Abstract
Dyspnea and fear of suffocation are burdensome to patients with respiratory disease. Inspiratory resistive loads offer an experimental respiratory stimulus to quantify the discriminative domain of respiratory perception. Resistive (R) load magnitude estimation (ME) and subjective ratings were measured over sustained multiple breaths in healthy subjects. There was no significant group difference between the ME for breath 1 and 20 for small R loads, but a significant gender difference for large R loads. Subjective responses of fear, fear of suffocation, displeasure, chest pressure, faintness, dizziness, fear of losing control, trembling, and tingling were significantly greater for females. These results demonstrate that ME of large resistive sustained loads elicits non-significant increases in ME in females, but a significant decrease in ME for males. The maintenance of ME in females co-occurs with increased aversive processing relative to males.
Introduction
Sensations of dyspnea and fear of suffocation negatively affect patients with respiratory diseases such as chronic obstructive pulmonary disease (COPD) and asthma and can contribute to an overall more negative quality of life. Chronic respiratory disease limits quality of life by preventing every day activities such as working, normal physical exertion, household chores, and participation in family activities (ALA, 2013). Health-related quality of life (HRQoL) has historically referred to the more subjective experience of the impact of the disease on the quality of life (Ketelaars et al, 1996). Asthmatics and COPD patients rate dyspnea as one of the most significant HRQoL contributors on rating scales (Nishimura et al, 2008).
Studies reducing airflow mechanically for single inspired loads, administered by adding uniform and controlled airflow resistance in an experimental setting, have traditionally offered a related measure of primarily the discriminative component of respiratory sensitivity (Davenport and Vovk, 2009). There are two primary cognitive components to the perception of increased respiratory loads: discriminative and affective (Davenport and Vovk, 2009). Perceptual discrimination refers to the somatosensory event and cognitive awareness of breathing disruption. During affective processing, the individual determines if the respiratory sensation (or load) is pleasant or unpleasant. Subjects seldom report unpleasant evaluations of single breath loads (Alexander-Miller & Davenport, 2010). However, it is likely that as a person increases the duration of breathing time against a load, the magnitude estimation of the load will increase (Alexander-Miller & Davenport, 2010), along with unpleasant sensations.
The specific comparison of the cognitive response to magnitude estimation of loads while breathing against a variety of sustained inspiratory loads has not been investigated, although single breath, large resistive loads have been shown to induce fear of suffocation (Pappens, Smets, Van de Bergh, and Van Diest, 2012; Alius, Pane-Farre, von Leupoldt, and Hamm, 2013). In line with this, research is increasingly demonstrating the relationship between fear, anxiety and respiratory disruption in animal and human models (Ren, Ding, Funk & Greer, 2012; Ritz, Meuret, Bhaskara & Peterson, 2013; Trueba, et al., 2013; Pate & Davenport, 2012). This is clinically related to the high incidence of anxiety in patients suffering from asthma and COPD (Bhandari et al, 2013; Maurer et al., 2008; Hill, Geist & Goldstein 2008; Ritz et al., 2012).
Variability in respiratory somatosensation is increasingly evident. Some subjects, such as females, magnify their perception of extended loads (Alexander-Miller and Davenport, 2010), while high-anxious subjects have reduced respiratory sensory gating leading to altered respiratory perception (Chan, et al., 2012). Individual variation in the processing and subsequent perception of respiratory somatosensation may be a result modulation of the affective domain. Individual variability has been attributed to “behavioral influences” of load compensation responses (Younes, 1995), and was more recently found to be correlated with fear of suffocation (Pappens, Smets, Van den Bergh, & van Diest, 2012). Perceptual discrimination of respiratory loads varies among subjects, such as in children with life threatening asthma, who have reduced magnitude estimation of inspiratory loads (Davenport and Kifle, 2001).
It is important to note that the individual differences in the perception of extrinsic loads do not correlate with differences in age or measures of lung function (Freedman and Campbell, 1970; Julius 2002). Several studies have determined that intolerance of the loads could not be explained as being due to any of the following variables reaching a critical or limiting value: ventilation, tidal volume, frequency, peak mouth pressure, peak inspiratory flow rate, added inspiratory work or power and end-tidal PCO2 (Freedman and Campbell, 1970; Julius et al., 2002). Since simple ventilatory and mechanical parameters to explain the subjects’ tolerance (or intolerance) of increased levels of loading were insufficient, subjective psychological factors may be important in determining each person’s load compensation response.
Psychophysical studies of respiratory load perception have been conducted in healthy individuals and patients using external resistances (Kifle, Seng & Davenport, 1997; Davenport & Kifle, 2001; Julius et al., 2002). These studies have shown considerable variation among subjects in the detection and magnitude estimation (ME) of resistive loads and the perceived effort of breathing. Hudgel et al. (1982) reported that anxious, non-independent subjects required higher inspiratory resistances for load detection than non-anxious subjects. It has also been demonstrated that subjects with generalized anxiety or panic disorder were unable to grade the magnitude of a series of inspiratory resistances (Tiller, 1987). This suggests that psychological state significantly contributes to or alters the perception and scaling of loads. Giardino et al (2010) found that while patients with COPD and panic disorder did not show heightened interoceptive accuracy, they did report greater dyspnea in response to inspiratory resistive loads. This could be attributed to the emotional response to dyspnea. Emotional states have been shown to affect the perception of respiratory efforts and magnitude estimation of resistive loads (Tsai et al, 2013). Previously, we have demonstrated significant group differences between males and females while rating prolonged inspiratory loads (Alexander-Miller and Davenport, 2010). Thus we hypothesize that females and males will differ in the processing and cognitive analysis of sustained breathing against resistive loads.
The perception of altered respiratory function by women may be more sensitive but less specific than by men (Becklake and Knauffman, 1999). Dyspnea is perceived as more important in women’s quality of life (QOL) scales than in men’s (Jones et. al. 1992) and is more of a contributor to QOL than it is for men (de Torres et al., 2006). Gender differences have been seen in forced expiratory volume (FEV1)-matched patients, with females reporting more dyspnea and lower HRQoL than males (Katsura, Yamada, Wakabayashi & Kida, 2007). Becklake and Knauffman (1999) also reported psychological factors such as depression have been linked to the reporting of respiratory symptoms, though gender differences in rates of reporting by psychological status have not been examined. Takano et. al. (1997) reported that differences may exist between males and females in the perception of respiratory discomfort. Our current study further expands this research area by examining psychological measures during loaded breathing and expands upon our previous study, which only examined ME of sustained loads. The purpose of this study was to determine if there was a relationship between gender differences in magnitude estimation and subjective, or affective, evaluation. We hypothesized that increasing the duration and strength of resistive loads would result in increases in the affective component of respiratory sensation measured by individual subjective responses. Furthermore, we hypothesized that this response will be greater in females than in males.
