Abstract
Lead (Pb) exposure during development impairs a variety of cognitive, behavioral and neurochemical processes resulting in deficits in learning, memory, attention, impulsivity and executive function. Numerous studies have attempted to model this effect of Pb in rodents, with the majority of studies focusing on hippocampus-associated spatial learning and memory processes. Using a different paradigm, trace fear conditioning, a process requiring coordinated integration of both the medial prefrontal cortex and the hippocampus, we have assessed the effects of Pb exposure on associative learning and memory. The present study examined both female and male Long Evans rats exposed to three environmentally relevant levels of Pb (150 ppm, 375 ppm and 750 ppm) during different developmental periods: perinatal (PERI; gestation – postnatal day 21), early postnatal (EPN; postnatal days 1–21) and late postnatal (LPN; postnatal days 1–55). Testing began at postnatal day 55 and consisted of a single day of acquisition training, and three post training time points (1, 2 and 10 days) to assess memory consolidation and recall. All animals, regardless of sex, developmental window or level of Pb-exposure, successfully acquired conditioned-unconditioned stimulus association during training. However, there were significant effects of Pb-exposure on consolidation and memory recall at days 1–10 post training. In females, EPN and LPN exposure to 150 ppm Pb (but not PERI exposure) significantly impaired recall. In contrast, only PERI 150 ppm and 750 ppm-exposed males had significant recall deficits. These data suggest a complex interaction between sex, developmental window of exposure and Pb-exposure level on consolidation and recall of associative memories.
Keywords: Developmental Lead Exposure, Fear Conditioning, Sexual Dimorphism
Introduction
Lead (Pb) exposure during development impairs a variety of cognitive processes in children including learning, memory, attention, and various aspects of executive function, and, these deficits can persist into adulthood (Cecil et al., 2008; Cecil and Kos, 2006; Mazumdar et al., 2011; Nigg et al., 2010; Surkan et al., 2007). The cognitive/behavioral effects of Pb exposure have been modeled in numerous animal studies, with the majority of studies focusing on hippocampal-associated spatial learning and memory processes in rodents, most often using the Morris water maze (ex., (Jett et al., 1997; Toscano and Guilarte, 2005). Although these studies have provided useful information on the effects of Pb on hippocampus-associated learning and memory, the water maze task may not be optimal for detecting more subtle behavioral disturbances as might occur with lower level exposures to Pb (Anderson et al., 2012; Jett et al., 1997). Other studies have examined the effects of low level developmental Pb exposures (i.e., blood Pb levels < 10μg/dl) on frontal cortex-associated attention and impulsivity problems using fixed-interval/fixed ratio testing or performance on a delay discounting task (Brockel and Cory-Slechta, 1998; Weston et al., 2014). While these studies have provided important information regarding the neurotoxic effects of low level developmental Pb exposure on clinically relevant cognitive processes and brain neurochemistry, studies using other experimental paradigms are also needed in order to better understand some of the more complex cognitive/behavioral issues reported in Pb-exposed children.
Children exposed to low levels of Pb experience a variety of behavioral disorders that impact cognitive performance and academic achievement (Bellinger, 2008a, b; Lanphear et al., 2005; Needleman, 2004; Zhang et al., 2013). Of the numerous adverse behavioral outcomes from developmental Pb exposure, anti-social and aggressive behaviors are amongst the most common and troublesome. Although anti-social and aggressive/violent behavior can be attributed to a variety of social factors, increased frequency of anti-social and aggressive behaviors as well as other behavioral disorders such as oppositional defiant behavior and anxiety have been shown to be related to developmental Pb exposure (Carpenter and Nevin, 2010; Chiodo et al., 2004; Liu et al., 2014; Nevin, 2007; Nigg et al., 2008). There has been little basic research on these aspects of developmental Pb neurotoxicity and their relationship to disturbed cognitive outcomes. Alterations in neural circuitry or neural functioning consequent to developmental Pb exposure that result in maladapted behavioral reactions to perceived threats or inappropriate fear responses in non-threatening situations may play a role in at least some of the behavioral/cognitive abnormalities seen in Pb-exposed populations.
Fear conditioning is a valuable tool with which to study the neural systems involved in the acquisition, consolidation, recall and extinction of memories (Gilmartin and Helmstetter, 2010). In particular, trace fear conditioning, in which a neutral conditioned stimulus (CS) is paired with an aversive unconditioned stimulus (US) and a “trace” interval of several seconds is incorporated between the CS and US, has shown the importance of attentional mechanisms and the role of the prefrontal cortex in associative learning and memory (Gilmartin and Helmstetter, 2010; Han et al., 2003). Using fear conditioning tasks, a direct role for the prefrontal cortex in associative memory storage has been suggested (Runyan et al., 2004) as have potential epigenetic mechanisms controlling associative learning and memory formation (Lubin et al., 2008; Miller et al., 2010). Although effects of Pb exposure on fear conditioning have been examined previously, those studies used only males with blood Pb levels in excess of 30 μg/dl (Jaako-Movits et al., 2005); (Salinas and Huff, 2002) and none used a trace conditioning paradigm. Understanding effects of developmental Pb exposure on trace fear conditioning could potentially provide important information on how Pb interferes with the functioning of prefrontal circuits involved in complex associative behaviors. Such studies could also provide insight into how developmental Pb exposure may adversely influence the subjective assessment of and behavioral response to threat and the learning, memory, and expression of fear responses in threatening and non-threatening situations (Gilmartin et al., 2014). Dysfunction in prefrontal-hippocampal-amygdala circuits consequent to developmental Pb exposure could at least in part contribute to various forms of behavioral pathology that have been associated with Pb exposure and trace fear conditioning paradigms may be valuable tools with which to study this in the context of associative learning and memory.
We have recently reported significant differences in hippocampus-based spatial learning and memory in males and females following developmental Pb exposure (Anderson et al., 2012). The developmental period during which Pb exposure occurs can also significantly influence cognitive and neurobiological outcomes (Anderson et al., 2012; Schneider et al., 2013). Thus, the present study was performed to assess the effects of low level developmental exposure to Pb during different developmental periods on associative learning and memory in males and females, using a trace fear conditioning paradigm.
