Abstract
Tau proteins play a role in the stabilization of microtubules, but in pathological conditions, tauopathies, tau is modified by phosphorylation and can aggregate into aberrant aggregates. These aggregates could be toxic to cells, and different cell models have been used to test for compounds that might prevent these tau modifications. Here, we have used a cell model involving the overexpression of human tau in human embryonic kidney 293 cells. In human embryonic kidney 293 cells expressing tau in a stable manner, we have been able to replicate the phosphorylation of intracellular tau. This intracellular tau increases its own level of phosphorylation and aggregates, likely due to the regulatory effect of some growth factors on specific tau kinases such as GSK3. In these conditions, a change in secreted tau was observed. Reversal of phosphorylation and aggregation of tau was found by the use of lithium, a GSK3 inhibitor. Thus, we propose this as a simple cell model to study tau pathology in nonneuronal cells due to their viability and ease to work with.
Keywords: tau, phosphorylation, aggregation, model, pathology, Alzheimer’s disease, HEK293 cells
Introduction
Tau is modified by phosphorylation in pathological conditions known as tauopathies. Then, phosphorylated tau can aggregate into aberrant insoluble polymers called neurofibrillary tangles (NFTs).1 NFTs consist of paired helical filaments and straight filaments of tau and can be formed from all six isoforms of tau.2 In vitro, numerous kinases have been shown to phosphorylate tau, although the number that is physiologically relevant is significantly lower.3 Dephosphorylated tau has been demonstrated to promote microtubule assembly and is, therefore, tau in its natural, healthy state.4,5 The amount of NFTs in the brain has been shown to correlate with cognitive decline in diseases such as Alzheimer disease (AD).6 In some tauopathies such as AD, there is an increase not only in the amount of phosphorylated tau but, to a lesser extent, also in the amount of total tau.7
Among scholars, there is a debate whether NFTs themselves are toxic or, rather, if toxicity is caused mainly by the hyperphosphorylated tau.8,9 What is known is that NFTs are formed by hyperphosphorylated tau, but the process by which aggregation occurs has yet to be described. Numerous factors have been shown to promote aggregation, but the initial trigger of aggregation is still unknown. Because phosphorylated tau is a structural feature of NFTs, it is possible that their existence in cells may trigger the tangle-forming process. Some mouse models have demonstrated a link between phosphorylated tau and the ensuing tau aggregates.5,10
While NFTs are the most common products of tau aggregation and are associated with many different tauopathies, other classes of tau aggregates do exist. Abnormally phosphorylated tau with four repeats can aggregate to form argyrophilic grains and coiled bodies, as seen in argyrophilic grain disease.11 Hirano bodies, which are associated with various neurodegenerative diseases including AD, are intracellular aggregates containing actin, tau, and other microtubule-associated proteins.12 Three-repeat isoforms of tau can accumulate in spherical aggregations in limbic and cortical neurons in the form of Pick bodies.13
Although tauopathies comprise various forms of tau, it is evident that all tauopathies are characterized by the accumulation, phosphorylation, and aggregation of tau. This overexpression and accumulation of tau can also lead to release of tau to the extracellular medium, which is known to be toxic to cells.14,15
The goal of this study is to create a simple model to study tau pathology. This model will be a nonneuronal cell model expressing tau in high amounts, exemplifying the accumulation of tau. We hope to observe the modification of tau by phosphorylation and ultimately the formation of aggregates. If accomplished, we would have created a simple model emulating the three major characteristics of all tauopathies, namely, accumulation, phosphorylation, and aggregation of tau. We propose to use human embryonic kidney 293 (HEK293) cells due to their general stability, viability, and ease of transfection.
Material and Methods
Materials
Antibodies are PHF-1, Tau-7.51, Tau-1, Tau-12, GSK-3β, P-GSK3, and β-actin.
PHF-1 recognizes tau at the phosphorylated residues of serines 396 and 404 and was a kind gift from Dr. Peter Davies (Scotland, UK).16 Tau-7.51 is a generic tau marker as it recognizes all PHF core-derived tau, native soluble tau, and recombinant tau.17 The Tau-7.51 antibody was a kind gift from Dr. Claude Wischik (New York, USA). Tau-1 recognizes tau protein when the serine residues 195, 198, 199, and 202 are dephosphorylated.18 It was obtained from Chemicon. Tau-12 also detects total tau by binding to an epitope that lies between amino acids 9 and 18 on human tau. It was obtained from Abcam. GSK-3β antibody recognizes nonphosphorylated GSK3. It was obtained from transduction. P-GSK3 antibody recognizes both GSK-3α phosphorylated at serine 21 and GSK-3β phosphorylated at serine 9.19 It was obtained from Cell Signaling Technology. β-Actin is used as a loading control. It was obtained from Sigma-Aldrich.
