Summary
Objective
To use deep sequencing to identify novel microRNAs (miRNAs) in human osteoarthritic cartilage which have a functional role in chondrocyte phenotype or function.
Design
A small RNA library was prepared from human osteoarthritic primary chondrocytes using in-house adaptors and analysed by Illumina sequencing. Novel candidate miRNAs were validated by northern blot and qRT-PCR. Expression was measured in cartilage models. Targets of novel candidates were identified by microarray and computational analysis, validated using 3′-UTR-luciferase reporter plasmids. Protein levels were assessed by western blot and functional analysis by cell adhesion.
Results
We identified 990 known miRNAs and 1621 potential novel miRNAs in human osteoarthritic chondrocytes, 60 of the latter were expressed in all samples assayed. MicroRNA-140-3p was the most highly expressed microRNA in osteoarthritic cartilage. Sixteen novel candidate miRNAs were analysed further, of which six remained after northern blot analysis. Three novel miRNAs were regulated across models of chondrogenesis, chondrocyte differentiation or cartilage injury. One sequence (novel #11), annotated in rodents as microRNA-3085-3p, was preferentially expressed in cartilage, dependent on chondrocyte differentiation and, in man, is located in an intron of the cartilage-expressed gene CRTAC-1. This microRNA was shown to target the ITGA5 gene directly (which encodes integrin alpha5) and inhibited adhesion to fibronectin (dependent on alpha5beta1 integrin).
Conclusion
Deep sequencing has uncovered many potential microRNA candidates expressed in human cartilage. At least three of these show potential functional interest in cartilage homeostasis and osteoarthritis (OA). Particularly, novel #11 (microRNA-3085-3p) which has been identified for the first time in man.
Keywords: Osteoarthritis, Cartilage, microRNA, Integrin
Introduction
Osteoarthritis (OA) is a degenerative joint disease characterised by degradation of articular cartilage as well as thickening of subchondral bone and formation of osteophytes at the joint margin1. The chondrocyte is the only cell in cartilage, is crucial to tissue function2 and must express appropriate genes to achieve tissue homeostasis; this is altered in OA3.
Small non-coding RNAs, microRNAs (miRNAs), are important regulators of gene expression in human cells. miRNAs are transcribed as primary transcripts and processed to short stem-loop structures (pre-miRNA) in the nucleus. The pre-miRNA is processed by Dicer, forming two complementary short RNA molecules one of which (the guide strand) is integrated into the RNA-induced silencing complex (RISC), the other of which (the passenger strand or miRNA*) is degraded4. After integration into RISC, miRNAs base pair with their mRNA targets, usually in the 3′UTR, but sometimes in promoter or coding regions. Depending on the level of sequence complementarity between miRNA and target mRNA, RISC either cleaves the target mRNA (if complementarity is high) or suppresses translation and mainly promotes more rapid mRNA degradation (if complementarity is lower) the latter being predominant in animals5, 6.
Many miRNAs are regulated during cartilage development, including by the cartilage-specifying transcription factor Sox9 (e.g., miR-140 and miR-455) (7, 8, Barter et al., unpublished), or regulating Sox9 expression (e.g., miR-675 and miR-145)9, 10. Many miRNAs are expressed differentially during OA11, though with high variability. These include miR-9, miR-98, miR-146a12, 13, miR-483, miR-149, miR-582, miR-1227, miR-634, miR-576, miR-64114 and miR-27a and b15, though miRNA-140 is the most studied to date. MicroRNA-140 null mice are predisposed to the development of age-related OA-like changes16, 17 and increased cartilage destruction in surgically-induced OA. Conversely, in an antigen-induced arthritis model, transgenic over-expression of miR-140 in chondrocytes protected against cartilage damage16.
There are 1881 precursors and 2588 mature human miRNAs in Release 21 of miRBase (http://www.mirbase.org). Deep sequencing has been used to identify potential new miRNAs in a number of cells and tissues, including rat cartilage during development18, however, this has not been applied to human chondrocytes to date. In this study, we aimed to use deep sequencing to identify novel miRNAs in human osteoarthritic cartilage and identify their possible functional roles. Osteoarthritic cartilage was used to reveal the maximum number of disease-associated miRNAs. We sequenced libraries of small RNAs purified from human osteoarthritic chondrocytes using a recently developed approach19. Potential novel miRNAs were then validated and characterised further. Of these, miRNA-3085, previously annotated in the mouse and rat, was identified as a human miRNA, selectively expressed in cartilage, which affects chondrocyte function via decreased expression of integrin alpha5.
Method
Cell culture
SW1353 chondrosarcoma cells were from American Type Culture Collection8. Primary human articular chondrocytes (HACs) were isolated from osteoarthritic cartilage20. DF1 cells (a gift from Prof. Andrea Münsterberg, University of East Anglia, UK) are spontaneously transformed chicken fibroblasts21. Parental and DLD-1 Dicer null cell lines were from Horizon Discovery (Cambridge UK) and originated from a colorectal adenocarcinoma.
Tissue collection
Femoral head and knee cartilage were obtained from OA [HOA (Hip OA) age 61–89 years, 3M, 3F; KOA (Knee OA) age 55–71, 2F, 3M] and trauma [NOF (neck of femur), age 71–92 years, 3F, 3M] patients undergoing total joint replacement surgery at the Norfolk and Norwich University Hospital, UK. OA was diagnosed using clinical history, examination and X-ray; confirmation of gross pathology was made at time of joint removal. Fracture patients had no known history of joint disease and cartilage was free of lesions. This study was performed with Ethical Committee approval and all patients provided informed consent. Cartilage was dissected and snap frozen in liquid nitrogen within 30 min of surgery.
RNA purification and quantitative real time PCR (qRT-PCR)
RNA was purified from cartilage or HACs using mirVana™ miRNA Isolation Kit (Life Technologies). For mRNA analysis by qRT-PCR from cultured cells, cDNA synthesis used the ‘Cells-to-cDNA’ method as described20. For direct reverse transcription from purified RNA, Superscript II and qRT-PCR for mRNA expression was performed as described8. Primer sequences are listed in Supplementary Table 1. Primers to measure novel miRNAs were designed using the Exiqon web-based assay tool, sequences for these and primers for known miRNAs are proprietary.
Northern blot
RNA was separated on a 12% (w/v) polyacrylamide gel and transferred to Hybond-NX membrane (Amersham Biosciences). The blot was hybridised in ULTRAHyb-Oligo buffer (Life Technologies) with a γATP-labelled probe complementary to the miRNA at 37°C overnight. Membranes were exposed to a Kodak Phosphor Screen SD230 and scanned on a Molecular Imager FX reader (Bio-Rad) for quantification.
Next generation sequencing
Total RNA was extracted from primary HACs and small RNAs enriched from 10 μg total RNA using the mirVana™ miRNA Isolation Kit. The small RNA library was prepared using the Illumina Small RNA V1.5 Sample Preparation Guide, however sRNA adaptors were substituted with High Definition (HD) adaptors19. Approximately 200 ng RNA enriched for small RNA was ligated to adenylated 3′ HD adaptor with truncated T4 RNA ligase 2 (New England Biolabs). The ligated fragment was then ligated to 5′ HD adaptor using T4 RNA ligase 1 (New England Biolabs). The ligated fragment was reverse transcribed followed by PCR amplification and size fractionated on an 8% (w/v) PAGE gel. A band corresponding to 145–150bp was gel purified and analysed on an Illumina Genome Analyzer IIX with 50 nt read length (Baseclear, Netherlands). Reads were trimmed for 4 nt barcodes on both ends and for Illumina adaptors on the 3′ end. Resulting reads longer than 16 nt were mapped to the human genome (version GRCh38) using Patman software22, no mismatches were allowed. Reads mapping to more than 100 loci were discarded. The remaining reads were inputted to miRCat23 with default parameters. miRCat novel miRNA candidates were separated from known miRNAs using in-house scripts.
