Abstract
Background
Approximately 30% of all cases of nonsmall-cell lung cancer (NSCLC) are of a locally advanced (IIIA or IIIB) stage. However, surgical therapy for patients with stage IIIA (N2) NSCLC is associated with a disappointing 5-year survival rate. The optimal treatment for stage IIIA (N2) NSCLC is still in dispute.
Methods
A literature search was performed in the PubMed, Embase, and MEDLINE databases (last search updated in March 2015), and a meta-analysis of the available data was conducted. Two authors independently extracted data from each eligible study.
Results
A total of nine studies, including five randomized controlled trials and four retrospective studies, were enrolled in this meta-analysis. Significant homogeneity (χ2=49.62, P=0.000, I2=81.9%) was detected between four of the studies, including a total of 11,948 selected cases. Among the nine studies that investigated overall survival, the pooled hazard ratio (HR) was 0.70 (95% confidence interval (CI): 0.56–0.87; P=0.000). Subgroup analyses were performed according to the study design and the extent of resection. We observed a statistically significant better outcome after lobectomy (pooled HR: 0.52; 95% CI: 0.47–0.58; P=0.000) than after pneumonectomy (pooled HR: 0.82; 95% CI: 0.69–0.98; P=0.028). Unfortunately, there was no significant difference between the randomized controlled studies, as the pooled HR was 0.94 (95% CI: 0.81–1.09; P=0.440).
Conclusion
Neoadjuvant chemoradiotherapy or chemotherapy followed by surgery (particularly lobectomy) is superior to following these therapies with definitive chemoradiation or radiotherapy, particularly in patients undergoing lobectomy.
Keywords: nonsmall cell lung carcinoma, N2 stage, therapy, surgery, chemoradiotherapy, lobectomy
Introduction
Lung cancer is the leading cause of cancer-related death worldwide and accounts for more than 80% of lung cancer diagnoses.1 In general, surgery provides the best chance for a cure in patients with stage I or stage II disease. In advanced-stage (stages III and IV) nonsmall-cell lung cancer (NSCLC), 5-year survival rate varies widely (3%–50%) depending on the number of lymph nodes involved, resectability, and tumor histology.2 At present, surgery as a potential option for patients with lung cancer is considered acceptable for patients with N2 disease. However, as a result of local recurrences and the presence of distant metastatic disease, surgical therapy in patients with stage IIIA NSCLC is associated with a 5-year survival rate of only 15%–30%.2
Induction chemotherapy followed by surgery has been demonstrated to improve survival in selected patients with stage IIIA NSCLC.3,4 Additionally, radiation therapy has been shown to prolong the overall survival (OS) of patients with stage III NSCLC. A retrospective study performed using the Surveillance, Epidemiology, and End Results database including more than 48,000 patients with stage III NSCLC revealed that OS in those who received neoadjuvant radiotherapy plus surgery was significantly better compared with radiation therapy alone, postoperative radiation therapy, or surgery alone.5 Thus, chemoradiotherapy or chemotherapy, with or without resection (preferably lobectomy), is an option for patients with stage IIIA (N2) NSCLC.
In a previous study,6 we identified four randomized controlled trials that compared neoadjuvant chemotherapy or chemoradiotherapy before surgical resection (n=414) with neoadjuvant chemotherapy or chemoradiotherapy before radical radiotherapy (n=406) in patients with NSCLC. However, we found that the former therapeutic strategy did not appear to be clinically superior to the latter therapeutic strategy in patients with stage IIIA (N2) NSCLC. In recent years, three large-scale retrospective studies7–9 were published that drew opposite conclusions. Thus, whether neoadjuvant chemoradiotherapy or chemotherapy followed by surgery is better than following these therapies with definitive radiotherapy for locoregionally advanced disease remains controversial.
The examination and synthesis of the limited available data comparing groups undergoing surgical resection or definitive radiotherapy after neoadjuvant chemoradiotherapy or chemotherapy may allow clinicians to determine the optimal treatment for patients with stage IIIA (N2) disease. The objective of this study is to perform a systematic review and meta-analysis of the available data to determine whether surgery is superior to definitive radiotherapy after neoadjuvant chemoradiotherapy or chemotherapy in patients with operable stage IIIA (N2) NSCLC.
