Abstract
Many patients with Alzheimer’s disease will develop agitation at later stages of the disease, which constitutes one of the most challenging and distressing aspects of dementia. Recently, nonpharmacological therapies have become increasingly popular and have been proven to be effective in managing the behavioral symptoms (including agitation) that are common in the middle or later stages of dementia. These therapies seem to be a good alternative to pharmacological treatment to avoid unpleasant side effects. We present a systematic review of randomized controlled trials (RCTs) focused on the nonpharmacological management of agitation in Alzheimer’s disease (AD) patients aged 65 years and above. Of the 754 studies found, eight met the inclusion criteria. This review suggests that music therapy is optimal for the management of agitation in institutionalized patients with moderately severe and severe AD, particularly when the intervention includes individualized and interactive music. Bright light therapy has little and possibly no clinically significant effects with respect to observational ratings of agitation but decreases caregiver ratings of physical and verbal agitation. Therapeutic touch is effective for reducing physical nonaggressive behaviors but is not superior to simulated therapeutic touch or usual care for reducing physically aggressive and verbally agitated behaviors. Melissa oil aromatherapy and behavioral management techniques are not superior to placebo or pharmacological therapies for managing agitation in AD. Further research in clinical trials is required to confirm the effectiveness and long-term effects of nonpharmacological interventions for managing agitation in AD. These types of studies may lead to the development of future intervention protocols to improve the well-being and daily functioning of these patients, thereby avoiding residential care placement.
Keywords: dementia, nonpharmacological, behavioral and psychological symptoms
Introduction
Dementia is one of the most prevalent diseases in older people and constitutes the largest global public health care challenge. According to the 2015 World Alzheimer Report data,1 nearly 47 million people are living with dementia in 2015, a number that will nearly double every 20 years. Alzheimer’s disease (AD) is a specific form of dementia that causes as many as 50%–70% of all dementia cases.2 Although cognitive impairment is the central symptom of AD, behavioral and psychological symptoms, such as agitation, often coexist and are a common cause of patient and caregiver distress, institutionalization, and impairment in quality of life.3,4 Frequencies of agitation between 13.0% and 50.4% have been reported across studies and settings,5–7 and these increase as the severity of dementia progresses. Agitation has been defined as “inappropriate verbal, vocal, or motor activity that is not explained by needs or confusion per se”.8 Although there is no consensus regarding specific behaviors that integrate the concept,4 clinicians have identified three subtypes of agitation as follows: physically nonaggressive behavior, aggressive behavior, and verbally agitated behavior.9 Interestingly, there appears to be a relationship between agitation and unmet needs originated by a decreased ability to cope with environmental stimulation and to communicate these needs.10
Pharmacological interventions have been traditionally used in the treatment of agitation, but many studies have documented adverse effects of sedative and antipsychotic drugs, such as worsening cognitive function, higher cerebrovascular side effects, longer hospitalizations, and increased mortality.11 Thus, the use of a nonpharmacological approach as a first-line treatment for agitation in dementia patients has been increasingly recommended.12,13 Overall, well-conducted, evidence-based studies on nonpharmacological interventions are lacking. Nonpharmacological approaches address the contextual and/or psychosocial reasons for agitation and avoid the potentially negative side effects of pharmacological treatment. Interventions such as cognitive stimulation/training, behavioral interventions, physical exercise, therapeutic touch, aromatherapy, bright light therapy, music therapy, and multisensory stimulation have shown promising results in decreasing agitation and cognitive impairment in older adults with dementia.14,15
We performed a systematic review of randomized controlled trials (RCTs) focusing on the nonpharmacological management of agitation of AD patients, with the aim of making evidence-based recommendations about the use of specific intervention strategies. Previous systematic reviews on the topic have been published, including studies with dementia patients, but none of these studies analyzed their results according to dementia subtypes.12,14–17 Because patients with different subtypes of dementia or different levels of cognitive impairment may present with different levels of agitation and respond differently to interventions, we specifically focused this review on AD patients aged 65 years and above.
This paper had three specific aims as follows: 1) to review the literature on nonpharmacological therapies used to manage agitation in older AD patients over the past 20 years, 2) to assess the specific effectiveness of each nonpharmacological therapy, and 3) to provide evidence-based recommendations about the use of specific therapies and future research on this topic. Exploring these aspects is clinically relevant and has important implications for health service planning.
Methods
Data sources and search strategy
A systematic review of the literature published during the past 20 years (January 1996 to June 2015) was performed. Four computerized electronic databases were searched, including PubMed, Web of Science, PsycINFO, and Scopus, using the following keywords for AD, agitation, and non-pharmacological therapy: randomized controlled trial, Alzheimer*, agitat*, agress*, non-pharmacological, non-drug therap*, acupuncture, aromatherapy, light therap*, massage, touch, music*, group exercise*, activities, snoezelen, multisensory stimulation, social contact*, environmental modification*, caregiver training, behavioral or behavioral management, psychosocial, reminiscence therap*, validation therap*, reality orientation, space retrieval, alternative therap*, intervention*, and staff training.
Two independent reviewers evaluated appropriateness of inclusion, and conflicts were discussed until a consensus was reached. In cases in which a consensus was not reached, a third reviewer was involved.