Transfection with siRNA
Primary HACs were plated at 2.5 × 105 cells/well of a 6-well tissue culture plate and incubated overnight in complete medium. Cells were transfected in serum- and antibiotic-free DMEM using Lipofectamine 2000 (Invitrogen), miRNA mimics at 30 nM (Qiagen), miRNA inhibitors at 50 nM (Qiagen), or non-targetting controls (All Stars at 30 nM (Qiagen), miScript Inhibitor control at 50 nM (Qiagen)), or mock transfection. Cells were incubated for 6 h in serum-free and antibiotic-free media. Media was replaced with complete medium for a further 48 h.
For functional analysis, RNA (pooled from three samples per condition) was subjected to whole genome array using the Illumina Human HT12v4 platform (Source Bioscience). Whole genome array was normalised using R with the Lumi package24, background correction and normalisation used a between-array quantile methodology. Normalised data were analysed to measure fold-change expression. Target sequences for novel candidate miRNAs were identified using R with the Biostrings 2.28.0 package in 3′UTRs of the human genome.
Chondrogenic differentiation
Human mesenchymal stem cells (hMSC) were resuspended in chondrogenic culture medium consisting of high glucose Dulbecco's modified Eagle's medium containing 100 μg/ml sodium pyruvate (Lonza), 10 ng/ml TGF-β3 (Peprotech), 100 nM dexamethasone, 1× ITS-1 premix, 40 μg/ml proline, and 25 μg/ml ascorbate-2-phosphate (all from Sigma–Aldrich, Poole, UK). 5 × 105 hMSC in 100 μl medium were pipetted onto 6.5 mm diameter, 0.4-μm pore size polycarbonate Transwell filters (Merck Millipore), centrifuged in a 24-well plate (200 g, 5 min), then 0.5 ml of chondrogenic medium was added to the lower well as described25. Media were replaced every 2 or 3 days up to 14 days.
Mouse femoral head cartilage wounding assay
A mouse femoral head cartilage wounding assay was as previously described26. Briefly, the acetabulofemoral joint was exposed, the hip joint dislocated and the femoral cap was avulsed using forceps. Tissue was cultured in serum-free medium and harvested into 500 μl Trizol (Life Technologies) at the time points: 0, 1, 3, 6, 12, 24 and 48 h and stored at −80°C. Samples were homogenized with a disposable pestle, insoluble material removed by centrifugation and RNA purified as manufacturer's instructions.
Transient transfection
The 3′ UTR of mRNAs was amplified by PCR (using primers in Supplementary Table 1) and subcloned into the pmirGLO Dual-Luciferase Vector (Promega). Mutation of the miRNA seed sequence used QuikChange (Agilent). Constructs were sequence verified. DF1 cells were plated into a 96 well plate at 3.75 × 104 cells/cm2 overnight, transfected with 100 ng luciferase reporter plasmid, 50 nM miRNA mimic, inhibitor or control using Lipofectamine 2000 according to manufacturer's instructions (Invitrogen), and incubated for 48 h. Cell lysates were assayed using the Dual Luciferase Reporter Assay Kit (Promega) and luciferase was normalised to renilla to control for the transfection efficiency.
Western blot
Whole cell lysates were harvested into ice cold RIPA buffer (50 mM Tris-HCL pH7.6, 150 mM NaCl, 1% (v/v) Triton x-100, 1% (w/v) sodium deoxycholate, 0.1% (w/v) SDS, 10 mM NaF, 2 mM Na3VO4, 1x protease cocktail III tablet (Fisher Scientific)). Samples were separated on reducing SDS-PAGE, transferred to PVDF membrane and probed overnight at 4°C. The anti-integrin alpha5 antibody (Abcam ab150361) was detected using HRP-conjugated secondary antibodies (DAKO), visualised using LumiGLO reagent (New England Biolabs) and exposed to Kodak Biomax MS film (Sigma–Aldrich).
Cell adhesion
96-well plates were coated overnight with 10 μg/ml fibronectin (Sigma–Aldrich) at 4°C and blocked with 1% (w/v) BSA. SW1353 cells were transfected with 100 nM miRNA mimic, siRNA against ITGA5 or All Stars non-targetting control for 48 h as above. Cells were trypsinised and resuspended in serum-free medium at 4 × 105 cells/ml. Antibodies (anti-alpha5 integrin, Abcam ab25251 or control IgG) were added to cell suspension at 10 μg/ml and pre-incubated for 10 min at 37°C. Cells were added at 100 μl per well in at least triplicate for 15 min. Cells were fixed with 4% (v/v) paraformaldehyde and stained with 1% (w/v) methylene blue in 10 mM sodium borate. Cells were lysed with 50% (v/v) ethanol in 0.1M HCl for 15 min and absorbance measured at 590 nm.
Statistics
Data are plotted as mean with 95% confidence intervals. Data were tested for normality prior to further analysis using Student's t-test to compare between two samples, or one-way ANOVA with post-hoc Tukey's test to compare between multiple samples. All statistical analyses were performed using GraphPad Prism version 5 or PASW Statistics 18.
Results
Purification of RNA
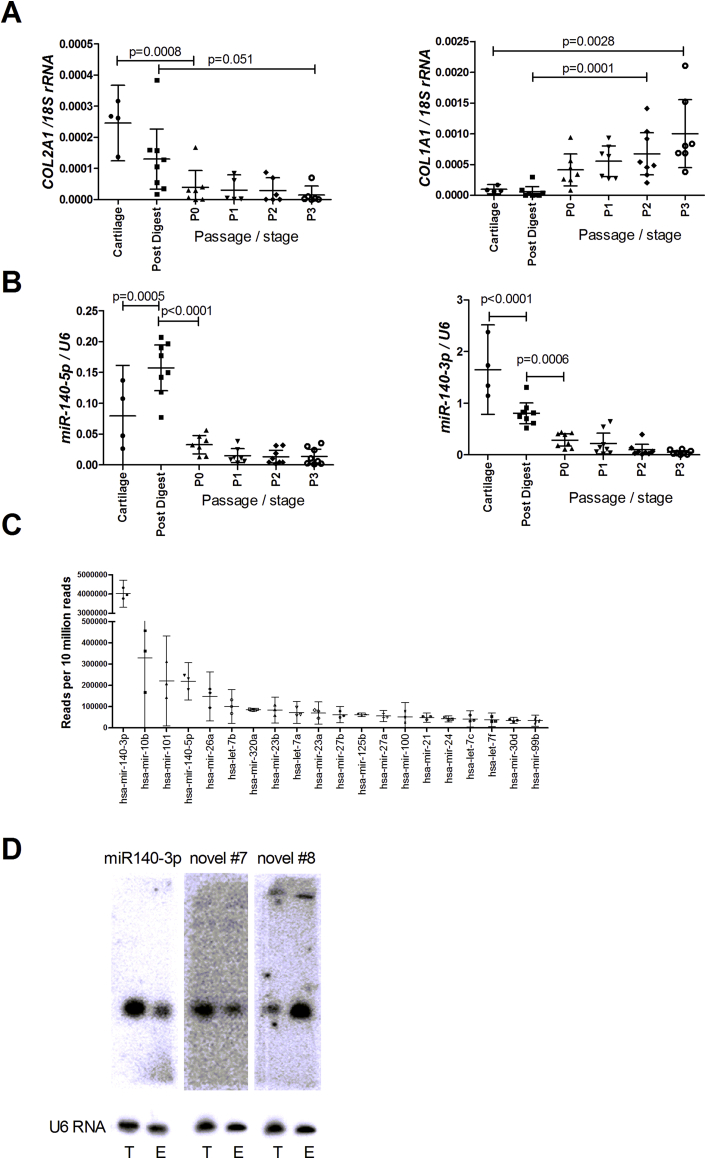
We sought to identify optimal samples for sequencing to detect all cartilage miRNAs. Total RNA was purified from articular cartilage taken at total knee replacement for OA, chondrocytes immediately after tissue digestion and cells across passage in monolayer culture. Fig. 1(A) shows that COL2A1 expression decreased and COL1A1 increased with chondrocyte isolation and culture as expected. MicroRNA-140-5p increased in expression (∼2-fold, p = 0.0005) in chondrocytes digested from cartilage [Fig. 1(B)], then decreased in monolayer. Interestingly, miR-140-3p was highest in cartilage and then decreased across isolation and passage [Fig. 1(B)]. Since miR-140-5p is strongly implicated in OA, we used RNA isolated from cells digested from cartilage (which also gives the highest quality RNA) as the starting point for sequencing studies.
