Abstract
In recent years, the prevalence of hypertension and pre-hypertension increased markedly among children and adolescents, highlighting the importance of identifying determinants of elevated blood pressure early in life. Low birthweight and rapid early childhood weight gain are associated with higher future blood pressure. However, few studies have examined the timing of postnatal weight gain in relation to later blood pressure, and little is known regarding the contribution of linear growth. We studied 957 participants in Project Viva, an ongoing U.S. pre-birth cohort. We examined the relations of gains in body mass index z-score and length/height z-score during four early life age intervals (birth–6mo, 6mo–1y, 1–2y, and 2–3y) with blood pressure during mid-childhood (6–10y), and evaluated whether these relations differed by birth size. After accounting for confounders, each additional z-score gain in body mass index during birth–6mo and 2–3y was associated with 0.81 (0.15, 1.46) and 1.61 (0.33, 2.89) mmHg higher systolic blood pressure, respectively. Length/height gain was unrelated to mid-childhood blood pressure, and there was no evidence of effect modification by birth size for body mass index or length/height z-score gain. Our findings suggest that more rapid gain in body mass index during the first 6 postnatal months and in the preschool years may lead to higher systolic blood pressure in mid-childhood, regardless of size at birth. Strategies to reduce accrual of excess adiposity during early life may reduce mid-childhood blood pressure, which may also impact adult blood pressure and cardiovascular health.
Keywords: blood pressure, weight gain, adiposity, length gain, linear growth, children
INTRODUCTION
In recent decades, hypertension and pre-hypertension – conditions formerly confined largely to adults – have pervaded pediatric populations. Although data from the National Health and Nutrition Examination Survey (NHANES) 2011–2012 indicate that the prevalence of hypertension (≥95th percentile age-, sex-, and height-specific systolic or diastolic BP1) and pre-hypertension (90–<95% percentile1) is leveling off2 following increases during earlier survey periods3 these conditions still affect more than 1 in 10 youth in the U.S. Considering that BP tracks from childhood into adulthood4 and that raised BP in early life is an independent risk factor for future cardiovascular disease,5 it is important to identify early life risk factors for elevated BP in children in order to inform timely strategies for cardiovascular disease prevention.
Pre- and postnatal growth may influence future BP. Larger size at birth, typically assessed by birthweight,6 and faster weight gain during the first two postnatal years are consistently associated with obesity in childhood and adulthood,6, 7 with some8, 9 investigations also showing relationships with higher BP. Yet, several issues remain to be clarified. First, although some studies suggest that weight gain patterns in early infancy – e.g. during the first 6 postnatal months9, 10 – are particularly important to future BP, two recent investigations11, 12 observed that weight gain during childhood was a stronger predictor of future BP than were gains in infancy, highlighting the need for additional research to reconcile mixed findings. Second, the role of adiposity gain as compared to linear growth has received little attention, but are each important to consider separately since weight contains fat mass and height, both of which are positively associated with BP in children.13, 14 Third, the extent to which birth size modifies the associations of postnatal growth with future BP remains unclear. We previously found that infants who were thinner at birth were more susceptible to the adverse effects of rapid gain in weight-for-length during the first 6 postnatal months on BP at age 3 years.9 Whether this finding persists into mid-childhood when BP is more highly correlated with adult BP4 is not known. Finally, some studies observed an association between early weight gain and future BP only after adjusting for current body size (e.g. weight status),15, 16 which has raised concerns that the associations are a statistical artifact rather than a true causal relationship.17
In this study, we investigated the associations of gain in body mass index (BMI) and linear growth during 4 early childhood age intervals, birth to 6 months, 6 months to 1 year, 1 to 2 years, and 2 to 3 years, with BP during mid-childhood, and assessed whether these relationships differed by birth size.
METHODS
Study population
We studied participants in Project Viva, an ongoing Boston-area pre-birth cohort. Details on recruitment and study design have been published.18 Of the 2128 live singleton births, 1708 mother-child pairs were eligible for the mid-childhood visit, of whom 65% (n=1116) attended an in-person visit at age 6–10 years (median 7.7). For this analysis, we further excluded children whose mothers had type 1 or 2 diabetes mellitus (n=16) and those with gestation length <34 weeks (n=45) since we were interested in patterns of growth among healthy, term infants of mothers without preexisting metabolic complications. After excluding an additional 159 participants with no data on birth size (birthweight-for-gestational-age z-score), early growth (BMI or length/height z-score change during at least one of the age intervals), or BP in mid-childhood, the final sample included 957 participants. Not all participants contributed to BMIZ and HAZ change during all age intervals (n=321 with BMIZ and HAZ data for all 4 timeframes), but the subsamples were similar with respect to sociodemographic characteristics, as well as the exposures and outcomes of interest (S1). The 957 children in this study did not differ from the 159 participants who attended the mid-childhood visit but were not included in terms of household income; father’s BMI; or maternal pre-pregnancy BMI, parity, or education. However, compared to children excluded from the analyses, the sample included a lower proportion of Black children (15.8% vs. 24.4%), higher proportion of mothers who smoked before pregnancy (20.3% vs. 12.0%), and lower proportion who smoked during pregnancy (8.9% vs. 15.8%). All mothers provided written informed consent at recruitment and at outcome assessment. All children provided verbal assent at the mid-childhood visit. The institutional review board of Harvard Pilgrim Health Care approved all study protocols.
