Abstract
An often employed strategy to enhance signals in 31P MR spectroscopy is the generation of the nuclear Overhauser effect (NOE) by saturating the water resonance. However, NOE allegedly increases the variability of the 31P data, because variation is reported in NOE enhancements. This would negate the SNR gain it generates. We hypothesized that variation in NOE enhancement values is not due to variability in the NOE itself, but that it is attributable to measurement uncertainties in the values used to calculate the enhancement. If true, the expected increase in SNR with NOE would improve the repeatability of 31P MR spectroscopy measurements. To verify this hypothesis, a repeatability study of native and NOE-enhanced 31P MRSI was performed in the brain of 7 healthy volunteers at 7T.
The repeatability coefficient (RC) and the coefficient of variation in repeated measurements (CoVrepeat) were determined per method, and the 95% limits of agreement (LoA) between native and NOE-enhanced signals were calculated. The variation between the methods, defined by the LoA, is at least as great as that predicted by the RC of each method. The sources of variation in NOE enhancements were determined using variance component analysis.
In the 7 metabolites with a positive NOE enhancement (9 metabolite resonances assessed), CoVrepeat improved on average by 15%. The LoAs could be explained by the RCs of the individual methods for the majority of the metabolites, generally confirming our hypothesis. Variation in NOE enhancement was mainly attributable to the factor repeat, but between-voxel effects were also present for phoshpoethanolamine and (glycero)phosphocholine. CoVrepeat and fitting error were strongly correlated and improved with positive NOE.
Our findings generally indicate that NOE enhances the signal of the metabolites, improving the repeatability of metabolite measurements. Additional variability due to NOE was minimal. These findings encourage the use of NOE-enhanced 31P MRSI.
Keywords: repeatability, reproducibility, 7 Tesla, ultra-high field, MR spectroscopy
Introduction
Phosphorus (31P) MR spectroscopy is a non-invasive technique to study tissue metabolism under various physiological and pathophysiological conditions. Human organs and tissues frequently investigated using 31P MRS include the liver (1,2), skeletal muscle (3,4), the heart (5) and the brain (6,7), and studies concerning the prostate (8), the placenta (9), and breast (10) have also been reported recently. Metabolic changes can be followed over time with dynamic 31P MRS, and MR spectroscopic imaging (MRSI) allows the two- or three-dimensional mapping of metabolites to study their spatial distribution.
The low intrinsic sensitivity of in vivo 31P MRS and MRSI however impedes the regular use of these techniques. Obviously, advances in radiofrequency (RF) coil design and increases in magnetic field strength have been embraced to gain 31P sensitivity enabling reduced overall acquisition times or increased spatial resolution. Sensitivity of 31P spectra can also be improved by adding proton (1H) irradiation to the MR sequence (11–13). Proton irradiation may activate two physical mechanisms: decoupling and the nuclear Overhauser effect (NOE).
The first process, proton decoupling, is particularly useful at clinical field strengths (1.5-3 T), where the line width of the resonances of interest after shimming can be of similar size as the weak heteronuclear 31P-1H J-couplings (5-7 Hz). Without proton decoupling the coupled metabolites split into doublets or triplets, giving rise to broad resonances and spectral overlap. Many metabolites present in an in vivo 31P spectrum contain one or more weak 31P-1H J-couplings, i.e. phosphoethanolamine (PE), phosphocholine (PC), glycerophosphoethanolamine (GPE), glycerophosphocholine (GPC), 2,3-diphosphoglycerate, α-adenosine triphosphate (αATP) and nicotinamide adenine dinucleotide (NAD+ and NADH). The improvement in spectral resolution obtained by decoupling reduces the spectral overlap of the 31P metabolites and thus allows better peak assignment and consequently better spectral quantification. Decoupling can be achieved by applying high-power broadband proton irradiation during 31P signal acquisition, creating high specific absorption rates (SAR) at higher field strengths.
The second process of signal improvement, the NOE, uses low-power proton irradiation to saturate the water resonance in between 31P signal acquisitions. The saturated water protons can increase the steady state magnetization of the 31P nuclei through dipolar interactions, resulting in enhanced 31P metabolite signal intensities. In vivo enhancements up to 80% have been reported (14), which encourages the use of NOE-enhanced 31P MRS or MRSI. Because NOE requires considerably less power than decoupling, it is also suited for higher magnetic field strengths.
Absolute NOE enhancement values depend on the extent to which the dipolar 31P-1H interaction mediates T1 relaxation of the 31P spins (15). The enhancement values therefore vary between metabolites, and are dependent on the mobility of the metabolites in the tissue and magnetic field strength (14,16–22). Sufficient saturation of the water resonance is essential to maximize the NOE enhancement (12,13), and needs to be optimized for each sequence. At ultrahigh magnetic field strengths it is even more important to determine the minimum transmit RF amplitude (B1+) for constant NOE, because of the known spatial inhomogeneities in the B1+ field (22).
The in vivo NOE signal enhancement indeed seemed very beneficial initially (14), but considerable variation was later reported in NOE enhancement values per metabolite, even if the measurements were calibrated well (19,20,22). This has raised concerns that using NOE might add variability to the 31P data, negating the gain in signal-to-noise ratio (SNR). We hypothesized that variation in NOE enhancement values is not due to variability in the NOE itself (i.e. non-constant NOE enhancements because of natural variation or inadequate NOE calibration), but that it is attributable to measurement uncertainties in the values used to calculate the NOE enhancement. If true, this would argue for the use of NOE as the SNR is expected to increase, which would potentially decrease measurement uncertainties. To test this hypothesis repeated measurements with both methods, i.e. native, non-enhanced 31P MRS/ MRSI and NOE-enhanced MRS/ MRSI are needed. The measurement uncertainties of the two methods, estimated by their repeatabilities, limit the amount of agreement possible between the two methods, and thus define a minimum variation in NOE enhancement values per metabolite (23).
