Abstract
Cytochrome P450 2C19 (CYP2C19) is a highly polymorphic gene, it codes for a protein responsible for the metabolism of multiple clinically important therapeutic agents. However, there is currently no available data on the distribution of CYP2C19 mutant alleles in the Tibetan population. The aim of the present study was to identify different CYP2C19 mutant alleles and determine their frequencies, along with genotypic frequencies, in the Tibetan population. The whole CYP2C19 gene was amplified and sequenced in 96 unrelated, healthy Tibetans from the Tibet Autonomous Region of China, the promoter region, exons, introns and the 3′-UTR were screened for genetic variants. Three novel genetic polymorphisms in CYP2C19 were detected among a total of 27 different mutations. The allele frequencies of CYP2C19*1A, *1B, *2A, *3A and *17 were 50, 28.13, 15.10, 5.21 and 1.56%, respectively. The most common genotype combinations were CYP2C19*1A/*1B (56.25%) and *1A/*2A (30.21%). One novel non-synonymous mutation (Asn to Lys) in CYP2C19 was identified, and this mutation was predicted to be intolerant and benign by SIFT and PolyPhen-2, respectively. The observations of the present study may have important clinical implications for the use of medications metabolized by CYP2C19 among Tibetans.
Keywords: CYP2C19, genetic polymorphism, genotype, phenotype, Tibetan
Introduction
The cytochrome P450 2C (CYP2C) subfamily of enzymes metabolizes ~20–30% of all pharmaceuticals in use today (1). CYP2C19 comprises 16% of the CYP2C subfamily (2) and is involved in the metabolism of a range of clinically important compounds, including the antiplatelet therapeutic agent clopidogrel; anticonvulsants such as phenytoin and diazepam; proton pump inhibitors such as omeprazole and lansoprazole; tricyclic antidepressants such as amitriptyline and clomipramine; and the selective serotonin reuptake inhibitor citalopram (3–7).
An association has been identified between CYP2C19 genetic variation and therapeutic outcomes, adverse drug reactions and treatment failures (8). Among the various characterized polymorphic variants of CYP2C19, the most common loss of function mutations are CYP2C19*2 (681G>A, rs4244285) and CYP2C19*3 (636G>A, rs4986893) (9). By contrast, the common CYP2C19*17 allele (-806CNT, rs12248560) has been associated with increased gene expression and enzyme activity (9). CYP2C19 exhibits genetic polymorphisms among different races, as demonstrated by variations in the metabolism of therapeutic agents (10). A previous study evaluated enzymatic activity and genotypic association of CYP2C19 among Chinese, Korean, Japanese and Caucasian populations (11).
Tibet is one of the oldest ethnic groups in China, with their own spoken and written language. To the best of our knowledge, no genotypic information on CYP2C19 mutants in this population is currently available. The aim of the present study was to determine CYP2C19 mutant allele and genotype frequencies in a healthy Tibetan population. The results were compared with CYP2C19 genetic polymorphisms in other populations. The results of the present study may assist in predicting adverse effects and optimization of dosage and treatment with medications metabolized by CYP2C19 in the Tibetan population.
Materials and methods
Participants
A group of 96 normal, healthy, non-related Tibetans (including 48 males and 48 females) were recruited between October and December 2009 from Xizang Minzu University in Xianyang. All participants were Tibetan Chinese living in the Tibet Autonomous Region of China, with a minimum of three generations of paternal ancestry within this ethnic group. Subjects with any type of medical illness, organ transplant, drug/alcohol addiction or those who were pregnant were excluded from the study. These exclusion criteria were used to minimize factors that may have influenced genetic variation in the genes of interest.
The present study was approved by The Human Research Committee of the Xizang Minzu University for Approval of Research Involving Human Subjects; all subjects were informed, verbally and in writing, about the experimental procedures, confidentiality and the purpose of the study. All participants gave their written informed consent prior to enrollment in the study.
