Abstract
Background and Purpose
We investigated the intra- and interrater reliability of ischemic lesion volumes measurements assessed by different MRI sequences at various times from onset.
Methods
Ischemic lesion volumes were measured for intrarater reliability using diffusion-weighted (DWI), mean transit time (MTT) perfusion and fluid-attenuated inversion recovery (FLAIR) MRI at chronic (>3 days from stroke onset) time points. A single intrarater reader, blind to clinical information and time point, repeated the volume measurements on two occasions separated by at least 1 week. Interrater reliability was also obtained in the second set of patients using acute DWI, MTT and chronic FLAIR MRI. Four blinded readers performed these volume measurements. Average deviations across repeat measurements per lesion and differences between sample means between the two measurements were calculated globally, ie, across all sequences and time points, and per reader type for each sequence at each time point.
Results
There was good concordance of the mean sample volumes of the 2 intrarater readings (deviations were <4% and 2 mL globally, <2% and 2 mL for DWI, <6% and 7 mL for MTT, and <2% and 1 mL for FLAIR). There was also good concordance of the interrater readings (<5% and 2 mL globally).
Conclusions
Repeat measurements of stroke lesion volumes show excellent intra- and interrater concordance for DWI, MTT and FLAIR at acute through chronic time points.
Keywords: acute stroke, brain imaging, magnetic resonance, neuroradiology, thrombolysis
Measurements of ischemic lesion volumes have been used in numerous imaging studies. Investigations of stroke outcomes and studies of the effects of therapeutic interventions including randomized clinical trials have included lesion volumes measured acutely with diffusion (DWI) and perfusion (PWI) weighted MRI and chronically with T2-weighted or fluid-attenuated inversion recovery (FLAIR) MRI.1–9 The use of volumetric measurements as an objective quantitative tool depends on intra- and interrater variability, but limited information is available on the reliability of these measurements across MRI sequence type and time from stroke onset. Reliabilities reported have been restricted to small sample sizes using manual or semiautomated techniques. Martel et al reported an intra- and interobserver repeatability coefficient defined as the 95% CI, of <6 mL for their semiautomatic method of measuring lesion volume using DWI (N = 10).10 Ritzl et al reported an interobserver error of <3 mL for measurement of chronic FLAIR volume (N = 8).11 Baird et al and Barber et al both reported an interrater variability of 5% with manual segmentation of lesion volumes on DWI.12,13 The current study provides intra- and interrater reliability estimates of lesion volume measurements, which may be relevant to stroke trial design and lesion data analysis.
Methods
Patients
This study is part of a prospective, natural history study of MRI in a consecutive series of tissue plasminogen activator (tPA)-treated patients at the National Institutes of Neurological Disorders and Stroke (NINDS) and Suburban Hospital, Bethesda, Md.14,15 The Institutional Review Board at NINDS and Suburban Hospital approved the study. From February 2000 through January 2005, 147 patients were treated with standard intravenous tPA; of those, 81 patients had an MRI before tPA treatment and were the subject of the intrarater analysis. Patients were eligible for this analysis if they received an MRI scan (“acute” scan) followed by standard intravenous tPA within 3 hours from stroke onset. The study design was to obtain serial MRI approximately 3 hours after the acute scan (“3-hour” scan), approximately 24 hours after the start of tPA (“24-hour” scan), and then at 5, 30, and 90 days after stroke onset (“chronic” scan). Chronic (>3 days from stroke onset) FLAIR volumes were taken from the latest available FLAIR for a given patient. Because of clinical care requirements, patient preferences, or death, all posttreatment MRI time points were not obtained in all patients. Only image time points and sequences with identifiable lesions were included.
A second confirmatory sample for intrarater analysis was obtained from August 1999 through July 2005 in 106 patients diagnosed with an acute cerebrovascular event, ischemic stroke, or transient ischemic attack. These patients had an acute MRI on average within 14 hours of onset and were not treated with tPA. The study design was identical to the tPA patients with the exception of the “3- and 24-hour” scans, which were not obtained. These same patients were also measured for the interrater analysis.
Imaging Sequences
MRI sequences were performed on a GE 1.5-T clinical scanner (Twinspeed; General Electric).
Diffusion-Weighted Imaging
In this study, the DWI spin-echo planar sequence included 20 contiguous axial oblique slices with b = 0 and b = 1000 s/mm2, isotropically weighted, using repetition time (TR)/echo time (TE) = 6000/72 ms, acquisition matrix of 128×128, 7-mm slice thickness, and 24-cm field of view (FOV).
