolic blood pressure using highly discordant siblings. Circulation. 1999;99:1407–1410. doi: 10.1161/01.cir.99.11.1407. [DOI] [PubMed] [Google Scholar]
- 164.Kunau RT, Jr, Hart JI, Walker KA. Effect of metabolic acidosis on proximal tubular total CO2 absorption. Am J Physiol. 1985;249:F62–F68. doi: 10.1152/ajprenal.1985.249.1.F62. [DOI] [PubMed] [Google Scholar]
- 165.Kunimi M, Muller-Berger S, Hara C, Samarzija I, Seki G, Fromter E. Incubation in tissue culture media allows isolated rabbit proximal tubules to regain in-vivo-like transport function: Response of HCO3− absorption to norepinephrine. Pflugers Arch. 2000;440:908–917. doi: 10.1007/s004240000361. [DOI] [PubMed] [Google Scholar]
- 166.Kwon TH, Fulton C, Wang W, Kurtz I, Frokiaer J, Aalkjaer C, Nielsen S. Chronic metabolic acidosis upregulates rat kidney Na+-HCO3− cotransporters NBCn1 and NBC3 but not NBC1. Am J Physiol Renal Physiol. 2002;282:F341–F351. doi: 10.1152/ajprenal.00104.2001. [DOI] [PubMed] [Google Scholar]
- 167.Kwon TH, Pushkin A, Abuladze N, Nielsen S, Kurtz I. Immunoelectron microscopic localization of NBC3 sodium-bicarbonate cotransporter in rat kidney. Am J Physiol Renal Physiol. 2000;278:F327–F336. doi: 10.1152/ajprenal.2000.278.2.F327. [DOI] [PubMed] [Google Scholar]
- 168.L’Allemain G, Paris S, Pouyssegur J. Role of a Na+-dependent Cl−/HCO3− exchange in regulation of intracellular pH in fibroblasts. J Biol Chem. 1985;260:4877–4883. [PubMed] [Google Scholar]
- 169.Lacruz RS, Nanci A, Kurtz I, Wright JT, Paine ML. Regulation of pH During Amelogenesis. Calcif Tissue Int. 2010;86:91–103. doi: 10.1007/s00223-009-9326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lacruz RS, Nanci A, White SN, Wen X, Wang H, Zalzal SF, Luong VQ, Schuetter VL, Conti PS, Kurtz I, Paine ML. The sodium bicarbonate cotransporter (NBCe1) is essential for normal development of mouse dentition. J Biol Chem. 2010;285:24432–24438. doi: 10.1074/jbc.M110.115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lacruz RS, Smith CE, Moffatt P, Chang EH, Bromage TG, Bringas P, Jr, Nanci A, Baniwal SK, Zabner J, Welsh MJ, Kurtz I, Paine ML. Requirements for ion and solute transport, and pH regulation during enamel maturation. J Cell Physiol. 2012;227:1776–1785. doi: 10.1002/jcp.22911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ladoux A, Krawice I, Cragoe EJ, Jr, Abita JP, Frelin C. Properties of the Na+-dependent Cl−/HCO3− exchange system in U937 human leukemic cells. Eur J Biochem. 1987;170:43–49. doi: 10.1111/j.1432-1033.1987.tb13665.x. [DOI] [PubMed] [Google Scholar]
- 173.Lagadic-Gossmann D, Buckler KJ, Vaughan-Jones RD. Role of bicarbonate in pH recovery from intracellular acidosis in the guinea-pig ventricular myocyte. J Physiol. 1992;458:361–384. doi: 10.1113/jphysiol.1992.sp019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lagadic-Gossmann D, Vaughan-Jones RD, Buckler KJ. Adrenaline and extracellular ATP switch between two modes of acid extrusion in the guinea-pig ventricular myocyte. J Physiol. 1992;458:385–407. doi: 10.1113/jphysiol.1992.sp019423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Larsen AM, Krogsgaard-Larsen N, Lauritzen G, Olesen CW, Honore HS, Boedtkjer E, Pedersen SF, Bunch L. Gram-scale solution-phase synthesis of selective sodium bicarbonate co-transport inhibitor S0859: In vitro efficacy studies in breast cancer cells. Chem Med Chem. 2012;7:1808–1814. doi: 10.1002/cmdc.201200335. [DOI] [PubMed] [Google Scholar]
- 176.Lauritzen G, Jensen MB, Boedtkjer E, Dybboe R, Aalkjaer C, Nylandsted J, Pedersen SF. NBCn1 and NHE1 expression and activity in ΔNErbB2 receptor-expressing MCF-7 breast cancer cells: Contributions to pHi regulation and chemotherapy resistance. Exp Cell Res. 2010;316:2538–2553. doi: 10.1016/j.yexcr.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 177.Lauritzen G, Stock CM, Lemaire J, Lund SF, Jensen MF, Damsgaard B, Petersen KS, Wiwel M, Ronnov-Jessen L, Schwab A, Pedersen SF. The Na+/H+ exchanger NHE1, but not the Na+, HCO3− cotransporter NBCn1, regulates motility of MCF7 breast cancer cells expressing constitutively active ErbB2. Cancer Lett. 2012;317:172–183. doi: 10.1016/j.canlet.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 178.Le Quesne SP, Saihan Z, Rangesh N, Steele-Stallard HB, Ambrose J, Coffey A, Emmerson J, Haralambous E, Hughes Y, Steel KP, Luxon LM, Webster AR, Bitner-Glindzicz M. Comprehensive sequence analysis of nine Usher syndrome genes in the UK National Collaborative Usher Study. J Med Genet. 2012;49:27–36. doi: 10.1136/jmedgenet-2011-100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lee HJ, Park HJ, Lee S, Kim YH, Choi I. The sodium-driven chloride/bicarbonate exchanger NDCBE in rat brain is upregulated by chronic metabolic acidosis. Brain Res. 2011;1377:13–20. doi: 10.1016/j.brainres.2010.12.062. [DOI] [PubMed] [Google Scholar]
- 180.Lee MG, Ohana E, Park HW, Yang D, Muallem S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol Rev. 2012;92:39–74. doi: 10.1152/physrev.00011.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lee S, Lee HJ, Yang HS, Thornell IM, Bevensee MO, Choi I. Sodium-bicarbonate cotransporter NBCn1 in the kidney medullary thick ascending limb cell line is upregulated under acidic conditions and enhances ammonium transport. Exp Physiol. 2010;95:926–937. doi: 10.1113/expphysiol.2010.053967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lee S, Yang HS, Kim E, Ju EJ, Kwon MH, Dudley RK, Smith Y, Yun CC, Choi I. PSD-95 Interacts with NBCn1 and Enhances Channel-like Activity without Affecting Na+/HCO3− Cotransport. Cell Physiol Biochem. 2012;30:1444–1455. doi: 10.1159/000343332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lee SK, Boron WF, Parker MD. Relief of autoinhibition of the electrogenic Na+-HCO3− cotransporter NBCe1-B: Role of IRBIT vs. amino-terminal truncation. Am J Physiol Cell Physiol. 2012;302:C518–C526. doi: 10.1152/ajpcell.00352.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lee SK, Grichtchenko II, Boron WF. Distinguishing HCO3− from CO3 2− transport by NBCe1-A. FASEB J. 2011;25:656.9. [Google Scholar]
- 185.Leem CH, Lagadic-Gossmann D, Vaughan-Jones RD. Characterization of intracellular pH regulation in the guinea-pig ventricular myocyte. J Physiol. 1999;517:159–180. doi: 10.1111/j.1469-7793.1999.0159z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Leviel F, Borensztein P, Houillier P, Paillard M, Bichara M. Electroneutral K+/HCO3− cotransport in cells of medullary thick ascending limb of rat kidney. J Clin Invest. 1992;90:869–878. doi: 10.1172/JCI115962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Leviel F, Hubner CA, Houillier P, Morla L, El MS, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest. 2010;120:1627–1635. doi: 10.1172/JCI40145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li CY, Zhou WZ, Zhang PW, Johnson C, Wei L, Uhl GR. Meta-analysis and genome-wide interpretation of genetic susceptibility to drug addiction. BMC Genomics. 2011;12:508. doi: 10.1186/1471-2164-12-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li HC, Collier JH, Shawki A, Rudra JS, Li EY, Mackenzie B, Soleimani M. Sequence- or position-specific mutations in the carboxyl-terminal FL motif of the kidney sodium bicarbonate cotransporter (NBC1) disrupt its basolateral targeting and alpha-helical structure. J Membr Biol. 2009;228:111–124. doi: 10.1007/s00232-009-9164-6. [DOI] [PubMed] [Google Scholar]
