Abstract
The present study aimed to investigate the effect of electroacupuncture stimulation at CV4 (also termed Guanyuan) on femoral osteocalcin also termed bone gla protein (BGP), alkaline phosphatase (ALP), bone mineral density (BMD) and biomechanics, as well as the Wnt-β-catenin signaling pathway in rats with postmenopausal osteoporosis. Female Sprague-Dawley rats (4.5-months old) were randomly divided into sham, Ovx, CV4 and mock groups (n=10/group). With the exception of those in the sham group, the rats were ovariectomized to induce postmenopausal osteoporosis. The rats in the CV4 and mock groups were given electroacupuncture at CV4 and non-acupoint, respectively. The rats in the Ovx model and sham groups underwent identical fixing procedures, but did not undergo electroacupuncture. Following treatment, hematoxylin and eosin staining was used to observe morphological changes in the left femoral trabecular bone, and a three-point-bending test was used to analyze femur biomechanics and determine the BMD. In addition, an enzyme-linked immunosorbent assay was used to measure the serum levels of ALP/BGP and reverse transcription-quantitative polymerase chain reaction was used detect the expression levels of Wnt3a, β-catenin and Runx2. In the present study, it was demonstrated that electroacupuncture at CV4 significantly improved the osteoporotic morphological changes that occurred in the ovariectomized rats, increased serum ALP and BGP levels, enhanced the maximum and fracture loads, increased BMD (P<0.01), and activated the Wnt-β-catenin signaling pathway. These findings demonstrated that electroacupuncture stimulation at CV4 affected bone formation and promoted bone metabolism in rats with postmenopausal osteoporosis, possibly by activating the Wnt-β-catenin signaling pathway.
Keywords: postmenopausal osteoporosis, ALP, BGP, β-catenin, Wnt3a, Runx2
Introduction
Postmenopausal osteoporosis (PMOP) refers to clinical manifestations of increased bone fragility and susceptibility to fracture in postmenopausal women, and is characterized by low bone mass and bone tissue microstructure degenerative changes. It is caused by greater bone resorption than bone formation in bone metabolism as a result of decreased estrogen level and ovarian function (1). In Traditional Chinese medicine (TCM), PMOP is classified as 'bone atrophy'. Patients with PMOP-induced fractures are difficult to treat and often become bed-ridden. Long-term inability of self-care, lack of exercise and other activities not only seriously affect the health and quality of life of elderly people, but also increase the incidence of consolidated respiratory and circulatory system diseases, leading to markedly increased risk of mortality (2). At present, Western medicine lacks effective measures to return bone mass to normal levels in patients with osteoporosis, and the existing drug treatment choices are limited and controversial. For example, although estrogen replacement therapy is effective, it can increase the incidence of breast cancer and has unavoidable side effects. Bisphosphonates can reduce the incidence of fractures by inhibiting bone turnover, however, long-term use may increase the incidence of fatigue fracture and intertrochanteric fractures (3–6). By contrast, as an effective alternative therapy for osteoporosis, acupuncture has been widely used in China and abroad. Investigating the mechanisms underlying its effects is important.
TCM hypothesizes that kidney deficiency is the basic pathogenesis for PMOP. Previous studies have revealed that patients with kidney deficiency often exhibit hypothalamic-pituitary-gonadal axis dysfunction and decreased gonadal hormone secretion, which leads to a decline in osteoblast functions, reduced bone tissue per unit volume and eventually osteoporosis (7). According to TCM, the connections of Yin and Yang meridians are vital to human life, and therefore so is CV4 (also termed Guanyuan), an acupoint of RenMia (one of meridians) (8), since it converges with the three Yin meridians of the foot and connects the primordial Yin and the primordial Yang (8). Acupuncture at CV4 can enhance kidney function, strengthen human health, compensate vitality (9), restore the Yang, improve physique, protect human innate libido and exert a therapeutic effect for PMOP by targeting the causes (8). Our previous study demonstrated that acupuncture therapy can improve PMOP induced by deficiency of kidney essence and bone nutrition, and enhanced bone resorption by upregulating bone morphogenetic protein-2 is one of the important molecular biological mechanisms (10–15), suggesting that improving bone metabolism is an important mechanism of acupuncture action. Numerous previous studies have shown that activation of the canonical Wnt signaling pathway is important in bone cell metabolism, particularly osteoblast differentiation and proliferation (16–18). In the present study, a single acupuncture point, CV4, was selected as the optimum acupoint, and the effect of electroacupuncture at CV4 on the canonical Wnt signaling pathway was observed, with the aim of clarifying the molecular biological mechanisms underlying the effects of acupuncture treatment on osteoporosis.
