Abstract
Asthma is an inflammatory disease that involves airway inflammation and remodeling. Sinomenine (SIN) has been demonstrated to have immunosuppressive and anti-inflammatory properties. The aim of the present study was to investigate the inhibitory effects of SIN on airway inflammation and remodeling in an asthma mouse model and observe the effects of SIN on the transforming growth factor-β1 (TGF-β1)/connective tissue growth factor (CTGF) pathway and oxidative stress. Female BALB/c mice were sensitized by repetitive ovalbumin (OVA) challenge for 6 weeks in order to develop a mouse model of asthma. OVA-sensitized animals received SIN (25, 50 and 75 mg/kg) or dexamethasone (2 mg/kg). A blank control group received saline only. The area of smooth muscle and collagen, levels of mucus secretion and inflammatory cell infiltration were evaluated 24 h subsequent to the final OVA challenge. mRNA and protein levels of TGF-β1 and CTGF were determined by reverse transcription-quantitative polymerase chain reaction and immunohistology, respectively. The indicators of oxidative stress were detected by spectrophotometry. SIN significantly reduced allergen-induced increases in smooth muscle thickness, mucous gland hypertrophy, goblet cell hyperplasia, collagen deposition and eosinophilic inflammation. The levels of TGF-β1 and CTGF mRNA and protein were significantly reduced in the lungs of mice treated with SIN. Additionally, the total antioxidant capacity was increased in lungs following treatment with SIN. The malondialdehyde content and myeloperoxidase activities in the lungs from OVA-sensitized mice were significantly inhibited by SIN. In conclusion, SIN may inhibit airway inflammation and remodeling in asthma mouse models, and may have therapeutic efficacy in the treatment of asthma.
Keywords: asthma, transforming growth factor-β1, connective tissue growth factor, sinomenine, oxidative stress
Introduction
Bronchial asthma is a chronic allergic disease characterized by airway inflammation and remodeling (1). The chronic airway inflammation can induce several structural alterations of the airway, eventually leading to airway remodeling (2,3). Remodeling is frequently observed in patients with refractory asthma, and can arise as a result of excessive repair or failure to resolve the inflammation (4). Eventually, remodeling can contribute to airway high reactivity and progressive decline of lung function (5).
Transforming growth factor-β (TGF-β) is the critical mediator in the regulation of airway inflammation and remodeling in asthma (6). Increased TGF-β1 levels can be detected in asthmatic bronchoalveolar lavage fluids (7), whilst increased levels of TGF-β1 in the asthmatic airway mucosa correlates with the thickness of the reticular basement membrane (8). Downstream of TGF-β, connective tissue growth factor (CTGF) modulates the cellular response to TGF-β (9). Furthermore, CTGF can upregulate the production of collagen type I and fibronectin in human lung fibroblasts (10). In asthma, TGF-β upregulates the levels of CTGF in airway smooth muscle cells (11). Thus, this pathway is important in the regulation of airway remodeling in asthma. Additionally, oxidative stress is of importance in airway inflammation, and determines the severity of asthma, whilst reduced antioxidant defense is also associated with asthma (12). Oxidative stress increases in the lungs following antigen stimulation, and antioxidant administration has been demonstrated to attenuate the regeneration of reactive oxidative stress, and inflammation in vivo (13).
Glucocorticoids are the current gold standard in treatment for asthma, however, their ability to modulate airway remodeling is limited (14). The combination of glucocorticoids and bronchodilators is also unable to revert asthmatic features (14), and results in significant adverse side-effects. Therefore, there is an urgent requirement for the identification of novel therapeutic agents for the treatment of asthma. Sinomenine (SIN) is an alkaloid that is isolated from the root and stem of the climbing plant Sinomenium acutum (chemical structure presented in Fig. 1), and demonstrates immunosuppressive, anti-inflammatory, anti-arrhythmic, analgesic and anti-arthritic properties (15,16). SIN has been observed to significantly suppress the production of inflammatory mediators in rats (17). However, the effects of SIN on asthma remain to be established. The aim of the present study was to evaluate the potential anti-inflammatory, anti-airway remodeling and antioxidant effects of SIN in an asthma animal model.
Figure 1.