Ultimately, one wishes to answer the question whether or not to use NOE in 31P MRS/MRSI studies in any tissue at any field strength. Because of the dependency of NOE enhancements on dipolar relaxation processes and saturation efficiency, spatial variations in NOE enhancement might be possible, and these may depend on the tissue type and state (healthy and diseased) and the field strength. To reduce complexity regarding all these issues, the research question in this study was narrowed down to the possible beneficial effect of NOE on 31P MRSI of the healthy human brain at 7 T. To answer this question we performed repeated measurements of native 31P MRSI and NOE-enhanced 31P MRSI in the brain of healthy volunteers at 7 T.
Experimental
Hardware
Measurements were performed on a 7 T whole-body MR research system (MAGNETOM, Siemens Healthcare, Erlangen, Germany). An eight-rung high?pass quadrature birdcage coil tuned to 31P (120.3 MHz) was used (24), which was designed to fit within a homebuilt eight-channel 1H array head coil with meander elements (25). The safety of the coil combination was assessed previously (24). 1H MR imaging and 1H saturation for NOE generation was performed with an add-on system for RF shimming, which also performed real-time SAR monitoring on both the 1H and the 31P channels (26,27).
Subjects
Seven healthy volunteers (6 male, 1 female, mean age 30 years, range 21-39 years) participated in this study. The study was conducted in accordance with all guidelines set forth by the approving institutional review board, and signed informed consent was obtained from all subjects before the measurements.
NOE generation
To ensure efficient water saturation for NOE, a wideband alternating-phase low-power technique for zero residual splitting (WALTZ-4, historically used for decoupling) (28) was adopted. The WALTZ-4 train was applied during the full TR, except during the 31P RF pulse and the 204 ms of signal acquisition. The durations of the individual pulses of one WALTZ sub-train were 2.5, 5.0 and 7.5 ms, with 2.5 ms pauses in between segments, requiring 14 WALTZ-4 trains of 90 ms each per repetition time. The minimum power needed to generate constant NOE enhancements for this setting of WALTZ-4 was determined to be 30 Hz in previous phantom experiments (partly presented in (22)).
MR measurement protocol
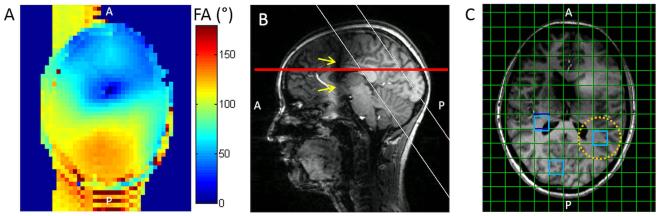
Anatomical images for planning of the 3D 31P MRSI measurements were acquired using a magnetization prepared 3D T1-weighted gradient echo (MPRAGE) sequence. Magnetic field (B0) phasemap shimming was performed to optimize the B0 homogeneity in the posterior region of the brain. 1H transmit RF amplitude (B1+) was maximized in the same region using RF shimming. The amplitude of WALTZ-4 pulses to generate a constant NOE was set to reach at least 30 Hz in the posterior part of the brain by using absolute B1+ maps (fig. 1a). These maps were acquired using a magnetization prepared gradient echo sequence (TR = 5000 ms, TE = 5 ms, magnetization preparation flip = 90°, excitation flip angle = 10°) (29).
Figure 1.
Overview of preparation steps for native and NOE-enhanced 31P MRSI and data analysis in one subject. A: Transversal absolute 1H B1+ map after B1+ calibration to obtain at least 90° flip angles in the posterior part of the brain, allowing minimally 30 Hz NOE power. B: Sagittal MPRAGE image. The location of the absolute 1H B1+ map is indicated by the red line and the slice selection for flip angle calibration of 31P is indicated by the white lines. The yellow arrows indicate signal dropout due to low B1+. C: Transversal MPRAGE images with the spectroscopic grid as an overlay. The voxels indicated in blue were used for data analysis. The approximate real voxel size is indicated by the yellow dotted circle.
Flip angle calibration for 31P was performed using a slice selective pulse acquire sequence (6 ms excitation pulse, TR = 15 s), selecting a 40 mm thick slice through the posterior brain (fig. 1b). The amplitude of the RF pulse was varied between shots to find the maximum magnitude of the phosphocreatine (PCr) peak. This maximum was assumed to correspond to 90° excitation, and all 31P RF amplitudes in the subsequent MRSI sequences were scaled relative to this value.
For the 3D 31P MRSI measurements, a pulse acquire sequence was used with a 300 μs block pulse for excitation (flip angle = 45°) and a TR of 1500 ms. The field of view (FOV) was set to 240 × 240 × 240 mm and the 3D phase encoding matrix to 12 × 12 × 8, creating isotropic dimensions in the transverse plane. The delay between the excitation pulse and the start of data acquisition was 410μs. An elliptical k-space acquisition scheme was applied (1 average) and k-space was Hamming filtered (100%). The nominal voxel size of 20 × 20 × 30 mm is therefore enlarged to a ellipsoid-shaped voxel volume of approximately 38 cm3 (30). The MRSI acquisition of 7 min 48 s was repeated four consecutive times. The first and third MRSI acquisitions were obtained without 1H irradiation (native), the second and the fourth with 1H irradiation to generate the NOE.
Data analysis – voxel selection and spectral fitting
Three non-neighboring MRSI voxels were selected per volunteer (fig. 1c). Care was taken to select only voxels that received a minimum 1H B1+ of 30 Hz in the case of NOE generation, that were situated completely within the brain, and that showed good spectral quality in all four MRSI datasets. Different voxel locations were chosen per volunteer. The average B1+ in each selected voxel was estimated using the absolute B1+ map.
The 31P spectra of the selected voxels were fitted with Metabolite Report, a work in progress package from Siemens Healthcare (Erlangen, Germany). Metabolite Report performs automated, prior knowledge based, complex fitting in the time domain and has previously been applied to 7 T 31P spectroscopy data of the prostate (8). The spectral resonances PE, PC, GPE, GPC, inorganic phosphate (Pi), α-, γ-, β-ATP and NAD+/ NADH were fitted using Gaussian lineshapes, while PCr was fitted with a Lorentzian lineshape. Prior knowledge of the relative peak frequencies and the 31P J-coupling patterns in the ATPs was used. The line widths of PE, PC, GPE, and GPC were assumed to be the same and thus constrained to each other. The quality of the spectral fit was assessed using the Cramér-Rao lower bound (CRLB) values and qualitatively by visual inspection by spectroscopist. The CRLB values of the fits with and without NOE were compared using a paired t-test per metabolite.