Method
Subjects
A total of 22 subjects were tested for this study. The average subject information is listed in Table 1. Subjects were required to be in good general heath with no neurological, cardiovascular, respiratory or any other major medical disease history. Subjects had to be free of any acute respiratory illness, including cough or nasal congestion. The study was approved by the University of Florida Institutional Review Board and consent was obtained from each subject prior to the beginning of the study. Subjects were divided into high and low negative affectivity (NA) participants, using a median split of the State-Trait-Anxiety-Index (STAI-state) scores. Subjects were required to comply with the study protocol by answering each question and reporting load magnitude and subjective responses following load presentations. Three subjects were excluded from final data inclusion for the following reasons: poor subject compliance, failure to self-report exclusion criteria (the subject was discovered to be a smoker after the experiment) and inability to complete the experiment.
Table 1.
Mean (± standard deviation) subject demographic data
| Male | Female | |
|---|---|---|
| Age (yrs) | 23.70 (± 3.4) | 32.56 (± 10.81) |
| Weight (lbs) | 170.10 (± 24.07) | 143.22 (± 21.01) |
| Height (in) | 70.55 (±3.01) | 67.56 (±1.72) |
| FVC (liter) | 4.91 (± 0.45) | 4.17 (±0.13) |
| FVC % Pred Avg | 1.06 (± 0.16) | 0.85 (± 0.00) |
| FEV1 (liter) | 4.59 (± 0.31) | 3.35 (±0.62) |
| FEV1 % Pred Avg | 1.11 (± 0.13) | 1.27 (±0.00) |
Experimental Protocol
The subjects were asked to refrain from strenuous physical activity, large meals and caffeine for at least four hours prior to the tests. Simple instructions were given to inform the subject how to complete the questionnaires, complete the pulmonary function test, stop at any time they felt uncomfortable, and breathe into the mouthpiece. A pulmonary function test (Forced vital capacity [FVC], FEV1 and FEV1/FVC) with forced expiratory maneuvers was performed. All subjects exceeded 70% predicted FVC, FEV1 and FEV1/FVC. The subject’s intrinsic respiratory resistance was measured using the forced oscillation method. The subjects were seated in front of the apparatus and breathed “normally” through the mouthpiece, with their cheeks supported by both hands. Approximately 10 tidal breaths were collected continuously to analyze respiratory resistance by computer (Jaeger Toennies, Medizintechnikmit System, V. 4.5). The test was repeated at least three times for each subject with a one-minute rest between repetitions. The average of three measures of the resistance at 5 Hz and 20 Hz were used as the subject’s respiratory system resistance. All subjects were within 80% predicted values for resistance.
Subjects were seated in a lounge chair in a sound isolated chamber separated from the experimenter and the experimental apparatus. Prior to load testing, each subject was given the State Trait Anxiety Inventory (STAI), a 20-items questionnaire measuring anxiety as a trait or as a state (Spielberger, Gorsuch, & Luchene, 1970). The STAI was repeated after the entire experiment was completed. The subject was then connected to the breathing apparatus and respired through a mouthpiece connected to a non-rebreathing valve with their nose clamped. The inspiratory port of the valve was connected to the resistive load manifold (Alexander-Miller and Davenport, 2010). The subjects were informed that at any time during the experiment they could remove the mouthpiece if they felt they could not breathe. They were also informed that at no time would they be at risk of injury due to lack of oxygen or airflow, and with the proper effort, could always maintain constant ventilation. At no time were they informed of the load magnitude.
Each subject was given a set of the subjective questions (survey), and followed along with the experimenter, who carefully explained each question, word meaning, and illustration. The subject was allowed to ask for clarification of the meaning of the subjective question. They were informed that the experimenter would give them a survey after each 20-breath load presentation, for a total of 15 surveys.
The subjects were instructed that when the light in front of them was illuminated, the next breath would be loaded. They were also given a verbal cue, “Please rate your next breath in.” This verbal cue was not changed, regardless of inspiratory load strength. The verbal cue was given and green light illuminated during expiration, cueing the subject that for the next 20 breaths they would be estimating their perception of their breathing which may or may not be loaded. A second red light was illuminated on the 1st, 10th and 20th loaded breath cueing the subject to estimate their perceived breathing. They estimated the perceived load magnitude using a 0–10 modified Borg category scale (Borg, 1982) according to how difficult it was to breathe against the inspired resistive load.
Prior to data recording, the subject targeted their airflow on the display monitor (Kellerman et al., 2000; Zhao et al., 2003; Alexander-Miller & Davenport, 2010). They were instructed to reach their targeted airflow during each breath. A series of loads was presented in a practice session to familiarize the subject with the tasks of airflow targeting, load perception and the range of loads. The trial began with the subject breathing normally. After a minimum of five normal breaths, they were given an example of a “small load” of 5 H2O/L s−1. They reported their perception of the low load. Then they were given an example of a “large load” of 30 H2O/L s−1. They reported their perception of the high load. They demonstrated comprehension of the magnitude estimation by pressing a button corresponding to the Borg scale (0–10) estimate of the sample loads. This also provided a reference for magnitude estimation results during data analysis. After the practice trial, the subject was given a 5-minute rest. This was followed by the 3 experimental trials. There were a total of 5 resistive load magnitudes: 0, 5, 15, 30, and 45 5 H2O/L s−1. Each load was applied to the inspiratory port for 20 consecutive inspirations (expiration remained unloaded). At loaded breath 1, 10, and 20, the subject provided a magnitude estimation of their perceived difficulty of breathing. The subject then had a three-minute breathing recovery period with no presented loads. There were 3 experimental trials with the 5 load magnitudes presented in an independently randomized order for each trial. A 5-minute break separated each experimental trial. This resulted in a total of three 20-breath presentations of each load magnitude over the 3 experimental trials. The subject was monitored by the experimenter with a video camera that did not record the subject’s image.
After each 20-breath load presentation, the subject was given a 4 page survey with subjective responses. They were asked to rate the following:
Fear of suffocation on a 0–10 modified Borg scale (Borg 1982)
General level of fear on a 0–10 modified Borg scale (Borg 1982)
SAM Ratings: The subject can select any of the 5 figures comprising each scale, resulting in a 5-point scale for each dimension. Ratings are scored so that 5 represents a high rating on each dimension (high displeasure, high arousal, high dominance) and 1 represents a low rating on each dimension (low displeasure, low arousal, and low dominance).
Body Sensation Questionnaire: The Diagnostic Symptoms Questionnaire (DSQ; Rapee et al., 1992) is a 15-item measure of the presence and intensity of 12 somatic and three cognitive panic symptoms. Intensity ratings for each endorsed symptom are made on a 4 point Likert-type scale (0 = not at all to 4 = very strongly felt). A Likert-type scale presents a set of attitude statements. The following composite measures can be derived from the DSQ: total number of physical symptoms, catastrophic and non-catastrophic thought, mean intensity of physical sensations, cognitive symptoms and reported fear.