Perfusion-Weighted Imaging
In this study, the PWI gradient-echo planar sequence included 20 contiguous axial oblique slices with single-dose gadolinium injection of 0.1 mmol/kg through a power injector using 25 phase measurements (2 seconds per phase measurement), TR/TE = 2000/45 ms, acquisition matrix of 64×64, 7-mm slice thickness, and 24-cm FOV.
Mean Transit Time
Mean transit time (MTT) maps were calculated from PWI using time concentration curves in this study as the first moment of the time concentration curves divided by the zeroeth moment.
Fluid-Attenuated Inversion Recovery
FLAIR images were used for chronic lesion measurement as the suppression of cerebrospinal fluid in these images is advantageous when delineating the ischemic lesions from the adjacent sulci or ventricles. In this study, the high-resolution FLAIR sequence included 66 contiguous axial oblique slices, TR/TE = 9000/92 ms, TI = 2200 ms, acquisition matrix of 256×128, 2-mm slice thickness, and 24-cm FOV.
Image Analysis
Image analysis software (Cheshire; Perceptive Informatics) was used for measurement of ischemic lesion volumes from DWI, MTT, and FLAIR MRI sequences. Lesion volumes were measured at acute, subacute (3 and 24 hours), and chronic time points. Readers blind to clinical characteristics and time point used a semiautomated technique for initial identification of all the lesions and a manual editing tool for final corrections to the lesion borders. All lesion areas on a slice-by-slice basis were segmented with a semiautomated segmentation tool followed by manual editing. The semiautomated segmentation tool is based on a watershed method dependent on a series of seed points and the subsequent sampled surrounding area placed by the reader. The volumes were automatically produced by the multiplication of the slice thickness times the total lesion area. The intrarater reader (M.L.) measured the volume of each lesion on two occasions separated by at least 1 week. The intrarater reader trained one medical student (J.B.) and two stroke neurologists (P.S., J.M.) to perform interrater measurements using the same semiautomated technique. For the interrater readings, one reader (M.L.) performed one complete set of these measurements; however, the second set was performed by any combination of two of the three additional interrater readers with no repeat measurements. Only one measurement was produced by one of the additional interrater readers to perform direct comparisons with the first set of measurements.
DWI lesion volumes were assessed on the affected slices with hyperintense areas visible from the b = 1000 mm/s2 images. The reader paid particular attention to the typical locations of bilateral artifact and produced apparent diffusion coefficient maps as necessary to identify positive DWI lesions. For MTT assessments, the reader paid particular attention to exclude hyperintensities attributable to the typical susceptibility artifacts adjacent to the paranasal sinuses. The reader assessed the MTT as not evaluable if the signal drop from the contrast did not produce at least a 10% drop or if there was significant patient motion causing inconsistency in the confirmation of the perfusion deficit. In evaluating FLAIR, the reader paid particular attention to preexisting chronic lesions present on the acute FLAIR images to avoid replication of these lesion areas in the current FLAIR volume calculations.
Measurements from each sequence were performed independently of the other time points except for the chronic FLAIR measurements in which reference to the acute FLAIR sequence aided identification of the index stroke. Within each time point, the reader had access to the other sequences to aid in accurate identification of the index lesion.
