Abstract
Purpose
To determine if pelvic soft tissue and bony dimensions on endorectal MRI influence recovery of continence after radical prostatectomy (RP) and whether adding significant MRI variables to a statistical model improves prediction of continence recovery.
Materials and Methods
Between 2001 and 2004, 967 men undergoing RP had preoperative MRI. Soft tissue and bony dimensions were retrospectively measured by two raters blinded to clinical and pathological data. Patients who received neoadjuvant therapy, were preoperatively incontinent, or had missing followup for continence were excluded, leaving 600 patients eligible for analysis. No pad usage defined continent. Logistic regression was used to identify variables associated with continence recovery at 6 and 12 months. We evaluated whether predictive accuracy of a base model improved by adding independently significant MRI variables.
Results
Urethral length and urethral volume were both significantly associated with recovery of continence at 6 and 12 months. Larger inner and outer levator distances were significantly associated with a decreased probability of regaining continence at either 6 or 12 months; they did not reach statistical significance for the other time point. Addition of these four MRI variables to a base model including age, clinical stage, PSA and comorbidities marginally improved the discrimination (12 months AUC improved from 0.587 to 0.634).
Conclusions
Membranous urethral length, urethral volume and an anatomically close relation between the levator muscle and membranous urethra on preoperative MRI are independent predictors of continence recovery after RP. Addition of MRI variables to a base model improved the predictive accuracy for continence recovery but predictive accuracy remains low.
Keywords: prostate cancer, radical prostatectomy, continence, MRI, pelvimetry
Introduction
Post operative loss of bladder control in patients undergoing radical prostatectomy remains one of the biggest concerns for prostate cancer patients treated surgically. Postoperative urinary incontinence can importantly affect quality of life as a physical and psychosocial burden 1. The likelihood of experiencing incontinence after radical prostatectomy in the published literature is variable and difficult to assess due to lack of consensus in regard to definition of continence as well as time and methodology of assessment 2. Multiple demographic, disease specific or surgical technique related risk factors have been implicated to impact recovery of continence 3. Anatomic variables, such as membranous urethral length (MUL), are suggested to correlate with recovery of urinary continence. Therefore they should be carefully evaluated as they have the potential to improve understanding of the anatomy and function, optimize surgical technique, and provide appropriate patient counseling. Preoperative pelvic MRI provides an optimal tool to visualize detailed anatomy of the prostate, periprostatic tissue, pelvic floor and bony dimensions to determine if these anatomic structures are associated with recovery of urinary continence after radical prostatectomy. In this pilot study, our aim was to evaluate various pelvic soft tissue and bony dimensions on preoperative endorectal MRI and to identify potential variables that influence recovery of continence.
Methods
After receiving institutional review board approval we identified 1048 men who had pelvic staging MRI prior to radical prostatectomy performed by one of five dedicated prostate surgeons (2 laparoscopic, 3 open surgeons) between 11/2001 and 12/2004. 81 patients were excluded due to MRI images which could retrospectively not be measured, on scanned films from outside institutions, or one of the three planes (axial, sagittal, coronal) was not available. Of the remaining 967 patients, men who received neoadjuvant therapy (n=3), were preoperatively incontinent (n=12), were missing follow up for continence (n=345), or other covariates (n=7) were excluded, leaving 600 patients eligible for analysis.
Due to interval censoring, we converted the time-to-event data into binary variables for the outcome of continence at 6- and 12-months. Patients who regained function before 6 months (12 months) after surgery were considered continent; patients who were incontinent before and after 6 months (12 months) were considered incontinent. Patients without eligible data were excluded from the analyses.
Outcome assessment
Continence was graded on an institutional not externally validated 5-point-scale to grade urinary continence. This scale is completed with the patient at each visit by a physician or proxy and recorded. No pad usage (no security pad) defined continent. Urinary continence levels 2 to 5 (defined as: level 2 = leaks only during heavy activity, 1-2 pads; level 3: leaks with moderate activity, 3-4 pads; level 4: leaks at normal activity, dry at night and rest; level 5: total incontinence) were considered incontinent.