Inclusion and exclusion criteria
We included original scientific articles in English that met the following criteria: 1) Population: AD patients (65 years of age or older, or a mean age of at least 75 if no age ranges were provided). Studies were selected if they reported a validated or medical diagnosis of AD, and they were excluded if they included patients with other subtypes of dementia; 2) Intervention: nonpharmacological interventions aimed at managing agitated behaviors were included; 3) Type of experimental design: RCTs comparing agitation before and after interventions were included. In terms of the level of evidence, all studies were considered to be level 2 based on the Oxford Centre for Evidence-Based Medicine (CEBM) criteria;18 4) Outcome: Only studies exploring nonpharmacological interventions for agitation as a primary outcome (measured quantitatively and with a validated scale) or studies including sufficient information to determine the effect of nonpharmacological intervention on agitation were included; 5) Type of study: Only original articles were included. Abstracts, reviews, descriptive studies, and studies based on the description of a protocol, as well as studies based on the perspective of the authors, books, short surveys, observational studies, comments on an article, and conference abstracts, were excluded.
Data extraction
Studies were synthesized according to the following characteristics: author and year, country, sample characteristics (age and sex), study design, level of cognitive impairment, type of intervention, agitation scale, and main findings. A narrative synthesis approach was performed to examine the results. Next, we made recommendations about the clinical use for each nonpharmacological intervention based on the main findings.
Results
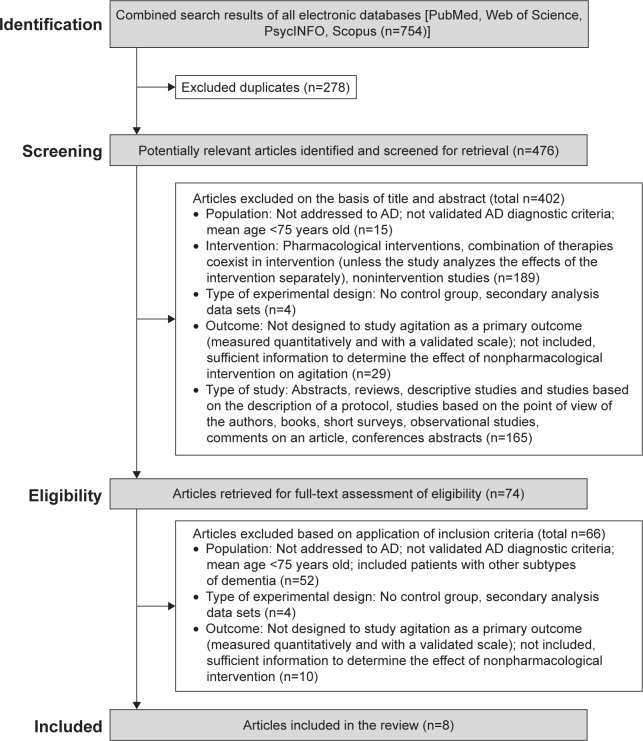
The review procedure is described in Figure 1. As shown in the figure, a total of 754 studies were identified; after the removal of duplicates, 476 were considered potentially relevant and were screened for relevant content. From these studies, 402 were excluded on the basis of the title and abstract, and 74 were retrieved for full-text assessment. In the next phase, 66 were excluded based on the inclusion criteria. Finally, eight studies met the inclusion criteria for the review (Figure 1).
Figure 1.
Flowchart of systematic literature search.
Abbreviation: AD, Alzheimer’s disease.
Data synthesis
Studies’ characteristics
The characteristics of the studies are listed in Table 1. The eight studies that were included provided data on the effectiveness of music therapy (n=3),19–21 bright light therapy (n=2),22,23 aromatherapy (n=1),24 therapeutic touch (n=1),25 and psychological interventions with caregivers using behavioral management techniques (BMT) (n=1).26
Table 1.
Studies included in the review (n=8)
| Study | Country | Study design | Level of cognitive impairment | Sample | Therapy | Agitation scale | Main findings |
|---|---|---|---|---|---|---|---|
| Ancoli-Israel et al22 | USA | RCT | MMSE =5.7±5.6 | Institutionalized patients with severe AD (n=92; 63 women; mean ± SD =82.3±7.6, range 61–99) Treatment groups: E1: Morning bright light (n=30) E2: Evening bright light (n=31) C: Control (n=31) |
Light therapy N sessions: 10 (1/day, 120 minutes) Duration: 10 days Follow-up: 5 days |
CMAI9 ABRS31 |
CMAI: Caregiver ratings of agitation significantly decreased after treatment in all groups ABRS: No significant effects on observational rating in agitation |
| Burns et al24 | UK | Multicenter RCT | CDR =3 | Institutionalized AD patients (n=94; 56 women; age range 63–98) Treatment groups: E1: Aromatherapy (Melissa oil; n=32; 21 women, mean age =85.6) E2: Donepezil (n=31; 21 women; mean age =84.6)C: Placebo (n=31; 15 women; mean age =85.1) |
Aromatherapy N sessions: 2/day (1–2 minutes) Duration: 4 weeks (n=94) Follow-up: week 12 (n=81) |
PAS32 NPI30 |
No significant differences between aromatherapy, donepezil, and placebo groups at week 4 and 12 Improvements in all three groups in the PAS and NPI over 12 weeks |
| Dowling et al23 | USA | RCT | MMSE =7.0±7.0 | Institutionalized AD patients (n=70; 57 women; mean age ± SD =84.0±10.0, range 58–98) Treatment groups: E1: Morning bright light (n=29) E2: Afternoon bright light (n=24) C: Control (n=17) |