Methods
Animals and Treatments
The use of animals was in compliance with NIH Guidelines for the Care and Use of Laboratory Animals and the study was approved by the institutional animal care and use committee at Thomas Jefferson University. Three Pb exposure paradigms were used: perinatal (PERI) exposure, early postnatal (EPN) exposure, and long-term postnatal (LPN) exposure. In the perinatal exposure group, Long Evans dams (Harlan Laboratories) were fed chow (RMH 1000) with or without added Pb acetate (0 ppm, 150 ppm, 375 ppm or 750 ppm) for ten days prior to breeding and remained on the same diet through weaning. These Pb exposure levels were chosen based on our previous experience showing that exposure to these levels of Pb during development result in environmentally relevant blood Pb levels (Schneider et al., 2013). Litters were culled to equal numbers of pups to standardize litter size at postnatal day 7, with an aim of having eight pups per litter. Equal numbers of males and females were maintained wherever possible and were exposed to Pb from gestation through lactation (i.e., to postnatal day 21). At weaning, a single male or female was randomly selected and assigned to a behavioral cohort which consisted of a single sex, rats were housed four to a standard cage (940 cm2) with ad lib access to chow (no added Pb) and water until behavioral testing, beginning at postnatal day 55, with each treatment arm having an n=8. For animals in the EPN group, dams were fed RMH 1000 chow with no added Pb during gestation and were then fed chow with or without added Pb acetate beginning at day of birth (postnatal day 1) and pups continued to receive the same exposure to Pb through weaning at postnatal day 21. The LPN group received Pb exposure similar to the EPN group but exposure continued to postnatal day 55 (Figure 1A). Animals were exposed to a 12 h:12 h light:dark cycle for the duration of the experiment. Blood samples were collected at the time of euthanasia (postnatal day 65) and analyzed for Pb levels using graphite furnace atomic absorption with Zeeman background correction (ESA Labs, MA).
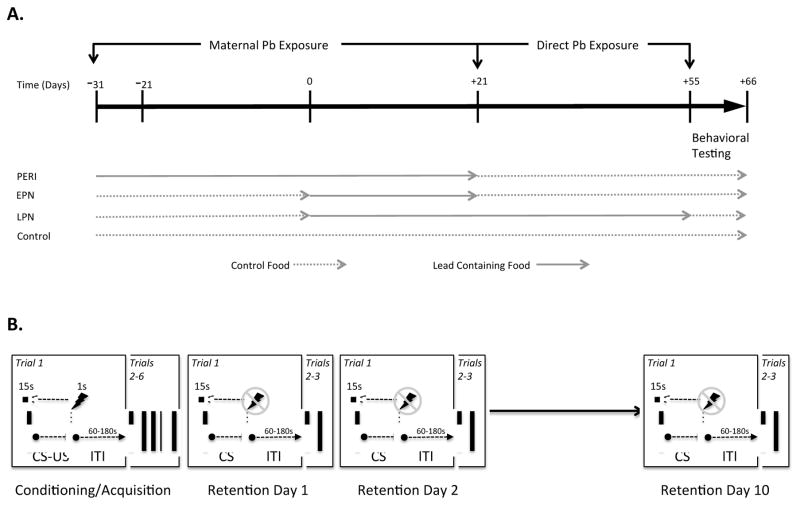
Figure 1.
A. Timelines for Lead Exposure. All animals were either exposed to lead (Pb) containing chow (RMH1000 containing 150, 375 or 750ppm lead acetate) and or control chow (RMH1000). Timing and duration of Pb exposure was either Perinatal (PERI), Early Postnatal (EPN) or Long-term Postnatal (LPN), as shown. All animals were behaviorally assesssed on a trace fear conditioning test at postnatal days 55–66. B. Fear Conditioning Experimental Design. All animals underwent 1 day of initial training using a pseudo-random CS (tone; 15 s duration)-US (shock, 1 s duration) combination (6 paired tones and shocks) separated by pseudo-random inter-trial intervals (ITI). The animals were then place in a novel context (novel visual, olfactory and tactile environment as described in detail in the methods) at 1, 2 and 10 days post training and presented with 3 cues (tone) separated by pseudo-random ITI’s with no subsequent shock, and the degree of freezing behavior was recorded (Anymaze, Stoelting Co.).
Trace Fear Conditioning
Trace fear conditioning was carried out using two Ugo Basile Fear Conditioning systems equipped with Anymaze software (Stoelting Co.) which was used to automatically measure the freezing response, based upon previously published methods (Wiltgen et al., 2005). Animals were habituated to the fear conditioning chamber which contained a test box with a grid floor through which shocks could be delivered, located within a dimly lit sound attenuating enclosure with white background noise, for 10 minutes one day prior to the start of fear conditioning. The animals were always placed in the same testing chamber for all training and testing sessions. The trace fear conditioning paradigm used for this study is shown schematically in Figure 1B. During conditioning/acquisition trials, animals were placed in a chamber with clear walls and given 120 seconds to habituate after which a series of 6 paired tone-shocks occurred (Tone: 3000 Hz, 80 dB for 15 seconds; Shock: 0.8mA for 1.0 second). Freezing behavior, defined by absence of all but respiratory movements, was measured every second for 20 seconds during the trace period. Each conditioning trial (CS-US pairing) was followed by a random inter-trial interval (ITI) that varied between 1 and 3 minutes. Conditioning trials were repeated 6 times during an 18 minute period. Retention testing occurred at 1, 2 and 10 days post conditioning. For retention testing, animals were placed back into the same chamber in which they were initially trained but with different visual and olfactory cues (i.e., the chamber was modified to have a solid opaque base, the walls were covered in a black and white checker board pattern and tubes containing vanilla extract were placed out of the reach of the animals, but allowed the scent to fill the chamber). On each retention testing day, animals were habituated to the chamber for 120 seconds followed by presentation of 3 tones for 15 seconds each, in the absence of foot shock, with novel pseudo random ITI’s between presentation of tones. Freezing was measured by the Anymaze software every second during the trace period (20 seconds) after tone presentation.
