Abstract
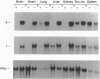
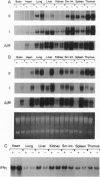
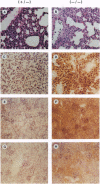
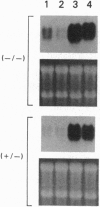
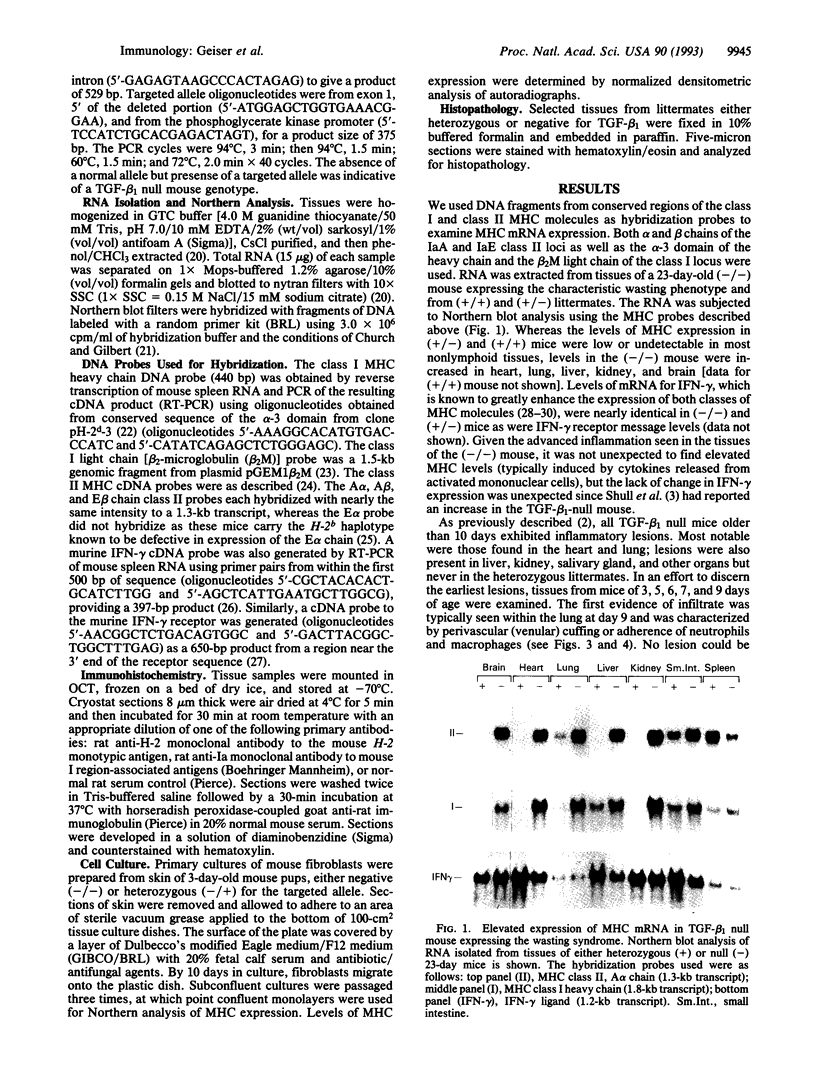
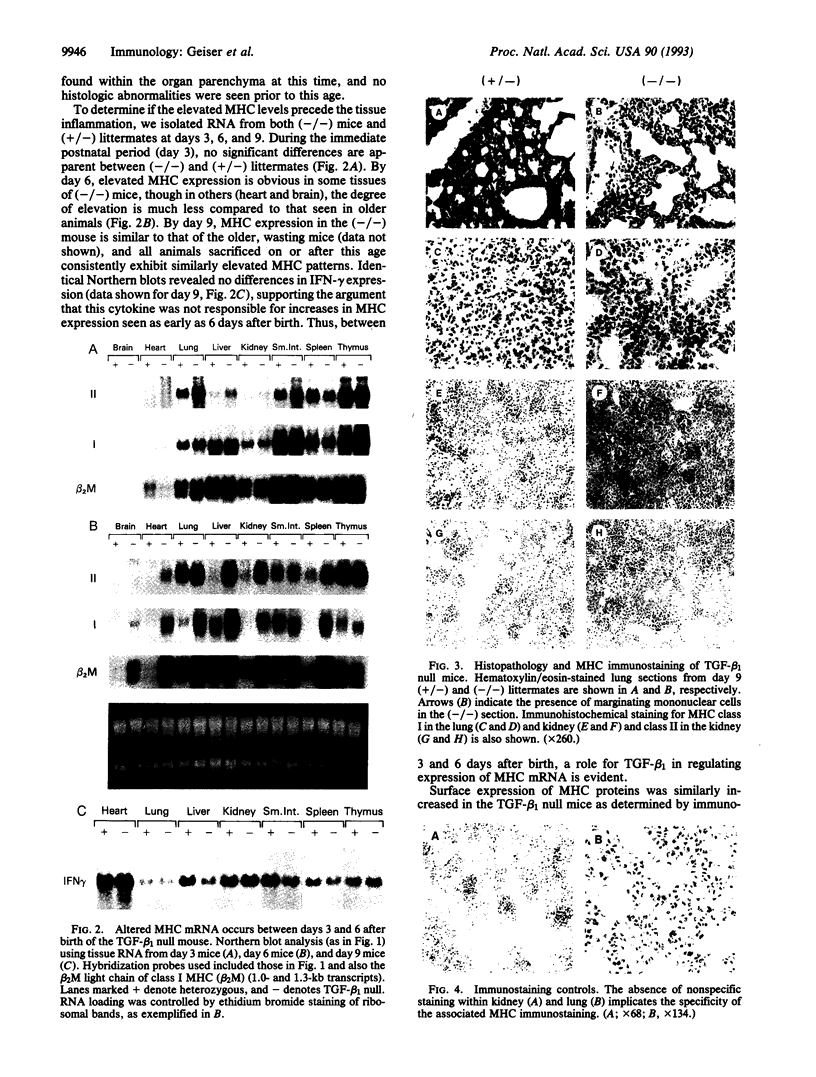
The phenotype of the transforming growth factor beta 1 (TGF-beta 1) null mouse has been previously described and is characterized by inflammatory infiltrates in multiple organs leading to a wasting syndrome and death as early as 3 weeks after birth. Since this phenotype occurs in the absence of any detectable pathogen, potential autoimmune disease mechanisms were investigated. We examined major histocompatibility complex (MHC) mRNA expression in tissues of the TGF-beta 1 null mouse and found levels of both the class I and class II MHC mRNA elevated compared to normal or TGF-beta 1 heterozygous littermates. This elevated expression was seen prior to any evidence of inflammatory infiltrates, suggesting a causal relationship between increased MHC expression and activation of immune cell populations. Cell surface expression of MHC molecules was detected by immunohistochemistry and correlated well with mRNA levels. Expression of mRNA for interferon gamma and its receptor was unchanged at the ages when increased MHC expression became apparent. Down-regulation of class I MHC expression by TGF-beta 1 was also demonstrated in vitro in fibroblasts isolated from TGF-beta 1 null mice. These findings suggest that one natural function of TGF-beta 1 is to control expression of both MHC classes. Altered regulation of MHC expression may be a critical step leading to the multifocal inflammation and wasting syndrome seen in the TGF-beta 1 null mouse. These results suggest potential applications for TGF-beta in the management of autoimmune disease, allograft rejection, and other problems associated with altered MHC expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold B., Schönrich G., Hämmerling G. J. Multiple levels of peripheral tolerance. Immunol Today. 1993 Jan;14(1):12–14. doi: 10.1016/0167-5699(93)90317-E. [DOI] [PubMed] [Google Scholar]
- Basham T. Y., Merigan T. C. Recombinant interferon-gamma increases HLA-DR synthesis and expression. J Immunol. 1983 Apr;130(4):1492–1494. [PubMed] [Google Scholar]