Fig. 1.
Gene expression in articular chondrocyte isolation and expansion. Expression levels of (A) COL2A, COL1A1, (B) miR-140-5p, miR-140-3p were measured by qRT-PCR from RNA isolated from: osteoarthritic knee cartilage tissue, isolated chondrocytes (post digest) and subsequent P0, P1, P2, and P3 passaged chondrocytes from monolayer culture. Data were normalised to 18S rRNA (mRNA) or U6 RNA (miRNA) expression. (RNA was obtained from 8 patients for chondrocytes and 4 patients for tissue; a one way Anova analysis with a post hoc Tukey test was used to test for significance; data are plotted as mean ± 95% confidence interval. (C) Deep sequencing of a small RNA library of post-digest chondrocyte RNA showed 630 known miRNA in all three samples sequenced, the 20 miRNA with the highest read numbers are shown; n = 3, data are plotted as mean ± 95% confidence interval. (D) Expression of candidate novel miRNA #7 and #8 was measured by northern blot using 10 μg SW1353 total (T) RNA or 2 μg of RNA enriched (E) for small RNAs. MicroRNA-140-3p was used as a size control and the small RNA U6 was used as a standard reference for the northern blot.
Small RNA high-throughput sequencing
cDNA libraries were generated from small RNA isolated from chondrocytes taken from three independent osteoarthritic knees. High definition adaptors were used (see Methods) to reduce bias and reveal maximum sequence19. Approximately 20 million sequencing reads were obtained per sample and analysed using miRCat23. Known miRNAs were confirmed using miRBase (Release 20, www.mirbase.org/27) with 990 individual known miRNAs within the dataset, of which 630 were present in all three patients. The 20 miRNAs with highest read number per 107 sequencing reads are shown in Fig. 1(C). Surprisingly, the greatest number of sequencing reads came from miR-140-3p, the so-called passenger strand of miR-140 and investigation of its function is the subject to another manuscript (Wheeler et al. in preparation). Most of the top 20 miRNAs have previously been linked with cartilage or arthritis, apart from miR-23a, miR-100 and miR-99a11.
MiRCat generated 60 candidate novel miRNAs in all three samples (Supplementary Table 2). These 60 candidates were further selected (using the presence of both miRNA strands, number of genomic locations; read number; level in Dicer 1 null cells), reducing the number of candidate miRNAs to 16.
Measurement of candidate miRNAs
Initially, candidate miRNAs were measured by northern blot in total RNA purified from SW1353 chondrosarcoma cells. A number of novel candidates gave bands at high molecular weight, larger than miRNAs (data not shown; novel #1, #3, #4, #5, #9, #12, #13, #15, #16). Novel #7 and #8 gave an appropriately sized band similar to miR-140-3p [Fig. 1(D)]. Novel #6 gave multiple small bands. Novel #2, #10, #11 and #14 gave no signal (data not shown) and were included in further analyses.
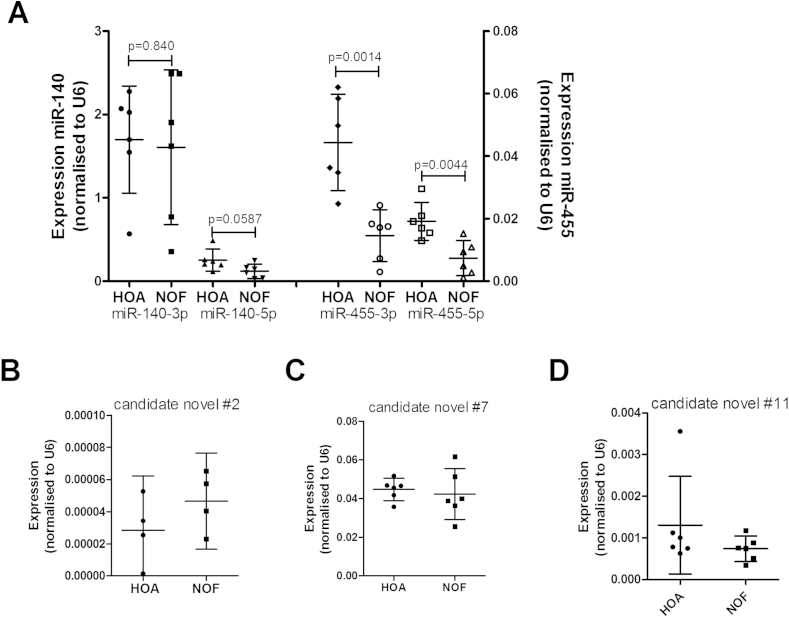
Incorrect size on northern therefore triaged 10 miRNAs. The six remaining candidate novel miRNAs, including those with no detectable signal on northern blot, were measured by qRT-PCR in hip cartilage from OA patients and patients fracturing their neck of femur (NOF) [novel #2, #7 and #11, miR-140 and miR-455 shown in Fig. 2(A)–(D)]. No candidate novel miRNAs showed a significant difference between hip OA cartilage compared to NOF. MicroRNA-140-5p showed a trend to increased expression (p = 0.0587) in OA cartilage compared to NOF as we have previously reported8; the more highly expressed miR-140-3p showed no difference between groups.
Fig. 2.
MicroRNA expression in cartilage tissue from hip osteoarthritis (HOA) and hip cartilage following fracture to the neck of femur (NOF). Expression levels of (A) miR-140, miR-455, (B) candidate novel miRNA #2, (C) candidate novel miRNA #7, (D) candidate novel miRNA #11 were obtained by qRT-PCR from RNA isolated from cartilage tissue. Data were normalised to U6 RNA expression. Graphs show mean ± 95% confidence interval. n = 6, analysed by Student's t-test.
During the course of this project, a number of our novel miRNAs were annotated on miRBase. Novel #2, hsa-miR-6509-5p; #7, hsa-miR-664b-3p; #8 is a truncated form of hsa-miR-1277-5p; #10, hsa-miR-487a-5p; #11, mmu/rno-miR-3085-3p (though not annotated as a human miRNA). Since none of these have been further characterised, they remained in downstream analyses.