Data Analysis
Statistical comparisons were conducted with both SigmaStat and SPSS software. Statistical analysis of the magnitude estimation was done by blinding the analyst to group. Differences between groups were evaluated with analysis of variance for repeated measures for ventilatory and ME results, with a significance level of p<0.05. Subjective measures were examined with a univariate analysis of variance, with a significance level of p<0.05. Ventilatory pattern was measured for all 20 breaths of each inspiratory resistive-loaded trial for each subject. Mouth pressure (cm H2O), inspiratory time (s), and maximum inspiratory airflow (L/S) were measured for the same breath as the subject’s self-reported magnitude estimation. Breaths 1, 10, and 20 were averaged for each load magnitude trial. A total of 60 breaths from all 3 trials for each load were analyzed for each subject, resulting in a total of 300 loaded breaths per subject. A multiple factor ANOVA was performed for airflow, load magnitude, breath number, time, load magnitude, and breath number, and pressure, load magnitude and breath number. The results are presented in Tables 2 and 3.
Table 2.
The peak airflow for each resistive load magnitude, breath the ME was made and gender. There was there no significant difference between genders for airflow.
| Female Airflow (L/sec) |
Mean | Std Dev | Male Airflow (L/sec) |
Mean | STD DEV | q Value | P | ||
|---|---|---|---|---|---|---|---|---|---|
| R=0 | Breath 1 | 0.10 | 0.02 | R=0 | Breath 1 | 0.12 | 0.03 | 0.39 | >0.05 |
| Breath 10 | 0.09 | 0.02 | Breath 10 | 0.10 | 0.03 | 0.34 | >0.05 | ||
| Breath 20 | 0.10 | 0.02 | Breath 20 | 0.10 | 0.03 | 0.02 | >0.05 | ||
| R=5 | Breath 1 | 0.10 | 0.02 | R=5 | Breath 1 | 0.13 | 0.07 | Ns | >0.05 |
| Breath 10 | 0.09 | 0.02 | Breath 10 | 0.10 | 0.04 | Ns | >0.05 | ||
| Breath 20 | 0.09 | 0.02 | Breath 20 | 0.11 | 0.05 | Ns | >0.05 | ||
| R=15 | Breath 1 | 0.09 | 0.02 | R=15 | Breath 1 | 0.09 | 0.03 | Ns | >0.05 |
| Breath 10 | 0.08 | 0.02 | Breath 10 | 0.07 | 0.01 | Ns | >0.05 | ||
| Breath 20 | 0.07 | 0.02 | Breath 20 | 0.08 | 0.02 | Ns | >0.05 | ||
| R=30 | Breath 1 | 0.08 | 0.02 | R=30 | Breath 1 | 0.07 | 0.02 | Ns | >0.05 |
| Breath 10 | 0.07 | 0.02 | Breath 10 | 0.06 | 0.02 | Ns | >0.05 | ||
| Breath 20 | 0.07 | 0.02 | Breath 20 | 0.07 | 0.02 | Ns | >0.05 | ||
| R=45 | Breath 1 | 0.06 | 0.02 | R=45 | Breath 1 | 0.07 | 0.02 | 0.49 | >0.05 |
| Breath 10 | 0.06 | 0.02 | Breath 10 | 0.05 | 0.01 | 0.28 | >0.05 | ||
| Breath 20 | 0.06 | 0.01 | Breath 20 | 0.06 | 0.03 | 0.80 | >0.05 |
Table 3.
The inspiratory time for each resistive load magnitude, breath the ME was made and gender. There was no significant difference between genders for inspiratory time.
| Female Time (sec) |
Mean | STD DEV | Male Time (sec) |
Mean | STD DEV | q Value | P | ||
|---|---|---|---|---|---|---|---|---|---|
| R=0 | Breath 1 | 3.34 | 0.70 | R=0 | Breath 1 | 2.85 | 0.55 | 0.90 | >0.05 |
| Breath 10 | 3.02 | 0.75 | Breath 10 | 3.03 | 0.96 | 0.49 | >0.05 | ||
| Breath 20 | 2.91 | 0.63 | Breath 20 | 2.73 | 1.10 | 0.66 | >0.05 | ||
| R=5 | Breath 1 | 3.87 | 0.84 | R=5 | Breath 1 | 3.29 | 0.98 | Ns | >0.05 |
| Breath 10 | 3.58 | 0.63 | Breath 10 | 3.29 | 0.62 | Ns | >0.05 | ||
| Breath 20 | 3.67 | 0.61 | Breath 20 | 2.68 | 0.81 | Ns | >0.05 | ||
| R=15 | Breath 1 | 4.55 | 1.27 | R=15 | Breath 1 | 3.93 | 1.06 | Ns | >0.05 |
| Breath 10 | 4.01 | 0.92 | Breath 10 | 3.58 | 0.80 | Ns | >0.05 | ||
| Breath 20 | 4.09 | 0.89 | Breath 20 | 3.76 | 0.93 | Ns | >0.05 | ||
| R=30 | Breath 1 | 4.85 | 1.14 | R=30 | Breath 1 | 4.24 | 1.16 | Ns | >0.05 |
| Breath 10 | 3.97 | 1.31 | Breath 10 | 4.02 | 1.19 | Ns | >0.05 | ||
| Breath 20 | 4.16 | 0.99 | Breath 20 | 4.26 | 1.10 | Ns | >0.05 | ||
| R=45 | Breath 1 | 5.56 | 1.46 | R=45 | Breath 1 | 4.62 | 1.06 | 0.59 | >0.05 |
| Breath 10 | 5.00 | 1.63 | Breath 10 | 4.32 | 0.91 | 0.10 | >0.05 | ||
| Breath 20 | 4.99 | 1.48 | Breath 20 | 4.38 | 1.12 | 0.37 | >0.05 |
The subject estimated the load magnitude for each load magnitude at breaths 1, 10, and 20. The magnitude estimation was analyzed using a 3-way repeated measure ANOVA (Tables 4 and 5). Post-hoc analysis was performed using a Tukeys HSD test. Subjective measures self-reported after each load magnitude (a total of 15 subjective reports, 4 pages each), were transferred into SPSS and grouped by gender and load magnitude (level), then analyzed using a one-way ANOVA. Post-hoc analysis for ANOVA was performed using Tukeys HSD test.
Table 4.