Only metabolite fits with a CRLB below 30% were considered reliable. The original spectra and the fitting results were visually inspected, and if a metabolite peak was visually present and its fit was assigned to the correct resonance, giving a minimal residue, the fitting result was accepted. Only metabolites which passed both quality checks were used in further analyses.
The NOE enhancement (η) per metabolite in each selected voxel was calculated by
| (1) |
with representing the mean metabolite peak integral of the two repeated measurements using NOE-enhanced and native MRSI respectively.
Theory of repeatability and method comparison
To be able to answer our research question, we used an approach presented by Bland and Altman to assess the agreement between two methods of clinical measurement (23). The term “agreement” might be misleading here, since a difference between the native and NOE-enhanced method is expected because of the NOE enhancements for most metabolites. However, the agreement between methods, or the lack of it, has two components: the bias, i.e. the mean difference between the methods, and the random variation. The random variation is at least as great as that predicted by the repeatability of each method, but may be larger because of heterogeneity between individual measurement units (e.g. subjects, but in this study individual voxels as well). For correct interpretation of the measure of agreement between methods it is thus essential to first determine the repeatability of each individual method.
Statistical analyses of repeatability and method comparison
Useful measures for the repeatability of a method are the repeatability coefficient (RC) and the coefficient of variation in repeated measurements (CoVrepeat). The RC defines the width of the interval within which we expect the absolute difference between two measurements with the same method to lie in 95% of the cases. It is defined as , where σrepeat is the standard deviation of repeated measurements. Once the RCs are known for two methods, their repeatability intervals (2*RC) can be directly compared to the interval of the 95% limits of agreement (LoA) between the two methods (see below), allowing to assess how much of the observed variation between the methods can be explained by the repeatability of the individual methods (23).
In the present work, σrepeat was estimated for each metabolite and each method (native and NOE-enhanced) by decomposing the total observed variance into the factors “between-subject”, “between-voxel” (or “within-subject”), and “repeat” (or “within-voxel”):
| (2) |
This was done by fitting a 3-level no-predictors linear mixed model to the data of each metabolite and method, in which “subject” and “voxel” were treated as level-3 and level-2 random intercepts, respectively.
The CoVrepeat is a normalized measure of σrepeat to study relative repeatability:
| (3) |
with μ the mean of the metabolite integrals of all voxels and repeated measurements, without or with NOE (μnative and μNOEenh). The native and NOE-enhanced CoVrepeat were determined per metabolite and for metabolite ratios, using standard error propagation methods. The apparent CoVrepeat of NOE-enhanced metabolite intensities corrected for their enhancement (which would be needed for absolute quantification) was calculated using error propagation as well. A relation between quality of the metabolite fits and the repeatabilities of the metabolites was explored by the Pearson correlation between CRLB and COVrepeat.
The agreement between native and NOE-enhanced 31P MRSI was quantified using their 95% limits of agreement (LoA). The interval of 95% LoA is determined by the overall bias between the two methods and the variation around this mean difference, expressed by the standard deviation of the differences (σd):
| (4) |
The LoA were calculated using (the mean integrals of the two repeated measurements per voxel with each method). The overall bias was estimated using
| (5) |
where the brackets denote the average over all voxels. Since we include all data in calculating the agreement, using the two repeated measures for , the observed standard deviation of the differences between pairs of means of repeated measures needs an adaptation to attain the standard deviation of the differences between the methods σd (23):
| (6) |
The 95% LoA and the repeatability intervals were compared for each metabolite. If these ranges are similar, then the (lack of) agreement is fully explained by the (lack of) repeatability. If the range of the 95% LoA is considerably wider than the repeatability intervals of the individual methods, then (some) additional heterogeneity exists between the individual voxels and subjects in NOE enhancement (i.e. NOE would introduce additional signal variation).
The presence of NOE-induced additional signal variation between voxels and subjects was also assessed by variance components analysis of measured NOE enhancements, using the same model as described above (Eq. 2).
The effect of NOE on the peak integrals of individual metabolites was tested for significance using similar 3-level mixed models as above, with “NOE” as an additional fixed factor. To test whether the minimum 1H power criterion of 30 Hz for constant NOE was sufficient in vivo, linear mixed models of the NOE enhancements of the individual metabolites were constructed, which included local B1+ as a fixed covariate. The possible effect of local B1+ on NOE enhancement was assessed using these models. Statistical test outcomes with p-values < 0.05 were considered significant.
Results
All subjects completed the full scan protocol, without having to repeat any measurements. The minimum power condition of 30 Hz for NOE was reached in every subject in the posterior part of the brain (example in figure 1). All measurements could be performed within the simulation-based SAR limits (15W/6 min into the coil), governed by local SAR limits and an additional 40% safety margin. The power on the 1H channel had the highest contribution to the total SAR. High quality spectra were obtained throughout large parts of the brain, but to meet the 30 Hz criterion on 1H, only voxels from the posterior brain region were selected for quantification. The βATP intensity was strongly attenuated or not present because it was outside the effective bandwidth of the excitation pulse and it was therefore excluded from further analysis. The PC and NAD resonances were sometimes only visible as a small shoulder on the right side of respectively PE or αATP. An example of a spectral fit is presented in the supplementary material.