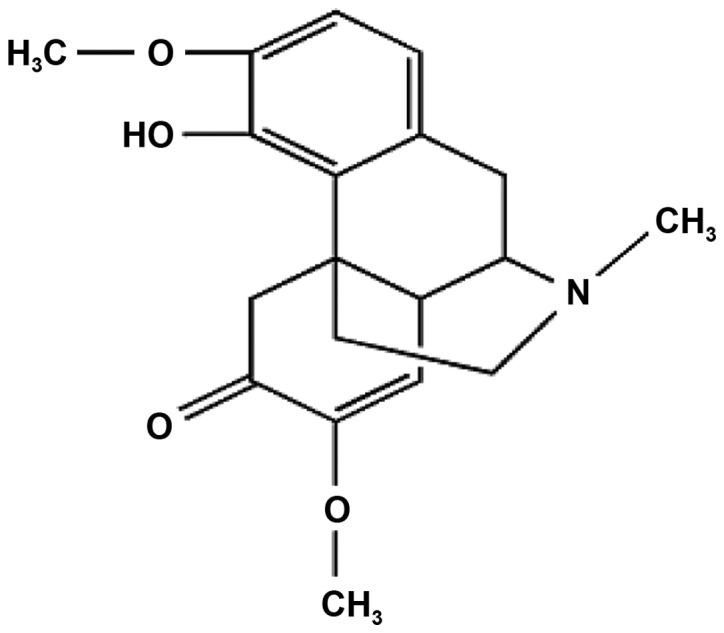
Chemical structure of sinomenine. Systematic name, (9α,13α, 14α)-7,8-didehydro-4-hydroxy-3,7-dimethoxy-17-methylmorphinan-6-one; formula, C19H23NO4.
Materials and methods
Animals
A total of 60 female BALB/c mice aged 6–8 weeks (18–22 g) were purchased from the animal medical center of Lanzhou University (Lanzhou, China). All animals received humane care and were maintained with a 12 h light/dark cycle in a temperature-controlled room (21–23°C), with free access to food and water. All procedures described in the present study were approved by the internal ethical committee of the First Hospital of Lanzhou University (LDYYLL2014-0058).
Experimental asthma models and intervention
Mice were divided randomly into six groups (ten animals per group): i) Blank control group; ii) asthma model; iii) asthma treated with dexamethasone (DEX; 2 mg/kg; Lianshui Pharmaceutical Co., Ltd., Jiangsu, China); iv) asthma model with low-dose SIN (25 mg/kg; Zelang Medical Technology Co., Ltd. Nanjing, China); v) asthma treated with moderate-dose SIN (50 mg/kg); and vi) asthma treated with high-dose SIN (75 mg/kg). DEX and SIN were administered by gavage.
Asthma was induced by ovalbumin (OVA; Sigma-Aldrich, St. Louis, MO, USA) using a previously described method (18). Animals were immunized by intraperitoneal injection (i.p.) of 20 µg OVA in the presence of 2 mg of Al(OH)3 adjuvant (Pierce Biotechnology, Inc., Rockford, IL, USA) diluted in 0.2 ml saline solution on days 0, 7 and 14. Mice were exposed to 2.5% (w/v) OVA solution in phosphate-buffered saline (PBS) for 30 min, using an pump nebulizer (Pari TurboBoy N; Pari GmbH, Starnberg, Germany) on days 21–28 subsequent to initial sensitization. Subsequently, mice were exposed to aerosolized 2.5% OVA for 30 min, once every 2 days, from days 29–70. DEX and SIN were administered for 1 h prior to the OVA inhalation. Control mice received the adjuvant (i.p.) and were exposed to nebulized aerosol of 0.9% NaCl (Gansu Fuzheng Pharmaceutical Technology Co., Ltd., Lanzhou, China) at the same time points. Mice were sacrificed via cervical dislocation at 24 h subsequent to the final challenge.
Histochemical analyses
The left lobe of the lung (which was fixed in 10% formalin (Shanghai Jianxin Chemical Co., Ltd., Shanghai, China) and embedded in paraffin (Shanghai Yongye Biological Technology Co., Ltd., Shanghai, China) was cut into 3-µm sections using a Leica RM 2135 microtome (Leica Microsystems GmbH, Wetzlar, Germany) for histological and immunohistochemical analysis. The remainder of the lung was triturated using a mortar and pestle for reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and oxidative stress detection.
The sections were stained with hematoxylin and eosin (HE) in order to observe the infiltration of cells. The degree of inflammatory cell infiltration was determined using the established scoring system (19). The point-counting technique was used to evaluate levels of eosinophil (20). To determine the eosinophils/unit area (mm2) (21), the number of points of the integrating eyepiece falling on areas of peribronchiolar inflammation in three areas of each airway wall and the number of eosinophils in the same area were counted using a Olympus DP71 microscope (Olympus Corporation, Tokyo, Japan). Goblet cells and mucus expression were determined by staining the sections with periodic acid Schiff's reagent (PAS). PAS-positive cells were counted as goblet cells in four airways from one section. The degree of mucus plugging of the airways (0.5–0.8 mm in diameter) was classified by a semiquantitative system (22). Airway smooth muscle thickness was determined in HE-stained lung sections using MetaMorph 6.1 image software (Universal Imaging Corporation, West Chester, PA, USA) by measuring the thickness of the smooth muscle cell layer per micrometer perimeter of basement membrane (Wam/Pbm). Masson's trichrome (Sigma-Aldrich) was used to evaluate collagen deposition by analyzing the positive area per micrometer perimeter of basement membrane (Wac/Pbm) by MetaMorph 6.1 image software (Universal Imaging Corporation) (23).