Methods
We conducted this meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.10 This statement helps authors to report the results of systematic reviews and meta-analyses in an accurate and reliable manner.
Identification and eligibility of relevant studies
A literature search was performed in the PubMed, Embase, and MEDLINE databases (last search updated in March 2015) using the following keywords or MeSH terms: (Chemoradiotherapy OR Chemotherapy OR Radiotherapy OR Chemoradiation) AND NSCLC AND N2 AND surgery. The titles and abstracts were reviewed by two authors independently as a primary screen of the potential literature. Disagreements were solved by discussion between the two authors. Then, we determined the final studies to be included by reading the full text of the remaining articles. When several studies reported repetitious data, only the most complete study was included. The electronic searches were supplemented by scanning the reference lists from the retrieved articles to identify additional studies. To identify unpublished studies, we also searched abstracts from conference proceedings of the European Society for Medical Oncology, the American Society of Clinical Oncology, and the World Lung Cancer Conference. We contacted the authors via email if the conference presentation slides were unavailable.
Inclusion criteria
The studies should 1) compare chemoradiotherapy or chemotherapy followed by surgery with chemoradiotherapy or chemotherapy followed by definitive radiotherapy; 2) include stage IIIA (N2) NSCLC cases; 3) provide survival data, such as survival curves, hazard ratios (HRs), and the associated 95% confidence interval (CI) for OS or progression-free survival (PFS); and 4) be published in English.
Data extraction
A data extraction sheet was created to capture all of the data needed to assess the quality and eligibility of the studies and perform a systematic review and meta-analysis. Two authors independently extracted the following data from each eligible study: the name of the first author, year of publication, source of patients, study design, sample size, chemoradiotherapy or chemotherapy regimen before random assignment to two groups, and the HR for the PFS and OS of the two groups.
Statistical analysis
The HR calculation spreadsheet provided by Tierney et al11 was used to calculate the HR and its 95% CI for survival data, and the results were consistent with our previous studies.12,13 In our meta-analysis, fixed or random effects models were used depending on the heterogeneity between studies. Heterogeneity between studies was estimated using the χ2-based Q-test,14 whose significance was set at P<0.10. Begg’s funnel plots15 and Egger’s linear regression test16 were used to assess publication bias. In addition, sensitivity analysis was conducted to confirm that the results were stable and reliable. All statistical analyses were performed with Stata Version 11.0 (StataCorp LP, College Station, TX, USA).
Results
Literature search and summary of studies
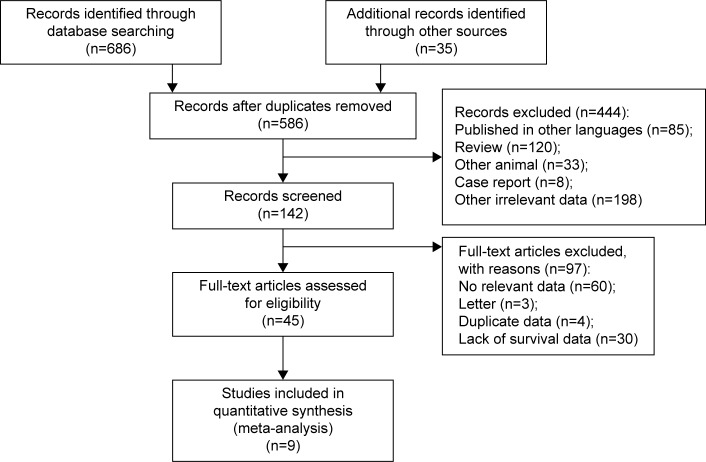
A total of 586 articles addressing neoadjuvant therapy in patients with stage IIIA (N2) NSCLC were identified. After screening the titles and abstracts, 444 articles were excluded because they were published in other languages, were review articles or case reports, involved other animals, or were irrelevant to the current study, leaving 45 potential articles, whose full text was carefully reviewed according to the inclusion criteria (shown earlier). Finally, nine studies were included in this meta-analysis. A brief flow diagram is shown in Figure 1.
Figure 1.
Brief flowchart.