Light therapy N sessions: 1 hour/day (Monday–Friday) Duration: 10 weeks (50 hours) |
NPI-NH30 | Significant but possibly potentially not clinically relevant worsening on agitation/aggression after treatment |
| Hawraniket al25 | Canada | RCT | MMSE =5.5±6.6 | Residents of a long-term care facility with AD (n=51) Treatment groups: E1: Therapeutic Touch (TT; n=17; 10 women; mean age ± SD =83.3±8.3) E2: Simulated TT (n=16; 14 women; mean age ± SD =84.2±6.2) C: Usual care (n=18; 12 women; mean age ± SD =80.9±7.4) |
Therapeutic touch N sessions: 5 (1/day; 30–40 minutes) Duration: 5 days |
CMAI9 | Physical nonaggressive behaviors decreased in E1 (therapeutic touch) No differences across groups in physically aggressive and verbally agitated behaviors |
| Narme et al19 | France | RCT | Music group: MMSE =9.6±5.3 Cooking group: 10.8±8.4 |
Nursing home AD patients (n=37; 32 women) Treatment groups: E: Music (n=18; 15 women; mean age ± SD =86.7±6.4) C: Cooking (n=19; 17 women; mean age ± SD =87.5±6.0) |
Music therapy N sessions: 2/week (1 hour each) Duration: 4 weeks (8 hours total) Follow-up: 2 and 4 weeks |
CMAI9 | Music decreased severity of agitated behaviors during the treatment (fourth session) but not at the end (eighth session) or at follow-up |
| Sakamoto et al20 | Japan | RCT | CDR =3 | Patients with severe AD from group homes and a special dementia hospital (n=39) Treatment groups: E1: Passive music (n=13; 10 women; mean age ± SD =78.7±12.1) E2: Interactive music (n=13; 11 women; mean age ± SD =81.2±7.5) C: No-music control (n=13) |
Music therapy N sessions: 10 (1/week; 30 minutes) Duration: 10 weeks Follow-up: 3 weeks |
BEHAVE-AD29 | Reduction in behavioral and psychological symptoms following interactive music intervention. The interactive music intervention reduced aggressiveness toward caregivers. Effects disappeared at follow-up |
| Svansdottir and Snaedal21 | Iceland | Case-control study | GDS =5–7 | Nursing home patients with moderate or severe AD (n=38; age range 71–87) Treatment groups: E: Music therapy (n=20) C: Control (n=18) |
Music therapy N sessions: 18 (30 minutes). 3 times/week Duration: 6 weeks Follow-up: 4 weeks |
BEHAVE-AD29 | Significant reduction in activity disturbances in the music therapy group |
| Teri et al26 | USA | Multicenter RCT | BMT group: MMSE =12.0±7.0 Haloperidol group: MMSE =13.0±8.0 Trazodone group: MMSE =14.0±7.0 Placebo group: MMSE =13.0±8.0 |
AD outpatients (n=148) Treatment groups: E1: BMT (n=41, mean age ± SD =74.8±8.4) E2: Haloperidol (n=34; mean age ± SD =75.3±6.9) E3: Trazodone (n=37; mean age ± SD =73.2±6.6) C: Placebo (n=36; mean age ± SD =75.8±6.2) |
Behavioral Management Training (BMT) N sessions: 11 (8 weekly & 3 biweekly) Duration: 16 weeks |
CMAI9 ABID33 |
No significant differences on agitation across groups. Significantly fewer adverse events in the BMT group |
Abbreviations: AD, Alzheimer’s disease; ABID, Agitated Behavior Inventory for Dementia; ABRS, Agitated Behavior Rating Scale; BEHAVE-AD, Behavioral Pathology in Alzheimer’s Disease Rating Scale; BMT, Behavior Management Techniques; C, Control group; CDR, Clinical Dementia Rating Scale; CMAI, Cohen-Mansfield Agitation Inventory; E, Experimental group; GDS, Global Deterioration Scale; MMSE, Mini–Mental State Examination; NPI, Neuropsychiatric Inventory; NPI-NH, Neuropsychiatric Inventory-Nursing Home; PAS, Pittsburgh Agitation Scale; RCT, Randomized Controlled Trial; N sessions, Number of sessions.
The sample size of the included studies ranged from 3719 to 14826 AD participants. Three studies were from Europe,19,21,24 whereas three studies were from the United States,22,23,26 one study was from Asia,20 and one study was from Canada.25 All studies except one26 included institutionalized AD patients in care homes. Two were multicenter studies.24,26
Of the eight included studies, four studies22–24,26 used the National Institute of Neurological and Communication Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA) clinical criteria for AD,27 whereas two studies19,20 used the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria for AD,28 one study21 diagnosed AD according to International Classification of Diseases, Tenth Edition (ICD-10), and one study25 did not report to specific criteria, but the AD diagnosis was confirmed from the medical records of each patient.