Data Analyses
Behavior data were analyzed using a within-subjects repeated-measures ANOVA followed by a Tukey test for post hoc analyses using Graphpad Prism Statistical Analysis software. Analyses of acquisition data included all data points, whereas analysis of all subsequent cued-retention trials limited the data to the first trial only. Statistical significance was defined at p < 0.05.
Results
Animal Characteristics
As we have previously reported (Schneider et al., 2013), there were no significant differences in the body weights of Pb exposed animals and control animals at the end of the study (age 65 days old) (data not shown). Blood lead levels (BLLs) in all control animals were below the minimum detectable limit (<1.0 μg/dl, Table 1). Mean BLLs for Pb-exposed animals, measured at the end of the study, were not significantly different between males and females within any exposure level or developmental window (Table 1). Due to the differences in exposure paradigms and differences in time from last day of Pb exposure to day of euthanasia (approximately 44 days for PERI and EPN exposed animals, vs 10 days for LPN exposed animals), statistical comparisons of BLLs across the different exposure groups was not performed. Comparison of BLL’s for littermates at weaning have previously been published by us (Schneider et al., 2013), with EPN BLL’s at weaning relevant to both EPN and LPN exposures. Blood Pb levels at weaning for EPN males ranged from 8.73 μg/dl (150ppm) to 15.75 ug/dl (750ppm), EPN females ranged from 9.67 μg/dl (150ppm) to 15.65 μg/dl (750ppm). Blood Pb levels at weaning for PERI males ranged from 15.38 μg/dl (150ppm) to 28.97 ug/dl (750ppm), PERI females ranged from 17.23 μg/dl (150ppm) to 27.35 μg/dl (750ppm).
Table 1.
Blood Lead Levels of Behaviorally Tested Animals.
| 0ppm | Perinatal | Early Postnatal | Late Postnatal | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 150ppm | 375ppm | 750ppm | 150ppm | 375ppm | 750ppm | 150ppm | 375ppm | 750ppm | ||
| Female | <1.00 | 1.36 ± 0.15 | 2.13 ± 0.13 | 2.08 ± 0.14 | 2.11 ± 0.39 | 2.00 ± 0.21 | 3.09 ± 0.21 | 4.50 ± 0.65 | 5.75 ± 0.48 | 9.58 ± 0.62 |
| Male | <1.00 | 1.25 ± 0.25 | 2.42 ± 0.38 | 2.47 ± 0.17 | 1.64 ± 0.25 | 1.95 ± 0.24 | 2.83 ± 0.21 | 2.01 ± 0.10 | 8.00 ± 0.02 | 7.46 ± 0.38 |
All values are reported as μg/dl mean blood lead level ± SEM, with an n=8 animals per group.
Behavioral Analyses: Control Animals (No Pb Exposure)
There were no differences in baseline locomotor behavior assessed during the initial 3 minutes spent in the testing chamber prior to the initiation of the test or freezing behavior assessed during the 120s habituation period prior to the first shock being administered) in any group of animals regardless of sex, Pb exposure or Pb exposure level, or developmental window of exposure (data not shown). Furthermore, there was no evidence of auditory or somatosensory impairments in any of the Pb-exposed animals. There was no effect of sex, Pb exposure level or developmental window of exposure on sensory functioning and the ability to sense the tone used in the trace conditioning paradigm or the ability to sense the electrical shock (data not shown).
The main effects for Sex and Trial x Sex during acquisition, were not significantly different between control males and females. That is, control males and females displayed similar percentages of freezing time following the presentation of the first CS-US and in both sexes, while the percent of time freezing increased during acquisition training (F(5, 348)=41.05, P=0.0001), with all animals achieving near-maximal response by the second trial. This effect, however, was driven by a significant difference between freezing time at Trial 1 versus all subsequent trials. There was no main effect of either Sex or Sex x Day for post acquisition memory testing, however there was a significant main effect of Day on post acquisition freezing when comparing control females and males (F(2, 180)=12.10, P<0.0001). That is, mean percent freezing for control females and males was not significantly different at post acquisition days 1 and 2, but at post acquisition day 10, control females showed a reduced amount of freezing compared to control males (t=2.831, p<0.05).
Behavioral Analyses: Perinatal (PERI) Pb Exposure
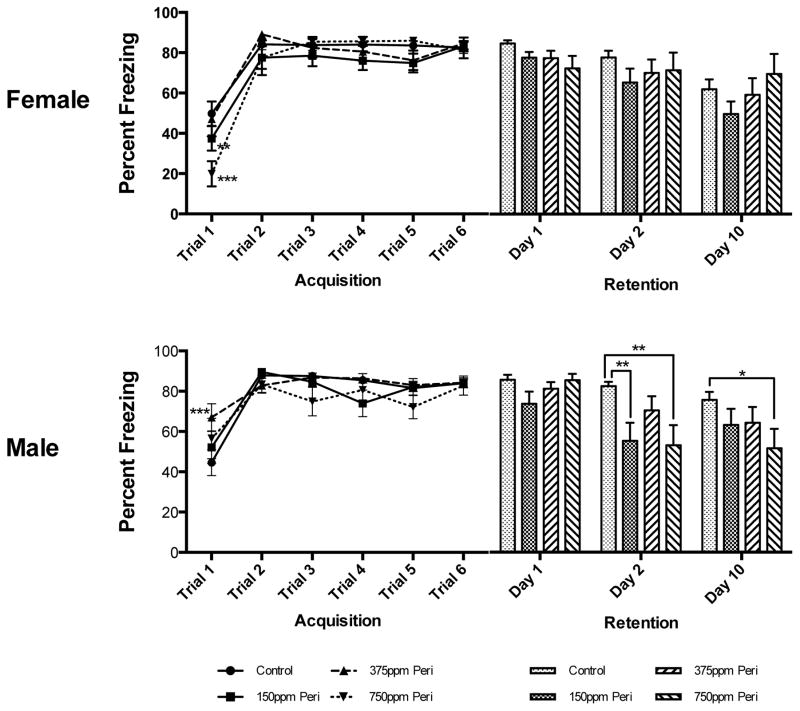
Fear Learning in Females: There was no main effect of Pb Exposure Level alone or Trial x Pb Exposure Level on learning (percent freezing) during trace fear conditioning in females, however Trial had a significant main effect on the percent of freezing (F(5,370)=61.63, p<0.0001). On the first acquisition trial, PERI females in the 750 ppm exposure group had significantly lower freezing responses than both control and 375 ppm PERI exposed females (P< 0.01 and P<0.001, respectively, Figure 2). By the end of the training period, there were no differences in the freezing response across any of the groups. Memory Testing in Females: There was no main effect of either Pb Exposure Level alone or Day x Pb-exposure level on post acquisition retention of the freezing response in females, while Day alone had a significant main effect on the percent of freezing (F(2,150)=14.31, p<0.0001). At no time post acquisition were there any significant differences between PERI females with different amounts of Pb exposure in the retention of the freezing response; all groups (with the exception of the 750 ppm exposure group) tended to have a reduced freezing response over time but these differences did not reach statistical significance (Figure 2).