Genotyping of CYP2C19
A blood sample (5 ml) was taken from each subject in an EDTA tube (Jiangsu Kangjie Medical Devices Co., Ltd. Jiangyan, China) and DNA was extracted using the GoldMag-Mini Whole Blood Genomic DNA Purification kit (GoldMag Co., Ltd., Hainan, China), according to the manufacturer's instructions. Primers (presented in Table I; Sangon Biotech, Shanghai, China) were designed to amplify the 5′-flanking regions, all exons, and all introns of the CYP2C19 gene. Polymerase chain reaction (PCR) was performed for all single nucleotide polymorphisms (SNPs) in 10 µl reactions with 1 µl template DNA, 5 µl HotStar Taq Master mix (Qiagen, Germantown, MD, USA), 0.5 µl each primer (5 µM) and 3 µl deionized water. The thermal cycling conditions were as follows: Denaturation step of 15 min at 95°C; followed by 35 cycles of denaturation at 95°C for 30 sec, 55–64°C for 30 sec and 72°C for 1 min; and a final extension step at 72°C for 3 min. PCR products were incubated with 0.5 µl shrimp alkaline phosphatase (Roche Diagnostics, Basel, Switzerland), 8 µl HotStar PCR product, and 1.5 µl deionized water (to a total volume of 10 µl), at 37°C for 30 min, followed by heat inactivation at 80°C for 15 min. Purified PCR products were sequenced directly using the ABI Prism BigDye Terminator Cycle Sequencing kit version 3.1 (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA), using an ABI Prism 3100 sequencer (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Table I.
Primers and amplicon sizes for cytochrome P450 2C19.
| Primer | Primer sequence (5′→3′) | Polymerase chain reaction product size (bp) |
|---|---|---|
| Promoter | F: GCCTGTTTTATGAACAGGATGA | 918 |
| Promoter | R: TAAGACAACCGTGAGCTTGC | |
| Exon1 | F: ACAGAGTGGGCACTGGGACGA | 844 |
| Exon1 | R: GGTCCTAAACCCACAGCTGCTTCC | |
| Exon2_3 | F: TTGTCTGACCATTGCCTTGA | 833 |
| Exon2_3 | R: TCTCAGCTTCAAACCCTGCT | |
| Exon4 | F: CCCCAACTATTCTCACCCTTT | 916 |
| Exon4 | R: AAAGTGTGAATTGAAGGACAAGC | |
| Exon5 | F: TCAGGTTGTGCAAACTCTTTT | 908 |
| Exon5 | R: CCTTCACTCACTTTTTGATGGA | |
| Exon6 | F: ATGTTGGTAAGTATACAATGTGAGT | 386 |
| Exon6 | R: TCACACCATTAAATTGGGACAGA | |
| Exon7 | F: TTTTGATTGGAAATTTTAGTCCATT | 921 |
| Exon7 | R: TCAGTTCTTTCCAAACTGACCT | |
| Exon8 | F: GTCACTGGCCTTAAGCTCATGCCT | 718 |
| Exon8 | R: CCCAGCCTAGGGGGTGAGGG | |
| Exon9 | F: TGAGAGTAGGGGAGGTGAAGA | 907 |
| Exon9 | R: GATGACGGGTCAGAAGAAGC | |
| 3′-UTR | F: ACGGATTTGTGTGGGAGAGGGC | 674 |
| 3′-UTR | R: AATGCTCAGCCAAAATAGCTTCCCT |
bp, base pairs; UTR, untranslated region.
Statistical analysis
Sequencher 4.10.1 (http://www.genecodes.com/) software was used to analyze the sequences, including manual curation, fragment assembly and mutation detection. CYP2C19 variants were designed based on the nucleotide reference sequence NG_008384.2, which is searched from the National Center for Biotechnology Information database (NCBI database, http://www.ncbi.nlm.nih.gov/). The CYP allele nomenclature is quoted from the Human Cytochrome P450 Allele Nomenclature Committee tables (http://www.cypalleles.ki.se/). The χ2 test was used to compare allele and genotype frequencies, with descriptive analysis used to compare allele frequencies between the Tibetan and other populations (12). Haploview 4.1 (http://broad.mit.edu/mpg/haploview) was used to assess linkage disequilibrium (LD) and Hardy-Weinberg equilibrium for each genetic variant (13). Haplotypes were constructed from the selected tag SNPs and haplotype frequencies were derived for the Tibetan population.
Translational prediction
Non-synonymous SNPs in the CYP2C19 coding regions were analyzed to predict the coded protein function. Two algorithms, Sorting Intolerant From Tolerant (SIFT; http://sift.bii.a-star.edu.sg/) and Polymorphism Phenotyping version 2 (PolyPhen-2; http://genetics.bwh.harvard.edu/pph2/), were used to perform the functional prediction of non-synonymous SNPs (14). Depending on the metabolic activity of CYP2C19, the subjects were divided into three phenotypic groups: Poor metabolizer (PM), extensive metabolizer and ultra-rapid metabolizer, based on CYP allele nomenclature (http://www.cypalleles.ki.se/) (15).
