Abstract
Calcium is an important second messenger and it is widely recognized that acute lung injury (ALI) is often caused by oscillations of cytosolic free Ca2+. Previous studies have indicated that the activation of transient receptor potential-vanilloid (TRPV) channels and subsequent Ca2+ entry initiates an acute calcium-dependent permeability increase during ALI. However, whether seawater exposure induces such an effect through the activation of TRPV channels remains unknown. In the current study, the effect of calcium, a component of seawater, on the inflammatory reactions that occur during seawater drowning-induced ALI, was examined. The results demonstrated that a high concentration of calcium ions in seawater increased lung tissue myeloperoxidase activity and the secretion of inflammatory mediators, such as tumor necrosis factor-α (TNF-α) and interleukin (IL)-1β and IL-6. Further study demonstrated that the seawater challenge elevated cytosolic Ca2+ concentration, indicated by [Ca2+]c, by inducing calcium influx from the extracellular medium via TRPV1 channels. The elevated [Ca2+c] may have resulted in the increased release of TNF-α and IL-1β via increased phosphorylation of nuclear factor-κB (NF-κB). It was concluded that a high concentration of calcium in seawater exacerbated lung injury, and TRPV1 channels were notable mediators of the calcium increase initiated by the seawater challenge. Calcium influx through TRPV1 may have led to greater phosphorylation of NF-κB and increased release of TNF-α and IL-1β.
Keywords: transient receptor potential-vanilloid 1, lung injury, calcium oscillation, inflammation
Introduction
Drowning is a major, but often neglected, public health problem (1). Drowning is the third leading cause of accidental mortality, with >388,000 mortalities per year worldwide (2). Water inhalation can induce acute lung injury (ALI) and acute respiratory distress syndrome by disturbing the barrier function of alveolar epithelium, leading to lung edema and inflammatory reactions (3–5). Previous research has demonstrated that intracellular calcium (Ca2+) oscillations are vital in ALI, as they lead to reduced integrity of the blood-air barrier (6,7), increased NF-κB activation and lung inflammation (7). It was also indicated that intracellular Ca2+ oscillations are dependent on extracellular Ca2+ (7), and an increased infiltration coefficient can be prevented in low-Ca2+ lung perfusate (8). Therefore, modulating calcium signaling may provide beneficial effects in cases of ALI induced by seawater aspiration.
Transient receptor potential-vanilloid (TRPV) is a family of plasma membrane ion channels consisting of seven members (TRPV1-7) (9). They are a notable receptor family that respond to a wide variety of endogenous and exogenous stimuli, including temperature (10,11), proinflammatory mediators (11–13), pH (11,14), stretch (15) and osmolality (11,16,17). Two members of the TRPV family (TRPV1 and 4) have been identified to participate in ALI (15,18). As a cell membrane-bound Ca2+ channel, activation of TRPV1 is an important factor in intracellular Ca2+ oscillations following exposure to cytokines, abnormal pH and osmolality (12,18–20). TRPV4 is also a Ca2+-permeable cation channel gated by a variety of external factors (17), including heat (21), membrane stretch, osmotic changes (22) and mechanical stimuli (23). Previous studies have indicated that the activation of TRPV4 and subsequent Ca2+ entry initiated an acute calcium-dependent permeability increase following lung injury resulting from 14,15-epoxyeicosatrienoic acid (14,15-EET), 5,6-EET and 8,9-EET (6) exposure, in addition to ventilator-induced lung injury (15). These results imply that TRPV is important during ALI and is a potential therapeutic target for the treatment of lung injury. Whether seawater exposure induces ALI through the activation of TRPV remains unknown. The role of the interaction between hypertonia and high calcium concentration in seawater instillation-induced ALI required further investigation.
In the present study, the hypothesis that seawater instillation induces ALI through the activation of TRPV and subsequent intracellular calcium oscillations was analyzed and the interaction between hypertonia and high calcium concentration during ALI was examined.
Materials and methods
Reagents
The following reagents were used in the current study: Monoclonal mouse β-actin (1:8,000 dilution; cat. no. A5441; Sigma-Aldrich, St. Louis, MO, USA); monoclonal rabbit phospho-NF-κB p65 (Ser536; 1:500 dilution; cat. no. 3033; Cell Signaling Technology, Inc., Danvers, MA, USA) and monoclonal rabbit NF-κB p65 (1:500 dilution; cat. no. 8242; Cell Signaling Technology, Inc.) antibodies. Acti-stain 488 phalloidin (Cytoskeleton, Inc., Denver, USA); enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Inc., Minneapolis, MN, USA); myeloperoxidase (MPO) activity assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China); Fluo-3,AM and Pluronic F-127 (Biotium, Inc., Hayward, CA, USA); ruthenium red, capsazepine and BAPTA-AM (Abcam); HC067047 and EGTA (Sigma-Aldrich). Seawater was prepared according to the major compositions of the East China Sea provided by the Chinese Ocean Bureau (osmolality, 1,300 mmol/l; pH 8.2; NaCl, 26.518 g/l; MgSO4, 3.305 g/l; MgCl2, 2.447 g/l; CaCl2, 1.141 g/l; KCl, 0.725 g/l; NaHCO3, 0.202 g/l; NaBr, 0.083 g/l).
Cell culture and treatment
The epithelial cell line A549 (American Type Culture Collection, Rockville, MD, USA), derived from lung adenocarcinoma was cultured in RPMI 1640 medium (HyClone Laboratories, Inc., Logan, UT, USA) supplemented with 10% fetal bovine serum (Zhejiang Tianhang Biotechnology Co., Ltd., Huzhou, China) at 37°C and in a 5% CO2 atmosphere. The cells were treated with seawater by addition to the culture medium at a 25% volume ratio. The cells and supernatant were harvested 4 h after exposure to seawater.