Figure 2. Effects of Perinatal (PERI) Lead Exposure on Associative Learning and Memory in Female and Male Long Evans Rats.
There were significant initial differences in freezing time during acquisition in female 750ppm PERI Pb-exposed animals when compared to both Control (***p<0.001) and 375ppm PERI Pb-exposed females (**p<0.01), however there were no significant differences between any groups of females at the end of acquisition. In female animals there were also no significant effects of Pb exposure on post acquisition retention the CS-US association (i.e., no effect on percent of time freezing during the trace period) at any time point or at any Pb exposure level. In males, there was a significant difference in freezing between control and 375ppm PERI Pb-exposed animals only in Trial 1 of acquisition (***p<0.001). There were no differences between groups by the end of the acquisition period. Males had significant differences in post acquisition recall of the CS-US association at Day 2 only, with both 150ppm and 750ppm PERI Pb-exposed animals being significantly different from Controls (**p<0.01). This effect was maintained in 750ppm PERI Pb-exposed animals at 10 days post acquisition (*p<0.05 vs. Control). All data are represented as mean ± S.E.M.
Fear Learning in Males: There was no main effect of Pb Exposure Level alone on learning (percent freezing) in PERI Pb-exposed males. There was both a main effect of Trial alone (F(5,295)=29.43, p<0.0001) and a significant interaction between Trial x Pb Exposure Level (F(15,295)=1.81, p=0.0324) on freezing during learning in males. In particular, on the first acquisition trial, PERI 375 ppm-exposed males had a significantly higher percentage of freezing than did males in any of the other groups (P< 0.01, Figure 2). By the end of the training period, there were no differences in the freezing response across any of the groups. Memory Testing in Males: There were main effects of Pb Exposure Level alone (F(3,62)=4.48, p=0.0066), Day alone (F(2,124)=16.97, p<0.0001), and Day x Pb Exposure Level (F(6,124)=2.74, p=0.0270) on post-acquisition retention of the freezing response in males. On the second day of post-acquisition memory testing, the freezing response was significantly reduced in both 150 ppm and 750 ppm PERI males compared to non-Pb exposed controls (p<0.01 for both comparisons, Figure 2). Ten days post-acquisition, the freezing response was lower than in control animals in all groups but only significantly so in 750 ppm exposure group (p<0.05) (Figure 2).
Behavioral Analyses: Early Postnatal Lead (EPN) Exposure
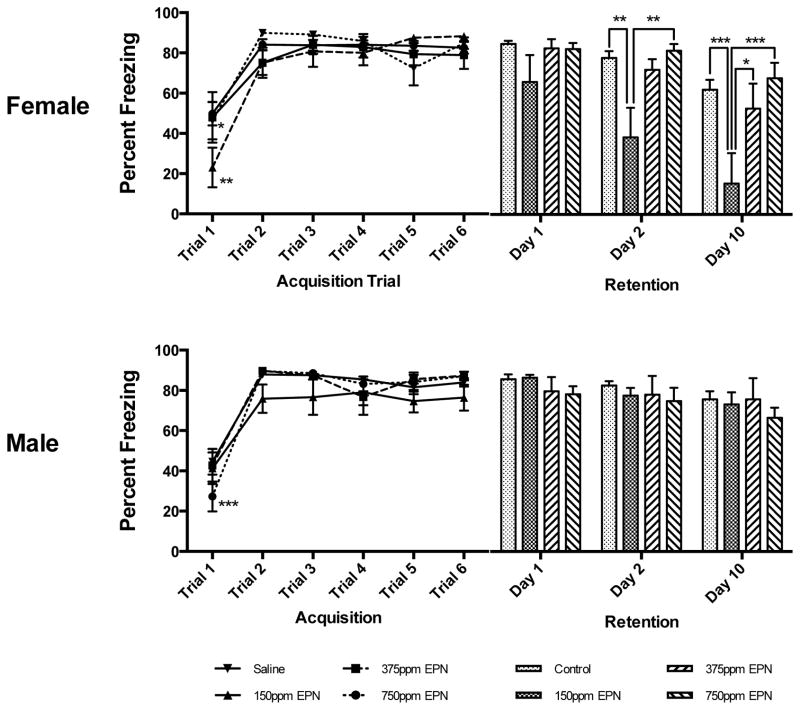
Fear Learning in Females: There was no main effect of either Pb Exposure Level alone or Trial x Pb Exposure Level on acquisition of the CS-US association (i.e., percent freezing) in females, while Trial alone had a significant main effect on freezing (F(5,285)=38.48, p<0.0001). On the first acquisition trial, 375 ppm EPN Pb-exposed females had a significantly lower percent of freezing compared to both control and 750 ppm EPN Pb-exposed females (P< 0.01 and P<0.05, respectively, Figure 3). There was no effect of 150 ppm EPN Pb exposure at any time during acquisition. By the end of the training period, there were no differences in the freezing response of any of the groups. Memory Testing in Females: There were both main effects of Trial alone (F(2,110)=24.30, p=0.0001) and Pb Exposure Level alone (F(3,55)=6.31, p=0.0009) but no interaction between Day x Pb Exposure Level on post-acquisition retention of the CS-US association in EPN females (Figure 3). There were no significant differences between control animals and EPN Pb-exposed females on the first post-acquisition testing day. When tested two days post-acquisition, the freezing response was significantly reduced in 150 ppm EPN females compared to both Control and 750 ppm EPN females (p<0.01 for both comparisons, Figure 3), but not 375 ppm EPN Pb-exposed animals. Memory for the CS-US association was further impaired in 150 ppm EPN females at day 10 with no differences in retention between control animals and animals with 375 ppm or 750 ppm Pb exposures (p<0.001 vs. control; Figure 3).
