Abstract
Objectives
Preterm premature rupture of membranes (PROM) accounts for 30–40% of all preterm births. The objectives of this study were to determine whether matrix metalloproteinase-9 (MMP-9) is increased in preterm PROM fetal membranes, whether labor or gestational age affects expression, and whether the increase is localized to the rupture site or is membrane-wide.
Methods
Fetal membranes were collected from 15 pregnancies complicated by preterm PROM and 26 control cases, which delivered at term or preterm without PROM. The preterm PROM cases represented both patients who labored and those who did not. Membrane samples at the rupture site and a remote site (approximately > 5 cm) were analyzed for MMP-9 protein and enzymatic activity by Western blot and gelatin zymography, respectively.
Results
MMP-9 levels in fetal membranes were similar at both the rupture and the remote sites. The highest levels of total MMP-9 protein were found in preterm PROM patients with labor (p < 0.05) and were increased four-fold over protein levels in non-laboring preterm PROM patients delivered by Cesarean section (p < 0.001). In preterm PROM patients without labor, levels of MMP-9 protein were similar to those of non-laboring patients at term and preterm. Zymography correlated with protein results in all membranes.
Conclusions
Preterm PROM without labor is not associated with increased membrane levels of MMP-9 protein, suggesting that its local elevation does not play a role in early membrane rupture.
Keywords: PRETERM BIRTH, MMP-9, PRETERM PROM, FETAL MEMBRANES, INFLAMMATION
INTRODUCTION
Preterm premature rupture of membranes (PROM) results in 30–40% of all preterm births1. It is a significant cause of infant morbidity and mortality, as well as high medical costs2. Many obstetric factors and maternal disease states are associated with a significantly increased risk for preterm PROM. The most significant independent risk factors include smoking, infection and previous preterm delivery3.
The mechanism causing membrane rupture is unknown. One proposed theory is that of an increase in endogenous proteinase activity4. Potential candidates include the matrix metalloproteinases (MMPs) which are involved in the degradation of endogenous, structural collagens within the fetal membrane5. The MMPs are a large group of related collagenases and gelatinases which function in the regulation and turnover of extracellular collagens found in fetal membranes (types I, III, IV and V)6–8. Matrix metalloproteinase-9 (MMP-9/collagenase IV, or gelatinase B) degrades type IV collagen, proteoglycans, elastin and collagen type I that has already been denatured by MMP-18,9.
MMP expression is central to any extracellular matrix remodelling. Immunohistochemical studies of normal, term membranes indicate an increase in MMP-9 at the site of rupture6,10. Both preterm labor and chorioamnionitis have been associated with elevated membrane and amniotic fluid levels of MMP-97,11,12. Our previous studies on fetal mem branes from preterm PROM have demonstrated an increase in general protease activity as well as a 92-kDa gelatinase identified by zymography4.
The purpose of our study was, first, to confirm the identity of the 92-kDa protease activity we had previously described in preterm PROM membranes as MMP-9; second, to determine whether the elevation of MMP-9 was correlated with the presence of labor or preterm gestational age in our preterm PROM patients; and, third, to determine whether MMP-9 protein was elevated only at the rupture site compared to the midpoint site, or whether it was globally elevated across the membrane.
METHODS
Patient population and delivery information
Fetal membranes were collected from placentas of women attending the labor and delivery suites at Magee-Women’s Hospital from August 1994 to June 1999. A clinical investigator or research nurse attended the delivery to collect the membranes and to process them within 15 min of delivery. The membranes were collected under a protocol approved by the Human Experimentation and Review Committee of Magee-Women’s Hospital (IRB no. MWH 94-092). At the time of delivery, clinical information was collected on delivery route, presence of labor, gestational age, duration of ruptured membranes and meconium staining.