Single cell [Ca2+]c measurement
A549 cells grown in culture were exposed to culture medium containing the Ca2+-sensitive fluorescent indicator, Fluo-3,AM (5 µM), and a nonionic dispersing agent, Pluronic F-127 (0.025%), for 30 min at 37°C. Following the loading period, the medium was replaced, and the cells were incubated for a further 30 min. Fluorescence intensity, reflecting the concentration of [Ca2+]c, was recorded by confocal laser-scanning microscopy (FV10; Olympus, Tokyo, Japan). The re-addition of calcium was performed by adding CaCl2. The liquid above the cells was replaced with the seawater with a normal concentration of CaCl2 (1.141 g/l). Images were captured for quantification. The groups and their treatments were as follows: Control group: no extra treatment with the exception of loading the Fluo-3; seawater group: Following loading with Fluo-3 AM (5 µM), the cells were exposed to seawater challenge at the predetermined time; BAPTA-AM group: Cells were loaded with Flou-3 AM (5 µM) and BAPTA-AM (5 µM) 30 min prior to exposure to seawater challenge at the predetermined time; EGTA group: Following loading with Fluo-3 AM (5 µM), the cells were exposed to Ca2+-free seawater with 1 mM EGTA at the predetermined time; ruthenium red group: Cells were loaded with Flou-3 AM (5 µM) and ruthenium red (3 µM) 30 min prior to exposure to seawater challenge at the predetermined time; HCO67047 group: Cells were loaded with Flou-3 AM (5 µM) and HCO67047 (1 µM) 30 min prior to exposure to seawater challenge at the predetermined time; capsazepine group: Cells were loaded with Flou-3 AM (5 µM) and capsazepine (10 µM) 30 min prior to exposure to seawater challenge at the predetermined time.
ELISA assay
Levels of TNF-α, IL-1β and IL-6 in lung tissues were determined using the ELISA kits. Lung tissues were homogenized in cool phosphate-buffered saline (PBS) at a 1:5 ratio of lung tissue to PBS. Assays were conducted according to the manufacturer's instructions. The groups and their treatments were as follows: Control group: Cells were treated with 25% PBS and 75% RPMI-1640 for 4 h; seawater group: Cells were exposed to 25% seawater and 75% RPMI-1640 for 4 h; BAPTA-AM group: Cells were loaded with BAPTA-AM (5 µM) for 30 min and then exposed to 25% seawater and 75% RPMI-1640 for 4 h; EGTA group: Cells were exposed to 25% Ca2+-free seawater with 1 mM EGTA and 75% RPMI-1640 for 4 h; seawater + PDTC group: Cells were loaded with PDTC (200 µM) for 30 min and then exposed to 25% seawater and 75% RPMI-1640 for 4 h; seawater + HCO67047 group: Cells were loaded with HCO67047 (1 µM) for 30 min and then exposed to 25% seawater and 75% RPMI-1640 for 4 h; seawater + capsazepine group: Cells were loaded with capsazepine (10 µM) for 30 min and then exposed to 25% seawater and 75% RPMI-1640 for 4 h.
Animal procedures
Adult male Sprague-Dawley (SD) rats weighing 180–200 g were purchased from the Laboratory Animal Centre of the Fourth Military Medical University (Xi'an, China) and housed under a light/dark cycle of 12/12 h, with standard food and water provided ad libitum. All procedures used in the present study were approved by the Animal Care and Use Committee of the Fourth Military Medical University. Rats were anesthetized with 1.5% sodium pentobarbital (50 mg/kg; Sigma-Aldrich) followed by intratracheal administration of seawater (4 ml/kg body weight) into the lungs within 4 min via a 20-gauge intravenous catheter through the trachea. The rats were maintained in a supine position with a 30° head-up tilt during the experiments. The rats were euthanized with a sodium pentobarbital overdose (500 mg/kg) at the predetermined points of time and then the lungs were harvested for further experiments. SD rats were randomly assigned into the following four groups (n=4): Control group: Rats with no intervention; seawater group: Rats were intratracheally administered seawater (4 ml/kg body weight) into the lungs within 4 min via a 20-gauge intravenous catheter through the trachea; Ca2+-free seawater group: Rats were intratracheally administered Ca2+-free seawater (4 ml/kg body weight) into the lungs; isotonic seawater group: Rats were intratracheally administered isotonic seawater with no change in calcium concentration.
Western analysis
Protein was extracted from the lower right lung by homogenization and centrifugation at 10,000 × g for 20 min at 4°C. Proteins were separated by 10% SDS-PAGE (120 v; Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and were transferred onto a nitrocellulose membrane (Pall Corp., Washington, NY, USA). The membranes were blocked with 5% non-fat dry milk in Tris-buffered saline (TBS) and probed with the antibodies against phospho-NF-κB p65 (Ser536; dilution, 1:500), NF-κB p65 (dilution, 1:500) and β-actin (dilution, 1:8,000). Following incubation with the primary antibody overnight, the membranes were washed with TBS-Tween 20 and incubated with horseradish peroxidase-conjugated secondary antibody (dilution, 1:10,000). Target proteins were detected by the enhanced chemiluminescence detection system (WesternBright ECL-spray ; Advansta, Inc., Menlo Park, CA, USA). Four samples from each group were used for densitometry analysis (version 4.6.2; Quantity One software; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Confocal visualization of F-actin
A549 cells were cultured on coverslips and exposed to the different treatment conditions, including the control (no treatment), seawater-treated, seawater + BAPTA-AM and sewater + capsazepine groups. Following treatments, cells were fixed and permeabilized at room temperature, washed with PBS and incubated with 100 nM Acti-stain 488 phalloidin in the dark for 30 min. The nuclei were visualized by DAPI (4′,6-diamidino-2-phenylindole; Beyotime Institute of Biotechnology, Shanghai, China) staining This stain was excited using a 340 nm laser and detected by confocal laser-scanning microscopy. The groups were treated as described above.
Histopathological evaluation
The lung tissues of the lower lobe of the right lung harvested from each rat were fixed with 4% paraformaldehyde (Sigma-Aldrich) for 24 h and embedded in paraffin and (Sigma-Aldrich) cut into 5-µm sections. The sections were stained with hematoxylin and eosin (Sigma-Aldrich) prior to visualization at ×200 magnification under a light microscope (CX41; Olympus).
MPO activity assay
The extent of neutrophil accumulation in the lung samples was measured by assaying MPO activity. Following homogenization and centrifugation (10,000 × g) of these lung tissues in cool PBS, MPO activity was determined by colorimetric analysis using a SmartSpec Plus spectrophotometer (Bio-Rad, Laboratories, Inc.), according to the manufacturer's instructions. The MPO activity was expressed as U/mg protein.
