Abstract
Changes in pulmonary microvascular permeability following cardiopulmonary bypass (CPB) and the underlying mechanisms have not yet been established. Therefore, the aim of the present study was to elucidate the alterations in pulmonary microvascular permeability following CPB and the underlying mechanism. The pulmonary microvascular permeability was measured using Evans Blue dye (EBD) exclusion, and the neutrophil infiltration and proinflammatory cytokine secretion was investigated. In addition, the activation of Src kinase and the phosphorylation of caveolin-1 and vascular endothelial cadherin (VE-cadherin) was examined. The results revealed that CPB increased pulmonary microvascular leakage, neutrophil count and proinflammatory cytokines in the bronchoalveolar lavage fluid, and activated Src kinase. The administration of PP2, an inhibitor of Src kinase, decreased the activation of Src kinase and attenuated the increase in pulmonary microvascular permeability observed following CPB. Two important proteins associated with vascular permeability, caveolin-1 and VE-cadherin, were significantly activated at 24 h in the lung tissues following CPB, which correlated with the alterations in pulmonary microvascular permeability and Src kinase. PP2 administration inhibited their activation, suggesting that they are downstream factors of Src kinase activation. The data indicated that the Src kinase pathway increased pulmonary microvascular permeability following CPB, and the activation of caveolin-1 and VE-cadherin may be involved. Inhibition of this pathway may provide a potential therapy for acute lung injury following cardiac surgery.
Keywords: pulmonary microvascular permeability, Src kinase pathway, caveolin-1, vascular endothelial-cadherin, cardiopulmonary bypass
Introduction
Cardiopulmonary bypass (CPB) has been widely used in open heart surgery in the last six decades since John H. Gibbon invented the artificial heart and lung machine (1). With improvements in medical equipment and biomaterial technologies, including smaller prime volume circuits, more biocompatible surfaces and gas-permeable microporous membranes, the incidence of CPB-induced complications have significantly decreased (2,3). However, CPB is known to activate systemic inflammatory response syndrome with acute lung injury that is associated with microvascular barrier injury (4). Numerous factors, including pulmonary hypoperfusion, induction of inflammatory mediators, hypothermia and blood contact with foreign surfaces during CPB, contribute to the etiology of lung injury (5). Post-surgical lung injury predominantly consists of lung edema and hypoxia, which are associated with CPB-induced neutrophil infiltration and increased microvascular permeability (6).
The Src family is important in intracellular signal transduction in acute inflammatory responses (7,8). Src is widely expressed by macrophages, monocytes, neutrophils, alveolar epithelial cells, endothelial cells and fibroblasts in the lung. It has been reported that Src is involved in the increase of lung vascular permeability in mice exposed to mechanical ventilation and hyperoxia-augmented ventilation (9,10). Thus, the present study aimed to determine whether the Src kinase pathway is involved in CPB-induced proinflammatory cytokine secretion, neutrophil infiltration and microvascular hyperpermeability.
Caveolin-1, a member of the caveolin family, exists primarily in lung endothelial cells and type I epithelial cells and functions as a structural and signaling protein (11). It is required for the formation and trafficking of caveolae, the primary vesicular carriers and mechanism of transcellular macromolecule transport through the vascular endothelial barrier (12,13). Vascular endothelial cadherin (VE-cadherin) is a cell-specific member of the cadherin protein family, which regulates endothelial adherens junctions (14,15). The present study initially investigated the effects of CPB on pulmonary microvascular permeability, neutrophil infiltration and secretion of proinflammatory cytokines. Subsequently, the role of Src kinase activation, caveolin-1 and VE-cadherin phosphorylation in CPB was examined.
Materials and methods
Animals and drugs
A total of 460 male Sprague-Dawley rats, weighing 250±10 g, age 10 weeks, were obtained from the Shanghai Experimental Animal Center (Shanghai, China) and used in all experiments. Animals were raised under standard conditions (22°C, 33% humidity) with a 12 h light/dark cycle. The study was performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (16) and with the approval of the research committee at Shanghai Jiaotong University (Shanghai, China). The non-specific Src kinase inhibitor 4-amino-5-(4-chlorophen yl)-7-(dimethylethyl)pyrazolo[3,4-d]pyrimidine (PP2) was purchased from Cayman Chemical Co. (Ann Arbor, MI, USA).
Experimental design
In order to examine the time course of pulmonary microvascular permeability, neutrophil infiltration and proinflammatory cytokine secretion, rats were randomly assigned to the following four groups (n=10 in each group): Sham group, CPB group, CPB + PP2 group and the untreated group. Rats in the sham group received similar surgery to the CPB and CPB + PP2 group, however, no blood was drained from the rats. Rats in the CPB group received CPB surgery as described below. Rats in the CPB + PP2 group received PP2 administration (1 mg/kg, intraperitoneal injection) 30 min prior to CPB surgery. Rats in the untreated group received no treatment. In order to investigate the time course of Src kinase phosphorylation, rats were randomly assigned to five groups (n=10 in each group): Pre-CPB (baseline), 0 h after CPB, 12 h after CPB, 24 h after CPB and 48 h after CPB. To determine the alterations in VE-cadherin and caveolin-1 phosphorylation, rats were randomly assigned to five groups (n=10 in each group): Sham, 24 h after CPB, 24 h after CPB + 1 mg/kg PP2, 24 h after CPB + 2 mg/kg PP2 and 24 h after CPB + 4 mg/kg PP2.