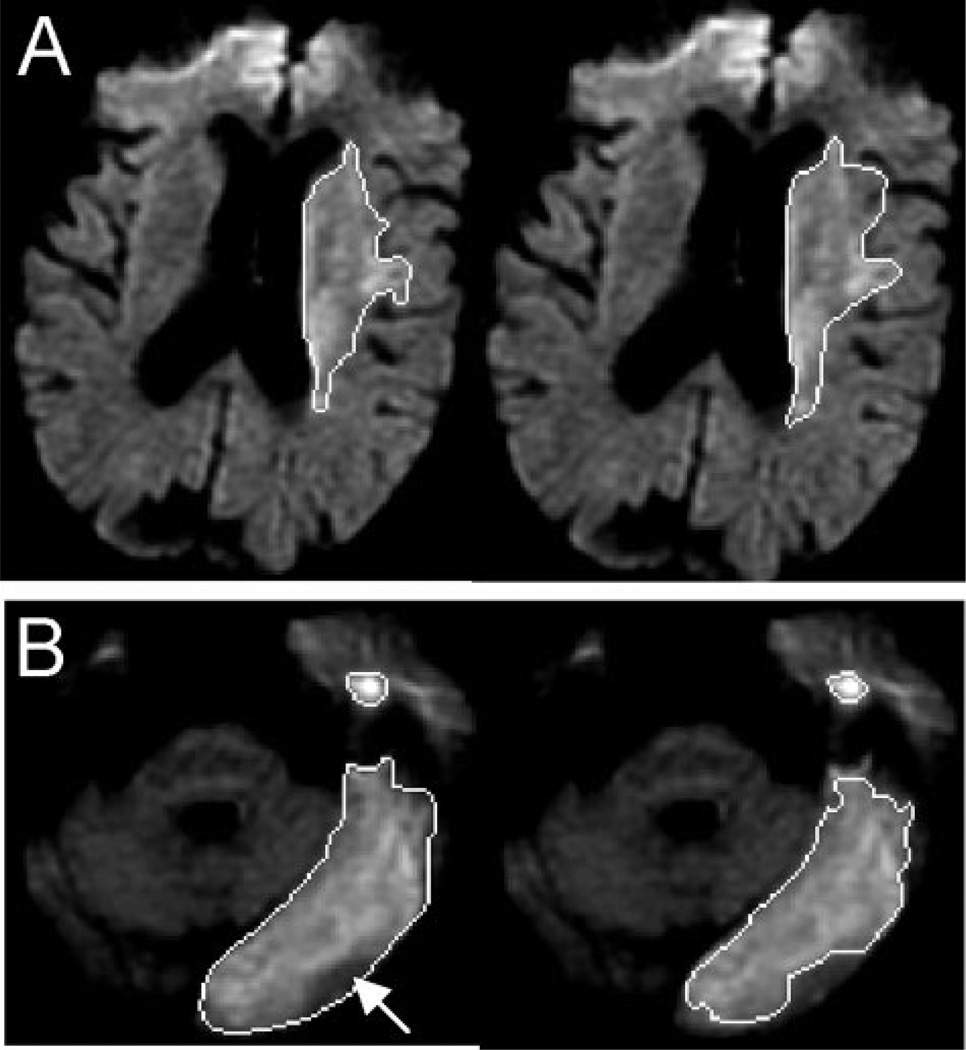
Patients’ intra- and interrater measurements from single slices of the acute DWI volumes are displayed in Figure 1. These same patients’ acute MTT and chronic FLAIR measurements from the corresponding single slices are displayed in Figures 2 and 3.
Figure 1.
(A) Intrarater volumetric measurements on single DWI image (top left region of interest [ROI])—(top right ROI). (B) Interrater volumetric measurements on single DWI image (bottom left ROI—(bottom right ROI) with arrow indicating main difference between readings.
Figure 2.
(A) Intrarater volumetric measurements on single MTT image (top left ROI)—(top right ROI). (B) Interrater volumetric measurements on single MTT image (bottom left ROI)—(bottom right ROI) with arrows indicating main differences between readings.
Figure 3.
(A) Intrarater volumetric measurements on single FLAIR image (top left ROI)—(top right ROI). (B) Interrater volumetric measurements on single FLAIR image (bottom left ROI)— (bottom right ROI) with arrows indicating main differences between readings.
Statistical Analysis
Deviations between measurements were evaluated in two ways. The sample means, globally (across all sequences and time points) and within each reader type (intra- or interrater), time point, and sequence were compared for the two reads. The deviation per lesion was computed as the absolute value of the difference between the two readings; percent deviation per lesion was the per lesion absolute deviation divided by the average of the two readings. All volume data were skewed heavily toward mild. Cube root transformation of the raw volumes was performed to normalize the volumetric data. For example, for intrarater, acute DWI volume sample had a skewness of 2.1 and a kurtosis of 3.9; after transformation, the skewness was 0.7 and the kurtosis was −0.3. The absolute volume of the difference and the percentage deviation of the two readings, raw volume and cube root of volume, were calculated for each reader type and sequence and then averaged across all patients.