Evans blue extravasation assessment
Barrier permeability of the lungs was measured by Evans blue extravasation analysis, and 30 min prior to instillation of seawater, Evans blue dye (Sigma-Aldrich; 20 mg/kg) was injected into the rats through the tail vein. Normal saline was injected into the right ventricle immediately prior to euthanization. Once clear fluid was effused from the left atrium, the lower lobe of the right lung was removed. Evans blue dye was extracted from the tissue by incubation of the lung lobes in formamide (Sigma-Aldrich; 3 ml/100 mg) for 24 h. Total Evans blue (µg/g) was calculated using spectrophotometry (620 nm; SmartSpec Plus; Bio-Rad, Laboratories, Inc.).
Bronchoalveolar lavage fluid (BALF) analysis
Following anesthetization of the rats, the lungs were lavaged with 1 ml ice-cold PBS three times. The number of total cells and neutrophils in the BALF was calculated using Wright's staining (Sigma-Aldrich). The cells in the BALF were collected by centrifugation at 2,500 × g, stained with Wright's stain according the manufacturer's instructions and then neutrophils that were dyed pale purple were counted using a cell counting plate.
Statistical analysis
All data are expressed as the mean ± standard deviation. Statistically significant differences between the different groups were assessed using analysis of variance with a Bonferroni post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Seawater challenge elevated cytosolic [Ca2+]c by inducing calcium entry from extracellular medium
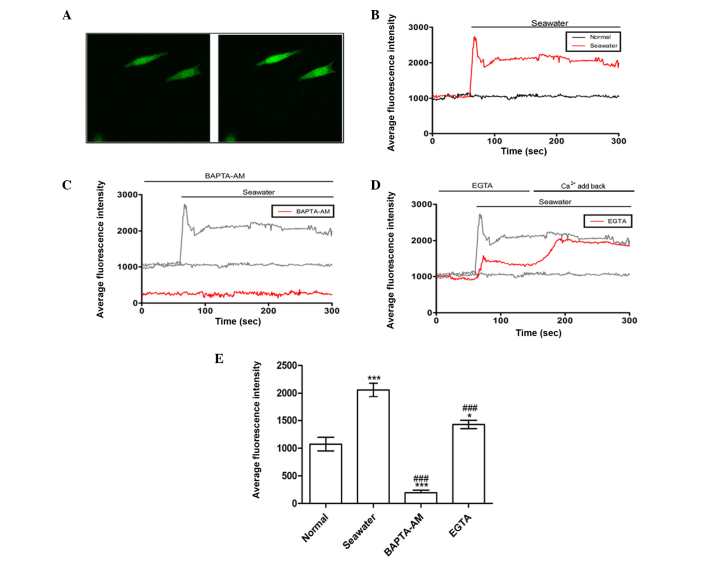
It was hypothesized that seawater inhalation leads to ALI by stimulating Ca2+ entry into the cytosol. In order to address this issue, a series of experiments were performed to examine the effects of seawater composed of modified components on the [Ca2+]c in A549 cells. The effects of seawater challenge on the [Ca2+]c were recorded using confocal laser-scanning microscopy to measure the fluorescent Ca2+-sensitive indicator, Fluo-3,AM, and images were captured for quantitative analysis. A rise in the fluorescence intensity indicated an increase in [Ca2+]c. As presented in Fig. 1A and B, seawater exposure evoked a rapid [Ca2+]c increase and the increase reached a maximal value within 30 sec, followed by a trifling recovery and a sustained plateau. Subsequently, a parallel experiment in which cells were treated with 5 µM BAPTA-AM (a selective intracellular Ca2+ chelator) for 40 min prior to seawater exposure demonstrated that pretreatment with a chelator completely abolished the elevation of [Ca2+]c induced by seawater (Fig. 1C).
Figure 1.
Effect of seawater exposure on the average fluorescence intensity in A549 cells. (A) Seawater exposure evoked a rapid fluorescence intensity increase that represented [Ca2+]c elevation. Left panel, A549 cells without stimulation; right panel, A549 cells exposed to seawater. (B) Quantification of fluorescence intensity following seawater treatment. (C) Pretreatment with BAPTA-AM may reverse the elevation of [Ca2+]c. (D) Chelation of calcium with EGTA reduced the rise of [Ca2+]c triggered by seawater. (E) Average fluorescence intensities for all groups. *P<0.05 and ***P<0.001 vs. the normal group; ###P<0.001 vs. the seawater group.
Next, to clarify the source of Ca2+ ions, experiments were performed to evaluate whether seawater challenge elevated [Ca2+]c via release of Ca2+ from intracellular stores or influx of extracellular Ca2+. As presented in Fig. 1D, an extracellular Ca2+ chelator, EGTA, decreased the increase of [Ca2+]c induced by seawater. The inhibitory effect was cancelled by re-addition of Ca2+ (Fig. 1D). Thus, it was concluded that the elevation of [Ca2+]c evoked by seawater exposure was predominantly accomplished by increasing Ca2+ influx from extracellular sources. The effects of the different treatments on [Ca2+]c are summarized in Fig. 1E.
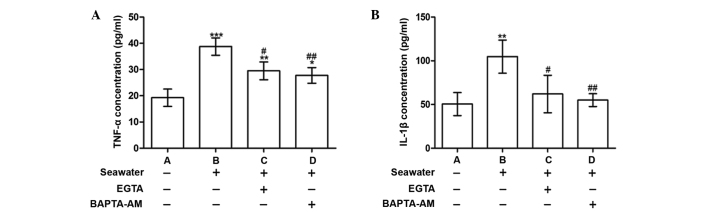
Calcium chelation by EGTA or BAPTA-AM reduced the release of inflammatory mediators following seawater exposure
To explore the consequences of [Ca2+]c elevation, the concentrations of the proinflammatory cytokines, TNF-α and IL-1β, were measured in the supernatant. Following seawater exposure, the levels of TNF-α and IL-1β were significantly increased compared with the control group (P<0.001 and P<0.01, respectively; Fig. 2). These alterations were alleviated when cells were treated with BAPTA-AM or EGTA (Fig. 2).
Figure 2.
Effect of chelation of calcium on the secretion of TNF-α and IL-1β in A549 cells. (A) TNF-α and (B) IL-1β concentrations following control, seawater, EGTA and BAPTA-AM treatments. *P<0.05, **P<0.01 and ***P<0.001 vs. the untreated control; #P<0.05 and ##P<0.01 vs. seawater treatment. TNF-α, tumor necrosis factor α; IL-1β; interleukin 1β.