- 190.Li HC, Kucher V, Li EY, Conforti L, Zahedi KA, Soleimani M. The role of aspartic acid residues 405 and 416 of the kidney isotype of sodium-bicarbonate cotransporter 1 in its targeting to the plasma membrane. Am J Physiol Cell Physiol. 2012;302:C1713–C1730. doi: 10.1152/ajpcell.00147.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Li HC, Li EY, Neumeier L, Conforti L, Soleimani M. Identification of a novel signal in the cytoplasmic tail of the Na+:HCO3− cotransporter NBC1 that mediates basolateral targeting. Am J Physiol Renal Physiol. 2007;292:F1245–F1255. doi: 10.1152/ajprenal.00410.2006. [DOI] [PubMed] [Google Scholar]
- 192.Li HC, Szigligeti P, Worrell RT, Matthews JB, Conforti L, Soleimani M. Missense mutations in Na+:HCO3− cotransporter NBC1 show abnormal trafficking in polarized kidney cells: A asis of proximal renal tubular acidosis. Am J Physiol Renal Physiol. 2005;289:F61–F71. doi: 10.1152/ajprenal.00032.2005. [DOI] [PubMed] [Google Scholar]
- 193.Li HC, Worrell RT, Matthews JB, Husseinzadeh H, Neumeier L, Petrovic S, Conforti L, Soleimani M. Identification of a carboxyl-terminal motif essential for the targeting of Na+-HCO3− cotransporter NBC1 to the basolateral membrane. J Biol Chem. 2004;279:43190–43197. doi: 10.1074/jbc.M405780200. [DOI] [PubMed] [Google Scholar]
- 194.Li J, Sun XC, Bonanno JA. Role of NBC1 in apical and basolateral HCO3− permeabilities and transendothelial HCO3− fluxes in bovine corneal endothelium. Am J Physiol Cell Physiol. 2005;288:C739–C746. doi: 10.1152/ajpcell.00405.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lin CY, Ho CM, Bau DT, Yang SF, Liu SH, Lin PH, Lin TH, Tien N, Shih MC, Lu JJ. Evaluation of breast cancer susceptibility loci on 2q35, 3p24, 17q23 and FGFR2 genes in Taiwanese women with breast cancer. Anticancer Res. 2012;32:475–482. [PubMed] [Google Scholar]
- 196.Liu X, Williams JB, Sumpter BR, Bevensee MO. Inhibition of the Na/bicarbonate cotransporter NBCe1-A by diBAC oxonol dyes relative to niflumic acid and a stilbene. J Membr Biol. 2007;215:195–204. doi: 10.1007/s00232-007-9018-z. [DOI] [PubMed] [Google Scholar]
- 197.Liu Y, Xu JY, Wang DK, Wang L, Chen LM. Cloning and identification of two novel NBCe1 splice variants from mouse reproductive tract tissues: A comparative study of NCBT genes. Genomics. 2011;98:112–119. doi: 10.1016/j.ygeno.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 198.Liu Y, Xu K, Chen LM, Sun X, Parker MD, Kelly ML, LaManna JC, Boron WF. Distribution of NBCn2 (SLC4A10) splice variants in mouse brain. Neuroscience. 2010;169:951–964. doi: 10.1016/j.neuroscience.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lo YF, Yang SS, Seki G, Yamada H, Horita S, Yamazaki O, Fujita T, Usui T, Tsai JD, Yu IS, Lin SW, Lin SH. Severe metabolic acidosis causes early lethality in NBC1 W516X knock-in mice as a model of human isolated proximal renal tubular acidosis. Kidney Int. 2011;79:730–741. doi: 10.1038/ki.2010.523. [DOI] [PubMed] [Google Scholar]
- 200.Loiselle FB, Morgan PE, Alvarez BV, Casey JR. Regulation of the human NBC3 Na+/HCO3− cotransporter by carbonic anhydrase II and PKA. Am J Physiol Cell Physiol. 2004;286:C1423–C1433. doi: 10.1152/ajpcell.00382.2003. [DOI] [PubMed] [Google Scholar]
- 201.Long J, Shu XO, Cai Q, Gao YT, Zheng Y, Li G, Li C, Gu K, Wen W, Xiang YB, Lu W, Zheng W. Evaluation of breast cancer susceptibility loci in Chinese women. Cancer Epidemiol Biomarkers Prev. 2010;19:2357–2365. doi: 10.1158/1055-9965.EPI-10-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lopez IA, Acuna D, Galbraith G, Bok D, Ishiyama A, Liu W, Kurtz I. Time course of auditory impairment in mice lacking the electroneutral sodium bicarbonate cotransporter NBC3 (slc4a7) Brain Res Dev Brain Res. 2005;160:63–77. doi: 10.1016/j.devbrainres.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 203.Lu J, Boron WF. Reversible and irreversible interactions of DIDS with the human electrogenic Na+/HCO3− cotransporter NBCe1-A: Role of lysines in the KKMIK motif of TM5. Am J Physiol Cell Physiol. 2007;292:C1787–C1798. doi: 10.1152/ajpcell.00267.2006. [DOI] [PubMed] [Google Scholar]
- 204.Lu J, Daly CM, Parker MD, Gill HS, Piermarini PM, Pelletier MF, Boron WF. Effect of human carbonic anhydrase II on the activity of the human electrogenic Na+/HCO3− cotransporter NBCe1-A in Xenopus oocytes. J Biol Chem. 2006;281:19241–19250. doi: 10.1074/jbc.M602181200. [DOI] [PubMed] [Google Scholar]
- 205.Luo X, Choi JY, Ko SB, Pushkin A, Kurtz I, Ahn W, Lee MG, Muallem S. HCO3− salvage mechanisms in the submandibular gland acinar and duct cells. J Biol Chem. 2001;276:9808–9816. doi: 10.1074/jbc.M008548200. [DOI] [PubMed] [Google Scholar]
- 206.Majumdar D, Bevensee MO. Na+-coupled bicarbonate transporters of the solute carrier 4 family in the nervous system: Function, localization, and relevance to neurologic function. Neuroscience. 2010;171:951–972. doi: 10.1016/j.neuroscience.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Majumdar D, Maunsbach AB, Shacka JJ, Williams JB, Berger UV, Schultz KP, Harkins LE, Boron WF, Roth KA, Bevensee MO. Localization of electrogenic Na/bicarbonate cotransporter NBCe1 variants in rat brain. Neuroscience. 2008;155:818–832. doi: 10.1016/j.neuroscience.2008.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Marino CR, Jeanes V, Boron WF, Schmitt BM. Expression and distribution of the Na+-HCO3− cotransporter in human pancreas. Am J Physiol. 1999;277:G487–G494. doi: 10.1152/ajpgi.1999.277.2.G487. [DOI] [PubMed] [Google Scholar]
- 209.Maunsbach AB, Vorum H, Kwon TH, Nielsen S, Simonsen B, Choi I, Schmitt BM, Boron WF, Aalkjaer C. Immunoelectron microscopic localization of the electrogenic Na+/HCO3− cotransporter in rat and ambystoma kidney. J Am Soc Nephrol. 2000;11:2179–2189. doi: 10.1681/ASN.V11122179. [DOI] [PubMed] [Google Scholar]
- 210.McAlear SD, Bevensee MO. A cysteine-scanning mutagenesis study of transmembrane domain 8 of the electrogenic sodium/bicarbonate cotransporter NBCe1. J Biol Chem. 2006;281:32417–32427. doi: 10.1074/jbc.M607253200. [DOI] [PubMed] [Google Scholar]
- 211.McAlear SD, Liu X, Williams JB, McNicholas-Bevensee CM, Bevensee MO. Electrogenic Na+/HCO3− cotransporter (NBCe1) variants expressed in Xenopus oocytes: Functional comparison and roles of the amino and carboxy termini. J Gen Physiol. 2006;127:639–658. doi: 10.1085/jgp.200609520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.McLean LA, Roscoe J, Jorgensen NK, Gorin FA, Cala PM. Malignant gliomas display altered pH regulation by NHE1 compared with non-transformed astrocytes. Am J Physiol Cell Physiol. 2000;278:C676–C688. doi: 10.1152/ajpcell.2000.278.4.C676. [DOI] [PubMed] [Google Scholar]
- 213.McMurtrie HL, Cleary HJ, Alvarez BV, Loiselle FB, Sterling D, Morgan PE, Johnson DE, Casey JR. The bicarbonate transport metabolon. J Enzyme Inhib Med Chem. 2004;19:231–236. doi: 10.1080/14756360410001704443. [DOI] [PubMed] [Google Scholar]
- 214.Millar ID, Brown PD. NBCe2 exhibits a 3HCO3−:1 Na+ stoichiometry in mouse choroid plexus epithelial cells. Biochem Biophys Res Commun. 2008;373:550–554. doi: 10.1016/j.bbrc.2008.06.053. [DOI] [PubMed] [Google Scholar]