Exposures
Birth size
Medical records provided information on perinatal characteristics including the child’s sex, birthweight, delivery method, and delivery date. We calculated gestational-age-at-birth from the date of the last menstrual period or from a second trimester ultrasound if the estimated delivery date differed by >10 days. We determined sex-specific birthweight-for-gestational-age z-scores and percentiles using U.S. reference data.19 We defined small-for-gestational age (SGA) as <10th percentile, and large-for-gestational-age (LGA) as ≥90th percentile.
BMI z-score (BMIZ) and length/height-for-age z-score (HAZ) change
We obtained data on weight and length/height from medical records and research measures. Weight at birth, 1 year, and 2 years were abstracted from medical records. Weight at 6 months and 3 years was assessed at research visits, where research assistants (RAs) weighed participants to the nearest 0.1 kg using an electronic scale (Tanita, Inc., Arlington Heights, IL). For length at birth and 6 months, we used the recumbent length board technique.20 Length at 1 and 2 years was abstracted from clinical records. Because the pediatric clinics used the paper-and-pencil method to measure length, we applied a corrective algorithm to the clinical lengths to account for the systematic overestimation.20 At the 3 year research visit, RAs measured standing height to the nearest 0.1 cm with a calibrated stadiometer (Shorr Productions, Olney, MD).
We calculated BMI (kg/m2) and determined age- and sex-specific z-scores for BMI (BMIZ) and length/height (HAZ) using the World Health Organization (W.H.O.) growth reference.21, 22 We quantified change in BMIZ and HAZ during each age interval as the difference in values between the beginning and end of each timeframe. Our previous study examined weight-for-length, which is highly correlated with BMIZ (Spearman’s ρ: 0.81, 0.99, 0.98, and 0.99 at birth, 6 months, 1 year, and 2 years, respectively), which additional incorporates age at measurement. Given this and the fact that BMIZ captures fat growth in mid-childhood, 23, 24 we decided to use BMIZ change as an indicator for weight gain for all age intervals.25
Outcome: mid-childhood BP
We measured blood pressure using biannually-calibrated automated oscillometric monitors (Dinamap Pro100, Tampa, Florida). Trained research assistants recorded BP on the child’s upper arm up to 5 times at 1-minute intervals, while also taking note of the cuff size, child’s position (sitting, semi-reclining, reclining, standing), activity level during measurement (still, moving, quiet, talking), time of day, and ambient temperature. Although the first measurement tended to be higher than the 2nd through 5th measurements, we included all 5 in the analysis as it improves precision when quantifying between-person differences rather than absolute levels, particularly in children.26 We used the average of the 5 measurements since the intra-class coefficient was high (ICC=0.74). We categorized children as pre-hypertensive if SBP or DBP ≥90th and <95th percentile, and as hypertensive if SBP or DBP ≥95th percentile according to the NHANES 1999–2000 BP reference for children and adolescents.1 However, there were only 3 cases of pre-hypertension and 2 cases of hypertension in mid-childhood, thus we present associations with continuous BP only.
Additional participant characteristics
Mothers reported on their age, race/ethnicity, education, parity, household income, smoking habits in pregnancy, pre-pregnancy weight, and paternal weight and height via interviews and questionnaires administered at enrollment. Duration in postpartum questionnaires, women reported on breastfeeding duration. Medical records provided information on prenatal glucose tolerance. We calculated maternal pre-pregnancy BMI using self-reported pre-pregnancy weight and height, and used standard adult classifications for weight status.27 To identify women with hypertensive disorders of pregnancy, we reviewed outpatient charts for blood pressure and urine protein results, and created a 4-level variable with the categories normotensive, chronic hypertension, gestational hypertension, and preeclampsia as previously described.28
At the mid-childhood visit, RAs measured children’s weight to the nearest 0.1 kg with an electronic scale, and height to the nearest 0.1 cm with a calibrated stadiometer. We calculated age- and sex-specific height z-scores using the Centers for Disease Control (CDC) 2–20 year growth reference.29
Data analysis
We first examined bivariate associations of SBP and DBP across categories of sociodemographic and perinatal characteristics to identify potential confounders. We then examined the unadjusted associations of birth size using conventional categories (SGA, AGA, LGA) and continuously (birthweight-for-gestational-age z-score), and BMIZ and HAZ change during each age interval with mid-childhood BP. We assessed BMIZ and HAZ change in quartiles to ensure linear associations before examining them as continuous variables.
Next, we used multivariable linear regression models that accounted for maternal education, age, pre-pregnancy BMI, and smoking habits in pregnancy; birthweight-for-gestational-age z-score; and the child’s age, sex, race/ethnicity to investigate associations of BMIZ and HAZ change during each age interval with BP in mid-childhood. For the later growth periods, we also adjusted for growth during prior age intervals to account for the influence of previous growth on growth during the timeframe of interest. Because correlations between BMIZ and HAZ change were moderately high for certain periods (S2), we evaluated for multicollinearity using the variance inflation factor (VIF). VIF for all models were <2.5, which is below the standard cut-off of 10 that indicates high multicollinearity.30
We conducted a formal test for an interaction between BMIZ and HAZ change during each period with birthweight-for-gestational age z-score and sex, separately, and found no indication of effect modification by either variable (P-interaction >0.05).
