Abstract
Prematurity and perinatal hypoxia-ischemia are common problems that result in significant neurodevelopmental morbidity and high mortality worldwide. The Vannucci model of unilateral brain injury was developed to model perinatal brain injury due to hypoxia-ischemia. Because the rodent brain is altricial, i.e., it develops postnatally, investigators can model either preterm or term brain injury by varying the age at which injury is induced. This model has allowed investigators to better understand developmental changes that occur in susceptibility of the brain to injury, evolution of brain injury over time, and response to potential neuroprotective treatments. The Vannucci model combines unilateral common carotid artery ligation with a hypoxic insult. This produces injury of the cerebral cortex, basal ganglia, hippocampus, and periventricular white matter ipsilateral to the ligated artery. Varying degrees of injury can be obtained by varying the depth and duration of the hypoxic insult. This chapter details one approach to the Vannucci model and also reviews the neuroprotective effects of erythropoietin (Epo), a neuroprotective treatment that has been extensively investigated using this model and others.
Keywords: Vannucci model, Erythropoietin, Hypoxic-ischemia, Common carotid artery ligation
1 Introduction
1.1 Neonatal Brain Injury
Extreme prematurity and neonatal encephalopathy significantly impair the outcomes of affected infants. Even with optimal care, both conditions carry a 50% risk of death, or in survivors, mental retardation, cerebral palsy, hydrocephalus, and seizures. One of eight babies is born preterm, with 1 in 100 born at the extreme end of the spectrum (less than 28 weeks of gestation). Neonatal encephalopathy occurs in 3–5 of every 1,000 live births. Consequences of these conditions bring heartbreak for the child and family as well as expense. Loss of productivity, dependency, recurrent use of medical and rehabilitation services, and reduced life expectancy all exacerbate the burden (1–4). Many factors influence the outcomes of these vulnerable neonates, including the duration and severity of brain injury, brain maturity at time of injury, as well as the general condition of the maternal–infant pair prior to the injury (e.g., nutrition, hypoxic preconditioning, infection, stress). In term infants (>36 weeks of gestation) the neurons of the cerebral cortex, hippocampus, and basal ganglia–thalamus are most often affected, while preterm infants most often develop diffuse white matter injury (5), followed by impaired development of gray as well as white matter (6).
Animal models have been used to study the pathophysiology of neonatal brain injury and how vulnerability to specific injury changes as the brain matures. Animal models of neonatal hypoxic-ischemic brain injury are essential for the development and testing of novel therapeutic approaches. Rodent models are commonly used because they are inexpensive, easy to work with, and the gene expression pathways are readily translated to human relevance. Models of neonatal brain injury include the application of prolonged hypoxia alone (7, 8), transient middle cerebral artery occlusion (9, 10), and unilateral ligation of the common carotid artery followed by exposure of the neonatal rat to hypoxia (11–15). Hyperoxia may also play an important role in brain injury, by increasing oxidative injury in vulnerable tissues (16–18). Depending on the precise nature of the investigator’s question, postnatal age at the time of insult can be varied, and the severity of injury can be adjusted by varying the length of hypoxia, the degree of hypoxia, and the environmental temperature. Although rodent models are valuable, there are significant limitations to their use, including substantial morphological differences between human and rodent brains (e.g., the degree of encephalization and gray–white matter ratios). Thus, while rodent models are ideal for developing a framework for therapies, and the genetic and biochemical pathways by which they function, prior to clinical trials, it is important to test these approaches in larger mammals such as fetal sheep, piglets or nonhuman primates (19–24).
1.2 Erythropoietin Neuroprotection
Erythropoietin (Epo) is a circulating glycoprotein first identified for its role in erythropoiesis. More recently, the neurodevelopmental and neuroprotective functions of Epo, acting via cell-specific Epo receptors (EpoR) in the brain, have been the subject of extensive investigation. EpoR are expressed in brain throughout embryology and fetal development (25), and Epo has trophic effects both on the vascular and nervous systems (26, 27). As the fetal brain development proceeds, EpoR become increasingly regional and cell-specific (28). The function of Epo during brain development is thought to be both trophic and protective: Epo and EpoR knockout mice have smaller brains and decreased tolerance to hypoxic insults with increased neuronal apoptosis (26). To date, hundreds of studies have been published using Epo as a neuroprotective strategy in adult and neonatal models of brain injury. Significant neuroprotection has been identified in brain injury caused by hypoxia-ischemia, stroke, trauma, kainate-induced seizures, and subarachnoid hemorrhage (29–32).
EpoR are present on neural progenitor cells (33), select populations of mature neurons (34), astrocytes (35), oligodendrocytes (36), microglia (37), and endothelial cells (33) within the brain. Anti-apoptotic pathways are activated when Epo binds to cell surface EpoRs, which dimerize to activate phosphorylation of Janus kinase 2 (JAK2), phosphorylation and activation of the mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (ERK1/2), as well as the phosphatidylinositol 3-kinase (PI3K)/Akt (protein kinase B) pathway, and signal transducer and activator of transcription 5 (STAT5) (38). Epo protects oligodendrocytes from interferon-γ and LPS toxicity (39), improves white matter survival in vivo (40) and promotes oligodendrocyte maturation and differentiation in culture (35).