Results
Genetic variants
CYP2C19 was sequenced in the group of 96 Tibetan participants (48 males and 48 females) and the results successfully identified a total of 27 CYP2C19 polymorphisms in this population. Three of the polymorphisms had not previously been reported in either the National Center for Biotechnology Information database or the Human Cytochrome P450 Allele Nomenclature Committee tables (Table II), two of the novel polymorphisms were synonymous mutations within exon five and one was a non-synonymous mutation in exon eight. No duplications or deletions within the sequenced CYP genes were observed.
Table II.
Positions and frequencies of cytochrome P450 2C19 genetic variants in the Tibetan study population.
| No. | SNP | Position | Region | Nucleotide change | Allele | Frequency | Percentage (%) | Amino-acid effect |
|---|---|---|---|---|---|---|---|---|
| 1 | rs576566073 | −844 | Promoter | G>T | 1/96 | 1.042 | Not translated | |
| 2 | rs12248560 | −806 | Promoter | C>T | CYP2C19*17 | 3/96 | 3.125 | Not translated |
| 3 | / | −643 | Promoter | C>T | 1/96 | 1.042 | Not translated | |
| 4 | / | −597 | Promoter | A>G | 1/96 | 1.042 | Not translated | |
| 5 | rs17885098 | 99 | Exon 1 | C>T | 86/96 | 89.583 | Pro33a | |
| 6 | / | 483 | Intron 1 | G>A | 1/96 | 1.042 | Not translated | |
| 7 | rs7918461 | 527 | Intron 1 | A>T | 3/96 | 3.125 | Not translated | |
| 8 | rs4986893 | 17948 | Exon 4 | G>A | CYP2C19*3A | 11/86 | 12.644 | Trp212Ter |
| 9 | rs7088784 | 18911 | Intron 4 | A>G | 22/96 | 22.917 | Not translated | |
| 10 | rs4244285 | 19154 | Exon 5 | G>A | CYP2C19*2A | 39/96 | 40.625 | Pro227a |
| 11 | 19184 | Exon 5 | T>C | Novel 1 | 1/96 | 1.042 | Leu237a | |
| 12 | 19280 | Exon 5 | C>A | Novel 2 | 1/96 | 1.042 | IlE269a | |
| 13 | rs12571421 | 19520 | Intron 5 | A>G | 39/96 | 40.625 | Not translated | |
| 14 | rs557466494 | 58017 | Intron 6 | G>A | 1/96 | 1.042 | Not translated | |
| 15 | rs28399513 | 79936 | Intron 6 | T>A | 37/94 | 39.362 | Not translated | |
| 16 | rs374366253 | 79980 | Intron 6 | T>C | 1/94 | 1.064 | Not translated | |
| 17 | rs3758580 | 80160 | Exon 7 | C>T | CYP2C19*2A | 37/94 | 39.362 | Val330a |
| 18 | rs3758581 | 80161 | Exon 7 | A>G | 93/94 | 98.936 | Ile331Valb | |
| 19 | rs4917623 | 87106 | Intron 7 | T>C | 79/96 | 82.292 | Not translated | |
| 20 | rs17886522 | 87313 | Exon 8 | A>C | CYP2C19*3A | 12/96 | 12.500 | Gly417a |
| 21 | 87331 | Exon 8 | C>A | Novel 3 | 2/96 | 2.083 | Asn423Lysb | |
| 22 | rs17882572 | 87594 | Intron 8 | G>T | 13/96 | 12.500 | Not translated | |
| 23 | rs17885052 | 87620 | Intron 8 | A>T | 22/96 | 22.917 | Not translated | |
| 24 | rs12268020 | 89909 | Intron 8 | C>T | 3/96 | 3.125 | Not translated | |
| 25 | / | 90302 | 3′-UTR | C>T | 1/96 | 1.042 | Not translated | |
| 26 | / | 90325 | 3′-UTR | C>T | 2/96 | 2.083 | Not translated | |
| 27 | rs185030154 | 90581 | 3′-UTR | T>C | 3/96 | 3.125 | Not translated |
Synonymous mutations.
Non-synonymous mutations. UTR, untranslated region; SNP, single nucleotide polymorphism.
Allele and genotype frequency
A total of five CYP2C19 alleles were detected in the Tibetan study population (Table III). The CYP2C19*1A allele had the highest frequency (50%), followed by the CYP2C19*1B allele (28.13%) and the CYP2C19*2A allele (15.10%). The other two identified alleles, CYP2C19*3A and *17, were relatively rare with frequencies of 5.21% and 1.56%, respectively. These results indicate that alleles that do not affect the function of CYP2C19 (CYP2C19*1A and *1B), are the most prevalent with a combined frequency of 68.13%, followed by alleles that inactivate enzyme function (CYP2C19*2A and *3A) with a combined frequency of 20.31%. The CYP2C19*17 allele, which may increase the activity of CYP2C19, had the lowest frequency of 1.56%.