RT-qPCR
Total RNA was prepared using the RNAiso Plus reagent (Takara Bio, Inc., Otsu, Japan) according to the manufacturer's instructions. RNA (2 µg per sample) was reverse-transcribed into cDNA using PrimeScript™ Reverse Transcriptase (Takara Bio, Inc.). qPCR was performed with a Rotor-gene 6000 Thermal Cycler (Corbett Research Australia, Mortlake, Australia) and the SYBR Premix Ex Taq II kit (Takara Bio, Inc.) in accordance with the manufacturer's protocol. The sequences for the primers were as follows: TGF-β1, F 5′-GTGTGGAGCAACATGTGGAACTCTA-3′ and R 5′-TTGGTTCAGCCACTGCCGTA-3′; CTGF, F 5′-CCTGTGCCTGCCATTA-3′ and R 5′-TTCCTCCCACGGTAGTT-3′; β-actin, F 5′-CATCCGTAAAGACCTCTATGCCAAC-3′ and R 5′-ATGGAGCCACCGATCCACA-3′. Gene expression was normalized to β-actin. The experiments were repeated twice. Data are expressed as the fold increase in RNA expression compared with control animals.
Immunohistochemistry (IHC)
TGF-β1 and CTGF-induced protein expression was determined by immunohistochemical staining with a Strept Actividin-Biotin Complex kit (Zhongshan Biological Technology, Beijing, China). IHC was performed using the specific rabbit polyclonal anti-TGF-β1 (sc-146) and anti-CTGF immunoglobulin G (sc-25440) antibodies (1:150; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Images were obtained using a Leica DM1000 optical microscope (Leica Microsystems GmbH) and were analyzed using Image-Pro Plus software, version 6.0 (Media Cybernetics, Inc., Silver Spring, MD, USA). Five unduplicated fields were selected in one slide.
Measurement of the levels of oxidative stress
Subsequent to thawing, the lung tissues were weighed and homogenized in ice-cold PBS (20 mM, pH 7.4, w/v=1:9) using a glass homogenizer on ice. The homogenate was centrifuged at 3,000 × g for 20 min at 4°C. The obtained supernatant was used for measurement of the total antioxidant capacity (TAC), malondialdehyde (MDA) content and myeloperoxidase (MPO) activities. Detection of TAC, MDA and MPO were measured using commercially available assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer's protocol.
Statistical analysis
SPSS software, version 19.0 (IBM SPSS, Armonk, NY, USA) was used for the statistical analysis. All data are expressed as the mean ± standard deviation. The measurements were subjected to one-way analysis of variance. Differences between experimental groups were determined by Fisher's least significant differences post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
SIN reduced airway inflammation and remodeling
Compared with the control, the OVA asthma group presented significantly increased airway eosinophil infiltration (P<0.01; Fig. 2A). DEX and SIN treatment reduced the number of eosinophils in sensitized animals compared with the model group (P<0.01), and the inhibitory effects of SIN (25, 50 and 75 mg/kg) were not as effective as DEX treatment (Fig. 2A). The OVA challenge resulted in an increase in the number of goblet cells. While compared with the control (P<0.01), therapeutic administration of DEX and SIN significantly reduced the number of goblet cells compared with model mice (Fig. 2B). Additionally, OVA challenge led to an increase in the peribronchial and perivascular inflammatory cell infiltration score, compared with that of the blank control. DEX and various doses of SIN significantly reduced inflammation score. (P<0.05 compared with model) Compared to the DEX group, the inflammation scores were higher in the low-dose SIN group (25 mg/kg), however, no significant differences between moderate (50 mg/kg) and high-dose (75 mg/kg) SIN, and the DEX group were identified (Fig. 2C). A marked increase in airway mucus secretion was observed in OVA-treated mice compared with the control animals. The DEX treatment group displayed airway occlusion mucus scores which were significantly lower than those of the model mice. However, the SIN (25, 50 and 75 mg/kg) groups demonstrated no alterations in mucus secretion compared with the model group (Fig. 2D).
Figure 2.
The alteration of indicators of airway inflammation in the lungs. (A) Eosinophilic inflammation in the lungs. (B) Goblet cell hyperplasia in lung. (C) Score of inflammatory cell infiltration. (D) Score of mucus secretion. Blank control group, non-sensitized and nontreated; model group, sensitized and vehicle-treated; DEX group, sensitized and DEX-treated; SIN groups, sensitized and SIN-treated (25, 50 or 75 mg/kg). ##P<0.01 vs. the control group; **P<0.01 and *P<0.05 vs. the model group; ΔP<0.05 and ΔΔP<0.01 vs. the DEX group. The data are presented as the mean ± standard deviation. DEX, dexamethasone; SIN, sinomenine.