Bland-Altman plots were generated for the raw volume data to display the spread of data and the limits of agreement, specifically to illustrate how many of the averaged data points lie within 2 SDs from the mean difference.16 The Bland-Altman plots were used to address the key question of whether one set of volumetric measurements is sufficiently representative or if two sets of measurements are required for providing the most accurate results. The 95% confidence limits are proposed as the repeatability coefficients of one type of measurement for another, ie, one set of volumetric measurement is sufficient rather than requiring two sets in this particular study.16
Results
In the intrarater analysis, DWI had measurable lesions in 68 patients at the acute time point, 62 at 3 hours and 41 at 24 hours. MTT images were measured in 62 patients at the acute time point, 38 at 3 hours and 24 at 24 hours. Chronic FLAIR images were measured in 46 patients. The volume statistics by sequence, DWI, MTT, and FLAIR are listed in Table 1. Only DWI, MTT, and FLAIR measurements in which at least one volume measurement is nonzero are reported in Table 1 and all subsequent tables and figures. The corresponding sample sizes are provided for each reader type, sequence, and time point. The correlation coefficients and cube root of the raw volume data are presented in supplemental Table I, available online at http://stroke.ahajournals.org.
TABLE 1.
Intrarater Volume (mL) Statistics by Time Point and Sequence
| Sequence | Time Point | N | Read 1 Average (mL) median (IQR) |
Read 2 Average (mL) median (IQR) |
Absolute Volume Difference (mL) Mean±SD median (IQR) |
Percent Deviation Mean±SD median (IQR) |
|---|---|---|---|---|---|---|
| DWI | Acute | 68 | 21.09 7.74 (1.97–27.35) | 20.19 6.47 (1.8–23.7) | 3.48±5.41 0.87 (0.19–4.69) | 30.11±48.42 12.69 (5–27) |
| 3-hour | 62 | 25.64 9.35 (2.8–34.0) | 26.09 10.9 (3.35–38.22) | 3.21 ±4.71 1.58 (0.43–4.5) | 23.75±34.17 12.71 (7–24) | |
| 24-hour | 41 | 61.81 16.26 (2.47–57.83) | 60.17 15.85 (2.34–54.82) | 3.31 ±6.15 0.74 (0.21–3.13) | 29.02±54.15 8.99 (2–24) | |
| MTT | Acute | 62 | 119.22 94.72 (31.85–187.42) | 112.38 97.88 (28.71–180.79) | 13.98±23.09 6.0 (1.58–17.87) | 18.13±29.77 9.08 (4–19) |
| 3-hour | 38 | 111.44 88.71 (22.18–184.27) | 110.57 86.57 (11.77–164.32) | 11.27±16.13 3.86 (0.98–17.13) | 18.52±35.58 8.56 (2–15) | |
| 24-hour | 24 | 93.89 74.99 (18.26–102.15) | 88.5 62.16 (14.98–109.77) | 11.32 ±14.61 7.74 (2.43–16.24) | 43.91±65.5 14.67 (4–56) | |
| FLAIR | Days 4–145 | 46 | 44.43 12.1 (1.06–52.98) | 43.82 11.4 (0.98–53.66) | 2.02 ±3.13 0.81 (0.14–2.33) | 34.68±61.76 9.4 (3–20) |
| Confirmatory intrarater sample statistics | ||||||
| DWI | Acute | 98 | 15.45 2.37 (0.8–12.72) | 14.48 3.25 (0.79–13.29) | 2.7±7.2 0.73 (0.16–2.1) | 41.55±59.2 17.88 (7–40) |
| MTT | Acute | 72 | 60.51 19.28 (1.78–76.69) | 56.0 17.26 (2.18–80.32) | 11.89±42.65 3.49 (0.93–8.15) | 58.64±76.2 19.37 (7–93) |
| FLAIR | Days 5–90 | 28 | 6.3 1.82 (0.76–5.64) | 6.08 1.76 (0.66–4.9) | 1.31±2.32 0.53 (0.11–0.95) | 45.82±697 17.73 (8–62) |
The confirmatory intrarater sample had measurable lesions in 98 patients at the acute DWI, 72 patients at the acute MTT, and 28 patients at the chronic FLAIR time points. The volume statistics for the confirmatory sample are presented in Table 1.
In the interrater analysis, there were measurable lesions in 103 patients at the acute DWI, 77 patients at the acute MTT, and 29 patients at the chronic FLAIR time points. The volume statistics by sequence, DWI, MTT, and FLAIR are listed in Table 2 for the interrater analysis.
TABLE 2.
