ABSTRACT
Background
Although mortality associated with atrial fibrillation (AF) has been reported to decrease over prior decades, the mortality risk of asymptomatic, nonhospitalized AF has not been examined.
Hypothesis
Asymptomatic, nonhospitalized AF is associated with an increased risk of death.
Methods
This analysis included 25 976 participants (mean age, 65 ± 9.4 years; 55% female; 38% black) from the Reasons for Geographic And Racial Differences (REGARDS) study. Atrial fibrillation was detected on the baseline electrocardiogram (ECG AF) or by self‐reported history. Atrial fibrillation unawareness was defined as present if ECG evidence of the arrhythmia was detected but no self‐reported history was reported. All‐cause mortality was confirmed during follow‐up through March 31, 2014.
Results
A total of 2208 (8.5%) participants had AF at baseline (ECG: n = 371/17%; self‐reported: n = 1837/83%). Over a median follow‐up of 7.6 years, 3481 deaths occurred. In a multivariable Cox regression model, AF was associated with a 32% increased risk of mortality (95% confidence interval [CI]: 1.19‐1.46). Risk of death was higher among those with ECG AF (hazard ratio: 1.71, 95% CI: 1.42‐2.07) compared with self‐reported cases (hazard ratio: 1.15, 95% CI: 1.03‐1.29). Those who were unaware of their AF diagnosis had a 94% increased risk of death (95% CI: 1.50‐2.52) compared with AF participants who were aware of their diagnosis.
Conclusions
Asymptomatic, nonhospitalized AF is associated with an increased risk of mortality in the general population. Mortality is higher in those with ECG‐confirmed cases and among those who are unaware of their diagnosis.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia encountered in clinical practice, affecting nearly 3 million Americans.1 Risk factors for AF include diabetes mellitus (DM), hypertension, smoking, and coronary heart disease (CHD).2, 3 The importance of AF as a public health problem is related not only to the arrhythmia itself, but also its associations with several adverse cardiovascular outcomes, including stroke, heart failure (HF), and myocardial infarction (MI).4, 5, 6
Several population‐based studies have shown that AF carries an increased mortality risk.7, 8, 9, 10, 11, 12 This increased mortality risk has been attributed to comorbid conditions (eg, HF and stroke) that often accompany AF.10 This is supported by data showing that the mortality risk associated with AF is dramatically decreased after the first year of diagnosis.11 However, these reports have been limited in racial diversity, as the populations examined were largely Caucasian.10, 11, 12 Additionally, prior studies ascertained AF events by self‐report with subsequent medical review,10 and from hospitalization data.11 Thus, the mortality risk of asymptomatic, nonhospitalized AF from racially diverse registries is not well established. Therefore, the purpose of this analysis was to examine the association between AF and all‐cause mortality in the Reasons for Geographic And Racial Differences in Stroke (REGARDS) study, a racially diverse population‐based cohort study.
Methods
Study Population and Design
Details of REGARDS have been published previously.13 Briefly, REGARDS was designed to identify causes of regional and racial disparities in stroke mortality. The study oversampled blacks and residents of the stroke belt (North Carolina, South Carolina, Georgia, Alabama, Mississippi, Tennessee, Arkansas, and Louisiana) between January 2003 and October 2007. A total of 30 239 participants were recruited from a commercially available list of residents using postal mailings and telephone data. Demographic information and medical histories were obtained using a computer‐assisted telephone interview system that was conducted by trained interviewers. Additionally, a brief in‐home physical examination was performed 3 to 4 weeks after the telephone interview. During the in‐home visit, trained staff collected information regarding medications, blood and urine samples, and a resting electrocardiogram (ECG). The study was approved by the institutional review boards at all participating universities, and all participants provided written informed consent.
For the purpose of this analysis, participants were excluded with data anomalies (n = 56), missing follow‐up data (n = 490), missing AF data (n = 691), and missing baseline characteristics (n = 3026). A total of 25 976 participants (mean age, 65 ± 9.4 years; 55% female; 38% black) remained and were included in the final analysis.