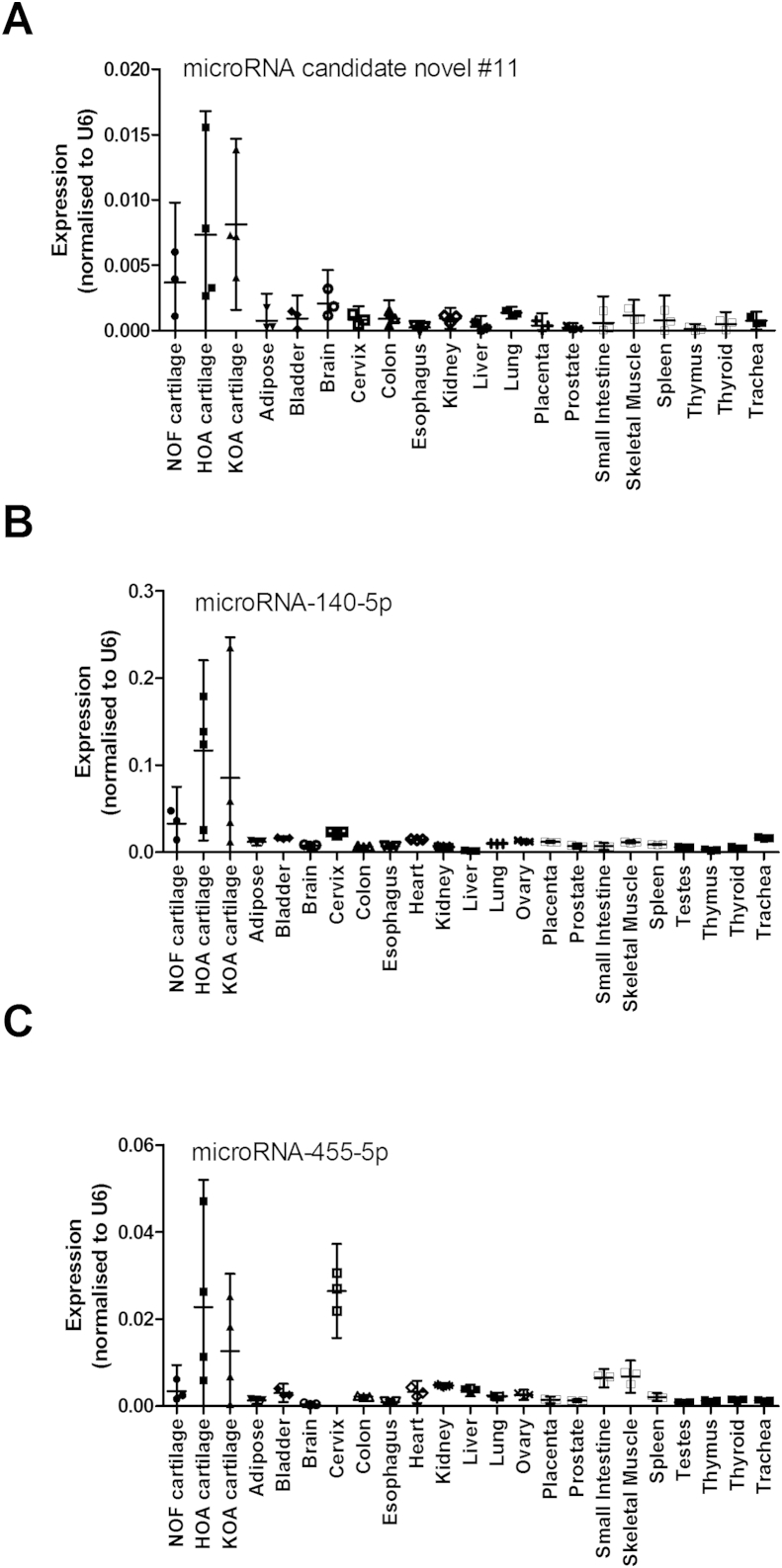
All novel miRNAs were measured in articular cartilage and across human tissue panels. Novel #11 showed selectivity of expression in cartilage compared to any other tissue [Fig. 3(A)]. This was equivalent to miRNA-140-5p [Fig. 3(B)] or miR-140-3p (data not shown) and similar to miRNA-455-3p or miR-455-5p [Fig. 3(C)] though this miRNA showed strong expression in cervix. Expression patterns of the other candidates were widespread and not predominant in cartilage (data not shown).
Fig. 3.
MicroRNA expression across a human tissue panel. Expression levels of miRNAs (A) candidate novel miRNA #11, (B) miRNA-140-5p, (C) miRNA-455-5p were obtained by qRT-PCR from RNA isolated from 23 human tissues as shown. NOF, hip cartilage following fracture to the neck of femur; HOA, hip osteoarthritis; KOA, knee osteoarthritis. Data were normalised to U6 RNA expression. RNA of all cartilage samples was from 3 patients. RNA from all remaining tissues was from a pool of three patients with n = 3 technical replicates. Data are plotted as mean ± 95% confidence interval.
Further characterisation of candidate miRNAs
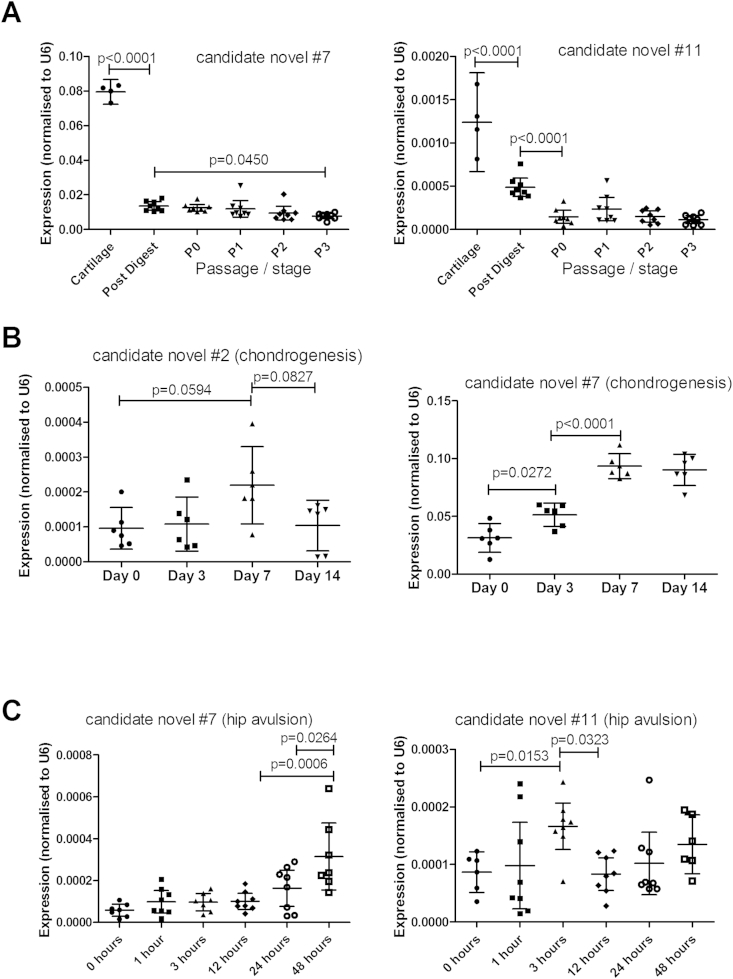
Three miRNAs, novel #2, #7 and #11, were all expressed at a statistically significantly lower level in a Dicer null cell line (DLD-1) than in an isogenic wild type control line (data not shown), and these were analysed further.
Fig. 4(A) shows that novels #7 and #11 have significantly higher expression in cartilage tissue than extracted cells, with further decrease in monolayer or with increasing passage. Novel #2 is not modulated by cell extraction or culture (data not shown).