CPB procedure
The CPB procedure was performed according to the method described in our previous study (17). Initially, animals were anesthetized by intraperitoneal administration of butylone (60 mg/kg; Shanghai Experimental Animal Center) and then pentobarbital (3%; 1.5 mg/kg body weight; Shanghai Experimental Animal Center) was continuously provided to maintain anesthesia. The right femoral artery was cannulated with a 24-gauge catheter (heparinized with polytetrafluoroethylene) to monitor arterial pressure. Following administration of heparin (250 U/kg), a 16-gauge catheter was advanced to the right atrium through the right jugular vein. A 22-gauge catheter was cannulated to the tail artery as an arterial infusion line.
As described in our previous study (17), the mini-CPB circuit consisted of a venous reservoir, a specially designed membrane oxygenator, a roller pump and sterile polyvinyl chloride tubing with an internal diameter of 3 mm for the venous and arterial lines. The roller pump was equipped with a silicone tube 15 cm in length with an internal diameter of 5 mm. The membrane oxygenator was specially designed with a surface area for gas exchange of 0.05 m2 (Micro-1; Dongguan Kewei Medical Instrument Co., Ltd., Dongguan, China), with a total assembly dynamic priming volume of ~2 ml. The CPB circuit was primed with 12 ml of a solution of heparin (250 U/kg) and hetastarch. The blood was drained from the right atrium through the jugular vein catheter to a 5-ml sterile open reservoir using a siphon. A roller pump (BT00-300M; Baoding Lange Co., Ltd., Baoding, China) was used to drive the blood through silicone arterial inflow tubing and then return it to the tail artery.
Evans blue dye (EBD) exclusion analysis
Pulmonary microvascular injury was assessed by the extravasation of EBD into the lung parenchyma as described by Cavriani et al (18). EBD solution (100 mg/ml) was prepared in phosphate-buffered saline (PBS; pH 7.4) and intravenously injected at a dose of 30 mg/kg, then allowed to circulate for 30 min prior to sacrifice by decapitation. The right lungs were then excised and flushed with cold PBS three times. Two samples of lung parenchyma were resected and weighed. One sample was dried in an oven (60°C) for 72 h to obtain the dry weight. The other sample was homogenized in 5 ml of formamide to extract EBD. This homogenate was then incubated at 60°C for 24 h and centrifuged at 4,000 × g for 30 min. The supernatant was then collected. The EBD optical density was measured at a wavelength of 620 nm using an EAR 340 mictrotiter plate reader (SLT-Lab Instruments, Salzburg, Austria). The concentration of EBD was calculated from a standard curve of EBD-formamide solutions. The dry/wet ratio of each lung sample was calculated and used in the final calculation of Evans blue extravasation. EBD was expressed as µg Evans blue/g dry weight.
Bronchoalveolar lavage fluid (BALF) collection and assays
At the time point of sample collection, animals were sacrificed by decapitation and the chest was opened. A cannula was then inserted into the left trachea. The left lung cavity was gently flushed with 500 µl saline (4°C) up to a total volume of 2 ml to obtain BALF, which was then centrifuged at 400 × g for 10 min. The supernatant was used for the proinflammatory cytokine assay. The pelleted cells were re-suspended in PBS and then the neutrophil count was determined using a Hemovet HV950FS (CDC Technologies Inc., Oxford, CT, USA). ELISA kits (BioLegend, Inc., San Diego, CA, USA) were used to measure the levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 in the supernatants of BALF according to the manufacturer's protocol. The results were expressed as pg/ml BALF.