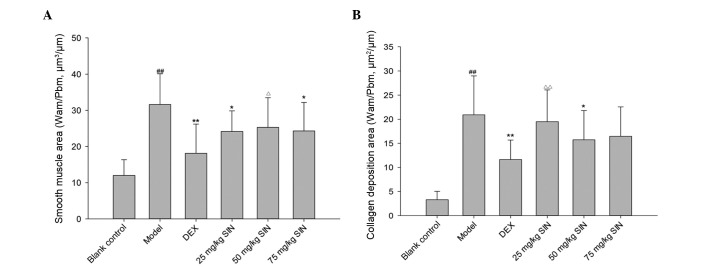
The OVA-challenged mice presented with a thicker smooth muscle layer compared with the control group subsequent to correction for the airway basement perimeter. DEX and SIN (25, 75 mg/kg) were effective in reducing myocyte hyperplasia (Fig. 3A). OVA challenge led to an increase in collagen deposition compared with control mice, whilst DEX and SIN (50 mg/kg) inhibited this collagen deposition (Fig. 3B).
Figure 3.
Alteration of indicators of airway remodeling. (A) Smooth muscle thickness of lung tissues. (B) Collagen deposition in the lungs. Blank control group, non-sensitized and nontreated; model group, sensitized and vehicle-treated; DEX group, sensitized and DEX-treated; SIN groups, sensitized and SIN-treated (25, 50 and 75 mg/kg). All results are presented as the mean ± standard deviation. ##P<0.01 vs. the control group; **P<0.01 or *P<0.05 vs. the model group; ΔΔP<0.01 or ΔP<0.05 vs. the DEX group. DEX, dexamethasone; SIN, sinomenine.
SIN modulated the TGF-β1 pathway
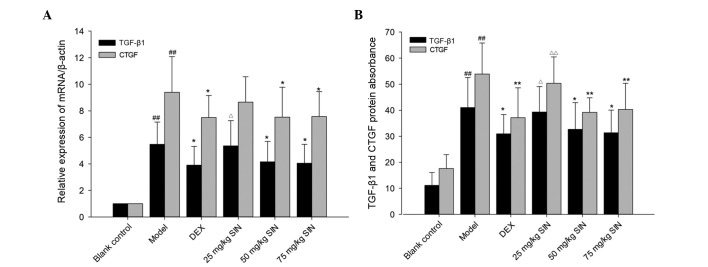
As demonstrated in Fig. 4, following the OVA-challenge, TGF-β1 and CTGF mRNA expression was observed to be significantly increased compared with the blank control group (P<0.01). TGF-β1 and CTGF mRNA expression levels in the SIN (50 and 75 mg/kg) and DEX groups was significantly reduced compared with those in the OVA group (P<0.05). No significant differences in TGF-β1 and CTGF mRNA expression between mice treated with SIN (50 and 75 mg/kg), and DEX were identified (Fig. 4).
Figure 4.
The levels of mRNA and protein of TGF-β1 and CTGF in the lungs. (A) Relative expression of TGF-β1 and CTGF mRNA. (B) Expression of TGF-β1 and CTGF protein in the lungs. Blank control group, non-sensitized and nontreated; model group, sensitized and vehicle-treated; DEX group, sensitized and DEX-treated; SIN groups, sensitized and SIN-treated (25, 50 and 75 mg/kg). All results are presented as the mean ± standard deviation. ##P<0.01 vs. the control group; **P<0.01 or *P<0.05 vs. the model group; ΔΔP<0.01 or ΔP<0.05 vs. the DEX group. TGF-β1, transforming growth factor-β1; CTGF, connective tissue growth factor; DEX, dexamethasone; SIN, sinomenine.
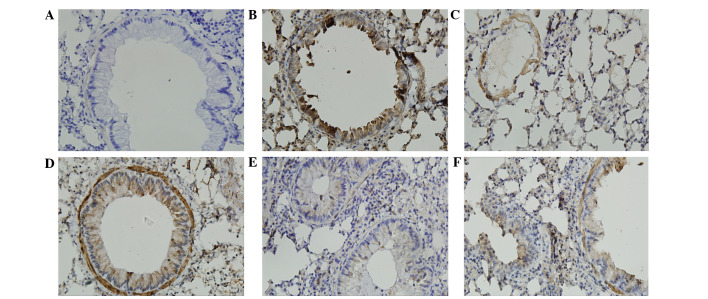
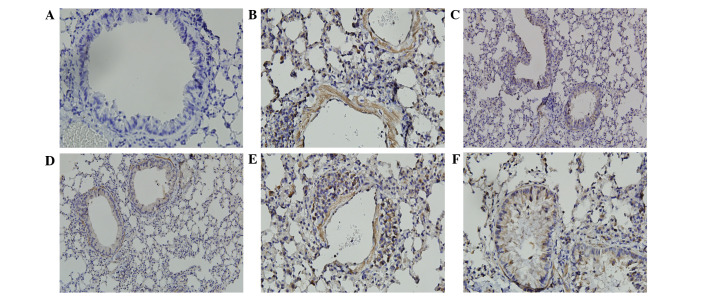
The immunostained areas of peribronchial TGF-β1 and CTGF in the OVA group were greater than those in the control group (Figs. 5 and 6). Administration of SIN (50 and 75 mg/kg) and DEX in OVA-challenged mice reduced the immunostained area of TGF-β1 and CTGF compared with the OVA group. There were no differences in TGF-β1 and CTGF expression levels between mice treated with SIN (50 and 75 mg/kg) and DEX (Figs. 5 and 6).