MRI measurements and imaging
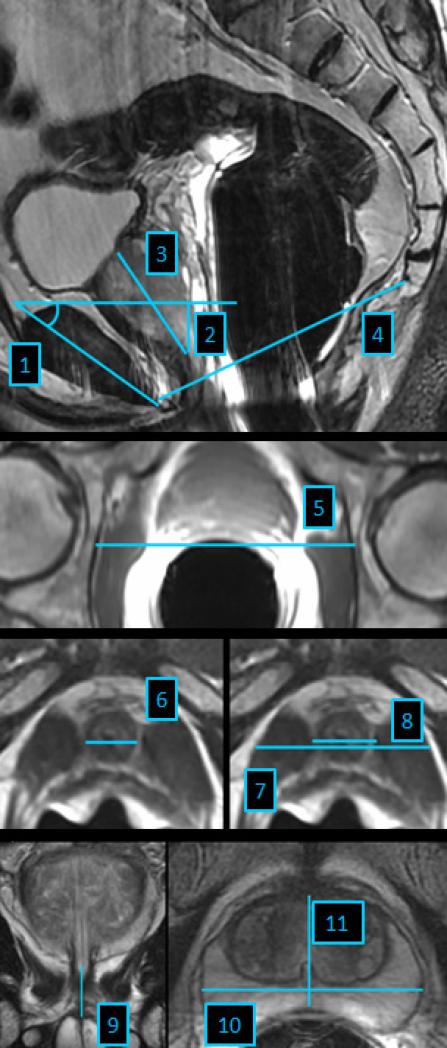
MRI measurements were performed on the axial, sagittal and coronal plane by two raters on preoperative endorectal MRI. Both raters were blinded to all clinical and pathological data. All measurements were defined by an experienced radiologist. Training was performed under supervision and the accuracy of the measurements was confirmed by the radiologist. Figure 1 demonstrates each defined measurement performed. The Mid-Pelvic-Area (MPA), Levator thickness at the height of apical dissection, the Outer and Inner Levator Distance (OLD, ILD), Urethra volume and Prostate volume were calculated (Table1).
Figure 1.
1. Symphysis angle defined as angle between the long axis of the symphysis pubis and the horizontal (Mid-sagittal T2-weighted image)
2. Apical depth of prostate: Vertical measurement from the most proximal margin of the symphysis pubis to the level of the distal margin of the prostatic apex (Mid-sagittal T2-weighted image)
3. Maximum height of the prostate measured from prostate base to apex at any level (Mid-sagittal T2-weighted image)
4. Lower conjugate of pelvic midplane defined as distance from lower inner symphysis pubis to sacrococcygeal junction (Mid-sagittal T2-weighted image)
5. Bony femoral width defined as bony width of the pelvis at the mid femoral head level (Axial T1-weighted image)
6. Urethra width defined as maximal diameter of the urethra (Axial T2-weighted image)
7. Outer levator distance defined as distance from the outer border of the levator muscles measured at the same level as inner levator distance (Axial T2-weighted image)
8. Inner levator distance defined as narrowest distance from the inner border of the levator muscle to the urethra below the caudal margin of the prostatic apex (Axial T2-weighted image)
9. Urethral length measured from the apex of the prostate to the base of the urethral bulbus (Coronal T2-weighted image)
10. Maximal prostate width (Axial T2-weighted image)
11. Maximal prostate length measured at the same level as maximal width measurement (Axial T2-weighted image)
Table 1.
Summary of patient characteristics and measured dimensions on MRI. All values are median (IQR) or frequency (proportion).