Fig. 4.
MicroRNA expression in models of chondrocyte differentiation or injury. (A) MicroRNA expression in articular chondrocyte isolation and expansion. Expression levels of candidate miRNA novel #7 and novel #11 were measured by qRT-PCR from RNA isolated from: osteoarthritic knee cartilage tissue, isolated chondrocytes (post digest) and subsequent P0, P1, P2, and P3 chondrocytes passaged through monolayer culture. Data were normalised to U6 RNA expression. (RNA was obtained from 8 patients for chondrocytes and 4 patients for tissue; a one way Anova analysis with a post hoc Tukey test was used to test for significance; data are plotted as mean ± 95% confidence interval.). (B) MicroRNA expression in a mesenchymal stem cell chondrogenesis assay. Expression levels of candidate miRNA novel #2 and novel #7 were measured by qRT-PCR from RNA obtained from mesenchymal stem cells induced through chondrogenesis to form cartilage discs. RNA samples were taken at time points during the assay: day 0, 3, 7, 14. Data shows mean ± 95% confidence interval, n = 3, data were normalised to U6 RNA expression and analysed by a one way ANOVA with a post hoc Tukey test. (C) MicroRNA expression in a cartilage injury model. Expression levels of candidate miRNA novel #7 and novel #11 were obtained by qRT-PCR from RNA isolated from hip cartilage removed from 3 to 5 week old mice and incubated in culture medium for 48 h. RNA was isolated at the time points; 0, 1, 3, 6, 12, 24 and 48 h. Data shows mean ± 95% confidence interval, n = 8. Data were normalised to U6 RNA expression and analysed by a one way ANOVA with a post hoc Tukey test.
In an assay of cartilage formation from human bone-marrow derived MSC, expression of novel #2 increased at day 7, but decreased to later time points [Fig. 4(B)], similarly to Sox925. Novel #7 increased throughout cartilage formation [Fig. 4(B)], more similarly to COL2A125. Novel #11 showed no significant change across the 14 days (data not shown).
In the mouse hip avulsion assay of cartilage wounding, novel #7 increased in expression across 48 h, whilst candidate #11 was expressed in a biphasic pattern, with two peaks across 48 h [Fig. 4(C)]. Novel #2 is not found in the mouse genome. MicroRNA-140 (both strands) and miR-455 (both strands) increased in expression across the time course similarly to novel #7 (data not shown).
ITGA5 is a direct target of novel #11 (miR-3085-3p)
Primary HACs (from three separate patients) were transfected with either miRNA mimics or inhibitors for novels #2, #7 and #11 or negative controls for 48 h, followed by microarray. Genes which are true targets of the miRNA will decrease in expression in cells treated with miRNA mimics, increase in expression in cells treated with miRNA inhibitors and contain a seed sequence for the miRNA in their 3′UTR. Supplementary Table 3(A)-(C) show the top genes for each candidate which fit these criteria.
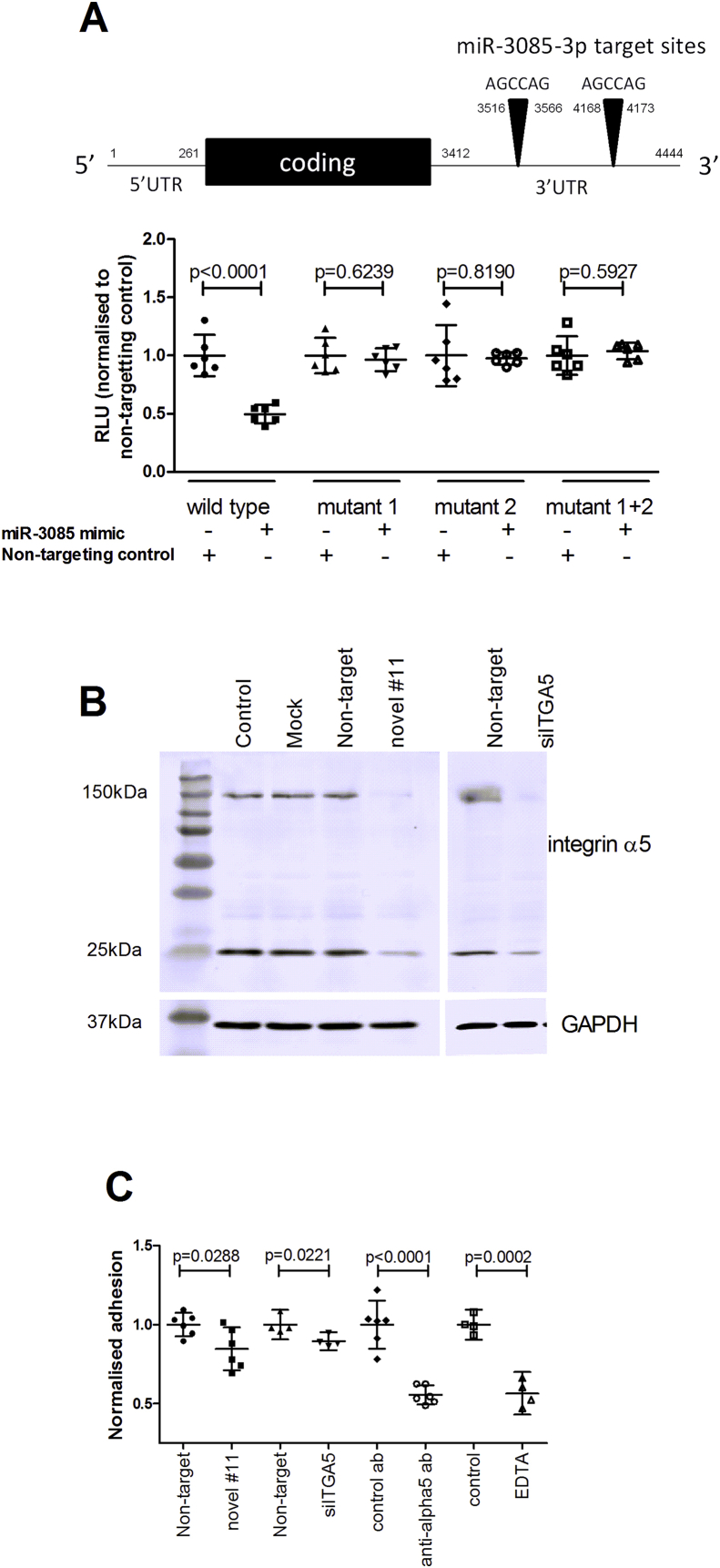
Since novel #11 was expressed in a cartilage-selective manner, and qRT-PCR verified ITGA5 as a potential target (data not shown), this gene was further assessed. Fig. 5(A) shows that expression of luciferase controlled by the 3′UTR of ITGA5 was decreased by novel #11 and that this effect was abrogated by mutation of the seed sites (from AGCCAG to GAGCTC). This demonstrates that novel #11 directly targets the ITGA5 gene. Western blot shows that the level of integrin alpha5 (encoded by ITGA5) is reduced by transfection with the novel #11 miRNA mimic [Fig. 5(B)], with the same bands reduced by an ITGA5 targeting siRNA. The functional potential of novel #11 was probed by measuring adhesion to fibronectin, mediated by integrin alpha5beta1. A function blocking antibody against integrin alpha5 reduces adhesion, as does EDTA. Transfection of cells with candidate #11 inhibited adhesion to a similar extent to siITGA5 [Fig. 5(C)].
Fig. 5.