- 215.Milne RL, Gaudet MM, Spurdle AB, Fasching PA, Couch FJ, Benitez J, Arias Perez JI, Zamora MP, Malats N, dos SSI, Gibson LJ, Fletcher O, Johnson N, Anton-Culver H, Ziogas A, Figueroa J, Brinton L, Sherman ME, Lissowska J, Hopper JL, Dite GS, Apicella C, Southey MC, Sigurdson AJ, Linet MS, Schonfeld SJ, Freedman DM, Mannermaa A, Kosma VM, Kataja V, Auvinen P, Andrulis IL, Glendon G, Knight JA, Weerasooriya N, Cox A, Reed MW, Cross SS, Dunning AM, Ahmed S, Shah M, Brauch H, Ko YD, Bruning T, Lambrechts D, Reumers J, Smeets A, Wang-Gohrke S, Hall P, Czene K, Liu J, Irwanto AK, Chenevix-Trench G, Holland H, Giles GG, Baglietto L, Severi G, Bojensen SE, Nordestgaard BG, Flyger H, John EM, West DW, Whittemore AS, Vachon C, Olson JE, Fredericksen Z, Kosel M, Hein R, Vrieling A, Flesch-Janys D, Heinz J, Beckmann MW, Heusinger K, Ekici AB, Haeberle L, Humphreys MK, Morrison J, Easton DF, Pharoah PD, Garcia-Closas M, Goode EL, Chang-Claude J. Assessing interactions between the associations of common genetic susceptibility variants, reproductive history and body mass index with breast cancer risk in the breast cancer association consortium: A combined case-control study. Breast Cancer Res. 2010;12:R110. doi: 10.1186/bcr2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mohebbi N, Mihailova M, Wagner CA. The calcineurin inhibitor FK506 (tacrolimus) is associated with transient metabolic acidosis and altered expression of renal acid-base transport proteins. Am J Physiol Renal Physiol. 2009;297:F499–F509. doi: 10.1152/ajprenal.90489.2008. [DOI] [PubMed] [Google Scholar]
- 217.Moody WJ., Jr The ionic mechanism of intracellular pH regulation in crayfish neurones. J Physiol. 1981;316:293–308. doi: 10.1113/jphysiol.1981.sp013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Moser H. Intracellular pH regulation in the sensory neurone of the stretch receptor of the crayfish (Astacus fluviatilis) J Physiol. 1985;362:23–38. doi: 10.1113/jphysiol.1985.sp015660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflugers Arch. 2004;447:710–721. doi: 10.1007/s00424-003-1090-3. [DOI] [PubMed] [Google Scholar]
- 220.Muller-Berger S, Coppola S, Samarzija I, Seki G, Fromter E. Partial recovery of in vivo function by improved incubation conditions of isolated renal proximal tubule. I. Change of amiloride-inhibitable K+ conductance. Pflugers Arch. 1997;434:373–382. doi: 10.1007/s004240050410. [DOI] [PubMed] [Google Scholar]
- 221.Muller-Berger S, Ducoudret O, Diakov A, Fromter E. The renal Na+-HCO3− cotransporter expressed in Xenopus laevis oocytes: Change in stoichiometry in response to elevation of cytosolic Ca2+ concentration. Pflugers Arch. 2001;442:718–728. doi: 10.1007/s004240100592. [DOI] [PubMed] [Google Scholar]
- 222.Muller-Berger S, Nesterov VV, Fromter E. Partial recovery of in vivo function by improved incubation conditions of isolated renal proximal tubule. II. Change of Na+-HCO3− cotransport stoichiometry and of response to acetazolamide. Pflugers Arch. 1997;434:383–391. doi: 10.1007/s004240050411. [DOI] [PubMed] [Google Scholar]
- 223.Mulligan AM, Couch FJ, Barrowdale D, Domchek SM, Eccles D, Nevanlinna H, Ramus SJ, Robson M, Sherman M, Spurdle AB, Wappenschmidt B, Lee A, McGuffog L, Healey S, Sinilnikova OM, Janavicius R, Hansen T, Nielsen FC, Ejlertsen B, Osorio A, Munoz-Repeto I, Duran M, Godino J, Pertesi M, Benitez J, Peterlongo P, Manoukian S, Peissel B, Zaffaroni D, Cattaneo E, Bonanni B, Viel A, Pasini B, Papi L, Ottini L, Savarese A, Bernard L, Radice P, Hamann U, Verheus M, Meijers-Heijboer HE, Wijnen J, Gomez Garcia EB, Nelen MR, Kets CM, Seynaeve C, Tilanus-Linthorst MM, van der Luijt RB, van OT, Rookus M, Frost D, Jones JL, Evans DG, Lalloo F, Eeles R, Izatt L, Adlard J, Davidson R, Cook J, Donaldson A, Dorkins H, Gregory H, Eason J, Houghton C, Barwell J, Side LE, McCann E, Murray A, Peock S, Godwin AK, Schmutzler RK, Rhiem K, Engel C, Meindl A, Ruehl I, Arnold N, Niederacher D, Sutter C, Deissler H, Gadzicki D, Kast K, Preisler-Adams S, Varon-Mateeva R, Schoenbuchner I, Fiebig B, Heinritz W, Schafer D, Gevensleben H, Caux-Moncoutier V, Fassy-Colcombet M, Cornelis F, Mazoyer S, Leone M, Boutry-Kryza N, Hardouin A, Berthet P, Muller D, Fricker JP, Mortemousque I, Pujol P, Coupier I, Lebrun M, Kientz C, Longy M, Sevenet N, Stoppa-Lyonnet D, Isaacs C, Caldes T, de la Hoya M, Heikkinen T, Aittomaki K, Blanco I, Lazaro C, Barkardottir RB, Soucy P, Dumont M, Simard J, Montagna M, Tognazzo S, D’Andrea E, Fox S, Yan M, Rebbeck T, Olopade O, Weitzel JN, Lynch HT, Ganz PA, Tomlinson GE, Wang X, Fredericksen Z, Pankratz VS, Lindor NM, Szabo C, Offit K, Sakr R, Gaudet M, Bhatia J, Kauff N, Singer CF, Tea MK, Gschwantler-Kaulich D, Fink-Retter A, Mai PL, Greene MH, Imyanitov E, O’Malley FP, Ozcelik H, Glendon G, Toland AE, Gerdes AM, Thomassen M, Kruse TA, Jensen UB, Skytte AB, Caligo MA, Soller M, Henriksson K, Wachenfeldt v, Arver B, Stenmark-Askmalm M, Karlsson P, Ding YC, Neuhausen SL, Beattie M, Pharoah PD, Moysich KB, Nathanson KL, Karlan BY, Gross J, John EM, Daly MB, Buys SM, Southey MC, Hopper JL, Terry MB, Chung W, Miron AF, Goldgar D, Chenevix-Trench G, Easton DF, Andrulis IL, Antoniou AC. Common breast cancer susceptibility alleles are associated with tumour subtypes in BRCA1 and BRCA2 mutation carriers: Results from the Consortium of Investigators of Modifiers of BRCA1/2. Breast Cancer Res. 2011;13:R110. doi: 10.1186/bcr3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Odgaard E, Jakobsen JK, Frische S, Praetorius J, Nielsen S, Aalkjaer C, Leipziger J. Basolateral Na+-dependent HCO3− transporter NBCn1-mediated HCO3− influx in rat medullary thick ascending limb. J Physiol. 2004;555:205–218. doi: 10.1113/jphysiol.2003.046474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Okubo K, Kang D, Hamasaki N, Jennings ML. Red blood cell band 3. Lysine 539 and lysine 851 react with the same H2DIDS (4,4′-diisothiocyanodihydrostilbene-2,2′-disulfonic acid) molecule. J Biol Chem. 1994;269:1918–1926. [PubMed] [Google Scholar]
- 226.Orlowski A, De Giusti VC, Morgan PE, Aiello EA, Alvarez BV. Binding of carbonic anhydrase IX to extracellular loop 4 of the NBCe1 Na+/HCO3− cotransporter enhances NBCe1-mediated. Am J Physiol Cell Physiol. 2012;303:C69–C80. doi: 10.1152/ajpcell.00431.2011. [DOI] [PubMed] [Google Scholar]
- 227.Orlowski J, Grinstein S. Na+/H+ exchangers of mammalian cells. J Biol Chem. 1997;272:22373–22376. doi: 10.1074/jbc.272.36.22373. [DOI] [PubMed] [Google Scholar]
- 228.Paillard M. H+ and HCO3− transporters in the medullary thick ascending limb of the kidney: Molecular mechanisms, function and regulation. Kidney Int Suppl. 1998;65:S36–S41. [PubMed] [Google Scholar]
- 229.Paine ML, Snead ML, Wang HJ, Abuladze N, Pushkin A, Liu W, Kao LY, Wall SM, Kim YH, Kurtz I. Role of NBCe1 and AE2 in secretory ameloblasts. J Dent Res. 2008;87:391–395. doi: 10.1177/154405910808700415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Park HJ, Rajbhandari I, Yang HS, Lee S, Cucoranu D, Cooper DS, Klein JD, Sands JM, Choi I. Neuronal expression of sodium/bicarbonate cotransporter NBCn1 (SLC4A7) and its response to chronic metabolic acidosis. Am J Physiol Cell Physiol. 2010;298:C1018–C1028. doi: 10.1152/ajpcell.00492.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Park K, Hurley PT, Roussa E, Cooper GJ, Smith CP, Thevenod F, Steward MC, Case RM. Expression of a sodium bicarbonate cotransporter in human parotid salivary glands. Arch Oral Biol. 2002;47:1–9. doi: 10.1016/s0003-9969(01)00098-x. [DOI] [PubMed] [Google Scholar]