- Benoist C., Mathis D. Regulation of major histocompatibility complex class-II genes: X, Y and other letters of the alphabet. Annu Rev Immunol. 1990;8:681–715. doi: 10.1146/annurev.iy.08.040190.003341. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Dean B. M., McNally J. M., MacKay E. H., Swift P. G., Gamble D. R. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985 Aug 8;313(6):353–360. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- Brégégère F., Abastado J. P., Kvist S., Rask L., Lalanne J. L., Garoff H., Cami B., Wiman K., Larhammar D., Peterson P. A. Structure of C-terminal half of two H-2 antigens from cloned mRNA. Nature. 1981 Jul 2;292(5818):78–81. doi: 10.1038/292078a0. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T., Korman A. J., Wake C. T., Boss J. M., Kappes D. J., Fiers W., Ault K. A., Gimbrone M. A., Jr, Strominger J. L., Pober J. S. Immune interferon activates multiple class II major histocompatibility complex genes and the associated invariant chain gene in human endothelial cells and dermal fibroblasts. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4917–4921. doi: 10.1073/pnas.81.15.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarniecki C. W., Chiu H. H., Wong G. H., McCabe S. M., Palladino M. A. Transforming growth factor-beta 1 modulates the expression of class II histocompatibility antigens on human cells. J Immunol. 1988 Jun 15;140(12):4217–4223. [PubMed] [Google Scholar]
- Daar A. S., Fuggle S. V., Fabre J. W., Ting A., Morris P. J. The detailed distribution of MHC Class II antigens in normal human organs. Transplantation. 1984 Sep;38(3):293–298. doi: 10.1097/00007890-198409000-00019. [DOI] [PubMed] [Google Scholar]
- David-Watine B., Israël A., Kourilsky P. The regulation and expression of MHC class I genes. Immunol Today. 1990 Aug;11(8):286–292. doi: 10.1016/0167-5699(90)90114-o. [DOI] [PubMed] [Google Scholar]
- Decker T., Lew D. J., Mirkovitch J., Darnell J. E., Jr Cytoplasmic activation of GAF, an IFN-gamma-regulated DNA-binding factor. EMBO J. 1991 Apr;10(4):927–932. doi: 10.1002/j.1460-2075.1991.tb08026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. A., Allen H., Burkly L. C., Sherman D. H., Waneck G. L., Widera G. Molecular biology of the H-2 histocompatibility complex. Science. 1986 Jul 25;233(4762):437–443. doi: 10.1126/science.3726537. [DOI] [PubMed] [Google Scholar]
- Foulis A. K., Farquharson M. A. Aberrant expression of HLA-DR antigens by insulin-containing beta-cells in recent-onset type I diabetes mellitus. Diabetes. 1986 Nov;35(11):1215–1224. doi: 10.2337/diab.35.11.1215. [DOI] [PubMed] [Google Scholar]
- Frauman A. G., Chu P., Harrison L. C. Nonimmune thyroid destruction results from transgenic overexpression of an allogeneic major histocompatibility complex class I protein. Mol Cell Biol. 1993 Mar;13(3):1554–1564. doi: 10.1128/mcb.13.3.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble J. R., Vadas M. A. Endothelial adhesiveness for blood neutrophils is inhibited by transforming growth factor-beta. Science. 