Functional analysis of integrin alpha5 as a target of candidate microRNA #11. (A) The schematic shows the ITGA5 cDNA with the location of target sites numbered. Cells (DF1) were transfected with the 3′UTR of ITGA5 cloned into pmiRGLO, or a construct with either or both miR-3085-3p seed sites mutated (to GAGCTC) (mutant 1, 2 or 1 + 2), with or without candidate miRNA #11 (miR-3085-3p) mimic or non-targeting control. Firefly luciferase relative light units were normalised to Renilla relative light units. Data are mean ± 95% confidence interval, n = 6, analysed by a Student's t test. (B) SW1353 cells had no treatment (control), mock transfected, or transfected with a miR-3085 mimic, an siRNA against ITGA5 (siITGA5) or non-targeting siRNA; protein was extracted and separated on 12.5% (w/v) SDS-PAGE, blotted onto PVDF and probed with an anti-integrin alpha5 antibody. The blot was stripped and re-probed with a GAPDH antibody to assess loading. (C) SW1353 cells were transfected with a miR-3085 mimic, siITGA5 or a non-targeting control (100 nM) for 48 h. Cells were pre-incubated in serum-free medium with an anti-integrin alpha5 antibody or control antibody (10 μg/ml) or EDTA (2 mM) for 10 min if required and then allowed to adhere to fibronectin for 15 min. After washing cells were fixed and stained with methylene blue, lysed and absorbance at 590 nm measured. Data are normalised to the control and presented as mean ± 95% confidence interval, n = 4–6, analysed by a Student's t test).
Discussion
We initially sought to identify the best source of chondrocyte RNA from which to identify miRNAs. We measured a number of miRNA and mRNA known to be important in OA across cartilage tissue and cells derived from it across passage in culture. For miRNA-140-5p, the miRNA most implicated in cartilage homeostasis and OA, expression was highest in cells digested from cartilage rather than in the tissue itself. This increased expression of miR-140-5p may be a response to injury, a known phenomenon for miRNAs in several areas of physiology and pathology, including cartilage e.g.,28, 29. Expression of both MMP1 and MMP13 is also significantly higher in cells digested from cartilage compared to the tissue itself (data not shown).
We sequenced libraries from three OA patients using so-called ‘high definition’ adaptors19 which have been shown to approximately double read coverage, finding 60 potential new miRNA sequences in all three. Sun et al. undertook a deep sequencing analysis of rat cartilage across development and uncovered 86 novel candidate miRNAs18, however, further validation of these sequences was not reported.
The miRCat software23 was used to designate miRNAs by factors including, location, abundance, secondary structure, number of mismatches between 5p and 3p strand and hairpin length. These sequences were then searched in miRBase27 to discount miRNAs already known and novel sequences further triaged as described. This led to 16 potential novel miRNAs being further investigated. New releases of miRBase have included some of the candidate novel miRNAs, identified in other sequencing projects.
The levels of each miRNA using either deep sequencing, northern blot, or qRT-PCR were not comparable. It has previously been reported that measurement between platforms shows high variability30. miRNAs vastly different in read number within the sequencing library, where bias is known, showed much closer expression in northern blot analysis (e.g., miR-140, miR-455 and novel #7 or #8) or in qRT-PCR (e.g., mean Ct for miR-455-5p in hip OA cartilage = 25.11 and for novel #11 = 28.2), where Ct < 30 is robust expression.
Northern blot showed that two candidate novel miRNAs, #7 and #8 gave an appropriately sized band and can be assigned as miRNAs; this confirms recent sequence identification for novel #7 as miR-664b-3p and novel #8 as miR-1277-5p. Four candidate novel miRNAs, #2, #10, #11 and #14, gave no signal, and may be more weakly expressed in the SW1353 chondrosarcoma cell line used. Three of these have since been described as miRNAs in recent versions of miRBASE (#2, #10 and #11). Ten candidate novel miRNAs were discounted on the basis of their size.
Expression across a broad tissue panel showed that only novel miRNA #11 was selectivity expressed in articular cartilage. This pattern was similar for miR-140, with miR-455 showing high expression in cervix as well as articular cartilage. Novel #11 is annotated in miRBase as miR-3085 but only in the mouse or rat. In these species, the miRNA is 3′ to cartilage acidic protein 1 (CRTAC1, previously called CEP-68), however, in man, it is within a final intron of the gene, though these longer human transcripts have not yet been annotated in rodents. The miRNA is conserved in mammals (data not shown). Many intronic miRNAs are functionally related to the genes they are within31. Cartilage acidic protein 1 is a protein of unknown function, which was originally described as a marker of chondrocytes in culture, able to delineate between MSC, chondrocytes and osteoblast-like cells32. The gene is expressed in both cartilage and bone tissue, but rapidly lost from osteoblasts upon culture32. Its expression is also markedly decreased when chondrocytes are digested from cartilage, but increased again in 3D culture33. CRTAC1 is also reported to be increased in expression in microarrays of human OA cartilage (e.g.,3, 34). CRTAC-1 protein has been identified in the plasma of patients with long-bone fracture, where it was suggested either to indicate normal callus formation and healing or concomitant joint injury35.
Novel miRNA #2 (not found in mouse) is located in an intron of the gene WDR91 which has no known function, whilst novel #7 comes from the snoRNA SNORA36A. It has been shown that miRNAs can be derived from snoRNAs36, and indeed miR-140 has been described as snoRNA-like miRNA37.
The pattern of expression of each miRNA in a variety of cell models was different. Broadly, novel miRNA #7 follows the same pattern as miR-140-5p and miR-455-5p and -3p. Novel #11 decreases with chondrocyte dedifferentiation, but does not reproducibly increase in the MSC chondrogenesis model.
We used overexpression of a miRNA mimic or inhibitor in primary HACs to define direct targets of the novel miRNAs. A number of possible targets are identified bioinformatically, though these remain to be validated in future studies. Several potential targets have been implicated in cartilage and OA. For novel miRNA #2, PDGF-D is a recently discovered platelet-derived growth factor isoform, where PDGF has been shown to regulate chondrocyte proliferation38; secreted frizzled-related protein 4 (sFRP4) is a Wnt antagonist with the Wnt pathway key in cartilage homeostasis and OA39; TGFBR3 encodes betaglycan, a co-receptor for TGFβ, another pathway key in OA40. For novel miRNA #7, cartilage intermediate layer protein has been implicated in cartilage structure, but also in calcification and can interact with key growth factor pathways (e.g., TGFβ or IGF)41. Sox4 is a SoxC gene, shown to be involved in skeletogenesis, but also in Wnt signalling42. TIMP-3 is an inhibitor of cartilage-degrading proteinases43. Versican, a cartilage proteoglycan has been mooted as a biomarker, along with TIMP-3, in shoulder OA44. For novel miRNA #11 (miR-3085-3p), IL-18 and Myd88 are known to be involved in tissue inflammation45 and TGFBR3 is also a predicted target for this miRNA as well as novel miRNA #2 above.
The ITGA5 gene was shown to be a direct target of novel #11 (miR-3085-3p) and the miRNA strongly reduced the level of integrin alpha5 protein in chondrocytes. Integrin alpha5beta1 has been implicated in cartilage homeostasis and destruction46, e.g., mediating signalling through matrix fragments and mechanotransduction. Functionally, novel #11 (miR-3085-3p) was shown to inhibit chondrocyte binding to fibronectin to the same extent as a specific siRNA against ITGA5 [Fig. 5(C)]. Fibronectin is a glycoprotein found in the superficial zone of cartilage and is increased in OA; functionally is has a role in lubrication, matrix organisation and in cartilage development47, 48, 49, 50. The impact of novel #11 (miR-3085-3p) on the reported fibronectin fragment-induced expression of MMP13 through alpha5beta1 was measured, but this miRNA also reduces the expression of basal MMP13, even though this gene does not have a seed sequence for this miRNA (data not shown). This effect is likely indirect: our microarray data show that MMP13 expression decreases with the mimic and increases with the inhibitor of novel #11, potentially via the NFκB, STAT and retinoic acid receptor pathways which are also targeted.