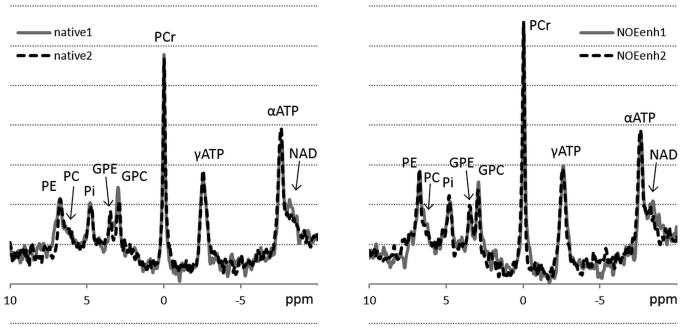
Small variations in spectral patterns between repeated measurements using the same method were observed, and NOE enhancements could also be observed visually (fig. 2). For all metabolites except αATP the quality of the spectral fit was significantly better in the NOE-enhanced 31P MRSI data than in the native 31P MRSI data (table 1). From 378 metabolite signals (7 volunteers * 2 measurements * 3 voxels * 9 signals) in the native 31P MRSI data, 15 metabolite fits had a CRLB above 30% (PC: 2, NAD: 13), while from 378 signals in the NOE-enhanced 31P MRSI data, this occurred 2 times (PC: 1, NAD: 1). None of the metabolite signals with a CRLB below 30% was discarded by visual inspection.
Figure 2.
Example of repeated spectra of the same voxel in one volunteer using native 31P MRSI (left, native) and using NOE-enhanced 31P MRSI (right, NOEenh). Variations between the individual measurements per method are visible, and the NOE-enhanced spectra show a clear increase in signal for several metabolites. PE: phosphoethanolamine, PC: phosphocholine, Pi: inorganic phosphate, GPE: glycerophosphoethanolamine, GPC: glycerophosphocholine, PCr: phosphocreatine, γ- and α-ATP: γ- and α-adenosine triphosphate, NAD: nicotinamide adenine dinucleotide.
Table 1.
NOE enhancements (±, mean ± SD) and quality of the spectral fit expressed in percentage Cramér-Rao Lower Bounds (CRLB, mean ± SD) of native and NOE-enhanced spectra.
| PE | PC | Pi | GPE | GPC | PCr | γATP | αATP | NAD | |
|---|---|---|---|---|---|---|---|---|---|
| η | 0.39±0.22 | 0.19±0.31 | 0.21±0.20 | 0.30±0.22 | 0.36±0.20 | 0.22±0.05 | 0.07±0.07 | 0.00±0.05 | 0.21±0.23 |
|
| |||||||||
|
CRLB
native |
9.4±1.7 | 20.2±5.8 | 13.2±2.6 | 15.0±3.4 | 11.0±2.7 | 3.3±0.8 | 2.4±0.4 | 2.9±0.7 | 26.5±7.4 |
|
| |||||||||
|
CRLB
NOEenh |
6.8±1.0 | 16.7±4.1 | 10.8±1.8 | 11.7±2.6 | 8.1±1.8 | 2.7±0.6 | 2.3±0.3 | 2.8±0.7 | 21.2±5.5 |
|
| |||||||||
| p-value | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | 0.56 | <0.001 |
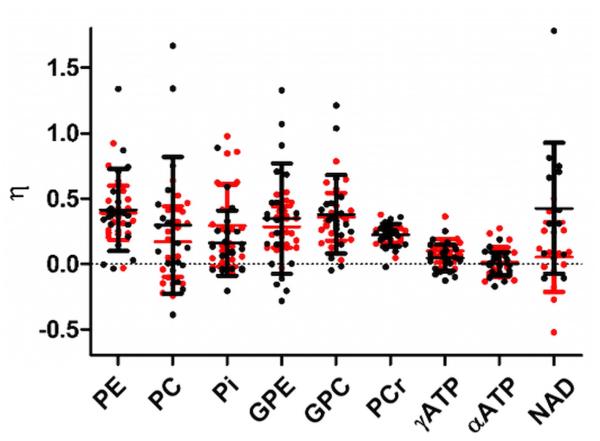
NOE enhancement factors varied between metabolites (table 1, fig. 3), with mean enhancements up to 39%. The fitted 31P metabolite integrals were significantly higher (p≤0.003) using NOE for all metabolites except αATP. PE, PC, Pi, GPE, GPC and NAD showed considerable variation in NOE enhancement between the voxels, but outliers did not result from the same voxels.
Figure 3.
Nuclear Overhauser Effect (NOE) enhancements (η) per voxel of 31P metabolite resonances in human brain at 7 T. NOE enhancement resulting from the first and second set of native and NOE-enhanced measurements are shown in black and red, respectively.
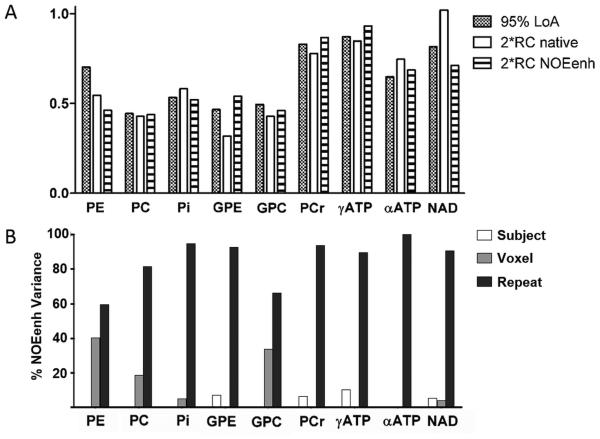
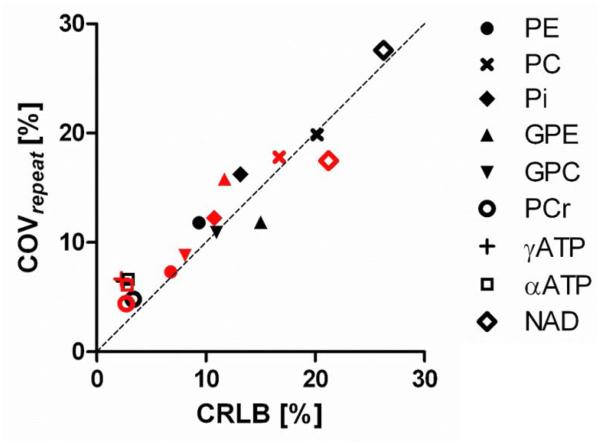
The repeatability coefficients of native and NOE-enhanced 31P MRSI were comparable for all metabolites except for GPE and NAD (fig. 4a). GPE was considerably less repeatable with NOE, whereas NAD was better repeatable with NOE. Because of the signal increase with NOE, the CoVrepeat (relative repeatability within voxels) was better in the NOE-enhanced data for PE, PC, Pi, GPC, PCr, and NAD (table 2, fig. 4b). Similarly, the relative repeatability of metabolite ratios containing these metabolites improved with NOE (table 2). In general, CoVrepeat was best for PCr, γATP and αATP, i.e. metabolites with the highest signal integrals and smallest fitting errors (table 2). Correcting NOE-enhanced metabolite intensities for their enhancement factors for absolute quantification purposes causes larger variation in all metabolites compared to the native or NOE-enhanced data (table 2). A strong correlation existed between mean CRLB and COVrepeat (R2=0.91, p<0.0001, fig. 5).