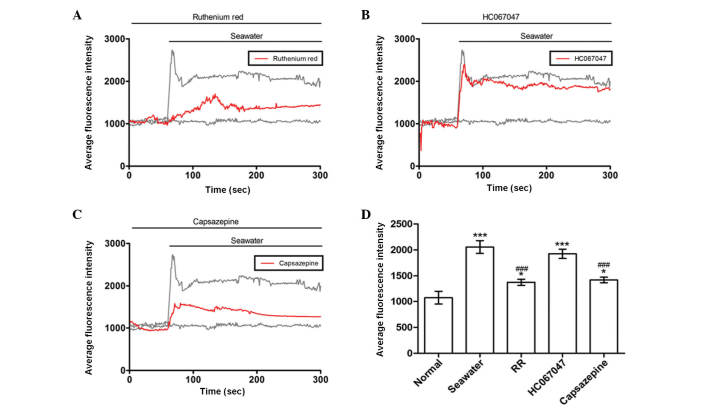
Seawater challenge evoked extracellular Ca2+ influx by activating TRPV1 channels
It was reported that TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs (6). The current study investigated whether similar membrane transport pathways were required for seawater exposure-induced extracellular Ca2+ influx. Subsequent experiments were then performed to identify the membrane transport pathway that mediated Ca2+ influx. Cells were treated with a range of inhibitors of potential Ca2+ entry channels 30 min prior to seawater exposure. The TRPV1-6 inhibitor ruthenium red significantly reduced the Ca2+ response to seawater challenge but did not abolish it (Fig. 3A). However, a potent selective TRPV4 antagonist, HC067047, had no observed effect on the seawater-induced increase of [Ca2+]c (Fig. 3B). Notably, the elevated level of the [Ca2+]c response to seawater exposure was reduced by treatment with the TRPV1-specific inhibitor, capsazepine (Fig. 3C). These results suggested that, unlike ventilator-induced lung injury, extracellular Ca2+ influx through TRPV1 channels was crucial to the increase of [Ca2+]c observed in A549 cells following exposure to seawater.
Figure 3.
Fluorescence intensity in A549 cells. Effect of (A) RR, (B) HC067047 and (C) capsazepine treatments on average fluorescence intensity in A549 cells. (D) Summary of average fluorescence intensities of each treatment group. *P<0.05 and ***P<0.001 vs. the normal group, ###P<0.001 vs. the seawater group. RR, ruthenium red.
Seawater exposure induced TNF-α and IL-1β release through TRPV1 activation and NF-κB phosphorylation
To establish the role of TRPV1 in mediating Ca2+ influx and the subsequent inflammatory reactions, the phosphorylation of NF-κB and the concentration of TNF-α and IL-1β in the supernatant were measured. Fig. 4 indicates that NF-κB phosphorylation was increased following seawater stimulation (P<0.001, compared with the control group), whereas capsazepine abolished this effect (P<0.05, compared with the seawater treatment).
Figure 4.
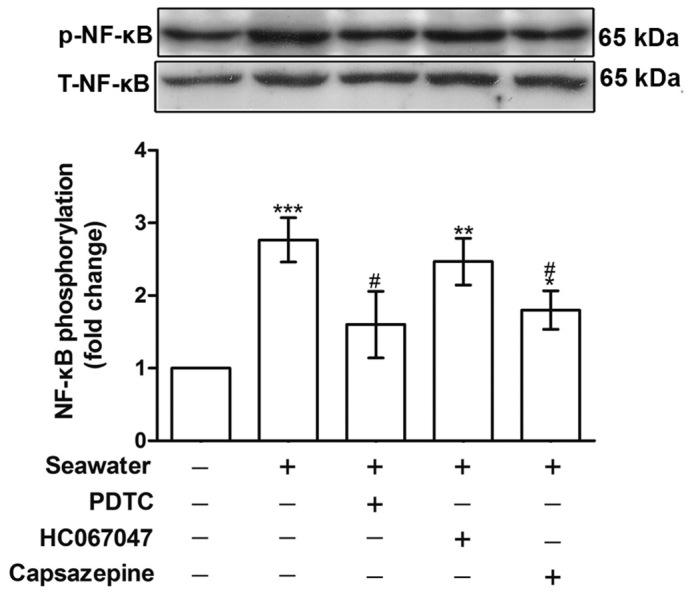
Effect of PDTC, HC067047 and capsazepine treatments on the phosphorylation of NF-κB. *P<0.05, **P<0.01 and ***P<0.001 vs. the control group, #P<0.05 vs. the seawater group. PDTC, pyrrolidine dithiocarbamate; NF-κB, nuclear factor κB; p, phospho; T, total.
To understand how seawater stimulation induces proinflammatory cytokine production, the effects of NF-κB and TRPV1 inhibition were compared. As presented in Fig. 5, cells pretreated with either PDTC (NF-κB inhibitor) or, capsazepine (TRPV1 inhibitor) attenuated the release of TNF-α and IL-1β elicited by the seawater challenge. PDTC and capsazepine inhibited the increase in TNF-α and IL-1β concentrations observed following seawater challenge. By contrast, blockage of TRPV4 channels using HC067047 exhibited no effect on the levels of these cytokines (Fig. 5). These results were consistent with the finding that seawater challenge evoked extracellular Ca2+ influx by activating TRPV1 channels rather than TRPV4.
Figure 5.