A variety of membranes were collected for analysis (n = 41). The groups of interest included membranes from deliveries at term, for patients with repeat Cesarean sections without labor, term vaginal deliveries, patients with preterm PROM and Cesarean sections without labor, preterm PROM vaginal deliveries, non-laboring patients with preterm Cesarean sections, and preterm patients in labor without PROM (Table 1). Assignment to groups for analysis was confirmed by a final chart review by an investigator blinded to the biochemical assay values. Preterm delivery was defined as delivery at a gestational age of less than 34 weeks. For these comparison studies, an effort was made to control for gestational age, route of delivery and presence of labor. All preterm PROM patients were tested at presentation to the labor and delivery ward for Chlamydia trachomatis, Neisseria gonorrhoeae and Trichomonas vaginalis and were found to be uninfected. The term controls were not tested but were negative at entry into prenatal care. Amniocentesis was not performed in any preterm PROM patient, as no signs or symptoms of infection were evident at presentation.
Table 1.
Relative amounts of matrix metalloproteinase-9(MMP-9) protein in human fetal membrane sites from different patient delivery types
| Patient group |
Delivery type | Labor | Rupture site, MMP-9 relative ODU (median ± SE) |
Midpoint site, MMP-9 relative ODU (median ± SE) |
Number of patients |
Gestational age (weeks) |
|
|---|---|---|---|---|---|---|---|
| Range | Median | ||||||
| 1 | term C/S | no | 298 ± 128 | 344 ± 165 | 8 | 38–40 | 39 |
| 2 | term Vag | yes | 686 ± 279 | 654 ± 80 | 10 | 38–42 | 40 |
| 3 | pPROM C/S | no | 394 ± 111 | 301 ± 100 | 5 | 27–33 | 30 |
| 4 | pPROM Vag | yes | 1528 ± 347 | 1643 ± 258 | 10 | 25–33 | 27 |
| 5 | preterm C/S | no | 513 ± 333 | 687 ± 279 | 4 | 30–33 | 30 |
| 6 | PTD non-PROM | yes | 1059 ± 181 | 740 ± 305 | 4 | 28–33 | 29 |
ODU, optical density units; C/S, Cesarean section; Vag, vaginal delivery (labored); pPROM, preterm premature rupture of membranes; PTD, preterm delivery Relative membrane levels of MMP-9 protein were compared between the two sampling sites (rupture and midpoint) and no significant differences were found. Medians for rupture sites from each group were compared and significant differences were found between the term Cesareanno-labor group (C/S NL) and the pPROM vaginal delivery group (p < 0.001). Comparisons of midpoint levels were made and significant differences were found between pPROM NL vs. pPROM with labor; term C/S NL vs. pPROM with labor; and pPROM with labor vs. term vaginal delivery
Processing of membranes
Membranes were collected from the placenta using universal precautions and a sterile field. A 5-cm wide strip of membrane was cut from the rupture (or incision) site to the placental rim. While maintaining orientation, the strip was further cut into two sections: rupture site and midpoint. The midpoint was at least 5 cm from the last edge of the rupture site tissue. Samples were immediately placed into labelled plastic bags and flash frozen in liquid nitrogen for transport to the laboratory. They were either processed immediately for biochemical studies or placed into a −80°C freezer for batch analysis. Membranes were defrosted, ground at 4°C in a Power Gen 125 tissue homogenizer (Fisher/SP Products, Pittsburgh, PA, USA) with 2 ml ice-cold grinding buffer (phosphate-buffered saline with 1 mmol/l EDTA, pH 7.4), clarified by centrifugation in the Sorvall Microfuge at 10 000 rpm for 5 min at 4°C, and the supernatant used in the assays.
Protein assay
Protein concentration was determined by the BCA protein assay (Pierce Chemical Company, Rockford, IL, USA) in a 96-well microtiter plate format. Samples were diluted 1 : 20 and 10-μl aliquots were analyzed according to the manufacturer’s directions. The colored end-product was read in a Bio Tek EL-312 miniplate spectrophotometer (Bio Tek Inc., Winooski, VT, USA) at 550 nm. Samples were run in duplicate, concentrations were averaged, and bovine serum albumin (BSA) (Pierce Chemical) was used as the standard.