Figure 4.
A: 95% Limits of agreement (LoA) between the two methods and repeatability intervals (2*RC) of each method. B: Variance components in NOE enhancement.
Table 2.
Coefficients of Variation in repeated measurement (CoVrepeat) of native and NOE-enhanced data per metabolite and for metabolite ratios, as well as the apparent CoVrepeat of NOE-enhanced metabolite intensities corrected for their enhancement.
| CoVrepeat | ||||||
|---|---|---|---|---|---|---|
| Native | NOEenh | Corrected | Native | NOEenh | ||
| PE | 11,8% | 7,3% | 17.4% | PE/PC | 23,1% | 19,3% |
|
| ||||||
| PC | 19,9% | 17,8% | 31.6% | GPE/GPC | 16,1% | 18,1% |
|
| ||||||
| Pi | 16,2% | 12,2% | 20.6% | PE/PCr | 12,8% | 8,5% |
|
| ||||||
| GPE | 11,9% | 15,8% | 23.3% | Pi/PCr | 16,9% | 13,0% |
|
| ||||||
| GPC | 10,9% | 8,8% | 17.0% | (PE+PC)/PCr | 23,2% | 17,0% |
|
| ||||||
| PCr | 4,8% | 4,4% | 6.2% | PCr/ γATP | 8,1% | 8,0% |
|
| ||||||
| γATP | 6,5% | 6,7% | 9.5% | NAD/ αATP | 28,4% | 18,5% |
|
| ||||||
| αATP | 6,6% | 6,1% | 7.8% | |||
|
| ||||||
| NAD | 27,6% | 17,5% | 25.7% | |||
Figure 5.
Relation between fitting error of the metabolites (expressed in percentage Crémer-Rao Lower Bound, CRLB, average across subjects) and the relative repeatability (expressed in the coefficient of variation in repeated measurements, COVrepeat) of the metabolites. Black symbols: native. Red symbols: NOE-enhanced.
The agreement between native and NOE-enhanced 31P MRSI, expressed by the 95% LoA, was in the range of the repeatability intervals of the individual methods for all metabolites except PE (fig. 4a). The variation in NOE enhancements between subjects was dominated by the “repeat” component for all metabolites (mean 85%, minimum 60% of total variance for PE, fig. 4b). Between-subjects variations were negligible (mean 3%, maximum 10% of total variance), while between-voxel differences explained a noticeable fraction of the variance only for PE (40%), GPC (34%) and PC (19%). Absolute 1H B1+ (≥30 Hz) was not a significant predictor for NOE enhancement levels (p>0.11) for any of the metabolites except PC (p=0.04). For the latter, 1H B1+ only partially explained the between-voxel variations: controlling for this factor still yielded unexplained between-voxel variations of 10% of the total variation.
Discussion
This study was performed to answer the question “should we use NOE in 31P MRS(I) or not?”. To a great extent, this question can be answered with yes, but we will discuss several aspects which should be taken into consideration in deciding upon using NOE or not.
Significantly higher 31P signals were measured using NOE for most metabolites, while none of the metabolites showed a (significant) decrease in signal. Moreover, the signal enhancement significantly improved fitting quality of the metabolite resonances with a positive NOE enhancement. These favorable aspects of NOE at 7T have been reported before (19,22) and especially the first reason has historically led to the use of NOE in in vivo studies. However, when quantifying NOE enhancements per metabolite by using native and NOE-enhanced 31P MRSI data, considerable variation in enhancement values was observed in this study, reproducing findings in many previous studies of different tissues at different field strengths (16–19,21,22). This variation may indicate lack of agreement between both methods, and in unreplicated studies this can be interpreted as NOE adding variability to the data. However, it might also be caused by poor repeatability of (one of) the methods. The design of this study allowed us to put this variation into the perspective of the repeatability of the individual methods. For a good repeatability study, all measurements need to be performed with the same system and method, within a short period of time. This is in contrast to reproducibility studies, in which the variation in measurements is observed under changing conditions (31). Our hypothesis was that variation in NOE enhancement values is caused by the limited repeatability of the underlying methods (native and NOE-enhanced 31P MRSI). The measurement uncertainties of the 31P metabolite values will propagate in the calculation of the NOE enhancement.
The similarity of the 95% LoA of both methods and the 95% repeatability intervals of each method as well as the dominance of the factor repeat in the NOE enhancement confirmed our hypothesis. The metabolites PC, Pi, GPE, GPC and NAD showed considerable variation in NOE enhancements, but this apparent lack of agreement between the methods could be traced back to the repeatabilities of both methods for these metabolites. It depends upon the application of the method what relative repeatability can be considered sufficient, but for all these metabolites the CoVrepeat was above 10% with native 31P MRSI, and improved for all but GPE with NOE. Similarly, the CoVrepeat of ratios containing these metabolites improved with NOE. GPE showed both a higher absolute and relative repeatability with NOE, although a NOE enhancement of 30.1% was observed. We suspect this finding to be incidental, resulting from the limited sample size and low SNR of this metabolite. NOE enhancements are known to be dependent on dipolar relaxation time (15), which might be affected by pH or temperature, but all circumstances were kept the same between the repeated measurements, so it is unlikely that the dipolar relaxation time has changed within a voxel between the two repeated NOE-enhanced 31P MRSI measurements. The relative repeatability of metabolites with endogenously higher signal intensities and smaller fitting errors was better (PCr, γATP and αATP) compared to the metabolites with lower signal intensities, even though their absolute repeatability was slightly worse. These metabolites also showed the smallest variation in their NOE enhancement values. The strong linear correlation between quality of fit, expressed by the CRLB, and the relative repeatability indicated that CRLB values present a good measure of relative repeatability of 31P MRSI.