Epo promotes neuroprotection by stimulation and interaction with other protective factors such as brain-derived neurotrophic factor (BDNF) and glial cell derived neurotrophic factor (GDNF) (33, 41). Epo actively participates in the prevention of oxidative stress with generation of antioxidant enzymes, inhibition of nitric oxide production, and decrease of lipid peroxidation. Long-term survival of injured or newly generated cells may be fostered by Epo together with vascular endothelial growth factor (VEGF) to promote angiogenesis and repair (42, 43). Enhanced migration of neural progenitor cells occurs after treatment with Epo (44). Thus, some protective effects of Epo are the result of direct neuronal receptor-mediated interaction, and others are indirect. In fact, a recent study shows there to be neuroprotective effects of Epo in the absence of neural EpoR (45).
Epo crosses the intact blood–brain barrier at high dosages (5000 U/kg i.p.) (46) and may cross at lower doses in the setting of acute brain injury associated with disruption of the blood–brain barrier (47). Neuroprotective Epo doses range from 300 U/kg/dose to 30,000 U/kg, depending on the species studied, the injury model, severity of injury, and the number of doses given (47, 48). Multiple Epo treatments provide superior neuroprotection when compared to single Epo doses following brain injury in rodent models (48, 49). This is likely due to the multiple effects of Epo that are important during the evolving injury response: Epo decreases the early inflammatory response; decreases both early and late neuronal apoptosis (50); and stimulates late repair processes such as neurogenesis, angiogenesis, and migration of regenerating neurons (51). Higher Epo dosages (20–30,000 U/kg), particularly when given repeatedly, lose protective properties, may cause harm, and are not recommended (52). Of note circulating blood concentrations in neonates given 500–1,000 U/kg/dose are similar to those achieved in rat pups given 5,000 U/kg/dose (48, 53). Complications of Epo treatment in adults include polycythemia, rash, seizures, hypertension, shortened time to death, myocardial infarction, congestive heart failure, progression of tumors, and stroke. These complications have not been observed in neonates.
Through animal experimentation, it is known that a defined injury produced by a discrete hypoxic-ischemic insult will evolve through discrete stages including acute necrosis, inflammation, apoptosis, and late repair (54). However, there is tremendous variability between individual responses to a defined injury. It is not understood why one individual will respond to an injury with an injurious cascade of inflammation and ongoing damage while another individual can curtail this response and mobilize a repair pathway, leading to healing. These responses are likely mediated by the sequential activation of specific proteins and corresponding metabolic response. Investigating these responses in a systematic manner can provide insight into new therapeutic approaches to neonatal brain injury. There is potential for therapeutic intervention at each stage in the evolution of injury; however, the efficacy of treatment is dependent on the timing of directed interventions. For example, one could target acute injury, decreasing the initial damage caused by the inflammatory response to injury, or, one could target the later repair phase. To date, hypothermia is the only proven therapy for neonatal hypoxic-ischemic brain injury, and this is only appropriate for term infants. Translational Epo studies are now underway, and there is hope that this therapy might benefit both preterm brain injury and, in conjunction with hypothermia, improve outcomes for term infants with neonatal encephalopathy.
1.3 Unilateral Brain Injury in a Neonatal Rodent Model
The most commonly used small animal model of neonatal brain injury is the Vannucci model of unilateral brain injury (11). It is modified from the Levine preparation of adult rats (55) and is created by unilateral common carotid artery ligation or electrocauterization followed by a defined hypoxic exposure, generally using 8% oxygen. Ipsilateral brain damage can include the cerebral cortex, basal ganglia, hippocampus, and periventricular white matter injury (14). The brain hemisphere contralateral to the ligated carotid artery is generally uninjured. Severity of brain injury can be modified by adjusting the duration of hypoxia (usually ranging between 30 min and 3.5 h), with the resulting injury ranging from selective neuronal necrosis to gross infarction. The postnatal (P) age at which the experiment is performed determines whether the model is targeting premature brain injury, or term brain injury. A Web-based program is available to translate brain maturity from one species to another (56). The Vannucci procedure was first described in rat pups, but has since been modified for mice. Of note, mice are generally more sensitive to hypoxia than are rats, and significant strain differences exist, and brain maturation is slightly different than in rats (57, 58).
1.4 Overview of Procedure
Neonatal rat pups are anesthetized, undergo isolation and electro-cauterization of the right common carotid artery, followed by exposure to 8% oxygen for 90 min to produce a moderate unilateral hypoxic-ischemic brain injury. Depending on the length of the hypoxic episode, the timing of peak cerebral edema ranges from 4 to 24 h (23, 59). The blood–brain barrier is altered during the recovery from the hypoxic injury as evidenced by extravasation of horseradish peroxidase from the vascular space into the brain parenchyma (60). Cerebral blood flow to the ipsilateral brain is unaffected by the unilateral common carotid ligation, but is decreased during the hypoxia-ischemic episode (12) (see Note 1).
2 Materials
Personal protective equipment (lab coat, sterile gloves, mask).
Instrument sterilizer.
Inhalant anesthetic (e.g., Isoflurane), matching vaporizer with regulated oxygen supply and adjustable flow control.
Anesthesia induction chamber.
Anesthetic exhaust or trap.
Dissecting microscope with light source.