- 232.Park M, Ko SB, Choi JY, Muallem G, Thomas PJ, Pushkin A, Lee MS, Kim JY, Lee MG, Muallem S, Kurtz I. The cystic fibrosis transmembrane conductance regulator interacts with and regulates the activity of the HCO3− salvage transporter human Na+-HCO3− cotransporter isoform 3. J Biol Chem. 2002;277:50503–50509. doi: 10.1074/jbc.M201862200. [DOI] [PubMed] [Google Scholar]
- 233.Park M, Li Q, Shcheynikov N, Zeng W, Muallem S. NaBC1 is a ubiquitous electrogenic Na+-coupled borate transporter essential for cellular boron homeostasis and cell growth and proliferation. Mol Cell. 2004;16:331–341. doi: 10.1016/j.molcel.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 234.Parker MD, Boron WF, Tanner MJA. Characterization of human “AE4” as an electroneutral sodium-dependent bicarbonate transporter. FASEB J. 2002 [Google Scholar]
- 235.Parker MD, Bouyer P, Daly CM, Boron WF. Cloning and characterization of novel human SLC4A8 gene products encoding Na+- driven Cl−/HCO3− exchanger variants NDCBE-A, -C, and -D. Physiol Genomics. 2008;34:265–276. doi: 10.1152/physiolgenomics.90259.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Parker MD, Musa-Aziz R, Rojas JD, Choi I, Daly CM, Boron WF. Characterization of human SLC4A10 as an electroneutral Na+/HCO3− cotransporter (NBCn2) with Cl− self-exchange activity. J Biol Chem. 2008;283:12777–12788. doi: 10.1074/jbc.M707829200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Parker MD, Qin X, Williamson RC, Toye AM, Boron WF. HCO3−- independent conductance with a mutant Na+/HCO3− cotransporter (SLC4A4) in a case of proximal renal tubular acidosis with hypokalaemic paralysis. J Physiol. 2012;590:2009–2034. doi: 10.1113/jphysiol.2011.224733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Parks SK, Chiche J, Pouyssegur J. pH control mechanisms of tumor survival and growth. J Cell Physiol. 2011;226:299–308. doi: 10.1002/jcp.22400. [DOI] [PubMed] [Google Scholar]
- 239.Pedrosa R, Goncalves N, Hopfer U, Jose PA, Soares-da-Silva P. Activity and regulation of Na+-HCO3− cotransporter in immortalized spontaneously hypertensive rat and Wistar-Kyoto rat proximal tubular epithelial cells. Hypertension. 2007;49:1186–1193. doi: 10.1161/HYPERTENSIONAHA.106.083444. [DOI] [PubMed] [Google Scholar]
- 240.Perry C, Baker OJ, Reyland ME, Grichtchenko II. PKCαβγ- and PKCδ-dependent endocytosis of NBCe1-A and NBCe1-B in salivary parotid acinar cells. Am J Physiol Cell Physiol. 2009;297:C1409–C1423. doi: 10.1152/ajpcell.00028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Perry C, Blaine J, Le H, Grichtchenko II. PMA- and ANG II-induced PKC regulation of the renal Na+-HCO3− cotransporter (hkNBCe1) Am J Physiol Renal Physiol. 2006;290:F417–F427. doi: 10.1152/ajprenal.00395.2004. [DOI] [PubMed] [Google Scholar]
- 242.Perry C, Quissell DO, Reyland ME, Grichtchenko II. Electrogenic NBCe1 (SLC4A4), but not electroneutral NBCn1 (SLC4A7), cotransporter undergoes cholinergic-stimulated endocytosis in salivary ParC5 cells. Am J Physiol Cell Physiol. 2008;295:C1385–C1398. doi: 10.1152/ajpcell.00153.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Piermarini PM, Choi I, Boron WF. Cloning and characterization of an electrogenic Na/HCO3− cotransporter from the squid giant fiber lobe. Am J Physiol Cell Physiol. 2007;292:C2032–C2045. doi: 10.1152/ajpcell.00544.2006. [DOI] [PubMed] [Google Scholar]
- 244.Piermarini PM, Kim EY, Boron WF. Evidence against a direct interaction between intracellular carbonic anhydrase II and pure C-terminal domains of SLC4 bicarbonate transporters. J Biol Chem. 2007;282:1409–1421. doi: 10.1074/jbc.M608261200. [DOI] [PubMed] [Google Scholar]
- 245.Planelles G, Thomas SR, Anagnostopoulos T. Change of apparent stoichiometry of proximal-tubule Na+-HCO3− cotransport upon experimental reversal of its orientation. Proc Natl Acad Sci U S A. 1993;90:7406–7410. doi: 10.1073/pnas.90.15.7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Poronnik P, Schumann SY, Cook DI. HCO3− -dependent ACh-activated Na+ influx in sheep parotid secretory endpieces. Pflugers Arch. 1995;429:852–858. doi: 10.1007/BF00374810. [DOI] [PubMed] [Google Scholar]
- 247.Praetorius J, Hager H, Nielsen S, Aalkjaer C, Friis UG, Ainsworth MA, Johansen T. Molecular and functional evidence for electrogenic and electroneutral Na+-HCO3− cotransporters in murine duodenum. Am J Physiol Gastrointest Liver Physiol. 2001;280:G332–G343. doi: 10.1152/ajpgi.2001.280.3.G332. [DOI] [PubMed] [Google Scholar]
- 248.Praetorius J, Kim YH, Bouzinova EV, Frische S, Rojek A, Aalkjaer C, Nielsen S. NBCn1 is a basolateral Na+-HCO3− cotransporter in rat kidney inner medullary collecting ducts. Am J Physiol Renal Physiol. 2004;286:F903–F912. doi: 10.1152/ajprenal.00437.2002. [DOI] [PubMed] [Google Scholar]
- 249.Praetorius J, Nejsum LN, Nielsen S. A SCL4A10 gene product maps selectively to the basolateral plasma membrane of choroid plexus epithelial cells. Am J Physiol Cell Physiol. 2004;286:C601–C610. doi: 10.1152/ajpcell.00240.2003. [DOI] [PubMed] [Google Scholar]
- 250.Preisig PA, Alpern RJ. Chronic metabolic acidosis causes an adaptation in the apical membrane Na+/H+ antiporter and basolateral membrane Na(HCO3)3 symporter in the rat proximal convoluted tubule. J Clin Invest. 1988;82:1445–1453. doi: 10.1172/JCI113750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Pushkin A, Abuladze N, Gross E, Newman D, Tatishchev S, Lee I, Fedotoff O, Bondar G, Azimov R, Ngyuen M, Kurtz I. Molecular mechanism of kNBC1-carbonic anhydrase II interaction in proximal tubule cells. J Physiol. 2004;559:55–65. doi: 10.1113/jphysiol.2004.065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I. Cloning, tissue distribution, genomic organization, and functional characterization of NBC3, a new member of the sodium bicarbonate cotransporter family. J Biol Chem. 1999;274:16569–16575. doi: 10.1074/jbc.274.23.16569. [DOI] [PubMed] [Google Scholar]
- 253.Pushkin A, Abuladze N, Newman D, Lee I, Xu G, Kurtz I. Cloning, characterization and chromosomal assignment of NBC4, a new member of the sodium bicarbonate cotransporter family. Biochim Biophys Acta. 2000;1493:215–218. doi: 10.1016/s0167-4781(00)00149-4. [DOI] [PubMed] [Google Scholar]
- 254.Pushkin A, Abuladze N, Newman D, Muronets V, Sassani P, Tatishchev S, Kurtz I. The COOH termini of NBC3 and the 56-kDa H+-ATPase subunit are PDZ motifs involved in their interaction. Am J Physiol Cell Physiol. 2003;284:C667–C673. doi: 10.1152/ajpcell.00225.2002. [DOI] [PubMed] [Google Scholar]
- 255.Pushkin A, Kurtz I. SLC4 base (HCO3−, CO32−) transporters: Classification, function, structure, genetic diseases, and knockout models. Am J Physiol Renal Physiol. 2006;290:F580–F599. doi: 10.1152/ajprenal.00252.2005. [DOI] [PubMed] [Google Scholar]
- 256.Pushkin A, Yip KP, Clark I, Abuladze N, Kwon TH, Tsuruoka S, Schwartz GJ, Nielsen S, Kurtz I. NBC3 expression in rabbit collecting duct: Colocalization with vacuolar H+-ATPase. Am J Physiol. 1999;277:F974–F981. doi: 10.1152/ajprenal.1999.277.6.F974. [DOI] [PubMed] [Google Scholar]