Interrater Volume (mL) Statistics by Time Point and Sequence
| Review Type |
Sequence | Time Point | N | Read 1 Average (mL) median (IQR) |
Read 2 Average (mL) median (IQR) |
Absolute Volume Difference (mL) Mean±SD median (IQR) |
Percent Deviation Mean±SD median (IQR) |
|---|---|---|---|---|---|---|---|
| Inter | DWI | Acute | 103 | 14.7 1.9 (0.67–11.73) | 13.0 1.4 (0.61–8.47) | 2.4±4.67 0.63 (0.23–2.17) | 51.83±59.0 29.79 (15–60) |
| Inter | DWI—cubed root | Acute | 103 | 0.26±0.32 0.15 (0.06–0.29) | 31.98±59.37 9.99 (5–21) | ||
| Inter | MTT | Acute | 77 | 56.61 14.1 (1.25–75.0) | 55.81 18.11 (2.19–75.17) | 19.42±34.57 5.65 (1.14–18.74) | 82.96±78.87 39.66 (19–200) |
| Inter | MTT—cubed root | Acute | 77 | 0.72±0.73 0.42 (0.21–0.96) | 64.75±84.34 13.38 (6–200) | ||
| Inter | FLAIR | Days 5–90 | 29 | 6.08 1.77 (0.73–5.51) | 4.28 1.75 (0.39–2.82) | 2.55±4.93 0.51 (0.31–1.94) | 78.77±63.09 62.52 (30–84) |
| Inter | FLAIR—cubed root | Days 5–90 | 29 | 0.33±0.25 0.27 (0.16–0.53) | 46.84±65.14 21.48 (10–30) |
Sequence: Diffusion-Weighted Image
The mean volumes, absolute volume (mL) differences, and percent deviations between the two intrarater readings (N = 68, 62, and 41) for DWI at acute, 3-hour, and 24-hour time points are reported in Table 1. Supplemental Figure I (panels A through C; available online at http://stroke.ahajournals.org) display the Bland-Altman plots for the DWI raw volume data for the intraacute, 3-hour, and 24-hour time points. The upper and lower limits, shown as thick black solid lines, were calculated for each plot to represent ±2 SD from the mean. A total of 90%, 92%, and 90% data points were within these boundary limits for the DWI data, respectively. There were no significant changes with respect to time point in the reliability of the DWI data.
The confirmatory intrarater sample statistics are reported in Table 1. Supplemental Figure I (panel D) contains the Bland-Altman plot for the DWI raw volume data. At total of 96% data points were within the boundary limits.
The mean volumes, absolute volume (mL) differences, and percent deviations along with the cube root statistics between the two interrater readings at acute DWI (N = 103) time points are reported in Table 2. Supplemental Figure I (panel E) displays the interrater acute DWI Bland-Altman plot. A total of 94% data points were within the boundary limits. By excluding DWI lesions <10 mL in volume, the percent deviations (average, median, and SD of 25.01, 18.47, and 26.81, respectively) were comparable to those of the intrarater readings.
In addition to the measured samples, 11 and 12 acute DWI sequences were evaluated separately as normal, zero volumes, for the intra- and interrater readings. Included in the measured samples, there were 4 and 11 acute DWI sequences with one intrarater and interrater readings as zero volume. An additional five and four DWI sequences at 3 and 24 hours were evaluated separately as normal for both intrarater readings. There was one DWI sequence at 3 hours with one intrarater read as normal. There were three DWI sequences at 24 hours with one intrarater reading as normal.
Sequence: Mean Transit Time
The mean volumes, absolute volume (mL) differences, and percent deviations between the two intrarater readings (N = 62, 38, and 24) for MTT at acute, 3 hour, and 24 hour time points are reported in Table 1. Supplemental Figure I (panels F through H) display the Bland-Altman plots for the MTT raw volume data for the intra- acute, 3-hour, and 24-hour time points. A total of 94%, 92%, and 92% data points were within the boundary lines for the MTT data, respectively. There were no significant changes with respect to time point in the reliability of the MTT data.
The confirmatory intrarater sample statistics are reported in Table 1. Supplemental Figure I (panel I) displays the Bland- Altman plot for the MTT raw volume data. At total of 99% data points were within the boundary limits.
The mean volumes, absolute volume (mL) differences, and percent deviations along with the cube root statistics between the two interrater readings at acute MTT (N = 77) time points are reported in Table 2. Supplemental Figure I (panel J) displays the interrater acute MTT Bland-Altman plot. A total of 95% data points were within the boundary limits. When excluding the MTT deficits <10 mL in volume, the percent deviation statistics improved but were still not comparable to the intrarater readings.