Cell culture and transfection for model of tau expression in HEK293 cells
HEK293 cells were cultured in a supplemented Dulbecco’s modified Eagle’s medium (DMEM) as previously described,20 supplemented with 10% fetal bovine serum, 2 mM glutamine, 1 mM piruvate, 100 U/mL penicillin, 100 U/mL streptomycin, and 0.2 mg/mL Zeocin in a humidified atmosphere of 5% CO2/95% air at 37°C. The supplemented DMEM without the serum or a slight modification of either [addition of lithium chloride (LiCl)] was used in some experiments. Transient transfection with three-repeat tau was done as previously described.20 At varying time intervals after transfection, cells were harvested by scraping, washed in phosphate-buffered saline (PBS), resuspended and homogenized in buffer for Western blotting, or fixed for immunofluorescence analysis. HEK293 tau-expressing cells (expressing tau 3R isoform, a kind gift from Dr. Miguel Medina)20 in a stable manner were grown in DMEM with 0.2 mg/mL Zeocin.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blotting
Proteins for cell extracts (15 µg) were boiled at 100°C for five minutes to denature the proteins. Later, the samples were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis on 10% resolving gels in trisglycine buffer and transferred to a nitrocellulose membrane via electroblotting according to standard procedures. Total amount of transferred proteins was determined by Ponceau S staining. All nitrocellulose membranes were blocked with a buffer comprising 5% nonfat powdered milk in PBS with 0.1% Tween-20. The membranes were then probed with the appropriate monoclonal antibodies to identify the fractionated proteins. The blots were subjected to chemiluminescence and visualized.
Immunocytochemistry
Immunocytochemistry was performed as previously described.21 Briefly, cells fixed on crystals with 4% paraformaldehyde in PBS were washed and preincubated with PBS containing 0.1% Triton X-100 and 1% serum. Monoclonal primary antibodies were added, and the cells were incubated overnight. The cells were then washed with PBS and incubated with fluorescent secondary antibody conjugates. To visualize the nuclei, cells were stained with 4′-6dianino-2-fenilindol (DAPI) (Molecular Probes). Slides were mounted with Fluoromount, and fluorescent images were captured using appropriate filters in a ZEISS vertical microscope Axioskop 2 Plus.
Data analysis
Quantification of immunoreactivity of tau bands obtained from Western blotting was obtained by densitometric scanning using a GS-800 calibrated densitometer and Quantity One for quantification. All the analyses were performed using SPSS for Windows version 17.0. The ratios of protein levels among the treated samples to the controls are expressed as mean ± standard error of the mean, n = 3. The results were analyzed by Student’s unpaired t-test.
Results
Model
In the central nervous system, tau exists in six isoforms with either three or four repeats.5 HEK293 cells were transfected with three-repeat tau (3 + 0) that can be expressed under cytomegalovirus promoter to induce high constitutive expression of tau. Recent literature has demonstrated that tau expressed in HEK293 cells can be modified by phosphorylation in these proliferating cells.22 This study aims to expand this knowledge by exemplifying that a model can be created to mimic the three factors for tau pathology, namely, accumulation, phosphorylation, and aggregation. This model is proposed to be HEK293 cells expressing tau. We propose that this model can be used to study compounds preventing tau pathology.
Transient transfection of 3 + 0 tau in HEK293 cells leads to tau phosphorylation
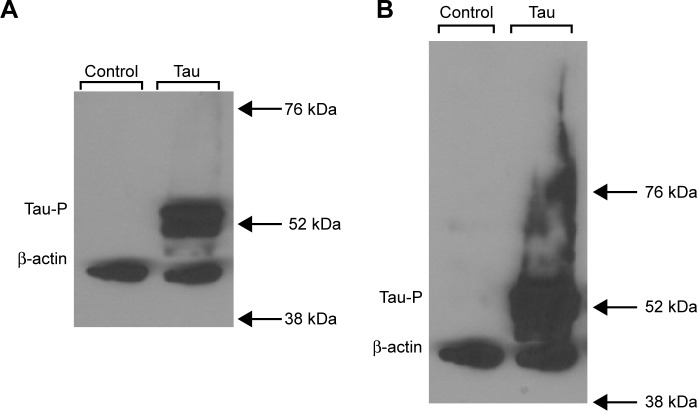
The goal of this experiment was merely to determine if HEK293 cells are able to express tau protein when transfected transiently with tau. In order to this, transient transfection assays were performed with varying quantities of tau (3 + 0) cDNA up to 2.0 µg, and tau expression was determined by subjecting the isolated proteins to Western blot analysis using the primary antibodies PHF-1 and β-actin. PHF-1 is an antibody isolated from aggregated human tau isolated in Dr. Davies’ laboratory23 that could recognize phosphotau not only in disease but also in other states.24 Figure 1 depicts the results of the Western blot analysis. As can be seen, control HEK293 cells do not express tau at all. Transiently transfected cells with tau are able to express phosphorylated tau (Fig. 1A), and this tau also aggregates, as seen by the elongated signal when the Western blot is exposed for a longer period of time (Fig. 1B). Knowing that HEK293 cells are capable of expressing Tau3R, HEK293 cells can be stably transfected with three-repeat tau as previously described (HEK293-Tau3R),20 and this cell line was maintained and used for all future experiments.