Western blot analysis
Following collection of BALF, the lung tissues were washed in ice-cold saline, then homogenized in 4°C RIPA lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) with 1 mM phenylmethanesulfonyl fluoride and centrifuged at 3,000 × g and 4°C for 15 min. The supernatants were collected and the protein concentration was determined using a BCA protein assay kit (Beyotime Institute of Biotechnology). Protein samples (40 µg) were loaded per lane and separated using 10% SDS-PAGE (Beyotime Institute of Biotechnology). The target proteins, including phosphorylated Src, VE-cadherin and caveolin-1, were then electrophoretically transferred onto nitrocellulose membranes (Beyotime Institute of Biotechnology). The protein blots were blocked in Tris-Buffered Saline and Tween 20 (TBST; 5% non-fat milk, 10 mM Tris, 150 mM NaCl, 0.05% Tweek-20) for 1 h, followed by incubation with primary antibodies against phosphorylated Src (monoclonal; 1:200, rabbit anti-mouse; ab4816; Oncogene Research Products; La Jolla, CA, USA), phosphorylated VE-cadherin (polyclonal, 1:400; rabbit anti-mouse; SAB4504676; BD Biosciences, Franklin Lakes, NJ, USA), VE-cadherin (monoclonal; 1:400; rabbit anti-mouse; V1514; BD Biosciences), phosphorylated caveolin-1 (polyclonal, 1:400; rabbit anti-mouse; sc-14037; Santa Cruz Biotechnology Inc., Dallas, TX, USA) or caveolin-1 (monoclonal; 1:400; rabbit anti-mouse; sc-53564; Santa Cruz Biotechnology Inc.) overnight at 4°C. Blots were then treated with the following secondary antibodies in TBST solution for 1 h: Secondary Src antibody [polyclonal; 1:4000; chicken anti-rabbit immunoglobulin G (IgG); ab6829; Abcam, Cambridge, MA, USA], phosphorylated VE-cadherin (polyclonal), VE-cadherin (monoclonal), phosphorylated caveolin-1 (polyclonal) or caveolin-1 (monoclonal; all 1:3,000; chicken anti-rat; ab112448; Abcam). Each sample was also probed with β-actin antibody (1:30,000; rabbit anti-mouse; A5316l; Sigma-Aldrich, St. Louis, MO, USA) as a loading control, and β-actin secondary antibody (monoclonal; 1:3,000; chicken anti-mouse IgG; ab131368, Abcam). Finally, blots were washed with PBS with Tween 20 and then examined using the ECL Plus Western Blotting Detection System (Amersham Life Science, Little Chalfont, Buckinghamshire, UK).
Statistical analysis
Values are presented as the mean ± standard error of the mean. Statistical analysis was performed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) with one-way analysis of variance followed by Student-Newman-Keuls post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
PP2 attenuates the increase in pulmonary microvascular leakage in BALF following CPB
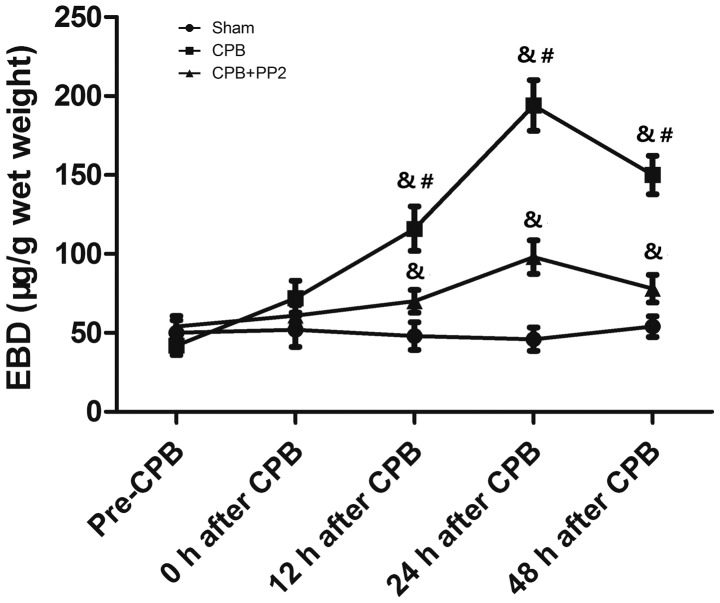
Fig. 1 shows alterations in pulmonary microvascular leakage over time demonstrated by the concentration of EBD. Pulmonary microvascular leakage increased up to 24 h after CPB, but decreased at 48 h after CPB. Treatment with PP2 significantly inhibited the increase in pulmonary microvascular leakage compared with the CPB group (P<0.05). However, the CPB + PP2 group exhibited increased pulmonary microvascular leakage (P<0.05) compared with the sham group with the exception of at 0 h after CPB.
Figure 1.
EBD concentration in BALF following CPB. Animals were sacrificed at different time points prior to or following CPB surgery (Pre-CPB, 0 h after CPB, 12 h after CPB, 24 h after CPB and 48 h after CPB). In the CPB + PP2 group, 1 mg/kg PP2 was intraperitoneally injected 30 min prior to CPB. &P<0.05, compared with the sham group; #P<0.05, compared with the CPB + PP2 group. EBD, Evans Blue Dye; BALF, bronchoalveolar lavage fluid; CPB, cardiopulmonary bypass; PP2, 4-amino-5-(4-chlorophenyl)-7-(di methylethyl)pyrazolo[3,4-d]pyrimidine.