Regarding the scales used to assess agitation, two studies19,25 used the Cohen-Mansfield Agitation Inventory (CMAI),9 which assesses the frequency of agitated behavior in a 7-point rating scale, with a higher score indicating more agitation. Two studies20,21 used the Behavioral Pathology in Alzheimer’s Disease rating scale (BEHAVE-AD),29 which covers behavioral symptoms in seven categories: paranoid and delusional ideations, hallucinations, activity disturbances, aggressiveness, diurnal rhythm disturbances, affective disturbances, and anxieties and phobias, with a higher score indicating more severity. One study23 used the Neuropsychiatric Inventory-Nursing Home (NPI-NH),30 which assesses either ten or 12 behavioral disturbances common in dementia as follows: delusions, hallucinations, dysphoria, anxiety, agitation or aggression, euphoria, disinhibition, irritability or lability, apathy, aberrant motor activity, sleep and nighttime behavior disturbances, and appetite and eating changes, with a higher score indicating greater severity. In the study by Ancoli-Israel et al,22 agitation was assessed both with the CMAI9 and the Agitated Behavior Rating Scale (ABRS).31 The ABRS is a behavioral observation rating scale of the following five major behaviors: manual manipulation, restraint escape, searching or wandering, tapping or banging, and vocalization, with a higher score indicating more severity. In the study by Burns et al,24 agitation was assessed using both the Pittsburg Agitation Scale (PAS)32 and the NPI.30 The PAS is a 16-item observational scale that rates the severity of agitation from 0 to 4 using the following four general behavior groups: aberrant vocalization, motor agitation, aggressiveness, and resisting, with a higher score indicating more agitation. In the study by Teri et al,26 agitation was assessed with the CMAI9 and Agitated Behavior Inventory for Dementia (ABID).33 The ABID consists of 16 items designed specifically to evaluate the frequency of common agitated behaviors and caregiver reactions in community-residing dementia patients. Most studies19,22,23,25,26 evaluated cognitive status by administering the Mini–Mental State Examination (MMSE).34
Description of the studies
Studies were organized according to the types of nonpharmacological therapy, which are briefly described using Medical Subject Headings (MeSH) definitions.
Music therapy
Music therapy is the use of music as an adjunctive therapy in the treatment of neurological, mental, or behavioral disorders. Three studies19–21 provided evidence of the effectiveness of music therapy for reducing agitation in institutionalized patients with moderately severe and severe AD. This result was particularly strong when the intervention included individualized (related to special positive memories of the participants) and interactive (including clapping, singing, and dancing) music.20
One RCT19 compared the short- and long-term effects of participative music and cooking interventions lasting 4 weeks and reported that both types of pleasant intervention decreased the severity of agitation measured using the CMAI9 and NPI.30 The intervention group (n=18) received two 1-hour sessions a week of participatory music therapy (listening to music, singing, and playing percussion instruments), and the control group (n=19) received a cooking intervention (preparing a recipe). Importantly, in the music therapy group, the decrease in agitated behaviors was significant during the intervention (after the fourth session) but not at the end or at the follow-up evaluations (2 and 4 weeks after the intervention). Moreover, this study did not include a no-contact group to ensure that behavioral improvement did not result from increasing familiarity with the supervisor, who had no previous education in music therapy. Because the authors did not take into account individual music preferences, and the supervisor (psychologist) was not a qualified music therapist (and he/she was more comfortable in cooking), the benefit of music therapy observed in this study may have been reduced. Importantly, the improvement in agitation was stronger in the cooking intervention, suggesting that the observed benefits were not specific to musical interventions.
The other RCT20 explored the long-term effects of passive (listening to music via a CD player) or interactive (including clapping, singing, and dancing) music therapy lasting 10 weeks using the BEHAVE-AD29 and reported a higher, long-term reduction in behavioral symptoms in the interactive music group (n=13) compared with the passive music group (n=13) and no-music control group (n=13). Particularly, the scores of five items of the BEHAVE-AD were significantly reduced in the interactive group: paranoid and delusional ideations, activity disturbances, aggressiveness, affective disturbances, and anxieties and phobias; however, this effect disappeared 3 weeks after the music intervention, indicating the need for regularly conducted music interventions to maintain the beneficial effects. The music facilitators included two music therapists, four occupational therapists, and six nurses. Each intervention was performed once a week and lasted 30 minutes, and individualized music related to specific positive memories for each participant was selected.
In a case-control study,21 a significant reduction was observed in activity disturbances, aggressiveness, and anxiety measured using the unpublished Icelandic version of the BEHAVE-AD29 in a music therapy group during a 6-week period, although this effect had mostly disappeared 1 month later. In this study, the music intervention (n=20) was 30 minutes of music therapy delivered three times a week, whereas the control (n=18) was usual care. A qualified music therapist performed the intervention after the selection of a collection of songs familiar to the elderly. Each song was sung twice during the sessions, and every patient participated actively (singing, playing instruments, or dancing) or passively (holding the songbook and listening).
Bright light therapy
Light therapy is the exposure to light, particularly by variously concentrated light rays or specific wavelengths. Two studies22,23 examined the efficacy of bright light therapy in decreasing agitated behaviors in AD patients, and these results showed that this therapy had little and possibly no clinically significant effects. One of the RCTs22 including AD patients with significant agitation at baseline examined both caregiver perceptions of the agitated behavior using the CMAI9 and the direct observation of agitated behaviors using the ABRS.31 The researchers found that increasing light exposure (2,500 lux) significantly decreased caregiver ratings of physical and verbal agitation after intervention, but caregiver ratings also decreased in the control group. They also reported that bright light had no significant effects on the observational ratings of agitation in any of the light-treatment groups, although morning bright light treatment delayed the timing (but not the strength) of agitation.22 Importantly, they also observed a worsening of verbal agitation in the evening bright light group.