Western blot analyses
Western blot analyses on membrane samples were performed by a modified Towbin technique13 following Lammeli SDS gel electrophoresis14. Samples were mixed 1 : 1 with 2 × Tris-Glycine SDS sample buffer with β-mercaptoethanol and heated at 37°C for 10 min12. A 30-μl volume containing 50 μg protein per lane was loaded on a 4% SDS-PAGE stacking gel over a 10% SDS-PAGE resolving gel and a constant voltage of 135 V was applied for 60 min. Prior to blotting, the gels and the 0.45-μm nitrocellulose membranes (BioRad, Richmond, CA, USA) were equilibrated in blotting buffer for 15 min. Once the gels were equilibrated, transfer to nitrocellulose was performed overnight (16.5 h) at 30 V constant voltage at room temperature.
Western blots were blocked in 100 mmol/l Tris (Sigma Chemical Co., St Louis, MO, USA) buffered saline (TBS) containing 1% purified BSA at pH 7.2, and allowed to incubate at room temperature for 1 h with shaking. For detection, the primary antibody was mouse MMP-9 monoclonal antibody 2 (Oncogene Research Products, Cambridge, MA, USA), diluted 1 : 100 in TBS with 1% BSA. This mouse monoclonal antibody recognizes both active and latent forms of MMP-9 as well as MMP-9 in complexes with protease inhibitors. This antibody was chosen to give the best detection of total MMP-9 protein present in membrane samples. Western blots were developed according to ProtoBlot® Western Blot AP System protocol (Promega Corporation, Madison, WI, USA) utilizing an alkaline phosphatase-labelled, anti-mouse IgG antibody as the secondary antibody. Blots were rinsed in water to stop the reaction and dried flat between two absorbent filter papers. Each sample was analyzed twice on different blots, and pre-stained molecular weight markers (BioRad) were used as standards.
Scanning densitometry
Western blot band signals were analyzed for total optical density per lane using a Sony camera imaging system, IBM-compatible PC and Harmony™ integrating software (Tim Mahoney, Pittsburgh, PA, USA). The integration area for analysis was standardized for size, and then applied to each lane of the blot image. Background readings of the same size were taken from the four edges of each blot, averaged, and subtracted from the test reading. The molecular weight marker for the 97-kDa standard was used as an internal signal transfer control.
Zymography analysis
Zymography was performed as previously described12. Commercially prepared, pre-cast gelatin gels (0.1%) were purchased from Novex, Inc. (Del Mar, CA, USA) and used according to the manufacturer’s directions. Gelatin zymograms were performed on preterm prematurely ruptured membranes to demonstrate any difference in MMP-9 activity forms between membrane sites and membrane types as well as to correlate with quantity of protein.
Statistical analyses
Membranes were grouped by delivery route and labor status into six comparison groups. MMP-9 protein values were analyzed by a non-parametric, repeated measures analysis of variance (SPSS statistical package). Following any significant omnibus tests, post-hoc comparisons were carried out to determine pair-wise group differences. Non-parametric techniques were used, owing to the violation of the homogeneity of variance and normality assumptions necessary for the parametric tests. Significance was defined as p ≤ 0.05.
RESULTS
Patients
Patient numbers, gestational ages and delivery status for membrane samples are reported in Table 1. The range of gestational ages in each group is indicated and all of our preterm PROM patients were ≤ 33 weeks. The range for preterm PROM patients without labor was similar, but the median values were not. Preterm PROM patients without labor were the most important control group for controlling the effect of labor on MMP-9 levels. However, these patients were fewer in number, because they were rare and the most difficult to identify. Culture results were not available for most of our membranes, because these are not routinely carried out, but all preterm PROM patients received prophylactic antibiotics (ampicillin). Histology of tissue inflammation indicated the presence of polymorphonuclear leukocytes in the membranes of all patients with preterm PROM.