Atrial Fibrillation
Atrial fibrillation was identified in study participants at baseline by the scheduled ECG and also from self‐reported history of a physician diagnosis during the computer‐assisted telephone interview surveys. The ECGs were read and coded at a central reading center by electrocardiographers who were blind to other REGARDS data. Self‐reported AF was defined as an affirmative response to the following question: “Has a physician or a health professional ever told you that you had atrial fibrillation?”14
Mortality
All‐cause mortality was assessed by semiannual telephone follow‐up and contact with proxies provided by the participant on recruitment. Subsequently, the date of death was confirmed through linkage with the Social Security and National Death Index or by death certificates. Mortality data were complete through March 31, 2014.
Covariates
Age, sex, race, income, education, and smoking status were self‐reported. Annual household income was dichotomized at $20 000. Education was categorized into “high school or less” or “some college or more.” Smoking was defined as ever (eg, current and former) or never smoker. Fasting blood samples were obtained and assayed for serum glucose, total cholesterol (TC), and high‐density lipoprotein cholesterol (HDL‐C). Diabetes mellitus was defined as a fasting glucose level ≥126 mg/dL (or a nonfasting glucose ≥200 mg/dL among those failing to fast) or by self‐reported use of DM medications. The current use of aspirin, antihypertensive medications, and lipid‐lowering therapies was self‐reported. The use of warfarin, β‐blockers, nondihydropyridine calcium channel blockers, digoxin, and other antiarrhythmic agents (class I and III) was ascertained during the in‐home visit by pill‐bottle review. After the participant rested for 5 minutes in a seated position, blood pressure was measured using a sphygmomanometer. Two values were obtained following a standardized protocol and averaged. Body mass index (BMI) was computed as the weight in kilograms divided by the square of the height in meters. Weight and height were measured during the in‐home examination. Using baseline ECG data, left ventricular hypertrophy (LVH) was defined by the Sokolow‐Lyon criteria.15 Coronary heart disease was ascertained by self‐reported history of MI, coronary artery bypass grafting, coronary angioplasty or stenting, or if evidence of prior MI was present on the baseline ECG. Prior stroke and transient ischemic attack (TIA) were ascertained by participant self‐reported history. Heart failure was defined as present if participants reported the use of ≥1 pillow to sleep at night or nighttime awakening due to trouble breathing. Additionally, the above data were used to calculate the CHADS2 score (1 point each for HF, hypertension, age ≥75 years, and DM; 2 points for stroke/TIA history).16
Statistical Analysis
Categorical variables were reported as frequency and percentages, and continuous variables were reported as mean ± SD. Statistical significance of differences for categorical variables was tested using the χ2 method and the Wilcoxon rank‐sum procedure for continuous variables. Incidence rates per 1000 person‐years for all‐cause mortality were calculated for those with and without AF. Kaplan‐Meier estimates were used to compute the cumulative incidence of all‐cause mortality by AF status and the differences in estimates were compared using the log‐rank procedure.17 Follow‐up time was defined as the time from the in‐home visit until death, loss to follow‐up, or end of follow‐up (March 31, 2014). Cox regression was used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between AF and all‐cause mortality. Multivariable models included the following covariates: Model 1 adjusted for age, sex, race, education, income, and geographic region; Model 2 adjusted for Model 1 covariates with the addition of systolic blood pressure (SBP), HDL‐C, TC, BMI, smoking, DM, antihypertensive medications, lipid‐lowering medications, warfarin, LVH, CHD, stroke/TIA, and HF. Additionally, subgroup analyses were performed to evaluate effect modification by age (dichotomized at 75 years), sex, and race.
Several secondary analyses were performed. To examine whether the mortality risk differs between ECG AF and self‐reported cases, we constructed separate Cox models limited to each exposure. We also constructed multivariable Cox models using Model 2 covariates to identify predictors of death in AF and included medications (eg, β‐blockers, calcium channel blockers, digoxin, antiarrhythmic medications) and the CHADS2 score as predictors. Due to differences reported in all‐cause mortality in those with paroxysmal compared with other cases of AF,10 we examined predictors in those with ECG‐confirmed cases and self‐reported AF separately. Additionally, we were able to determine if a higher mortality risk existed in those who were unaware of their AF diagnosis (ie, present on the baseline ECG but no self‐reported history) compared with AF participants who were aware of their diagnosis (ie, self‐reported history). The proportional hazards assumption was not violated in our analyses. Statistical significance for all comparisons including interactions was defined as P < 0.05. SAS version 9.3 (SAS Institute, Inc., Cary, NC) was used for all analyses.