The other RCT23 explored the effect of timed morning (n=29) or afternoon bright light (n=24) exposure at 2,500 lux for 1 hour daily compared with usual indoor light levels (n=17; 150–200 lux) in AD patients who experienced rest–activity disruption at baseline. They found a significant but clinically small worsening in agitation/aggression as measured with the NPI-NH30 after 10 weeks of treatment, particularly in the morning-light group. Importantly, the nursing staff was not unaware of the experimental condition of the participants, and the effect of interrater variability on the observed results was impossible to determine in this study.
Aromatherapy
Aromatherapy is the use of fragrances and essences from plants to affect or alter a person’s mood or behavior and facilitate physical, mental, and emotional well-being. A multicenter, double-blind, placebo RCT24 explored the effects of aromatherapy with Melissa officinalis oil in the treatment of agitation in institutionalized AD patients with a previous history of significant agitation, and found that intervention with Melissa oil (n=32) was not superior to placebo (n=31) or pharmacological therapy with donepezil (5 mg/day, n=31) after 4 or 12 weeks of treatment. Agitation was assessed by the PAS32 and NPI.30 The application of Melissa oil occurred twice a day and involved massaging the oil into the hands and upper arms for 1–2 minutes. Given the substantial improvement in the placebo group, the authors emphasized the potential benefits of meeting regularly (touch and social interaction) with care staff in the management of agitation in AD patients.
Therapeutic touch
Therapeutic touch is an intentionally directed process during which the practitioner places his or her hands upon the person to be cured with the intent of spiritual energetic healing. One RCT25 compared the effectiveness of therapeutic touch intervention (n=17), which was administered daily for 30–40 minutes for 5 consecutive days, with a simulated therapeutic touch intervention (n=16) and with usual care (n=18) in institutionalized AD patients with a previous history of agitated behaviors. The therapeutic touch intervention was conducted by nurses who had completed the advanced level of a therapeutic touch and healing program, and the simulated therapeutic touch intervention was conducted by nursing and health-related volunteer students. Agitation levels were measured at various times after treatment and then compared. Therapeutic touch was effective in reducing the frequency of physical nonaggressive behaviors (pacing, repetitious movements, and general restlessness) but was not superior to simulated therapeutic touch or usual care in reducing physically aggressive and verbally agitated behaviors. It is important to note, however, that a significant decrease in the number of all analyzed agitated behaviors was observed in the three groups from baseline to the end of intervention, although this reduction was significantly lower in the usual care group.
Psychological intervention with family caregivers
Psychological therapies imply the use of specific psychological techniques or approaches for the reduction of behavioral symptoms in dementia. One multicenter, placebo RCT26 reported that a psychological intervention with family caregivers (spouses or adult relatives) by training in BMT (n=41) was not superior to pharmacological therapy with haloperidol (mean dose, 1.8 mg/d; n=34), trazodone (mean dose, 200 mg/d; n=37), or placebo (n=36) in reducing agitation in AD outpatients with a previous history of significant agitation and moderate-to-severe cognitive impairment, although it slightly reduced the incidence of adverse events such as bradykinesia and parkinsonian gait after 16 weeks of treatment. No long-term effects of the intervention were found at 3-, 6-, and 12-month follow-ups. In this study, the intervention was performed by expert therapists with master’s degrees and consisted of eleven structured sessions that provided information about AD strategies to decrease agitation, and structured in- and out-of-session assignments, including a videotaped training program. The treatment protocol was general due to the diversity of the participants, and the authors suggest that a more individualized BMT may have yielded better results.
Discussion
The purpose of this review was to identify and synthesize available research evidence on the efficacy of the nonpharmacological management of agitation in older adults with AD and to make evidence-based recommendations about the clinical use of different interventions in this population. The focus on AD is relevant, because the treatment effects on agitation may be different depending on the subtype and the severity of dementia. There is also evidence of more agitation in patients with AD than in patients with vascular dementia or other types of dementia.35
Effective nonpharmacological therapies to manage agitation in AD
On the basis of the methodological quality of the three included studies exploring the effectiveness of music therapy in AD,19–21 we concluded that music therapy performed by qualified music therapists is an optimal intervention in institutionalized AD patients. The effectiveness of this type of therapy in reducing agitated behaviors has been reported in previous systematic reviews with dementia patients,14,15,17,36–38 particularly when based on individual music choices and when complemented by group interventions. Thus, it appears that music therapy is effective for managing agitation in institutionalized dementia patients, potentially because it can engage interest and provides an opportunity for social interaction.
It is important to note that in the three studies, agitated behaviors returned to baseline levels at follow-up, suggesting that music therapy interventions must be implemented on a long-term basis to maintain their beneficial effects. Moreover, the long-term benefits of music therapy remain unclear and require further investigation.
One of the three studies19 included participants with some previous agitation, whereas the other two studies20,21 did not clearly report the previous history of agitation. Thus, the effect of music therapy in AD patients with severe baseline agitation has not been specifically investigated.
Low number and variability among the included studies makes it difficult to identify the most effective duration and frequency of the music intervention. The researchers completed therapy over several weeks (4–10 weeks), with the sessions lasting 30–60 minutes and occurring 1, 2, or 3 times a week; thus, a minimum of 30 minutes of interactive and individualized music therapy twice a week appears reasonable in AD patients. The three trials were small; thus, to strengthen the above evidence, future studies should include larger samples. Finally, it is important to note that the sample was predominantly female, making it difficult to explore differences between the sexes with respect to the effectiveness of music therapy.