Mean magnitude estimation for each resistive load at breath 1, 10, and 20 for males and females. The mean (± standard deviation) and corresponding p-values between load magnitude and between genders is presented when significantly different.
| Female ME | Breath 1 | Breath 10 | Breath 20 | t (M vs F) | P-value | P-value between genders |
| R=0 | 0.08 (±0.16) | 0.18 (±0.23) | 0.18 (±0.20) | 0.212 | ||
| R=5 | 1.37 (±1.18) | 1.44 (±1.10) | 1.47 (±1.14) | 2.262 | ||
| R=15 | 3.35 (±1.54) | 3.47 (±1.92) | 3.48 (±1.90) | 2.472 | p<0.02 (Breath 10) | |
| R=30 | 5.73 (±1.48) | 6.1 (±1.39) | 6.27 (±1.30) | 5.981 | p<0.005 (Breath 10) p<0.00006 (Breath 20) |
|
| R=45 | 6.37 (±1.98) | 7.42 (±1.44) | 7.62 (±0.78) | 5.077 | *p=0.06 | p<0.005 (Breath 10) p<0.0001(Breath 20) |
| Male ME | Breath 1 | Breath 10 | Breath 20 | P-value | P-value | P-value between genders |
| R=0 | 0.02 (±0.05) | 0.07 (±0.09) | 0.17 (±0.17) | 0.212 | ||
| R=5 | 1.37 (±0.95) | 1.44 (±0.59) | 1.47 (±0.26) | 2.262 | ||
| R=15 | 3.19 (±0.81) | 2.51 (±1.07) | 2.34 (±0.65) | 2.472 | p<0.01 | p<0.02 (Breath 10) |
| R=30 | 5.05 (±1.37) | 4.18 (±1.36) | 3.38 (±1.17) | 5.981 | p<0.009 | p<0.005 (Breath 10) p<0.00006 (Breath 20) |
| R=45 | 6.49 (±1.37) | 5.57 (±1.36) | 4.68 (±1.17) | 5.077 | p<0.02 | p<0.005 (Breath 10) p<0.0001(Breath 20) |
Table 5.
Mean (± standard deviation) log ME, log resistive load and log-log slope are presented for breath 1, 10, and 20, along with the corresponding between load p-values.
| Female ME logs | Breath 1 | Breath 10 | Breath 20 | P-val |
| R=0 | 0.62 (±0.28) | 0.60 (±0.28) | 0.57 (±0.24) | |
| R=5 | 0.03 (±0.42) | 0.02 (±0.39) | 0.10 (±0.37) | |
| R=15 | 0.49 (±0.18) | 0.48 (±0.24) | 0.47 (±0.27) | |
| R=30 | 0.75 (±0.11) | 0.78 (±0.10) | 0.79 (±0.10) | p<0.05 |
| R=45 | 0.78 (±0.14) | 0.86 (±0.09) | 0.88 (±0.08) | p<0.05 |
| ME Log-Log Slope | 0.89 (±0.41) | 0.91 (±0.39) | 0.79 (±0.44) | p<0.05 |
| Male ME logs | Breath 1 | Breath 10 | Breath 20 | P-val |
| R=0 | 0.78 (±0.00) | 0.78 (±0.00) | 0.66 (±0.21) | |
| R=5 | 0.09 (±0.32) | 0.23 (±0.28) | 0.36 (±0.23) | p<0.05 |
| R=15 | 0.49 (±0.13) | 0.36 (±0.20) | 0.35 (±0.15) | p<0.05 |
| R=30 | 0.69 (±0.13) | 0.60 (±0.14) | 0.51 (±0.15) | p<0.05 |
| R=45 | 0.80 (±0.10) | 0.73 (±0.11) | 0.66 (±0.12) | p<0.05 |
| ME Log-Log Slope | 0.94 (±0.34) | 0.94 (±0.41) | 1.05 (±0.30) | |
Results
Ventilatory Response
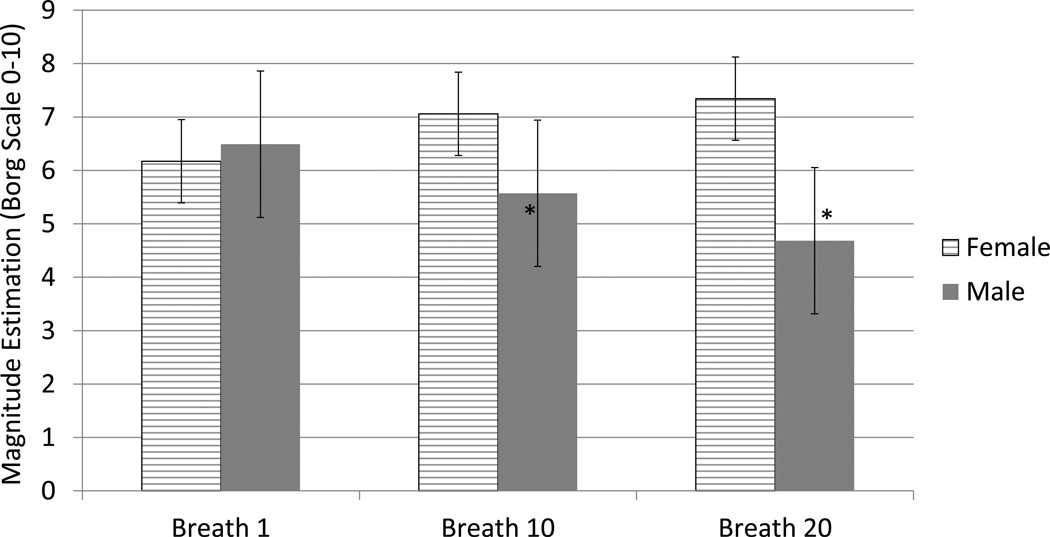
The results of the 3-way ANOVA indicated a main effect on load duration on magnitude estimation and subjective response. There was a significant interaction between gender and load (F= 12.469, p<0.001), gender and symptom magnitude estimation (F=2.764, p<0.001) and load and symptom magnitude estimation (F=5.738, p<0.001). These results are presented in tables 4 and 5 and Figure 1. Although females demonstrated no significant difference between breaths 1 and 20 within their group, the figures in the raw ME data (Figures 3–4, 3–5, and 3–6) demonstrate an increase in the absolute ME for all load magnitudes with raw ME p=0.06 for R 45 cmH2O/l*sec−1 between breath 1 and 20. As load magnitude increased, magnitude estimation increased accordingly (tables 4 and 5). Although the first breath was not significantly different, breaths 10 and 20 were. Significant differences were found for the ME of all loads between breaths 1 and 20, demonstrating an alteration in perception of each load after sustained presentation of load. Female subjects had a lower slope indicating a compression of the perceptual score range. Males had a significant decrease in the magnitude estimation between breaths 1 and 20 in the opposite direction of the females.
Figure 1.
Magnitude Estimation (Borg Scale 0–10).
Analyses performed to compare the group, male and female responses for airflow, expiratory, and inspiratory time showed no significant difference for gender (Tables 2 and 3). Within load magnitude, breath 1, 10 and 20 were compared. Breath number between load magnitudes was compared for each load (R=0 5 H2O/Ls−1, 5 5 H2O/Ls−1, 15 5 H2O/Ls−1, 30 5 H2O/Ls−1 and 45 5 H2O/Ls−1) and breath 1, 10, and 20. Non-similar breath numbers across load magnitude were not compared (i.e. breath 10, R=5 5 H2O/Ls−1 with breath 20, R=15 5 H2O/Ls−1). The group differences related to load magnitude are reflected in both males and females. Table 2 demonstrates the lack of significance between gender for airflow, and table 3 demonstrates the lack of significant difference between genders for time. The greatest difference in time and airflow is seen between the smallest (R=55 H2O/Ls−1) and largest resistances (R=45 5 H2O/Ls−1). Inspiratory time (Ti) lengthened as load magnitude increased and airflow decreased as load magnitude increased.