Table III.
Allele and genotype frequencies of CYP2C19 in the Tibetan study population.
| A, Allele frequencies
| |||
|---|---|---|---|
| CYP2C19 | Total (n=96) | Phenotype | Frequency (%) |
| *1A | 96 | Normal | 50 |
| *1B | 54 | Normal | 28.13 |
| *2A | 29 | None | 15.10 |
| *3A | 10 | None | 5.21 |
| *17 | 3 | Increased | 1.56 |
| B, Genotype frequencies
| |||
|---|---|---|---|
| CYP2C19 | Total (n=96) | Phenotype | Frequency (%) |
| *1A/*1B | 54 | Normal enzyme activity | 56.25 |
| *1A/*2A | 29 | Reduced enzymatic activity | 30.21 |
| *1A/*3A | 10 | Reduced enzymatic activity | 10.42 |
| *1A/17 | 3 | Increased enzyme activity | 3.13 |
CYP2C19, cytochrome P450 2C19.
Four CYP2C19 genotypes were identified, with frequencies ranging from 3.13 to 56.25% in the Tibetan population under study (Table III). Of the identified genotypes, one exhibits normal enzymatic activity, two exhibit reduced enzymatic activity and one exhibits increased enzymatic activity. The CYP2C19 allele frequencies were further compared with previously published data from different countries and ethnic groups in Eastern Asia (16–19), Southern Asia (20), Europe (21–25) and Africa (26–28) (Table IV). The results of the present study demonstrated that the frequency of the wild-type allele, CYP2C19*1, in the Tibetan group was significantly lower than in Caucasian populations (P<0.05), however was highest in Asian populations. Furthermore, the frequencies of CYP2C19*2 and CYP2C19*3 were significantly higher (P<0.05) among those of Tibetan descent compared with Caucasian and African populations.
Table IV.
CYP2C19 allele frequencies in different populations.
| Population | n | Allele frequency (%)
|
Ref. | ||||
|---|---|---|---|---|---|---|---|
| CYP2C19*1 | CYP2C19*2 | CYP2C19*3 | CYP2C19*4 | CYP2C19*17 | |||
| Asian subjects | |||||||
| Tibetan | 96 | 78.13 | 15.10 | 5.21 | / | 1.56 | Present |
| Chinese Han | 100 | 67.50 | 25.50 | 2.00 | 0.50 | 3.00 | (16) |
| Chinese Dai | 193 | 66.30a | 30.30b | 3.40 | / | / | (17) |
| Japanese | 140 | 53.90b | 35.00b | 11.10 | / | / | (18) |
| Korean | 103 | 67.00 | 21.00 | 12.00 | / | / | (19) |
| Vietnamese | 90 | 62.00a | 24.00 | 14.00a | / | / | (20) |
| Thai | 121 | 59.90b | 35.10b | 5.00 | / | / | (20) |
| Caucasian subjects | |||||||
| Swedish | 175 | 76.60 | 23.10 | 0.30b | / | / | (21) |
| Russian | 290 | 88.30a | 11.40 | 0.30b | / | / | (22) |
| Italian | 360 | 88.90b | 11.10 | 0.00b | / | / | (23) |
| Bolivian | 778 | 92.10b | 7.80a | 0.10b | / | / | (24) |
| Faroese | 312 | 97.10b | 2.90b | 0.00b | / | / | (25) |
| African subjects | |||||||
| Tanzanian | 251 | 81.50 | 17.90 | 0.60b | / | / | (26) |
| Ethiopian | 114 | 84.00 | 14.00 | 2.00 | / | / | (27) |
| Zimbabwean | 84 | 86.90 | 13.10 | 0.00a | / | / | (28) |
P<0.01, compared with the data of the present study;
P<0.05, compared with the data of the present study. / indicates synonymous SNP mutations that have no effects on protein sequences. CYP2C19, cytochrome P450 2C19.