- 257.Putnam RW. pH regulatory transport systems in a smooth muscle-like cell line. Am J Physiol. 1990;258:C470–C479. doi: 10.1152/ajpcell.1990.258.3.C470. [DOI] [PubMed] [Google Scholar]
- 258.Rabon EC, Reuben MA. The mechanism and structure of the gastric H+,K+-ATPase. Annu Rev Physiol. 1990;52:321–344. doi: 10.1146/annurev.ph.52.030190.001541. [DOI] [PubMed] [Google Scholar]
- 259.Reiners J, van WE, Marker T, Zimmermann U, Jurgens K, te BH, Overlack N, Roepman R, Knipper M, Kremer H, Wolfrum U. Scaffold protein harmonin (USH1C) provides molecular links between Usher syndrome type 1 and type 2. Hum Mol Genet. 2005;14:3933–3943. doi: 10.1093/hmg/ddi417. [DOI] [PubMed] [Google Scholar]
- 260.Reinertsen KV, Tonnessen TI, Jacobsen J, Sandvig K, Olsnes S. Role of chloride/bicarbonate antiport in the control of cytosolic pH. Cell-line differences in activity and regulation of antiport. J Biol Chem. 1988;263:11117–11125. [PubMed] [Google Scholar]
- 261.Rickmann M, Orlowski B, Heupel K, Roussa E. Distinct expression and subcellular localization patterns of Na+/HCO3− cotransporter (SLC 4A4) variants NBCe1-A and NBCe1-B in mouse brain. Neuroscience. 2007;146:1220–1231. doi: 10.1016/j.neuroscience.2007.02.061. [DOI] [PubMed] [Google Scholar]
- 262.Riihonen R, Nielsen S, Vaananen HK, Laitala-Leinonen T, Kwon TH. Degradation of hydroxyapatite in vivo and in vitro requires osteoclastic sodium-bicarbonate co-transporter NBCn1. Matrix Biol. 2010;29:287–294. doi: 10.1016/j.matbio.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 263.Ro HA, Carson JH. pH microdomains in oligodendrocytes. J Biol Chem. 2004;279:37115–37123. doi: 10.1074/jbc.M403099200. [DOI] [PubMed] [Google Scholar]
- 264.Robey RB, Ruiz OS, Espiritu DJ, Ibanez VC, Kear FT, Noboa OA, Bernardo AA, Arruda JA. Angiotensin II stimulation of renal epithelial cell Na+/HCO3− cotransport activity: A central role for Src family kinase/classic MAPK pathway coupling. J Membr Biol. 2002;187:135–145. doi: 10.1007/s00232-001-0157-3. [DOI] [PubMed] [Google Scholar]
- 265.Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. Cloning and functional expression of rNBC, an electrogenic Na+-HCO3− cotransporter from rat kidney. Am J Physiol. 1998;274:F425–F432. doi: 10.1152/ajprenal.1998.274.2.F425. [DOI] [PubMed] [Google Scholar]
- 266.Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/HCO3− cotransporter. Nature. 1997;387:409–413. doi: 10.1038/387409a0. [DOI] [PubMed] [Google Scholar]
- 267.Romero MF, Henry D, Nelson S, Harte PJ, Dillon AK, Sciortino CM. Cloning and characterization of a Na+-driven anion exchanger (NDAE1). A new bicarbonate transporter. J Biol Chem. 2000;275:24552–24559. doi: 10.1074/jbc.M003476200. [DOI] [PubMed] [Google Scholar]
- 268.Roos A, Boron WF. Intracellular pH. Physiol Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- 269.Rose CR, Deitmer JW. Evidence that glial cells modulate extracellular pH transients induced by neuronal activity in the leech central nervous system. J Physiol. 1994;481:1–5. doi: 10.1113/jphysiol.1994.sp020413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Roussa E, Nastainczyk W, Thevenod F. Differential expression of electrogenic NBC1 (SLC4A4) variants in rat kidney and pancreas. Biochem Biophys Res Commun. 2004;314:382–389. doi: 10.1016/j.bbrc.2003.12.099. [DOI] [PubMed] [Google Scholar]
- 271.Roussa E, Romero MF, Schmitt BM, Boron WF, Alper SL, Thevenod F. Immunolocalization of anion exchanger AE2 and Na+-HCO3− cotransporter in rat parotid and submandibular glands. Am J Physiol. 1999;277:G1288–G1296. doi: 10.1152/ajpgi.1999.277.6.G1288. [DOI] [PubMed] [Google Scholar]
- 272.Ruiz OS, Arruda JA. Regulation of the renal Na+-HCO3− cotransporter by cAMP and Ca2+-dependent protein kinases. Am J Physiol. 1992;262:F560–F565. doi: 10.1152/ajprenal.1992.262.4.F560. [DOI] [PubMed] [Google Scholar]
- 273.Ruiz OS, Robey RB, Qiu YY, Wang LJ, Li CJ, Ma J, Arruda JA. Regulation of the renal Na+-HCO3− cotransporter. XI. Signal transduction underlying CO2 stimulation. Am J Physiol. 1999;277:F580–F586. doi: 10.1152/ajprenal.1999.277.4.F580. [DOI] [PubMed] [Google Scholar]
- 274.Ruminot I, Gutierrez R, Pena-Munzenmayer G, Anazco C, Sotelo-Hitschfeld T, Lerchundi R, Niemeyer MI, Shull GE, Barros LF. NBCe1 mediates the acute stimulation of astrocytic glycolysis by extracellular K+ J Neurosci. 2011;31:14264–14271. doi: 10.1523/JNEUROSCI.2310-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Russell JM, Boron WF. Role of chloride transport in regulation of intracellular pH. Nature. 1976;264:73–74. doi: 10.1038/264073a0. [DOI] [PubMed] [Google Scholar]
- 276.Russell JM, Boron WF. Intracellular pH regulation in squid giant axons. Kroc Found Ser. 1981;15:221–237. [PubMed] [Google Scholar]
- 277.Saito Y, Wright EM. Bicarbonate transport across the frog choroid plexus and its control by cyclic nucleotides. J Physiol. 1983;336:635–648. doi: 10.1113/jphysiol.1983.sp014602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Sandmann S, Yu M, Kaschina E, Blume A, Bouzinova E, Aalkjaer C, Unger T. Differential effects of angiotensin AT1 and AT2 receptors on the expression, translation and function of the Na+-H+ exchanger and Na+-HCO3− symporter in the rat heart after myocardial infarction. J Am Coll Cardiol. 2001;37:2154–2165. doi: 10.1016/s0735-1097(01)01287-6. [DOI] [PubMed] [Google Scholar]
- 279.Santella RN, Gennari FJ, Maddox DA. Metabolic acidosis stimulates bicarbonate reabsorption in the early proximal tubule. Am J Physiol. 1989;257:F35–F42. doi: 10.1152/ajprenal.1989.257.1.F35. [DOI] [PubMed] [Google Scholar]
- 280.Sassani P, Pushkin A, Gross E, Gomer A, Abuladze N, Dukkipati R, Carpenito G, Kurtz I. Functional characterization of NBC4:Anewelectrogenic sodium-bicarbonate cotransporter. Am J Physiol Cell Physiol. 2002;282:C408–C416. doi: 10.1152/ajpcell.00409.2001. [DOI] [PubMed] [Google Scholar]
- 281.Schlue WR, Deitmer JW. Ionic mechanisms of intracellular pH regulation in the nervous system. Ciba Found Symp. 1988;139:47–69. doi: 10.1002/9780470513699.ch4. [DOI] [PubMed] [Google Scholar]
- 282.Schlue WR, Thomas RC. A dual mechanism for intracellular pH regulation by leech neurones. J Physiol. 1985;364:327–338. doi: 10.1113/jphysiol.1985.sp015748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Schmitt BM, Biemesderfer D, Romero MF, Boulpaep EL, Boron WF. Immunolocalization of the electrogenic Na+-HCO3− cotransporter in mammalian and amphibian kidney. Am J Physiol. 1999;276:F27–F38. doi: 10.1152/ajprenal.1999.276.1.F27. [DOI] [PubMed] [Google Scholar]
- 284.Schosser A, Gaysina D, Cohen-Woods S, Domenici E, Perry J, Tozzi F, Korszun A, Gunasinghe C, Gray J, Jones L, Binder EB, Holsboer F, Craddock N, Owen MJ, Craig IW, Farmer AE, Muglia P, McGuffin P. A follow-up case-control association study of tractable (druggable) genes in recurrent major depression. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:640–650. doi: 10.1002/ajmg.b.31204. [DOI] [PubMed] [Google Scholar]