Results
Baseline Characteristics
A total of 2208 (8.5%) participants had AF at baseline (ECG: n = 371/17%; self‐reported: n = 1837/83%). Participants with AF were more likely to be older, white, and to have a history of smoking, DM, CHD, stroke/TIA, HF, lower educational attainment, and lower income than those without AF. Also, those with AF were more likely to have higher values for SBP and lower values for TC and HDL‐C, and were more likely to report taking aspirin, antihypertensive medications, lipid‐lowering therapies, warfarin, β‐blockers, nondihydropyridine calcium channel blockers, digoxin, and antiarrhythmic medications compared with those without AF. Baseline characteristics for the study population are shown in Table 1.
Table 1.
Baseline Characteristics Stratified by AF (N = 25 976)
| Characteristic | No AF, n = 23 768 | AF, n = 2208 | P Valuea | ECG AF, n = 371 | Self‐Reported AF, n = 1837 | P Valuea |
|---|---|---|---|---|---|---|
| Age, y, mean (SD) | 65 (9.3) | 68 (9.7) | <0.001 | 74 (7.9) | 66 (9.5) | <0.001 |
| Male sex | 10 790 (45) | 1030 (47) | 0.26 | 269 (73) | 761 (41) | <0.001 |
| Black race | 9691 (41) | 780 (35) | <0.001 | 68 (18) | 712 (39) | <0.001 |
| Region | 0.13 | 0.0042 | ||||
| Stroke Buckle | 4988 (21) | 504 (23) | 69 (19) | 435 (24) | ||
| Stroke Belt | 8242 (35) | 753 (34) | 114 (30) | 639 (35) | ||
| Nonbelt | 10 538 (44) | 951 (43) | 188 (51) | 763 (42) | ||
| Education, high school or less | 8912 (38) | 923 (42) | <0.001 | 146 (39) | 777 (42) | 0.29 |
| Annual income, <$20 000 | 4008 (17) | 482 (22) | <0.001 | 62 (17) | 420 (23) | 0.0089 |
| Ever smoker | 12 846 (54) | 1295 (59) | <0.001 | 219 (59) | 1076 (59) | 0.87 |
| DM | 4868 (20) | 559 (25) | <0.001 | 90 (24) | 469 (26) | 0.61 |
| SBP, mm Hg, mean (SD) | 127 (16) | 128 (18) | 0.016 | 126 (16) | 129 (18) | 0.0078 |
| BMI, kg/m2, mean (SD) | 29 (6.1) | 29 (6.5) | 0.59 | 29 (5.9) | 30 (6.6) | 0.0082 |
| TC, mg/dL, mean (SD) | 192 (40) | 185 (41) | <0.001 | 175 (41) | 187 (41) | <0.001 |
| HDL‐C, mg/dL, mean (SD) | 52 (16) | 50 (16) | <0.001 | 46 (15) | 51 (17) | <0.001 |
| ASA | 10 211 (43) | 1125 (51) | <0.001 | 118 (32) | 1007 (55) | <0.001 |
| Antihypertensive medications | 12 350 (52) | 1454 (66) | <0.001 | 216 (58) | 1238 (67) | <0.001 |
| Lipid‐lowering medications | 7732 (33) | 931 (42) | <0.001 | 129 (35) | 802 (44) | 0.0016 |
| Warfarin | 434 (1.8) | 484 (22) | <0.001 | 249 (67) | 235 (13) | <0.001 |
| β‐Blockers | 4910 (21) | 955 (43) | <0.001 | 173 (47) | 782 (43) | 0.15 |
| Nondihydropyridine CCBs | 1353 (5.7) | 274 (12) | <0.001 | 55 (15) | 219 (12) | 0.12 |
| Digoxin | 302 (1.3) | 350 (16) | <0.001 | 147 (40) | 203 (11) | <0.001 |
| Antiarrhythmic medications | 82 (0.4) | 170 (7.7) | <0.001 | 19 (5.1) | 151 (8.2) | 0.041 |