LD analysis
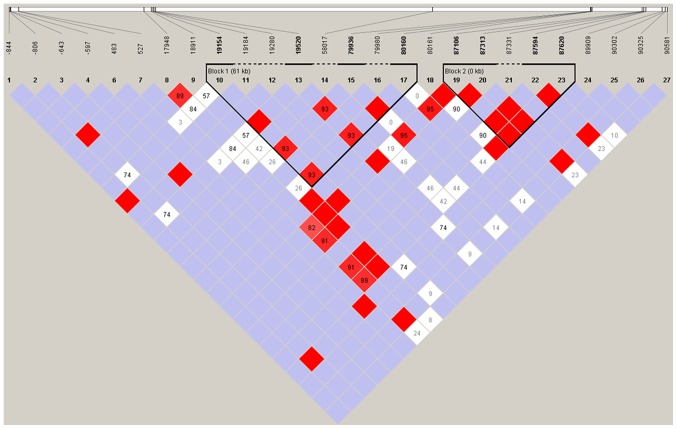
LD analysis was performed using Haploview with confidence intervals to define LD blocks (Fig. 1). The LD was determined for CYP2C19 using those SNPs whose minor allele frequencies were >0.05, as SNPs with low frequency have little power to detect LD. The deviation from the expected (D') was calculated, in addition to the correlation of alleles at two loci (r2) and the LD statistic for all possible pairs of SNPs. Two LD blocks across CYP2C19 were identified. Block 1, the larger of the two blocks, spans a 61 kb region from nucleotide 19154 (rs4244285) in the promoter region to nucleotide 80160 (rs3758580). Block 2 includes five tightly clustered SNPs (rs4917623, rs17886522, 2C19_87331, rs17882572 and rs17885052), each with a D' value equal to 1 (indicating complete LD).
Figure 1.
LD analysis of cytochrome P450, family 2, subfamily C, polypeptide 19. LD is presented with standard color schemes, bright red for very strong LD (LOD>2, D'=1), pink-red and blue for intermediate LD (LOD>2, D'<1 and LOD<2, D'=1, respectively) and white for no LD (LOD<2, D<1). Block 1 spans a 61 kb region from the nucleotide 19154 (rs4244285) in the promoter region to nucleotide 80160 (rs3758580). Block 2 includes five tightly clustered SNPs (rs4917623, rs17886522, 2C19_87331, rs17882572 and rs17885052), each with a D' value equal to 1 (indicating complete LD). LD, linkage disequilibrium, LOD, logarithm (base 10) of odds.
Protein function prediction
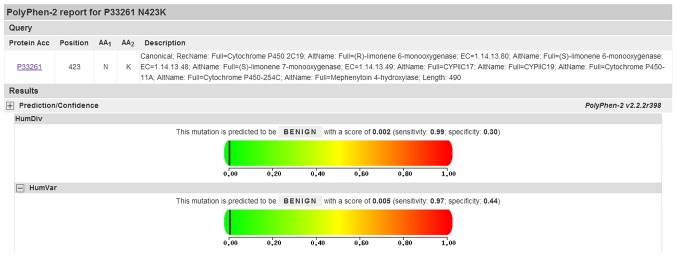
Three novel CYP2C19 variants were identified with only one of these being a non-synonymous mutation. SIFT was used to predict the effect of mutations on CYPC219 function, with the Ala423Lys substitution predicted to affect protein function with a score of 0.01 (results are divided into four levels: Intolerant, 0.00–0.05; potentially intolerant, 0.051–0.10; borderline, 0.101–0.2; tolerant, 0.201–1.00). By contrast, PolyPhen-2 analysis predicted that this mutation is benign with a score of 0.005 (Fig. 2, the results here were divided into five levels: Benign, 0.000–0.999; borderline, 1.000–1.249; potentially damaging, 1.250–1.499; possibly damaging, 1.500–1.999; probably damaging, >2.000; and when the closer the score is to zero, the less damage is predicted).
Figure 2.
PolyPhen-2 prediction of functional alterations resulting from an amino acid mutation at position 423.
Discussion
The polymorphic isoenzyme CYP2C19 is responsible for the metabolism of various important therapeutic agents and CYP2C19 polymorphisms result in inter-individual and inter-ethnic variation in the break-down of multiple therapeutic agents (29). To the best of our knowledge, the present study is the first to systematically screen a group of Tibetan individuals for CYP2C19 variants by direct sequencing and compare these results with ethnic populations from different continents. The present study observed 27 genetic variants, including three novel polymorphisms, five alleles and four genotypes. Only one of the novel genetic variants, within exon eight, was a non-synonymous mutation.