A total of nine studies,7–9,17–22 consisting of five randomized controlled trials, comprising two large randomized controlled trials (the European Organisation for Research and Treatment of Cancer [EORTC] trial 0894120 and the North American Intergroup Study 0139 [INT 0139]),21 three small randomized controlled trials, and four retrospective studies, were included in our meta-analysis. All of the patients received chemotherapy or chemoradiotherapy before being allocated to a surgery group or radiotherapy group. The studies were published from 1998 to 2015. EORTC 08941 was performed at multiple academic and community hospitals in the Netherlands, while INT 0139 was conducted in the USA and Canada. The other studies were carried out in the USA, England, Spain, and Canada. The patients included in the INT 0139 trial21 received chemoradiotherapy before being randomly assigned to two groups, while the patients in the other three studies only received induction chemotherapy. Most of the patients had T1, T2, or T3 primary NSCLC with pathological proof of N2 involvement (ie, from endobronchial ultrasound-guided procedures, mediastinoscopy, or thoracoscopic procedures). Most of the studies provided the HR and its 95% CI for PFS and OS. The three small studies8,18,19 and the subgroup analyses of two studies9,21 only provided survival curves. Thus, we obtained the HR and its 95% CI using the HR calculations spreadsheet provided by Tierney et al.11 The minimum follow-ups of all studies ranged from 14.0 to 124.8 months.
Meta-analysis and evaluation of heterogeneity
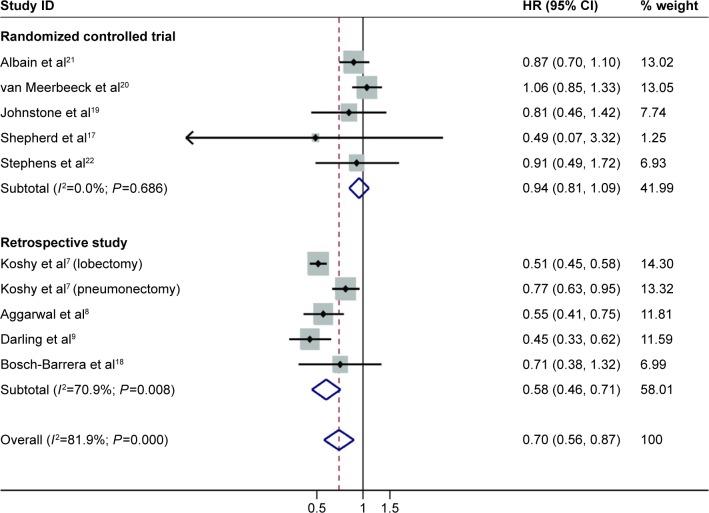
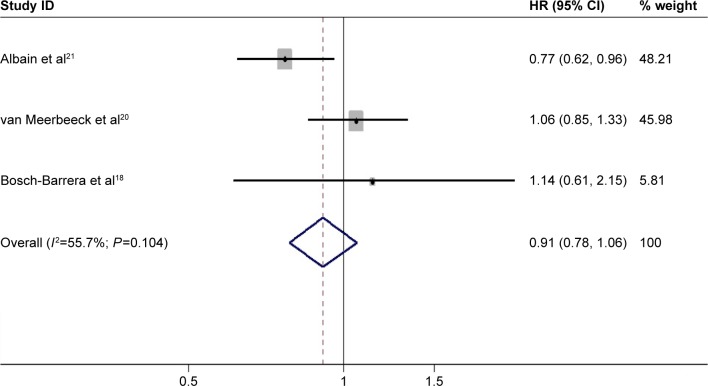
A total of nine studies, including five randomized controlled trials and four retrospective studies, were enrolled in this meta-analysis. Significant homogeneity (χ2=49.62, P=0.000, I2=81.9%) was detected between four of the studies, including a total of 11,948 selected cases. Among the nine studies that investigated OS, a random effects model was used to conduct the meta-analysis. The pooled HR was 0.70 (95% CI: 0.56–0.87; P=0.000) (Figure 2), which implied that the surgery group was superior to the definitive radiotherapy group of patients with stage IIIA (N2) NSCLC. PFS was investigated in three studies. Unfortunately, there was no significant difference in PFS between the three groups, as the pooled HR was 0.91 (95% CI: 0.78–1.06; P=0.190) (Figure 3). A fixed effects model was used because modest heterogeneity (χ2=4.52, P=0.104, I2=55.7%) was detected.