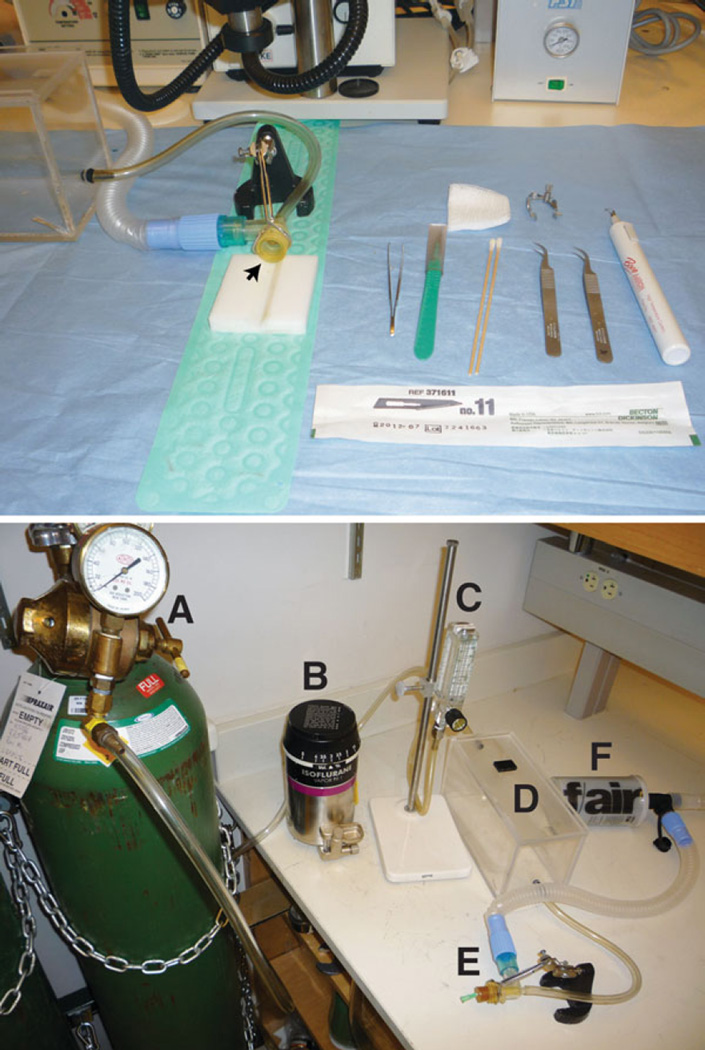
Plastic animal cradle. Should contain a V-shaped indentation in which to hold the rat pup supine during surgery (Fig. 1).
Heating pad × 2.
Small animal incubator (Veterinary warmer).
Fine rat-tooth forceps.
Scalpel with #11 blade.
Microretractor or suitable substitute.
Sharp fine forceps × 2.
Cauterizer.
Skin staples, skin glue, or suture material for wound closure.
Gauze or cotton swabs.
Pentobarbital (or other approved euthanizing agent, to euthanize failed surgeries).
Body bag.
Hypoxia chamber (sealed container with ports for gas delivery).
Thermoregulated water bath.
Thermometer.
8% oxygen tank with regulator.
Oxygen meter.
Fig. 1.
Setup. The top panel illustrates the surgical setup, including the animal cradle, anesthetic outlet (arrow), and surgical instruments on a sterile field. The bottom panel shows the setup for anesthesia. The oxygen tank (A) is connected to the anesthesia vaporizer (B), a flow meter (C), the induction chamber (D), anesthetic outlet (E), and gas scavenger (F)
3 Methods
3.1 Setup
3.1.1 Animal Warming Station
Turn on heating pad or veterinary warmer.
Place a home cage on top of a heating pad or inside a veterinary warmer. This will provide a warmed area to keep the pups when they are not with their dam.
3.1.2 Surgical Station (Fig. 1)
Prepare a sterile field in the area adjacent to the dissecting microscope. Tools are autoclaved prior to use, and a hot bead sterilizer is used between animals. Surgeons wear sterile gloves, face masks, and clean lab coats.
Lay out sterilized surgical instruments, warming pad and animal cradle (see Fig. 1, top panel).
Prepare dissecting microscope by turning on light source, and checking focal range.
Prepare anesthesia delivery system by connecting regulated oxygen supply to the anesthetic vaporizer. Connect the vaporizer to the induction chamber. The induction chamber connects to the anesthesia outlet (Fig. 1, bottom panel, arrow) which is connected to an exhaust or trap. A rubber dam can be secured to the anesthesia outlet to decrease the escape of anesthetic gas into the environment. To do this, cut a 3 in. square from a laboratory glove, secure to the outlet with a rubber band, and cut a small hole in the taut surface. The pup’s nose will be inserted into this hole.
3.1.3 Hypoxia Station
Obtain an 8% O2 tank and regulator, then connect tubing to channel the gas supply through a humidifier followed by a water trap then run the line to the hypoxia chamber. The humidifier and gas trap are readily made using two Erlenmeyer flasks, the humidifier contains dH2O and is immersed in the water bath to warm and humidify the gas supply.
The hypoxia chamber must have an input and one-way exhaust port to prevent pressure build up. The optimal chamber is transparent acrylic or plastic with an air-tight opening. In advance, the minimal flow rate needed to quickly flush the chamber with 8% oxygen should be determined using an oxygen meter.
Prior to gas exposure, heat the water bath/chamber apparatus so that the humidifier and chamber will be at the desired temperatures.
3.2 Hypoxic-Ischemic Brain Injury
Select, identify, weigh, and assign animals to treatment groups as appropriate for the experimental protocol.