| N=600 | |
|---|---|
| Age at surgery (years) | 66 (61, 70) |
| Body Mass Index | 27 (25, 30) |
| PSA | 5.4 (4.1, 7.0) |
| Clinical Stage | |
| T1 | 358 (60%) |
| T2A | 116 (19%) |
| T2B+ | 126 (21%) |
| Biopsy Gleason Score | |
| <=6 | 345 (57%) |
| 7 | 214 (36%) |
| >=8 | 41 (7%) |
| Nerve Sparing | |
| No | 90 (15%) |
| Unilateral | 169 (28%) |
| Bilateral | 340 (57%) |
| Year of surgery | |
| 2001 | 13 (2%) |
| 2002 | 168 (28%) |
| 2003 | 193 (32%) |
| 2004 | 226 (38%) |
| Comorbidities | |
| 0 | 262 (44%) |
| 1 | 238 (40%) |
| 2 | 83 (14%) |
| 3+ | 17 (3%) |
| MRI variables | |
| Urethra width (mm) | 12 (11, 14) |
| Inner levator (mm) | 17 (15, 19) |
| Outer levator (mm) | 40 (37, 42) |
| Levator thickness (mm) * | 12 (10, 13) |
| Urethra length (mm) | 13 (11, 16) |
| Urethra volume (mm3) ** | 1577 (1235, 1998) |
| Prostate width (mm) | 50 (46, 55) |
| Prostate length (mm) | 28 (25, 33) |
| Prostate height (mm) | 44 (39, 50) |
| Prostate volume (cm3) *** | 32 (25, 44) |
| Prostate depth (mm) | 26 (21, 31) |
| Bony width (mm) | 102 (98, 107) |
| Lower conjugate (mm) | 103 (97, 109) |
| Midpelvic area (cm2) **** | 82 (77, 89) |
| Symphysis angle (degree) | 41 (37, 44) |
| Continence outcomes | |
| Continent at 6 months | 375 (63%) |
| Continent at 12 months | 457 (76%) |
calculated: (Outer levator distance – Inner levator distance) /2 (mm)
calculated: Urethra length * pi * (Urethra width/2)2 (mm3)
calculated: Prostate width × Prostate length × Prostate height × π/6/1000 (cm3)
calculated: (Bony width × Lower conjugate × π)/400 (cm2)
MR imaging was performed on a 1.5-Tesla system (Signa;GE Medical Systems, Milwaukee, WI). A body coil for excitation and a pelvic phased-array coil were used in combination with an endorectal coil (Medrad, Pittsburgh, Pa) for signal reception. Transverse spin-echo T1-weighted images were obtained through the pelvis with the following parameters: repetition time /echo time, 400–600 msec/8–10 msec; section thickness, 5 mm; intersection gap, 1 mm; field of view, 24 cm; matrix, 256 × 192; and two signals acquired. Thin-section, high-spatial-resolution transverse, coronal, and sagittal T2-weighted fast spin-echo images were obtained through the prostate and the seminal vesicles with the following parameters: repetition time /echo time, 4000–6000 msec/96–120 msec; echo train length, 12–16; section thickness, 3 mm; intersection gap, 0 mm; field of view, 12–14 cm; matrix, 256 × 192; and four signals acquired. Automated correction was applied to the T1- and T2-weighted images for the reception profile of the endorectal and pelvic phased-array coils.
Statistical methods
Our first aim was to identify MRI features that were independently associated with recovery of continence. We used logistic regression to evaluate the association between each MRI variable and continence at either 6 or 12 months, adjusting for age, comorbidities (0, 1, 2 or 3 or more), clinical stage (T1, T2A or ≥T2B), PSA and year of surgery (continuous). MRI variables that were found to be statistically independently significant (p<0.05) were included together in a multivariable model that included the same patient and clinical variables as above. We used this form of stepwise regression because of the large number of MRI variables that were investigated – too many to include together in a multivariable model – and there were no prior data from which to base our choice of specific variables a priori.
Our second aim was to evaluate the discrimination of the multivariable model including MRI features and patient and clinical variables (the “full model”) with that of a model including only patient and clinical variables (the “base model”). Discrimination was measured using the area-under-the-curve (AUC), and corrected for statistical optimism using bootstrap methods.
Lastly, inter-rater agreement of the various MRI measurements were evaluated using a weighted kappa statistic (with linear weights) and 95% limits of agreement on a random subgroup of 100 patients. All analyses were performed using Stata10.1 (StataCorp, College Station, Texas).
Results
Patient characteristics, including preoperative MRI measurements, are presented in table 1. The median age of patients was 66. The majority of patients had clinical stage T1 (60%) and Gleason score 6 or less (57%).