Western blots
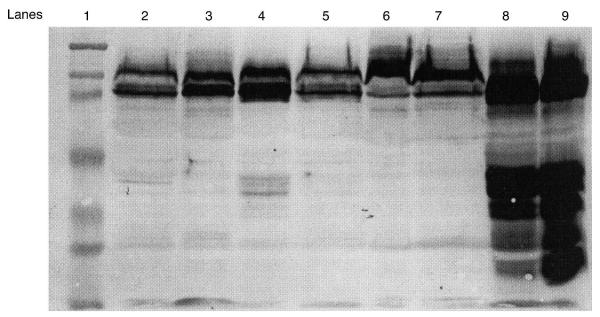
Western blots were performed on all membranes from the two sites (midpoint and rupture). In the case of Cesarean-delivered membranes, the incision site was considered the rupture site. Abundant MMP-9 was identified in preterm prematurely ruptured membranes. Representative Western immunoblots of the different preterm PROM delivery groups are seen in Figure 1, and membrane sites from three non-laboring patients with preterm PROM and one laboring patient with preterm PROM are seen. Abundant MMP-9 protein was detected in all laboring patients with preterm PROM, with the most seen in patients with long labors. Term Cesarean patients without labor had the least amount of MMP-9 protein, and the signals were barely detectable (blots not shown).
Figure 1.
A Western blot demonstrating matrix metalloproteinase-9protein signals in fetal membrane samples from rupture and mipoint locations, respectively, in membrane from three patients without labor and preterm premature rupture of membranes (PROM) (lanes 2–7) and one preterm PROM patient with labor (lanes 8 and 9). Lane 1 contains the molecular weight standards: from the lane top to bottom 205, 119, 82, 52, 32, 27 and 14 kDa; lanes 2 and 3 are patient 1 samples; lanes 4 and 5 are patient 2 samples; and lanes 6 and 7 are patient 3 samples
In Figure 1, note the 97- and 83-kDa molecular weight bands indicating proenzyme and active forms of MMP-9, respectively, and the many similarities in Western signals between midpoint and rupture sites for each patient. Note the complex signal pattern in the last two lanes including several dense signals at approximately 47 kDa. These lanes contained membrane samples from a patient with preterm PROM and labor. There are several abundant lower molecular weight bands of MMP-9 protein in these lanes which probably represent degradation forms of the enzyme. This last MMP-9 Western pattern is typical of preterm prematurely ruptured membranes with labor in our patient population and the differences between the laboring and non-laboring preterm PROM samples was consistent.
MMP-9 protein levels
Patients were separated into labor and delivery categories and the levels of MMP-9 were compared. In Table 1, the median levels of MMP-9 protein in fetal membrane tissues are presented as measured by scanning densitometry of Western blots at two different membrane sites. There was no significant difference in MMP-9 levels between midpoint and rupture sites within a membrane for each delivery group. Several samples from placental rim membrane sites were also measured for MMP-9, and there were no significant differences when these values were compared to midpoint membrane values (data not shown).
However, between groups, significant omnibus differences were found in medians when rupture-to-rupture (p < 0.006), or midpoint-to-midpoint sites (p < 0.0025) were compared. Post-hoc analysis revealed significant differences (p < 0.05) between rupture sites of groups 1 (term Cesarean section) and 4 (preterm PROM and vaginal delivery). For midpoint comparisons, significant differences (p < 0.05) were found between group 4 and groups 1, 2 (term vaginal and Cesarean deliveries) and 3 (preterm PROM and Cesarean section).
When the influence of labor on MMP-9 protein levels was analyzed, there was a striking difference between membranes from patients who labored compared to those from patients who did not (Table 1). All three of the non-laboring groups (term Cesarean section, preterm Cesarean section and preterm PROM with Cesarean section) showed lower levels of MMP-9 than membranes from laboring patients. Membranes from normal, term, spontaneous vaginal deliveries following labor (group 2) generally had MMP-9 protein levels 1.9–2.3 times higher than those from patients with repeat, term Cesarean sections without labor (group 1), but the difference did not reach significance. In preterm PROM laboring patients the increase in MMP-9 over non-laboring patients was 3.8–5.4-fold (p < 0.05). In general, the groups of membranes from patients without labor did not have elevated amounts of MMP-9 protein, but had protein levels in the 300–500 optical density unit (ODU) range. When the mean level for all rupture sites combined (905 ± 900 ODU) was compared to the mean for all midpoint sites (897 ± 887 ODU), no significant differences were found.