In summary, we have identified and validated several miRNA candidates from human osteoarthritic cartilage. Most interestingly, novel #11 is annotated in mouse and rat as miRNA-3085-3p, but is 3′ of a cartilage expressed gene CRTAC1. In man, longer transcripts of this gene have been annotated and it is located in the last intron of the gene. This miRNA has high expression in cartilage which decreases on chondrocyte dedifferentiation, is expressed in a cartilage-selective manner and we have shown it to target the ITGA5 gene directly, functionally disrupting integrin alpha5. These data will allow the further functional characterisation of identified miRNAs. It is clear that a number of miRNAs function in cartilage and are dysregulated in OA. A detailed understanding of the role of miRNAs in OA will allow their targeting to prevent or slow the progression of the disease.
Author contributions
-
•
Conception and design: IMC, DAY, TD
-
•
Analysis and interpretation of the data: NC, HP, TD, IMC
-
•
Drafting of the article: NC, IMC
-
•
Critical revision of the article for important intellectual content: All authors
-
•
Final approval of the article: All authors
-
•
Statistical expertise: HP, IMC
-
•
Obtaining of funding: IMC, TES, TD
-
•
Collection and assembly of data: NC, TES, LL, MJB, GW, HP, STD
Conflict of interests
There are no conflicts of interest.
Acknowledgements
This work was supported by grants from Arthritis Research UK (#19321 and #19424); LL was supported by a grant from the Vietnam Ministry of Education (Project 322). We would like to thank Dr Simon Tew (University of Liverpool) for his guidance in measuring gene expression in newly extracted chondrocytes; Prof Tonia Vincent (University of Oxford) for her help and advice with the murine hip avulsion model; Dr Emma Blain (University of Cardiff) for bringing chondrocyte function of alpha5beta1 integrin to our attention; Dr Jelena Gavrilovic (University of East Anglia) for her help and advice with cell adhesion assays to assess integrin function.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.joca.2015.10.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Goldring S.R., Goldring M.B. Clinical aspects, pathology and pathophysiology of osteoarthritis. J Musculoskelet Neuronal Interact. 2006;6:376–378. [PubMed] [Google Scholar]
- 2.Goldring M.B. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Pract Res Clin Rheumatol. 2006;20:1003–1025. doi: 10.1016/j.berh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Aigner T., Fundel K., Saas J., Gebhard P.M., Haag J., Weiss T. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum. 2006;54:3533–3544. doi: 10.1002/art.22174. [DOI] [PubMed] [Google Scholar]
- 4.Winter J., Jung S., Keller S., Gregory R.I., Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 5.Guo H., Ingolia N.T., Weissman J.S., Bartel D.P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X., Fortin K., Mourelatos Z. MicroRNAs: biogenesis and molecular functions. Brain Pathol. 2008;18:113–121. doi: 10.1111/j.1750-3639.2007.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura Y., He X., Kato H., Wakitani S., Kobayashi T., Watanabe S. Sox9 is upstream of microRNA-140 in cartilage. Appl Biochem Biotechnol. 2012;166:64–71. doi: 10.1007/s12010-011-9404-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swingler T.E., Wheeler G., Carmont V., Elliott H.R., Barter M.J., Abu-Elmagd M. The expression and function of microRNAs in chondrogenesis and osteoarthritis. Arthritis Rheum. 2012;64:1909–1919. doi: 10.1002/art.34314. [DOI] [PubMed] [Google Scholar]
- 9.Dudek K.A., Lafont J.E., Martinez-Sanchez A., Murphy C.L. Type II collagen expression is regulated by tissue-specific miR-675 in human articular chondrocytes. J Biol Chem. 2010;285:24381–24387. doi: 10.1074/jbc.M110.111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Sanchez A., Dudek K.A., Murphy C.L. Regulation of human chondrocyte function through direct inhibition of cartilage master regulator SOX9 by microRNA-145 (miRNA-145) J Biol Chem. 2012;287:916–924. doi: 10.1074/jbc.M111.302430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le L.T., Swingler T.E., Clark I.M. Review: the role of microRNAs in osteoarthritis and chondrogenesis. Arthritis Rheum. 2013;65:1963–1974. doi: 10.1002/art.37990. [DOI] [PubMed] [Google Scholar]
- 12.Iliopoulos D., Malizos K.N., Oikonomou P., Tsezou A. Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks. PLoS One. 2008;3:e3740. doi: 10.1371/journal.pone.0003740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones S.W., Watkins G., Le Good N., Roberts S., Murphy C.L., Brockbank S.M. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-alpha and MMP13. Osteoarthritis Cartilage. 2009;17:464–472. doi: 10.1016/j.joca.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Diaz-Prado S., Cicione C., Muinos-Lopez E., Hermida-Gomez T., Oreiro N., Fernandez-Lopez C. Characterization of microRNA expression profiles in normal and osteoarthritic human chondrocytes. BMC Musculoskelet Disord. 2012;13:144. doi: 10.1186/1471-2474-13-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tardif G., Hum D., Pelletier J.P., Duval N., Martel-Pelletier J. Regulation of the IGFBP-5 and MMP-13 genes by the microRNAs miR-140 and miR-27a in human osteoarthritic chondrocytes. BMC Musculoskelet Disord. 2009;10:148. doi: 10.1186/1471-2474-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyaki S., Sato T., Inoue A., Otsuki S., Ito Y., Yokoyama S. MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Genes Dev. 2010;24:1173–1185. doi: 10.1101/gad.1915510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura Y., Inloes J.B., Katagiri T., Kobayashi T. Chondrocyte-specific microRNA-140 regulates endochondral bone development and targets Dnpep to modulate bone morphogenetic protein signaling. Mol Cell Biol. 2011;31:3019–3028. doi: 10.1128/MCB.05178-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun J., Zhong N., Li Q., Min Z., Zhao W., Sun Q. MicroRNAs of rat articular cartilage at different developmental stages identified by Solexa sequencing. Osteoarthritis Cartilage. 2011;19:1237–1245. doi: 10.1016/j.joca.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Sorefan K., Pais H., Hall A.E., Kozomara A., Griffiths-Jones S., Moulton V. Reducing ligation bias of small RNAs in libraries for next generation sequencing. Silence. 2012;3:4. doi: 10.1186/1758-907X-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Culley K.L., Hui W., Barter M.J., Davidson R.K., Swingler T.E., Destrument A.P. Class I histone deacetylase inhibition modulates metalloproteinase expression and blocks cytokine-induced cartilage degradation. Arthritis Rheum. 2013;65:1822–1830. doi: 10.1002/art.37965. [DOI] [PubMed] [Google Scholar]
- 21.Himly M., Foster dN., Bottoli I., Iacovoni J.S., Vogt P.K. The DF-1 chicken fibroblast cell line: transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses. Virology. 1998;248:295–304. doi: 10.1006/viro.1998.9290. [DOI] [PubMed] [Google Scholar]