The frequency of the wild-type CYP2C19 allele (CYP2C19*1) in the Tibetan study population was notably lower than in Caucasian populations, which was consistent with findings in a previous study on the Chinese Han population (30). A previous study has demonstrated that CYP2C19*2 and CYP2C19*3 are null alleles, which result in the total absence of enzyme activity and these two alleles account for >99% of oriental PMs and ~87% of Caucasian PMs (31). The occurrence of CYP2C19*3 in the Tibetan subjects in the present study was significantly higher (P<0.01) than the frequency reported for Caucasian and African populations. These results suggest that the toxicological or pharmacological properties of medications that are metabolized by CYP2C19 are likely to differ among Tibetans, other Asians, Caucasian and African populations. CYP2C19*17 is within the promoter region of CYP2C19 and this mutation is frequently reported in oriental races (16,30,32). Amongst the Tibetan study population CYP2C19*17 had a frequency of 1.56%, which is not significantly different compared with a previous study on the Chinese Han population (30).
Various clinically important therapeutic agents are substrates for CYP2C19, as outlined earlier. As different mutant alleles are associated with different phenotypes and enzyme activities, CYP2C19 genotypic and phenotypic analysis may be used to optimize dosages, improve treatment efficacy and optimize the cost effectiveness of certain treatments (33). Clopidogrel is an inactive prodrug that requires hepatic bioactivation via CYP2C19 to exert its effects (34). This process is impaired in PMs, such as individuals with the CYP2C19*2 allele and, consequently, the production of the active metabolite in these individuals is reduced (35). The frequency value of CYP2C19*2 in the present Tibetan study population was between that of the other Asian groups and Caucasians, thus, it may be recommended that the dosage of clopidogrel should also lie between those used for the two populations.
An analysis of novel genetic variants in the coding region demonstrated only one non-synonymous mutation, which results in an amino acid change from asparagine to lysine. The results of the protein function analysis were determined to be intolerant and benign by SIFT and PolyPhen-2, respectively. The prediction accuracy of SIFT and PolyPhen-2 is 63 and 75%, while the false positive rate is 19 and 9%, respectively (14,36). The novel genetic variants identified in the present study should be further elucidated by other means in future studies.
In conclusion, results from the present study provide a basic profile of CYP2C19 in the Tibetan population, and may be used to determine optimal dosage recommendations, leading to individualized medicine.
Acknowledgments
The present study was supported by the Major Science and Technology Research Projects of Xizang (Tibet) Autonomous Region (2015), The National Natural Science Foundation (grant no. 81560516) and the Major Training Program of Tibet University for Nationalities (grant no. 13myZP06).
References
- 1.Lee CR, Goldstein JA, Pieper JA. Cytochrome P450 2C9 polymorphisms: A comprehensive review of the in-vitro and human data. Pharmacogenetics. 2002;12:251–263. doi: 10.1097/00008571-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Gerbal-Chaloin S, Pascussi JM, Pichard-Garcia L, Daujat M, Waechter F, Fabre JM, Carrère N, Maurel P. Induction of CYP2C genes in human hepatocytes in primary culture. Drug Metab Dispos. 2001;29:242–251. [PubMed] [Google Scholar]
- 3.Andersson T, Holmberg J, Röhss K, Walan A. Pharmacokinetics and effect on caffeine metabolism of the proton pump inhibitors, omeprazole, lansoprazole and pantoprazole. Br J Clin Pharmacol. 1998;45:369–375. doi: 10.1046/j.1365-2125.1998.t01-1-00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardiner SJ, Begg EJ. Pharmacogenetics, drug-metabolizing enzymes, and clinical practice. Pharmacol Rev. 2006;58:521–590. doi: 10.1124/pr.58.3.6. [DOI] [PubMed] [Google Scholar]