1988 Oct 7;242(4875):97–99. doi: 10.1126/science.3175638. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Goeddel D. V. Cloning and expression of murine immune interferon cDNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5842–5846. doi: 10.1073/pnas.80.19.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Pujol-Borrell R., Chiovato L., Russell R. C., Doniach D., Bottazzo G. F. Aberrant expression of HLA-DR antigen on thyrocytes in Graves' disease: relevance for autoimmunity. Lancet. 1983 Nov 12;2(8359):1111–1115. doi: 10.1016/s0140-6736(83)90628-1. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Campbell I. L., Allison J., Miller J. F. MHC molecules and beta-cell destruction. Immune and nonimmune mechanisms. Diabetes. 1989 Jul;38(7):815–818. doi: 10.2337/diab.38.7.815. [DOI] [PubMed] [Google Scholar]
- Hedfors E., Lindahl G. Variation of MHC class I and II antigen expression in relation to lymphocytic infiltrates and interferon-gamma positive cells. J Rheumatol. 1990 Jun;17(6):743–750. [PubMed] [Google Scholar]
- Hom J. T., Bendele A. M., Carlson D. G. In vivo administration with IL-1 accelerates the development of collagen-induced arthritis in mice. J Immunol. 1988 Aug 1;141(3):834–841. [PubMed] [Google Scholar]
- Hopkins S. J., Meager A. Cytokines in synovial fluid: II. The presence of tumour necrosis factor and interferon. Clin Exp Immunol. 1988 Jul;73(1):88–92. [PMC free article] [PubMed] [Google Scholar]
- Ignotz R. A., Heino J., Massagué J. Regulation of cell adhesion receptors by transforming growth factor-beta. Regulation of vitronectin receptor and LFA-1. J Biol Chem. 1989 Jan 5;264(1):389–392. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Cell adhesion protein receptors as targets for transforming growth factor-beta action. Cell. 1987 Oct 23;51(2):189–197. doi: 10.1016/0092-8674(87)90146-2. [DOI] [PubMed] [Google Scholar]
- Inge T. H., McCoy K. M., Susskind B. M., Barrett S. K., Zhao G., Bear H. D. Immunomodulatory effects of transforming growth factor-beta on T lymphocytes. Induction of CD8 expression in the CTLL-2 cell line and in normal thymocytes. J Immunol. 1992 Jun 15;148(12):3847–3856. [PubMed] [Google Scholar]
- Johns L. D., Flanders K. C., Ranges G. E., Sriram S. Successful treatment of experimental allergic encephalomyelitis with transforming growth factor-beta 1. J Immunol. 1991 Sep 15;147(6):1792–1796. [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Scheynius A., Kabelitz D., Wigzell H. Evidence in support of a self-perpetuating HLA-DR-dependent delayed-type cell reaction in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3632–3636. doi: 10.1073/pnas.79.11.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A. B., Huh C. G., Becker D., Geiser A., Lyght M., Flanders K. C., Roberts A. B., Sporn M. B., Ward J. M., Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar C. S., Muthukumaran G., Frost L. J., Noe M., Ahn Y. H., Mariano T. M., Pestka S. Molecular characterization of the murine interferon gamma receptor cDNA. J Biol Chem. 1989 Oct 25;264(30):17939–17946. [PubMed] [Google Scholar]