Figure 3. Effects of Early Postnatal (EPN) Lead Exposure on Associative Learning and Memory in Female and Male Long Evans Rats.
Female 375ppm EPN Pb-exposed animals were significantly different from control (**p<0.01) and 750ppm EPN (*p<0.05) animals in the percent of freezing on the 1st trial of acquisition only. There were no significant differences between control and Pb-exposed females in freezing behavior at the end of acquisition. In female rats exposed to 150ppm Pb, there were significant deficits (i.e., decreased freezing responses) at 2 days (when compared to Control (**p<0.01) and 750ppm (**p<0.01)) and at 10 days (when compared to all Control (***p<0.001), 375ppm (*p<0.05) and 750ppm (***p<0.001)) for post acquisition recall. In males, only 750ppm animals were significantly different from control during the first trial of acquisition (***p<0.001), and by the completion of acquisition, there were no differences between any exposure groups. In males there was no effect of Pb exposure at any time or exposure level on post acquisition recall. All data are represented as mean ± S.E.M.
Fear Learning in Males: There was no main effect of Pb Exposure Level alone on learning the CS-US association in EPN males nor an interaction between Trial x Pb Exposure Level during acquisition training. There was however a main effect of Trial alone (F(5,265)=64.48, p<0.0001) on the percent of freezing during task acquisition in males. On the first acquisition trial, 750 ppm Pb-exposed EPN males showed significantly less freezing than did control males (P< 0.05, Figure 3). By the end of the training period, there were no differences in the freezing response across any of the groups. Memory Testing in Males: There were no main effects of Pb Exposure Level alone, Day alone, nor an interaction between Day x Pb Exposure Level in EPN males on retention testing results. At no time during retention testing were there any significant differences between controls and any of the EPN Pb-exposed males (Figure 3).
Behavioral Analyses: Long-term Postnatal Lead (LPN) Exposure
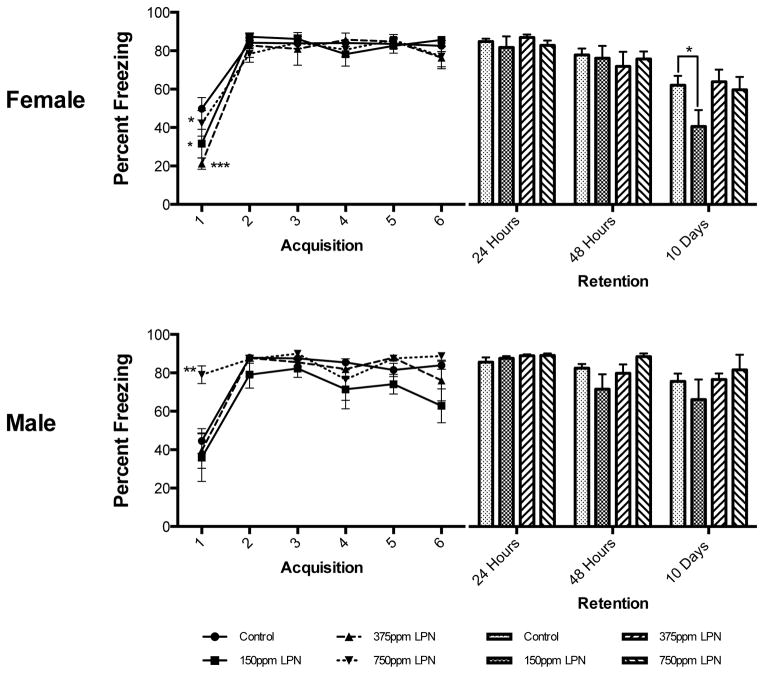
Fear Learning in Females: There was no main effect of Pb Exposure Level alone or Trial x Pb Exposure Level on learning in females, but a significant main effect of Trial alone on learning (F(5,315)=62.81, p<0.0001). On the first acquisition trial both 150 ppm and 375 ppm LPN Pb-exposed females showed significantly less freezing than controls (P<0.05 and P<0.001 respectively) and the 375 ppm LPN Pb-exposed females showed significantly less freezing than 750 ppm LPN Pb-exposed females (P<0.05, Figure 4). Memory Testing in Females: There was no main effect of either Pb Exposure Level alone nor an interaction between Day x Pb Exposure Level on retention of the CS-US association in LPN females. However, Day alone had a significant main effect on retention (F(2,126)=32.51, p<0.0001). There were no significant differences between any groups at retention testing days 1 and 2. However, at 10 days post-acquisition, there was a significant reduction in freezing in 150 ppm LPN females compared to controls (P<0.05, Figure 4).
Figure 4. Effects of Late Postnatal (LPN) Lead Exposure on Associative Learning and Memory in Female and Male Long Evans Rats.
There were significant differences in freezing duration during the first trial of acquisition only for 150ppm (*p<0.05) and 375ppm (***p<0.001) LPN Pb-exposed females compared to controls. Furthermore, the 375ppm LPN Pb-exposed group was significantly different from the 750ppm LPN Pb-exposed group (*p<0.05). There were no significant differences between any groups of LPN females in freezing behavior at post acquisition days 1 and 2. At 10 days post acquisition, there was a significant deficit in post acquisition recall (i.e., decreased freezing) in 150ppm LPN females only, compared to controls (*p<0.05). There was a significant difference between 750ppm LPN Pb-exposed males and all other males during the first acquisition trial, with 750ppm LPN Pb-exposed males having greater amounts of freezing at the outset (**p<0.01 vs. what?). At the end of acquisition training there were no significant differences in any male experimental group. There were also no significant differences between any of the male groups at any of the post acquisition recall trials. All data are represented as mean ± S.E.M.