Materials and methods
Experimental animals and grouping
A total of 40 female Sprague-Dawley rats (age, 4.5 months; weight, 250–290 g) were purchased from Fujian Medical University Experimental Animal Center (Fujian, China; lot no. 2007000530580). The rats were randomly assigned to sham, Ovx, CV4 and mock groups (n=10/group). With the exception of those in the sham group, the rats were ovariectomized to induce PMOP. The rats in the CV4 and mock groups subsequently received electroacupuncture at CV4 and non-acupoint, respectively, as described below. All animal treatments were strictly in accordance with international ethical guidelines and the National Institutes of Health Guide concerning the Care and Use of Laboratory Animals, and all experiments were approved by the Institutional Animal Care and Use Committee of Fujian University of TCM (Fujian, China).
Materials and reagents
The major instruments used in the present study were universal material testing machine (AG-IC 20KN; Shimadzu, Kyoto, Japan), optical microscope (YS2-H; Nikon Corporation, Tokyo, Japan), MOTIC6.0 image analysis software (Motic China, Xiamen, China), Olympus 1X70 inverted microscope (Olympus, Tokyo, Japan), enzyme-linked immunosorbent assay (ELISA) instrument and microplate washing machine (Bio-Tek Instruments, Inc., Winooski, VT, USA), electric incubator (ZW-A; Shanghai Jinghong experimental Equipment Co., Ltd., Shanghai, China), centrifuge (LXJ-II; Shanghai Anting scientific Instrument Factory, Shanghai, China), 0.5 inch acupuncture needles (0.30×13 mm, Suzhou Medical Supplies Co., Ltd., Suzhou, China), electroacupuncture device (Hua SDZ-V; Suzhou Medical Supplies Co., Ltd.), slides, syringes, cotton swabs, gauze, scalpels, scissors, plastic gloves and cotton gloves (Behrman Biotechnology Co., Ltd., Fuzhou, China).
Wnt3a (C64F2) rabbit mAb monoclonal (cat. no. 2721S; dilution, 1:1000) and β-catenin (D10A8) XP® rabbit mAb (cat. no. 8480S; dilution, 1:1000) antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA), and ready-to-use SABC immunohistochemical staining kit and diaminobenzidine kit were purchased from Wuhan Boster Biotechnology Co., Ltd., (Wuhan, China). The anesthetics, 2% pentobarbital sodium (Sigma-Aldrich, St. Louis, MO, USA) and estradiol valerate, were obtained from Guangzhou Schering Pharmaceutical Co., Ltd. (Guangzhou, China). The 0.9% sodium chloride solution, penicillin (40,000 U/ml), 75% ethanol and 4% paraformaldehyde were purchased from Behrman Biotechnology Co., Ltd.
Preparation of the rat osteoporosis model
The rat osteoporosis model was established by ovariectomy (OVx; estrogen withdrawal), as previously described (19). Briefly, the rats were anesthetized by intraperitoneally injecting 2% sodium pentobarbital, at a dose of 0.2 ml/100 g body weight, and an incision was made along the abdominal midline. Following the opening of the abdominopelvic cavity, the ovaries were identified and their pedicles were clamped and immediately ligated with 4-0 nylon suture. Subsequently, the ovaries were removed bilaterally at the junction of the uterine horns and hemostasis was checked. The excised ovaries were then washed in physiological solution (0.9% NaCl). Following this, the muscles and skin were sutured layer by layer. Following surgery, the rats were intraperitoneally injected with 1 ml penicillin saline (40,000 U/ml) daily for 3 consecutive days to prevent inflammation. All rats were maintained in clean, pathogen-free rooms, in an environment with controlled temperature (22°C), humidity (60%) and noise (<50 db), under a 12 h/12 h light/dark cycle with free access to water and a standard laboratory diet. One month following modeling, vaginal cytological examination was conducted as follows to observe vaginal changes and determine whether rats were successfully neutered: The rats were restrained and the tip of a plastic pipette, filled with PBS (HyClone Laboratories Inc.; GE Healthcare, Logan, UT, USA) or 0.9% saline (10 µl) was inserted into the vagina. The final flush, containing vaginal fluid, was collected in the pipette tip and was then placed on a glass slid. The characterization of the cell types was performed under light microscopy (magnification, ×10; YS2-H, Nikon Corporation, Tokyo, Japan). The predominant cell type following ovariectomy was found to be basal cells. At 90 days post-modeling, the PMOP model was established. Rats in the sham group underwent the same surgery as described above, with the exception that their ovaries were not resected. At 91 days post-modeling, rats were used for subsequent experiments.