Figure 5.
Sinomenine treatment reduced TGF-β1 expression in lung tissue from ovalbumin-challenged mice. Positive staining is depicted in brown and the nuclei are stained blue. Positive staining highlighted TGF-β1 expression in the epithelium, macrophages, leukocytes and smooth muscle. (A) Control group; (B) model group; (C) dexamethasone group; (D) 25 mg/kg sinomenine group; (E) 50 mg/kg sinomenine group; (F) 75 mg/kg sinomenine group. TGF-β1, transforming growth factor-β1.
Figure 6.
Sinomenine treatment reduced CTGF expression in lung tissue from ovalbumin-challenged mice. Positive staining is depicted in brown and nuclei are stained blue. Positive staining highlighted CTGF expression in the epithelium, macrophages, leukocytes and smooth muscle. (A) Control group; (B) model group; (C) dexamethasone group; (D) 25 mg/kg sinomenine group; (E) 50 mg/kg sinomenine group; (F) 75 mg/kg sinomenine group. CTGF, connective tissue growth factor.
SIN attenuated pulmonary oxidative stress
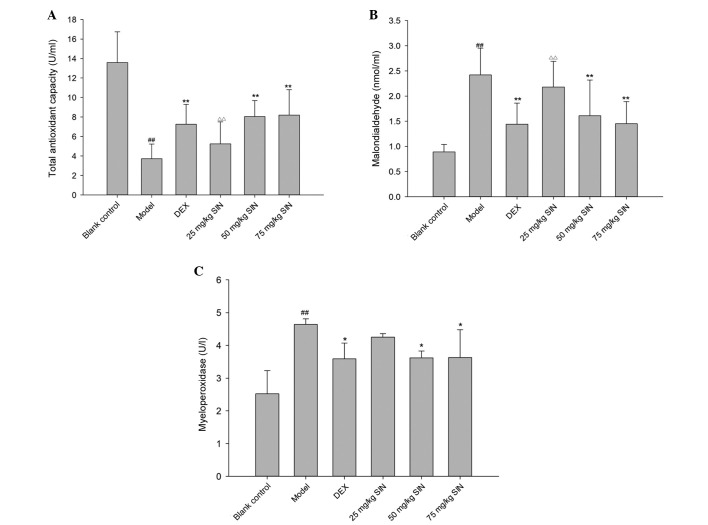
The model group presented lower TAC compared with the blank control group (P<0.01; Fig. 7A). TAC was increased significantly in the SIN (50 and 75 mg/kg) and DEX groups, compared with the model group (Fig. 7A). The concentration of lung MDA increased in the model group compared with the blank control. (P<0.01; Fig. 7B) However, the increase was significantly attenuated in the DEX and SIN (50 and 75 mg/kg) treatment groups (P<0.01; Fig. 7B). Lung MPO activity was increased in the model group compared with the blank control group, and reduced in the SIN (50 and 75 mg/kg) and DEX treatment groups compared with the model group (Fig. 7C).
Figure 7.
Alterations in levels of of oxidative stress indicators in the lungs. (A) Total antioxidant capacity, (B) malondialdehyde and (C) myeloperoxidase levels. Blank control group, non-sensitized and nontreated; model group, sensitized and vehicle-treated; DEX group, sensitized and DEX treated; SIN groups, sensitized and SIN-treated (25, 50 or 75 mg/kg). All results are presented as the mean ± standard deviation. ##P<0.01 vs. the control group; **P<0.01 or *P<0.05 vs. the model group; ΔΔP<0.01 vs. the DEX group. DEX, dexamethasone; SIN, sinomenine.
Discussion
The present study investigated the effects of SIN on airway inflammation and remodeling in a mouse model of asthma. The principle observation of the current study was that SIN was able to inhibit airway inflammation and remodeling in an asthma mouse model, however, there remains a limited amount of data regarding the treatment of human patients. The results of the present study do however demonstrate that SIN is able to reduce airway inflammation and remodeling, perhaps by modulation of TGF-β1 pathways and pulmonary oxidative stress. Thus, SIN treatment may have the potential to treat asthma.