3.2.1 Anesthesia
Fill vaporizer with anesthetic.
Open gas supply and adjust gas flow to 2–3 LPM, depending on size of induction chamber.
Turn on vaporizer to 2.5–3%.
Place animal in chamber. Before the start of any surgical steps, ensure adequate sedation by checking for loss of spontaneous and reflexive movements (including lack of postural/geotaxis orientation or response to touch), increased muscle relaxation, and decreased respiration. 3–5 min of pre-anesthesia is generally sufficient.
3.2.2 Common Carotid Artery Electrocautery
Aseptic precautions (must include method of instrument sterilization prior to initial use and between animals, if applicable)—Tools are autoclaved prior to use, and a hot bead sterilizer is used between animals. Surgeons wear sterile gloves, face masks, and clean lab coats. Turn on heating pad under the animal cradle.
Place the sedated animal supine onto the animal cradle with its nose in the anesthesia outlet (Fig. 1, arrow). Adjust the focus of the dissecting scope as necessary. Place a piece of tape across the animal’s chest and forelimbs to gently secure the animal to the cradle. See Note 2 for additional comments.
Prepare the neck with Betadine surgical scrub. After a small midline neck incision the right CCA is isolated and permanently cauterized and the incision is closed with skin adhesive or wound clips as appropriate and topical Betadine solution is applied.
Use a #11 scalpel blade to make a 1 cm incision in skin of the mid anterior neck. Retract skin to improve access.
Use blunt dissection with fine curved forceps (repeated opening of the forceps) to retract the muscle layers and expose the underlying veins and arteries. The veins are dark, avoid them. The artery will be a pulsatile structure with bright red blood, surrounded by a white muscular layer. Dissect down to expose the strap muscles, then laterally to expose the sterno-cleidomastoid muscles, and the carotid is deep in the cleft between those muscles.
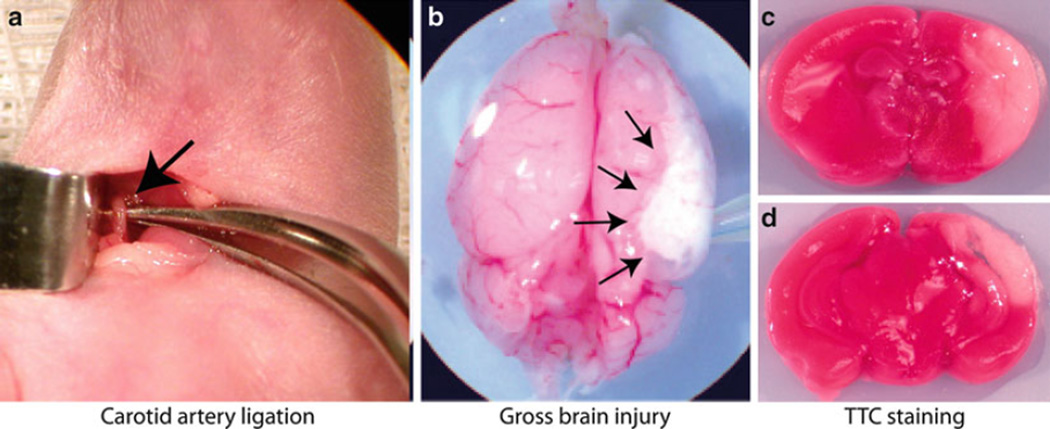
Gently lift the exposed common carotid artery using a curved forceps (Fig. 2a).
To cauterize the artery, begin by touching the cauterizer to the forceps (not the artery). The heat will be transferred from the forceps to the artery. Observe the color change in the artery as it coagulates. Once it becomes opaque, you may touch the cauterizer to the artery to completely sever the structure.
Align the skin and apply a skin staple to close the incision. Alternatively, the incision can be sutured closed or glued using Vetbond.
Return the pups to the warming station and monitor recovery. Once the animal has recovered from anesthesia, it may be placed back in the home cage with the dam for 2 h before hypoxia exposure (see Note 3).
Fig. 2.
Brain injury. Panel (a) shows the isolated common carotid artery (arrow). Panel (b) shows a brain with severe gross injury 48 h after ligation. Arrows outline the injured region. Panels (c) and (d) show TTC staining of injured brain from an anterior section (c), and posterior (d)
3.2.3 Hypoxia Exposure
Place pups in the preheated hypoxia chamber (heated to 36°C) (see Note 4).
Turn on the 8% oxygen tank at 15 L/min for 5 min to purge the tank. Decrease the flow to 5 L/min for duration of hypoxic event (30 min to 3.5 h, depending on severity of brain injury desired) (see Note 5).
When hypoxia exposure is completed and animals are stable, return pups to the dams.
3.3 Evaluation of Injury
3.3.1 Gross Evaluation of Injury
Weight gain, growth, timing, and cause of mortality and morbidity should be recorded. All brains should be scored for gross injury at the time of sacrifice. Gross brain injury is visually apparent 24 h after injury (Fig. 2b), and can be evaluated using an ordinal scale modified from Vannucci (61) as shown in Table 1. If animals are not perfusion-fixed, tetrazolium chloride (TTC) staining, which is incorporated into actively respiring tissues, can be used to clearly demarcate areas of non-vital tissue (Fig. 2c, d). The intensity of the color is proportional to the rate of respiration in the tissues, so living tissue stains red, while infarcted areas appear white.