In addition to the measured samples, seven and 34 acute MTT sequences were evaluated separately as normal, zero volumes, for the intra- and interrater readings. Included in the measured samples, there were one and 21 acute MTT sequences with one intrarater and interrater readings as zero volume. An additional five and nine MTT sequences at 3 and 24 hours were evaluated separately as normal for both intrarater readings. There were three MTT sequences at 3 hours with one intrarater read as normal.
Sequence: Fluid-Attenuated Inversion Recovery
The mean volumes, absolute volume (mL) differences, and percent deviations between the two intrareadings (N = 46) for chronic FLAIR time points are reported in Table 1. Supplemental Figure I (panel K) displays the Bland-Altman plot for the intrarater chronic FLAIR time points. A total of 91% data points were within the boundary lines.
The confirmatory intrarater sample statistics are reported in Table 1. Supplemental Figure I (panel L) contains the Bland-Altman plot for the FLAIR raw volume data. A total of 89% data points were within the boundary limits.
The mean volumes, absolute volume (mL) differences, and percent deviations along with the cube root statistics between the two interrater readings at chronic FLAIR (N = 29) time points are reported in Table 2. Supplemental Figure I (panel M) displays the interrater FLAIR Bland-Altman plot. A total of 93% data points were within the boundary limits. By excluding the FLAIR lesions <10 mL, the sample size was reduced to N = 6; however, the percent deviations (average, median, and SD of 39.74, 39.73, and 27.69, respectively) were then comparable to those of the intrarater readings.
In addition to the measured sample, 12 chronic FLAIR sequences were evaluated separately as normal, zero volumes, for both intrarater readings. Included in the measured sample, there were five FLAIR sequences with only one intrarater reading as zero volume. There were no FLAIR sequences evaluated separately as normal, zero volumes, for both interrater readings. Included in the measured sample, there were four FLAIR sequences with only one interrater reading as zero volume.
A subset of nine intrarater patients with all sequences and time points was calculated for comparison purposes to Table 1. The mean volumes (N = 9) of the two intrarater readings for DWI were 35.3 mL and 35.9 mL, 46.47 mL and 42.14 mL, and 78.53 mL and 75.89 mL at acute, 3 hours, and 24 hour time points, respectively. The mean volumes (N = 9) of the two intrarater readings for MTT were 164.56 mL and 162.04 mL, 134.73 mL and 138.07 mL, and 86.39 mL and 83.83 mL at acute, 3 hours, and 24 hour time points, respectively. The mean volumes (N = 9) of the two intrarater readings for FLAIR were 58.05 mL and 58.59 mL. The volume statistics of this subset of nine patients was consistent with those of the larger group of patients contained in Table 1.
The global mean volumes (N = 341) of the two intrarater readings were 63.0 mL and 60.9 mL. The absolute volume (mL) differences between the reads were 6.54±13.27/1.92 (0.4 to 6.86) (mean±SD/median [interquartile range {IQR} 25% to 75%]). The percent deviations between the reads were 26.94±46.51/11.14 (0.04 to 0.24).
The global mean volumes (N = 198) of the two confirmatory intrarater readings were 30.5 mL and 28.4 mL. The absolute volume (mL) differences between the two readings were 5.84±26.52/1.06 (0.24 to 4.3) (mean±SD/median [IQR 25% to 75%]). The percent deviations between the reads were 48.37±66.26/18.28 (0.07 to 0.51).
The global mean volumes (N = 209) of the two interrater readings were 28.9 mL and 27.6 mL. The absolute volume (mL) differences between the reads were 8.69±22.76/1.31 (0.34 to 5.98) (mean±SD/median [IQR 25% to 75%]). The percent deviations between the reads were 67.04±68.87/ 35.64 (0.18 to 0.87).
Discussion
The volume of injury, infarction, and ischemia has assumed an increasingly important role in stroke research in general and in stroke clinical trials in particular. Yet, relatively little attention has been paid to the reliability of volumetric measures and the contribution of measurement error to the overall variance of these outcomes in stroke studies. Because measurement error may potentially obscure true biologic effects and is potentially controllable by the investigator, it is important to understand the sources and magnitude of measurement error.