Allergic asthma is characterized by allergen-induced airway bronchoconstriction, inflammation involving eosinophils and mucus hypersecretion and airway remodeling (24). Infiltrating inflammatory cells entering the lung strongly contribute to the development of allergic airway inflammation and remodeling (25). SIN, which is an alkaloid and a morphinan derivative, has anti-inflammatory, antiarrhythmic, antirheumatic, analgesic and immunosuppressive properties (16,26,27). However, the effects of SIN on airway inflammation and remodeling remain unclear. Although SIN is known to trigger release of histamine, none of the mice with asthma died following its administration in the present study. Utilizing this safe dose of SIN, the present study demonstrated that models of OVA exposure for 6 weeks induced leukocyte infiltration to the lungs in mice. Additionally, following exposure to OVA, mice displayed greater eosinophil infiltration in the airway compared with mice in the control group, a significant feature of airway inflammation in asthma (28). SIN significantly reduced the size and extent of leukocyte infiltration to the lung, and also reduced the number of eosinophils surrounding airways in the model. These effects were similar to those of mice treated with DEX, suggesting that SIN was able to inhibit eosinophilic inflammation.
Distinctive features of airway remodeling include the thickening of the airway wall, increased smooth muscle mass, goblet cell hyperplasia and mucous gland hypertrophy (29). The experimental model in the present study reproduced several of the features observed in asthmatic patients. It was indicated that OVA-sensitized animals presented eosinophilic inflammation and airway remodeling in lungs, including airway mucus expression, goblet cell hyperplasia, collagen deposition and airway smooth muscle layer thickening. Furthermore, it was observed that treatment with SIN reduced collagen deposition and smooth muscle layer thickening, in addition to mucus hypersecretion in a mouse model. It was demonstrated that SIN has potential as a therapeutic agent for asthma through its anti-remodeling properties. Compared with DEX, SIN has a similar ability to prevent asthma airway remodeling. These observations indicate potential for the use of this small molecular compound in the treatment of asthma airway remodeling.
TGF-β is a profibrotic cytokine important in promoting the structural alterations of airway remodeling, and is highly expressed in asthma (30). It has been demonstrated that airway wall remodeling is closely associated with TGF-β in asthma (27,31). CTGF is responsible for modulating the cellular response to TGF-β1, and contributes to airway remodeling and the production of collagen smooth muscle cells (11). The present study demonstrated that OVA-challenge resulted in a significant increase in TGF-β1 and CTGF levels, however, SIN was able to block the TGF-β1 pathway by inhibiting TGF-β1 and CTGF expression. No statistically significant differences between SIN and DEX in TGF-β1 and CTGF expression levels were identified. These observations suggest that the therapeutic action of SIN against OVA-induced asthma may be associated with the inhibition of the TGF-β1 pathway.
Oxidative stress is characterized by the excessive production of reactive oxygen species (ROS) or reactive nitrogen species (RNS) and the imbalance of oxidant/antioxidant response (32). A large number of studies have confirmed that ROS and RNS may lead to airway inflammation and remodeling (33). Excessive ROS and oxidative stress-mediated expression of TGF-β1 promote airway remodeling in asthma (34). TAC is an indicator possessing enzymatic and non-enzymatic properties, allowing it to reflect the antioxidant status (35). MDA is the lipid peroxidation product of a chain reaction of oxygen free radicals, and is involved in amplification of injury by ROS (36). MPO can activate the matrix metalloproteinases and is involved in asthmatic airway remodeling (37). It has been elucidated that increased MDA and MPO levels and reduced levels of TAC are present in the peripheral blood, induced sputum and bronchoalveolar lavage fluid of patients with asthma. These features are closely associated with the severity of asthma (12,35). The current study demonstrated that increased levels of TAC and reduced levels of MDA or MPO were observed subsequent to SIN administration, suggesting that SIN may inhibit oxidative stress in asthma. This antioxidant effect of SIN may be associated with the TGF-β1 pathway.
In conclusion, the current study reports that SIN ameliorates airway inflammation and remodeling in vivo, which may be associated with an ability to modulate the TGF-β1/CTGF pathway and oxidative stress. These observations support the hypothesis that SIN has therapeutic value for the treatment of asthma.