PP2 attenuates increases in the neutrophil count and proinflammatory cytokines, IL-1β and IL-6, in BALF following CPB
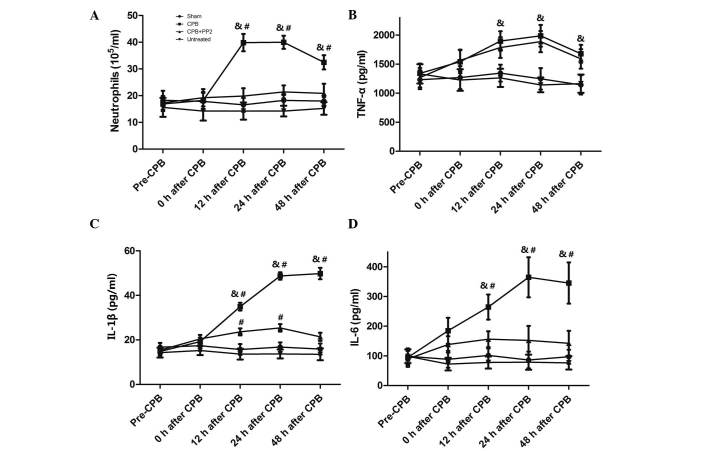
Fig. 2A shows alterations in the neutrophil count in BALF following CPB. The neutrophil count was increased following CPB surgery over time until 12 h after CPB. It peaked at 12 and 24 h after CPB and then decreased at 48 h after CPB. Treatment with PP2 significantly inhibited the increase in neutrophil count in BALF compared with the CPB group (P<0.05, compared with the CPB group; P>0.05, compared to the sham group). No significant difference was observed between the sham group and the untreated group.
Figure 2.
Neutrophil count and proinflammatory cytokines in BALF following CPB (treatments are described in Fig. 1). (A) Changes in the number of neutrophils in BALF. Expression levels of (B) TNF-α, (C) IL-1β and (D) IL-6 in BALF. Values are expressed as the mean ± standard error. N=10 per group. &P<0.05, compared with the sham group; #P<0.05, compared with the CPB + PP2 group. BALF, bronchoalveolar lavage fluid; CPB, cardiopulmonary bypass; IL, interleukin; TNF, tumor necrosis factor; PP2, 4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazolo[3,4-d]pyrimidine.
Fig. 2B–D show the results of TNF-α, IL-1β and IL-6 in BALF. TNF-α partially increased following CPB surgery, continued increasing in the first 24 h and then decreased at 48 h after CPB. Treatment with PP2 did not alter post-surgical increases in TNF-α. Unlike TNF-α, IL-1β increased over time until 48 h after CPB. PP2 significantly inhibited the level of IL-1β compared with the CPB group (P<0.05), although it remained higher than the sham group (P<0.05). Alterations in IL-6 concentration demonstrated a similar pattern with TNF-α and peaked at 24 h after CPB. PP2 significantly ameliorated the increase in IL-6 in BALF (P<0.05, compared with the CPB group; P>0.05 compared with the sham group). No significant difference was identified between the sham group and untreated group.
Src phosphorylation increases in the lung tissues following CPB surgery
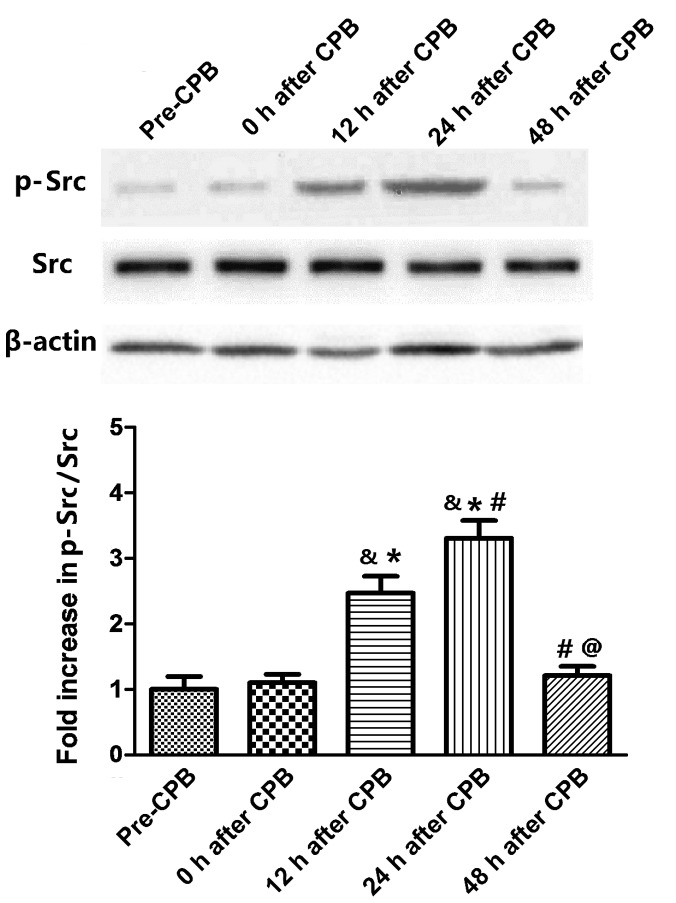
Fig. 3 reveals the time course of Src phosphory-lation in lung tissues. Src phosphorylation was not altered immediately following CPB surgery. However, Src phos-phorylation increased at 12 h after CPB and peaked at 24 h after CPB (P<0.05, compared with the 12 h after CPB group). At 48 h after CPB, it regressed to the normal level (P<0.05, compared with 12 and 24 h after CPB).
Figure 3.