Nonpharmacological therapies requiring more research
The inconclusive results of the two studies exploring the effectiveness of bright light therapy22,23 and the fact that it may slightly worsen symptomatic agitation indicate that future research is necessary to examine the actual effect of bright light exposure on agitated behaviors in AD patients.
The study by Burns et al24 provided strong evidence indicating that there is no benefit for aromatherapy with Melissa oil in the management of agitation in AD, at least over a 12-week period of intervention. Consistent with this finding, a recent systematic review39 of RCTs including dementia patients of any type and severity reported equivocal benefits with aromatherapy.
Regarding the effectiveness of therapeutic touch, the results obtained by Hawranick et al25 provided evidence of the modest potential for this therapy in treating agitated but not physically aggressive behaviors in AD patients with severe cognitive impairment, but additional research is required to obtain evidence about its long-term effects because it constitutes an inexpensive method that can be implemented by family caregivers or staff.
Regarding psychological interventions involving training of family caregivers to use BMT, the study by Teri et al26 provides strong evidence of modest and comparable reductions in agitation with placebo and active treatments (both pharmacological and nonpharmacological), suggesting that training family caregivers with this technique is not an optimal clinical alternative for the management of agitation in AD outpatients.
Consistent with these findings, a recent systematic review found inconclusive evidence regarding the efficacy of these therapies in improving agitation in dementia and recommended further research on the topic.15
Challenges and solutions
Optimal management of agitation in AD constitutes a major clinical challenge to avoid or delay institutionalization and to improve quality of life for patients and caregivers. On the basis of the present literature review, there are some questions that merit further investigation. First, there is insufficient evidence in the literature about the effectiveness of nonpharmacological therapies in noninstitutionalized AD patients and the long-term effects of these interventions. Future studies in home and community settings are particularly relevant to determine if nonpharmacological therapies can delay or avoid residential care placement. Second, all included studies implemented interventions in AD patients with significant cognitive impairment, but the benefits of nonpharmacological therapy on patients with milder forms of AD may be underestimated. Third, future studies should include a detailed description of the previous history of agitation to distinguish between spontaneous or reactive agitation. Indeed, in our opinion, the identification of the underlying causes (and biological mechanisms) and the severity of agitation in AD patients is crucial and may assist clinicians and researchers in designing effective nonpharmacological interventions. AD patients usually present multiple comorbidities that may act as behavioral risk factors, and these can have an important impact on the effectiveness of the nonpharmacological therapies. Evaluating for their presence and addressing them is equally important to implement personalized interventions for agitation management.
Given the limited evidence currently available regarding the efficacy of nonpharmacological therapies to manage agitation in AD, there is a strong need for further and more rigorous research. In recent years, multisensory stimulation (MSS) has become a commonly used approach to manage behavioral disturbances and to promote positive mood in dementia patients. MSS actively stimulates the senses of hearing, touch, vision, and smell in an individual-oriented, nonthreatening environment. It is intended to provide individualized, sensory stimulation without the need for higher intellectual activity to achieve or maintain a state of well-being and may be useful in the management of agitation in AD. In previous studies and reviews, it has been demonstrated that MSS produces immediate or short-term positive effects on behavior and mood.38,40–46 Future studies are required to assess its effectiveness in this dementia subtype.
Importantly, in the present study, we included only RCTs; it was recently suggested that this likely excludes potent interventions in dementia patients, which limits conclusions.36 Finally, we would like to highlight that although we have made some recommendations providing valuable practical information about the optimal management of agitation in AD patients based on the level of evidence of the reviewed studies, these recommendations are not definitive because clinicians should take into consideration the specific characteristics of their patients or alternative/complementary treatments before using a specific intervention.47
Conclusion
This review found that music therapy is an effective nonpharmacological intervention for reducing agitation in institutionalized AD patients, particularly when the intervention implies individualized and interactive music. However, more evidence regarding the long-term effects of this therapy is needed. Bright light therapy has little and potentially no clinically significant effects on agitation levels. Therapeutic touch is effective for reducing physical nonaggressive behaviors but is not superior to simulated therapeutic touch or usual care in reducing physically aggressive and verbally agitated behaviors. Melissa aromatherapy and BMT do not appear to be superior to pharmacological therapies or placebo in managing agitation in AD patients; more evidence about their effects on agitation is needed to make definitive clinical recommendations. In general, there is a severe paucity of research into the effects of nonpharmacological therapies in managing agitation in older AD patients.