- 285.Schueler C, Becker HM, McKenna R, Deitmer JW. Transport activity of the sodium bicarbonate cotransporter NBCe1 is enhanced by different isoforms of carbonic anhydrase. PLoS One. 2011;6:e27167. doi: 10.1371/journal.pone.0027167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Schwab A, Rossmann H, Klein M, Dieterich P, Gassner B, Neff C, Stock C, Seidler U. Functional role of Na+-HCO3− cotransport in migration of transformed renal epithelial cells. J Physiol. 2005;568:445–458. doi: 10.1113/jphysiol.2005.092957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Schwiening CJ, Boron WF. Regulation of intracellular pH in pyramidal neurones from the rat hippocampus by Na+-dependent Cl−-HCO3− exchange. J Physiol. 1994;475:59–67. doi: 10.1113/jphysiol.1994.sp020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Sciortino CM, Romero MF. Cation and voltage dependence of rat kidney electrogenic Na+-HCO3− cotransporter, rkNBC, expressed in oocytes. Am J Physiol. 1999;277:F611–F623. doi: 10.1152/ajprenal.1999.277.4.F611. [DOI] [PubMed] [Google Scholar]
- 289.Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, Leotta A, Pai D, Zhang R, Lee YH, Hicks J, Spence SJ, Lee AT, Puura K, Lehtimaki T, Ledbetter D, Gregersen PK, Bregman J, Sutcliffe JS, Jobanputra V, Chung W, Warburton D, King MC, Skuse D, Geschwind DH, Gilliam TC, Ye K, Wigler M. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Seidler U, Bachmann O, Jacob P, Christiani S, Blumenstein I, Rossmann H. Na+/HCO3− cotransport in normal and cystic fibrosis intestine. JOP. 2001;2:247–256. [PubMed] [Google Scholar]
- 291.Seki G, Coppola S, Fromter E. The Na+-HCO3− cotransporter operates with a coupling ratio of 2 HCO3− to 1 Na+ in isolated rabbit renal proximal tubule. Pflugers Arch. 1993;425:409–416. doi: 10.1007/BF00374866. [DOI] [PubMed] [Google Scholar]
- 292.Seki G, Coppola S, Yoshitomi K, Burckhardt BC, Samarzija I, Muller-Berger S, Fromter E. On the mechanism of bicarbonate exit from renal proximal tubular cells. Kidney Int. 1996;49:1671–1677. doi: 10.1038/ki.1996.244. [DOI] [PubMed] [Google Scholar]
- 293.Shiohara M, Igarashi T, Mori T, Komiyama A. Genetic and long-term data on a patient with permanent isolated proximal renal tubular acidosis. Eur J Pediatr. 2000;159:892–894. doi: 10.1007/pl00008363. [DOI] [PubMed] [Google Scholar]
- 294.Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G, Mikoshiba K. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3− cotransporter 1 (pNBC1) Proc Natl Acad Sci U S A. 2006;103:9542–9547. doi: 10.1073/pnas.0602250103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Sindic A, Chang MH, Mount DB, Romero MF. Renal physiology of SLC26 anion exchangers. Curr Opin Nephrol Hypertens. 2007;16:484–490. doi: 10.1097/MNH.0b013e3282e7d7d0. [DOI] [PubMed] [Google Scholar]
- 296.Sinning A, Liebmann L, Kougioumtzes A, Westermann M, Bruehl C, Hubner CA. Synaptic glutamate release ismodulated by the Na+-driven Cl−/HCO3− exchanger Slc4a8. J Neurosci. 2011;31:7300–7311. doi: 10.1523/JNEUROSCI.0269-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Soleimani M, Aronson PS. Ionic mechanism of Na+-HCO3− cotransport in rabbit renal basolateral membrane vesicles. J Biol Chem. 1989;264:18302–18308. [PubMed] [Google Scholar]
- 298.Soleimani M, Grassi SM, Aronson PS. Stoichiometry of Na+-HCO3− cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. J Clin Invest. 1987;79:1276–1280. doi: 10.1172/JCI112948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Soleimani M, Lesoine GA, Bergman JA, McKinney TD. A pH modifier site regulates activity of the Na+:HCO3− cotransporter in basolateral membranes of kidney proximal tubules. J Clin Invest. 1991;88:1135–1140. doi: 10.1172/JCI115413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Sonalker PA, Tofovic SP, Bastacky SI, Jackson EK. Chronic noradrenaline increases renal expression of NHE-3, NBC-1, BSC-1 and aquaporin-2. Clin Exp Pharmacol Physiol. 2008;35:594–600. doi: 10.1111/j.1440-1681.2007.04846.x. [DOI] [PubMed] [Google Scholar]
- 301.Sonalker PA, Tofovic SP, Jackson EK. Increased expression of the sodium transporter BSC-1 in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2004;311:1052–1061. doi: 10.1124/jpet.104.071209. [DOI] [PubMed] [Google Scholar]
- 302.Sterling D, Reithmeier RA, Casey JR. A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J Biol Chem. 2001;276:47886–47894. doi: 10.1074/jbc.M105959200. [DOI] [PubMed] [Google Scholar]
- 303.Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol. 2005;67:377–409. doi: 10.1146/annurev.physiol.67.031103.153247. [DOI] [PubMed] [Google Scholar]
- 304.Steward MC, Poronnik P, Cook DI. Bicarbonate transport in sheep parotid secretory cells. J Physiol. 1996;494:819–830. doi: 10.1113/jphysiol.1996.sp021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Stutz AM, Teran-Garcia M, Rao DC, Rice T, Bouchard C, Rankinen T. Functional identification of the promoter of SLC4A5, a gene associated with cardiovascular and metabolic phenotypes in the HERITAGE Family Study. Eur J Hum Genet. 2009;17:1481–1489. doi: 10.1038/ejhg.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Sueta A, Ito H, Kawase T, Hirose K, Hosono S, Yatabe Y, Tajima K, Tanaka H, Iwata H, Iwase H, Matsuo K. A genetic risk predictor for breast cancer using a combination of low-penetrance polymorphisms in a Japanese population. Breast Cancer Res Treat. 2012;132:711–721. doi: 10.1007/s10549-011-1904-5. [DOI] [PubMed] [Google Scholar]
- 307.Suzuki M, Vaisbich MH, Yamada H, Horita S, Li Y, Sekine T, Moriyama N, Igarashi T, Endo Y, Cardoso TP, de Sa LC, Koch VH, Seki G, Fujita T. Functional analysis of a novel missense NBC1 mutation and of other mutations causing proximal renal tubular acidosis. Pflugers Arch. 2008;455:583–593. doi: 10.1007/s00424-007-0319-y. [DOI] [PubMed] [Google Scholar]
- 308.Suzuki M, Van PW, Stalmans I, Horita S, Yamada H, Bergmans BA, Legius E, Riant F, De JP, Li Y, Sekine T, Igarashi T, Fujimoto I, Mikoshiba K, Shimadzu M, Shiohara M, Braverman N, Al-Gazali L, Fujita T, Seki G. Defective membrane expression of the Na+-HCO3− cotransporter NBCe1 is associated with familial migraine. Proc Natl Acad Sci U S A. 2010;107:15963–15968. doi: 10.1073/pnas.1008705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Svastova E, Witarski W, Csaderova L, Kosik I, Skvarkova L, Hulikova A, Zatovicova M, Barathova M, Kopacek J, Pastorek J, Pastorekova S. Carbonic anhydrase IX interacts with bicarbonate transporters in lamellipodia and increases cell migration via its catalytic domain. J Biol Chem. 2012;287:3392–3402. doi: 10.1074/jbc.M111.286062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Svichar N, Esquenazi S, Chen HY, Chesler M. Preemptive regulation of intracellular pH in hippocampal neurons by a dual mechanism of depolarization-induced alkalinization. J Neurosci. 2011;31:6997–7004. doi: 10.1523/JNEUROSCI.6088-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Taylor JY, Maddox R, Wu CY. Genetic and environmental risks for high blood pressure among African American mothers and daughters. Biol Res Nurs. 2009;11:53–65. doi: 10.1177/1099800409334817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Thevenod F, Roussa E, Schmitt BM, Romero MF. Cloning and immunolocalization of a rat pancreatic Na+ bicarbonate cotransporter. Biochem Biophys Res Commun. 1999;264:291–298. doi: 10.1006/bbrc.1999.1484. [DOI] [PubMed] [Google Scholar]
- 313.Thomas RC. Ionic mechanism of the H+ pump in a snail neurone. Nature. 1976a;262:54–55. doi: 10.1038/262054a0. [DOI] [PubMed] [Google Scholar]