Fear Learning in Males: There were main effects of both Trial alone (F(5,210)=20.86, p<0.0001) and Pb Exposure Level (F(3,42)=3.63, p=0.0205) on learning the CS-US association in LPN males, however there was no interaction between Trial x Pb Exposure Level. There was a significant difference between 750 ppm LPN Pb-exposed males and all other males on the first acquisition trial, with 750 ppm LPN Pb-exposed males showing higher levels of freezing (Figure 4). Memory Testing in Males: There were no main effects of Pb Exposure Level alone nor an interaction between Day x Pb Exposure Level EPN on retention of the CS-US association in LPN males. There was a main effect of Day alone (F(2,86)=6.0, p=0.0036) on freezing during retention testing in males but there were no significant differences between retention responses of control animals and any of the LPN Pb-exposed males on any of the retention testing days (Figure 4).
Discussion
We and others have previously reported sex-related differences in hippocampus-based learning and memory (Anderson et al., 2012), gene expression patterns in both the frontal cortex and hippocampus of littermates (Anderson et al., 2013; Schneider et al., 2011) and modulation of epigenetic control elements including DNA methyltransferases of littermates (Schneider et al., 2013) following Pb-exposure. The results of the current study suggest that there are also complex effects of sex, developmental window of exposure and level of Pb-exposure on associative memory as assessed by trace fear conditioning. Similar to previous reports by us and others (Schneider et al., 2012a; Schneider et al., 2013); (Leasure et al., 2008), greater effects were observed at lower levels of Pb exposure in several of the experimental groups and in the present study, this was observed more frequently in females. This type of nonlinear dose-response effect of Pb is consistent with other work (e.g., Lanphear et al., 2005; Leasure et al., 2008) that suggested that the impact from exposure to low levels of Pb may be greater than the proportional impact from higher level exposures.
Comparison of control males and females identified no significant sex-related differences in either the ability to acquire, consolidate, or recall the associative memory until the 10th day post training, at which time females had a greater reduction in overall freezing than did males. This suggests that there were no intrinsic sex-related differences in the ability to learn and consolidate the associative memory, but that females may have a more rapid extinction of the learned association with time. A rapid extinction of the learned association over time may also explain the decrease in the amount of freezing observed in PERI 150 ppm Pb-exposed males at 10 days compared to the response at 2 days. In both females and males, regardless of developmental window or level of Pb-exposure, all Pb-exposed animals were able to similarly learn the association between CS-US as evidenced by the lack of differences in the degree of freezing between the second and last acquisition trials when compared with sex-matched non-Pb-exposed controls. The initial differences in freezing response observed on Trial 1 in some Pb-exposed animals may reflect differences in the response to a novel stimulus, as on Trial 1, the animals hear the CS sound for the first time and have yet to experience the US. Following training and acquisition of the CS-US association, there was a specific pattern of deficits in recall of the learned association dependent upon sex, developmental window of Pb-exposure and amount of Pb-exposure. These data suggest an intact ability to acquire and consolidate associative memories in Pb-exposed females and males, regardless of the developmental timing of exposure or level of Pb-exposure, however long-term retention or recall of the memory, at least in the present trace fear conditioning paradigm, can be negatively affected in both sexes following Pb-exposure. The effects discussed above also argue against an aberrant anxiety-related effect driving the outcomes in Pb-exposed animals.
Associative memory storage, as assessed by trace fear conditioning, requires functional integration of both the medial prefrontal cortex and the hippocampus (Gilmartin and Helmstetter, 2010; Gilmartin et al., 2012; Runyan et al., 2004). Altering the function of this circuit by modification of medial prefrontal cortex activity during CS-US acquisition, through inhibition of either GABAA receptor activity by muscimol injection or N-methyl-D-aspartate receptor (NMDA-R) activity by 2-amino-5-phophonovaleric acid injection (APV), impairs formation of associative memory (defined by CS recall 24 hours post acquisition) without affecting acquisition (Gilmartin and Helmstetter, 2010). Temporary inhibition of NMDA-R activation in the ventral or dorsal hippocampus via APV infusion prior to trace fear conditioning training diminished but did not inhibit CS-US acquisition, while infusion of APV post CS-US acquisition into the ventral hippocampus, but not the dorsal hippocampus inhibited CS recall (Czerniawski et al., 2012). Furthermore, disconnection of the medial prefrontal cortex and dorsal hippocampus via excitotoxic lesion of either nuclei following trace fear conditioning training impaired recall at 11 days post training, with the dorsal hippocampal lesion producing the more profound deficit. However, when animals were retested at 200 days post training, dorsal hippocampal-lesioned animals had normal recall and animals with medial prefrontal lesions had significant recall deficits (Quinn et al., 2008). These data suggest a complex temporal interaction between the medial prefrontal cortex and dorsal hippocampus with regard to consolidation and associative memory recall, regardless of an animal’s capacity to initially acquire the CS-US association. Our data suggest that short term memory necessary for acquisition is not impaired in either males or females following developmental Pb-exposure, but that consolidation and longer-term recall of associative memory is negatively affected in the Pb-exposed animals and this may result from a potential decoupling of the prefrontal cortex from the dorsal hippocampus (Czerniawski et al., 2012; Gilmartin et al., 2012). The possibility exhists that developmental timing of differences in functional maturation of frontal cortex and ventral hippocampus expression of NMDA-R and/or GABAA receptors in males and females could potentially impact the influences of Pb exposure occurring during different developmental periods, and could in part explain why males exposed to Pb during the perinatal period had associative memory negatively impacted by Pb exposure whereas early postnatal and long-term postnatal Pb-exposed females were negatively impacted by Pb-exposure.