Zymography
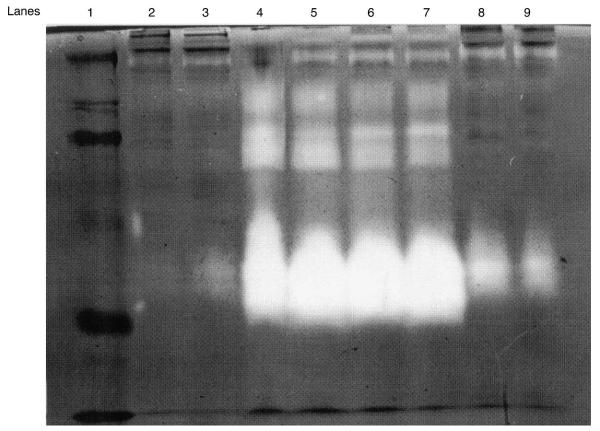
Figure 2 shows a gelatin zymogram representative of preterm PROM patients matched for gestational age (24–26 weeks). The white band in each lane represents gelatinase activity. These patients were either with or without labor, and delivered either vaginally or by Cesarean section, respectively. The patients who delivered vaginally (lanes 4–7) had the most abundant forms of gelatinase activity. There was a significant amount of 47-kDa gelatinase activity, which corresponded to the 47-kDa Western blot signal, suggesting a cleavage product of MMP-9 during labor. There was also a faint 47-kDa activity in the rupture sample of a preterm PROM patient without labor (in lane 3) and slightly more activity in the samples of the last two lanes (lanes 8 and 9) from another preterm PROM patient delivered by Cesarean section but with several hours of labor. This suggests that the appearance of the 47-kDa band in this sample is an effect of labor.
Figure 2.
Representative gelatin zymography of proteinase activity (gelatinase) found in human fetal membranes from preterm premature rupture of membranes (PROM) patients with and without labor. Membrane midpoint and rupture sites are compared in lanes side by side. Lane 1: molecular weight standards are 205, 119, 82, 52, 32, 27 and 14 kDa. Lanes 2 and 3: patient 1, with preterm PROM and no labor, delivered by Cesarean section. Lanes 4 and 5: patient 2, with preterm PROM and vaginal delivery. Lanes 6 and 7: patient 3, with preterm PROM and vaginal delivery. Lanes 8 and 9: patient 4, with preterm PROM and 4 h of labor and Cesarean delivery. Note the similarity in patterns between the two sites in each patient and the increase in gelatinase activity (white bands) in patients with labor (lanes 4–7)
DISCUSSION
Increased expression of MMP-9 protein and activity has been demonstrated as a marker for preterm as well as term labor7. Several studies have demonstrated the increase in this MMP activity in fetal membranes as well as amniotic fluid from patients undergoing preterm labor who deliver vaginally5,7,11. Our work expands on those studies, in that we compared MMP-9 protein in fetal membranes from preterm PROM patients with and without labor. The unique aspect of these data is that they describe MMP-9 levels specifically in membranes from preterm PROM patients who did not labor and in whom the membranes were delivered by Cesarean section. These are rare obstetric cases limited to a few patients per year and only available in large hospitals with high numbers of patients. The study’s impact is significant for studies of preterm PROM pathogenesis in that these non-laboring patients did not demonstrate an increase in MMP-9. Previous studies have all associated increased levels of MMP-9 with the occurrence or pathogenesis of preterm PROM, but these studies were not controlled for labor effects on the enzymatic activity of the membranes. When gestational age, labor and route of delivery were controlled for, elevated levels of MMP-9 did not occur. Thus, if one defines preterm PROM as membrane rupture occurring in the absense of labor, MMP-9 may not play a significant direct role in membrane rupture.
Preterm prematurely ruptured membranes delivered with labor had significantly elevated MMP-9 levels and the highest levels of protein in all the groups. Even when the contribution of labor was controlled for, levels of MMP-9 protein in preterm PROM patients were greater than those found in membranes from patients with normal term labor. This finding provides potential insight into the pathogenesis of preterm PROM. The fact that MMP-9 protein was very high in preterm prematurely ruptured membranes indicates that there is an increasing source of the enzyme during labor. The source may be enzyme transported into the membranes within the granules of migrating maternal polymorphonuclear leukocytes or macrophages, or enzyme synthesized by amniocytes, trophoblasts, or fibroblasts15, for there are several fetal membrane cell types that could be the source of synthesized MMP-9 protein16.