- 314.Thomas RC. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol. 1976b;255:715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Thomas RC. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977;273:317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Thouverey C, Malinowska A, Balcerzak M, Strzelecka-Kiliszek A, Buchet R, Dadlez M, Pikula S. Proteomic characterization of biogenesis and functions of matrix vesicles released from mineralizing human osteoblast-like cells. J Proteomics. 2011;74:1123–1134. doi: 10.1016/j.jprot.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 317.Tombola F, Del GG, Papini E, Zoratti M. Blockers of VacA provide insights into the structure of the pore. Biophys J. 2000;79:863–873. doi: 10.1016/S0006-3495(00)76342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Tonnessen TI, Ludt J, Sandvig K, Olsnes S. Bicarbonate/chloride antiport in Vero cells: I. Evidence for both sodium-linked and sodium-independent exchange. J Cell Physiol. 1987;132:183–191. doi: 10.1002/jcp.1041320202. [DOI] [PubMed] [Google Scholar]
- 319.Tonnessen TI, Sandvig K, Olsnes S. Role of Na+-H+ and Cl−-HCO3− antiports in the regulation of cytosolic pH near neutrality. Am J Physiol. 1990;258:C1117–C1126. doi: 10.1152/ajpcell.1990.258.6.C1117. [DOI] [PubMed] [Google Scholar]
- 320.Tsuganezawa H, Kobayashi K, Iyori M, Araki T, Koizumi A, Watanabe S, Kaneko A, Fukao T, Monkawa T, Yoshida T, Kim DK, Kanai Y, Endou H, Hayashi M, Saruta T. A new member of the HCO3− transporter superfamily is an apical anion exchanger of beta-intercalated cells in the kidney. J Biol Chem. 2001;276:8180–8189. doi: 10.1074/jbc.M004513200. [DOI] [PubMed] [Google Scholar]
- 321.Uhl GR, Liu QR, Walther D, Hess J, Naiman D. Polysubstance abuse-vulnerability genes: Genome scans for association, using 1,004 subjects and 1,494 single-nucleotide polymorphisms. Am J Hum Genet. 2001;69:1290–1300. doi: 10.1086/324467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Usui T, Hara M, Satoh H, Moriyama N, Kagaya H, Amano S, Oshika T, Ishii Y, Ibaraki N, Hara C, Kunimi M, Noiri E, Tsukamoto K, Inatomi J, Kawakami H, Endou H, Igarashi T, Goto A, Fujita T, Araie M, Seki G. Molecular basis of ocular abnormalities associated with proximal renal tubular acidosis. J Clin Invest. 2001;108:107–115. doi: 10.1172/JCI11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Villanger O, Veel T, Raeder MG. Secretin causes H+/HCO3− secretion from pig pancreatic ductules by vacuolar-type H+ -adenosine triphosphatase. Gastroenterology. 1995;108:850–859. doi: 10.1016/0016-5085(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 324.Vince JW, Reithmeier RA. Identification of the carbonic anhydrase II binding site in the Cl−/HCO3− anion exchanger AE1. Biochemistry. 2000;39:5527–5533. doi: 10.1021/bi992564p. [DOI] [PubMed] [Google Scholar]
- 325.Virkki LV, Choi I, Davis BA, Boron WF. Cloning of a Na+-driven Cl/HCO3 exchanger from squid giant fiber lobe. Am J Physiol Cell Physiol. 2003;285:C771–C780. doi: 10.1152/ajpcell.00439.2002. [DOI] [PubMed] [Google Scholar]
- 326.Virkki LV, Wilson DA, Vaughan-Jones RD, Boron WF. Functional characterization of human NBC4 as an electrogenic Na+-HCO3− cotransporter (NBCe2) Am J Physiol Cell Physiol. 2002;282:C1278–C1289. doi: 10.1152/ajpcell.00589.2001. [DOI] [PubMed] [Google Scholar]
- 327.Vorum H, Kwon TH, Fulton C, Simonsen B, Choi I, Boron W, Maunsbach AB, Nielsen S, Aalkjaer C. Immunolocalization of electroneutral Na+-HCO3− cotransporter in rat kidney. Am J Physiol Renal Physiol. 2000;279:F901–F909. doi: 10.1152/ajprenal.2000.279.5.F901. [DOI] [PubMed] [Google Scholar]
- 328.Wahl M, Deetjen P, Thurau K, Ingvar DH, Lassen NA. Micropuncture evaluation of the importance of perivascular pH for the arteriolar diameter on the brain surface. Pflugers Arch. 1970;316:152–163. doi: 10.1007/BF00586483. [DOI] [PubMed] [Google Scholar]
- 329.Wakabayashi S, Shigekawa M, Pouyssegur J. Molecular physiology of vertebrate Na+/H+ exchangers. Physiol Rev. 1997;77:51–74. doi: 10.1152/physrev.1997.77.1.51. [DOI] [PubMed] [Google Scholar]
- 330.Wang CZ, Yano H, Nagashima K, Seino S. The Na+-driven Cl−/HCO3− exchanger: Cloning, tissue distribution, and functional characterization. J Biol Chem. 2000;275:35486–35490. doi: 10.1074/jbc.C000456200. [DOI] [PubMed] [Google Scholar]
- 331.Wang DN, Sarabia VE, Reithmeier RA, Kuhlbrandt W. Three-dimensional map of the dimeric membrane domain of the human erythrocyte anion exchanger, Band 3. EMBO J. 1994;13:3230–3235. doi: 10.1002/j.1460-2075.1994.tb06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Wang G, Li C, Kim SW, Ring T, Wen J, Djurhuus JC, Wang W, Nielsen S, Frokiaer J. Ureter obstruction alters expression of renal acid-base transport proteins in rat kidney. Am J Physiol Renal Physiol. 2008;295:F497–F506. doi: 10.1152/ajprenal.00425.2007. [DOI] [PubMed] [Google Scholar]
- 333.Wang G, Topcu SO, Ring T, Wen J, Djurhuus JC, Kwon TH, Nielsen S, Frokiaer J. Age-dependent renal expression of acid-base transporters in neonatal ureter obstruction. Pediatr Nephrol. 2009;24:1487–1500. doi: 10.1007/s00467-009-1193-y. [DOI] [PubMed] [Google Scholar]
- 334.Wang W, Praetorius J, Li C, Praetorius HA, Kwon TH, Frokiaer J, Nielsen S. Vacuolar H+-ATPase expression is increased in acid-secreting intercalated cells in kidneys of rats with hypercalcaemia-induced alkalosis. Acta Physiol (Oxf) 2007;189:359–368. doi: 10.1111/j.1748-1716.2007.01672.x. [DOI] [PubMed] [Google Scholar]
- 335.Wendel C, Becker HM, Deitmer JW. The sodium-bicarbonate cotransporter NBCe1 supports glutamine efflux via SNAT3 (SLC38A3) co-expressed in Xenopus oocytes. Pflugers Arch. 2008;455:885–893. doi: 10.1007/s00424-007-0351-y. [DOI] [PubMed] [Google Scholar]
- 336.Whitfield JB, Dy V, McQuilty R, Zhu G, Heath AC, Montgomery GW, Martin NG. Genetic effects on toxic and essential elements in humans: Arsenic, cadmium, copper, lead, mercury, selenium, and zinc in erythrocytes. Environ Health Perspect. 2010;118:776–782. doi: 10.1289/ehp.0901541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Whitfield JB, Dy V, McQuilty R, Zhu G, Montgomery GW, Ferreira MA, Duffy DL, Neale MC, Heijmans BT, Heath AC, Martin NG. Evidence of genetic effects on blood lead concentration. Environ Health Perspect. 2007;115:1224–1230. doi: 10.1289/ehp.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Wieth JO, Andersen OS, Brahm J, Bjerrum PJ, Borders CL., Jr Chloride-bicarbonate exchange in red blood cells: Physiology of transport and chemical modification of binding sites. Philos Trans R Soc Lond B Biol Sci. 1982;299:383–399. doi: 10.1098/rstb.1982.0139. [DOI] [PubMed] [Google Scholar]
- 339.Wostyn P, van DD, Audenaert K, de Deyn PP. Genes involved in cerebrospinal fluid production as candidate genes for late-onset Alzheimer’s disease: A hypothesis. J Neurogenet. 2011;25:195–200. doi: 10.3109/01677063.2011.620191. [DOI] [PubMed] [Google Scholar]
- 340.Wu J, McNicholas CM, Bevensee MO. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates the electrogenic Na+/HCO3− cotransporter NBCe1-A expressed in Xenopus oocytes. Proc Natl Acad Sci U S A. 2009;106:14150–14155. doi: 10.1073/pnas.0906303106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Xu J, Barone S, Petrovic S, Wang Z, Seidler U, Riederer B, Ramaswamy K, Dudeja PK, Shull GE, Soleimani M. Identification of an apical Cl−/HCO3− exchanger in gastric surface mucous and duodenal villus cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1225–G1234. doi: 10.1152/ajpgi.00236.2003. [DOI] [PubMed] [Google Scholar]