Table 1.
Ordinal scale
| Brain injury scale | ||
|---|---|---|
| Scale | Acute injury (24–48 h) | Chronic injury (>1 week) |
| 0 | Normal ipsilateral hemisphere | Normal ipsilateral hemisphere |
| 1 | Mild edema with <25% ipsi/contra size difference | Mild atrophy with <25% ipsi/contra size difference |
| 2 | Moderate edema (25–50%) difference | Moderate atrophy (25–50% difference) |
| 3 | Liquefaction of 50–75% hemisphere | Cystic cavitation 50–75% hemisphere |
| 4 | Liquefaction of ≥75% hemisphere | Cystic cavitation ≥75% hemisphere |
3.3.2 Triphenyltetrazolium Chloride Staining
When TTC diffuses into actively respiring tissues it accepts electrons from the mitochondrial electron transport chain and the stain is reduced to the pink compound formazan. The accumulation of this pink compound stains actively metabolizing tissues red, and the intensity of the red is proportional to the rate of respiration in those tissues. This method can therefore be used to distinguish between live (stain, red) and infarcted (no stain, white) areas on 2 mm thick brain sections.
Using a mouse brain grid, rat pup brains are coronally sliced into 2 mm sections. Sections are incubated in 2% TTC in 0.9% Sodium Chloride for 30 min at room temperature. TTC is removed, and samples washed with fixative (4% Paraformaldehyde). Sections are removed from the solution for photographing.
Further investigation into specific effects of injury or neuroprotective treatments can be done using mRNA protein measurement or immunohistochemical approaches (see Note 6).
4 Notes
There are many confounders that may affect the extent of injury with this model. The age of the rodent changes which cell lines and brain regions are more at risk from hypoxic-ischemic injury (62, 63). Rodent pups should be sexed since gender differences have been seen in response to hypoxic-ischemic injury (64). These animals are exquisitely sensitive to temperature variation, and hypothermia can attenuate hypoxic-ischemic injury; therefore, body temperature should be constant during hypoxia-ischemia and throughout the experimental period. The hypoxic exposure should occur within 2–3 h after ligation of the carotid artery to allow suckling of the pups, thus avoiding the attenuation of injury secondary to fasting hypoglycemia (65). If larger experiments are planned, pups can be randomly assigned to dams and will be cross fostered. This decreases the maternal influence on experimental outcomes. Culling the litter size to 8 pups per litter will increase uniformity of pup growth and improve survival.
Anesthetic choice may affect outcomes, so a consistent anesthetic must be used across groups and when comparing groups over time. For example, isoflurane is a vasodilator and also may have neurotoxic effects, while other anesthetics such as xenon may be neuroprotective.
Rat dams will eat the pups if disturbed. This occurs most commonly in the first days of life, but can still occur at P7. To minimize this, make sure there is no blood on the pups when they are returned to the dam after surgery. We have found that wiping the incision with a Betadine swab after incision closure decreases the likelihood that the dam will interfere.
Brain injury in this model can be highly variable, and is extremely temperature dependent. It is critical to monitor environmental temperatures closely before, during, and after the experiment. Small decreases in temperature will produce neuroprotection, and increases in temperature will increase brain injury. One solution is to use a plastic hypoxia chamber immersed in a temperature-controlled water bath. In advance, determine the optimal water bath temperature for the chosen chamber. We have also found that separating the pups during the hypoxia exposure will help ensure uniform temperature, and providing a heating pad turned to “low” after the experiment for several days will increase the uniformity of brain injury.
The depth and duration of hypoxia will determine the severity of brain injury. 6–12% oxygen exposure for 30 min to 3.5 h has been reported. Younger animals are more tolerant of hypoxia and require longer hypoxia exposures to create injury. Mice are more susceptible to hypoxic brain injury than are rats, and this is strain dependent (57), so caution must be used when beginning this experimental procedure in new strains. Despite this, working with mice can be advantageous because they have a more homogeneous background than rats, and transgenic strains are available.
For short-term experiments, animals can be identified using permanent marker numbers that are renewed daily. For long-term experiments, permanent identifiers must be used. Ear punches or paw tattoos work equally well. For long-term experiments, weaning at 21–28 days and separation of sexes is required.