References
- 1.Manuyakorn W, Howarth PH, Holgate ST. Airway remodelling in asthma and novel therapy. Asian Pac J Allergy Immunol. 2013;31:3–10. [PubMed] [Google Scholar]
- 2.Al-Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. J Allergy Clin Immunol. 2011;128:451–462. doi: 10.1016/j.jaci.2011.04.047. quiz 463–464. [DOI] [PubMed] [Google Scholar]
- 3.Lederlin M, Ozier A, Montaudon M, Begueret H, Ousova O, Marthan R, Berger P, Laurent F. Airway remodeling in a mouse asthma model assessed by in-vivo respiratory-gated micro-computed tomography. Eur Radiol. 2010;20:128–137. doi: 10.1007/s00330-009-1541-0. [DOI] [PubMed] [Google Scholar]
- 4.Kearley J, Buckland KF, Mathie SA, Lloyd CM. Resolution of allergic inflammation and airway hyperreactivity is dependent upon disruption of the T1/ST2-IL-33 pathway. Am J Respir Crit Care Med. 2009;179:772–781. doi: 10.1164/rccm.200805-666OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King GG, Farah CS. Targeting airway remodelling in asthma: The how, what and where? Respirology. 2012;17:585–587. doi: 10.1111/j.1440-1843.2012.02160.x. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt-Weber CB, Blaser K. The role of TGF-beta in allergic inflammation. Immunol Allergy Clin North Am. 2006;26:233–244. doi: 10.1016/j.iac.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Redington AE, Madden J, Frew AJ, Djukanovic R, Roche WR, Holgate ST, Howarth PH. Transforming growth factor-beta 1 in asthma. Measurement in bronchoalveolar lavage fluid. Am Respir Crit Care Med. 1997;156:642–647. doi: 10.1164/ajrccm.156.2.9605065. [DOI] [PubMed] [Google Scholar]
- 8.Vignola AM, Chanez P, Chiappara G, Merendino A, Pace E, Rizzo A, la Rocca AM, Bellia V, Bonsignore G, Bousquet J. Transforming growth factor-beta expression in mucosal biopsies in asthma and chronic bronchitis. Am J Respir Crit Care Med. 1997;156:591–599. doi: 10.1164/ajrccm.156.2.9609066. [DOI] [PubMed] [Google Scholar]
- 9.Grotendorst GR. Connective tissue growth factor: A mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997;8:171–179. doi: 10.1016/S1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 10.Frazier K, Williams S, Kothapalli D, Klapper H, Grotendorst GR. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Dermatol. 1996;107:404–411. doi: 10.1111/1523-1747.ep12363389. [DOI] [PubMed] [Google Scholar]
- 11.Johnson PR, Burgess JK, Ge Q, Poniris M, Boustany S, Twigg SM, Black JL. Connective tissue growth factor induces extracellular matrix in asthmatic airway smooth muscle. Am J Respir Crit Care Med. 2006;173:32–41. doi: 10.1164/rccm.200406-703OC. [DOI] [PubMed] [Google Scholar]
- 12.Sahiner UM, Birben E, Erzurum S, Sackesen C, Kalayci O. Oxidative stress in asthma. World Allergy Organ J. 2011;4:151–158. doi: 10.1097/WOX.0b013e318232389e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park CS, Kim TB, Lee KY, Moon KA, Bae YJ, Jang MK, Cho YS, Moon HB. Increased oxidative stress in the airway and development of allergic inflammation in a mouse model of asthma. Ann Allergy Asthma Immunol. 2009;103:238–247. doi: 10.1016/S1081-1206(10)60188-3. [DOI] [PubMed] [Google Scholar]
- 14.Baraket M, Oliver BG, Burgess JK, Lim S, King GG, Black JL. Is low dose inhaled corticosteroid therapy as effective for inflammation and remodeling in asthma? A randomized, parallel group study. Respir Res. 2012;13:11. doi: 10.1186/1465-9921-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao T, Hao J, Wiesenfeld-Hallin Z, Wang DQ, Xu XJ. Analgesic effect of sinomenine in rodents after inflammation and nerve injury. Eur J Pharmacol. 2013;721:5–11. doi: 10.1016/j.ejphar.2013.09.062. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Li XK. Immunosuppressive and anti-inflammatory activities of sinomenine. Int Immunopharmacol. 2011;11:373–376. doi: 10.1016/j.intimp.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Zhou H, Wong YF, Wang J, Cai X, Liu L. Sinomenine ameliorates arthritis via MMPs, TIMPs, and cytokines in rats. Biochem Biophys Res Commun. 2008;376:352–357. doi: 10.1016/j.bbrc.2008.08.153. [DOI] [PubMed] [Google Scholar]
- 18.Temelkovski J, Hogan SP, Shepherd DP, Foster PS, Kumar RK. An improved murine model of asthma: Selective airway inflammation, epithelial lesions and increased methacholine responsiveness following chronic exposure to aerosolised allergen. Thorax. 1998;3:849–856. doi: 10.1136/thx.53.10.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duan W, Chan JH, Wong CH, Leung BP, Wong WS. Anti-inflammatory effects of mitogen-activated protein kinase kinase inhibitor U0126 in an asthma mouse model. J Immunol. 2004;172:7053–7059. doi: 10.4049/jimmunol.172.11.7053. [DOI] [PubMed] [Google Scholar]