Intervention methods
CV4 point in the rats was selected as described previously (20). Following the removal of hair at CV4, an alcohol swab (70–80% alcohol) was used to disinfect the penetration site (CV4 point) by scrubbing in a rotary motion starting at the centre of the site. Pre-packaged, disposable sterile 0.5-inch acupuncture needles (Suzhou Medical Supplies Factory Co., Ltd., Suzhou, China) were vertically inserted into the acupoints at a depth of 5 mm. An SDZ-V Electronic Acupuncture Treatment Instrument (hwato brand; Shanghai Xinhua E-General Merchandise Co., Ltd., Shanghai, China) was adopted with dilatational wave of AC PULSE current and connected to the acupuncture needles. The current intensity was 1 mA and the frequency 2 Hz. Needles were kept in the acupoints for 20 min once daily between 9:00 and 11:00 am, for three courses (10 days per course). The interval between courses was 1 day.
The rats in the mock group were treated similarly to the rats in the CV4 group, with the exception that a random point (non-acupoint), which was not located on the meridians of this study, was selected for acupuncture (20). The rats in the model and sham groups were treated as the rats in the CV4 group, with the exception that they were not administered any acupuncture.
One month following the intervention, the rats were sacrificed under anesthesia with pentobarbital sodium (100 mg/kg body weight) injected intraperitoneally to collect their bilateral femurs. Following the removal of muscle ligaments attached to the femoral head end, the left femur was stored in 10% neutral formalin solution at 4°C for future immunohistochemical examination. The right femur was wrapped in saline-buffered gauze and stored at −20°C.
Hematoxylin and eosin (H&E) staining
The rat femoral head was fixed in 10% paraformaldehyde for 48 h, and was decalcified with 10% EDTA (pH 7.2) for ~42 days. Following washing with running water for 24 h, the decalcified femur was dehydrated with an upward gradient alcohol solution, transparented with xylene, embedded in paraffin, sliced to 5 mm, mounted onto poly-lysine-coated slides, incubated overnight at 60°C, dewaxed and subsequently washed with water and phosphate-buffered saline (PBS) twice. The samples were then stained with hematoxylin for 7 min, soaked in 70% ethanol for 30 min to remove cytoplasm staining, alkalized using alkali solution, rinsed with distilled water for 1 min, stained with eosin for 40 sec, dehydrated with graded ethanol solution, transparented twice with xylene, dried, mounted and observed under an optical microscope for femoral morphology to determine whether the rats suffered from osteoporosis.
Measurement of bone biomechanical properties
Right femurs were placed in a Tsushima AG-20KNA universal test machine for measurement of biomechanical properties using a three-point bending method. Briefly, the femurs were soaked in saline at 23°C and placed on the test machine with the concave of the femoral head downward, femur head end forward and pressure head just over the mid femur. The pressure head was adjusted to as close as possible to the femur without touching, to avoid pressure on the femur. The load was applied to the femur with a load span of 24 mm and loading speed of 2 mm/min until the femur fractured. The maximum load and fracture load were recorded as the maximum femur withstand load and the maximum femur fracture load.
Bone mineral density (BMD) testing
The rats were anesthetized using 2 ml/kg of 2% sodium pentobarbital and fixed in the supine position on the board. Their BMD was measured using DEXL dual energy X-ray absorptiometry (Norland XR-26 Mark II, Norland Corp., Fort Atkinson, WI, USA). All scans were performed using the flexible accompanying software for small animals (version 2.5.0; Norland Corp.).
Measurement of alkaline phosphatase (ALP) and bone gla protein (BGP)
The activity of ALP was measured using a biochemical analyzer (MY-B010; Guangzhou Maya Medical Equipment Co., Ltd., Guangzhou, China) and the serum concentration of BGP was measured using an ELISA, according to the manufacturer's protocol.
Reverse transcription-polymerase chain reaction (RT-PCR)
The total RNA was extracted from the right femur using TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Oligo(dT)-primed RNA (1 µg) was reverse transcribed using SuperScript II Reverse Transcriptase Promega (Madison, WI, USA) according to the manufacturer's protocol. The obtained cDNA was used to determine the mRNA levels of Wnt3a, β-catenin and Runx2 using Taq DNA polymerase (Fermentas; Thermo Fisher Scientific, Inc., Waltham, MA, USA). GAPDH or β-actin was used as an internal control. The primers used were as follows: Wnt3a, 5′-TCCGACTCTTGGCAGAACTT-3′ forward and 5′-AATGGAATAGGTCCCGAACA-3′ reverse [annealing temperature (At)=59°C; 307 bp]; β-catenin, 5′-TGCTGAAGGTGCTGTCTGTC-3′ forward and 5′-TCGGTAATGTCCTCCCTGTC-3′ reverse (At=57°C; 394 bp); Runx2, forward: 5′-TCCAGCCACCTTCACTTACAC-3′ and reverse: 5′-GCGTCAACACCATCATTCTG-3′ (At= 55°C; 687 bp); β-actin, forward: 5′-ACTGGCATTGTGATGGACTC-3′ and reverse: 5′-CAGCACTGTGTTGGCATAGA-3′ (At= 55°C; 453 bp). The amplification consisted of 35 cycles of: 5 min pre-denaturation at 95°C, 30 sec denaturation at 94°C, 40 sec annealing at 72°C, 30 sec of extension at 72°C and 7 min final extension at 72°C, using a S1000™ Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Samples were analyzed by 1.5% agarose gel electrophoresis. The DNA bands were examined using a Gel Documentation System (Gel Doc 2000) Bio-Rad Laboratories, Inc., Hercules, CA, USA) and Image lab 3.0 (both from Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used for the analysis of the gray value data.