References
- 1.Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volpe JJ. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol. 2009;24:1085–1104. doi: 10.1177/0883073809338067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyson JE, Parikh NA, Langer J, Green C, Higgins RD. Intensive care for extreme prematurity—moving beyond gestational age. N Engl J Med. 2008;358:1672–1681. doi: 10.1056/NEJMoa073059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang B, Chen Y, Zhang J, Li J, Guo Y, Hailey D. A preliminary study into the economic burden of cerebral palsy in China. Health Policy. 2008;87:223–234. doi: 10.1016/j.healthpol.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Back SA. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev. 2006;12:129–140. doi: 10.1002/mrdd.20107. [DOI] [PubMed] [Google Scholar]
- 6.Kapellou O, Counsell SJ, Kennea N, Dyet L, Saeed N, Stark J, Maalouf E, Duggan P, Ajayi-Obe M, Hajnal J, Allsop JM, Boardman J, Rutherford MA, Cowan F, Edwards AD. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006;3:e265. doi: 10.1371/journal.pmed.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart WB, Ment LR, Schwartz M. Chronic postnatal hypoxia increases the numbers of cortical neurons. Brain Res. 1997;760:17–21. doi: 10.1016/s0006-8993(97)00271-0. [DOI] [PubMed] [Google Scholar]
- 8.Ment LR, Schwartz M, Makuch RW, Stewart WB. Association of chronic sublethal hypoxia with ventriculomegaly in the developing rat brain. Brain Res Dev Brain Res. 1998;111:197–203. doi: 10.1016/s0165-3806(98)00139-4. [DOI] [PubMed] [Google Scholar]
- 9.Ashwal S, Cole DJ, Osborne S, Osborne TN, Pearce WJ. A new model of neonatal stroke: reversible middle cerebral artery occlusion in the rat pup. Pediatr Neurol. 1995;12:191–196. doi: 10.1016/0887-8994(95)00006-2. [DOI] [PubMed] [Google Scholar]
- 10.Sola A, Rogido M, Lee BH, Genetta T, Wen TC. Erythropoietin after focal cerebral ischemia activates the janus kinase-signal transducer and activator of transcription signaling pathway and improves brain injury in postnatal day 7 rats. Pediatr Res. 2005;57(4):481–487. doi: 10.1203/01.PDR.0000155760.88664.06. [DOI] [PubMed] [Google Scholar]
- 11.Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 12.Vannucci RC, Lyons DT, Vasta F. Regional cerebral blood flow during hypoxiaischemia in immature rats. Stroke. 1988;19:245–250. doi: 10.1161/01.str.19.2.245. [DOI] [PubMed] [Google Scholar]
- 13.Yager JY, Brucklacher RM, Vannucci RC. Cerebral energy metabolism during hypoxia-ischemia and early recovery in immature rats. Am J Physiol. 1992;262:H672–H677. doi: 10.1152/ajpheart.1992.262.3.H672. [DOI] [PubMed] [Google Scholar]
- 14.Towfighi J, Zec N, Yager J, Housman C, Vannucci RC. Temporal evolution of neuropathologic changes in an immature rat model of cerebral hypoxia: a light microscopic study. Acta Neuropathol. 1995;90:375–386. doi: 10.1007/BF00315011. [DOI] [PubMed] [Google Scholar]
- 15.Vannicci RC, Connor JR, Mauger DT, Palmer C, Smith MB, Towfighi J, Vannucci SJ. Rat model of perinatal hypoxic-ischemic brain damage. J Neurosci Res. 1999;55:158–163. doi: 10.1002/(SICI)1097-4547(19990115)55:2<158::AID-JNR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Felderhoff-Mueser U, Sifringer M, Polley O, Dzietko M, Leineweber B, Mahler L, Baier M, Bittigau P, Obladen M, Ikonomidou C, Buhrer C. Caspase-1-processed interleukins in hyperoxia-induced cell death in the developing brain. Ann Neurol. 2005;57:50–59. doi: 10.1002/ana.20322. [DOI] [PubMed] [Google Scholar]
- 17.Saugstad OD. Oxygen toxicity in the neonatal period. Acta Paediatr Scand. 1990;79:881–892. doi: 10.1111/j.1651-2227.1990.tb11348.x. [DOI] [PubMed] [Google Scholar]
- 18.Munkeby BH, Borke WB, Bjornland K, Sikkeland LI, Borge GI, Halvorsen B, Saugstad OD. Resuscitation with 100% O2 increases cerebral injury in hypoxemic piglets. Pediatr Res. 2004;56:783–790. doi: 10.1203/01.PDR.0000141988.89820.E3. [DOI] [PubMed] [Google Scholar]
- 19.Ment LR, Stewart WB, Fronc R, Seashore C, Mahooti S, Scaramuzzino D, Madri JA. Vascular endothelial growth factor mediates reactive angiogenesis in the postnatal developing brain. Brain Res Dev Brain Res. 1997;100:52–61. doi: 10.1016/s0165-3806(97)00012-6. [DOI] [PubMed] [Google Scholar]
- 20.Ment LR, Stewart WB, Duncan CC, Pitt BR, Cole JS. Beagle puppy model of perinatal cerebral infarction. Regional cerebral prostaglandin changes during acute hypoxemia. J Neurosurg. 1986;65:851–855. doi: 10.3171/jns.1986.65.6.0851. [DOI] [PubMed] [Google Scholar]
- 21.Adcock LM, Yamashita Y, Goddard-Finegold J, Smith CV. Cerebral hypoxia-ischemia increases microsomal iron in newborn piglets. Metab Brain Dis. 1996;11:359–367. doi: 10.1007/BF02029496. [DOI] [PubMed] [Google Scholar]