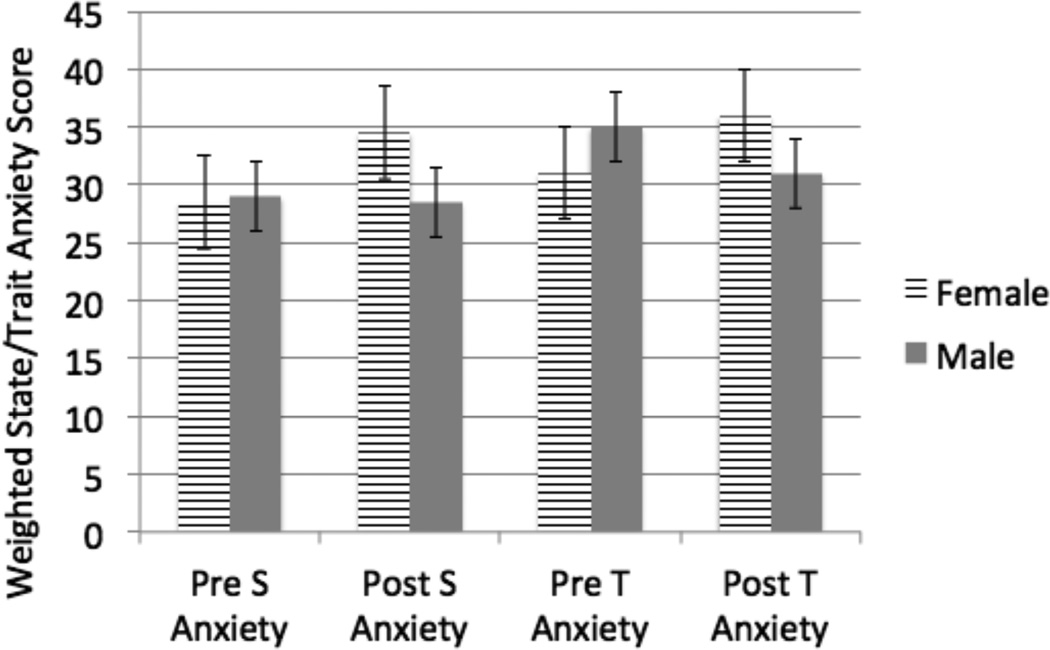
As a group, load levels resulted in subjective increases in fear, fear of suffocation, faintness, dizziness, trembling, and dyspnea (Tables 6 and 7). There were no differences between high and low NA groups. Significant gender differences were found for the following subjective responses: fear, fear of suffocation, displeasure, chest pressure, faintness, dizziness, trembling, sense of unreality, and tingling (Tables 6 and 7). There were no significant group differences for sense of control, dyspnea, and palpations. A gender difference was found in the fear of losing control, but not the sense of control. A repeated-measures ANOVA showed non-significant effects for gender in the mean scores of the State-Trait Anxiety Index (STAI) for subjects pre-experiment and post-experiment (Q=6.6 female, Q=17.6 male). Kruskal-Wallis One Way Analysis of Variance on Ranks of the delta-scores of the difference pre- and post-experiment demonstrated significant differences for men in trait anxiety post experiment (p<0.05). A multiple pairwise comparison’s procedure (Dunn’s method) was used. Females had no significant change. These results are presented in tables 6 and 7.
Table 6.
Mean (± standard deviation) of emotional subjective estimation responses to questionnaires.
| Fear | Female | Male | F | *P= <.05 |
|---|---|---|---|---|
| R=0 | 0.06(±0.19) | 0(±0) | 0.81 | |
| R=5 | 0.15(±0.22) | 0.04(±0.10) | 1.87 | |
| R=15 | 0.67(±0.57) | 0.07(±0.20) | 7.92 | * |
| R=30 | 1.18(±1.07) | 0.11(±0.15) | 8.01 | * |
| R=45 | 1.70(±1.77) | 0.59(±0.51) | 2.92 | |
| Fear of Suffocation | ||||
| R=0 | 0.12(±0.31) | 0(±0) | 1.38 | |
| R=5 | 0.58(±0.65) | 0.30(±0.39) | 1.28 | |
| R=15 | 1.42(±0.75) | 0.56(±0.58) | 8.16 | * |
| R=30 | 3(±1.40) | 1.30(±1.20) | 8.35 | * |
| R=45 | 3.59(±1.45) | 2.37(±1.31) | 3.83 | |
| Displeasure | ||||
| R=0 | 1.4(±0.66) | 1.41(±0.97) | 0.00 | |
| R=5 | 1.5(±0.71) | 1.48(±0.96) | 0.00 | |
| R=15 | 2.13(±0.55) | 1.52(±1.04) | 2.67 | |
| R=30 | 2.78(±0.74) | 1.67(±0.99) | 7.93 | * |
| R=45 | 3.08(±0.80) | 2(±1.09) | 6.18 | * |
| Sense of Control | ||||
| R=0 | 1.27(±0.64) | 1.33(±0.78) | 0.04 | |
| R=5 | 1.4(±0.75) | 1.41(±0.76) | 0.00 | |
| R=15 | 1.9(±0.79) | 1.63(±0.90) | 0.49 | |
| R=30 | 2.4(±0.64) | 1.82(±0.87) | 2.83 | |
| R=45 | 2.75(±0.69) | 2.56(±0.83) | 0.31 | |
| Chest Pressure | ||||
| R=0 | 1.13(±0.32) | 0.96(±0.11) | 2.27 | |
| R=5 | 1.4(±0.66) | 1.11(±0.17) | 1.61 | |
| R=15 | 1.77(±0.74) | 1.30(±0.51) | 2.55 | |
| R=30 | 2.32(±0.79) | 1.48(±0.50) | 7.39 | * |
| R=45 | 2.68(±0.93) | 2.11(±1.09) | 1.47 |
Significance between genders is indicated by the asterisks.
Table 7.