Two separate readers, blinded to clinical and pathological data, evaluated MRI measurements in a subgroup of patients (n=100). Overall, readers showed moderate or better agreement for all MRI variables (weighted kappa ≥0.48 for all MRI measurements). Prostate width and bony width (weighted kappa 0.80 and 0.78, respectively) showed better agreement than membranous urethra length (weighted kappa 0.48) or inner levator distance (weighted kappa 0.49). The 95% limits of agreement indicated that measurements were in general relatively consistent, but could differ by more than 30% for some measurements. In total, 63% (n=375) and 76% (n=457) regained function at 6 and 12 months, respectively. Inter-rater agreement results and the association between each MRI measurements and continence at either 6 or 12 months after surgery are published as supplementary material on the institutional website (Table 3,4). After adjusting for age, comorbidities, clinical stage, clinical grade, PSA and year of surgery, we found that larger urethra length and urethra volume were significantly associated with an increased probability of regaining continence at both 6 and 12 months. Larger inner and outer levator distance were significantly associated with a decreased probability of regaining continence at one of the two time points assessed (12 and 6 months, respectively); they did not reach conventional levels of statistical significance for the other. We did not find any evidence that urethra width; prostate width, length, height, volume or depth; bony width, symphysis angle, or midpelvic area were significantly associated with recovery of continence at either 6 or 12 months (all p ≥0.2).
Our last aim was to compare the discrimination of a base model that included age, comorbidities, clinical stage, clinical grade, PSA and year or surgery to that of a model that additionally included the MRI variables found to be significant above. The discrimination of the base model was low (bootstrap corrected AUC of 0.606 and 0.587 for continence at 6 and 12 months, respectively), and was marginally increased with the addition of the MRI variables (to 0.636 and 0.634, respectively) (Table 2).
Table 2.
Area under the curve (95% CI) for a base model that includes age, comorbidities, clinical stage, clinical grade, PSA and year of surgery, plus each MRI variable that was found to be independently significant separately and a full model including all MRI variables. All values were corrected for statistical optimism using bootstrap methods.
| Continence at 6 months | Continence at 12 months | |
|---|---|---|
| Base model | 0.606 | 0.587 |
| +Urethra length | 0.622 | 0.628 |
| +Outer levator | 0.613 | 0.595 |
| +Inner levator | 0.611 | 0.602 |
| +Urethra volume | 0.622 | 0.613 |
| Full model (base + all MRI variables) | 0.636 | 0.634 |
As a sensitivity analysis, we repeated all of our results stratified by procedure type – open or laparoscopic radical prostatectomy – in order to evaluate whether predictors differed by approach. Overall we did not find any evidence that predictors were importantly different by surgical technique. For example, the association between urethra length and continence at 6 months was similar for patients treated with open (OR: 1.07; 1.01, 1.13; p=0.026) and laparoscopic (OR: 1.15; 95% CI: 1.02, 1.29; p=0.025) surgery.
Discussion
Detailed knowledge and understanding of pelvic anatomy is crucial in optimizing surgical technique and preserving functional structures that might impact early recovery of urinary continence. Anatomical open radical prostatectomy has been optimized over the last 30 years and continues to undergo constant refinement aiming to improve oncologic outcomes while at the same time preserving urinary and sexual function 4-7. Minimal invasive laparoscopic radical prostatectomy and more recently robotic assisted approaches have been developed to duplicate and potentially improve the outcomes of open surgery 8-10.
We performed a pilot study in a large cohort of patients with the aim of identifying pelvic bony and soft tissue dimensions on preoperative MRI that are associated with continence recovery. Our subjective impression that surgery in a narrower pelvis with a deep situated prostate is more challenging and might be associated with adverse recovery of continence led to the use of pelvic bony dimensions, symphysis angle, apical depth and prostate volume. Hong et al and Matikainen et al suggested that surgery in a narrower and steep pelvis with an anatomically deep located prostate might be associated with a technically more difficult procedure that might impact outcomes 11, 12. In a cohort including more than 2000 patients Konety et all reported an association between ultrasound measured prostate volume and continence recovery 13. In contrast to Konety 13 but consistent with Eastham et al 2 and Pettus et al 14, prostate volume measured on MRI in our study, was not associated with recovery of continence at 6 or 12 months. This is likely due to the fact that we controlled for age of patient, a variable known to be closely correlated with prostate volume. The midpelvic area, reflecting bony pelvic size at the approximate height of the apical dissection, apical depth, and symphysis angle were not found to be associated with recovery of continence in our cohort. This suggests that surgical technique in experienced hands can compensate for a subjectively more challenging procedure in a steep or narrow pelvis.