Epigenetic modulation of cognition is currently an area of intense focus, with numerous papers showing that dynamic changes in DNA methylation, histone acetylation/methylation and microRNA expression play critical roles in the formation, consolidation and recall of memories, as assessed using cued or context fear conditioning paradigms (Feng et al., 2010; Miller et al., 2008; Miller et al., 2010; Miller and Sweatt, 2007; Wang et al., 2013). Trace fear conditioning, however, has not been specifically assessed for dynamic modulation of either DNA methylation or histone acetylation/methylation. However, using a variant of associative hippocampal memory testing, contextual fear conditioning, Miller et al (Miller and Sweatt, 2007) found increased expression of DNA methyltransferase 3a mRNA (DNMT3a), the enzyme responsible for de novo methylation of DNA, but not DNA methyltransferase 1 mRNA (DNMT1a), the enzyme responsible for homeostatic control of DNA methylation, in the CA1 region of the hippocampus. These authors suggested that their findings reflected a dynamic regulation of DNMT activity in response to environmental sensory stimulation (Miller and Sweatt, 2007). Previously we identified changes in basal or resting state expression of DNMT1 and DNMT3a expression in the hippocampus of Pb-exposed animals that were dependent on sex, developmental window of Pb-exposure and amount of Pb-exposure (Schneider et al., 2013). For example, expression of DNMT3a was increased in the hippocampus of males exposed to 150 ppm and 750 ppm Pb perinatally while DNMT1 was unaffected. Expression of DNMT3a was decreased in females with early postnatal exposure to 150 ppm and 375 ppm Pb while DNMT1 was unaffected in all early postnatally exposed females (Schneider et al., 2013). While available data suggest that DNA methylation is dynamically regulated in various brain regions in response to experience and that this is important for memory formation, memory formation involves both increased methylation at memory suppressor genes and decreased methylation at memory promoting genes (Day and Sweatt, 2010). Thus, memory function might be driven by either hypermethylation or hypomethylation. At this point, it is not entirely clear how our previous data on resting state DNMT expression fit with the current behavioral findings, however, studies in progress are aimed at better defining the potential dynamic epigenetic regulatory mechanisms that might underlie the current behavioral findings.
In conclusion, the current work adds to our understanding of the complex interactions between sex, level of Pb exposure and developmental window of Pb exposure on cognitive processing. Considering the known sensitivity of the frontal cortex and hippocampus to the effects of Pb (Schneider et al., 2012b), it is likely that the current effects are related to complex effects of Pb on fronto-hippocampal circuits involved in acquisition, consolidation and recall of memories (Miller et al., 2010), and in particular, emotional memories. In addition to adversely affecting the formation, stabilization and recall of memories, Pb-induced damage to prefrontal/hippocampal circuits may also underlie emotional and behavioral dysregulation frequently seen in Pb-exposed children (Carpenter and Nevin, 2010, Chiodo et al., 2004, Liu et al., 2014, Nevin, 2007, Nigg et al., 2008). Additional work in progress will better define the extent to which low level Pb exposure during different developmental periods affects the dynamic modulation of DNA methylation and post-translational histone modifications critical to normal cognitive and behavioral regulation.
Acknowledgments
This research was supported by NIEHS grant R01 ES015295.
Footnotes
Conflict of Interest Statement.
The authors declare that there are no conflicts of interest.
References
- Anderson DW, Mettil WA, Schneider JS. Rearing environment, sex and developmental lead exposure modify gene expression in the hippocampus of behaviorally naive animals. Neurochem Int. 2013;62:510–520. doi: 10.1016/j.neuint.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DW, Pothakos K, Schneider JS. Sex and rearing condition modify the effects of perinatal lead exposure on learning and memory. NeuroToxicol. 2012;33:985–995. doi: 10.1016/j.neuro.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC. Neurological and behavioral consequences of childhood lead exposure. PLoS Med. 2008a;5:e115. doi: 10.1371/journal.pmed.0050115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC. Very low lead exposures and children’s neurodevelopment. Curr Opin Pediatr. 2008b;20:172–177. doi: 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- Brockel BJ, Cory-Slechta DA. Lead, attention, and impulsive behavior: changes in a fixed-ratio waiting-for-reward paradigm. Pharmacol Biochem Behav. 1998;60:545–552. doi: 10.1016/s0091-3057(98)00023-9. [DOI] [PubMed] [Google Scholar]
- Carpenter DO, Nevin R. Environmental causes of violence. Physiol Behav. 2010;99:260–268. doi: 10.1016/j.physbeh.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Cecil KM, Brubaker CJ, Adler CM, Dietrich KN, Altaye M, Egelhoff JC, Wessel S, Elangovan I, Hornung R, Jarvis K, Lanphear BP. Decreased brain volume in adults with childhood lead exposure. PLoS Med. 2008;5:e112. doi: 10.1371/journal.pmed.0050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecil KM, Kos RS. Magnetic resonance spectroscopy and metabolic imaging in white matter diseases and pediatric disorders. Top Magn Reson Imaging. 2006;17:275–293. doi: 10.1097/RMR.0b013e318033787e. [DOI] [PubMed] [Google Scholar]
- Chiodo LM, Jacobson SW, Jacobson JL. Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicol Teratol. 2004;26:359–371. doi: 10.1016/j.ntt.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Czerniawski J, Ree F, Chia C, Otto T. Dorsal versus ventral hippocampal contributions to trace and contextual conditioning: differential effects of regionally selective NMDA receptor antagonism on acquisition and expression. Hippocampus. 2012;22:1528–1539. doi: 10.1002/hipo.20992. [DOI] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. DNA methylation and memory formation. Nat Neurosci. 2010;13:1319–1323. doi: 10.1038/nn.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD, Silva AJ, Fan G. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci. 2010;13:423–430. doi: 10.1038/nn.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Balderston NL, Helmstetter FJ. Prefrontal cortical regulation of fear learning. Trends Neurosci. 2014;37:455–464. doi: 10.1016/j.tins.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Helmstetter FJ. Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learn Mem. 2010;17:289–296. doi: 10.1101/lm.1597410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Kwapis JL, Helmstetter FJ. Trace and contextual fear conditioning are impaired following unilateral microinjection of muscimol in the ventral hippocampus or amygdala, but not the medial prefrontal cortex. Neurobiol Learn Mem. 2012;97:452–464. doi: 10.1016/j.nlm.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CJ, O’Tuathaigh CM, van Trigt L, Quinn JJ, Fanselow MS, Mongeau R, Koch C, Anderson DJ. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proc Nat Acad Sci USA. 2003;100:13087–13092. doi: 10.1073/pnas.2132313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaako-Movits K, Zharkovsky T, Romantchik O, Jurgenson M, Merisalu E, Heidmets LT, Zharkovsky A. Developmental lead exposure impairs contextual fear conditioning and reduces adult hippocampal neurogenesis in the rat brain. Int J Dev Neurosci. 2005;23:627–635. doi: 10.1016/j.ijdevneu.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Jett DA, Kuhlmann AC, Farmer SJ, Guilarte TR. Age-dependent effects of developmental lead exposure on performance in the Morris water maze. Pharmacol Biochem Behav. 1997;57:271–279. doi: 10.1016/s0091-3057(96)00350-4. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure JL, Giddabasappa A, Chaney S, Johnson JE, Jr, Pothakos K, Lau YS, Fox DA. Low-level human equivalent gestational lead exposure produces sex-specific motor and coordination abnormalities and late-onset obesity in year-old mice. Environ Health Perspect. 2008;116:355–361. doi: 10.1289/ehp.10862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu X, Wang W, et al. Blood lead concentrations and children’s behavioral and emotional problems: A cohort study. JAMA Pediatrics. 2014 doi: 10.1001/jamapediatrics.2014.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar M, Bellinger DC, Gregas M, Abanilla K, Bacic J, Needleman HL. Low-level environmental lead exposure in childhood and adult intellectual function: a follow-up study. Environ Health. 2011;10:24. doi: 10.1186/1476-069X-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Campbell SL, Sweatt JD. DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem. 2008;89:599–603. doi: 10.1016/j.nlm.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Gavin CF, White JA, Parrish RR, Honasoge A, Yancey CR, Rivera IM, Rubio MD, Rumbaugh G, Sweatt JD. Cortical DNA methylation maintains remote memory. Nat Neurosci. 2010;13:664–666. doi: 10.1038/nn.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Needleman HL. Low level lead exposure and the development of children. The Southeast Asian J Trop Med Public Health. 2004;35:252–254. [PubMed] [Google Scholar]
- Nevin R. Understanding international crime trends: the legacy of preschool lead exposure. Environ Res. 2007;104:315–336. doi: 10.1016/j.envres.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Knottnerus GM, Martel MM, Nikolas M, Cavanagh K, Karmaus W, Rappley MD. Low blood lead levels associated with clinically diagnosed attention-deficit/hyperactivity disorder and mediated by weak cognitive control. Biol Psychiatry. 2008;63:325–331. doi: 10.1016/j.biopsych.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Nikolas M, Mark Knottnerus G, Cavanagh K, Friderici K. Confirmation and extension of association of blood lead with attention-deficit/hyperactivity disorder (ADHD) and ADHD symptom domains at population-typical exposure levels. J Child Psychol Psychiatry. 2010;51:58–65. doi: 10.1111/j.1469-7610.2009.02135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Ma QD, Tinsley MR, Koch C, Fanselow MS. Inverse temporal contributions of the dorsal hippocampus and medial prefrontal cortex to the expression of long-term fear memories. Learn Mem. 2008;15:368–372. doi: 10.1101/lm.813608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan JD, Moore AN, Dash PK. A role for prefrontal cortex in memory storage for trace fear conditioning. J Neusrosci. 2004;24:1288–1295. doi: 10.1523/JNEUROSCI.4880-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas JA, Huff NC. Lead and conditioned fear to contextual and discrete cues. Neurotoxicol Teratol. 2002;24:541–550. doi: 10.1016/s0892-0362(02)00265-9. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Anderson DW, Sonnenahalli H, Vadigepalli R. Sex-based differences in gene expression in hippocampus following postnatal lead exposure. Toxicol Appl Pharmacol. 2011;256:179–190. doi: 10.1016/j.taap.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Anderson DW, Talsania K, Mettil W, Vadigepalli R. Effects of developmental lead exposure on the hippocampal transcriptome: Influences of sex, developmental period and lead exposure level. Toxicol Sci. 2012a;129:108–125. doi: 10.1093/toxsci/kfs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Kidd SK, Anderson DW. Influence of developmental lead exposure on expression of DNA methyltransferases and methyl cytosine-binding proteins in hippocampus. Toxicol Lett. 2013;217:75–81. doi: 10.1016/j.toxlet.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Mettil W, Anderson DW. Differential effect of postnatal lead exposure on gene expression in the hippocampus and frontal cortex. J Mol Neurosci: MN. 2012b;47:76–88. doi: 10.1007/s12031-011-9686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surkan PJ, Zhang A, Trachtenberg F, Daniel DB, McKinlay S, Bellinger DC. Neuropsychological function in children with blood lead levels <10 microg/dL. Neurotoxicology. 2007;28:1170–1177. doi: 10.1016/j.neuro.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano CD, Guilarte TR. Lead neurotoxicity: from exposure to molecular effects. Brain Res Rev. 2005;49:529–554. doi: 10.1016/j.brainresrev.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Wang RY, Phang RZ, Hsu PH, Wang WH, Huang HT, Liu IY. In vivo knockdown of hippocampal miR-132 expression impairs memory acquisition of trace fear conditioning. Hippocampus. 2013;23:625–633. doi: 10.1002/hipo.22123. [DOI] [PubMed] [Google Scholar]
- Weston HI, Weston DD, Allen JL, Cory-Slechta DA. Sex-dependent impacts of low-level lead exposure and prenatal stress on impulsive choice behavior and associated biochemical and neurochemical manifestations. Neurotoxicology. 2014;44:169–183. doi: 10.1016/j.neuro.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Ferguson C, Homanics GE, Fanselow MS. Trace fear conditioning is enhanced in mice lacking the delta subunit of the GABAA receptor. Learn Mem. 2005;12:327–333. doi: 10.1101/lm.89705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Baker HW, Tufts M, Raymond RE, Salihu H, Elliott MR. Early childhood lead exposure and academic achievement: evidence from Detroit public schools, 2008–2010. Am J Public Health. 2013;103:e72–77. doi: 10.2105/AJPH.2012.301164. [DOI] [PMC free article] [PubMed] [Google Scholar]