Western blot analysis of Src phosphorylation in the lung tissues. Treatments have been described in Fig. 1. &P<0.05, compared with the pre-CPB group; *P<0.05, compared with 0 h after CPB; #P<0.05, compared with 12 h after CPB; @P<0.05 compared with 24 h after CPB. CPB, cardiopulmonary bypass.
PP2 attenuates increases in caveolin-1 and VE-cadherin phosphorylation in the lung tissues following CPB
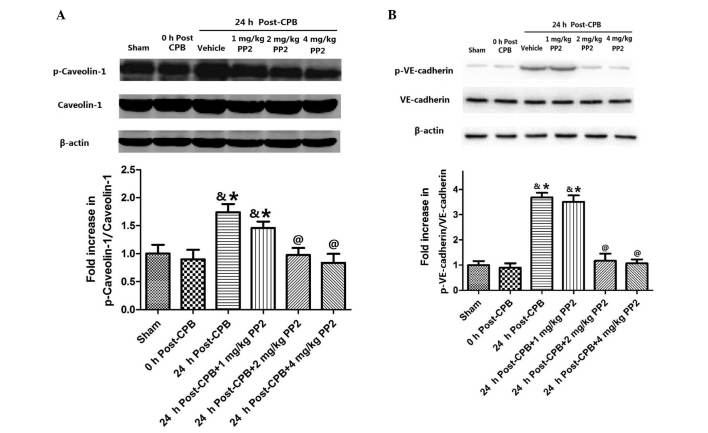
In order to examine the effect of Src kinase activation on caveolin-1 and VE-cadherin, caveolin-1 and VE-cadherin phosphorylation in the lung tissues was measured (Fig. 4). Caveolin-1 phosphorylation did not immediately change following CPB surgery, but significantly increased (P<0.05, compared with the sham group) at 24 h after CPB. Treatment with three doses of PP2 (1, 2 and 4 mg/kg) inhibited caveolin-1 phosphorylation in a dose-dependent manner (Fig. 4A). No significant difference was observed in VE-cadherin phosphorylation between the sham group and at 0 h after CPB surgery. However, at 24 h after CPB, VE-cadherin phosphorylation significantly increased (P<0.05) compared with the sham group. Treatment with high doses of PP2 (2 and 4 mg/kg) significantly inhibited VE-cadherin phosphorylation (Fig. 4B).
Figure 4.
Caveolin-1 and VE-cadherin phosphorylation in the lung tissues. (A) Changes of caveolin-1 and (B) VE-cadherin photophosphorylation. Values are expressed as the mean ± standard error. N=10 per group. &P<0.05, compared with the sham group; *P<0.05, compared with the 0 h post CPB group; @P<0.05, compared with the 24 h post CPB + 1 mg/kg PP2 group. VE-cadherin, vascular endothelial cadherin; CPB, cardiopulmonary bypass; PP2, 4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazolo[3,4-d]pyrimidine.
Discussion
Acute lung injury is among the leading cause of morbidity and mortality in patients who have undergone cardiac surgery necessitating CPB (19,20). Numerous factors may contribute to acute lung injury, including the exposure of blood to the artificial surface of the CPB machine, ischemia-reperfusion and lung ventilator-elicited inflammatory reactions (21). Certain previous studies have suggested that pulmonary microvascular permeability is a major contributor to acute lung injury (22,23), however, the mechanisms have not yet been investigated. In the present study, the EBD results indicated impaired pulmonary microvascular permeability following CPB surgery. The microvascular permeability started to increase following CPB surgery and then peaked 24 h later. CPB also induced significant increases in the neutrophil count and TNF-α, IL-1β and IL-6 in BALF, indicating the provoked inflammatory reaction and increased pulmonary microvascular permeability. Neutrophils, TNF-α and IL-6 in BALF reached a peak at 24 h post CPB, while IL-1β in BALF continued to increase until 48 h post CPB. These data revealed the time course of the inflammatory reaction, which emerged following CPB surgery and peaked at 24–48 h after CPB surgery. The time course of these changes correlates with the alterations in pulmonary microvascular permeability.
Src kinases belong to the non-receptor tyrosine kinase family, which contains c-Src, Fyn, Yes, Yrk, Blk, Fgr, Hck, Lck and Lyn (24). In response to stimulation of a variety of cell surface receptors, including tyrosine kinase receptors, integrin receptors and G protein-coupled receptors, the activity of Src can be upregulated by phosphorylation at Tyr 416, located in the catalytic domain (24). It has been demonstrated that Src mediates vascular endothelial permeability responses to TNF, reactive oxygen species, angiogenesis and vascular leakage (25–27). Inhibition of the Src family reduces cerebral edema and eradicates the increase in albumin permeability caused by C5α-activated neutrophils in venules (28,29). Neutrophil activation stimulates Src phosphorylation at Tyr 416 and decreases phosphorylation at Tyr 527, which upregulates Src activity (30). The results demonstrated that Src phosphorylation (activation) accompanied the increase in pulmonary microvascular leakage, while the administration of PP2, an inhibitor of the Src kinase, attenuated the alterations in pulmonary microvascular leakage, neutrophil count and proinflammatory cytokines (with the exception of TNF-α) in BALF caused by CPB, indicating that Src kinase has an important role in the effects of CPB on pulmonary microvascular permeability. For neutrophils and IL-6 in BALF, PP2 administration reduced their values to a level equivalent to the sham group. However, the fact that TNF-α was unaltered by PP2 suggests that the induction of TNF-α may not be regulated by Src kinase.