Figure 1.
HEK293 cells transiently transfected with three-repeat tau are capable of expressing phosphorylated tau. (A) Western blot analysis of intracellular presence of phosphorylated tau (PHF-1) and total cell count (β-actin) in untransfected (control) HEK293 cells (left) and HEK293 cells transiently transfected with three-repeat isoform tau (tau). No phosphorylated tau is present in control cells, while phosphorylated tau can be seen in transfected cells. (B) Longer exposure of Western blot analysis from (A). It is evident that the phosphorylated tau aggregates, as seen by the long, elongated signal.
Time-course analysis of total and phosphorylated tau in stable HEK293-Tau3R cells
The behavior of tau expressed in a stable manner (HEK293-Tau3R) was tested during cell growth. HEK293-Tau3R cells were placed in dishes (104 cells/dish), and after 12 and 24 hours, levels of tau protein were analyzed by Western blot. Figure 2 shows that tau protein levels (using 7.51 antibody) as well as phosphorylated tau (modified at the site recognized by PHF-1 antibody) are maintained during cell proliferation.
Figure 2.
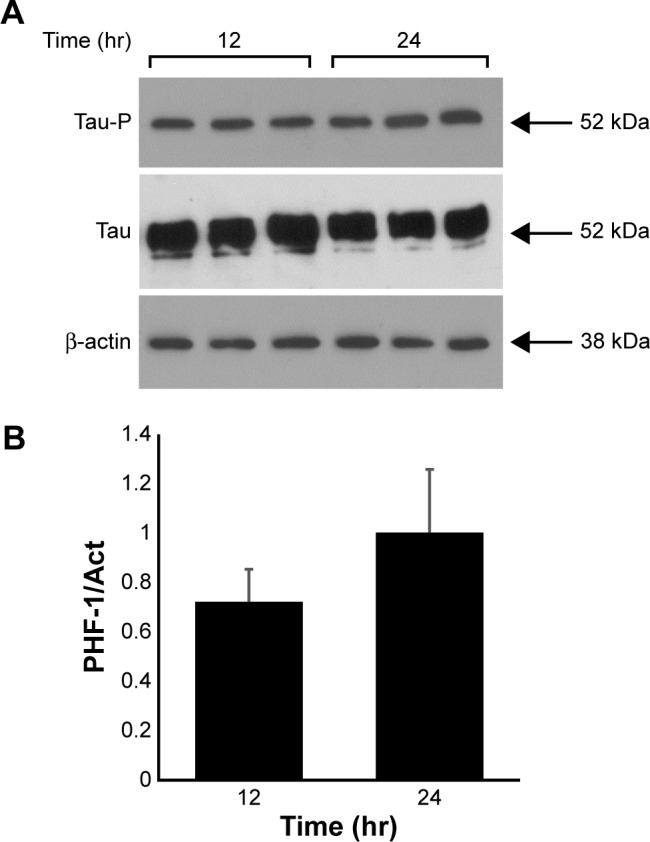
Total tau expression is maintained during cell proliferation in a time-dependent manner in stable transfected HEK293-Tau3R cells. (A) Growth of HEK293-Tau3R cells showing their proliferation rate after 12 and 24 hours after platting. Western blot analysis to test for the presence of total tau protein, using abTau 7.51, and phosphorylated tau, using abPHF-1. (B) Quantification, by densitometry, of the Western blot shown in (A). Overall, it can be seen that the increase in tau amount correlates with the number of HEK293-Tau3R cells.
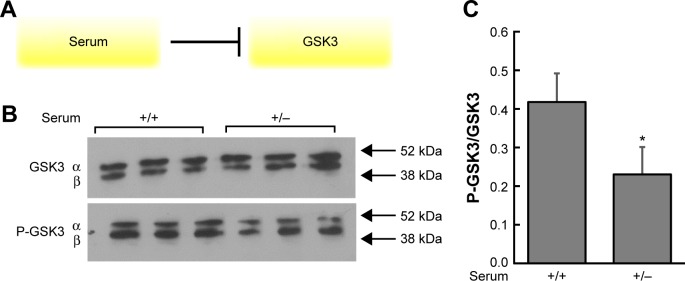
Depletion of serum in cultured cells causes changes in GSK3 phosphorylation
Cells were incubated in two different conditions: (1) nutrient-rich medium with serum for 48 hours (+) and (2) nutrient-rich medium with serum for 24 hours, medium removed and changed to serum-depleted medium for 24 hours (without serum) (−). Figure 3 depicts the Western blot results using primary antibodies to detect GSK-3β and phosphorylated GSK3 in inhibitory domains. The results indicate there are less P-GSK3 and more active (unphosphorylated) GSK3 in the (−) condition, which should lead to higher levels of phosphorylated tau. These results are what one would expect with respect to GSK3 phosphorylation, given the inhibition diagram shown in Figure 3A.