- 5.Onof S, Hatanaka T, Miyazawa S, Tsutsui M, Aoyama T, Gonzalez FJ, Satoh T. Human liver microsomal diazepam metabolism using cDNA-expressed cytochrome P450s: Role of CYP2B6, 2C19 and the 3A subfamily. Xenobiotica. 1996;26:1155–1166. doi: 10.3109/00498259609050260. [DOI] [PubMed] [Google Scholar]
- 6.Paveliu MS, Bengea S, Paveliu FS. Individualized drug response related to genetic variations of cytochrome P450 isoforms and other enzymes. Rev Farmacia. 2010;58:245–254. [Google Scholar]
- 7.Adrian A, Daniela C, Axente GI, Ionut O, Flavian R, Anamaria G. Detection of CYP2D6*6 allele by real time polymerase chain reaction in Romanian population. Rev Farmacia. 2010;58:353–361. [Google Scholar]
- 8.Goldstein JA. Clinical relevance of genetic polymorphisms in the human CYP2C subfamily. Br J Clin Pharmacol. 2001;52:349–355. doi: 10.1046/j.0306-5251.2001.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang M, Tybring G, Dahl ML, Lindh JD. Impact of cytochrome P450 2C19 polymorphisms on citalopram/escitalopram exposure: A systematic review and meta-analysis. Clin Pharmacokinet. 2014;53:801–811. doi: 10.1007/s40262-014-0162-1. [DOI] [PubMed] [Google Scholar]
- 10.Serrano D, Torrado S, Torrado-Santiago S, Gisbert JP. The influence of CYP2C19 genetic polymorphism on the pharmacokinetics/-pharmacodynamics of proton pump inhibitor-containing Helicobacter pylori treatments. Curr Drug Metab. 2012;13:1303–1312. doi: 10.2174/138920012803341393. [DOI] [PubMed] [Google Scholar]
- 11.Myrand SP, Sekiguchi K, Man MZ, Lin X, Tzeng RY, Teng CH, Hee B, Garrett M, Kikkawa H, Lin CY, et al. Pharmacokinetics/genotype associations for major cytochrome P450 enzymes in native and first-and third-generation Japanese populations: Comparison with Korean, Chinese and Caucasian populations. Clin Pharmacol Ther. 2008;84:347–361. doi: 10.1038/sj.clpt.6100482. [DOI] [PubMed] [Google Scholar]
- 12.Adamec C. Example of the use of the nonparametric test. Test X2 for comparison of 2 independent examples. Cesk Zdrav. 1964;12:613–619. In Czech. [PubMed] [Google Scholar]
- 13.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 14.Ng PC, Henikoff S. Accounting for human polymorphisms predicted to affect protein function. Genome Res. 2002;12:436–446. doi: 10.1101/gr.212802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer UA. Pharmacogenetics and adverse drug reactions. Lancet. 2000;356:1667–1671. doi: 10.1016/S0140-6736(00)03167-6. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Q, Yu XM, Lin HB, Wang L, Yun QZ, Hu SN, Wang DM. Genetic polymorphism, linkage disequilibrium, haplotype structure and novel allele analysis of CYP2C19 and CYP2D6 in Han Chinese. Pharmacogenomics J. 2009;9:380–394. doi: 10.1038/tpj.2009.31. [DOI] [PubMed] [Google Scholar]
- 17.He N, Yan FX, Huang SL, Wang W, Xiao ZS, Liu ZQ, Zhou HH. CYP2C19 genotype and S-mephenytoin 4′-hydroxylation phenotype in a Chinese Dai population. Eur J Clin Pharmacol. 2002;58:15–18. doi: 10.1007/s00228-002-0425-x. [DOI] [PubMed] [Google Scholar]
- 18.Kimura M, Ieiri I, Mamiya K, Urae A, Higuchi S. Genetic polymorphism of cytochrome P450s, CYP2C19, and CYP2C9 in a Japanese population. Ther Drug Monit. 1998;20:243–247. doi: 10.1097/00007691-199806000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Roh HK, Dahl ML, Tybring G, Yamada H, Cha YN, Bertilsson L. CYP2C19 genotype and phenotype determined by omeprazole in a Korean population. Pharmacogenetics. 1996;6:547–551. doi: 10.1097/00008571-199612000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Yamada S, Onda M, Kato S, Matsuda N, Matsuhisa T, Yamada N, Miki M, Matsukura N. Genetic differences in CYP2C19 single nucleotide polymorphisms among four Asian populations. J Gastroenterol. 2001;36:669–672. doi: 10.1007/s005350170029. [DOI] [PubMed] [Google Scholar]
- 21.Chang M, Dahl ML, Tybring G, Götharson E, Bertilsson L. Use of omeprazole as a probe drug for CYP2C19 phenotype in Swedish Caucasians: Comparison with S-mephenytoin hydroxylation phenotype and CYP2C19 genotype. Pharmacogenetics. 1995;5:358–363. doi: 10.1097/00008571-199512000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Gaikovitch EA, Cascorbi I, Mrozikiewicz PM, Brockmöller J, Frötschl R, Köpke K, Gerloff T, Chernov JN, Roots I. Polymorphisms of drug-metabolizing enzymes CYP2C9, CYP2C19, CYP2D6, CYP1A1, NAT2 and of P-glycoprotein in a Russian population. Eur J Clin Pharmacol. 2003;59:303–312. doi: 10.1007/s00228-003-0606-2. [DOI] [PubMed] [Google Scholar]