Src kinase may regulate the microvascular permeability and endothelial barrier structure through multiple pathways, including mitogen-activated protein kinase, myosin light chain kinase, β-catenin, or focal adhesion proteins (24,31,32). The present study focused on caveolin-1 and VE-cadherin, two important proteins in the regulation of pulmonary microvascular permeability. Caveolae were originally identified as 50–100 nm flask-shaped, non-clathrin-coated invaginations of the plasma membrane, which are important in transendothelial vesicular transport. Caveolin-1 is a critical protein for caveolae-mediated endocytosis and transcytosis in endothelial cells (33). It contains a scaffolding domain and acts as an inhibitory regulator of endothelial Rac1 signaling in the regulation of endothelial permeability (34,35). Tyrosine phosphorylation of caveolin-1 is important in the pathogenesis of oxidant-induced pulmonary vascular hyperpermeability (36). Previous studies have demonstrated that an increase in transcellular permeability was dependent on Src-mediated phosphorylation of caveolin-1 (13,34,37,38). The pulmonary vascular hyperpermeability induced by activation of neutrophils adherent to the vessel wall is dependent on signaling via caveolin-1 and increased caveolae-mediated transcytosis (39). Sun et al demonstrated that phosphorylation of caveolin-1 is an important mechanism mediating oxidant-induced vascular hyperpermeability by stimulating paracellular and caveolae-mediated transcellular permeability (36). The results from the present study demonstrated that caveolin-1 phosphorylation was not altered immediately following CPB surgery, but was significantly increased at 24 h after CPB. Treatment with 1 mg/kg PP2 did not alter caveolin-1 phosphorylation, however, 2 and 4 mg/kg PP2 inhibited caveolin-1 phosphorylation, indicating that Src kinase may function via the activation of caveolin-1.
VE-cadherin is a classical cadherin from the cadherin family, which is critical in endothelial cell biology and vascular permeability through homophilic binding to other VE-cadherins expressed on adjacent endothelial cells (40). Numerous stimuli, including TNF and vascular endothelial growth factor, may cause the phosphorylation of VE-cadherin, in which Src kinase acts as a key pathway mediator (14,41). It was also demonstrated that proinflammatory cytokines could induce the phosphorylation of VE-cadherin and the endocytosis of VE-cadherin in a β-arrestin-dependent manner through the Src kinase pathway (42). Src activation could also cause the phosphorylation of VE-cadherin by stimulation of H2O2 (43,44). The present study demonstrated that VE-cadherin phosphorylation was not altered immediately following CPB surgery, however, it significantly increased at 24 h post CPB. VE-cadherin phosphorylation was inhibited by treatment with PP2 (2 and 4 mg/kg). The results revealed that Src was involved in the phosphorylation of VE-cadherin, however, whether caveolin-1 mediated this process or not remains to be elucidated. A previous study demonstrated that the knockdown of caveolin-1 induced a decrease in VE-cadherin localized at inter-endothelial junctions (45). The interaction of caveolin-1 and VE-cadherin activation in Src-mediated pulmonary vascular hyperpermeability requires further investigation.
In conclusion, the present study demonstrated that pulmonary microvascular permeability was increased following CPB through the Src kinase pathway. The activation of caveolin-1 and VE-cadherin appears to be the downstream effect of Src kinase phosphorylation. Inhibition of this pathway may provide a potential therapy for acute lung injury following cardiac surgery.
Acknowledgments
This study was funded by the Science and Technology Commission of Shanghai Municipality (grant no. 11ZR1423700).