Figure 2.
Forest plot for overall survival (subgroup by study design) associated with neoadjuvant chemoradiotherapy or chemotherapy followed by surgery compared with following these therapies with definitive radiotherapy in stage IIIA (N2) NSCLC.
Note: Weights are from random effects analysis.
Abbreviations: CI, confidence interval; HR, hazard ratio; NSCLC, nonsmall-cell lung carcinoma; ID, identification.
Figure 3.
Forest plot for progression-free survival-associated neoadjuvant chemoradiotherapy or chemotherapy followed by surgery compared with following these therapies with definitive radiotherapy in stage IIIA (N2) NSCLC.
Abbreviations: CI, confidence interval; HR, hazard ratio; NSCLC, nonsmall-cell lung carcinoma; ID, identification.
Subgroup analyses
Subgroup analyses were performed according to the study design and the extent of resection. We observed a statistically significantly better outcome after lobectomy (pooled HR: 0.52; 95% CI: 0.47–0.58; P=0.000) than after pneumonectomy compared with definitive chemoradiation or radiotherapy (pooled HR: 0.82; 95% CI: 0.69–0.98; P=0.028). Fine homogeneity was detected in both subgroups. In the retrospective studies, neoadjuvant chemoradiotherapy or chemotherapy followed by surgery was associated with an approximately 50% benefit compared with definitive chemoradiation or radiotherapy. The combined HR for PFS was 0.58 (95% CI: 0.46–0.71; P=0.000). A random effects model was used because significant heterogeneity (χ2=13.74, P=0.008, I2=70.9%) was observed. However, there was no significant difference between the randomized controlled studies, as the pooled HR was 0.94 (95% CI: 0.81–1.09; P=0.440) (Figure 2), with fine homogeneity being observed.
Publication bias and sensitivity analysis
Both Begg’s funnel plot (Figure 4) and Egger’s test were performed to assess the publication bias of the studies. The results of Begg’s test (P=0.860) and Egger’s test (P=0.490) suggested no evidence of publication bias in this meta-analysis when all studies were included. There was also no significant publication bias observed in the subgroup analysis. Moreover, a sensitivity analysis was performed by excluding studies with small samples, and the results showed no significant changes.
Figure 4.
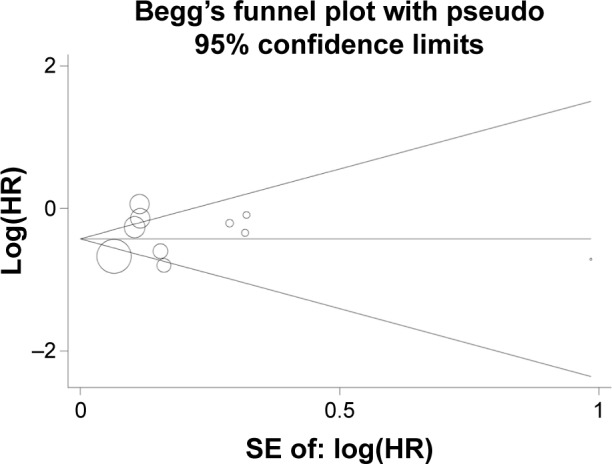
Begg’s funnel plot for overall survival associated with neoadjuvant chemoradiotherapy or chemotherapy followed surgery compared with following these therapies with definitive radiotherapy in stage IIIA (N2) NSCLC.
Abbreviations: NSCLC, nonsmall-cell lung carcinoma; SE, standard error.
Discussion
Patients with stage IIIA NSCLC with mediastinal lymph node involvement represent a large and heterogeneous subgroup of patients with NSCLC. Concurrent chemoradiation is considered the standard care for inoperable or unresectable patients at present. However, the role of surgery in stage IIIA (N2) NSCLC remains controversial. In the present study, we found that neoadjuvant chemoradiotherapy or chemotherapy followed by surgery improves survival compared with definitive chemoradiation or radiotherapy alone, as OS was close to reaching a statistically significant difference. Some observations from this study warrant further attention.