- 23.Scordo MG, Caputi AP, D'Arrigo C, Fava G, Spina E. Allele and genotype frequencies of CYP2C9, CYP2C19 and CYP2D6 in an Italian population. Pharmacol Res. 2004;50:195–200. doi: 10.1016/j.phrs.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Bravo-Villalta HV, Yamamoto K, Nakamura K, Bayá A, Okada Y, Horiuchi R. Genetic polymorphism of CYP2C9 and CYP2C19 in a Bolivian population: An investigative and comparative study. Eur J Clin Pharmacol. 2005;61:179–184. doi: 10.1007/s00228-004-0890-5. [DOI] [PubMed] [Google Scholar]
- 25.Halling J, Petersen MS, Damkier P, Nielsen F, Grandjean P, Weihe P, Lundgren S, Lundblad MS, Brøsen K. Polymorphism of CYP2D6, CYP2C19, CYP2C9 and CYP2C8 in the Faroese population. Eur J Clin Pharmacol. 2005;61:491–497. doi: 10.1007/s00228-005-0938-1. [DOI] [PubMed] [Google Scholar]
- 26.Herrlin K, Massele AY, Jande M, Alm C, Tybring G, Abdi YA, Wennerholm A, Johansson I, Dahl ML, Bertilsson L, Gustafsson LL. Bantu tanzanians have a decreased capacity to metabolize omeprazole and mephenytoin in relation to their CYP2C19 genotype. Clin Pharmacol Ther. 1998;64:391–401. doi: 10.1016/S0009-9236(98)90070-4. [DOI] [PubMed] [Google Scholar]
- 27.Persson I, Aklillu E, Rodrigues F, Bertilsson L, Ingelman-Sundberg M. S-mephenytoin hydroxylation phenotype and CYP2C19 genotype among Ethiopians. Pharmacogenetics. 1996;6:521–526. doi: 10.1097/00008571-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Masimirembwa C, Bertilsson L, Johansson I, Hasler JA, Ingelman-Sundberg M. Phenotyping and genotyping of S-mephenytoin hydroxylase (cytochrome P450 2C19) in a Shona population of Zimbabwe. Clin Pharmacol Ther. 1995;57:656–661. doi: 10.1016/0009-9236(95)90228-7. [DOI] [PubMed] [Google Scholar]
- 29.Kóbori L, Kõhalmy K, Porrogi P, Sárváry E, Gerlei Z, Fazakas J, Nagy P, Járay J, Monostory K. Drug-induced liver graft toxicity caused by cytochrome P450 poor metabolism. Br J Clin Pharmacol. 2008;65:428–436. doi: 10.1111/j.1365-2125.2007.03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, Qin S, Xie J, Tang J, Yang L, Shen W, Zhao X, Du J, He G, Feng G, et al. Genetic polymorphism analysis of CYP2C19 in Chinese Han populations from different geographic areas of mainland China. Pharmacogenomics. 2008;9:691–702. doi: 10.2217/14622416.9.6.691. [DOI] [PubMed] [Google Scholar]
- 31.Desta Z, Zhao X, Shin JG, Flockhart DA. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet. 2002;41:913–958. doi: 10.2165/00003088-200241120-00002. [DOI] [PubMed] [Google Scholar]
- 32.Anichavezhi D, Chakradhara Rao US, Shewade DG, Krishnamoorthy R, Adithan C. Distribution of CYP2C19*17 allele and genotypes in an Indian population. J Clin Pharm Ther. 2012;37:313–318. doi: 10.1111/j.1365-2710.2011.01294.x. [DOI] [PubMed] [Google Scholar]
- 33.Gurbel PA, Shuldiner AR, Bliden KP, Ryan K, Pakyz RE, Tantry US. The relation between CYP2C19 genotype and phenotype in stented patients on maintenance dual antiplatelet therapy. Am Heart J. 2011;161:598–604. doi: 10.1016/j.ahj.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, Cannon CP, Danchin N, Giusti B, Gurbel P, Horne BD, Hulot JS, Kastrati A, Montalescot G, Neumann FJ, Shen L, Sibbing D, Steg PG, Trenk D, Wiviott SD, Sabatine MS. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–1830. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collet JP, Hulot JS, Pena A, Villard E, Esteve JB, Silvain J, Payot L, Brugier D, Cayla G, Beygui F, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: A cohort study. Lancet. 2009;373:309–317. doi: 10.1016/S0140-6736(08)61845-0. [DOI] [PubMed] [Google Scholar]
- 36.Ng PC, Henikoff S. Predicting the effects of amino acid substitutions on protein function. Annu Rev Genomics Hum Genet. 2006;7:61–80. doi: 10.1146/annurev.genom.7.080505.115630. [DOI] [PubMed] [Google Scholar]