- 22.Stave U. Age-dependent changes of metabolism. II. Influences of hypoxia on tissue enzyme patterns of newborn and adult rabbits. Biol Neonat. 1965;8:114–130. [PubMed] [Google Scholar]
- 23.Vannucci RC. Experimental models of perinatal hypoxic-ischemic brain damage. APMIS Suppl. 1993;40:89–95. [PubMed] [Google Scholar]
- 24.Myers RE. Four patterns of perinatal brain damage and their conditions of occurrence in primates. Adv Neurol. 1975;10:223–234. [PubMed] [Google Scholar]
- 25.Juul SE, Anderson DK, Li Y, Christensen RD. Erythropoietin and erythropoietin receptor in the developing human central nervous system. Pediatr Res. 1998;43:40–49. doi: 10.1203/00006450-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Yu X, Shacka JJ, Eells JB, Suarez-Quian C, Przygodzki RM, Beleslin-Cokic B, Lin CS, Nikodem VM, Hempstead B, Flanders KC, Costantini F, Noguchi CT. Erythropoietin receptor signalling is required for normal brain development. Development. 2002;129:505–516. doi: 10.1242/dev.129.2.505. [DOI] [PubMed] [Google Scholar]
- 27.Chen ZY, Asavaritikrai P, Prchal JT, Noguchi CT. Endogenous erythropoietin signaling is required for normal neural progenitor cell proliferation. J Biol Chem. 2007;282:25875–25883. doi: 10.1074/jbc.M701988200. [DOI] [PubMed] [Google Scholar]
- 28.Juul SE, Yachnis AT, Rojiani AM, Christensen RD. Immunohistochemical localization of erythropoietin and its receptor in the developing human brain. Pediatr Dev Pathol. 1999;2:148–158. doi: 10.1007/s100249900103. [DOI] [PubMed] [Google Scholar]
- 29.Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the blood–brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juul S. Erythropoietin in the central nervous system, and its use to prevent hypoxic-ischemic brain damage. Acta Paediatr Suppl. 2002;91:36–42. doi: 10.1111/j.1651-2227.2002.tb02904.x. [DOI] [PubMed] [Google Scholar]
- 31.McPherson RJ, Juul SE. Recent trends in erythropoietin-mediated neuroprotection. Int J Dev Neurosci. 2008;26:103–111. doi: 10.1016/j.ijdevneu.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiong T, Qu Y, Mu D, Ferriero D. Erythropoietin for neonatal brain injury: opportunity and challenge. Int J Dev Neurosci. 2011;29(6):583–591. doi: 10.1016/j.ijdevneu.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 34.Wallach I, Zhang J, Hartmann A, van Landeghem FK, Ivanova A, Klar M, Dame C. Erythropoietin-receptor gene regulation in neuronal cells. Pediatr Res. 2009;65:619–624. doi: 10.1203/PDR.0b013e31819ea3b8. [DOI] [PubMed] [Google Scholar]
- 35.Sugawa M, Sakurai Y, Ishikawa-Ieda Y, Suzuki H, Asou H. Effects of erythropoietin on glial cell development; oligodendrocyte maturation and astrocyte proliferation. Neurosci Res. 2002;44:391–403. doi: 10.1016/s0168-0102(02)00161-x. [DOI] [PubMed] [Google Scholar]
- 36.Nagai A, Nakagawa E, Choi HB, Hatori K, Kobayashi S, Kim SU. Erythropoietin and erythropoietin receptors in human CNS neurons, astrocytes, microglia, and oligodendrocytes grown in culture. J Neuropathol Exp Neurol. 2001;60:386–392. doi: 10.1093/jnen/60.4.386. [DOI] [PubMed] [Google Scholar]
- 37.Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003;138:1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 39.Genc K, Genc S, Baskin H, Semin I. Erythropoietin decreases cytotoxicity and nitric oxide formation induced by inflammatory stimuli in rat oligodendrocytes. Physiol Res. 2006;55:33–38. doi: 10.33549/physiolres.930749. [DOI] [PubMed] [Google Scholar]
- 40.Vitellaro-Zuccarello L, Mazzetti S, Madaschi L, Bosisio P, Fontana E, Gorio A, De Biasi S. Chronic erythropoietin-mediated effects on the expression of astrocyte markers in a rat model of contusive spinal cord injury. Neuroscience. 2008;151:452–466. doi: 10.1016/j.neuroscience.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Dzietko M, Felderhoff-Mueser U, Sifringer M, Krutz B, Bittigau P, Thor F, Heumann R, Buhrer C, Ikonomidou C, Hansen HH. Erythropoietin protects the developing brain against N-methyl-d-aspartate receptor antagonist neurotoxicity. Neurobiol Dis. 2004;15:177–187. doi: 10.1016/j.nbd.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Chopp M, Gregg SR, Zhang RL, Teng H, Jiang A, Feng Y, Zhang ZG. Neural progenitor cells treated with EPO induce angiogenesis through the production of VEGF. J Cereb Blood Flow Metab. 2008;28:1361–1368. doi: 10.1038/jcbfm.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bocker-Meffert S, Rosenstiel P, Rohl C, Warneke N, Held-Feindt J, Sievers J, Lucius R. Erythropoietin and VEGF promote neural outgrowth from retinal explants in postnatal rats. Invest Ophthalmol Vis Sci. 2002;43:2021–2026. [PubMed] [Google Scholar]
- 44.Wang KK, Larner SF, Robinson G, Hayes RL. Neuroprotection targets after traumatic brain injury. Curr Opin Neurol. 2006;19:514–519. doi: 10.1097/WCO.0b013e3280102b10. [DOI] [PubMed] [Google Scholar]