The characteristics of the nine studies are shown in Table 1. In the INT 0139 trial, the patients received induction chemotherapy (cisplatin and etoposide) with concurrent radiotherapy, and regardless of their response to this therapy, they were randomly allocated to a surgery or radiotherapy group. However, in the EORTC 8941 trial, the patients only received induction chemotherapy, and only patients stratified for the type of response, histological subtype, and institution were randomly allocated to two groups. The induction chemotherapy applied in the EORTC 8941 trial consisted mainly of a combination of platinum/gemcitabine (40%) or platinum/taxane (21%). The patients in the other studies mainly received a combination of other platinum-based chemotherapy. These differences may be the source of heterogeneity between the studies. However, a previous meta-analysis demonstrated that induction chemoradiation is not superior to induction chemotherapy alone in stage IIIA lung cancer,23 as the obtained HR was 0.93 (95% CI: 0.54–1.62; P=0.810). Thus, it was still appropriate to combine the results of the two large studies.
Table 1.
The characteristics of the studies
| First author | Year | Study years | Country | Study design | Treatment for two groups
|
Number of patients
|
Stage | PFS (HR and 95% CI) | OS (HR and 95% CI) | Subgroup (number of patients) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery group | Definitive RT group | Surgery group | Definitive RT group | |||||||||
| Albain et al21 | 2009 | 1994–2001 | USA and Canada | RCT (INT 0139) | Neo concurrent CRT + surgery | Neo concurrent CRT + definitive RT | 202 | 194 | T1–3N2M0 | 0.77 (0.62–0.96) | Total: 0.87 (0.70–1.10); L vs CRT: 0.66 (0.46–0.94) P vs CRT: 1.12 (0.70–1.79) | L vs CRT (90 vs 90); P vs CRT (51 vs 51) |
| van Meerbeeck et al20 | 2007 | 1994–2002 | the Netherlands | RCT (EORTC 08941) | Neo chemo + surgery | Neo chemo + definitive RT | 167 | 165 | T1–T3N2M0 | 1.06 (0.85–1.33) | 1.06 (0.84–1.35) | |
| Johnstone et al19 | 2002 | 1990–1994 | USA | RCT | Neo chemo + surgery | Neo chemo + definitive RT | 29 | 15 | T1–T3N2M0 | NR | 0.81 (0.46–1.42) | |
| Shepherd et al17 | 1998 | NR | Canada | RCT | Neo chemo + surgery | Definitive RT | 15 | 16 | T1–T3N2M0 | NR | 0.49 (0.07–3.32) | |
| Stephens et al22 | 2005 | 1995–1999 | England | RCT | Neo chemo + surgery | Definitive RT | 24 | 24 | T3N1M0/T1–3N2M0 | NR | 0.91 (0.49–1.72) | |
| Koshy et al7 | 2013 | 1998–2004 | USA | Retrospective | Neo CRT + surgery | Definitive RT | 752 | 9,857 | T1–T3N2M0 | NR | L vs CRT: 0.51 (0.45–0.58) P vs CRT: 0.77 (0.63–0.95) | L vs CRT (564 vs 9,857); P vs CRT (188 vs 9,857) |
| Aggarwal et al8 | 2014 | 2000–2008 | USA | Retrospective | Neo CRT + surgery | Definitive RT | 155 | 103 | T3N1M0/T1–3N2M0 | NR | L vs CRT: 0.57 (0.45–0.58); P vs CRT: 0.77 (0.63–0.95) | L vs CRT (105 vs 103); P vs CRT (41 vs 103) |
| Darling et al9 | 2015 | 1997–2007 | Canada | Retrospective | Neo CRT or chemo + surgery | Definitive CRT | 104 | 111 | T1–T3N2M0 | NR | Total: 0.45 (0.33–0.62) L vs CRT: 0.48 (0.29–0.80) | L vs CRT (28 vs 34) |
| Bosch-Barrera et al18 | 2012 | 1996–2006 | Spain | Retrospective | Neo chemo + surgery | Neo chemo + definitive RT | 38 | 34 | T1–T4N2M0 | 1.14 (0.61–2.15) | 0.71 (0.38–1.32) | |
Abbreviations: Chemo, chemotherapy; CI, confidence interval; CRT, chemoradiation; HR, hazard ratio; L, lobectomy; Neo, neoadjuvant; NR, not reported; OS, overall survival; P, pneumonectomy; PFS, progression-free survival; RCT, randomized controlled trial; RT, radiotherapy.