In addition to bony pelvic size and anatomical location of the prostate we focused on soft tissue measurements of the distal sphincteric unit. The male sphincteric complex consists of the proximal sphincter unit, bladder neck, prostate and prostatic urethra while the distal sphincter unit consists of rhabdosphincter, paraurethral skeletal musculature and supporting fascial investments 15. After radical prostatectomy, bladder control is likely determined by the integrity of the remaining distal sphincteric unit, of which paraurethral support by the levator ani and its voluntary contractile pressure are suggested to have the most important effect 16. Coakley at al measured membranous urethral length (MUL) on preoperative MRI in 211 consecutive patients who underwent radical prostatectomy and reported that increasing MUL was associated with more rapid recovery of continence 17. Paparel et al measured preand postoperative MUL on MRI in 64 men and showed an association between increasing MUL and continence recovery after RP in regard to time to recovery and degree of recovery 18. Both studies were limited due to a small study cohort. Including several hundred patients in our study, we confirm that increasing MUL is associated with urinary recovery. In addition, increasing urethral volume was found to be an independent predictor of urinary recovery at 6 and 12 months. Song et al measured levator thickness immediately caudal to the apex of the prostate on the axial plane in 94 patients 16. He concluded that patients with better developed pelvic floor muscles, especially in relation to the size of the prostate, can be expected to achieve earlier recovery of continence after RP 16. Levator thickness immediately caudal to the apex was not significantly associated with recovery of continence but an anatomically close related levator muscle to the urethra was identified as an independent predictor in our study with decreased distance between the urethra and the levator being associated with increased urinary continence. In some men the levator muscles are anatomically located tightly adjacent to the apex and membranous urethra compared to others where the levator fibers are looser and anatomically distant. Our data suggests that not the thickness of the levator ani, but the close relation to the membranous urethra importantly impacts recovery of continence.
The addition of identified MRI variables to a preoperative base model for prediction of continence recovery increased predictive accuracy, but marginal improvement of discrimination in our study cohort was too low to suggest implementation into clinical counseling.
Our study has several limitations and strengths. MRI imaging was performed using an endorectal probe which might impact the soft tissue dimensions depending on the exact position of the probe. MRI was preoperatively performed, while MRI measurements were performed retrospectively. Not all patients treated with radical prostatectomy in the study period were staged with MRI. However, the distribution of pelvic dimensions is not expected to be different in patients who underwent MRI versus those who did not. In this pilot study we included all patients between 2001 and 2004 in order to design a confirmatory study including identified MRI variables and all other known preoperative variables. In a more current cohort preoperative MRI staging was applied in more patients, MRI technically improved after 2004 and surgical technique is in a constant refinement process. Another potential weakness is that we did not use externally validated questionnaires to measure urinary continence, but the fact that both continent and incontinent men were measured using the same methodology means that this is unlikely to influence predictors of urinary continence. In contrast to other studies we carefully performed an inter-rater agreement subanalysis showing moderate agreement between two trained raters but also to highlight the fact that variability exists, particularly for non-osseous variables, even on pelvic MRI which is presumably the best available tool to image soft tissue structures. Our cohort still might not be large enough to show small differences in outcome, but to the best of our knowledge, this is the largest cohort to date where the association between various pelvic bony and soft tissue dimensions on recovery of continence after radical prostatectomy measured on MRI was investigated.
Preoperative variables to predict continence recovery after surgical treatment of prostate cancer need to be developed and new variables should be explored to improve individual patient counseling. MRI imaging variables only marginally improved current prediction models which limits the use in clinical practice.
Conclusion
Membranous urethral length, urethral volume and an anatomically close related levator muscle to the membranous urethra on preoperative MRI are independent predictors of continence recovery after radical prostatectomy. Addition of these variables to a base model marginally improved predictive accuracy over that from standard patient and clinical variables. Further analysis and modeling of all known and potential preoperative variables is needed to carefully create improved prediction models prior to implementing into clinical practice.