| LVH | 2330 (9.8) | 235 (11) | 0.21 | 19 (5.1) | 216 (12) | <0.001 |
| CHD | 3745 (16) | 807 (37) | <0.001 | 141 (38) | 666 (36) | 0.52 |
| Stroke/TIA | 2128 (9.0) | 384 (17) | <0.001 | 67 (18) | 317 (17) | 0.71 |
| HF | 3302 (14) | 666 (30) | <0.001 | 76 (20) | 590 (32) | <0.001 |
| CHADS2 score | 0.36 | |||||
| 0 | 339 (15) | 48 (13) | 291 (16) | |||
| 1 | 640 (29) | 109 (29) | 531 (29) | |||
| ≥2 | 1229 (56) | 214 (58) | 1015 (55) |
Abbreviations: AF, atrial fibrillation; ASA, aspirin; BMI, body mass index; CCB, calcium channel blocker; CHADS2, congestive HF, hypertension, age ≥75 y, DM, prior stroke/TIA/TE; CHD, coronary heart disease; DM, diabetes mellitus; ECG, electrocardiogram; HDL‐C, high‐density lipoprotein cholesterol; HF, heart failure; LVH, left ventricular hypertrophy; SBP, systolic blood pressure; SD, standard deviation; TC, total cholesterol; TE, thromboembolism; TIA, transient ischemic attack.
Data are presented as n (%) or mean (SD).
Statistical significance for categorical variables was tested using the χ2 method, and for continuous variables the Wilcoxon rank‐sum test was used.
Differences were observed between those with ECG AF and self‐reported AF (Table 1). Those with ECG AF were more likely to be older, white, and to report higher educational attainment and income compared with those with self‐reported AF. Lower values for SBP, BMI, TC, and HDL‐C also were observed for those with ECG AF compared with self‐reported cases. Participants with ECG AF were more likely to report warfarin and digoxin use, but less likely than participants with self‐reported cases to report the use of aspirin, antihypertensive medications, lipid‐lowering therapies, and antiarrhythmic medications. Those with self‐reported AF were more likely to have baseline LVH and HF than ECG AF.
AF and Mortality
Over a median follow‐up of 7.6 years, a total of 3481 (13%) deaths occurred. A higher number of deaths occurred in those with AF (n = 530/24%) compared with those without AF (n = 2951/12%; P < 0.0001). The incidence rates for all‐cause mortality by AF status are shown in Table 2. The survival probabilities for those with and without AF are shown in Figure 1A and were statistically different (log‐rank P < 0.0001). Figure 1B shows the survival probabilities of ECG AF, self‐reported AF, and those without AF (log‐rank P < 0.0001). After adjustment for sociodemographics, cardiovascular risk factors, and potential confounders, AF was associated with a 32% increased risk of death (Table 2). The results remained consistent when stratified by age, sex, and race. The mortality risk was higher among those with ECG AF (HR: 1.71, 95% CI: 1.42‐2.07) than self‐reported cases (HR: 1.15, 95% CI: 1.03‐1.29).
Table 2.