Because height is a strong determinant of BP during childhood,14 and because clinical assessments of pediatric BP are based on age-, sex-, and height-specific values,1 we re-ran analyses with BP z-score as the outcome. In sensitivity analyses, we further adjusted for gestational diabetes, delivery method, breastfeeding, and paternal BMI; accounting for these characteristics did not change our findings. Since the age interval timeframes were not of equal length, we assessed BMIZ and HAZ change as velocities. Using velocities yielded no change in the direction, magnitude, or precision of the results; therefore we present results in terms of BMIZ and HAZ change for interpretability.
All analyses were performed with Statistical Analyses System software (v9.3; SAS Institute Inc., Cary, NC).
RESULTS
Among the 957 participants, mean±SD birthweight-for-gestational age z-score was 0.21±0.96 and birthweight was 3516±511 g. Children were 7.9±0.8 years old at the mid-childhood visit, and average SBP and DBP were 94.4±8.8 mmHg and 54.3±5.7 mmHg, respectively.
In bivariate analyses, maternal age and parity were inversely related to offspring DBP in mid-childhood, whereas maternal pre-pregnancy BMI and smoking during pregnancy were associated with higher offspring SBP and DBP (Table 1). We observed differences in mid-childhood BP with respect to race/ethnicity. While Black and White children had similar BP levels (P=0.32), Hispanic children had 4.03 (95% CI: 1.24, 6.81) mmHg lower SBP than their White peers (P=0.005). Children whose mothers had chronic hypertension and gestational hypertension had 6.52 (1.74, 11.31) mmHg and 2.61 (0.33, 4.90) mmHg higher SBP, respectively, as compared to those of women with normal blood pressure; similar but weaker associations were observed for DBP. Birthweight-for-gestational age z-score was inversely associated with DBP, but not SBP (Table 1).
Table 1.
Mid-childhood BP according to background characteristics of 957 Project Viva children
| Study sample characteristics | N (%) | Mean±SD BP in mid-childhood | |||
|---|---|---|---|---|---|
| SBP (mmHg) | P* | DBP (mmHg) | P* | ||
| Overall | 957 | 94.4±8.8 | 54.3±5.7 | ||
| Maternal/family characteristics | |||||
| Maternal age at enrollment | 0.24 | 0.01 | |||
| 15–24 y | 87 (9.1) | 94.4±7.9 | 54.6±5.6 | ||
| 25–34 y | 560 (58.5) | 94.8±9.1 | 54.7±5.7 | ||
| 35–44 y | 310 (32.4) | 93.8±8.5 | 53.5±5.6 | ||
| Mother’s marital status | 0.52 | 0.13 | |||
| Married/cohabiting | 879 (92.0) | 94.4±8.6 | 54.2±5.7 | ||
| Single | 76 (8.0) | 95.0±11.1 | 55.3±6.1 | ||
| Mother’s education | 0.93 | 0.99 | |||
| Primary | 89 (9.3) | 93.3±9.5 | 54.6±5.4 | ||
| Secondary | 533 (55.8) | 94.8±8.7 | 54.2±5.8 | ||
| University | 333 (34.9) | 94.1±8.7 | 54.4±5.6 | ||
| Annual household income | 0.40 | 0.15 | |||
| <$20,000 | 31 (3.5) | 91.9±8.8 | 52.8±5.1 | ||
| $20,000–$39,999 | 91 (10.4) | 95.9±9.0 | 55.3±5.6 | ||
| $40,000–$69,999 | 190 (21.6) | 95.4±9.3 | 55.3±5.9 | ||
| >$70,000 | 567 (64.5) | 94.1±8.4 | 54.0±5.7 | ||
| Parity | 0.27 | 0.004 | |||
| 0 | 461 (48.2) | 94.8±8.3 | 54.9±5.7 | ||
| 1 | 336 (35.1) | 94.1±9.3 | 53.9±5.8 | ||
| ≥2 | 160 (16.7) | 94.0±9.1 | 53.6±5.4 | ||
| Perinatal characteristics | |||||
| Mother’s pre-pregnancy BMI† | 0.0003 | 0.02 | |||
| Underweight (<18.5 kg/m2) | 33 (3.5) | 92.0±8.3 | 54.5±5.1 | ||
| Normal weight (18.5–24.9 kg/m2) | 589 (61.8) | 93.9±8.6 | 54.0±5.8 | ||
| Overweight (25.0–29.9 kg/m2) | 196 (20.6) | 94.9±9.0 | 54.6±5.5 | ||
| Obese (≥30 kg/m2) | 135 (142) | 96.6±9.1 | 55.3±5.8 | ||
| Maternal smoking in pregnancy | 0.009 | 0.08 | |||
| Never | 676 (70.9) | 93.9±8.8 | 54.1±5.7 | ||