Acknowledgments
Supported by: The Sidney Kimmel Center for Prostate and Urologic Cancers
Keys
- AUC
Area under the curve
- ILD
Inner levator distance
- IQR
Inter quartile range
- MPA
Midpelvic area
- MRI
Magnetic resonance imaging
- MUL
Membranous urethral length
- OLD
Outer levator distance
- PSA
Prostate specific antigen
- RP
Radical prostatectomy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Penson DF, McLerran D, Feng Z, et al. 5-year urinary and sexual outcomes after radical prostatectomy: results from the prostate cancer outcomes study. J Urol. 2005;173:1701. doi: 10.1097/01.ju.0000154637.38262.3a. [DOI] [PubMed] [Google Scholar]
- 2.Eastham JA, Kattan MW, Rogers E, et al. Risk factors for urinary incontinence after radical prostatectomy. J Urol. 1996;156:1707. [PubMed] [Google Scholar]
- 3.Sandhu JS, Eastham JA. Factors predicting early return of continence after radical prostatectomy. Curr Urol Rep. 2010;11:191. doi: 10.1007/s11934-010-0108-6. [DOI] [PubMed] [Google Scholar]
- 4.Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492. doi: 10.1016/s0022-5347(17)53012-8. [DOI] [PubMed] [Google Scholar]
- 5.Budaus L, Isbarn H, Schlomm T, et al. Current technique of open intrafascial nerve- sparing retropubic prostatectomy. Eur Urol. 2009;56:317. doi: 10.1016/j.eururo.2009.05.044. [DOI] [PubMed] [Google Scholar]
- 6.Montorsi F, Salonia A, Suardi N, et al. Improving the preservation of the urethral sphincter and neurovascular bundles during open radical retropubic prostatectomy. Eur Urol. 2005;48:938. doi: 10.1016/j.eururo.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Kessler TM, Burkhard FC, Studer UE. Nerve-sparing open radical retropubic prostatectomy. Eur Urol. 2007;51:90. doi: 10.1016/j.eururo.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Guillonneau B, Vallancien G. Laparoscopic radical prostatectomy: initial experience and preliminary assessment after 65 operations. Prostate. 1999;39:71. doi: 10.1002/(sici)1097-0045(19990401)39:1<71::aid-pros12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 9.Guillonneau B, Cathelineau X, Barret E, et al. Laparoscopic radical prostatectomy: technical and early oncological assessment of 40 operations. Eur Urol. 1999;36:14. doi: 10.1159/000019921. [DOI] [PubMed] [Google Scholar]
- 10.Binder J, Kramer W. Robotically-assisted laparoscopic radical prostatectomy. BJU Int. 2001;87:408. doi: 10.1046/j.1464-410x.2001.00115.x. [DOI] [PubMed] [Google Scholar]
- 11.Hong SK, Chang IH, Han BK, et al. Impact of variations in bony pelvic dimensions on performing radical retropubic prostatectomy. Urology. 2007;69:907. doi: 10.1016/j.urology.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 12.Matikainen MT, von Bodman C, Secin FP, et al. Depth of prostate apex is an independent predictor of positive apical margins at radical prostatectomy. BJU Int. 2010;106:622. doi: 10.1111/j.1464-410X.2009.09184.x. [DOI] [PubMed] [Google Scholar]
- 13.Konety BR, Sadetsky N, Carroll PR. Recovery of urinary continence following radical prostatectomy: the impact of prostate volume--analysis of data from the CaPSURE Database. J Urol. 2007;177:1423. doi: 10.1016/j.juro.2006.11.089. [DOI] [PubMed] [Google Scholar]
- 14.Pettus JA, Masterson T, Sokol A, et al. Prostate size is associated with surgical difficulty but not functional outcome at 1 year after radical prostatectomy. J Urol. 2009;182:949. doi: 10.1016/j.juro.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burnett AL, Mostwin JL. In situ anatomical study of the male urethral sphincteric complex: relevance to continence preservation following major pelvic surgery. J Urol. 1998;160:1301. [PubMed] [Google Scholar]
- 16.Song C, Doo CK, Hong JH, et al. Relationship between the integrity of the pelvic floor muscles and early recovery of continence after radical prostatectomy. J Urol. 2007;178:208. doi: 10.1016/j.juro.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 17.Coakley FV, Eberhardt S, Kattan MW, et al. Urinary continence after radical retropubic prostatectomy: Relationship with membranous urethral length on preoperative endorectal magnetic resonance imaging. Journal of Urology. 2002;168:1032. doi: 10.1016/S0022-5347(05)64568-5. [DOI] [PubMed] [Google Scholar]
- 18.Paparel P, Akin O, Sandhu JS, et al. Recovery of urinary continence after radical prostatectomy: association with urethral length and urethral fibrosis measured by preoperative and postoperative endorectal magnetic resonance imaging. Eur Urol. 2009;55:629. doi: 10.1016/j.eururo.2008.08.057. [DOI] [PubMed] [Google Scholar]