Risk of Mortality Associated With AF (N = 25 976)
| Events/No. at Risk | Incidence Rate per 1000 Person‐Years (95% CI) | Model 1, HR (95% CI)a | P Value | Model 2, HR (95% CI)b | P Value | Interaction P Valuec | |
|---|---|---|---|---|---|---|---|
| AF | |||||||
| No | 2951/23 768 | 17.3 (16.7‐18.0) | 1.0 | — | 1.0 | — | |
| Yes | 530/2208 | 36.7 (33.7‐40.0) | 1.74 (1.59‐1.91) | <0.001 | 1.32 (1.19‐1.46) | <0.001 | |
| ECG AF | |||||||
| No | 3320/25 605 | 18.2 (17.6‐18.8) | 1.0 | — | 1.0 | — | |
| Yes | 161/371 | 72.6 (62.2‐84.8) | 2.19 (1.87‐2.57) | <0.001 | 1.71 (1.42‐2.07) | <0.001 | |
| Self‐Reported AF | |||||||
| No | 3112/21 027 | 18.1 (17.4‐18.7) | 1.0 | — | 1.0 | — | |
| Yes | 369/1837 | 30.2 (27.3‐33.4) | 1.53 (1.37‐1.70) | <0.001 | 1.15 (1.03‐1.29) | 0.012 | |
| Age, yd | |||||||
| ≤75 | 2191/22 141 | 13.7 (13.1‐14.3) | 1.97 (1.74‐2.23) | <0.001 | 1.32 (1.16‐1.51) | <0.001 | 0.62 |
| >75 | 1290/3835 | 52.8 (50.0‐55.8) | 1.65 (1.43‐1.90) | <0.001 | 1.38 (1.17‐1.63) | <0.001 | |
| Sexd | |||||||
| F | 1405/14 156 | 14.2 (13.4‐14.9) | 1.78 (1.54‐2.07) | <0.001 | 1.32 (1.13‐1.55) | <0.001 | 0.89 |
| M | 2076/11 820 | 24.3 (23.3‐25.4) | 1.72 (1.53‐1.94) | <0.001 | 1.31 (1.14‐1.50) | <0.001 | |
| Raced | |||||||
| Black | 1481/10 471 | 20.6 (19.6‐21.7) | 1.64 (1.40‐1.93) | <0.001 | 1.23 (1.04‐1.46) | 0.015 | 0.23 |
| White | 2000/15 505 | 17.7 (16.9‐18.5) | 1.77 (1.58‐1.98) | <0.0001 | 1.39 (1.22‐1.58) | <0.001 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CHD, coronary heart disease; CI, confidence interval; DM, diabetes mellitus; ECG, electrocardiogram; F, female; HDL‐C, high‐density lipoprotein cholesterol; HF, heart failure; HR, hazard ratio; LVH, left ventricular hypertrophy; M, male; SBP, systolic blood pressure; TC, total cholesterol; TIA, transient ischemic attack.
Model 1 adjusted for age, sex, race, education, income, and geographic region.
Model 2 adjusted for Model 1 covariates with the addition of SBP, HDL‐C, TC, BMI, smoking, DM, antihypertensive medications, lipid‐lowering medications, warfarin, LVH, CHD, stroke/TIA, and HF.
Interactions tested using Model 2.
The reference group for comparison is No AF.
Figure 1.

Unadjusted Kaplan‐Meier survival curves with (A) survival estimates for those with and without AF and (B) survival estimates for those with ECG AF and self‐reported AF. Kaplan‐Meier estimates for both are statistically different (log‐rank P < 0.0001). Abbreviations: AF, atrial fibrillation; ECG, electrocardiogram.
Predictors of Mortality
Predictors of mortality in those with ECG AF and self‐reported AF are shown in Figure 2 and Figure 3, respectively. Age, male sex, smoking, DM, history of stroke/TIA, and HF were predictors of death in both ECG AF and self‐reported cases. Increasing BMI was protective in both AF types. Warfarin was protective in ECG AF and a trend for significance was observed in self‐reported AF. Education was a predictor of mortality in ECG AF, whereas income, LVH, CHD, and digoxin use were predictive of mortality in self‐reported AF.
Figure 2.