- 22.Prufer K., Stenzel U., Dannemann M., Green R.E., Lachmann M., Kelso J. PatMaN: rapid alignment of short sequences to large databases. Bioinformatics. 2008;24:1530–1531. doi: 10.1093/bioinformatics/btn223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moxon S., Schwach F., Dalmay T., Maclean D., Studholme D.J., Moulton V. A toolkit for analysing large-scale plant small RNA datasets. Bioinformatics. 2008;24:2252–2253. doi: 10.1093/bioinformatics/btn428. [DOI] [PubMed] [Google Scholar]
- 24.Team R.C. 2014. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 25.Barter M.J., Tselepi M., Gomez R., Woods S., Hui W., Smith G.R. Genome-wide microRNA and gene analysis of mesenchymal stem cell chondrogenesis identifies an essential role and multiple targets for miR-140-5p. Stem Cells. 2015;33:3266–3280. doi: 10.1002/stem.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chong K.W., Chanalaris A., Burleigh A., Jin H., Watt F.E., Saklatvala J. Fibroblast growth factor 2 drives changes in gene expression following injury to murine cartilage in vitro and in vivo. Arthritis Rheum. 2013;65:2346–2355. doi: 10.1002/art.38039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozomara A., Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39:D152–D157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin L., Zhao J., Jing W., Yan S., Wang X., Xiao C. Role of miR-146a in human chondrocyte apoptosis in response to mechanical pressure injury in vitro. Int J Mol Med. 2014 doi: 10.3892/ijmm.2014.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang M., Zhang L., Gibson G.J. Chondrocyte miRNAs 221 and 483-5p respond to loss of matrix interaction by modulating proliferation and matrix synthesis. Connect Tissue Res. 2015:1–8. doi: 10.3109/03008207.2015.1018384. [DOI] [PubMed] [Google Scholar]
- 30.Git A., Dvinge H., Salmon-Divon M., Osborne M., Kutter C., Hadfield J. Systematic comparison of microarray profiling, real-time PCR, and next-generation sequencing technologies for measuring differential microRNA expression. RNA. 2010;16:991–1006. doi: 10.1261/rna.1947110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao X., Qiao Y., Han D., Zhang Y., Ma N. Enemy or partner: relationship between intronic micrornas and their host genes. IUBMB Life. 2012;64:835–840. doi: 10.1002/iub.1079. [DOI] [PubMed] [Google Scholar]
- 32.Steck E., Benz K., Lorenz H., Loew M., Gress T., Richter W. Chondrocyte expressed protein-68 (CEP-68), a novel human marker gene for cultured chondrocytes. Biochem J. 2001;353:169–174. doi: 10.1042/0264-6021:3530169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benz K., Breit S., Lukoschek M., Mau H., Richter W. Molecular analysis of expansion, differentiation, and growth factor treatment of human chondrocytes identifies differentiation markers and growth-related genes. Biochem Biophys Res Commun. 2002;293:284–292. doi: 10.1016/S0006-291X(02)00223-1. [DOI] [PubMed] [Google Scholar]
- 34.Ijiri K., Zerbini L.F., Peng H., Otu H.H., Tsuchimochi K., Otero M. Differential expression of GADD45beta in normal and osteoarthritic cartilage: potential role in homeostasis of articular chondrocytes. Arthritis Rheum. 2008;58:2075–2087. doi: 10.1002/art.23504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grgurevic L., Macek B., Durdevic D., Vukicevic S. Detection of bone and cartilage-related proteins in plasma of patients with a bone fracture using liquid chromatography-mass spectrometry. Int Orthop. 2007;31:743–751. doi: 10.1007/s00264-007-0404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott M.S., Ono M. From snoRNA to miRNA: dual function regulatory non-coding RNAs. Biochimie. 2011;93:1987–1992. doi: 10.1016/j.biochi.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott M.S., Avolio F., Ono M., Lamond A.I., Barton G.J. Human miRNA precursors with box H/ACA snoRNA features. PLoS Comput Biol. 2009;5:e1000507. doi: 10.1371/journal.pcbi.1000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao J., Chen X., Xu L., Zhang Y., Yin Q., Wang F. PDGF regulates chondrocyte proliferation through activation of the GIT1- and PLCgamma1-mediated ERK1/2 signaling pathway. Mol Med Rep. 2014;10:2409–2414. doi: 10.3892/mmr.2014.2506. [DOI] [PubMed] [Google Scholar]
- 39.Lories R.J., Corr M., Lane N.E. To Wnt or not to Wnt: the bone and joint health dilemma. Nat Rev Rheumatol. 2013;9:328–339. doi: 10.1038/nrrheum.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhai G., Dore J., Rahman P. TGF-beta signal transduction pathways and osteoarthritis. Rheumatol Int. 2015;35:1283–1292. doi: 10.1007/s00296-015-3251-z. [DOI] [PubMed] [Google Scholar]
- 41.Bernardo B.C., Belluoccio D., Rowley L., Little C.B., Hansen U., Bateman J.F. Cartilage intermediate layer protein 2 (CILP-2) is expressed in articular and meniscal cartilage and down-regulated in experimental osteoarthritis. J Biol Chem. 2011;286:37758–37767. doi: 10.1074/jbc.M111.248039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kato K., Bhattaram P., Penzo-Mendez A., Gadi A., Lefebvre V. SOXC transcription factors induce cartilage growth plate formation in mouse embryos by promoting noncanonical WNT signaling. J Bone Miner Res. 2015 doi: 10.1002/jbmr.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Troeberg L., Lazenbatt C., Anower E.K.M.F., Freeman C., Federov O., Habuchi H. Sulfated glycosaminoglycans control the extracellular trafficking and the activity of the metalloprotease inhibitor TIMP-3. Chem Biol. 2014;21:1300–1309. doi: 10.1016/j.chembiol.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casagrande D., Stains J.P., Murthi A.M. Identification of shoulder osteoarthritis biomarkers: comparison between shoulders with and without osteoarthritis. J Shoulder Elbow Surg. 2015;24:382–390. doi: 10.1016/j.jse.2014.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez R., Villalvilla A., Largo R., Gualillo O., Herrero-Beaumont G. TLR4 signalling in osteoarthritis–finding targets for candidate DMOADs. Nat Rev Rheumatol. 2015;11:159–170. doi: 10.1038/nrrheum.2014.209. [DOI] [PubMed] [Google Scholar]
- 46.Loeser R.F. Integrins and chondrocyte-matrix interactions in articular cartilage. Matrix Biol. 2014;39:11–16. doi: 10.1016/j.matbio.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andresen Eguiluz R.C., Cook S.G., Brown C.N., Wu F., Pacifici N.J., Bonassar L.J. Fibronectin mediates enhanced wear protection of lubricin during shear. Biomacromolecules. 2015 doi: 10.1021/acs.biomac.5b00810. [DOI] [PubMed] [Google Scholar]
- 48.Peffers M.J., Cillero-Pastor B., Eijkel G.B., Clegg P.D., Heeren R.M. Matrix assisted laser desorption ionization mass spectrometry imaging identifies markers of ageing and osteoarthritic cartilage. Arthritis Res Ther. 2014;16:R110. doi: 10.1186/ar4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scanzello C.R., Markova D.Z., Chee A., Xiu Y., Adams S.L., Anderson G. Fibronectin splice variation in human knee cartilage, meniscus and synovial membrane: observations in osteoarthritic knee. J Orthop Res. 2015;33:556–562. doi: 10.1002/jor.22787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh P., Schwarzbauer J.E. Fibronectin matrix assembly is essential for cell condensation during chondrogenesis. J Cell Sci. 2014;127:4420–4428. doi: 10.1242/jcs.150276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.