The molecular mechanism(s) for such increases are not clearly defined, but may extend to other MMPs as well. Large increases in MMP-2 (72-kDa gelatinase/type IV collagenase, gelatinase A) have also been described in preterm prematurely ruptured membranes5. It has been suggested that MMP-2 is the activator of proMMP-9 and cleaves it into active forms17. The cleavage and activation of increasing amounts of proMMP-9 would enhance its degradation of other extracellular matrix components including intact collagen I fibers5.
Another significant finding of our studies was the similarity of MMP-9 levels between rupture and midpoints sites within membranes from patients with preterm PROM who underwent labor. There did not appear to be any significant differences between rupture sites and no localized elevation. Previous models of preterm PROM pathogenesis have suggested unique alterations in the biochemistry of the rupture site18. Our data suggest that there is a consistent increase in MMP-9 levels across the entire membrane in preterm PROM. These findings indicate that MMP-9 elevation is not unique to the rupture site; they point, rather, to an underlying mechanism that predisposes the entire membrane to unusually high levels of MMPs. Such mechanisms may be infection and generalized inflammation with an influx of polymorphonuclear leukocytes bearing granules laden with proMMP-9. In such circumstances, any localized conversion of proMMP-9 to active MMP-9 would create an active area of degradation for susceptible membrane components.
The 47-kDa fragment of MMP-9 is of interest. It has been described as an active proteolytic fragment of MMP-919,20. Its importance is that it does not bind the tissue inhibitor of matrix metalloproteinase-1 (TIMP-1), which is the major inhibitor of MMP-9 activity in tissues5,8. In our preterm PROM patients, we found a large amount of a fragment in this size range within both Western blots and zymograms. Putatively, this finding suggests that inhibitory regulation of MMP-9 by TIMP-1 could be compromised in these tissues and may indicate an underlying mechanism of membrane degradation.
In summary, we found that, in preterm PROM laboring patients, the increase in MMP-9 over non-laboring patients was significant. These findings suggest that labor is an important factor in the elevation of MMP-9 protein in fetal membranes and that its influence must be controlled for in future biochemical studies of MMP-9 expression and regulation. Future studies are needed to evaluate the regulation of TIMPs and MMPs within these tissues and their contribution to fetal membrane rupture. We need to focus our efforts on a better understanding of the physiology of fetal membrane rupture and to learn the mechanisms by which exogenous risk factors such as genetic predisposition, smoking and maternal genital infections predispose to preterm PROM.
ACKNOWLEDGEMENTS
Financial support for this project was provided by an Irene McLenahan grant (to M. Kush, grant no. 9802) and March of Dimes Birth Defects Foundation grant (to D. Draper, grant no. 6-FY97–0533) and National Institutes of Health grant (U19 Al 38513-05 project no. 3 to D. Draper).