Figure 3.
Growth factors in serum inhibit GSK3 through phosphorylation, and cells with depleted serum display a decrease in P-GSK3. (A) In the presence of serum, GSK3 is phosphorylated. In a medium with serum removed, growth factors such as insulin are not present to phosphorylate GSK3 and unphosphorylated GSK3 is now free to phosphorylate substrates such as tau. (B) It can be seen that overall levels of GSK3 remain the same in both growth conditions. However, there is an increase in phosphorylated GSK3 (bands halfway between the 38 and 52 kDA markers) in cells in the (+, with serum) condition when compared with the (−, without serum) condition. This indicates that there is less P-GSK3 and more active (unphosphorylated) GSK-3β in the (−) condition, which should lead to higher levels of phosphorylated tau. (C) Densitometric analysis of overall levels of P-GSK3/GSK3 confirms that P-GSK3 is higher in the (+) condition relative to overall GSK-3β, compared with the (−) condition. *P < 0.05 versus medium with serum.
Stable overexpression of tau in HEK293 cells causes secretion of tau to the extracellular milieu
Once again, HEK293-Tau3R cells were grown in regular medium for 24 hours. In half of the cohort, the medium was removed and replenished (+), and in the other half, the medium was removed and changed for a serum-depleted medium lacking growth factors (−). After a second 24 hours, the supernatant of each cohort was collected. The supernatants were then subjected to Western blot analysis using the Tau-12 primary antibody to detect the presence of total tau (Fig. 4). The results in Figure 4 demonstrate that tau is secreted to the extracellular milieu in a nutrient-rich (+) medium, whereas tau is not secreted in a serum-depleted medium. These results align with the previous literature, which shows that tau overexpression leads to its secretion.15
Figure 4.
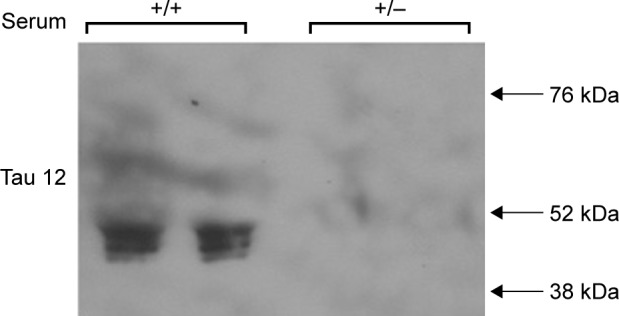
In culture, HEK293-Tau3R cells secrete tau to the extracellular milieu. Western blot analysis of aliquots of the cell media (supernatant) when HEK293-Tau3R cells are grown in either the presence (+) or absence (−) of serum. It is clear that in the (+) conditions, tau can be detected in the serum, indicating that it is secreted from within the cells. In the (−) condition, no tau is detected, indicating an inhibition in this secretion.
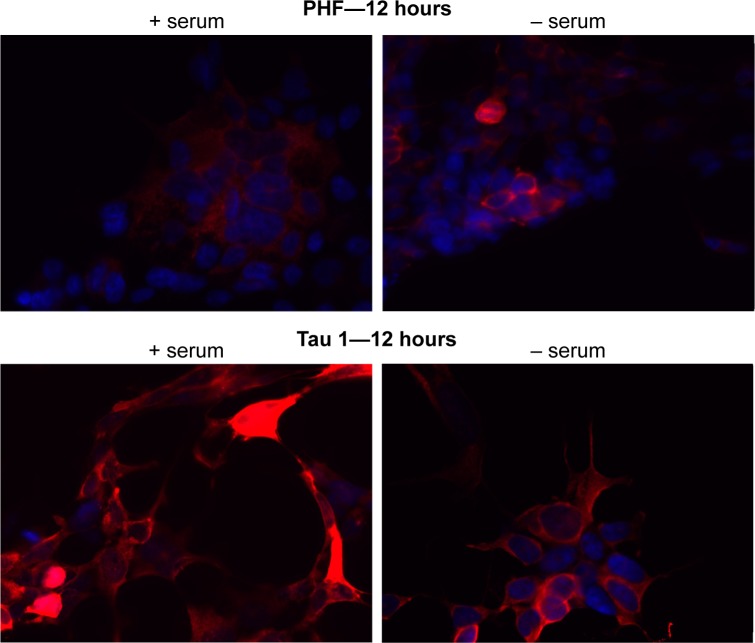
Levels of phosphorylated tau change in the presence or absence of serum
HEK293-Tau3R cells were again grown in vitro in either regular medium (+), which had serum, or a serum-depleted medium (−). At 12 hours, cells were collected and subjected to immunofluorescent staining and analyzed by optical microscopy, as depicted in Figure 5. Primary antibodies were used to detect phosphorylated tau (PHF-1) and dephosphorylated tau (Tau-1). It is clear that phosphorylation of tau is higher when cells are grown in the serum-depleted medium. The opposite is the case for dephosphorylated tau. Dephosphorylated tau is present in highest amounts when cells are grown in a regular nutrient-rich medium (Fig. 5). To confirm this observation, a Western blot analysis was performed.