Immunohistochemistry
Following fixation in 10% paraformal-dehyde for 48 h, the rat femoral head was decalcified with 10% EDTA (pH 7.2) for ~42 days, with refreshing once/week until no significant resistance to pin passing through. Following washing with running water for 24 h, the decalcified femur head was dehydrated with upward gradient alcohol solution, transparented with xylene, embedded in paraffin, sliced to 5 mm and mounted onto poly-lysine-coated slides. The tissue sections were stained for Wnt3a and β-catenin using 100-fold diluted primary antibodies provided in the SABC kit in strict accordance with the manufacturer's protocol. A negative control was performed by replacing primary antibodies with PBS buffer. A total of five randomly selected fields (magnifi-cation, x400) were observed and images were captured using an Olympus 1X70 inverted microscope. Wnt3a and β-catenin positive cells, which were defined by the appearance of a brown substance in the cell membrane, cytoplasm and nucleus, were counted, and their gray values were measured using MOTIC6.0 software (Motic China). The average gray value was used to represent staining intensity.
Statistical analysis
The data were analyzed using SPSS 16.0 statistical software (SPSS, Inc., Chicago, IL, USA). Since all the data are measurement data, and met the normality and homogeneity of variance, analysis was performed using single-factor analysis of variance and expressed as the mean ± standard deviation. Differences between groups were analyzed using a least significant difference t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Electroacupuncture stimulation at CV4 prevents bone loss in the estrogen deficiency model
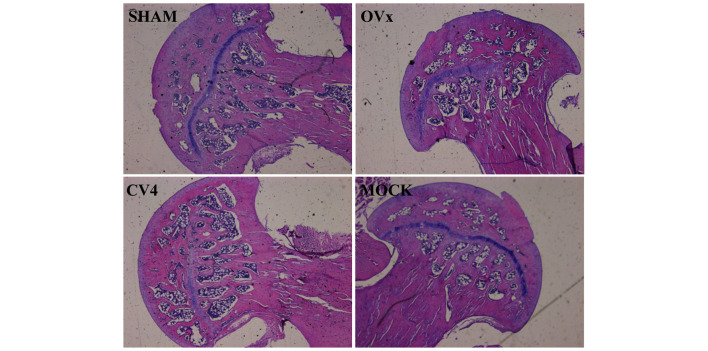
H&E staining revealed that 90 days after ovariectomy (Ovx group) a deterioration of the trabecular micro-architecture was observed, which was due to the destruction of the trabecular bone in the femoral head. Additionally, the group with electroacupuncture stimulation at CV4 (CV group) exhibited well-developed trabeculae, comparable with the control Sham group (Fig. 1). However, mock groups exhibited no improvement on the deterioration of trabeculae.
Figure 1.
Electroacupuncture stimulation at CV4 prevents bone loss in an estrogen deficiency model. Hematoxylin and eosin staining revealed ovariectomy-induced deterioration of the trabecular microarchitecture owing to the destruction of the trabecular bone in the femoral head (magnification, ×10). Electroacupuncture stimulation at CV4 led to well-developed trabeculae, compared with the sham group. However, the mock group did not exhibit an improvement in the deterioration of trabeculae. OVx, ovariectomy.
Electroacupuncture at CV4 improves biomechanical strength and BMD of rats
Biomechanical strength data revealed that ovariectomy in the OVx group decreased the maximum power and stiffness compared with the sham group (P<0.05; Table I). These decreases were reversed by electroacupuncture at CV4 (P<0.05), however, not at the non-acupoint (Table I). The BMD (Table II) of the OVx group was significantly decreased compared with that in the sham group (P<0.05). Electroacupuncture stimulation at CV4 (CV group) significantly increased the BMD. However, the mock group exhibited no significant change.
Table I.
Effects of various treatments on different bone parameters of osteopenic mice in different groups.
| Parameter | Group
|
|||
|---|---|---|---|---|
| Sham | Ovariectomy | CV4 | Mock | |
| Maximum power (N) | 32.16±2.89 | 20.66±4.07a | 28.31±5.12b | 23.67±4.24 |
| Stiffness (N/mm) | 58.51±2.26 | 45.76±3.11a | 54.11±3.98b | 47.96±5.21 |
Data are expressed as the mean ± standard deviation.
P<0.05 vs. the sham group and
P<0.05 vs. the ovariectomy group as determined using analysis of variance and a post hoc test. The experiment was performed in triplicate and similar results were obtained.