References
- 1.Gibbon JH., Jr Application of a mechanical heart and lung apparatus to cardiac surgery. Minn Med. 1954;37:171–185. [PubMed] [Google Scholar]
- 2.Edmunds LH., Jr Advances in the heart-lung machine after John and Mary Gibbon. Ann Thorac Surg. 2003;76:S2220–S2223. doi: 10.1016/j.athoracsur.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Wahba A. Centrifugal blood pump use in routine cardiac surgery. Interact Cardiovasc Thorac Surg. 2006;5:299–300. doi: 10.1510/icvts.2006.129502. [DOI] [PubMed] [Google Scholar]
- 4.Cox CS, Jr, Allen SJ, Brennan MS. Analysis of intestinal microvascular permeability associated with cardiopulmonary bypass. J Surg Res. 1999;83:19–26. doi: 10.1006/jsre.1998.5550. [DOI] [PubMed] [Google Scholar]
- 5.Ng CS, Wan S, Yim AP, Arifi AA. Pulmonary dysfunction after cardiac surgery. Chest. 2002;121:1269–1277. doi: 10.1378/chest.121.4.1269. [DOI] [PubMed] [Google Scholar]
- 6.Serraf A, Aznag H, Baudet B, Détruit H, Séccatore F, Mazmanian MG, Planché C. Pulmonary vascular endothelial growth factor and nitric oxide interaction during total cardiopulmonary bypass in neonatal pigs. J Thorac Cardiovasc Surg. 2003;125:1050–1057. doi: 10.1067/mtc.2003.402. [DOI] [PubMed] [Google Scholar]
- 7.Okutani D, Lodyga M, Han B, Liu M. Src protein tyrosine kinase family and acute inflammatory responses. Am J Physiol Lung Cell Mol Physiol. 2006;291:L129–L141. doi: 10.1152/ajplung.00261.2005. [DOI] [PubMed] [Google Scholar]
- 8.Oyaizu T, Fung SY, Shiozaki A, Guan Z, Zhang Q, dos Santos CC, Han B, Mura M, Keshavjee S, Liu M. Src tyrosine kinase inhibition prevents pulmonary ischemia-reperfusion-induced acute lung injury. Intensive Care Med. 2012;38:894–905. doi: 10.1007/s00134-012-2498-z. [DOI] [PubMed] [Google Scholar]
- 9.Miyahara T, Hamanaka K, Weber DS, Drake DA, Anghelescu M, Parker JC. Phosphoinositide 3-kinase, Src and Akt modulate acute ventilation-induced vascular permeability increases in mouse lungs. Am J Physiol Lung Cell Mol Physiol. 2007;293:L11–L21. doi: 10.1152/ajplung.00279.2005. [DOI] [PubMed] [Google Scholar]
- 10.Liu YY, Li LF, Fu JY, Kao KC, Huang CC, Chien Y, Liao YW, Chiou SH, Chang YL. Induced pluripotent stem cell therapy ameliorates hyperoxia-augmented ventilator-induced lung injury through suppressing the Src pathway. PLoS One. 2014;9:e109953. doi: 10.1371/journal.pone.0109953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 12.Cohen A, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 13.Minshall RD, Tiruppathi C, Vogel SM, Malik AB. Vesicle formation and trafficking in endothelial cells and regulation of endothelial barrier function. Histochem Cell Biol. 2002;117:105–112. doi: 10.1007/s00418-001-0367-x. [DOI] [PubMed] [Google Scholar]
- 14.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 15.Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, Ristagno G, Maddaluno L, Koh GY, Franco D, et al. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun. 2012;3:1208. doi: 10.1038/ncomms2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute of Laboratory Animal Resources (US) Committee on Care, Use of Laboratory Animals, and National Institutes of Health (US). Division of Research Resources: Guide for the care and use of laboratory animals. 7th edition. National Academies Press; Washington, DC: 1996. [Google Scholar]
- 17.Zhu J, Yin R, Shao H, Dong G, Luo L, Jing H. N-acetylcysteine to ameliorate acute renal injury in a rat cardiopulmonary bypass model. J Thorac Cardiovasc Surg. 2007;133:696–703. doi: 10.1016/j.jtcvs.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 18.Cavriani G, Oliveira-Filho RM, Trezena AG, da Silva ZL, Domingos HV, de Arruda MJ, Jancar S, Tavares de Lima W. Lung microvascular permeability and neutrophil recruitment are differently regulated by nitric oxide in a rat model of intestinal ischemia-reperfusion. Eur J Pharmacol. 2004;494:241–249. doi: 10.1016/j.ejphar.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 19.Altmay E, Karaca P, Yurtseven N, Ozkul V, Aksoy T, Ozler A, Canik S. Continuous positive airway pressure does not improve lung function after cardiac surgery. Can J Anaesth. 2006;53:919–925. doi: 10.1007/BF03022835. [DOI] [PubMed] [Google Scholar]
- 20.Apostolakis E, Filos KS, Koletsis E, Dougenis D. Lung dysfunction following cardiopulmonary bypass. J Card Surg. 2010;25:47–55. doi: 10.1111/j.1540-8191.2009.00823.x. [DOI] [PubMed] [Google Scholar]
- 21.Engels M, Bilgic E, Pinto A, Vasquez E, Wollschläger L, Steinbrenner H, Kellermann K, Akhyari P, Lichtenberg A, Boeken U. A cardiopulmonary bypass with deep hypothermic circulatory arrest rat model for the investigation of the systemic inflammation response and induced organ damage. J Inflamm (Lond) 2014;11:26. doi: 10.1186/s12950-014-0026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macnaughton PD, Braude S, Hunter DN, Denison DM, Evans TW. Changes in lung function and pulmonary microvascular permeability after cardiopulmonary bypass. Crit Care Med. 1992;20:1289–1294. doi: 10.1097/00003246-199209000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Messent M, Sinclair DG, Quinlan GJ, Mumby SE, Gutteridge JM, Evans TW. Pulmonary vascular permeability after cardiopulmonary bypass and its relationship to oxidative stress. Crit Care Med. 1997;25:425–429. doi: 10.1097/00003246-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 25.Kevil CG, Okayama N, Alexander JS. H(2)O(2)-mediated permeability II: Importance of tyrosine phosphatase and kinase activity. Am J Physiol Cell Physiol. 2001;281:C1940–C1947. doi: 10.1152/ajpcell.2001.281.6.C1940. [DOI] [PubMed] [Google Scholar]