PE, and to a smaller extent PC and GPC, showed an improvement in relative repeatability with NOE, but both the Bland-Altman based analysis and the decomposition of variance in NOE enhancements indicated the presence of additional heterogeneity between voxels next to repeatability variations. We tested and excluded local 1H B1+ to be the reason for additional variation with NOE. A different reason for additional variation between voxels with NOE could be spatial differences in dipolar interaction between the 1H and 31P spins related to tissue type. Because of the large true voxel size in this study, selected voxels contained a mixture of gray and white matter, and they were sometimes also contaminated with skull tissue or cerebral fluid. The diffuse border of the voxels (30,32) precluded analysis of the exact tissue distribution within the spectroscopic voxels by segmentation. To study the possible effect of tissue type on NOE enhancements would require a much higher spatial resolution and thus considerably longer acquisition times.
The effect of proton decoupling on the repeatability of 31P MRSI, solely or in combination with NOE, was not investigated in this study. The beneficial effect of proton decoupling decreases with field strength, and it adds considerably to the SAR burden. In this 7 T study we opted to saturate the water resonance to generate the NOE with a strategy well-known from decoupling; the WALTZ-4 irradiation scheme. This irradiation scheme provides a relatively broadband saturation, which reduces the sensitivity of water saturation to local B0 inhomogeneities. At clinical field strengths one could also use continuous wave irradiation to saturate the water resonance. Since the power needed for water saturation with WALTZ-4 contributed significantly to the SAR in our study, it would be of interest to determine if a lower 1H B1+ would be sufficient for in vivo experiments compared to phantom experiments.
In the current study, we homogenized B0 and 1H B1+ in the posterior part of the brain only, whereas for clinical studies homogenization over the whole brain is probably more often required. At clinical field strengths the 1H B1+ and B0 distributions are intrinsically more homogeneous than at 7 T, facilitating this prerequisite. Other coil configurations or parallel transmit techniques combined with tailored RF pulses may also increase the size of the 1H region that can be excited homogeneously at 7 T (33). Automatic scanner adjustments of clinical MR scanners obviate the need to calibrate the 1H pulse amplitudes for NOE, which was a relative time-consuming procedure in this study. The minimum 1H B1+ for constant NOE using a specific saturation strategy should however be determined in a pre-study. In cases where non-uniformities in the 1H RF field are expected, e.g. when using 1H surface coils or at ultra-high field strengths, careful examination of the field distribution and amplitude is needed before employing NOE. The necessity of 31P flip angle calibration in every single subject has to be assessed in a larger cohort. In the 7 subjects within this study, the 31P B1+ was very similar. It is very common to use a pre-determined, constant B1+ for multinuclear experiments at clinical field strengths, or a pre-determined fixed multiplication factor between the X-nucleus and the automated 1H B1+ calibration, because of the lack of automated procedures for multinuclear coils.
The results of this 31P MRSI study in the brain at 7T can guide human 31P MRS(I) investigations of the brain and of other tissues including those performed at different field strengths. The improvement of relative repeatability of 31P metabolites with positive NOE enhancements allows detection of smaller differences in metabolite intensities or ratios. This is of interest in follow-up studies or in the comparison of groups (e.g. healthy vs. patient). NOE-enhanced metabolite ratios obviously differ from native ratios, but they are more repeatable. When reporting NOE-enhanced metabolite ratios, it is of importance to clearly describe the way NOE is generated. This allows others to perform similar experiments, thus enabling between-site comparisons. When absolute quantification of 31P metabolite concentrations is preferred, it has to be realized that data should be corrected for the NOE enhancement, which introduces an additional source of variation to the quantification. Although it is known that NOE enhancements can vary between tissues (14,16–22), it remains uncertain if variations within an organ can exist as well. As discussed above we found an indication that this might be the case for NOE enhancements of PE, PC and GPC. It is similarly unknown if NOE is different in diseased tissue compared to normal tissue or during activation (exercise), or if there are changes in NOE with age. In diseased tissue, T1 relaxation times of 31P may change (34), which may have an effect on the absolute NOE enhancements. A larger repeatability study with a more heterogeneous study population, including patients, would be needed to confirm if the above-mentioned possible differences in NOE can be distinguished from the measurement uncertainties of native and NOE-enhanced 31P MRSI. It would also be of interest to study reproducibility of NOE enhanced 31P MR data by measuring subjects on different days (20,35). This allows calculation of sample sizes needed to reveal significant differences in certain metabolite ratios for paired (e.g. response to therapy) and independent (e.g. patient vs. control) studies.
In conclusion, the signal enhancement generated by NOE improved relative repeatability of brain 31P MRSI in healthy volunteers. Variations in NOE enhancements per metabolite could almost completely be explained by the repeatability of native and NOE-enhanced 31P MRSI. For these reasons, the use of NOE-enhanced 31P MRSI is encouraged.
Supplementary Material
Acknowledgement
We thank Arend Heerschap for constructive comments and Elisabeth Weiland from Siemens Healthcare for providing Metabolite Report.
Research grant: This work was supported by grant number 243115 from the European Research Council under the European Community’s Seventh Framework Programme (FP7/2007-2013). The work was partially supported by the intramural research programs of the NIMH and NINDS, NIH.