Footnotes
Author contributions
JCM made the conception of the study and design. CD and BA developed the search strategies, independently screened the title and abstracts of the 754 articles extracted from the literature search, applied the inclusion/exclusion criteria, and retrieved the full electronic text of the eight selected articles. LL and BA extracted the relevant data from the selected articles (independently). LL, BA, and JCM drafted the manuscript. AM and IG provided feedback on the protocol during its development and edited the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work. All authors read and approved the final version of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Alzheimer’s Disease International (AZ) World Alzheimer Report 2015. London, UK: Alzheimer’s Disease International; 2015. [Accessed September 28, 2015]. Available from: http://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf. [Google Scholar]
- 2.Quiu C, Kivipelto M, Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11:111–128. doi: 10.31887/DCNS.2009.11.2/cqiu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hay DP, Klein TT, Hay LK, Grossberg GT, Kennedy JS. Agitation in Patients with Dementia: A Practical Guide to Diagnosis and Management. 1st ed. Washington, DC: American Psychiatric Publishing; 2003. [Google Scholar]
- 4.Kong E. Agitation in dementia: concept clarification. J Adv Nurs. 2005;52:526–536. doi: 10.1111/j.1365-2648.2005.03613.x. [DOI] [PubMed] [Google Scholar]
- 5.Kverno KS, Rabins PV, Blass DM, Hicks K, Black BS. Prevalence and treatment of neuropsychiatric symptoms in hospice-eligible nursing home residents with advanced dementia. J Gerontol Nurs. 2008;34:8–17. doi: 10.3928/00989134-20081201-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okura T, Plassman BL, Steffens DC, Llewellyn DJ, Potter GG, Langa KM. Prevalence of neuropsychiatric symptoms and their association with functional limitations in older adults in the United States: the aging, demographics, and memory study. J Am Geriatr Soc. 2010;58:330–337. doi: 10.1111/j.1532-5415.2009.02680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinberg M, Shao H, Zandi P, et al. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County study. Int J Geriatr Psychiatry. 2008;23:170–177. doi: 10.1002/gps.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen-Mansfield J, Billing N. Agitated behaviors in the elderly. I. A conceptual review. J Am Geriatr Soc. 1986;34:711–721. doi: 10.1111/j.1532-5415.1986.tb04302.x. [DOI] [PubMed] [Google Scholar]
- 9.Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. J Gerontol. 1989;44:M77–M84. doi: 10.1093/geronj/44.3.m77. [DOI] [PubMed] [Google Scholar]
- 10.Cohen-Mansfield J, Dakheel-Ali M, Marx MS, Thein K, Regier NG. Which unmet needs contribute to behavior problems in persons with advanced dementia? Psychiatry Res. 2015;228:59–64. doi: 10.1016/j.psychres.2015.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry. 2006;14:191–210. doi: 10.1097/01.JGP.0000200589.01396.6d. [DOI] [PubMed] [Google Scholar]
- 12.Olazarán J, Reisberg B, Clare L, et al. Nonpharmacological therapies in Alzheimer’s disease: a systematic review of efficacy. Dement Geriatr Cogn Disord. 2010;30:161–178. doi: 10.1159/000316119. [DOI] [PubMed] [Google Scholar]
- 13.Panza F, Solfrizzi V, Seripa D, et al. Progresses in treating agitation: a major clinical challenge in Alzheimer’s disease. Expert Opin Pharmacother. 2015;16:1–8. doi: 10.1517/14656566.2015.1092520. [DOI] [PubMed] [Google Scholar]
- 14.Livingston G, Kelly L, Lewis-Holmes E, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess. 2014;18(39):1–226. doi: 10.3310/hta18390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livingston G, Kelly L, Lewis-Holmes E, et al. Non-pharmacological interventions for agitation in dementia: systematic review of randomised controlled trials. Br J Psychiatry. 2014;205:436–442. doi: 10.1192/bjp.bp.113.141119. [DOI] [PubMed] [Google Scholar]
- 16.Kong E, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13:512–520. doi: 10.1080/13607860902774394. [DOI] [PubMed] [Google Scholar]
- 17.Staedtler AV, Nunez D. Nonpharmacological therapy for the management of neuropsychiatric symptoms of Alzheimer’s disease: linking evidence to practice. Worldviews Evid Based Nurs. 2015;12:108–115. doi: 10.1111/wvn.12086. [DOI] [PubMed] [Google Scholar]
- 18.OCEBM Levels of Evidence Working Group . The Oxford 2011 Levels of Evidence. Oxford Centre for Evidence-Based Medicine; [Accessed September 28, 2015]. Available from: http://www.cebm.net/index.aspx?o=5653. [Google Scholar]
- 19.Narme P, Clément S, Ehrlé N, et al. Efficacy of musical interventions in dementia: evidence from a randomized controlled trial. J Alzheimers Dis. 2014;38:359–369. doi: 10.3233/JAD-130893. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto M, Ando H, Tsutou A. Comparing the effects of different individualized music interventions for elderly individuals with severe dementia. Int Psychogeriatr. 2013;25:775–784. doi: 10.1017/S1041610212002256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Svansdottir HB, Snaedal J. Music therapy in moderate and severe dementia of Alzheimer’s type: a case-control study. Int Psychogeriatr. 2006;18:613–621. doi: 10.1017/S1041610206003206. [DOI] [PubMed] [Google Scholar]
- 22.Ancoli-Israel S, Martin JL, Gehrman P, et al. Effect of light on agitation in institutionalized patients with severe Alzheimer disease. Am J Geriatr Psychiatry. 2003;11:194–203. [PubMed] [Google Scholar]