| Quit before pregnancy | 194 (20.3) | 95.6±8.6 | 54.8±5.5 | ||
| Smoked in early pregnancy | 84 (8.8) | 96.2±9.0 | 55.2±5.8 | ||
| Hypertensive disorders of pregnancy | 0.007 | 0.05 | |||
| None | 836 (89.6) | 94.1±8.6 | 54.2±5.7 | ||
| Chronic hypertension | 13 (1.4) | 100.7±9.8 | 55.3±5.4 | ||
| Gestational hypertension | 60 (6.4) | 96.8±10.1 | 56.3±5.4 | ||
| Preeclampsia | 24 (2.6) | 94.4±9.4 | 54.7±5.1 | ||
| Gestational glucose tolerance | 0.63 | 0.59 | |||
| Normoglycemic | 82.8 (791) | 94.4±8.8 | 54.4±5.7 | ||
| Isolated hyperglycemia | 9.0 (86) | 95.0±9.6 | 54.2±6.0 | ||
| Impaired glucose tolerance | 3.4 (32) | 91.5±7.0 | 52.5±5.1 | ||
| Gestational diabetes | 4.8 (46) | 96.1±8.5 | 54.7±5.8 | ||
| Delivery method | 0.80 | 0.33 | |||
| C-section | 22.2 (212) | 94.5±8.6 | 54.7±5.5 | ||
| Vaginal | 77.9 (745) | 94.4±8.8 | 54.2±5.8 | ||
| Child’s characteristics at birth | |||||
| Sex | 0.71 | 0.19 | |||
| Male | 49.6 (475) | 94.5±8.5 | 54.1±5.6 | ||
| Female | 50.4 (482) | 94.3±9.0 | 54.6±5.8 | ||
| Race/ethnicity | 0.01 | 0.008 | |||
| Black | 15.8 (151) | 95.1±10.4 | 54.7±6.0 | ||
| Hispanic | 4.2 (40) | 90.3±7.3 | 52.3±4.9 | ||
| White | 65.1 (622) | 94.3±8.5 | 54.1±5.6 | ||
| Asian | 2.9 (28) | 92.8±9.1 | 56.3±6.5 | ||
| Other | 12.0 (115) | 95.7±8.4 | 55.4±5.9 | ||
| Birth size‡ | 0.99 | 0.008 | |||
| SGA (<10th percentile) | 5.6 (54) | 93.4±10.4 | 55.3±6.0 | ||
| AGA (10-≤90th percentile) | 80.6 (771) | 94.6±8.8 | 54.4±5.7 | ||
| LGA (>90th percentile) | 13.8 (132) | 94.0±8.4 | 53.2±5.3 | ||
| Duration of any breastfeeding | 0.62 | 0.72 | |||
| <1 mo | 17.5 (159) | 94.6±10.2 | 54.4±5.6 | ||
| 1–6 mo | 33.6 (306) | 94.2±8.4 | 54.3±5.9 | ||
| 7–11 mo | 19.7 (179) | 94.5±8.2 | 54.2±5.5 | ||
| ≥12 mo | 29.2 (266) | 94.0±8.4 | 54.2±5.6 | ||
In unadjusted analysis, children born LGA had 1.23 (0.17, 2.28) mmHg lower DBP in mid-childhood than those who had been AGA newborns; the inverse relation with DBP was also apparent when we examined birth size continuously (−0.45 [−0.83, −0.07] mmHg per birthweight-for-gestational-age z-score; Table 2). Greater BMIZ gain from birth to 6 months was associated with higher SBP and DBP (Table 2). There were no consistent relations of HAZ change with mid-childhood BP.
Table 2.
Unadjusted associations of birth size and early life growth with mid-childhood blood pressure*
| Early life growth | N | Mean ± SD | β (95% CI) in mid-childhood BP | |
|---|---|---|---|---|
| SBP (mmHg) | DBP (mmHg) | |||
|
|
|
|||
| Birth size | ||||
| SGA (<10th percentile) | 54 | −1.66±0.30 | −1.14 (−3.57, 1.28) | 0.91 (−0.66, 2.48) |
| AGA (10 –≤90th percentile) | 771 | 0.07±0.65 | 0.00 (Reference) | Reference (0.00) |
| LGA (>90th percentile) | 132 | 1.75±0.39 | −0.53 (−2.16, 1.09) | −1.23 (−2.28, −0.17) |
| Per 1 unit BWZ† | 957 | 0.21±0.96 | 0.18 (−0.41, 0.76) | −0.45 (−0.83, −0.07) |
| BMI z-score change‡ | ||||
| Birth - 6 months | 475 | 0.11±1.30 | 0.88 (0.31, 1.46) | 0.42 (0.04, 0.81) |
| 6 months - 1 year | 661 | −0.08±0.83 | 0.59 (−0.19, 1.37) | 0.07 (−0.45, 0.58) |
| 1 – 2 years | 758 | −0.13±0.94 | −0.03 (−0.69, 0.62) | −0.09 (−0.52, 0.33) |
| 2 – 3 years | 703 | 0.28±0.88 | 0.58 (−0.14, 1.30) | 0.07 (−0.40, 0.55) |
| Length/height z-score change‡ | ||||
| Birth - 6 months | 470 | −0.50±1.02 | 0.29 (−0.45, 1.04) | 0.19 (−0.30, 0.69) |
| 6 months- 1 year | 674 | 0.38±0.76 | 0.61 (−0.24, 1.46) | 0.19 (−0.36, 0.75) |
| 1 – 2 years | 819 | 0.30±0.92 | 0.45 (−0.19, 1.10) | −0.21 (−0.63, 0.21) |
| 2 – 3 years | 762 | −0.45±0.73 | 0.12 (−0.71, 0.96) | −0.15 (−0.71, 0.40) |
Bolded estimate represent statistical significance at P<0.05.