In the present study, technical variables that could affect lesion volume measurement such as the MRI scanner type, pulse sequence parameters, image processing, and analysis software were held constant. Because the intrarater reader’s experience performing stroke volume measurements with this software has been extensive over a period of years, the skill and consistency in measurement technique can be assumed to be constant over the period of the intrarater study. Thus, we have characterized the intrarater reproducibility independent of other sources of measurement error.
In principle, programs to measure lesion volumes in a completely automated fashion could eliminate this source of error. However, because regions of abnormality on these MRI sequences often lack sharp contrast boundaries with the normal brain and may overlap in signal intensity with nonpathologic structures, investigator judgment is needed to avoid inaccuracies. The interrater study attempts to quantify reader subjectivity, the main concern with interpretations of the images by multiple readers. To reduce the measurement error, the intrarater reader trained the three additional interrater readers in the same image processing techniques and software. The interrater readers were also required to be familiar with the clinical reading of serial stroke MRI scans. Furthermore, the patients and imaging protocol used in the interrater analysis were from the same time period, hospital, and stroke population as the intrarater analysis.
The intrarater repeatability seen for DWI was <1 mL at the acute and 3-hour time points and <2 mL at the 24-hour time point. The overall intrarater percent difference ranged from 2% to 5% for DWI. The interrater percent difference was <5% for acute DWI. For DWI, the error of the lesion volume measurement was caused by two main sources. There were six outliers readily identified by the Bland-Altman plots in the interrater DWI readings caused by reader differences in interpretation of subtle changes, differences in exclusion of sulcal areas, and in one case, misidentification of a chronic lesion as acute. Measurement error was also attributable to significantly smaller lesions seen for the interrater readings.
The overall intrarater MTT percent difference ranged from 1% to 6%. The intrarater repeatability coefficient seen for MTT was <1 mL at the 3-hour time point but <7 mL and <6 mL at the acute and 24-hour time points, respectively. The interrater repeatability coefficient seen for acute MTT was <1 mL. The interrater MTT percent difference was <1%. The larger standard deviations seen with MTT are in part attributable to the larger lesions present in this sequence and the lower spatial resolution in the perfusion sequence acquisitions. This was most evidenced by the four outliers in the interrater MTT readings, which are most readily identified by the Bland-Altman plots.
The intrarater repeatability coefficient seen for FLAIR was <0.6 mL and the overall percent difference of <1.5%. The most consistent lesion volume was seen with FLAIR attributable in part to the increased spatial resolution of this sequence compared with DWI and MTT as well as the resolution of edema at the chronic time point allowing for a more stable measurement. The interrater repeatability coefficient seen for FLAIR was <2 mL but an overall percent difference of <50%. The decreased consistency in the interrater lesion volume measurement with FLAIR was mainly attributable to the significantly smaller lesions seen (approximately 6 mL on average). The two outliers displayed in the corresponding Bland-Altman plot in the interrater FLAIR readings were caused by one reader, including areas of cavitations that were excluded by the other reader. Overall, for the DWI, MTT, and FLAIR volumes by requiring a minimum lesion size, ie, 10 mL, the reproducibility of the measurements, especially percent deviation, is improved.
Quantitative volumes by a single expert reader can provide highly consistent and repeatable results of lesion volumes on DWI, MTT, and FLAIR. The variability seen with the interrater measurements increased compared with the intrarater measurements; however, they also demonstrated consistent and repeatable results. This study provides a resource of volumetric statistics from a large homogenous stroke population to researchers interested in potential selection of particular sequences and series of time points for further investigation.
Supplementary Material
Acknowledgments
Sources of Funding
This research was supported by the Division of Intramural Research of the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Footnotes
Disclosures
None.