Mean (± standard deviation) bodily subjective estimation responses to Diagnostic Symptom Questionnaire.
| Dyspnea | Female | Male | F | *P= <.05 |
|---|---|---|---|---|
| R=0 | 0.15(±0.31) | 0(±0) | 2.11 | |
| R=5 | 0.38(±0.57) | 0.19(±0.29) | 0.85 | |
| R=15 | 0.91(±0.75) | 0.56(±0.60) | 1.32 | |
| R=30 | 1.36(±0.74) | 0.96(±0.66) | 1.61 | |
| R=45 | 1.82(±0.94) | 1.41(±0.93) | 0.97 | |
| Faintness | ||||
| R=0 | 0.09(±0.22) | 0(±0) | 1.59 | |
| R=5 | 0.24(±0.52) | 0(±0) | 1.84 | |
| R=15 | 0.42(±0.50) | 0.04(±0.11) | 5.21 | * |
| R=30 | 0.70(±0.61) | 0.07(±0.15) | 9.03 | * |
| R=45 | 0.89(±0.63) | 0.52(±0.53) | 2.01 | |
| Dizziness | ||||
| R=0 | 0.39(±0.77) | 0(±0) | 2.32 | |
| R=5 | 0.51(±0.77) | 0(±0) | 3.89 | |
| R=15 | 0.89(±0.80) | 0.04(±0.11) | 10.15 | * |
| R=30 | 1.18(±0.87) | 0.11(±0.17) | 13.00 | * |
| R=45 | 1.35(±0.97) | 0.22(±0.29) | 11.15 | * |
| Tingling | ||||
| R=0 | 0.24(±0.53) | 0(±0) | 1.67 | |
| R=5 | 0.21(±0.46) | 0(±0) | 1.76 | |
| R=15 | 0.24(±0.40) | 0(±0) | 2.91 | |
| R=30 | 0.61(±0.99) | 0(±0) | 3.02 | |
| R=45 | 0.79(±0.87) | 0(±0) | 6.68 | * |
| Trembling | ||||
| R=0 | 0.09(±0.22) | 0(±0) | 1.59 | |
| R=5 | 0.15(±0.41) | 0(±0) | 1.25 | |
| R=15 | 0.18(±0.41) | 0(±0) | 1.8 | |
| R=30 | 0.32(±0.44) | 0.04(±0.11) | 3.50 | |
| R=45 | 0.52(±0.57) | 0.04(±0.11) | 6.19 | * |
| Unreality | ||||
| R=0 | 0(±0) | 0(±0) | . | |
| R=5 | 0(±0) | 0(±0) | . | |
| R=15 | 0.18(±0.35) | 0(±0) | 2.47 | |
| R=30 | 0.24(±0.40) | 0(±0) | 3.32 | |
| R=45 | 0.42(±0.56) | 0(±0) | 5.12 | * |
| Palpitations | Female | Male | F | |
| R=0 | 0(±0) | 0(±0) | . | |
| R=5 | 0(±0) | 0(±0) | . | |
| R=15 | 0.05(±0.15) | 0(±0) | 0.81 | |
| R=30 | 0.15(±0.23) | 0(±0) | 3.89 | |
| R=45 | 0.30(±0.64) | 0(±0) | 2.00 | |
| Fear of Losing Control | ||||
| R=0 | 0.27(±0.71) | 0(±0) | 1.31 | |
| R=5 | 0.27(±0.55) | 0.04(±0.11) | 1.56 | |
| R=15 | 0.35(±0.60) | 0.04(±0.11) | 2.35 | |
| R=30 | 0.52(±0.81) | 0.11(±0.17) | 2.16 | |
| R=45 | 0.94(±0.96) | 0.26(±0.32) | 4.07 | * |
Significance between genders is indicated by the asterisks.
Discussion
The results of this study demonstrate a significant gender difference in the evaluation, subjective ratings and affective response to sustained inspiratory resistive loads. This provides strong evidence for a gender effect, which may be attributed to males desensitizing with increasing breath number and females sensitizing with increasing breath number. Sustained loads over 15 cmH2O/L/s−1 elicited more negative affect responses in females, as demonstrated by increased ratings of fear, fear of suffocation, arousal, chest pressure, dizziness, fear of losing control, and sense of unreality. Sustained loads also elicit sensations of dyspnea in both males and females, indicating that subjects do not adapt or accommodate to large loads, but rather have to work harder to adjust to them. This is also evident in the ventilatory response of increasing airflow and time across both groups. Although there was no significant difference between groups for state anxiety scores, the delta scores for trait anxiety significantly decreased in males, and approached a significant increase in females. This finding was unexpected and merits further in-depth investigation in future research.
As previously mentioned in this article, respiratory perception is a 2-stage process including physical awareness (somatosensory cortical activation) in stage 1 and affective evaluation (including the amygdala and associated structures such as the anterior cingulate and insular cortex) in stage 2 (Davenport & Vovk, 2009). Fundamentally, this means that the first event makes the individual aware that their breathing has changed and the second component involves the determination of the load being neutral, pleasant or unpleasant. These results indicate that as an individual increases the duration of breathing time against a load, subsequent unpleasant sensations arise. This aversive processing is significantly greater in females in this study. Reconciling contraindicatory findings may be done by factoring gender as a determining factor in the processing of respiratory symptoms as unpleasant stimuli. Functional magnetic resonance imaging (fMRI) studies have shown women to be more responsive to unpleasant pictures (Lang et al., 1993; Lane et al., 1997; Lang et al., 1998). There are several limitations to this study. It is unclear how much of the change in magnitude estimation ratings in females were due to increased muscular fatigue. This should be addressed in future research by allowing individually determined recovery periods to minimize fatigue. Additionally, it is possible that the high and low NA groups did not sufficiently differ from one another enough to find group differences at this level.
The most likely source of the difference lies in the cognitive and emotional realm. Gender differences in the response and over-perception of symptoms are seen with many disease states, including asthma. Females tend to have a greater sensitivity to their perception of physical stimuli, particularly unpleasant experimental or chronic pain (Edwards, 2003). Women with chronic obstructive pulmonary disease have poorer health related quality of life than men (Kanervisto, 2010) and have a 2–3 fold higher suicide risk (Webb, 2012). Women with respiratory disorders, such as COPD, self-report more psychological distress than men (Laurin 2007; Di Marco et al. 2006). Female patients are more exposed to psychological impairment, which correlates well with the dyspneic component of chronic obstructive pulmonary disease (Di Marco et al. 2006). This may translate into the aversiveness demonstrated by females in this study after breathing against prolonged inspiratory loads. When females are affected by pulmonary disease, they report less confidence in their ability to control their respiratory symptoms and have less total and activity-related quality of life compared to men (Laurin 2007). This corresponds with the data demonstrating increase in fear of losing control reported by females. Socio-cultural factors (what a patient feels is socially acceptable or expected) also play a role in the gender differences in the perception, reporting, and diagnostic interpretation of respiratory symptoms (Becklake and Knauffman, 1999). A limitation of this study is that no personal belief or socio-cultural data tools were utilized. Future research should incorporate socio-cultural factors along with qualitative metrics to further evaluate these contributing factors.