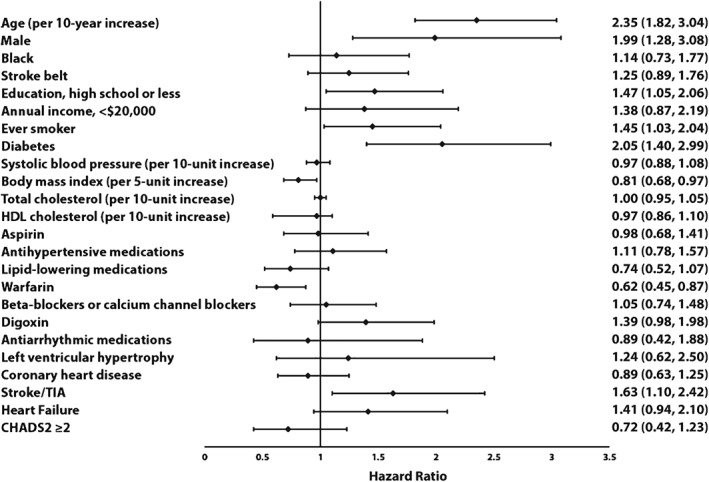
Predictors of mortality in ECG AF. Models adjusted for age, sex, race, education, income, geographic region, SBP, HDL‐C, total cholesterol, BMI, smoking, DM, antihypertensive medications, lipid‐lowering medications, warfarin, LVH, CHD, stroke/TIA, and HF. Abbreviations: AF, atrial fibrillation; BMI, body mass index; CHADS2, congestive HF, hypertension, age ≥75 y, DM, prior stroke/TIA/TE; CHD, coronary heart disease; DM, diabetes mellitus; ECG, electrocardiogram; HDL‐C, high‐density lipoprotein cholesterol; LVH, left ventricular hypertrophy; SBP, systolic blood pressure; TE, thromboembolism; TIA, transient ischemic attack.
Figure 3.

Predictors of mortality in self‐reported AF. Models adjusted for age, sex, race, education, income, geographic region, SBP, HDL‐C, total cholesterol, BMI, smoking, DM, antihypertensive medications, lipid‐lowering medications, warfarin, LVH, CHD, stroke/TIA, and HF. Abbreviations: AF, atrial fibrillation; BMI, body mass index; CHADS2, congestive HF, hypertension, age ≥75 y, DM, prior stroke/TIA/TE; CHD, coronary heart disease; DM, diabetes mellitus; HDL‐C, high‐density lipoprotein cholesterol; HF, heart failure; LVH, left ventricular hypertrophy; SBP, systolic blood pressure; TE, thromboembolism; TIA, transient ischemic attack.
Atrial Fibrillation Awareness
For the baseline characteristics of AF participants who were unaware of their diagnosis, see Supporting Information, Table, in the online version of this article. When we examined the mortality risk among those who were unaware of their AF diagnosis (n = 150) compared with participants who reported they had AF (n = 2058), a 94% higher risk of death was observed (HR: 1.94, 95% CI: 1.50‐2.52).
Discussion
In this large, racially diverse cohort, we found that asymptomatic, nonhospitalized AF is associated with an increased risk of death, and the excess mortality attributed to this arrhythmia does not vary by age, sex, or race. Additionally, ECG AF was associated with a higher risk of death compared with those who had self‐reported cases. We also identified several predictors of mortality in nonhospitalized AF and demonstrated that individuals who are unaware of their AF diagnosis have an increased mortality risk compared with persons who are aware of their diagnosis.
Several population‐based reports have examined the influence of AF on all‐cause mortality. In a matched‐cohort analysis from the Framingham Heart Study, men (odds ratio: 1.5, 95% CI: 1.2‐1.8) and women (odds ratio: 1.9, 95% CI: 1.5‐2.2) with new‐onset AF were found to have an increased risk of death.8 However, after excluding 30‐day mortality, AF was associated with a 50% greater mortality in women, but AF was no longer associated with mortality in men. Additionally, data from the Manitoba follow‐up study have shown that new‐onset AF is associated with a 31% increase in all‐cause mortality and a 37% increase in cardiovascular mortality.7 A report from the Renfrew/Paisley Study in Scotland also has shown that ECG‐detected AF is associated with increased mortality in men and women.9
Several recent reports also have examined the association between AF and mortality.10, 11, 12 In predominantly Caucasian women from the Women's Health Initiative study, new‐onset AF was associated with an increased risk of death (HR: 1.70, 95% CI: 1.30‐2.22).10 Incident AF events among patients of Olmsted County, Minnesota, also were shown to be associated with an excess risk of death within the first 90 days but not 1 year after AF diagnosis, suggesting that AF identified in hospitalized patients is a marker for terminal illness.11 Additionally, an analysis of participants from the original Framingham Heart Study and offspring cohort observed an increased AF‐related mortality risk despite a 25.4% decrease in multivariable adjusted mortality following a diagnosis of AF between 1958 and 1967 and 1998 and 2007.12