Figure 5.
Levels of dephosphorylated tau decrease as levels of phosphorylated tau increase. DAPI staining is depicted in blue and primary antibodies (either phosphorylated or dephosphorylated tau) in red. At 12 hours, in the presence of serum, there are high levels of dephosphorylated tau (Tau 1—bottom left panel) relative to phosphorylated tau (PHF-1—top left panel). In contrast, when cells are grown in serum-depleted medium, levels of phosphorylated tau increase (PHF-1—top right panel), and levels of dephosphorylated tau decrease (Tau 1—bottom right panel).
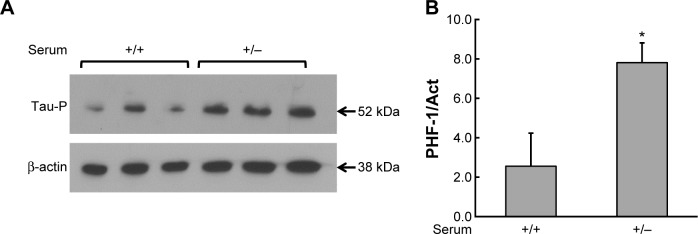
In the absence of serum, tau is phosphorylated by GSK-3β
Western blot was probed with the monoclonal primary PHF-1 antibody to detect tau phosphorylation. Figure 6 depicts the Western blot results, confirming the results shown in Figure 5, showing higher total tau levels of phosphorylated tau in the (+/−) condition than in the (+/+) condition. This suggests that, in this model, tau is indeed phosphorylated likely by GSK3. Additionally, traces of aggregate tau can be seen in the (+/−) condition by slight existence of signal between the 52 and 76 kDa markers.
Figure 6.
Tau phosphorylation increases in cells in a serum-depleted medium. (A) Western blot analysis of tau phosphorylation (PHF-1) when HEK293-Tau3R cells are grown in a nutrient-rich medium for 48 hours (+). Then, the medium was removed in some samples and changed to serum-depleted medium for another additional 24 hours (−). An increase in levels of phosphorylated tau can be seen in the (−) condition. (B) Densitometry analysis of phosphorylated tau of the Western blot bands from (A). There is an increase in phosphorylated tau in the (−) condition, relative to the (+) condition. *P < 0.05 versus medium with serum.
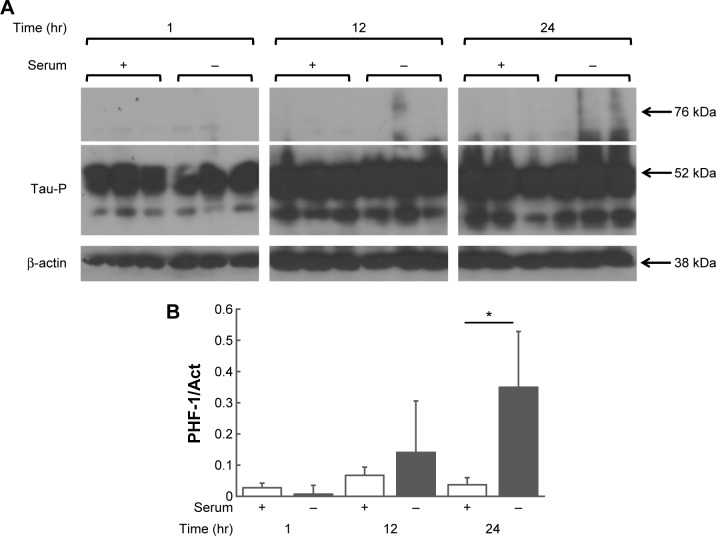
Intracellular aggregates increase when cells are grown in a serum-depleted medium in a time-dependent manner
The earlier experiments were combined and modified to assess the formation of tau aggregates in a time-dependent manner. HEK293-Tau3R cells were again grown in vitro in either regular medium (+), which had serum, or a serum-depleted medium (−). Cells were collected at 1, 12, and 24-hour time points and subjected to Western blot analysis using the primary PHF-1 antibody, which detects phosphorylated tau (Fig. 7). At 24 hours, it is evident that tau aggregates have been formed as shown by the elongated signal above the white line in Figure 7. There is a significant increase in aggregates at 24 hours compared with cells grown in serum-depleted medium.
Figure 7.