BWZ: Sex-specific birthweight-for-gestational-age z-score according to U.S. natality reference data.19
After accounting for confounders and previous growth, BMIZ change from birth to 6 months and 2 to 3 years were each associated with higher mid-childhood SBP. The magnitude of association was larger for the later period (1.40 [0.12, 2.68] vs. 0.73 [0.06, 1.39] vs. mmHg per z-score increment; Figure 1 and S3). The relation of BMIZ change from 6 months to 1 year with SBP was similar to that of birth to 6 months, but with wider CIs (0.86 [−0.14, 1.85] mmHg per z-score BMI gain). Associations with height-standardized BP z-scores paralleled those of raw BP.
Figure 1.
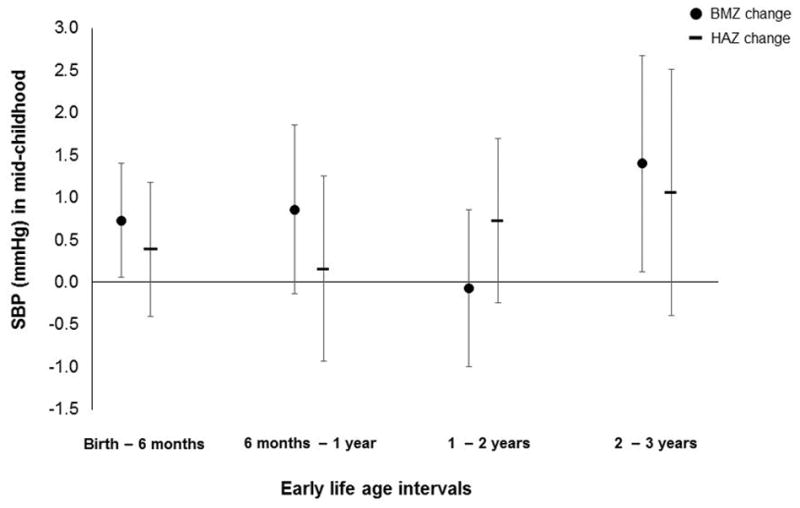
Associations of BMIZ and HAZ change during 4 age intervals with mid-childhood SBP. Estimates are adjusted for maternal pre-pregnancy BMI, age, smoking habits in pregnancy, hypertensive disorders of pregnancy, and education; birthweight-for-gestational-age z-score; and child’s age at mid-childhood visit, sex, race/ethnicity, and BMIZ or HAZ change during all previous age intervals.
DISCUSSION
In this study, each increment in BMI z-score gain during the first 6 postnatal months and 2 to 3 years of age corresponded with 0.7 and 1.5 mmHg higher SBP, respectively, in mid-childhood. In contrast to earlier studies15, 16 including one in Project Viva that examined BP at 3–5 years of age,9 we found no evidence of an interaction between birth size and BMIZ or length/height gain.
Previous studies have emphasized the first months of life as a sensitive period for development of cardiovascular risk.9, 31–33 In contrast, relatively little is known regarding the influence of adiposity accrual and linear growth during the toddler or preschool years. Our finding that BMIZ gain from 2 to 3 years had a larger influence on mid-childhood BP than did gains in infancy corroborates two recent investigations. In a study of 13,889 Belarusian participants of the Promotion of Breastfeeding Intervention Trial (PROBIT), Tilling et al. reported that in comparison to weight gain from birth to 3 months and 3 months to 1 year, weight gain from 1 to 5 years was a stronger determinant of BP at 6.5 years after adjustment for confounders and previous growth. In another study of 12,962 U.K. children in the Avon Longitudinal Study of Parents and Children (ALSPAC), Jones et al.11 used conditional growth modeling, a strategy comparable to previous growth adjustment, to explore how gains in weight, weight-for-height, and height correspond with BP at 10 years during two timeframes: birth to 17 months and 17 months to 10 years. Weight and weight-for-height gain during both periods predicted higher SBP, but a larger association was observed for 17 months to 10 years.11 Our findings add to this evidence base, especially since we examined more granular and evenly-spaced temporal windows. The fact that the relationship between BMI gain and future BP was most robust during the toddler/early preschool years is salient because this timeframe coincides with bi-annual well-child visits at which healthcare providers monitor growth and make recommendations to parents for optimal child health. Our findings also support the need for obesity-prevention and nutrition programs in preschool or childcare settings, as they may be feasible and effective community-based strategies to prevent excess early weight gain on a larger scale.34
We also found that BMIZ change during the birth to 6 month timeframe, and to some extent from 6 months to 1 year, corresponded with higher mid-childhood SBP - albeit at half the magnitude of what we observed for the 2 to 3 year interval. This finding aligns with that of Ben-Schlomo et al.,10 who reported that faster weight gain from birth to 5 months and 1 year 9 months to 5 year were stronger predictors of adult SBP than were gains from 5 months to 1 year 9 months, with larger effect sizes during the 1 year 9 months to 5 year period (1.29 vs. 1.44 mmHg).10 A potential reason for the null relation between 1 to 2 year BMIZ gain and mid-childhood BP in our study is that both weight and length/height measurements were derived from medical records, which may be less precise than research standard measures. For example, clinics used the paper-and-pencil method to measure length in infants, which leads to an overestimation of approximately 1.3±1.5 cm as compared to the research standard recumbent length board technique.20 Although we applied a length correction factor to account for this difference in technique, residual non-systematic bias could still obscure associations.