REFERENCES
- 1.American College of Obstetricians and Gynecologists . Premature rupture of membranes. ACOG Technical Bulletin No. 115. ACOG; Washington, DC: 1998. [Google Scholar]
- 2.Alger LS, Pupkin MJ. Etiology of preterm premature rupture of the membranes. Clin Obstet Gynecol. 1986;29:758–70. doi: 10.1097/00003081-198612000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Harger JH, Hsing AW, Tuomola RE, Gibbs RS, Mead PB, Eschenbach DE. Risk factors for preterm premature rupture of fetal membranes, a multi-center case–control study. Am J Obstet Gynecol. 1990;163:130–7. doi: 10.1016/s0002-9378(11)90686-3. [DOI] [PubMed] [Google Scholar]
- 4.Draper D, McGregor J, Hall J, Jones W, Beutz M, Heine RP, et al. Elevated protease activities in human amnion and chorion correlate with preterm premature rupture of membranes. Am J Obstet Gynecol. 1995;173:1506–12. doi: 10.1016/0002-9378(95)90640-1. [DOI] [PubMed] [Google Scholar]
- 5.Fortunato S, Menon R, Lombardi SJ. Collagenolytic enzymes (gelatinases) and their inhibitors in human amniochorionic membrane. Am J Obstet Gynecol. 1997;177:731–41. doi: 10.1016/s0002-9378(97)70260-6. [DOI] [PubMed] [Google Scholar]
- 6.Malak TM, Ockleford CD, Bell SC, Dalghleish R, Bright N, Macvicar J. Confocal immunofluorescence localization of collagen types I, III, IV, V and VI and their ultrastructural organization in term human fetal membranes. Placenta. 1993;14:385–406. doi: 10.1016/s0143-4004(05)80460-6. [DOI] [PubMed] [Google Scholar]
- 7.Vadillo-Ortega F, Gonzalez-Avila G, Furth EE, Lei H, Muschel RJ, Stetler-Stevenson WG, et al. 92 kDa type IV collagenase (matrix-matelloproteinase-9) activity in human amniochorion increases with labor. Am J Pathol. 1995;146:148–56. [PMC free article] [PubMed] [Google Scholar]
- 8.Matrisian L. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990;6:121–5. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- 9.Woessner JF. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–54. [PubMed] [Google Scholar]
- 10.Bell SC, Malak TM. Structural and cellular biology of the fetal membranes. In: Elder M, Romero R, Lamont R, editors. Preterm Labor. Churchill Livingstone; Edinburgh: 1997. pp. 401–28. [Google Scholar]
- 11.Vadillo-Ortega F, Hernandez A, Gonzalez-Avila G, Bermejo L, Iwata K, Strauss JF. Increased matrix metalloproteinase activity and reduced tissue inhibitor of metalloproteinase-1 levels in amniotic fluids from pregnancies complicated by premature rupture of the membranes. Am J Obstet Gynecol. 1996;174:1371–6. doi: 10.1016/s0002-9378(96)70687-7. [DOI] [PubMed] [Google Scholar]
- 12.Locksmith GJ, Clark P, Duff P, Schultz G. Amniotic fluid matrix metalloproteinase-9 levels in women with preterm labor and suspected intra-amniotic infection. Obstet Gynecol. 1999;94:1–6. doi: 10.1016/s0029-7844(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 13.Towbin HT, Staehlin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Parry S, Strauss JF. Premature rupture of the fetal membranes. N Engl J Med. 1998;338:663–70. doi: 10.1056/NEJM199803053381006. [DOI] [PubMed] [Google Scholar]
- 16.Bryant-Greenwood GD, Yamamoto Y. Control of peripartal collagenolysis in human amnion mesenchymal and epithelial cells. Am J Obstet Gynecol. 1995;172:63–70. doi: 10.1016/0002-9378(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 17.Lei H, Vadillo-Ortega F, Paavola LG, Strauss JF. 92 kDa gelatinase (matrix metalloproteinase-9) is induced in rat amnion immediately prior to parturition. Biol Reprod. 1995;53:339–43. doi: 10.1095/biolreprod53.2.339. [DOI] [PubMed] [Google Scholar]
- 18.Malak TM, Bell SC. Structural characteristics of term human fetal membranes: a novel zone of extreme morphological alteration within the rupture site. Br J Obstet Gynaecol. 1994;101:375–86. doi: 10.1111/j.1471-0528.1994.tb11908.x. [DOI] [PubMed] [Google Scholar]
- 19.Dittman KH, Lottspeiche F, Ries C, Petrides PE. Leukemic cells (HL-60) produce a novel extracellular matrix-degrading proteinase that is not inhibited by tissue inhibitors of matrix metalloproteinases (TIMPs) Exp Hematol. 1995;23:155–60. [PubMed] [Google Scholar]
- 20.Ries C, Lottspeiche F, Dittman KH, Petrides PE. HL-60 cells produce an autocatalytically truncated form of MMP-9 with impaired sensitivity to inhibition tissue inhibitors of metalloproteinases. Leukemia. 1996;10:1520–6. [PubMed] [Google Scholar]