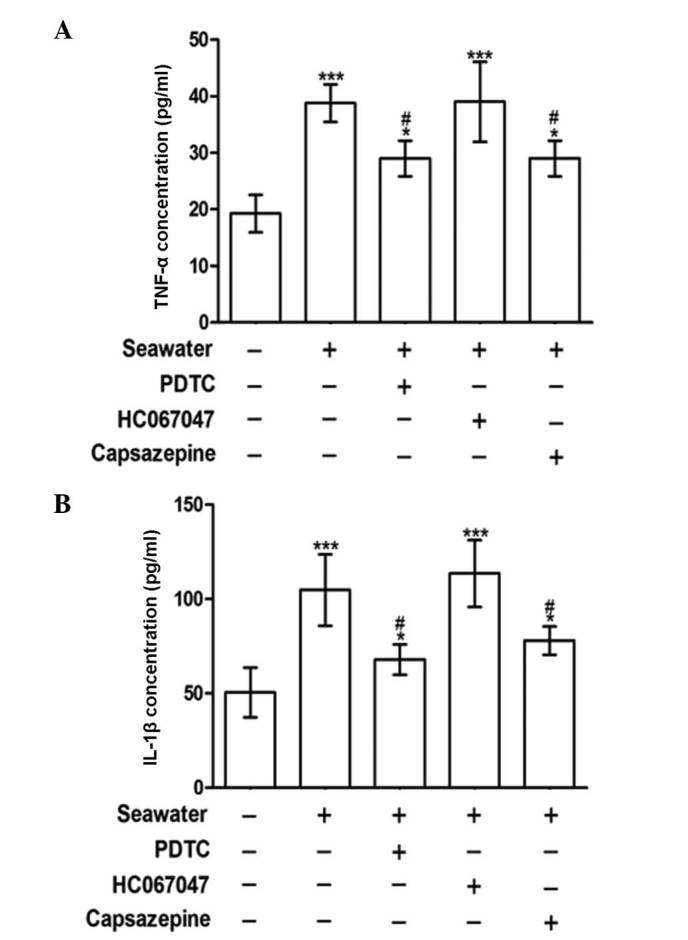
Effect of PDTC, HC067047 and capsazepine treatment on the secretion of TNF-α and IL-1β. (A) TNF-α and (B) IL-1β concentrations were measured following control, seawater and seawater + PDTC, HC067047 or capsazepine treatments. *P<0.05 and ***P<0.001 vs. the control group, #P<0.05 vs. the seawater group. PDTC, pyrrolidine dithiocarbamate; TNF-α, tumor necrosis factor α; IL-1β; interleukin 1β.
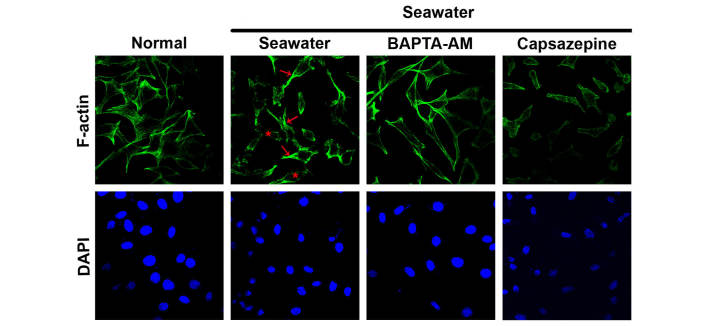
Changes in the actin cytoskeleton of A549 cells exposed to seawater were diminished by calcium chelation
To explore the effect of seawater exposure on the actin cytoskeleton, cells were fixed and stained to visualize the actin cytoskeleton using Acti-stain 488 phalloidin. In the control cells, actin filaments were observed to be in a regular arrangement and evenly distributed in the cytoplasm (Fig. 6). By contrast, following seawater challenge, cells exhibited a marked disorganization of actin filaments, formation of stress fibers under the plasma membrane and a dense ring of F-actin was located at the periphery of the cells (Fig. 6). It has previously been reported that cytosolic free Ca2+ oscillation can act as a mediator of actin reorganization (24). To verify whether intracellular calcium oscillation is a prerequisite for the remodeling of F-actin under these conditions, intracellular calcium was chelated by the preincubation of cells with BAPTA-AM or capsazepine. BAPTA-AM partially reversed the disruption of the actin cytoskeleton. However, the TRPV1-specific inhibitor, capsazepine, had no effect on the F-actin distribution (Fig. 6).
Figure 6.
Actin cytoskeleton analysis of A549 cells in the different treatment groups. The actin cytoskeleton was stained with phalloidin-Alexa488, and the nucleus was stained with DAPI, following control, seawater and seawater + BAPTA-AM or capsazepine treatments. Stress fibers are indicated by the red arrows, and the disorganization of actin filaments is indicated by the red asterisks.
Seawater instillation induced lung injury in a Ca2+-dependent manner
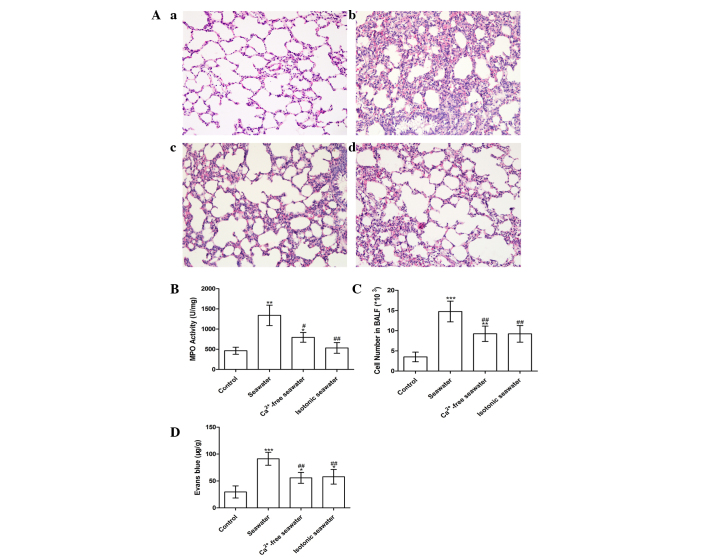
The pathology of seawater drowning-induced ALI is characterized by simultaneous neutrophil infiltration, pulmonary edema with hemorrhage, and production of inflammatory mediators (25,26). Therefore, to assess the severity of lung injury, TNF-α and IL-1β levels were examined, lung MPO activity was measured to determine the levels of neutrophil sequestration and histopathological examination of lung tissues was conducted. These results are presented in Fig. 7. In order to investigate the effect of high concentration of Ca2+ ions in seawater on the severity of lung injury, the Ca2+ in seawater was replaced with NaCl, followed by pH and osmotic pressure adjustment. Histopathological examination of lung tissues exposed to seawater displayed alveolar collapse, infiltration of inflammatory cells, septal thickening and interstitial edema, demonstrating that seawater challenge induced acute congestion in the lung tissues, in addition to edema and inflammation (Fig. 7A). However, fewer changes to the lung histoarchitecture were observed in the Ca2+-free and isotonic seawater groups (Fig. 7Ac and d).
Figure 7.