- Kuruvilla A. P., Shah R., Hochwald G. M., Liggitt H. D., Palladino M. A., Thorbecke G. J. Protective effect of transforming growth factor beta 1 on experimental autoimmune diseases in mice. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2918–2921. doi: 10.1073/pnas.88.7.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefer A. M., Ma X. L., Weyrich A. S., Scalia R. Mechanism of the cardioprotective effect of transforming growth factor beta 1 in feline myocardial ischemia and reperfusion. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1018–1022. doi: 10.1073/pnas.90.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C. Y., Calamai E. G., Unanue E. R. A defect in the antigen-presenting function of macrophages from neonatal mice. Nature. 1979 Nov 15;282(5736):327–329. doi: 10.1038/282327a0. [DOI] [PubMed] [Google Scholar]
- Margulies D. H., Parnes J. R., Johnson N. A., Seidman J. G. Linkage of beta 2-microglobulin and ly-m11 by molecular cloning and DNA-mediated gene transfer. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2328–2331. doi: 10.1073/pnas.80.8.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D. J., Benoist C., Williams V. E., 2nd, Kanter M., McDevitt H. O. Several mechanisms can account for defective E alpha gene expression in different mouse haplotypes. Proc Natl Acad Sci U S A. 1983 Jan;80(1):273–277. doi: 10.1073/pnas.80.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., Lider O., Roberts A. B., Sporn M. B., Weiner H. L. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton A. D., Fabre J. W. Massive induction of donor-type class I and class II major histocompatibility complex antigens in rejecting cardiac allografts in the rat. J Exp Med. 1985 Jan 1;161(1):98–112. doi: 10.1084/jem.161.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K., Wan Y. J., Orrison B. M. Mouse major histocompatibility class I gene expression begins at midsomite stage and is inducible in earlier-stage embryos by interferon. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2427–2431. doi: 10.1073/pnas.82.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M., Oldendorf W. H., Cancilla P., Frank H. J. Blood-brain barrier: interface between internal medicine and the brain. Ann Intern Med. 1986 Jul;105(1):82–95. doi: 10.7326/0003-4819-105-1-82. [DOI] [PubMed] [Google Scholar]