Albain et al21 (INT 0139) observed that PFS was longer in patients who underwent resection than in those who continued uninterrupted radiotherapy up to 61 Gy after concurrent chemoradiotherapy. Moreover, in the patients who showed downstaging (presenting an N0 status upon thoracotomy), the median OS (34.4 months) was significantly prolonged compared with the OS for all patients in the two groups.21 Pathological downstaging is a favorable prognostic factor in stage IIIA (N2) NSCLC.24,25 Decaluwe et al25 observed a trend of a better 5-year survival rate in patients with mediastinal nodal downstaging compared with patients with persistent N2 disease (49% vs 27%). These authors demonstrated that multilevel positive nodes at initial mediastinoscopy were related to a lower 5-year survival rate (17% vs 39%) compared with single level positive nodes. Standard resections include lobectomy, bilobectomy, and pneumonectomy. Several studies24,26 have shown that lobectomy leads to better long-term survival than pneumonectomy after induction chemotherapy, with no increase in postoperative complications or the recurrence rate. OS was improved for patients who underwent lobectomy, but not pneumonectomy, versus chemotherapy plus radiotherapy in the INT 0139 trial,21 possibly because of the high operative mortality associated with pneumonectomy. Furthermore, the pooled HR was less than 1, which implied that the surgery group was slightly superior to the group in which the initial treatment was followed by definitive radiotherapy, although PFS and OS did not reach a statistically significant difference. Interestingly, in the subgroup analysis, the patients who underwent lobectomy or pneumonectomy were shown to receive greater benefits compared with definitive chemoradiation or radiotherapy. Compared with radiation therapy, radical resection can completely remove the tumor and potential lymph node metastasis. Moreover, the incidence of long-term irreversible complications such as pulmonary interstitial fibrosis is lower in radical resection group. Although not all patients with locally advanced NSCLC are suitable for resection, surgery is clearly worth considering for a subpopulation of patients who may benefit from the procedure, such as those who exhibit downstaging or are suitable for lobectomy.
Radiation therapy is fully integrated in multimodality therapy for all stages of lung cancer, regardless of the application of definitive or palliative therapy. It is estimated that 50% of all patients with cancer will benefit from radiotherapy during the course of their disease. Rapid advances have occurred in radiation therapy technology, enabling conformal dose sculpting, dose intensification, and the sparing of normal tissue. These technologies include four-dimensional computed tomography simulation, three-dimensional radiotherapy, and intensity-modulated radiation therapy, stereotactic ablative radiotherapy, image guide radiation therapy, motion management strategies, and proton therapy, among others. In addition, positron emission tomography–computed tomography is increasingly being used for lung cancer diagnosis and staging as well as treatment planning, treatment, and the prediction of recurrence after radiation.27–29 These technologies are a potential way to improve the clinical curative effect of radiotherapy in patients with stage IIIA (N2) NSCLC.30,31 According to our results, definitive chemoradiotherapy may be recommended for patients with stage IIIA (N2) NSCLC, who require a pneumonectomy or exhibit multiple pathologically verified malignant nodes >3 cm that are unsuitable for resection.
One of the most significant strengths of this study is that we performed a comprehensive review using the most up-to-date published data. In addition, we contacted authors to obtain relevant unpublished data. It is noteworthy that subgroup and sensitivity analyses were conducted to reduce heterogeneity. However, as a meta-analysis based on the published literature, there are several limitations to this study. First, no individual patient data were used, and meta-analysis of individual patient data is the gold standard of meta-analysis. Second, there was significant heterogeneity between the studies. The treatments performed in the studies were not highly consistent (Table 1). The preoperative treatment applied in a few of the studies consisted of neoadjuvant concurrent chemoradiation,8,9,21 while in others, it was neoadjuvant chemotherapy.17,19,20,22 In addition, the postoperative therapy employed in some studies was definitive radiotherapy,7,8,17,22 while in others, it was definitive chemoradiation.9,18–21 Furthermore, in some studies, only responding patients were randomly assigned to a surgical resection or radiotherapy group.20,21 All of these issues could represent sources of heterogeneity. Other sources of heterogeneity may be the different research designs and study sample sizes involved. Third, potential publication bias is unavoidable because some reports with negative or controversial results may not be published. Finally, potential language bias existed because we only included literature published in the English language. Selection bias also cannot be ruled out in this study.