- 45.Xiong Y, Mahmood A, Lu D, Qu C, Kazmi H, Goussev A, Zhang ZG, Noguchi CT, Schallert T, Chopp M. Histological and functional outcomes after traumatic brain injury in mice null for the erythropoietin receptor in the central nervous system. Brain Res. 2008;1230:247–257. doi: 10.1016/j.brainres.2008.06.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juul SE, McPherson RJ, Farrell FX, Jolliffe L, Ness DJ, Gleason CA. Erytropoietin concentrations in cerebrospinal fluid of nonhuman primates and fetal sheep following high-dose recombinant erythropoietin. Biol Neonate. 2004;85:138–144. doi: 10.1159/000074970. [DOI] [PubMed] [Google Scholar]
- 47.Zhu C, Kang W, Xu F, Cheng X, Zhang Z, Jia L, Ji L, Guo X, Xiong H, Simbruner G, Blomgren K, Wang X. Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics. 2009;124:e218–e226. doi: 10.1542/peds.2008-3553. [DOI] [PubMed] [Google Scholar]
- 48.Kellert BA, McPherson RJ, Juul SE. A comparison of high-dose recombinant erythropoietin treatment regimens in brain-injured neonatal rats. Pediatr Res. 2007;61:451–455. doi: 10.1203/pdr.0b013e3180332cec. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez FF, Abel R, Almli CR, Mu D, Wendland M, Ferriero DM. Erythropoietin sustains cognitive function and brain volume after neonatal stroke. Dev Neurosci. 2009;31:403–411. doi: 10.1159/000232558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Juul SE, McPherson RJ, Bammler TK, Wilkerson J, Beyer RP, Farin FM. Recombinant erythropoietin is neuroprotective in a novel mouse oxidative injury model. Dev Neurosci. 2008;30:231–242. doi: 10.1159/000110348. [DOI] [PubMed] [Google Scholar]
- 51.Tsai PT, Ohab JJ, Kertesz N, Groszer M, Matter C, Gao J, Liu X, Wu H, Carmichael ST. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26:1269–1274. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weber A, Dzietko M, Berns M, Felderhoff-Mueser U, Heinemann U, Maier RF, Obladen M, Ikonomidou C, Buhrer C. Neuronal damage after moderate hypoxia and erythropoietin. Neurobiol Dis. 2005;20:594–600. doi: 10.1016/j.nbd.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 53.Juul SE, McPherson RJ, Bauer LA, Ledbetter KJ, Gleason CA, Mayock DE. A phase I/II trial of high-dose erythropoietin in extremely low birth weight infants: pharmacokinetics and safety. Pediatrics. 2008;122:383–391. doi: 10.1542/peds.2007-2711. [DOI] [PubMed] [Google Scholar]
- 54.Fan X, Kavelaars A, Heijnen CJ, Groenendaal F, van Bel F. Pharmacological neuroprotection after perinatal hypoxic-ischemic brain injury. Curr Neuropharmacol. 2010;8:324–334. doi: 10.2174/157015910793358150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levine S. Anoxic-ischemic encephalopathy in rats. Am J Pathol. 1960;36:1–17. [PMC free article] [PubMed] [Google Scholar]
- 56.Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- 57.Sheldon RA, Sedik C, Ferriero DM. Strain-related brain injury in neonatal mice subjected to hypoxia-ischemia. Brain Res. 1998;810:114–122. doi: 10.1016/s0006-8993(98)00892-0. [DOI] [PubMed] [Google Scholar]
- 58.Boasen JF, McPherson RJ, Hays SL, Juul SE, Gleason CA. Neonatal stress or morphine treatment alters adult mouse conditioned place preference. Neonatology. 2008;95:230–239. doi: 10.1159/000165379. [DOI] [PubMed] [Google Scholar]
- 59.Vannucci RC. Experimental biology of cerebral hypoxia-ischemia: relation to perinatal brain damage. Pediatr Res. 1990;27:317–326. doi: 10.1203/00006450-199004000-00001. [DOI] [PubMed] [Google Scholar]
- 60.Vannucci RC, Christensen MA, Yager JY. Nature, time-course, and extent of cerebral edema in perinatal hypoxic-ischemic brain damage. Pediatr Neurol. 1993;9:29–34. doi: 10.1016/0887-8994(93)90006-x. [DOI] [PubMed] [Google Scholar]
- 61.Vannucci RC, Towfighi J, Heitjan DF, Brucklacher RM. Carbon dioxide protects the perinatal brain from hypoxic-ischemic damage: an experimental study in the immature rat. Pediatrics. 1995;95:868–874. [PubMed] [Google Scholar]
- 62.Yager JY, Brucklacher RM, Vannucci RC. Paradoxical mitochondrial oxidation in perinatal hypoxic-ischemic brain damage. Brain Res. 1996;712:230–238. doi: 10.1016/0006-8993(95)01423-3. [DOI] [PubMed] [Google Scholar]
- 63.Towfighi J, Mauger D, Vannucci RC, Vannucci SJ. Influence of age on the cerebral lesions in an immature rat model of cerebral hypoxia-ischemia: a light microscopic study. Brain Res Dev Brain Res. 1997;100:149–160. doi: 10.1016/s0165-3806(97)00036-9. [DOI] [PubMed] [Google Scholar]
- 64.Vannucci SJ, Hagberg H. Hypoxiaischemia in the immature brain. J Exp Biol. 2004;207:3149–3154. doi: 10.1242/jeb.01064. [DOI] [PubMed] [Google Scholar]
- 65.Yager JY, Heitjan DF, Towfighi J, Vannucci RC. Effect of insulin-induced and fasting hypoglycemia on perinatal hypoxicischemic brain damage. Pediatr Res. 1992;31:138–142. doi: 10.1203/00006450-199202000-00009. [DOI] [PubMed] [Google Scholar]