- Pujol-Borrell R., Todd I., Londei M., Foulis A., Feldmann M., Bottazzo G. F. Inappropriate major histocompatibility complex class II expression by thyroid follicular cells in thyroid autoimmune disease and by pancreatic beta cells in type I diabetes. Mol Biol Med. 1986 Apr;3(2):159–165. [PubMed] [Google Scholar]
- Racke M. K., Cannella B., Albert P., Sporn M., Raine C. S., McFarlin D. E. Evidence of endogenous regulatory function of transforming growth factor-beta 1 in experimental allergic encephalomyelitis. Int Immunol. 1992 May;4(5):615–620. doi: 10.1093/intimm/4.5.615. [DOI] [PubMed] [Google Scholar]
- Racke M. K., Dhib-Jalbut S., Cannella B., Albert P. S., Raine C. S., McFarlin D. E. Prevention and treatment of chronic relapsing experimental allergic encephalomyelitis by transforming growth factor-beta 1. J Immunol. 1991 May 1;146(9):3012–3017. [PubMed] [Google Scholar]
- Rogers M. J., Germain R. N., Hare J., Long E., Singer D. S. Comparison of MHC genes among distantly related members of the genus Mus. J Immunol. 1985 Jan;134(1):630–636. [PubMed] [Google Scholar]
- Shull M. M., Ormsby I., Kier A. B., Pawlowski S., Diebold R. J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992 Oct 22;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitz L., Planz O., Bilzer T., Frei K., Fontana A. Transforming growth factor-beta modulates T cell-mediated encephalitis caused by Borna disease virus. Pathogenic importance of CD8+ cells and suppression of antibody formation. J Immunol. 1991 Nov 15;147(10):3581–3586. [PubMed] [Google Scholar]
- Strober S. Natural suppressor (NS) cells, neonatal tolerance, and total lymphoid irradiation: exploring obscure relationships. Annu Rev Immunol. 1984;2:219–237. doi: 10.1146/annurev.iy.02.040184.001251. [DOI] [PubMed] [Google Scholar]
- Suda T., Zlotnik A. In vitro induction of CD8 expression on thymic pre-T cells. I. Transforming growth factor-beta and tumor necrosis factor-alpha induce CD8 expression on CD8- thymic subsets including the CD25+CD3-CD4-CD8- pre-T cell subset. J Immunol. 1992 Mar 15;148(6):1737–1745. [PubMed] [Google Scholar]
- Wahl S. M. Transforming growth factor beta (TGF-beta) in inflammation: a cause and a cure. J Clin Immunol. 1992 Mar;12(2):61–74. doi: 10.1007/BF00918135. [DOI] [PubMed] [Google Scholar]
- Weisz A., Marx P., Sharf R., Appella E., Driggers P. H., Ozato K., Levi B. Z. Human interferon consensus sequence binding protein is a negative regulator of enhancer elements common to interferon-inducible genes. J Biol Chem. 1992 Dec 15;267(35):25589–25596. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]