Conclusion
Multimodality therapy offers the best chance for improved PFS and OS in stage IIIA (N2) NSCLC. Neoadjuvant chemoradiotherapy or chemotherapy followed by surgery is superior to following these therapies with definitive radiotherapy or chemoradiation. In patients with stage IIIA (N2) disease, surgery may be offered to patients who have achieved mediastinal downstaging or suitable to a lobectomy. In patients with stage IIIA (N2, bulky) NSCLC, definitive radiotherapy followed by adjuvant therapy might be the optimal choice. Further studies are needed to investigate the role of these therapies in the subgroup of patients who have undergone lobectomy.
Acknowledgments
This work was supported by Medical Science and Technology Project of Zhejiang Province (Nos 2015KYB054-01 and 2011KYA032) and the High-level Backbone Talent Project of Zhejiang Province Medical Platform (No 2011RCA014). This work was supported by the Key Research Project of Medicine of Zhejiang Province (2011c13039-1).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Walker S. Updates in non-small cell lung cancer. Clin J Oncol Nurs. 2008;12(4):587–596. doi: 10.1188/08.CJON.587-596. [DOI] [PubMed] [Google Scholar]
- 2.Mountain CF. A new international staging system for lung cancer. 1986. Chest. 2009;136(5 Suppl):e25. doi: 10.1378/chest.89.4_supplement.225s. [DOI] [PubMed] [Google Scholar]
- 3.Rosell R, Gomez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med. 1994;330(3):153–158. doi: 10.1056/NEJM199401203300301. [DOI] [PubMed] [Google Scholar]
- 4.Roth JA, Atkinson EN, Fossella F, et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer. 1998;21(1):1–6. doi: 10.1016/s0169-5002(98)00046-4. [DOI] [PubMed] [Google Scholar]
- 5.Koshy M, Goloubeva O, Suntharalingam M. Impact of neoadjuvant radiation on survival in stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;79(5):1388–1394. doi: 10.1016/j.ijrobp.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 6.Xu YP, Li B, Xu XL, Mao WM. Is there a survival benefit in patients with stage IIIA (N2) non-small cell lung cancer receiving neoadjuvant chemotherapy and/or radiotherapy prior to surgical resection: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94(23):e879. doi: 10.1097/MD.0000000000000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koshy M, Fedewa SA, Malik R, et al. Improved survival associated with neoadjuvant chemoradiation in patients with clinical stage IIIA(N2) non-small-cell lung cancer. J Thorac Oncol. 2013;8(7):915–922. doi: 10.1097/JTO.0b013e31828f68b4. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal C, Li L, Borghaei H, et al. Multidisciplinary therapy of stage IIIA non-small-cell lung cancer: long-term outcome of chemoradiation with or without surgery. Cancer Control. 2014;21(1):57–62. doi: 10.1177/107327481402100108. [DOI] [PubMed] [Google Scholar]
- 9.Darling GE, Li F, Patsios D, et al. Neoadjuvant chemoradiation and surgery improves survival outcomes compared with definitive chemoradiation in the treatment of stage IIIA N2 non-small-cell lung cancer. Eur J Cardiothorac Surg. 2015;48(5):684–690. doi: 10.1093/ejcts/ezu504. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu XL, Ling ZQ, Chen SZ, Li B, Ji WH, Mao WM. The impact of E-cadherin expression on the prognosis of esophageal cancer: a meta-analysis. Dis Esophagus. 2014;27(1):79–86. doi: 10.1111/dote.12024. [DOI] [PubMed] [Google Scholar]
- 13.Xu XL, Chen SZ, Chen W, et al. The impact of cyclin D1 overexpression on the prognosis of ER-positive breast cancers: a meta-analysis. Breast Cancer Res Treat. 2013;139(2):329–339. doi: 10.1007/s10549-013-2563-5. [DOI] [PubMed] [Google Scholar]