Abbreviations
- ATP
adenosine triphosphate
- B0
magnetic field
- B1+
transmit radiofrequency amplitude
- CoV
coefficient of variation
- CRLB
Cramér-Rao lower bound
mean bias between native and NOE-enhanced measurements
- η
NOE enhancement
- GPC
glycerophosphocholine
- GPE
glycerophosphoethanolamine
- LoA
limits of agreement
- NAD
nicotinamide adenine dinucleotide
mean integral of non-enhanced repeated measurements per voxel
- NOE
nuclear Overhauser effect
mean integral of NOE-enhanced repeated measurements per voxel
- MRSI
magnetic resonance spectroscopic imaging
- μ
mean metabolite integral over all voxels and measurements
- PC
phosphocholine
- PCr
phosphocreatine
- PE
phosphoethanolamine
- Pi
inorganic phosphate
- RC
repeatability coefficient
- RF
radiofrequency
- SAR
specific absorption rate
- σd
standard deviation of differences between methods
- σrepeat
standard deviation of repeated measurements
- SNR
signal-to-noise ratio
- WALTZ
wideband alternating-phase low-power technique for zero residual splitting
References
- 1.Solga SF, Horska A, Clark JM, Diehl AM. Hepatic 31P magnetic resonance spectroscopy: a hepatologist’s user guide. Liver Int. 2005;25:490–500. doi: 10.1111/j.1478-3231.2005.01131.x. [DOI] [PubMed] [Google Scholar]
- 2.Ter Voert EGW, Heijmen L, van Laarhoven HWM, Heerschap A. In vivo magnetic resonance spectroscopy of liver tumors and metastases. World J. Gastroenterol. 2011;17:5133–5149. doi: 10.3748/wjg.v17.i47.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Argov Z, Löfberg M, Arnold DL. Insights into muscle diseases gained by phosphorus magnetic resonance spectroscopy. Muscle Nerve. 2000;23:1316–1334. doi: 10.1002/1097-4598(200009)23:9<1316::aid-mus2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 4.Kemp GJ, Meyerspeer M, Moser E. Absolute quantification of phosphorus metabolite concentrations in human muscle in vivo by 31P MRS: a quantitative review. NMR Biomed. 2007;20:555–565. doi: 10.1002/nbm.1192. [DOI] [PubMed] [Google Scholar]
- 5.Hudsmith LE, Neubauer S. Magnetic resonance spectroscopy in myocardial disease. JACC Cardiovasc. Imaging. 2009;2:87–96. doi: 10.1016/j.jcmg.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Martin WRW. MR spectroscopy in neurodegenerative disease. Mol. Imaging Biol. 2007;9:196–203. doi: 10.1007/s11307-007-0087-2. [DOI] [PubMed] [Google Scholar]
- 7.Andrade CS, Otaduy MCG, Park EJ, Leite CC. Phosphorus-31 MR Spectroscopy of the Human Brain: Technical Aspects and Biomedical Applications. Int. J. Curr. Res. Rev. 2014;6:41–57. [Google Scholar]
- 8.Lagemaat MW, Vos EK, Maas MC, Bitz AK, Orzada S, van Uden MJ, Kobus T, Heerschap A, Scheenen TWJ. Phosphorus magnetic resonance spectroscopic imaging at 7 T in patients with prostate cancer. Invest. Radiol. 2014;49:363–372. doi: 10.1097/RLI.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 9.Sohlberg S, Wikström A-K, Olovsson M, Lindgren P, Axelsson O, Mulic-Lutvica A, Weis J, Wikström J. In vivo 31P-MR spectroscopy in normal pregnancy, early and late preeclampsia: A study of placental metabolism. Placenta. 2014;35:318–323. doi: 10.1016/j.placenta.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Stehouwer BL, van der Kemp WJM, Luijten PR, van den Bosch MAAJ, Veldhuis WB, Wijnen JP, Klomp DWJ. 31P magnetic resonance spectroscopy of the breast and the influence of the menstrual cycle. Breast Cancer Res. Treat. 2014;144:583–589. doi: 10.1007/s10549-014-2889-7. [DOI] [PubMed] [Google Scholar]
- 11.Luyten PR, Bruntink G, Sloff FM, Vermeulen JW, van der Heijden JI, den Hollander JA, Heerschap A. Broadband proton decoupling in human 31P NMR spectroscopy. NMR Biomed. 1989;1:177–183. doi: 10.1002/nbm.1940010405. [DOI] [PubMed] [Google Scholar]
- 12.Bachert-Baumann P, Ermark F, Zabel H-J, Sauter R, Semmler W, Lorenz WJ. In vivo nuclear overhauser effect in 31P- 1H double-resonance experiments in a 1.5-source whole-body MR system. Magn. Reson. Med. 1990;15:165–172. doi: 10.1002/mrm.1910150119. [DOI] [PubMed] [Google Scholar]
- 13.Freeman DM, Hurd R. Decoupling: theory and practice II. Sate of the art: in vivo applications of decoupling. NMR Biomed. 1997;10:381–393. doi: 10.1002/(sici)1099-1492(199712)10:8<381::aid-nbm495>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Brown TR, Stoyanova R, Greenberg T, Srinivasan R, Murphy-Boesch J. NOE Enhancements and T1 Relaxation Times of Phosphorylated Metabolites in Human Calf Muscle at 1.5 Tesla. Magn. Reson. Med. 1995;33:417–421. doi: 10.1002/mrm.1910330316. [DOI] [PubMed] [Google Scholar]
- 15.Mathur-De Vré R, Maerschalk C, Delporte C. Spin-lattice relaxation times and nuclear Overhauser enhancement effect for 31P metabolites in model solutions at two frequencies: implications for in vivo spectroscopy. Magn. Reson. Imaging. 1990;8:691–698. doi: 10.1016/0730-725x(90)90003-k. [DOI] [PubMed] [Google Scholar]
- 16.Bottomley PA, Hardy CJ. Proton Overhauser enhancements in human cardiac phosphorus NMR spectroscopy at 1.5 T. Magn. Reson. Med. 1992;24:384–390. doi: 10.1002/mrm.1910240220. [DOI] [PubMed] [Google Scholar]