Contrary to findings in ALSPAC that linear growth during infancy and childhood were positively associated with mid-childhood SBP, with effect sizes comparable to those for weight gain,11 early life HAZ change was unrelated to mid-childhood BP in our study. Given the positive relationship between age and BP in pre- and peri-pubertal children,35 the discrepancy in findings could be related to the older age at BP assessment in ALSPAC children (~10.5 years in ALSPAC vs. ~8 years in Project Viva).
There are a few biological mechanisms that could link early adiposity accrual with future BP. The first years of life represent a period of rapid growth and development, thus environmental and genetic factors that affect accrual of adiposity and/or linear growth may also influence cardiovascular system ontogeny. For example, the ratio of elastin to collagen fibers in cardiac tissue, a determinant of arterial stiffness and thus propensity for high BP, is established before birth.36 In this scenario, early life weight gain share an upstream cause with elevated BP, but is not a direct causal mechanisms. Another explanation relates to the fact that rapid postnatal and childhood weight gain is consistently associated with future obesity,6 possibly through early programming and establishment of adipocyte quantity.37 Higher adiposity13 - in particular, greater central fat mass38 - is a strong determinant of BP in both children and adults. Proposed mechanisms range from physical compression of the kidneys to endocrine effects of adipose tissue that cause dysregulation of hormones and cytokines involved in vascular function.39
This investigation has several strengths, including some improvements upon our previous study investigating relations of weight-for-length change from birth to 6 months with BP at 3–5 years.9 First, we assessed age-standardized measures of adiposity as well as length/height gain during multiple early life age intervals. Second, while associations with early childhood BP are informative, mid-childhood may be more relevant timeframe in light of stronger correlations between BP during this timeframe and adult BP.4 Additionally, as compared to findings from other contemporary cohorts, our results are more relevant to the current U.S. population. For example, ALSPAC and PROBIT recruitment date in the early-to-mid-1990s with 8.7%40 and 5.5%41 prevalence of obesity during mid-childhood, respectively, as compared to 12% in Project Viva. Considering 2012 estimates that 18 % of U.S. children 6–11 years of age are obese,42 our results are more applicable to current populations in terms of prevalence of obesity and related metabolic sequelae.
This study has some limitations. First, results can be impeded by unmeasured confounding, although we accounted for key sociodemographic and perinatal characteristics. Second, as with all cohort studies, there may be attrition bias as participants who remained in the study through mid-childhood follow-up tended to be older and White, with a larger proportion of mothers who are college graduates and non-smokers with higher annual income.18 Third, we have limited generalizability to lower socioeconomic populations and developing countries as Project Viva is a relatively well-educated population. It has also been suggested that compared with auscultation, oscillometric devices overestimate BP, leading to misclassification of pre- hypertension or hypertension.43 Because NHANES uses the auscultatory method, standardization of BP of Project Viva participants using this reference may lead to systematic overestimation of z-scores. However, we analyzed BP as a continuous variable and expect the relative rank of individual BP to be preserved. Finally, because BP assessments in formal settings may induce the “white coat effect,” it is possible that BP measurements were higher than usual. However, we took 5 repeated measurements to overcome the possibility, and again, because were interested in continuous BP, inter-individual differences were likely captured. Future studies might consider use of ambulatory BP monitoring, which is useful in evaluation of hypertension in children.44
PERSPECTIVES
An individual’s blood pressure relative to the population is established during the first decade of life,4 emphasizing the importance of identifying early predisposing factors for raised BP. The size of associations we detected with respect to early weight gain (differences of 0.7 to 1.4 mmHg SBP), while relatively small, may be relevant from a population prevention perspective45 considering that a 1 mmHg increment in childhood SBP predicted elevations in a cluster of metabolic risk factors in young adulthood including SBP, cholesterol, triglycerides, and fasting glucose in the Bogalusa Heart Study.46 Our findings suggest that a focus on strategies to reduce development of excess adiposity during the immediate postnatal period and in the preschool years may have lifelong benefits for cardiovascular health.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
We examined the associations of adiposity gain and linear growth during 4 early life age intervals with mid-childhood BP.
What is relevant?
An individual’s blood pressure rank within the population is determined by 10 years of age, thus understanding associations of growth during granular timeframes early in the life course with BP during mid-childhood could inform interventions to reduce risk of future elevated blood pressure.
Summary
More rapid adiposity gain during the first 6 postnatal months and in the toddler/preschool years is associated with higher SBP at age 6–10 years.
Acknowledgments
We are indebted to the mothers and children of Project Viva, and appreciate the assistance of Project Viva staff.
SOURCES OF FUNDING
US National Institutes of Health (K24 HD069408, R37 HD 034568, P30 DK092924).
Abbreviations
- BMIZ
body-mass-index-for-age z-score
- HAZ
height-for-age z-score
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
Footnotes
CONFLICTS/DISCLOSURES OF INTEREST
None.