Tau aggregation increases in a time-dependent manner, and even more when cells are grown in a serum-depleted medium. (A) Western blot analysis of intracellular presence of phosphorylated tau (PHF-1) in HEK293-Tau3R cells at 1, 12, and 24 hours in regular medium with serum (+) and a medium with serum removed (−). Aggregated tau can be seen at the 12 and 24-hour time points by the elongated signal above the white line. It is most noticeable in the cells grown in serum-depleted medium. (B) Densitometry analysis of aggregated phosphorylated tau of the Western blot bands from (A). There is a significant increase (*P < 0.05) in aggregates at 24 hours compared with cells grown in serum-depleted medium.
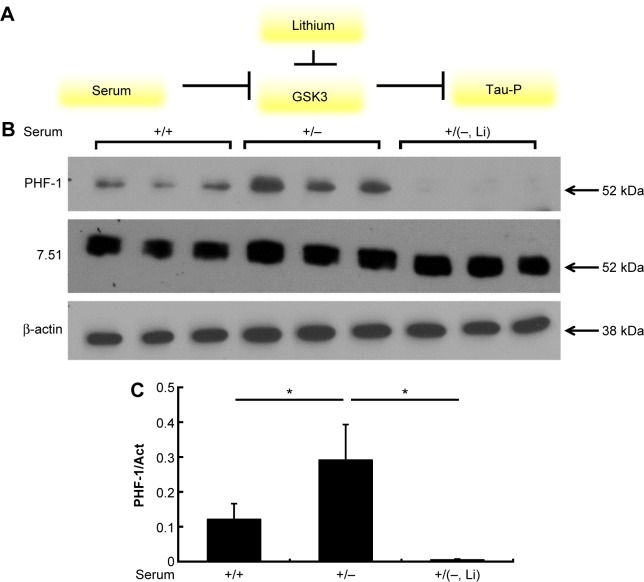
Introduction of lithium results in a decrease in tau phosphorylation and a decrease in tau aggregation
Similar to the previous experiments, two sets of HEK293-Tau3R cells were again grown in vitro for 24 hours in a regular medium. After 24 hours, the medium of the first group of cells was removed and changed for serum-depleted medium (−). In the second group of cells, the medium was also removed and changed for serum-depleted medium, but LiCl (20 mM) was added to the medium as well. Lithium is known to be an inhibiting agent of GSK3 in several ways, including directly or through complex signaling networks.25–27 This is depicted in Figure 8A. The addition of lithium should inhibit GSK3 and disallow for the phosphorylation that the lack of nutrients would generally provide. The Western blot results in Figure 8B and C confirm this. The addition of lithium to the cell medium nearly eradicates the tau phosphorylation that would have been induced by the serum-depleted medium.
Figure 8.
Introduction of lithium to the cell media nearly eradicates intracellular tau phosphorylation. (A) Lithium itself is an inhibitor of GSK3. Therefore, its presence should deactivate GSK3 and disallow for tau phosphorylation. This is similar to GSK3 being inhibited by phosphorylation when cells are grown in a nutrient-rich medium. (B) Western blot analysis of intracellular presence of phosphorylated tau (PHF-1), total tau (Ab7.51), and total cell count (β-actin). It is clear that the introduction of lithium (20 mM) to the cell medium blocks the tau phosphorylation that was induced by the lack of growth factors in the serum-depleted medium. (C) Densitometry analysis of phosphorylated tau of the Western blot bands from (B). *P < 0.05.
From these results, we can make several inferences based on some of our former experiments. As can be seen, tau pathology in cells grown in the medium with lithium should and does emulate tau changes in cells grown in normal (+) medium because lithium may have a similar inhibitory effect on GSK3 as the nutrients in the serum (insulin or related factors) do (Fig. 8). Based on these results, it can be assumed that lithium may cause a decrease in tau aggregation due to the fact that tau phosphorylation may lead to its own aggregation (Fig. 7).
Discussion
Tau proteins are integral to cell microtubule stabilization under healthy conditions, but in pathological conditions, tauopathies, tau becomes phosphorylated and can aggregate into aberrant polymers such as NFTs, which are found in AD. The goal of this project was to create a simple nonneuronal model to study tau pathology using HEK293 cells transfected with three-repeat tau.
Currently, while several animal models of AD exist, no stable cell line has been established to quickly test tau pathology. In our work, we transfected HEK293 cells with three-repeat tau and used Western blot analysis and immunocytochemistry to investigate tau phosphorylation in these cells. Three-repeat tau was used because we were unable to successfully produce a stable transfection expressing four-repeat tau in the same conditions of protein overexpression. A possible explanation is that Tau4R can bind to microtubules with a higher affinity, thus stabilizing the cells and impeding breakdown of microtubule polymers during interphase.28,29
A likely origin of the increase in tau phosphorylation observed in pathological conditions in neurons could be a decrease in the nutrients supplied by the surrounding non-neuronal cells. In cell culture, these nutrients are mainly supplied to the cells by the addition of serum to the cell medium. Because of this, we altered the medium in which the cells were grown by removing the growth factors and observing the changes in phosphorylation. Because specific kinase inhibitors such as lithium can prevent phosphorylation, we tested the effect of lithium addition to the cell medium on tau phosphorylation. If lithium decreased tau phosphorylation, we propose that this model could be extrapolated for other compounds and kinase inhibitors that affect tau phosphorylation.