(A) Histopathological changes in lung tissue in the (a) control; (b) seawater; (c) Ca2+-free seawater; and (d) isotonic seawater groups (hematoxylin and eosin staining; magnification, ×200). (B) MPO activity of lung tissue; (C) total cells in the BALF; and (D) leak index of Evans blue in the control, seawater, Ca2+-free and isotonic groups. *P<0.05, **P<0.01 and ***P<0.001 vs. the control group, #P<0.05 and ##P<0.01 vs. the seawater group. MPO, myeloperoxidase; BALF, bronchoalveolar lavage fluid.
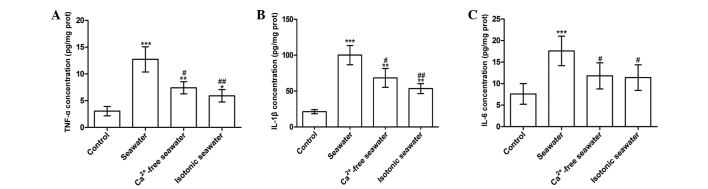
The seawater group demonstrated significantly increased MPO activity and number of the cells in the BALF, whereas the Ca2+-free seawater and isotonic seawater groups exhibited relatively lower levels compared with the seawater group (Fig. 7B and C). To assess the barrier permeability of the lung, the leak index of Evans blue dye was assessed. The seawater instillation significantly increased the barrier permeability. However, compared with the seawater group, the Ca2+-free group and isotonic groups exhibited significantly reduced barrier permeability (P<0.01; Fig. 7D). Consistent with these changes, seawater instillation resulted in a significant increase of TNF-α, IL-1β and IL-6 concentrations in lung tissues compared with control rats (P<0.001; Fig. 8). Compared with the seawater group, the levels of TNF-α and IL-1β were reduced by instilling either Ca2+-free seawater or isotonic seawater with no change in Ca2+ concentration (Fig. 8).
Figure 8.
Inflammatory mediators of lung tissue treated with seawater, Ca2+-free and isotonic seawater. (A) TNF-α; (B) IL-1β; and (C) IL-6 concentrations were measured in the control, seawater, Ca2+-free seawater and isotonic seawater groups. *P<0.05, **P<0.01 and ***P<0.001 vs. the control group, #P<0.05 and ##P<0.01 vs. the seawater group. TNF-α, tumor necrosis factor α; IL; interleukin.
TRPV1-mediated calcium oscillations connect hypertonia signals and alterations during seawater inhalation-induced lung injury
As summarized in Fig. 9, seawater challenge significantly increased Ca2+ oscillations in lung epithelial cells through the activation of TRPV1. Furthermore, increased [Ca2+]c gives rise to the breakdown of the alveolar-capillary barrier, release of proinflammatory molecules, accumulation of neutrophils and disorganization of actin filaments.
Figure 9.
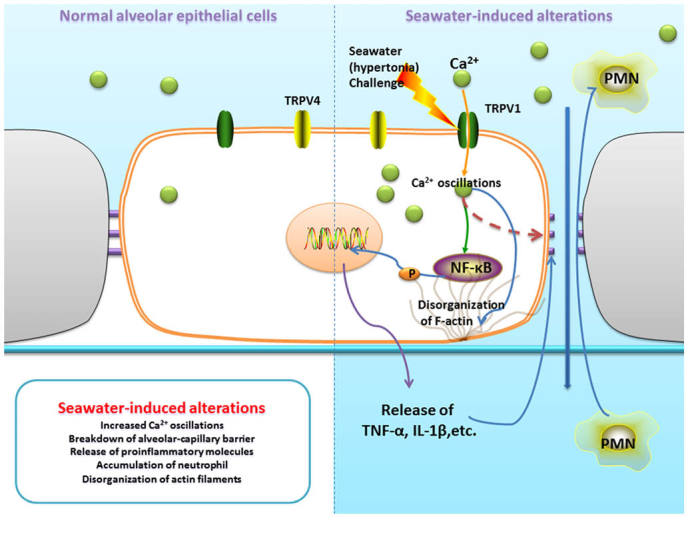
TRPV1-mediated calcium oscillations connect hypertonic signals and alterations during seawater inhalation-induced lung injury. TRPV1, transient receptor potential-vanilloid 1; PMN, polymorphonuclear cells; TNF-α, tumor necrosis factor α; IL-1β; interleukin 1β.
Discussion
Seawater drowning and associated ALI or respiratory failure remain a notable cause of accidental death (25,26). However, the underlying mechanism remains unclear, and requires further exploration. Similar to lipopolysaccharide (LPS) and cecalligation and puncture-induced lung injury, seawater instillation can also induce excessive release of inflammatory mediators, disturb the integrity of the alveolar septal network and increase blood-air barrier permeability (4). However, seawater exposure directly induces serious pulmonary interstitial edema, alveolar collapse, disturbance of lung blood-air barrier permeability and subsequent infiltration of inflammatory cells, in addition to the activation of the pulmonary inflammatory cascade.
In the current study, the effect of calcium, a component of seawater, on the inflammatory reactions in seawater drowning-induced ALI was investigated. It was demonstrated that high-concentration Ca2+ in seawater exacerbated lung injury. Further study revealed that seawater challenge elevated [Ca2+]c by inducing calcium entry from the extracellular medium via TRPV1 channels. Elevated [Ca2+]c may have induced the increased release of TNF-α and IL-1β. It was speculated that these inflammatory reactions were associated with the activation of NF-κB. Indeed, the study observed that the elevated [Ca2+]c led to greater phosphorylation of NF-κB (Fig. 4) and increased TNF-α and IL-1β levels. This was corroborated by the diminished inflammatory response following Ca2+ chelation, suggesting an important role for cytosolic Ca2+ in the regulation of lung inflammation.
Calcium is an important second messenger and regulates a variety of cellular functions (6). It is recognized that ALI is often dependent upon cytosolic free Ca2+ oscillation, and that Ca2+ entry into lung endothelium can participate in mediating microvascular-barrier permeability and the inflammatory response to high vascular pressure, hydrogen sulfide or LPS. Alvarez et al (6) reported that disruption of the alveolar septal barrier resulting from Ca2+ influx led to alveolar flooding and impaired gas exchange. Consistent with these findings, Jian et al (8) reported the HiPv-induced increases in Kf were attenuated by low extracellular Ca2+.