- 26.Nwariaku FE, Liu Z, Zhu X, Turnage RH, Sarosi GA, Terada LS. Tyrosine phosphorylation of vascular endothelial cadherin and the regulation of microvascular permeability. Surgery. 2002;132:180–185. doi: 10.1067/msy.2002.125305. [DOI] [PubMed] [Google Scholar]
- 27.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–924. doi: 10.1016/S1097-2765(00)80221-X. [DOI] [PubMed] [Google Scholar]
- 28.Paul R, Zhang ZG, Eliceiri BP, Jiang Q, Boccia AD, Zhang RL, Chopp M, Cheresh DA. Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat Med. 2001;7:222–227. doi: 10.1038/84675. [DOI] [PubMed] [Google Scholar]
- 29.Tinsley JH, Ustinova EE, Xu W, Yuan SY. Src-dependent, neutrophil-mediated vascular hyperpermeability and beta-catenin modification. Am J Physiol Cell Physiol. 2002;283:1745–1751. doi: 10.1152/ajpcell.00230.2002. [DOI] [PubMed] [Google Scholar]
- 30.Yuan SY. Protein kinase signaling in the modulation of microvascular permeability. Vascul Pharmacol. 2002;39:213–223. doi: 10.1016/S1537-1891(03)00010-7. [DOI] [PubMed] [Google Scholar]
- 31.Eliceiri BP, Puente XS, Hood JD, Stupack DG, Schlaepfer DD, Huang XZ, Sheppard D, Cheresh DA. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J Cell Biol. 2002;157:149–160. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi S, Garcia JG, Roy S, Parinandi NL, Natarajan V. Involvement of c-Src in diperoxovanadate-induced endothelial cell barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2000;279:L441–L451. doi: 10.1152/ajplung.2000.279.3.L441. [DOI] [PubMed] [Google Scholar]
- 33.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, et al. Loss of caveolae, vascular dysfunction and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 34.Minshall RD, Sessa WC, Stan RV, Anderson RG, Malik AB. Caveolin regulation of endothelial function. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1179–L1183. doi: 10.1152/ajplung.00242.2003. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez E, Nagiel A, Lin AJ, Golan DE, Michel T. Small interfering RNA-mediated down-regulation of caveolin-1 differentially modulates signaling pathways in endothelial cells. J Biol Chem. 2004;279:40659–40669. doi: 10.1074/jbc.M407051200. [DOI] [PubMed] [Google Scholar]
- 36.Sun Y, Hu G, Zhang X, Minshall RD. Phosphorylation of caveolin-1 regulates oxidant-induced pulmonary vascular permeability via paracellular and transcellular pathways. Circ Res. 2009;105:676–685. doi: 10.1161/CIRCRESAHA.109.201673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shajahan AN, Timblin BK, Sandoval R, Tiruppathi C, Malik AB, Minshall RD. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J Biol Chem. 2004;279:20392–20400. doi: 10.1074/jbc.M308710200. [DOI] [PubMed] [Google Scholar]
- 38.Minshall RD, Tiruppathi C, Vogel SM, Niles WD, Gilchrist A, Hamm HE, Malik AB. Endothelial cell-surface gp60 activates vesicle formation and trafficking via Gi-coupled Src kinase signaling pathway. J Cell Biol. 2000;150:1057–1070. doi: 10.1083/jcb.150.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu G, Vogel SM, Schwartz DE, Malik AB, Minshall RD. Intercellular adhesion molecule-1-dependent neutrophil adhesion to endothelial cells induces caveolae-mediated pulmonary vascular hyperpermeability. Circ Res. 2008;102:e120–e131. doi: 10.1161/CIRCRESAHA.107.167486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim MJ, Chiang ET, Hechtman HB, Shepro D. Inflammation-induced subcellular redistribution of VE-cadherin, actin and gamma-catenin in cultured human lung microvessel endothelial cells. Microvasc Res. 2001;62:366–382. doi: 10.1006/mvre.2001.2355. [DOI] [PubMed] [Google Scholar]
- 41.Gavard J, Hou X, Qu Y, Masedunskas A, Martin D, Weigert R, Li X, Gutkind JS. A role for a CXCR2/phosphatidylinositol 3-kinase gamma signaling axis in acute and chronic vascular permeability. Mol Cell Biol. 2009;29:2469–2480. doi: 10.1128/MCB.01304-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 43.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 44.Aberle H, Schwartz HR, Kemler R. Cadherin-catenin complex: Protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4<514::AID-JCB4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 45.Song L, Ge S, Pachter JS. Caveolin-1 regulates expression of junction-associated proteins in brain microvascular endothelial cells. Blood. 2007;109:1515–1523. doi: 10.1182/blood-2006-07-034009. [DOI] [PMC free article] [PubMed] [Google Scholar]