References
- 1.U.S. Department of Health and Human Services. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. 2005. [Google Scholar]
- 2.Kit BK, Kuklina E, Carroll MD, Ostchega Y, Freedman DS, Ogden CL. Prevalence of and trends in dyslipidemia and blood pressure among us children and adolescents, 1999–2012. JAMA Pediatr. 2015;169:272–279. doi: 10.1001/jamapediatrics.2014.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Din-Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488–1496. doi: 10.1161/CIRCULATIONAHA.106.683243. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: A systematic review and meta-regression analysis. Circulation. 2008;117:3171–3180. doi: 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, Jarvisalo MJ, Uhari M, Jokinen E, Ronnemaa T, Akerblom HK, Viikari JS. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: The cardiovascular risk in young finns study. JAMA. 2003;290:2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 6.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: Systematic review of size and growth in infancy and later obesity. BMJ. 2005;331:929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: Systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95:904–908. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- 8.Bowers K, Liu G, Wang P, Ye T, Tian Z, Liu E, Yu Z, Yang X, Klebanoff M, Yeung E, Hu G, Zhang C. Birth weight, postnatal weight change, and risk for high blood pressure among chinese children. Pediatrics. 2011;127:e1272–1279. doi: 10.1542/peds.2010-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belfort MB, Rifas-Shiman SL, Rich-Edwards J, Kleinman KP, Gillman MW. Size at birth, infant growth, and blood pressure at three years of age. J Pediatr. 2007;151:670–674. doi: 10.1016/j.jpeds.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-Shlomo Y, McCarthy A, Hughes R, Tilling K, Davies D, Smith GD. Immediate postnatal growth is associated with blood pressure in young adulthood: The barry caerphilly growth study. Hypertension. 2008;52:638–644. doi: 10.1161/HYPERTENSIONAHA.108.114256. [DOI] [PubMed] [Google Scholar]
- 11.Jones A, Charakida M, Falaschetti E, Hingorani AD, Finer N, Masi S, Donald AE, Lawlor DA, Smith GD, Deanfield JE. Adipose and height growth through childhood and blood pressure status in a large prospective cohort study. Hypertension. 2012;59:919–925. doi: 10.1161/HYPERTENSIONAHA.111.187716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tilling K, Davies N, Windmeijer F, Kramer MS, Bogdanovich N, Matush L, Patel R, Smith GD, Ben-Shlomo Y, Martin RM. Is infant weight associated with childhood blood pressure? Analysis of the promotion of breastfeeding intervention trial (probit) cohort. Int J Epidemiol. 2011;40:1227–1237. doi: 10.1093/ije/dyr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He Q, Ding ZY, Fong DY, Karlberg J. Blood pressure is associated with body mass index in both normal and obese children. Hypertension. 2000;36:165–170. doi: 10.1161/01.hyp.36.2.165. [DOI] [PubMed] [Google Scholar]
- 14.Regnault N, Kleinman KP, Rifas-Shiman SL, Langenberg C, Lipshultz SE, Gillman MW. Components of height and blood pressure in childhood. Int J Epidemiol. 2014;43:149–159. doi: 10.1093/ije/dyt248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adair LS, Cole TJ. Rapid child growth raises blood pressure in adolescent boys who were thin at birth. Hypertension. 2003;41:451–456. doi: 10.1161/01.HYP.0000054212.23528.B2. [DOI] [PubMed] [Google Scholar]
- 16.Adair LS, Martorell R, Stein AD, Hallal PC, Sachdev HS, Prabhakaran D, Wills AK, Norris SA, Dahly DL, Lee NR, Victora CG. Size at birth, weight gain in infancy and childhood, and adult blood pressure in 5 low- and middle-income-country cohorts: When does weight gain matter? Am J Clin Nutr. 2009;89:1383–1392. doi: 10.3945/ajcn.2008.27139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tu YK, West R, Ellison GT, Gilthorpe MS. Why evidence for the fetal origins of adult disease might be a statistical artifact: The “reversal paradox” for the relation between birth weight and blood pressure in later life. Am J Epidemiol. 2005;161:27–32. doi: 10.1093/aje/kwi002. [DOI] [PubMed] [Google Scholar]
- 18.Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, Rich-Edwards JW, Rifas-Shiman SL, Sagiv S, Taveras EM, Weiss ST, Belfort MB, Burris HH, Camargo CA, Jr, Huh SY, Mantzoros C, Parker MG, Gillman MW. Cohort profile: Project viva. Int J Epidemiol. 2015;44:37–38. doi: 10.1093/ije/dyu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a united states national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rifas-Shiman SL, Rich-Edwards JW, Scanlon KS, Kleinman KP, Gillman MW. Misdiagnosis of overweight and underweight children younger than 2 years of age due to length measurement bias. MedGenMed. 2005;7:56. [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organizaton. Who child growth standards: Height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index for-age: Methods and development. 2006. [Google Scholar]
- 22.de Onis M, Garza C, Victora C, Bhan M, Norum K. The who multicentre growth reference study (mgrs): Rationale, planning, and implementaton. Food Nutr Bull. 2004;25:S1–S89. doi: 10.1177/15648265040251S103. [DOI] [PubMed] [Google Scholar]