Overall, we were able to develop a nonneuronal cell model expressing tau that gives rise to tau phosphorylation and ultimately tau aggregation in the cells. As hypothesized, in this model, decreased factors (insulin and related factors) lead to the increase in tau phosphorylation and aggregation, as it is seen in AD. On the other hand, lithium, a GSK3 inhibitor, has been shown to inhibit this induced tau phosphorylation. This model has also been able to reproduce the secretion of tau from within the cells to the extracellular milieu, as has been seen in previous study.15 Recent literature has indicated that when tau is present outside the cells, it is more toxic in its dephosphorylated state; so, this model could be used for future experiments studying this phenomenon.30,31
Because NFTs are universally present in AD and many other tauopathies, this model can be used for more generalized large-scale studies. While we have shown that the model demonstrates the three key factors of tauopathies, namely, accumulation, aggregation, and phosphorylation, we have also shown that the model effectively replicates GSK3’s effects on tau via analysis of GSK3 itself as well as lithium, a GSK3 inhibitor. Therefore, this model can also be used to detect GSK3 inhibitors and potentially other proteins both upstream and downstream that could be involved in tau phosphorylation.
Future research is necessary to establish a clear link between tau aggregation and its phosphorylation. There may be factors upstream of tau aggregation, but downstream of GSK-3β phosphorylation, which may play a role in the forming of NFTs. It would also be prudent to further analyze how exactly tau phosphorylation and aggregation directly affects tau secretion. Overall, we believe this HEK293-Tau3R cell line to be a simple yet effective model to study tau pathology.
Acknowledgments
The authors thank Raquel Cuadros and Esther García García for their laboratory support.
Footnotes
ACADEMIC EDITOR: Lora Talley Watts, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1185 words, excluding any confidential comments to the academic editor.
FUNDING: Our laboratory is funded by grants from Ministerio de Educación y Ciencia, Comunidad de Madrid, and CIBERNED. The authors also acknowledge institutional support from Fundación Ramón Areces. ALH is a recipient of the Fulbright Scholarship. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived and designed the experiments: JÁ and FH. Analyzed the data: ALH. Wrote the first draft of the manuscript: ALH. Contributed to the writing of the manuscript: ALH, JÁ, and FH. Agreed with manuscript results and conclusions: ALH, JÁ, and FH. Jointly developed the structure and arguments for the paper: ALH, JÁ, and FH. Made critical revisions and approved the final version: ALH, JÁ, and FH. All the authors reviewed and approved the final manuscript.
REFERENCES
- 1.Alonso AdC, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K. Hyperphosphorylation induces self-assembly of τ into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci U S A. 2001;98(12):6923–6928. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goedert M, Spillantini M, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 3.Stoothoff WH, Johnson GV. Tau phosphorylation: physiological and pathological consequences. Biochim Biophys Acta. 2005;1739(2–3):280–297. doi: 10.1016/j.bbadis.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Cleveland DW, Hwo S-Y, Kirschner MW. Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol. 1977;116(2):227–247. doi: 10.1016/0022-2836(77)90214-5. [DOI] [PubMed] [Google Scholar]
- 5.Avila J, Lucas JJ, Perez M, Hernandez F. Role of tau protein in both physiological and pathological conditions. Physiol Rev. 2004;84(2):361–384. doi: 10.1152/physrev.00024.2003. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Braak E. Cognitive impairment in Parkinsons disease: amyloid plaques, neurofibrillary tangles, and neuropil threads in the cerebral cortex. J Neural Transm Park Dis Dement Sect. 1990;2(1):45–57. doi: 10.1007/BF02251245. [DOI] [PubMed] [Google Scholar]
- 7.Khatoon S, Grundke-Iqbal I, Iqbal K. Brain levels of microtubule-associated protein τ are elevated in Alzheimer’s disease: a radioimmuno-slot-blot assay for nanograms of the protein. J Neurochem. 1992;59(2):750–753. doi: 10.1111/j.1471-4159.1992.tb09432.x. [DOI] [PubMed] [Google Scholar]
- 8.Avila J. Alzheimer disease: caspases first. Nat Rev Neurol. 2010;6(11):587–588. doi: 10.1038/nrneurol.2010.157. [DOI] [PubMed] [Google Scholar]