These results suggest that addressing the emotional and subjective responses to breathing disruptions has important clinical implications. Clinically, the physical symptoms seen by women in this study (faintness, dizziness, trembling and tingling) may serve as an initial trigger to seek and utilize healthcare services to ease symptoms, overuse medications or avoid activities that may excacerbate dyspnea (Main et al., 2003; Ng et al., 2007; von Leupoldt and Dahme, 2007). These symptoms, which are associated with negative affect, may be mitigated with a holistic approach incorporating care of the respiratory disease and dyspnea along with the anxiety elicited from the sensation. Pharmacological interventions for asthma are no longer the exclusive treatment plan, with behavioral treatment integrating biopsychosocial approaches to complete disease management (Ritz, Meuret, Trueba, Fritzsche & von Leupoldt, 2012). Clinical priorities should include increased vigilance of mental health assessment by evaluating for previously undetected psychological distress in patients suffering from chronic respiratory disorders due to their increased suicide risk (Webb, 2012). As previously mentioned, women with pulmonary disease have less confidence in their ability to control their respiratory symptoms. This negatively affects quality of life, particularly related to activity engagement and treatment adherence. This also suggests that males may underestimate their respiratory symptoms placing them at risk of pulmonary complications due to delayed treatment. These results identify a target for development of a potential intervention to ease the negative affect of dyspnea in future research, with a focus on mitigating the over-processing of aversive respiratory stimuli. Management of psychosocial and perceptual effects of respiratory diseases should be considered to improve symptom processing, reduce related morbidity, and improve overall quality of life.
Figure 2.
Weighted State/Trait Anxiety Score.
Table 8.
Male and Female change in (Delta) STAI Scores before and after the load presentation trial.
| Group | N | Median | 25% | 75% | Q | P<0.05 |
|---|---|---|---|---|---|---|
| Female Delta State Anxiety | 12 | 2.5 | −1.5 | 9.5 | 6.6 | |
| Male Delta State Anxiety | 10 | −0.5 | −3 | 3 | 6.6 | |
| Female Delta Trait Anxiety | 12 | 3.5 | −0.5 | 7 | 17.6 | |
| Male Delta Trait Anxiety | 10 | −3 | −4 | −1 | 17.6 | * |
The * indicated significant difference (p<0.05) between gender.
Acknowledgements
NIH T32 HD043730
NIH HL48792
References
- Alexander-Miller S, Davenport PW. Perception of multiple-breath inspiratory resistive loads in males and females. Biol Psychol. 2010;84(1):147–149. doi: 10.1016/j.biopsycho.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alius MG, Pané-Farré CA, Von Leupoldt A, Hamm AO. Induction of dyspnea evokes increased anxiety and maladaptive breathing in individuals with high anxiety sensitivity and suffocation fear. Psychophysiology. 2013;50(5):488–497. doi: 10.1111/psyp.12028. [DOI] [PubMed] [Google Scholar]
- American Lung Association. COPD Fact Sheet. 2013 Retrieved from http://www.lung.org. [Google Scholar]
- Bhandari NJ, Jain T, Marolda C, Zuwallack RL. Comprehensive Pulmonary Rehabilitation Results in Clinically Meaningful Improvements in Anxiety and Depression in Patients With Chronic Obstructive Pulmonary Disease. J Cardiopulm Rehabil Prev. 2013;33(2):123–127. doi: 10.1097/HCR.0b013e31828254d4. [DOI] [PubMed] [Google Scholar]
- Becklake M, Kauffman F. Gender differences in airway behaviour over the human life span. Thorax. 1999:1119–1138. doi: 10.1136/thx.54.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G. Psychophysical bases of perceived exertion. Med Sci Sports Exercise. 1982;14:377–381. [PubMed] [Google Scholar]
- Chan PY, von Leupoldt A, Bradley MM, Lang PJ, Davenport PW. The effect of anxiety on respiratory sensory gating measured by respiratory-related evoked potentials. Biol Psychol. 2012;91(2):185–189. doi: 10.1016/j.biopsycho.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport PW, Kifle Y. Inspiratory Resistive Load Detection in Children with Life-Threatening Asthma. Pediatr Pulmonol. 2001;32:44–48. doi: 10.1002/ppul.1087. 2001. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Vovk A. Cortical and subcortical central neural pathways in respiratory sensations. Respir Physiol Neurobiol. 2009 May 30;167(1):72–86. doi: 10.1016/j.resp.2008.10.001. [DOI] [PubMed] [Google Scholar]
- de Torres JP, Casanova C, Hernandez C, Abreu J, Montejo de Garcini A, Aguirre-Jaime A, Celli BR. Gender associated differences in determinants of quality of life in patients with COPD: a case series study. Health Qual Life Outcomes. 2006;4:72. doi: 10.1186/1477-7525-4-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marco F, Verga M, Reggente M, Maria-Casanova F, Santus P, Blasi F, et al. Anxiety and depression in COPD patients: the roles of gender and disease severity. Respir Med. 2006:1767–1774. doi: 10.1016/j.rmed.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Edwards R, Augustson E, Fillingim R. Differential relationships between anxiety and treatment associated pain reduction among male and female chronic pain patients. Clin Journ of Pain. 2003;19:208–216. doi: 10.1097/00002508-200307000-00003. [DOI] [PubMed] [Google Scholar]
- Freedman S, Campbell EJM. The ability of normal subjects to tolerate added inspiratory loads. Resp Physiol. 1970;10:213. doi: 10.1016/0034-5687(70)90084-8. [DOI] [PubMed] [Google Scholar]
- Giardino ND, Curtis JL, Abelson JL, King AP, Pamp B, Liberzon I, Martinez FJ. The impact of panic disorder on interoception and dyspnea reports in chronic obstructive pulmonary disease. Biol Psychol. 2010 Apr;84(1):142–146. doi: 10.1016/j.biopsycho.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Hill K, Geist R, Goldstein RS, et al. Anxiety and depression in end-stage COPD. Eur Resp J. 2008;31:667–677. doi: 10.1183/09031936.00125707. [DOI] [PubMed] [Google Scholar]
- Hudgel DW, Cooperson DM, Kinsman RA. Recognition of added resistive loads in asthma. Am Rev. Respir Dis. 1982;126:121–125. doi: 10.1164/arrd.1982.126.1.121. [DOI] [PubMed] [Google Scholar]
- Jones P, Quirk F, Baveystock C, et al. A self-complete measure of health status for chronic airflow limitation. The St George's Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- Julius SM, Davenport KL, Davenport PW. Perception of intrinsic and extrinsic respiratory loads in children with life-threatening asthma. Ped Pulm. 2002;34:425–433. doi: 10.1002/ppul.10199. [DOI] [PubMed] [Google Scholar]