Table II.
Bone mineral density of rats in each group.
| Group | N | Bone mineral density |
|---|---|---|
| Sham | 10 | 0.206±0.006 |
| Ovariectomy | 10 | 0.192±0.009a |
| CV | 10 | 0.202±0.008b |
| Mock | 10 | 0.194±0.009 |
Data are expressed as the mean ± standard deviation.
P<0.05 vs. the sham group and
P<0.05 vs. the ovariectomy group as determined using analysis of variance and a post hoc test. The experiment was performed in triplicate and similar results were obtained.
Serum determination of ALP and BGP in rats
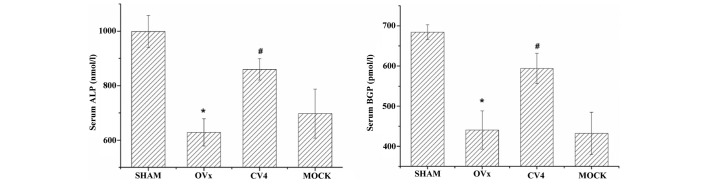
As shown in Fig. 2, the serum levels of ALP and BGP of rats in different groups were observed. Compared with those of the rats in the sham group, the serum levels of ALP and BGP were significantly reduced in rats in the model group (P<0.05), and this reduction was reversed by electroacupuncture at CV4 (P<0.05), however, not at the non-acupoint.
Figure 2.
Effects of various treatments on the serum levels of ALP and BGP in rats of different groups. The serum levels of ALP and BGP of rats in different groups were quantified. *P<0.05, compared with the sham group and #P<0.05, compared with the OVx group as determined using analysis of variance and a post hoc test. Experiments were performed in triplicate and similar results were obtained. OVx, ovariectomy group; ALP, alkaline phosphatase; BGP, bone gla protein.
Expression levels of Wnt3a, β-catenin and Runx2 of rats in different groups
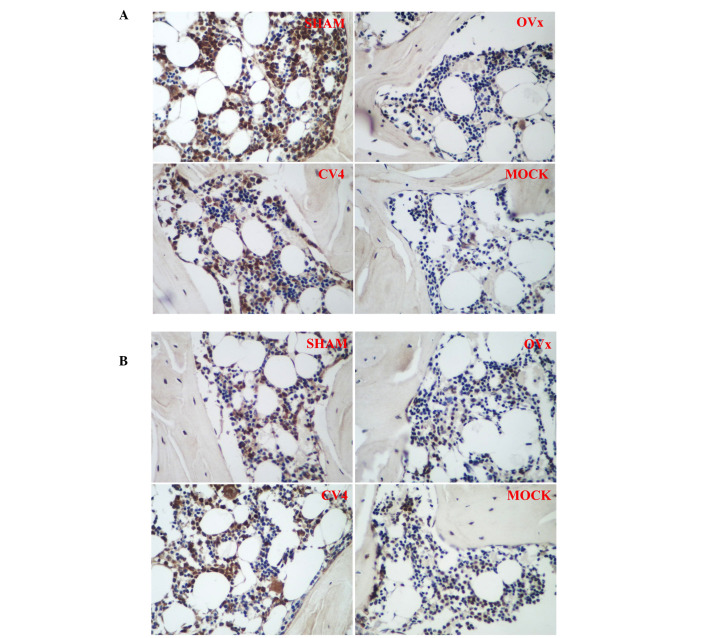
The RT-PCR results (Fig. 3) revealed that the expression levels of Wnt3a, β-catenin and Runx2 of the rats in the model group were significantly reduced compared with those of rats in the sham group (P<0.05), and these reductions were reversed by electroacupuncture at CV4 (P<0.05), however, not at the non-acupoint (P>0.05). Immunohistochemical results (Fig. 4; Table III) indicated that the expression levels of Wnt3a and β-catenin were significantly reduced in rats in the model group compared with the rats in the sham group (P<0.05), and this reduction was significantly reversed by electroacupuncture at CV4 (P<0.05), however, not at the non-acupoint (P>0.05).
Figure 3.