- 342.Yamada H, Horita S, Suzuki M, Fujita T, Seki G. Functional role of a putative carbonic anhydrase II-binding domain in the electrogenic Na+-HCO3− cotransporter NBCe1 expressed in Xenopus oocytes. Channels (Austin) 2011;5:106–109. doi: 10.4161/chan.5.2.14341. [DOI] [PubMed] [Google Scholar]
- 343.Yamaguchi S, Ishikawa T. Electrophysiological characterization of native Na+-HCO3− cotranspoter current in bovine parotid acinar cells. J Physiol. 2005;568:181–197. doi: 10.1113/jphysiol.2005.088633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Yamamoto T, Shirayama T, Sakatani T, Takahashi T, Tanaka H, Takamatsu T, Spitzer KW, Matsubara H. Enhanced activity of ventricular Na+-HCO3− cotransport in pressure overload hypertrophy. Am J Physiol Heart Circ Physiol. 2007;293:H1254–H1264. doi: 10.1152/ajpheart.00964.2006. [DOI] [PubMed] [Google Scholar]
- 345.Yamamoto T, Swietach P, Rossini A, Loh SH, Vaughan-Jones RD, Spitzer KW. Functional diversity of electrogenic Na+-HCO3− cotransport in ventricular myocytes from rat, rabbit and quinea pig. J Physiol. 2005;562:455–475. doi: 10.1113/jphysiol.2004.071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Yamazaki O, Yamada H, Suzuki M, Horita S, Shirai A, Nakamura M, Seki G, Fujita T. Functional characterization of nonsynonymous single nucleotide polymorphisms in the electrogenic Na+-HCO3− cotransporter NBCe1A. Pflugers Arch. 2011;461:249–259. doi: 10.1007/s00424-010-0918-x. [DOI] [PubMed] [Google Scholar]
- 347.Yang D, Li Q, So I, Huang CL, Ando H, Mizutani A, Seki G, Mikoshiba K, Thomas PJ, Muallem S. IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. J Clin Invest. 2011;121:956–965. doi: 10.1172/JCI43475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Yang D, Shcheynikov N, Zeng W, Ohana E, So I, Ando H, Mizutani A, Mikoshiba K, Muallem S. IRBIT coordinates epithelial fluid and HCO3− secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. J Clin Invest. 2009;119:193–202. doi: 10.1172/JCI36983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Yang HS, Cooper DS, Rajbhandari I, Park HJ, Lee S, Choi I. Inhibition of rat Na+-HCO3− cotransporter (NBCn1) function and expression by the alternative splice domain. Exp Physiol. 2009;94:1114–1123. doi: 10.1113/expphysiol.2009.048603. [DOI] [PubMed] [Google Scholar]
- 350.Yang HS, Kim E, Lee S, Park HJ, Cooper DS, Rajbhandari I, Choi I. Mutation of Aspartate 555 of the Sodium/Bicarbonate Transporter SLC4A4/NBCe1 Induces Chloride Transport. J Biol Chem. 2009;284:15970–15979. doi: 10.1074/jbc.M109.001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Yang Z, Alvarez BV, Chakarova C, Jiang L, Karan G, Frederick JM, Zhao Y, Sauve Y, Li X, Zrenner E, Wissinger B, Hollander AI, Katz B, Baehr W, Cremers FP, Casey JR, Bhattacharya SS, Zhang K. Mutant carbonic anhydrase 4 impairs pH regulation and causes retinal photoreceptor degeneration. Hum Mol Genet. 2005;14:255–265. doi: 10.1093/hmg/ddi023. [DOI] [PubMed] [Google Scholar]
- 352.Yoshitomi K, Fromter E. Cell pH of rat renal proximal tubule in vivo and the conductive nature of peritubular HCO3− (HO−) exit. Pflugers Arch. 1984;402:300–305. doi: 10.1007/BF00585513. [DOI] [PubMed] [Google Scholar]
- 353.Yoshitomi K, Fromter E. How big is the electrochemical potential difference of Na+ across rat renal proximal tubular cell membranes in vivo? Pflugers Arch. 1985a;405(Suppl 1):S121–S126. doi: 10.1007/BF00581792. [DOI] [PubMed] [Google Scholar]
- 354.Yoshitomi K, Burckhardt BC, Fromter E. Rheogenic sodium-bicarbonate cotransport in the peritubular cell membrane of rat renal proximal tubule. Pflugers Arch. 1985b;405:360–366. doi: 10.1007/BF00595689. [DOI] [PubMed] [Google Scholar]
- 355.Yu H, Riederer B, Stieger N, Boron WF, Shull GE, Manns MP, Seidler UE, Bachmann O. Secretagogue stimulation enhances NBCe1 (electrogenic Na+/HCO3− cotransporter) surface expression in murine colonic crypts. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1223–G1231. doi: 10.1152/ajpgi.00157.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Zhang D, Kiyatkin A, Bolin JT, Low PS. Crystallographic structure and functional interpretation of the cytoplasmic domain of erythrocyte membrane band 3. Blood. 2000;96:2925–2933. [PubMed] [Google Scholar]
- 357.Zhao H, Star RA, Muallem S. Membrane localization of H+ and HCO3− transporters in the rat pancreatic duct. J Gen Physiol. 1994;104:57–85. doi: 10.1085/jgp.104.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Zhao J, Hogan EM, Bevensee MO, Boron WF. Out-of-equilibrium CO2/HCO3− solutions and their use in characterizing a new K+/HCO3− cotransporter. Nature. 1995;374:636–639. doi: 10.1038/374636a0. [DOI] [PubMed] [Google Scholar]
- 359.Zhao J, Zhou Y, Boron WF. Effect of isolated removal of either basolateral HCO3− or basolateral CO2 on HCO3− reabsorption by rabbit S2 proximal tubule. Am J Physiol Renal Physiol. 2003;285:F359–F369. doi: 10.1152/ajprenal.00013.2003. [DOI] [PubMed] [Google Scholar]
- 360.Zhou Y, Bouyer P, Boron WF. Effects of angiotensin II on the CO2 dependence of HCO3− reabsorption by the rabbit S2 renal proximal tubule. Am J Physiol Renal Physiol. 2006a;290:F666–F673. doi: 10.1152/ajprenal.00287.2005. [DOI] [PubMed] [Google Scholar]
- 361.Zhou Y, Bouyer P, Boron WF. Role of a tyrosine kinase in the CO2-induced stimulation of HCO3− reabsorption by rabbit S2 proximal tubules. Am J Physiol Renal Physiol. 2006b;291:F358–F367. doi: 10.1152/ajprenal.00520.2005. [DOI] [PubMed] [Google Scholar]
- 362.Zhou Y, Bouyer P, Boron WF. Role of the AT1A receptor in the CO2-induced stimulation of HCO3− reabsorption by renal proximal tubule. Am J Physiol Renal Physiol. 2007;293:F110–F120. doi: 10.1152/ajprenal.00516.2006. [DOI] [PubMed] [Google Scholar]
- 363.Zhou Y, Zhao J, Bouyer P, Boron WF. Evidence from renal proximal tubules that HCO3− and solute reabsorption are acutely regulated not by pH but by basolateral HCO3− and CO2. Proc Natl Acad Sci U S A. 2005;102:3875–3880. doi: 10.1073/pnas.0500423102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Zhu Q, Azimov R, Kao L, Newman D, Liu W, Abuladze N, Pushkin A, Kurtz I. NBCe1-A Transmembrane Segment 1 Lines the Ion Translocation Pathway. J Biol Chem. 2009;284:8918–8929. doi: 10.1074/jbc.M806674200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Zhu Q, Kao L, Azimov R, Abuladze N, Newman D, Pushkin A, Liu W, Chang C, Kurtz I. Structural and functional characterization of the C-terminal transmembrane region of NBCe1-A. J Biol Chem. 2010;285:37178–37187. doi: 10.1074/jbc.M110.169201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Zhu Q, Kao L, Azimov R, Newman D, Liu W, Pushkin A, Abuladze N, Kurtz I. Topological location and structural importance of the NBCe1-A residues mutated in proximal renal tubular acidosis. J Biol Chem. 2010;285:13416–13426. doi: 10.1074/jbc.M109.093286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Zhu Q, Liu W, Kao L, Azimov R, Newman D, Abuladze N, Kurtz I. Topology of NBCe1 protein transmembrane segment 1 and structural effect of proximal renal tubular acidosis (pRTA) S427L mutation. J Biol Chem. 2013;288:7894–7906. doi: 10.1074/jbc.M112.404533. [DOI] [PMC free article] [PubMed] [Google Scholar]