- 17.Murphy-Boesch J, Stoyanova R, Srinivasan R, Willard T, Vigneron D, Nelson S, Taylor JS, Brown TR. Proton-decoupled 31P chemical shift imaging of the human brain in normal volunteers. NMR Biomed. 1993;6:173–180. doi: 10.1002/nbm.1940060302. [DOI] [PubMed] [Google Scholar]
- 18.Li CW, Negendank WG, Murphy-Boesch J, Padavic-Shaller K, Brown TR. Molar Quantitation of Hepatic Metabolites In Vivo in Proton-decoupled, Nuclear Overhauser Effect Enhanced 31P NMR Spectra Localized by Three-dimensional Chemical Shift Imaging. NMR Biomed. 1996;9:141–155. doi: 10.1002/(SICI)1099-1492(199606)9:4<141::AID-NBM403>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 19.Lei H, Zhu XH, Zhang XL, Ugurbil K, Chen W. In vivo 31P magnetic resonance spectroscopy of human brain at 7 T: an initial experience. Magn. Reson. Med. 2003;49:199–205. doi: 10.1002/mrm.10379. [DOI] [PubMed] [Google Scholar]
- 20.Tyler DJ, Emmanuel Y, Cochlin LE, Hudsmith LE, Holloway CJ, Neubauer S, Clarke K, Robson MD. Reproducibility of 31P cardiac magnetic resonance spectroscopy at 3 T. NMR Biomed. 2009;22:405–413. doi: 10.1002/nbm.1350. [DOI] [PubMed] [Google Scholar]
- 21.Wylezinska M, Cobbold JFL, Fitzpatrick J, McPhail MJW, Crossey MME, Thomas HC, Hajnal JV, Vennart W, Cox IJ, Taylor-Robinson SD. A comparison of single-voxel clinical in vivo hepatic 31P MR spectra acquired at 1.5 and 3.0 Tesla in health and diseased states. NMR Biomed. 2011;24:231–237. doi: 10.1002/nbm.1578. [DOI] [PubMed] [Google Scholar]
- 22.Lagemaat MW, Maas MC, Vos EK, Bitz AK, Orzada S, Weiland E, van Uden MJ, Kobus T, Heerschap A, Scheenen TWJ. 31P MR spectroscopic imaging of the human prostate at 7 T: T1 relaxation times, Nuclear Overhauser Effect, and spectral characterization. Magn. Reson. Med. 2014;73:909–920. doi: 10.1002/mrm.25209. [DOI] [PubMed] [Google Scholar]
- 23.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 24.Van de Bank B, Orzada S, Lagemaat MW, Bitz AK, Scheenen TWJ. Proceedings of the 22st Annual Meeting of ISMRM. Milan, Italy: 2014. 31P Birdcage Insert for an 8-Channel, Multi-Transmit, 1H Coil at 7T; p. 4810. [Google Scholar]
- 25.Orzada S, Kraff O, Schäfer LC, Brote I, Bahr A, Bolz T, Maderwald S, Ladd ME, Bitz AK. Proceedings of the 17th Annual Meeting of ISMRM. Honolulu, Hawaii, USA: 2009. 8-Channel Transmit/receive Head Coil for 7 T Human Imaging Using Intrinsically Decoupled Strip Line Elements with Meanders; p. 2999. [Google Scholar]
- 26.Bitz AK, Brote I, Orzada S, Kraff O, Maderwald S, Quick HH, Yazdanbakhsh P, Solbach K, Bahr A, Bolz T, Wicklow K, Schmitt F, Ladd ME. Proceedings of the 17th Annual Meeting of ISMRM. Honolulu, Hawaii, USA: 2009. An 8-channel add-on RF shimming system for whole-body 7 Tesla MRI including real-time SAR monitoring; p. 4767. [Google Scholar]
- 27.Kobus T, Bitz AK, van Uden MJ, Lagemaat MW, Rothgang E, Orzada S, Heerschap A, Scheenen TWJ. In vivo 31P MR spectroscopic imaging of the human prostate at 7 T: Safety and feasibility. Magn. Reson. Med. 2012;68:1683–1695. doi: 10.1002/mrm.24175. [DOI] [PubMed] [Google Scholar]
- 28.Shaka A, Keeler J, Freeman R. Evaluation of a new broadband decoupling sequence: WALTZ-16. J. Magn. Reson. 1969. 1983;53:313–340. [Google Scholar]
- 29.Fautz HP, Vogel MW, Gross P, Kerr AB, Zhu Y. Proceedings of the 16th Annual Meeting of ISMRM. Toronto, Ontario, Canada: 2008. B1 mapping of coil arrays for parallel transmission; p. 1247. [Google Scholar]
- 30.Scheenen TWJ, Klomp DWJ, Röll SA, Fütterer JJ, Barentsz JO, Heerschap A. Fast acquisition-weighted three-dimensional proton MR spectroscopic imaging of the human prostate. Magn. Reson. Med. 2004;52:80–88. doi: 10.1002/mrm.20103. [DOI] [PubMed] [Google Scholar]
- 31.Bartlett JW, Frost C. Reliability, repeatability and reproducibility: analysis of measurement errors in continuous variables. Ultrasound Obstet. Gynecol. 2008;31:466–475. doi: 10.1002/uog.5256. [DOI] [PubMed] [Google Scholar]
- 32.Vikhoff-Baaz B, Starck G, Ljungberg M, Lagerstrand K, Forssell-Aronsson E, Ekholm S. Effects of k-space filtering and image interpolation on image fidelity in 1H MRSI. Magn. Reson. Imaging. 2001;19:1227–1234. doi: 10.1016/s0730-725x(01)00456-8. [DOI] [PubMed] [Google Scholar]
- 33.Cloos MA, Boulant N, Luong M, Ferrand G, Giacomini E, Le Bihan D, Amadon A. kT -points: short three-dimensional tailored RF pulses for flip-angle homogenization over an extended volume. Magn. Reson. Med. 2012;67:72–80. doi: 10.1002/mrm.22978. [DOI] [PubMed] [Google Scholar]
- 34.Remy C, Albrand JP, Benabid AL, Decorps M, Jacrot M, Riondel J, Foray MF. In vivo 31P nuclear magnetic resonance studies of T1 and T2 relaxation times in rat brain and in rat brain tumors implanted to nude mice. Magn. Reson. Med. 1987;4:144–152. doi: 10.1002/mrm.1910040207. [DOI] [PubMed] [Google Scholar]
- 35.Edwards LM, Tyler DJ, Kemp GJ, Dwyer RM, Johnson A, Holloway CJ, Nevill AM, Clarke K. The reproducibility of 31-phosphorus MRS measures of muscle energetics at 3 Tesla in trained men. PloS One. 2012;7:e37237. doi: 10.1371/journal.pone.0037237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.