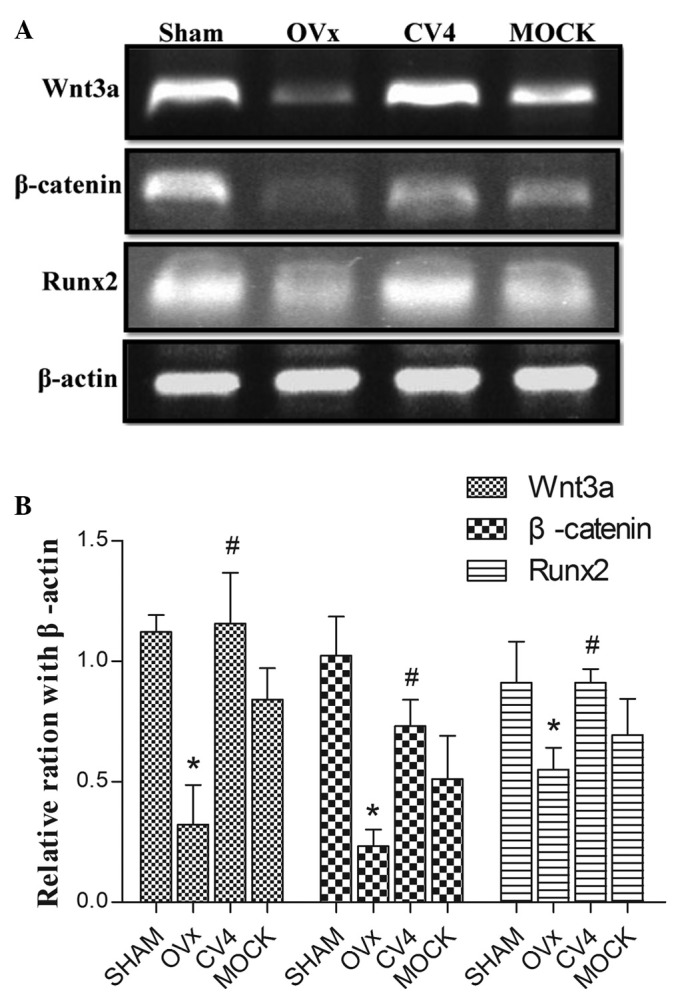
(A) mRNA expression levels of Wnt3a, β-catenin and Runx2 in different experimental groups. β-actin was used as an internal control for RT-PCR. (B) The quantified RT-PCR data are presented as the gray values of different groups. *P<0.05 versus Sham; #P<0.05 versus OVx. Experiments were performed in triplicate and similar results were obtained. OVx, ovariectomy; RT-PCR, reverse transcription-polymerase chain reaction.
Figure 4.
Expression and localization of Wnt3a and β-catenin in different experimental groups. Representative images are shown (magnification, ×400). The cells were stained for (A) Wnt3a or (B) β-catenin. Cells with brown or brownish yellow granules were positively stained cells, and deeper color indicates a more marked expression. Quantification of the immunohistochemistry assay are showed in Table III. OV, ovariectomy.
Table III.
Quantification of the immunohistochemistry assay as presented as the gray value of positively stained cells.
| Group | Wnt3a | β-catenin |
|---|---|---|
| Sham | 40.88±7.12 | 37.06±4.78 |
| Ovariectomy | 20.16±4,24a | 25.19±3.58a |
| CV | 43.35±8.11b | 36.93±5.18b |
| Mock | 25.29±6.93 | 28.13±2.21 |
Data are expressed as the mean ± standard deviation.
P<0.05 vs. the sham group and
P<0.05 vs. the ovariectomy group as determined using analysis of variance and a post hoc test. The experiment was performed in triplicate and similar results were obtained.
Discussion
Osteoblasts are the major functional cells involved in bone formation during bone metabolic processes. The Wnt signal transduction pathway is activated in the nucleus through β-catenin and is important in the osteoblast differentiation and proliferation processes. Among the involved proteins, Wnt3a as an initiation factor of the classic Wnt/β-catenin pathway, is pivotal to osteoblast proliferation and differentiation (21). β-catenin is key factor of the Wnt signaling pathway. It is predominantly present in the cytoplasm, but also in the cell membrane and nucleus. In the cytoplasm, when the Wnt signaling pathway is inactive, β-catenin is phosphorylated, which in turn activates the ubiquitin system, leading to its degradation through the proteasomal pathway (22–26). In the nucleus, β-catenin binds to intranuclear transcription factor, TCF/LEF, and promotes the transcription of its target genes, including c-myc and cyclin D1 (27,28), which allows the cells to enter S phase and differentiate and proliferate. Runx2 and c-myc are the two downstream target genes of the Wnt/β-catenin pathway (29), and c-myc regulates the cell cycle (30). When beta-catenin levels are elevated, it has been shown to translocate to the nucleus, where it complexes with Tcf-4 and regulates the expression of c-myc mediated through Tcf-4 binding sites in the c-myc promoter. This has been demonstrated to promote the proliferation and differentiation of osteoblasts (30). In addition, the activation of Runx2 in the nucleus significantly stimulates osteoblast differentiation and proliferation (31). The present results revealed that electroacupuncture at CV4 promoted the expression levels of β-catenin, Wnt3a and Runx2, suggesting that electroacupuncture at CV4 activated the classical Wnt/β-catenin pathway, and promoted the proliferation and differentiation of osteoblasts, resulting in treatment of osteoporosis. In addition, the femur bone tissue morphology revealed that rats in the model group exhibited a typical osteoporotic morphology suggesting that the experimental osteoporosis model was successfully established. Rats treated with electroacupuncture at CV4 exhibited well-developed trabeculae, suggesting that this significantly improved the pathological changes that occur in osteoporosis. The results also revealed that electroacupuncture at CV4 enhanced the levels of ALP and BGP, increased femur maximum load and fracture load, and increased BMD. The levels of ALP and BGP may affect bone formation during bone metabolism, which is indicated by improved bone strength, the specific marker for bone formation. Elevated serum levels of ALP and BGP indicated that electroacupuncture at CV4 improved bone metabolism, activated the Wnt/β-catenin pathway, and promoted osteoblast proliferation and differentiation, thereby improving osteoporosis induced by ovariectomy. However, the mock group showed no significant improvement in the deterioration of trabeculae and biomechanical change, the serum levels of ALP and BGP, or the activation of the Wnt-β-catenin signaling pathway. This indicated that electroacupuncture at CV4, however, not at the non-acupoint, significantly improved the ovariectomization-induced morphological changes of osteoporosis in rats.