In the present experimental model, seawater challenge resulted in a [Ca2+]c influx characterized by rapid increase to a maximum level within 30 sec, followed by a recovery period and sustained plateau. The source of Ca2+ was clarified by chelation of Ca2+ in the extracellular medium using EGTA, which resulted in a weakened increase in [Ca2+]c following exposure to seawater, and the result was confirmed by the re-addition of Ca2+ to the cells. These results indicated that elevation of [Ca2+]c evoked by seawater exposure was mainly accomplished by increase of Ca2+ entry.
Various membrane transport pathways have been identified as mediators of Ca2+ influx during ALI. Tauseef et al (7) demonstrated that endotoxins induce Ca2+ entry in endothelial cells through the activation of transient receptor potential canonical 6 channels in a Toll-like receptor 4-dependent manner. Alvarez et al (6) implicated TRPV4 in the Ca2+ entry-dependent regulation of endothelial permeability, and the permeability response to the TRPV4 agonist was abolished in lungs from TRPV4−/− mice. TRPV1 was also reported to participate in sepsis-evoked ALI (27). Pretreatment with capsazepine markedly attenuated pulmonary COX-2 expression in septic mice (27). To clarify which channels were predominantly responsible for mediating Ca2+ entry and the seawater-induced proinflammatory cytokine production in A549 cells, the present study focused on the role of TRPVs and blocked several potential pathways with the inhibitors ruthenium red, capsazepine and HC067047 (Fig. 3). The results revealed that extracellular Ca2+ influx required the activation of TRPV1 channels following seawater challenge and may be significantly reduced by the TRPV1-specific inhibitor, capsazepine, and the TRPV family inhibitor, ruthenium red.
TRPV1 is a cell membrane-bound Ca2+ channel highly expressed in primary sensory neurons (28) and numerous other cell types, including muscle cells, dendrites and airway epithelial cells (12,19,28). When cells are exposed to cytokines, abnormal pH, osmolality and other irritations, intracellular calcium oscillates by activating TRPV1 (12,18–20) and can initiate endoplasmic reticulum stress and cell death in human bronchial epithelial and alveolar cells (19). In cultured human lung cells, the activation of TRPV1 by various stimuli can also promote calcium-dependent cytokine release and acute respiratory inflammation, with similar results reported in human corneal epithelial cells (29). Additionally, other studies have demonstrated that hypertonic stress increased the levels of IL-6 and the chemoattractant IL-8 by eliciting NF-κB activation in a TRPV1-dependent manner (29), and that TRPV1 activation altered F-actin organization through extracellular regulated MAP kinase (ERK1/2) and myosin light chain 2 (MLC2) pathways (30).
In view of the pivotal role of TRPV1 and Ca2+ mobilization in the mediation of inflammation, endoplasmic reticulum stress, cell death and reorganization of the cytoskeleton, TRPV1 was selectively inhibited by capsazepine in vitro to elucidate the function of TRPV1 on seawater drowning-induced ALI. The results demonstrated that seawater exposure gave rise to NF-κB phosphorylation and capsazepine or Ca2+ chelation reduced the effect. Cells pretreated with either capsazepine or an NF-κB inhibitor, PDTC, attenuated the increase of TNF-α and IL-1β release elicited by seawater challenge. Thus, seawater challenge may increase the release of proinflammatory cytokines through the phosphorylation and activation of NF-κB. A549 cells exhibited a marked disorganization of actin filaments and formation of stress fibers following exposure to seawater, whereas changes to the actin cytoskeleton were diminished by pre-incubation of cells with the Ca2+ chelator, BAPTA-AM. However, pretreatment with the TRPV1-specific inhibitor, capsazepine, produced no observed effect on the F-actin distribution. It was surmised that this may be due to capsazepine only being able to diminish calcium influx and ERK1/2 and MLC2 activation, rather than abolish them completely, a slight change to Ca2+ influx may be sufficient to cause F-actin redistribution. Furthermore, seawater may initiate such changes through various other pathways. Further work is necessary to elucidate this mechanism.
In conclusion, these observations place cytosolic Ca2+ ions and TRPV1 at the center of the signaling pathways that mediate seawater drowning-induced ALI, due to their roles in modulating lung inflammation and the cytoskeleton. The present study observed that high-concentration Ca2+ in seawater exacerbated lung injury, and seawater challenge elevated [Ca2+]c by activating TRPV1 channels, potentially leading to the phosphorylation of NF-κB and subsequent increased release of TNF-α and IL-1β.
Acknowledgments
The current study was supported by the National Natural Science Foundation of China (grant no. 81270124) and the Military Key Projects in the 12th Five-year Plan of China (project no. CWS13J043).