- 9.de Calignon A, Fox LM, Pitstick R, et al. Caspase activation precedes and leads to tangles. Nature. 2010;464(7292):1201–1204. doi: 10.1038/nature08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noble W, Planel E, Zehr C, et al. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci U S A. 2005;102(19):6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrer I, Santpere G, van Leeuwen FW. Argyrophilic grain disease. Brain. 2008;131(6):1416–1432. doi: 10.1093/brain/awm305. [DOI] [PubMed] [Google Scholar]
- 12.Hirano A. Hirano bodies and related neuronal inclusions. Neuropathol Appl Neurobiol. 1994;20(1):3–11. doi: 10.1111/j.1365-2990.1994.tb00951.x. [DOI] [PubMed] [Google Scholar]
- 13.Goedert M. Neurofibrillary pathology of Alzheimer’s disease and other tauopathies. Prog Brain Res. 1998;117:287–306. doi: 10.1016/s0079-6123(08)64022-4. [DOI] [PubMed] [Google Scholar]
- 14.Avila J, Simon D, Diaz-Hernandez M, Pintor J, Hernández F. Sources of extracellular tau and its signaling. J Alzheimer Dis. 2014;40(suppl 1):S7–S15. doi: 10.3233/JAD-131832. [DOI] [PubMed] [Google Scholar]
- 15.Simon D, Garcia-Garcia E, Royo F, Falcón-Pérez JM, Avila J. Proteostasis of tau. Tau overexpression results in its secretion via membrane vesicles. FEBS Lett. 2012;586(1):47–54. doi: 10.1016/j.febslet.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Otvos L, Feiner L, Lang E, Szendrei GI, Goedert M, Lee VM. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res. 1994;39(6):669–673. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- 17.Novak M, Jakes R, Edwards PC, Milstein C, Wischik CM. Difference between the tau protein of Alzheimer paired helical filament core and normal tau revealed by epitope analysis of monoclonal antibodies 423 and 7.51. Proc Natl Acad Sci U S A. 1991;88(13):5837–5841. doi: 10.1073/pnas.88.13.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szendrei G, Lee VY, Otvos L. Recognition of the minimal epitope of monoclonal antibody Tau-1 depends upon the presence of a phosphate group but not its location. J Neurosci Res. 1993;34(2):243–249. doi: 10.1002/jnr.490340212. [DOI] [PubMed] [Google Scholar]
- 19.Fang X, Yu SX, Lu Y, Bast RC, Jr, Woodgett JR, Mills GB. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci U S A. 2000;97(22):11960–11965. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santa-Maria I, Hernandez F, Del Rio J, Moreno FJ, Avila J. Tramiprosate, a drug of potential interest for the treatment of Alzheimers disease, promotes an abnormal aggregation of tau. Mol Neurodegener. 2007;2(1):17. doi: 10.1186/1750-1326-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salinero O, Moreno-Flores M, Ceballos M, Wandosell F. β-Amyloid peptide induced cytoskeletal reorganization in cultured astrocytes. J Neurosci Res. 1997;47(2):216–223. [PubMed] [Google Scholar]
- 22.Camero S, Benítez MJ, Cuadros R, Hernández F, Avila J, Jiménez JS. Thermodynamics of the interaction between Alzheimer’s disease related tau protein and DNA. PLoS One. 2014;9(8):e104690. doi: 10.1371/journal.pone.0104690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberg SG, Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1990;87(15):5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Planel E, Bretteville A, Liu L, et al. Acceleration and persistence of neurofibrillary pathology in a mouse model of tauopathy following anesthesia. FASEB J. 2009;23(8):2595–2604. doi: 10.1096/fj.08-122424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freland L, Beaulieu JM. Inhibition of GSK3 by lithium, from single molecules to signaling networks. Front Mol Neurosci. 2012;5:14. doi: 10.3389/fnmol.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong M, Chen DC, Klein PS, Lee VM. Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J Biol Chem. 1997;272(40):25326–25332. doi: 10.1074/jbc.272.40.25326. [DOI] [PubMed] [Google Scholar]
- 27.Muñoz-Montaño JR, Moreno FJ, Avila J, Diaz-Nido J. Lithium inhibits Alzheimer’s disease-like tau protein phosphorylation in neurons. FEBS Lett. 1997;411(2):183–188. doi: 10.1016/s0014-5793(97)00688-1. [DOI] [PubMed] [Google Scholar]
- 28.Butner K, Kirschner MW. Tau protein binds to microtubules through a flexible array of distributed weak sites. J Cell Biol. 1991;115(3):717–730. doi: 10.1083/jcb.115.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gustke N, Trinczek B, Biernat J, Mandelkow EM, Mandelkow E. Domains of tau protein and interactions with microtubules. Biochemistry. 1994;33(32):9511–9522. doi: 10.1021/bi00198a017. [DOI] [PubMed] [Google Scholar]
- 30.Gómez-Ramos A, Díaz-Hernández M, Cuadros R, Hernández F, Avila J. Extracellular tau is toxic to neuronal cells. FEBS Lett. 2006;580(20):4842–4850. doi: 10.1016/j.febslet.2006.07.078. [DOI] [PubMed] [Google Scholar]
- 31.Avila J. Intracellular and extracellular tau. Front Neurosci. 2010;4:49. doi: 10.3389/fnins.2010.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]