In conclusion, these results showed that electroacupuncture at CV4 has a positive effect on the treatment of osteoporosis in postmenopausal rats, and the activation of the Wnt-β-catenin pathway may be one of the underlying mechanisms.
Acknowledgments
The present study was supported by the Natural Science Foundation of China (nos. 81173347 and 81102651).
Abbreviations
- PMOP
postmenopausal osteoporosis
- BGP
bone gla protein
- ALP
alkaline phosphatase
- BMD
bone mineral density
- OVx
ovariectomy
References
- 1.Xu ZY, Liang JG, Jiang NY, et al. Preliminary analysis of bone mineral mass variation in perimenopausal and postmenopausal women. Chinese Journal of Osteoporosis. 2006;12:378–396. In Chinese. [Google Scholar]
- 2.Semerano L, Guillot X, Rossini M, Avice E, Bégué T, Wargon M, Boissier MC, Saidenberg-Kermanac'h N. What predicts initiation of osteoporosis treatment after fractures: Education organisation or patients' characteristics? Clin Exp Rheumatol. 2011;29:89–92. [PubMed] [Google Scholar]
- 3.Ma J, Zheng HG. Cellular and molecular biological mechanisms for osteoporosis prevention and treatment using traditional Chinese medicine for kidney function improvement. Journal of Traditional Chinese Medicine. 2002;20:161–162. In Chinese. [Google Scholar]
- 4.Li J, Chen XG, Wu QF, et al. Experimental study on the role of Jiugulin Pill in retinoic acid-induced osteoporosis. Journal of First Military Medical University. 1999;19:242. In Chinese. [Google Scholar]
- 5.Armamento-Villareal R, Napoli N, Panwar V, Novack D. Suppressed bone turnover during alendronate therapy for high-turnover osteoporosis. N Engl J Med. 2006;355:2048–2050. doi: 10.1056/NEJMc062268. [DOI] [PubMed] [Google Scholar]
- 6.Kawai M, Mödder UI, Khosla S, Rosen CJ. Emerging therapeutic opportunities for skeletal restoration. Nat Rev Drug Discov. 2011;10:141–156. doi: 10.1038/nrd3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu MG, Wu BH, Liu XX, et al. experimental and clinical studies on the effects of Moxibustion on postmenopausal osteoporosis. Chinese acupuncture. 2002;22:335–336. In Chinese. [Google Scholar]
- 8.Lu JP, Cui YL, Shi RH. Chinese acupuncture and moxibustion. In: Zhang EQ, editor. A Practical English-Chinese Library of Traditional Chinese Medicine. 1st edition. Vol. 8. Publishing House of Shanghai College of Traditional Chinese Medicine; Shanghai: 1990. pp. 103–108. [Google Scholar]
- 9.Zhang F, Feng Y, Zhou R, Zhang YQ, Chen BY. Observation on the central afferent pathway of "Guanyuan" (CV 4) under normal and pathological states and the influence of electroacupuncture. Zhen Ci Yan Jiu. 2008;33:147–153. In Chinese. [PubMed] [Google Scholar]
- 10.Li P, Ji F, Lin Y, et al. Effects of acupuncture on 'Cuihui' point on the quality of life of patients with postmenopausal osteoporosis. Journal of Gansu College of Traditional Chinese Medicine. 2010;27:45–47. In Chinese. [Google Scholar]
- 11.Li P, Ji F, Chen GZ. Clinical observation of the effects of combined acupuncture and drug treatment on bone metabolism-related hormones in postmenopausal patients. Journal of Fujian College of Traditional Chinese Medicine. 2005;15:21–22. In Chinese. [Google Scholar]
- 12.Li P, Lin Y, Xu JB. Clinical studies on the effects of combined acupuncture and drug treatment on bone metabolism in postmenopausal women - analysis of 20 cases. Fujian Journal of Medicine. 2009;31:131–133. In Chinese. [Google Scholar]
- 13.Zhang ML, Ji F, Lin Y, et al. Effects of electroacupunture on Guanyuan point on IGF-1 and bone biomechanics in ovariectomized-induced rat osteoporosis model. Acupuncture Study. 2014;39:207–210. In Chinese. [PubMed] [Google Scholar]
- 14.Huang GR, Li P, Fan HL, et al. Effect of electroacupunture on Mingmeng Point on bone formation protein-2 in postmenopausal rat osteoporosis model. Acupuncture Study. 2014;39:130–4135. In Chinese. [Google Scholar]