The current study provides AF mortality estimates in an asymptomatic, nonhospitalized population. In comparison with prior reports, most studies have examined the mortality risk associated with new‐onset AF ascertained from hospitalization data. Such an approach would select for an AF population in which comorbid conditions, rather than the arrhythmia itself, possibly explain the increased mortality associated with AF. Therefore, our findings possibly provide an accurate assessment of the mortality risk associated with AF in the general population, as the AF cases included were from a racially diverse, nonhospitalized registry. We also were able to determine if the mortality risk associated with AF differed by race, as the aforementioned studies have been limited in racial diversity.7, 8, 9, 10, 11 Our results suggest that no racial differences exist in the risk of death associated with AF. Additionally, ECG‐confirmed cases of AF were associated with a higher risk of death compared with self‐reported cases. A similar phenomenon was observed in the Women's Health Initiative cohort, as nonpermanent AF cases were not associated with an increased risk of death (HR: 1.18, 95% CI: 0.80‐1.73).10 Potentially, participants with self‐reported AF had self‐limited cases and those with ECG AF were more likely to represent permanent AF. However, we were unable to determine if cases were permanent or nonpermanent, and this comparison is speculative regarding the time course of AF cases. Nonetheless, our data suggest that differences exist in the mortality risk associated with ECG‐confirmed cases and self‐reported AF, and further research is needed to explain our findings. Lastly, we have shown that individuals who are unaware of their AF diagnosis have a 94% higher risk of death compared with those who are aware.
Several predictors of mortality in those with ECG‐confirmed and self‐reported AF were identified. A recent report among Medicare beneficiaries undergoing catheter ablation for AF identified several predictors of survival that overlap with factors identified in this analysis (eg, older age, DM, and HF).18 Due to the numerous associations between AF and cardiovascular risk factors (eg, hypertension, DM), AF possibly represents a marker for an overall poor risk factor profile that is associated with excess mortality.2, 7 Additionally, AF is associated with an increased risk for the development of several cardiovascular diseases (eg, stroke/TIA, HF),4, 5, 6, 19 and these conditions were shown to negatively influence survival in our AF population. We also observed a survival benefit with increasing BMI, similar to obesity paradoxes that have been described in other cardiovascular diseases.20 A survival benefit was observed for those receiving warfarin with ECG AF, and this is consistent with a follow‐up analysis from the Atrial Fibrillation Follow‐up Investigation of Rhythm Management (AFFIRM) study.21 Also, a trend for significance was observed for the protective benefit of warfarin in self‐reported AF. The survival benefit likely comes from reductions in stroke morbidity and mortality.22 Similar to AFFIRM, we observed no difference in mortality with either rate‐ or rhythm‐control strategies, but we did observe a negative influence on survival with digoxin.21
Atrial fibrillation is becoming an increasingly common medical problem, especially among individuals older than 80 years of age, and the number of persons affected will increase with the growing elderly population.1, 23 Numerous clinical trials and guidelines have been published to determine the optimal medical management in AF, including anticoagulation strategies, and this has resulted in significant changes in clinical practice over the past 2 decades.24, 25 Agents used for ventricular rate control have changed considerably, with digoxin use significantly declining and the use of β‐blockers and nondihydropyridine calcium channel blockers increasing.25 Similarly, rates of amiodarone use have increased for rhythm control.25 The use of anticoagulants also has risen, but the rate in individuals 65 to 80 years of age has not increased at the rate expected for a group with a high risk for AF‐related thromboembolic complications.25 Despite these changes in clinical practice, our data confirm that AF remains a significant predictor of all‐cause mortality in a racially diverse, nonhospitalized cohort and suggest that anticoagulation strategies, in addition to risk factor modification, possibly will improve survival in those with AF. Additionally, our study identified AF participants who are unaware of their diagnosis as a high‐risk group for increased mortality. Increased patient and provider awareness of AF and the use of routine ECG screening possibly will enhance the detection of this arrhythmia and provide patients with medical therapies that improve survival (eg, anticoagulation).