- Kanervisto M, Saarelainen S, Vasankari T, Jousilahti P, Heistaro S, Heliövaara M, Luukkaala T, Paavilainen E. COPD, chronic bronchitis and capacity for day-to-day activities: negative impact of illness on the health-related quality of life. Chron Respir Dis. 2010;7(4):207–215. doi: 10.1177/1479972310368691. [DOI] [PubMed] [Google Scholar]
- Katsura H, Yamada K, Wakabayashi R, Kida K. Gender-associated differences in dyspnoea and health-related quality of life in patients with chronic obstructive pulmonary disease. Respirology. 2007;12(3):427–432. doi: 10.1111/j.1440-1843.2007.01075.x. [DOI] [PubMed] [Google Scholar]
- Kellerman BA, Martin AD, Davenport PW. Effect of Inspiratory Muscle Strengthening on Resistive Load Detection and Magnitude Estimation in Adults. Med Sci Sports Exer. 2000;32(11):1859–1867. doi: 10.1097/00005768-200011000-00007. [DOI] [PubMed] [Google Scholar]
- Ketelaars CA, Schlösser MA, Mostert R, Huyer Abu-Saad H, Halfens RJ, Wouters EF. Determinants of health-related quality of life in patients with chronic obstructive pulmonary disease. Thorax. 1996 Jan;51(1):39–43. doi: 10.1136/thx.51.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kifle Y, Seng V, Davenport PW. Magnitude estimation of inspiratory resistive loads in children with life-threatening asthma. Am J Respir Crit Care Med. 1997;156:1530–1535. doi: 10.1164/ajrccm.156.5.9703011. [DOI] [PubMed] [Google Scholar]
- Lane R, Reiman E, Bradley M, Lang P, Ahern G, Davidson R, Schwartz G. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997;35:1437–1444. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- Lang P. Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology. 1998;35:199–210. [PubMed] [Google Scholar]
- Lang P, Greenwald M, Bradley M, Hamm A. Looking at pictures: Affective, facial, visceral and behavioral variations. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Laurin C, Lavoie KL, Bacon SL, Dupuis G, Lacoste G, Cartier A, Labrecqu M. Sex differences in the prevalence of psychiatric disorders and psychological distress in patients with chronic obstructive pulmonary disease. Chest. 2007;132:148–155. doi: 10.1378/chest.07-0134. [DOI] [PubMed] [Google Scholar]
- Main J, Moss-Morris R, Booth R, Kaptein AA, Kolbe J. The use of reliever medication in asthma: the role of negative mood and symptom reports. J of Asthma. 2003;40:357–365. doi: 10.1081/jas-120018635. [DOI] [PubMed] [Google Scholar]
- Maurer J, et al. Anxiety and depression in COPD: current understanding, unanswered questions and research needs. Chest. 2008;134:43S–56S. doi: 10.1378/chest.08-0342. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TP, Niti M, Tan WC, Cao Z, Ong KC, Eng P. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Archives of Internal Medicine. 2007;167:60–67. doi: 10.1001/archinte.167.1.60. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Oga T, Ikeda A, Hajiro T, Tsukino M, Hiroshi K. Comparison of Health-Related Quality of Life Measurements Using a Single Value in Patients with Asthma and Chronic Obstructive Pulmonary Disease. J of Asthma. 2008;45:615–620. doi: 10.1080/02770900802127014. [DOI] [PubMed] [Google Scholar]
- Pappens M, Smets E, Vansteenwegen D, Van Den Bergh O, Van Diest I. Learning to fear suffocation: a new paradigm for interoceptive fear conditioning. Psychophysiology. 2012;49(6):821–828. doi: 10.1111/j.1469-8986.2012.01357.x. [DOI] [PubMed] [Google Scholar]
- Pate KM, Davenport PW. Tracheal Occlusion Conditioning Causes Stress, Anxiety and Neural State Changes in Conscious Rats. Exp Physiol. 2012 Nov 30; doi: 10.1113/expphysiol.2012.068924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapee RM, Sanderson WC, McCauley PA, Di Nardo P. Differences in reported symptom profile between panic disorder and other DSM-III-R anxiety disorders. Beh Res and Ther. 1992;30:45–52. doi: 10.1016/0005-7967(92)90095-x. [DOI] [PubMed] [Google Scholar]
- Ren J, Ding X, Funk GD, Greer JJ. Anxiety-related mechanisms of respiratory dysfunction in a mouse model of Rett syndrome. J Neurosci. 2012;32(48):17230–17240. doi: 10.1523/JNEUROSCI.2951-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T, Meuret AE, Bhaskara L, Petersen S. Respiratory muscle tension as symptom generator in individuals with high anxiety sensitivity. Psychosom Med. 2013;75(2):187–195. doi: 10.1097/PSY.0b013e31827d1072. [DOI] [PubMed] [Google Scholar]
- Ritz T, Meuret AE, Trueba AF, Fritzsche A, von Leupoldt A. Psychosocial Factors and Behavioral Medicine Interventions in Asthma. J Consult Clin Psychol. 2012;81(2):231–250. doi: 10.1037/a0030187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Luchene RE. The State–Trait Anxiety Inventory (STAI) test manual for Form X. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Takano N, Inaishi S, Zhang Y. Individual differences in breathlessness during exercise, as related to ventilatory chemosensitivities in human. J Physiol. 1997;499:843–848. doi: 10.1113/jphysiol.1997.sp021973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller J, Pain M, Biddle N. Anxiety disorder and perception of inspiratory resistive loads. Chest. 1987;91:547–551. doi: 10.1378/chest.91.4.547. [DOI] [PubMed] [Google Scholar]
- Trueba AF, Rosenfield D, Oberdörster E, Vogel PD, Ritz T. The effect of academic exam stress on mucosal and cellular airway immune markers among healthy and allergic individuals. Psychophysiology. 2013;50(1):5–14. doi: 10.1111/j.1469-8986.2012.01487.x. [DOI] [PubMed] [Google Scholar]
- Tsai HW, Chan PY, von Leupoldt A, Davenport PW. The impact of emotion on the perception of graded magnitudes of respiratory resistive loads. Biol Psych. 2013;93:220–224. doi: 10.1016/j.biopsycho.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Van den Bergh, Omer, Stegen K, Van de Woestijne KP. Memory effects on symptom reporting in a respiratory learning paradigm. Health Psychology. 1998;17(3):241–248. doi: 10.1037//0278-6133.17.3.241. [DOI] [PubMed] [Google Scholar]
- von Leupoldt A, Dahme B. Psychological aspects in the perception of dyspnea. Chest. 2007;128:345–354. doi: 10.1378/chest.128.1.345. [DOI] [PubMed] [Google Scholar]
- Webb RT, Kontopantelis E, Doran T, Qin P, Creed F, Kapur N. Suicide risk in primary care patients with major physical diseases: a case-control study. Arch Gen Psychiatry. 2012;69(3):256–264. doi: 10.1001/archgenpsychiatry.2011.1561. [DOI] [PubMed] [Google Scholar]
- Younes M. Mechanisms of respiratory load compensation. In: Dempsey JA, Pack AI, editors. Lung Biology in Health and Disease, Volume 79: Regulation of Breathing. NewYork: Marcel Dekker; 1995. pp. 867–922. [Google Scholar]
- Zhao W, Martin AD, Davenport PW. The Resistive Load Magnitude Estimation in Double Lung Transplant Recipients. J Appl Physiol. 2003;94(2):476–482. doi: 10.1152/japplphysiol.00564.2002. 2003. [DOI] [PubMed] [Google Scholar]