- 15.Liu F, Xiong J, Huang GY, Wang W. Study on the underlying mechanism of acupuncture in regulating neuroendocrine activity in dysmenorrhea rats. Zhen Ci Yan Jiu. 2009;34:3–8. [PubMed] [Google Scholar]
- 16.Zheng X, Wu G, Nie Y, Lin YL. Electroacupuncture at the governor vessel and bladder meridian acupoints improves postmenopausal osteoporosis through osteoprotegerin/RANKL/RANK and Wnt/β-catenin signaling pathways. Exp Ther Med. 2015;10:541–548. doi: 10.3892/etm.2015.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondo A, Tokuda H, Mizutani J, Matsushima-Nishiwaki R, Kozawa O, Otsuka T. Wnt3a upregulates prostaglandin F2α-stimulated vascular endothelial growth factor synthesis in osteoblasts. Mol Med Rep. 2012;6:421–425. doi: 10.3892/mmr.2012.916. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Peng J, Wu M, Ye H, Zheng C, Wu G, Xu H, Chen X, Liu X. BMP2 promotes chondrocyte proliferation via the Wnt/β-catenin signaling pathway. Mol Med Rep. 2011;4:621–626. doi: 10.3892/mmr.2011.474. [DOI] [PubMed] [Google Scholar]
- 19.Zhang HX, Mei H, Luo XD. Preventive effects of Xianlinggubao on ovariectomized-induce rats osteoporosis model. Chinese Journal of Traditional Medical Traumatology. 2011;19:672–675. In Chinese. [Google Scholar]
- 20.Liu F, Xiong J, Huang GY, Wang W. Study on the underlying mechanism of acupuncture in regulating neuroendocrine activity in dysmenorrhea rats. Zhen Ci Yan Jiu. 2009;34:3–8. In Chinese. [PubMed] [Google Scholar]
- 21.Rulifson EJ, Wu CH, Nusse R. Pathway specificity by the bifunctional receptor frizzled is determined by affinity for wingless. Mol Cell. 2000;6:117–126. doi: 10.1016/S1097-2765(05)00018-3. [DOI] [PubMed] [Google Scholar]
- 22.Kestler HA, Kühl M. From individual Wnt pathways towards a Wnt signaling network. Philos Trans R Soc Lond B Biol Sci. 2008;363:1333–1347. doi: 10.1098/rstb.2007.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kieslinger M, Folberth S, Dobreva G, Dorn T, Croci L, Erben R, Consalez GG, Grosschedl R. EBF2 regulates osteoblast-dependent differentiation of osteoclasts. Dev Cell. 2005;9:757–767. doi: 10.1016/j.devcel.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Shi YC, Worton L, Estehen L, Baldock P, Fong C, Eisman JA, Gardiner EM. Effects of continuous activation of vitamin D and Wnt response pathways on osteoblastic proliferation and differentiation. Bone. 2007;41:87–96. doi: 10.1016/j.bone.2007.04.174. [DOI] [PubMed] [Google Scholar]
- 25.Price MA. CKI, there's more than one: Casein kinase I family members in Wnt and Hedgehog signaling. Genes Dev. 2006;20:399–410. doi: 10.1101/gad.1394306. [DOI] [PubMed] [Google Scholar]
- 26.Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J Biol Chem. 2006;281:9971–9976. doi: 10.1074/jbc.M508778200. [DOI] [PubMed] [Google Scholar]
- 27.Takemaru KI, Moon RT. The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. J Cell Biol. 2000;149:249–254. doi: 10.1083/jcb.149.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shtutman M, Zhurinsky I, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaiswal AS, Marlow BP, Gupta N, Narayan S. Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (difemylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21:8414–8427. doi: 10.1038/sj.onc.1205947. [DOI] [PubMed] [Google Scholar]
- 30.Licchesi JD, Van Neste L, Tiwari VK, Cope L, Lin X, Baylin SB, Herman JG. Transcriptional regulation of Wnt inhibitory factor-1 by Miz-1/c-Myc. Oncogene. 2010;29:5923–5934. doi: 10.1038/onc.2010.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Canonical Wnt signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280:33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]