Study Limitations
Our results should be interpreted in the context of several limitations. Several baseline characteristics (eg, HF) were self‐reported and subjected our analysis to recall bias. Cases of AF were identified at baseline, and it is likely that participants developed AF during the study period. However, we sought to examine the mortality associated with asymptomatic, nonhospitalized AF. The inclusion of new‐onset AF possibly would detect events associated with comorbid conditions that negatively influence survival (eg, decompensated HF). Additionally, we adjusted for several potential confounders but acknowledge that residual confounding remains a possibility similar to other epidemiologic studies. For example, left ventricular ejection fraction was not measured in the REGARDS cohort, and this covariate possibly influenced survival among those with AF.
Conclusion
We have shown that asymptomatic, nonhospitalized AF is associated with an increased risk of mortality in REGARDS, a large, racially diverse cohort representative of the general United States population. Additionally, ECG‐confirmed AF was associated with a higher risk of mortality compared with self‐reported cases. We also have identified those with AF unawareness as a subgroup of AF who have an increased risk of death. Despite changes in medical management strategies for those with AF, the arrhythmia is associated with an increased risk for adverse outcomes. Potentially, widespread screening and increased awareness will identify those who will benefit from therapies that improve mortality, especially among nonhospitalized populations.
Supporting information
Supplemental Table. Baseline Characteristics for those with AF Unawareness (N=150)
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, US Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population‐based cohort: the Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 3. Heeringa J, Kors JA, Hofman A, et al. Cigarette smoking and risk of atrial fibrillation: the Rotterdam Study. Am Heart J. 2008;156:1163–1169. [DOI] [PubMed] [Google Scholar]
- 4. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 5. Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. [DOI] [PubMed] [Google Scholar]
- 6. Soliman EZ, Safford MM, Muntner P, et al. Atrial fibrillation and the risk of myocardial infarction [published correction appears in JAMA Intern Med. 2014;174:308]. JAMA Intern Med. 2014;174:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow‐Up Study. Am J Med. 1995;98:476–484. [DOI] [PubMed] [Google Scholar]
- 8. Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 9. Stewart S, Hart CL, Hole DJ, et al. A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. [DOI] [PubMed] [Google Scholar]
- 10. Conen D, Chae CU, Glynn RJ, et al. Risk of death and cardiovascular events in initially healthy women with new‐onset atrial fibrillation. JAMA. 2011;305:2080–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chamberlain AM, Gersh BJ, Alonso A, et al. Decade‐long trends in atrial fibrillation incidence and survival: a community study. Am J Med. 2015;128:260e1–267.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. [DOI] [PubMed] [Google Scholar]
- 14. Soliman EZ, Howard G, Meschia JF, et al. Self‐reported atrial fibrillation and risk of stroke in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2011;42:2950–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–186. [DOI] [PubMed] [Google Scholar]
- 16. Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 17. Gray RJ, Tsiatis AA. A linear rank test for use when the main interest is in differences in cure rates. Biometrics. 1989;45:899–904. [PubMed] [Google Scholar]
- 18. Piccini JP, Sinner MF, Greiner MA, et al. Outcomes of Medicare beneficiaries undergoing catheter ablation for atrial fibrillation. Circulation. 2012;126:2200–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Neal WT, Sangal K, Zhang ZM, et al. Atrial fibrillation and incident myocardial infarction in the elderly. Clin Cardiol. 2014;37:750–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Efird JT, O'Neal WT, Kennedy WL, et al. Grand challenge: understanding survival paradoxes in epidemiology. Front Public Health. 2013;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corley SD, Epstein AE, DiMarco JP, et al; AFFIRM Investigators . Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow‐Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109:1509–1513. [DOI] [PubMed] [Google Scholar]
- 22. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 23. Odden MC, Coxson PG, Moran A, et al. The impact of the aging population on coronary heart disease in the United States. Am J Med. 2011;124:827e5–833.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stafford RS, Robson DC, Misra B, et al. Rate control and sinus rhythm maintenance in atrial fibrillation: national trends in medication use, 1980–1996. Arch Intern Med. 1998;158:2144–2148. [DOI] [PubMed] [Google Scholar]
- 25. Fang MC, Stafford RS, Ruskin JN, et al. National trends in antiarrhythmic and antithrombotic medication use in atrial fibrillation. Arch Intern Med. 2004;164:55–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table. Baseline Characteristics for those with AF Unawareness (N=150)