- 20.Weibel ER. Principles and methods for the morphometric study of the lung and other organs. Lab Invest. 1963;12:131–155. [PubMed] [Google Scholar]
- 21.Angeli P, Prado CM, Xisto DG, Silva PL, Pássaro CP, Nakazato HD, Leick-Maldonado EA, Martins MA, Rocco PR, Tibério IF. Effects of chronic L-NAME treatment lung tissue mechanics, eosinophilic and extracellular matrix responses induced by chronic pulmonary inflammation. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1197–L1205. doi: 10.1152/ajplung.00199.2007. [DOI] [PubMed] [Google Scholar]
- 22.Henderson WR, Jr, Tang LO, Chu SJ, Tsao SM, Chiang GK, Jones F, Jonas M, Pae C, Wang H, Chi EY. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. Am J Respir Crit Care Med. 2002;165:108–116. doi: 10.1164/ajrccm.165.1.2105051. [DOI] [PubMed] [Google Scholar]
- 23.Zhou L, Qian ZX, Li F, Liu RY. The effect of melatonin on the regulation of collagen accumulation and matrix metal-loproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 mRNA and protein in a murine model of chronic asthma. Zhonghua Jie He He Hu Xi Za Zhi. 2007;30:527–532. In Chinese. [PubMed] [Google Scholar]
- 24.Toledo AC, Sakoda CP, Perini A, Pinheiro NM, Magalhães RM, Grecco S, Tibério IF, Câmara NO, Martins MA, Lago JH, Prado CM. Flavonone treatment reverses airway inflammation and remodelling in an asthma murine model. Br J Pharmacol. 2013;168:1736–1749. doi: 10.1111/bph.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oda H, Kawayama T, Imaoka H, Sakazaki Y, Kaku Y, Okamoto M, Kitasato Y, Edakuni N, Takenaka S, Yoshida M, et al. Interleukin-18 expression, CD8(+) T cells, and eosinophils in lungs of nonsmokers with fatal asthma. Ann Allergy Asthma Immunol. 2014;112:23–28. doi: 10.1016/j.anai.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Kok TW, Yue PY, Mak NK, Fan TP, Liu L, Wong RN. The anti-angiogenic effect of sinomenine. Angiogenesis. 2005;8:3–12. doi: 10.1007/s10456-005-2892-z. [DOI] [PubMed] [Google Scholar]
- 27.Chen DP, Wong CK, Leung PC, Fung KP, Lau CB, Lau CP, Li EK, Tam LS, Lam CW. Anti-inflammatory activities of Chinese herbal medicine sinomenine and Liang Miao San on tumor necrosis factor-α-activated human fibroblast-like synoviocytes in rheumatoid arthritis. J Ethnopharmacol. 2011;137:457–468. doi: 10.1016/j.jep.2011.05.048. [DOI] [PubMed] [Google Scholar]
- 28.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 29.Manuyakorn W. Airway remodelling in asthma: Role for mechanical forces. Asia Pac Allergy. 2014;4:19–24. doi: 10.5415/apallergy.2014.4.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holgate ST, Holloway J, Wilson S, Howarth PH, Haitchi HM, Babu S, Davies DE. Understanding the pathophysiology of severe asthma to generate new therapeutic opportunities. J Allergy Clin Immunol. 2006;117:496–506. doi: 10.1016/j.jaci.2006.01.039. quiz 507. [DOI] [PubMed] [Google Scholar]
- 31.Hardy CL, Nguyen HA, Mohamud R, Yao J, Oh DY, Plebanski M, Loveland KL, Harrison CA, Rolland JM, O'Hehir RE. The activin A antagonist follistatin inhibits asthmatic airway remodelling. Thorax. 2013;68:9–18. doi: 10.1136/thoraxjnl-2011-201128. [DOI] [PubMed] [Google Scholar]
- 32.Bowler RP, Crapo JD. Oxidative stress in allergic respiratory diseases. J Allergy Clin Immunol. 2002;110:349–356. doi: 10.1067/mai.2002.126780. [DOI] [PubMed] [Google Scholar]
- 33.Sugiura H, Ichinose M. Oxidative and nitrative stress in bronchial asthma. Antioxid Redox Signal. 2008;10:785–797. doi: 10.1089/ars.2007.1937. [DOI] [PubMed] [Google Scholar]
- 34.Brown SD, Baxter KM, Stephenson ST, Esper AM, Brown LA, Fitzpatrick AM. Airway TGF-β1 and oxidant stress in children with severe asthma: Association with airflow limitation. J Allergy Clin Immunol. 2012;129:388–396. doi: 10.1016/j.jaci.2011.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fatani SH. Biomarkers of oxidative stress in acute and chronic bronchial asthma. J Asthma. 2014;51:578–584. doi: 10.3109/02770903.2014.892965. [DOI] [PubMed] [Google Scholar]
- 36.Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360–438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vignola AM, Riccobono L, Mirabella A, Profita M, Chanez P, Bellia V, Mautino G, D'accardi P, Bousquet J, Bonsignore G. Sputum metalloproteinase-9/tissue inhibitor of metallopro-teinase-1 ratio correlates with airflow obstruction in asthma and chronic bronchitis. Am J Respir Crit Care Med. 1998;158:1945–1950. doi: 10.1164/ajrccm.158.6.9803014. [DOI] [PubMed] [Google Scholar]