References
- 1.Warach S. Tissue viability thresholds in acute stroke: the 4-factor model. Stroke. 2001;32:2460–2461. [PubMed] [Google Scholar]
- 2.Thijs VN, Lansberg MG, Beaulieu C, Marks MP, Moseley ME, Albers GW. Is early ischemic lesion volume on diffusion-weighted imaging an independent predictor of stroke outcome? A multivariate analysis. Stroke. 2000;31:2597–2602. doi: 10.1161/01.str.31.11.2597. [DOI] [PubMed] [Google Scholar]
- 3.Baird AE, Dambrosia J, Janket S, Eichbaum Q, Chaves C, Silver B, Barber PA, Parsons M, Darby D, Davis S, Caplan LR, Edelman RR, Warach S. A three-item scale for the early prediction of stroke recovery. Lancet. 2001;357:2095–2099. doi: 10.1016/s0140-6736(00)05183-7. [DOI] [PubMed] [Google Scholar]
- 4.Arenillas JF, Rovira A, Molina CA, Grive E, Montaner J, Alvarez-Sabin J. Prediction of early neurological deterioration using diffusion- and perfusion-weighted imaging in hyperacute middle cerebral artery ischemic stroke. Stroke. 2002;33:2197–2205. doi: 10.1161/01.str.0000027861.75884.df. [DOI] [PubMed] [Google Scholar]
- 5.Beaulieu C, de Crespigny A, Tong DC, Moseley ME, Albers GW, Marks MP. Longitudinal magnetic resonance imaging study of perfusion and diffusion in stroke: evolution of lesion volume and correlation with clinical outcome. Ann Neurol. 1999;46:568–578. doi: 10.1002/1531-8249(199910)46:4<568::aid-ana4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.van Everdingen KJ, van der Grond J, Kappelle LJ, Ramos LMP, Mali WPTM. Diffusion-weighted magnetic resonance imaging in acute stroke. Stroke. 1998;29:1783–1790. doi: 10.1161/01.str.29.9.1783. [DOI] [PubMed] [Google Scholar]
- 7.Warach S, Pettigrew LW, Dashe JF, Pullicino P, Lefkowitz DM, Sabounjian L, Harnett K, Schwiderski U, Gammans R Citicholine 010 Investigators. Effect of citicholine on ischemic lesions as measured by diffusion-weighted magnetic resonance imaging. Ann Neurol. 2000;48:713–722. [PubMed] [Google Scholar]
- 8.Hacke W, Albers G, Al-Rawi Y, Bogousslavsky J, Davalos A, Eliasziw M, Fischer M, Furlan A, Kaste M, Lees KR, Soehngen M, Warach S. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS) Stroke. 2005;36:66–73. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- 9.Warach S, Kaufman D, Chiu D, Devlin T, Luby M, Rashid A, Clayton L, Kaste M, Lees KR, Sacco R, Fisher M. Effect of the glycine antagonist gavestinel on cerebral infarcts in acute stroke patients, a randomized placebo-controlled trial: the GAIN MRI Substudy. Cerebrovasc Dis. 2006;21:106–111. doi: 10.1159/000090208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martel AL, Allder SJ, Delay GS, Morgan PS, Moody AR. Measurement of infarct volume in stroke patients using adaptive segmentation of diffusion weighted MR images. Medical Image Computing and Computer-Assisted Intervention—MICCAI ’99, LNCS. 1999;1679:22–31. [Google Scholar]
- 11.Ritzl A, Meisel S, Wittsack HJ, Fink GR, Siebler M, Modder U, Seitz RJ. Development of brain infarct volume as assessed by magnetic resonance imaging (MRI): follow-up of diffusion-weighted MRI lesions. J Magn Reson Imaging. 2004;20:201–207. doi: 10.1002/jmri.20096. [DOI] [PubMed] [Google Scholar]
- 12.Baird AE, Benfield A, Schlaug G, Siewert B, Lovblad KO, Edelman RR, Warach S. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol. 1997;41:581–589. doi: 10.1002/ana.410410506. [DOI] [PubMed] [Google Scholar]
- 13.Barber PA, Darby DG, Desmond PM, Yang Q, Gerraty RP, Jolley D, Donnan GA, Tress BM, Davis SM. Prediction of stroke outcome with echoplanar perfusion- and diffusion-weighted MRI. Neurology. 1998;51:418–426. doi: 10.1212/wnl.51.2.418. [DOI] [PubMed] [Google Scholar]
- 14.Chalela JA, Kang DW, Luby M, Ezzeddine M, Latour LL, Todd JW, Dunn B, Warach S. Early magnetic resonance imaging findings in patients receiving tissue plasminogen activator predict outcome: insights into the pathophysiology of acute stroke in the thrombolysis era. Ann Neurol. 2004;55:105–112. doi: 10.1002/ana.10781. [DOI] [PubMed] [Google Scholar]
- 15.Lee DK, Kim JS, Kwon SU, Yoo SH, Kang DW. Lesion patterns and stroke mechanism in atherosclerotic middle cerebral artery disease: early diffusion-weighted imaging study. Stroke. 2005;36:2583–2588. doi: 10.1161/01.STR.0000189999.19948.14. [DOI] [PubMed] [Google Scholar]
- 16.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;I:307–310. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.