- 23.Dowling GA, Graf CL, Hubbard EM, Luxenberg JS. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. West J Nurs Res. 2007;29:961–975. doi: 10.1177/0193945907303083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burns A, Perry E, Holmes C, et al. A double-blind placebo-controlled randomized trial of Melissa officinalis oil and donepezil for the treatment of agitation in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2011;31:158–164. doi: 10.1159/000324438. [DOI] [PubMed] [Google Scholar]
- 25.Hawranik P, Johnston P, Deatrich J. Therapeutic touch and agitation in individuals with Alzheimer’s disease. West J Nurs Res. 2008;30:417–434. doi: 10.1177/0193945907305126. [DOI] [PubMed] [Google Scholar]
- 26.Teri L, Logsdon RG, Peskind E, et al. Treatment of agitation in AD: a randomized, placebo-controlled clinical trial. Neurology. 2000;55:1271–1278. doi: 10.1212/wnl.55.9.1271. [DOI] [PubMed] [Google Scholar]
- 27.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 28.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, (DSM-IV) 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 29.Reisberg B, Borenstein B, Salob SP, Ferris SH, Franssen E, Georgotas A. Behavioral symptoms in Alzheimer’s disease: phenomenology and treatment. J Clin Psychiatry. 1987;48:9–15. [PubMed] [Google Scholar]
- 30.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gombein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 31.Bliwise DL, Lee KA. Development of an agitated behavior rating scale for discrete temporal observations. J Nurs Meas. 1993;1:115–124. [PubMed] [Google Scholar]
- 32.Rosen J, Burgio L, Kollar M, et al. The Pittsburg Agitation Scale: a user friendly instrument for rating agitation in dementia patients. Am J Geriatr Psychiatry. 1994;2:52–59. doi: 10.1097/00019442-199400210-00008. [DOI] [PubMed] [Google Scholar]
- 33.Logsdon RG, Teri L, Weiner MF, et al. Assessment of agitation in Alzheimer’s disease: the agitated behavior in dementia scale. Alzheimer’s disease cooperative study. J Am Geriatr Soc. 1999;47:1354–1358. doi: 10.1111/j.1532-5415.1999.tb07439.x. [DOI] [PubMed] [Google Scholar]
- 34.Folstein M, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 35.Zuidema S, Koopmans R, Verhey F. Prevalence and predictors of neuropsychiatric symptoms in cognitively impaired nursing home patients. J Geriatr Psychiatry Neurol. 2007;20:41–49. doi: 10.1177/0891988706292762. [DOI] [PubMed] [Google Scholar]
- 36.Cohen-Mansfield J. Non-pharmacological interventions for agitation in dementia: various strategies demonstrate effectiveness for care home residents; further research in home settings is needed. Evid Based Nurs. 2016;19(1):31. doi: 10.1136/eb-2015-102059. [DOI] [PubMed] [Google Scholar]
- 37.Forbes DA, Peacock S, Morgan D. Nonpharmacological management of agitated behaviours associated with Dementia. Geriatr Aging. 2005;8(4):26–30. [Google Scholar]
- 38.Livingston G, Johnston K, Katona C, Paton J, Lyketsos CG, Old Age Task Force of the World Federation of Biological Psychiatry Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry. 2005;162:1996–2021. doi: 10.1176/appi.ajp.162.11.1996. [DOI] [PubMed] [Google Scholar]
- 39.Forrester LT, Maayan N, Orrell M, Spector AE, Buchan LD, Soares-Weiser K. Aromatherapy for dementia. Cochrane Database Syst Rev. 2014;2:CD003150. doi: 10.1002/14651858.CD003150.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Baker R, Holloway J, Holtkamp CC, et al. Effects of multi-sensory stimulation for people with dementia. J Adv Nurs. 2003;43:465–477. doi: 10.1046/j.1365-2648.2003.02744.x. [DOI] [PubMed] [Google Scholar]
- 41.Collier L, McPherson K, Ellis-Hill C, Staal J, Bucks R. Multisensory stimulation to improve functional performance in moderate to severe dementia-interim results. Am J Alzheimers Dis Other Demen. 2010;25:698–703. doi: 10.1177/1533317510387582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maseda A, Sánchez A, Marante MP, González-Abraldes I, Buján A, Millán-Calenti JC. Effects of multisensory stimulation on a sample of institutionalized elderly people with dementia diagnosis: a controlled longitudinal trial. Am J Alzheimers Dis Other Demen. 2014;29:463–473. doi: 10.1177/1533317514522540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maseda A, Sánchez A, Marante MP, González-Abraldes I, de Labra C, Millán-Calenti JC. Multisensory stimulation on mood, behavior, and biomedical parameters in people with dementia: is it more effective than conventional one-to-one stimulation? Am J Alzheimers Dis Other Demen. 2014;29:637–647. doi: 10.1177/1533317514532823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sánchez A, Millán-Calenti JC, Lorenzo-López L, Maseda A. Multisensory stimulation for people with dementia: a review of the literature. Am J Alzheimers Dis Other Demen. 2012;28:7–14. doi: 10.1177/1533317512466693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sánchez A, Marante-Moar MP, Sarabia C, et al. Multisensory stimulation as an intervention strategy for elderly with severe dementia: a pilot randomized controlled trial. Am J Alzheimers Dis Other Demen. 2015 Dec 1; doi: 10.1177/1533317515618801. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staal JA, Sacks A, Matheis R, et al. The effects of Snoezelen (multi-sensory behavior therapy) and psychiatric care on agitation, apathy, and activities of daily living in dementia patients on a short term geriatric psychiatric inpatient unit. Int J Psychiatry Med. 2007;37:357–370. doi: 10.2190/PM.37.4.a. [DOI] [PubMed] [Google Scholar]
- 47.Howick J, Chalmers I, Glasziou P, et al. The 2011 Oxford CEBM Evidence Levels of Evidence (Introductory Document) Oxford Centre for Evidence-Based Medicine; [Accessed September 28, 2015]. Available from: http://www.cebm.net/index.aspx?o=5653. [Google Scholar]