- 14.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 15.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 16.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shepherd FA, Johnston MR, Payne D, et al. Randomized study of chemotherapy and surgery versus radiotherapy for stage IIIA non-small-cell lung cancer: a National Cancer Institute of Canada Clinical Trials Group Study. Br J Cancer. 1998;78(5):683–685. doi: 10.1038/bjc.1998.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosch-Barrera J, Garcia-Franco C, Guillen-Grima F, et al. The multimodal management of locally advanced N2 non-small cell lung cancer: is there a role for surgical resection? A single institution’s experience. Clin Transl Oncol. 2012;14(11):835–841. doi: 10.1007/s12094-012-0874-3. [DOI] [PubMed] [Google Scholar]
- 19.Johnstone DW, Byhardt RW, Ettinger D, Scott CB. Phase III study comparing chemotherapy and radiotherapy with preoperative chemotherapy and surgical resection in patients with non-small-cell lung cancer with spread to mediastinal lymph nodes (N2); final report of RTOG 89-01. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 2002;54(2):365–369. doi: 10.1016/s0360-3016(02)02943-7. [DOI] [PubMed] [Google Scholar]
- 20.van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst. 2007;99(6):442–450. doi: 10.1093/jnci/djk093. [DOI] [PubMed] [Google Scholar]
- 21.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374(9687):379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephens RJ, Girling DJ, Hopwood P, Thatcher N, Medical Research Council Lung Cancer Working Party A randomised controlled trial of pre-operative chemotherapy followed, if feasible, by resection versus radiotherapy in patients with inoperable stage T3, N1, M0 or T1-3, N2, M0 non-small cell lung cancer. Lung Cancer. 2005;49(3):395–400. doi: 10.1016/j.lungcan.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Shah AA, Berry MF, Tzao C, et al. Induction chemoradiation is not superior to induction chemotherapy alone in stage IIIA lung cancer. Ann Thorac Surg. 2012;93(6):1807–1812. doi: 10.1016/j.athoracsur.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Maurizi G, D’Andrilli A, Anile M, et al. Sleeve lobectomy compared with pneumonectomy after induction therapy for non-small-cell lung cancer. J Thorac Oncol. 2013;8(5):637–643. doi: 10.1097/JTO.0b013e318286d145. [DOI] [PubMed] [Google Scholar]
- 25.Decaluwe H, De Leyn P, Vansteenkiste J, et al. Surgical multimodality treatment for baseline resectable stage IIIA-N2 non-small cell lung cancer. Degree of mediastinal lymph node involvement and impact on survival. Eur J Cardiothorac Surg. 2009;36(3):433–439. doi: 10.1016/j.ejcts.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Shi W, Zhang W, Sun H, Shao Y. Sleeve lobectomy versus pneumonectomy for non-small cell lung cancer: a meta-analysis. World J Surg Oncol. 2012;10:265. doi: 10.1186/1477-7819-10-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eschmann SM, Friedel G, Paulsen F, et al. 18F-FDG PET for assessment of therapy response and preoperative re-evaluation after neoadjuvant radio-chemotherapy in stage III non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2007;34(4):463–471. doi: 10.1007/s00259-006-0273-5. [DOI] [PubMed] [Google Scholar]
- 28.Gregoire V, Haustermans K, Geets X, Roels S, Lonneux M. PET-based treatment planning in radiotherapy: a new standard? J Nucl Med. 2007;48(Suppl 1):68S–77S. [PubMed] [Google Scholar]
- 29.Asai N, Ohkuni Y, Shoji K, Kaneko N. Efficacy of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in evaluating lung cancer recurrence. J Bras Pneumol. 2013;39(2):242–244. doi: 10.1590/S1806-37132013000200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol. 2010;28(35):5153–5159. doi: 10.1200/JCO.2010.30.0731. [DOI] [PubMed] [Google Scholar]
- 31.Louie AV, Rodrigues G, Hannouf M, et al. Stereotactic body radiotherapy versus surgery for medically operable Stage I non-small-cell lung cancer: a Markov model-based decision analysis. Int J Radiat Oncol Biol Phys. 2011;81(4):964–973. doi: 10.1016/j.ijrobp.2010.06.040. [DOI] [PubMed] [Google Scholar]