- 23.Kakinami L, Henderson M, Chiolero A, Cole TJ, Paradis G. Identifying the best body mass index metric to assess adiposity change in children. Arch Dis Child. 2014;99:1020–1024. doi: 10.1136/archdischild-2013-305163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inokuchi M, Matsuo N, Takayama JI, Hasegawa T. Bmi z-score is the optimal measure of annual adiposity change in elementary school children. Ann Hum Biol. 2011;38:747–751. doi: 10.3109/03014460.2011.620625. [DOI] [PubMed] [Google Scholar]
- 25.Rifas-Shiman SL, Gillman MW, Oken E, Kleinman K, Taveras EM. Similarity of the cdc and who weight-for-length growth charts in predicting risk of obesity at age 5 years. Obesity (Silver Spring) 2012;20:1261–1265. doi: 10.1038/oby.2011.350. [DOI] [PubMed] [Google Scholar]
- 26.Gillman MW, Cook NR. Blood pressure measurement in childhood epidemiological studies. Circulation. 1995;92:1049–1057. doi: 10.1161/01.cir.92.4.1049. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. Global Database on Body Mass Index. 2012. Bmi classification. [Google Scholar]
- 28.Perng W, Stuart J, Rifas-Shiman SL, Rich-Edwards JW, Stuebe A, Oken E. Preterm birth and long-term maternal cardiovascular health. Ann Epidemiol. 2015;25:40–45. doi: 10.1016/j.annepidem.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 cdc growth charts for the united states: Methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 30.Kutner MH, Mactsheim CJ, Neter J. Applied linear regression models. McGraw-Hill Irwin; 2004. [Google Scholar]
- 31.Fernandes MT, Ferraro AA, Pires A, Santos E, Schvartsman C. Early-life weight and weight gain as predictors of obesity in brazilian adolescents. Clinics (Sao Paulo) 2013;68:1408–1412. doi: 10.6061/clinics/2013(11)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillman MW. The first months of life: A critical period for development of obesity. Am J Clin Nutr. 2008;87:1587–1589. doi: 10.1093/ajcn/87.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123:1177–1183. doi: 10.1542/peds.2008-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bluford DA, Sherry B, Scanlon KS. Interventions to prevent or treat obesity in preschool children: A review of evaluated programs. Obesity (Silver Spring) 2007;15:1356–1372. doi: 10.1038/oby.2007.163. [DOI] [PubMed] [Google Scholar]
- 35.Shankar RR, Eckert GJ, Saha C, Tu W, Pratt JH. The change in blood pressure during pubertal growth. J Clin Endocrinol Metab. 2005;90:163–167. doi: 10.1210/jc.2004-0926. [DOI] [PubMed] [Google Scholar]
- 36.Martyn CN, Greenwald SE. Impaired synthesis of elastin in walls of aorta and large conduit arteries during early development as an initiating event in pathogenesis of systemic hypertension. Lancet. 1997;350:953–955. doi: 10.1016/s0140-6736(96)10508-0. [DOI] [PubMed] [Google Scholar]
- 37.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J, Arner P. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 38.Zhang YX, Wang SR. Comparison of blood pressure levels among children and adolescents with different body mass index and waist circumference: Study in a large sample in shandong, china. Eur J Nutr. 2014;53:627–634. doi: 10.1007/s00394-013-0571-1. [DOI] [PubMed] [Google Scholar]
- 39.Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity-induced hypertension. Hypertens Res. 2010;33:386–393. doi: 10.1038/hr.2010.9. [DOI] [PubMed] [Google Scholar]
- 40.Hughes AR, Sherriff A, Lawlor DA, Ness AR, Reilly JJ. Incidence of obesity during childhood and adolescence in a large contemporary cohort. Prev Med. 2011;52:300–304. doi: 10.1016/j.ypmed.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kramer MS, Matush L, Vanilovich I, Platt RW, Bogdanovich N, Sevkovskaya Z, Dzikovich I, Shishko G, Collet JP, Martin RM, Davey Smith G, Gillman MW, Chalmers B, Hodnett E, Shapiro S. Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: Evidence from a large randomized trial. Am J Clin Nutr. 2007;86:1717–1721. doi: 10.1093/ajcn/86.5.1717. [DOI] [PubMed] [Google Scholar]
- 42.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the united states, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flynn JT, Pierce CB, Miller ER, 3rd, Charleston J, Samuels JA, Kupferman J, Furth SL, Warady BA. Reliability of resting blood pressure measurement and classification using an oscillometric device in children with chronic kidney disease. J Pediatr. 2012;160:434–440. e431. doi: 10.1016/j.jpeds.2011.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lurbe E, Sorof JM, Daniels SR. Clinical and research aspects of ambulatory blood pressure monitoring in children. J Pediatr. 2004;144:7–16. doi: 10.1016/j.jpeds.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 45.Rose G. Sick individuals and sick populations. Int J Epidemiol. 2001;30:427–432. doi: 10.1093/ije/30.3.427. discussion 433–424. [DOI] [PubMed] [Google Scholar]
- 46.Rademacher ER, Jacobs DR, Jr, Moran A, Steinberger J, Prineas RJ, Sinaiko A. Relation of blood pressure and body mass index during childhood to cardiovascular risk factor levels in young adults. J Hypertens. 2009;27:1766–1774. doi: 10.1097/HJH.0b013e32832e8cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