References
- 1.van Beeck EF, Branche CM, Szpilman D, Modell JH, Bierens JJ. A new definition of drowning: Towards documentation and prevention of a global public health problem. Bull World Health Organ. 2005;83:853–856. [PMC free article] [PubMed] [Google Scholar]
- 2.Engel SC. Drowning episodes: Prevention and resuscitation tips. J Fam Pract. 2015;64:E1–E6. [PubMed] [Google Scholar]
- 3.Salomez F, Vincent JL. Drowning: A review of epidemiology, pathophysiology, treatment and prevention. Resuscitation. 2004;63:261–268. doi: 10.1016/j.resuscitation.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Ma L, Li Y, Zhao Y, Wang Q, Nan Y, Mu D, Li W, Sun R, Jin F, Liu X. 3,5,4′-tri-O-acetylresveratrol ameliorates seawater exposure-induced lung injury by upregulating connexin 43 expression in lung. Mediators Inflamm. 2013;2013:182132. doi: 10.1155/2013/182132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Xu M, Fan Q, Xie X, Zhang Y, Mu D, Zhao P, Zhang B, Cao F, Wang Y, et al. Tanshinone IIA ameliorates seawater exposure-induced lung injury by inhibiting aquaporins (AQP) 1 and AQP5 expression in lung. Respir Physiol Neurobiol. 2011;176:39–49. doi: 10.1016/j.resp.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: A novel mechanism of acute lung injury. Circ Res. 2006;99:988–995. doi: 10.1161/01.RES.0000247065.11756.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tauseef M, Knezevic N, Chava KR, Smith M, Sukriti S, Gianaris N, Obukhov AG, Vogel SM, Schraufnagel DE, Dietrich A, et al. TLR4 activation of TRPC6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation. J Exp Med. 2012;209:1953–1968. doi: 10.1084/jem.20111355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jian MY, King JA, Al-Mehdi AB, Liedtke W, Townsley MI. High vascular pressure-induced lung injury requires P450 epoxygenase-dependent activation of TRPV4. Am J Respir Cell Mol Biol. 2008;38:386–392. doi: 10.1165/rcmb.2007-0192OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan Z, Wang Z, Yang H, Zhang F, Reinach PS. TRPV1 activation is required for hypertonicity-stimulated inflammatory cytokine release in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2011;52:485–493. doi: 10.1167/iovs.10-5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 11.Nishihara E, Hiyama TY, Noda M. Osmosensitivity of transient receptor potential vanilloid 1 is synergistically enhanced by distinct activating stimuli such as temperature and protons. PLoS One. 2011;6:e22246. doi: 10.1371/journal.pone.0022246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geppetti P, Materazzi S, Nicoletti P. The transient receptor potential vanilloid 1: Role in airway inflammation and disease. Eur J Pharmacol. 2006;533:207–214. doi: 10.1016/j.ejphar.2005.12.063. [DOI] [PubMed] [Google Scholar]
- 13.Sadofsky LR, Ramachandran R, Crow C, Cowen M, Compton SJ, Morice AH. Inflammatory stimuli up-regulate transient receptor potential vanilloid-1 expression in human bronchial fibroblasts. Exp Lung Res. 2012;38:75–81. doi: 10.3109/01902148.2011.644027. [DOI] [PubMed] [Google Scholar]
- 14.Thomas KC, Robers JK, Deering-Rice CE, Romero EG, Dull RO, Lee J, Yost GS, Reilly CA. Contributions of TRPV1, endovanilloids, and endoplasmic reticulum stress in lung cell death in vitro and lung injury. Am J Physiol Lung Cell Mol Physiol. 2012;302:L111–L119. doi: 10.1152/ajplung.00231.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamanaka K, Jian MY, Weber DS, Alvarez DF, Townsley MI, Al-Mehdi AB, King JA, Liedtke W, Parker JC. TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. Am J Physiol Lung Cell Mol Physiol. 2007;293:L923–L932. doi: 10.1152/ajplung.00221.2007. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Chen L, Liedtke W, Simon SA. Changes in osmolality sensitize the response to capsaicin in trigeminal sensory neurons. J Neurophysiol. 2007;97:2001–2015. doi: 10.1152/jn.00887.2006. [DOI] [PubMed] [Google Scholar]
- 17.Sidhaye VK, Guler AD, Schweitzer KS, D'Alessio F, Caterina MJ, King LS. Transient receptor potential vanilloid 4 regulates aquaporin-5 abundance under hypotonic conditions. Proc Natl Acad Sci USA. 2006;103:4747–4752. doi: 10.1073/pnas.0511211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansen ME, Reilly CA, Yost GS. TRPV1 antagonists elevate cell surface populations of receptor protein and exacerbate TRPV1-mediated toxicities in human lung epithelial cells. Toxicol Sci. 2006;89:278–286. doi: 10.1093/toxsci/kfi292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas KC, Sabnis AS, Johansen ME, Lanza DL, Moos PJ, Yost GS, Reilly CA. Transient receptor potential vanilloid 1 agonists cause endoplasmic reticulum stress and cell death in human lung cells. J Pharmacol Exp Ther. 2007;321:830–838. doi: 10.1124/jpet.107.119412. [DOI] [PubMed] [Google Scholar]
- 20.Lee LY, Gu Q. Role of TRPV1 in inflammation-induced airway hypersensitivity. Curr Opin Pharmacol. 2009;9:243–249. doi: 10.1016/j.coph.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/S0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Neil RG, Heller S. The mechanosensitive nature of TRPV channels. Pflugers Arch. 2005;451:193–203. doi: 10.1007/s00424-005-1424-4. [DOI] [PubMed] [Google Scholar]
- 24.Rosado JA, González A, Salido GM, Pariente JA. Effects of reactive oxygen species on actin filament polymerisation and amylase secretion in mouse pancreatic acinar cells. Cell Signal. 2002;14:547–556. doi: 10.1016/S0898-6568(01)00273-X. [DOI] [PubMed] [Google Scholar]
- 25.Ma L, Zhao Y, Li B, Wang Q, Liu X, Chen X, Nan Y, Liang L, Chang R, Liang L, et al. 3,5,4′-Tri-O-acetylresveratrol attenuates seawater aspiration-induced lung injury by inhibiting activation of nuclear factor-kappa B and hypoxia-inducible factor-1α. Respir Physiol Neurobiol. 2013;185:608–614. doi: 10.1016/j.resp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Fan Q, Zhao P, Li J, Xie X, Xu M, Zhang Y, Mu D, Li W, et al. 17β-Estradiol administration attenuates seawater aspiration-induced acute lung injury in rats. Pulm Pharmacol The. 2011;24:673–681. doi: 10.1016/j.pupt.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Ang SF, Sio SW, Moochhala SM, MacAry PA, Bhatia M. Hydrogen sulfide upregulates cyclooxygenase-2 and prostaglandin E metabolite in sepsis-evoked acute lung injury via transient receptor potential vanilloid type 1 channel activation. J Immunol. 2011;187:4778–4787. doi: 10.4049/jimmunol.1101559. [DOI] [PubMed] [Google Scholar]
- 28.Cortright DN, Szallasi A. Biochemical pharmacology of the vanilloid receptor TRPV1. An update. Eur J Biochem. 2004;271:1814–1819. doi: 10.1111/j.1432-1033.2004.04082.x. [DOI] [PubMed] [Google Scholar]
- 29.Reilly CA, Taylor JL, Lanza DL, Carr BA, Crouch DJ, Yost GS. Capsaicinoids cause inflammation and epithelial cell death through activation ofvanilloid receptors. Toxicol Sci. 2003;73:170–181. doi: 10.1093/toxsci/kfg044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cong X, Zhang Y, Yang NY, Li J, Ding C, Ding QW, Su QC, Mei M, Guo XH, Wu LL, Yu GY. Occludin is required for TRPV1-modulated paracellular permeability in the submandibular gland. J Cell Sci. 2013;26:1109–1121. doi: 10.1242/jcs.111781. [DOI] [PubMed] [Google Scholar]