Abstract
Neonatal cerebral hypoxia-ischemia (HI) commonly results in cognitive and sensory impairments. Early behavioral experience has been suggested to improve cognitive and sensory outcomes in children and animal models with perinatal neuropathology. In parallel, we previously showed that treatment with immunomodulator Inter-alpha Inhibitor Proteins (IAIPs) improves cellular and behavioral outcomes in neonatal HI injured rats. The purpose of the current study was to evaluate the influences of early experience and typical maturation in combination with IAIPs treatment on spatial working and reference memory after neonatal HI injury. A second aim was to determine the effects of these variables on hippocampal CA1 neuronal morphology. Subjects were divided into two groups that differed with respect to the time when exposed to eight arm radial water maze testing: Group one was tested as juveniles (early experience, Postnatal day (P) 36 to 61) and adults (P88 – 113), and Group two was tested in adulthood only (P88-113; without early experience). Three treatment conditions were included in each experience group (HI + Vehicle, HI + IAIPs, and Sham subjects). Incorrect arm entries (errors) were compared between treatment and experience groups across three error types (Reference memory (RM), Working memory incorrect (WMI), Working memory correct (WMC)). Early experience led to improved working memory performance regardless of treatment. Combining IAIPs intervention with early experience provided a long-term behavioral advantage on the WMI component of the task in HI animals. Anatomically, early experience led to a decrease in the average number of basal dendrites per CA1 pyramidal neuron for IAIP treated subjects and a significant reduction in basal dendritic length in control subjects, highlighting the importance of pruning in typical early life learning. Our results support the hypothesis that early behavioral experience combined with IAIPs improve outcome on a relativity demanding cognitive task, beyond that of a single intervention strategy, and appears to facilitate neuronal plasticity following neonatal brain injury.
Keywords: Working Memory, Water Maze, Hypoxia-Ischemia, Inter-alpha Inhibitor, Early experience, Neurobehavioral Maturation, Neuronal Plasticity
1. Introduction
Premature infants and high-risk full-term babies, exposed to abruptio placenta, prolonged labor, umbilical occlusion or prolapse, are at increased risk for cerebral oxygen deprivation (hypoxia) and insufficient blood flow (ischemia; [1-4]). Neonatal cerebral hypoxia-ischemia (HI) disrupts global physiological function often translating to long-term deficits in learning and memory both in humans [3-13] and in rodent models [12,14-20]. At this time, the only available treatment for humans following neonatal hypoxicischemic encephalopathy (HIE) is controlled hypothermia, which may reduce the body's inflammatory response to injury [21,22]. However, this treatment is only approved for use in full term neonates after HIE, and even after therapeutic hypothermia, over 40% of babies will suffer from death or moderate to severe physical or intellectual impairments [23-25]. Given limited treatment options, research has focused on developing alternative and/or complementary therapeutic strategies that can improve long-term cognitive function following neonatal HI injury.
Inter-alpha Inhibitor Proteins (IAIPs) are found in relatively high concentrations in human and rodent blood plasma and are thought to play an important role in inflammatory regulation [26-32]. Recent studies using rodent models have shown that IAIPs play a significant role in regulating the inflammatory response and increase cell survival in both the central nervous system and somatic cells following infection and brain injury [20,33,34]. Our group has shown that administration of human plasma derived IAIPs following neonatal HI brain injury in rats leads to neuronal and gross anatomical sparing across distinct developmental time windows (72 h post-injury (Postnatal day (P) 10) and adult (P80+)) and improves spatial and non-spatial learning outcomes in an age dependent manner in juvenile and adult rats [20].
In parallel, studies from our group and others have shown that early experience (enriched housing or more domain specific experience (e.g., auditory processing)) can lead to improved adult behavioral outcomes in rats with various forms of developmental brain injury [16,20,35]. Animals with early life exposure to domain-specific tasks show robust improvements in adult performance relative to inexperienced animals allowed to mature typically [35]. This is significant because, in humans with neurodevelopmental disorders, behavioral training/intervention has been shown to improve long-term cognitive and linguistic outcomes and appears to shift brain activation (using fMRI) patterns closer to that of typical individuals [36-41]. Furthermore, early life enriched housing or behavioral training has been shown to modify dendritic branching across divergent brain regions in rats (e.g., hippocampus, frontal cortex), relating to improved behavioral performance later in life [42,43]. Interestingly, the age at which behavioral experience takes place and the length of experience may influence whether a reduction/refinement of branching or dendritic expansion occurs following brain injury [44]. Taken together, the current literature suggests that early behavioral and/or sensory intervention is critical for improving long-term functional recovery in at risk developmental populations and is likely to facilitate neuronal plasticity.
Regardless of how effective new pharmacological treatments may be at improving neurological outcome, many infants will not meet criteria for treatment or will miss optimal timing windows required for treatment effectiveness. In addition, optimal drug dosage may be compromised due to difficulty predicting injury severity in human populations. Therefore, behavioral interventions will continue to be a valuable tool to improve long-term outcome, even in cases of severe birth trauma. However, given the central role of inflammation in neonatal HI injury and evidence for the benefits of early behavioral training, combining pharmacological and behavioral interventions will likely provide greater improvement in functional recovery than a single intervention alone. Thus, the purpose of the current study was to explore the efficacy of a multi-factorial intervention strategy to modulate inflammatory mediated brain injury with the use of Inter-alpha Inhibitor Proteins (IAIPs) in conjunction with early-life spatial working and reference memory experience in an effort to maximize cognitive recovery and facilitate neuronal plasticity. We hypothesized that administration of IAIPs in combination with early task-specific experience would significantly improve spatial working and reference learning in rats with neonatal HI injury beyond improvements from a single treatment. We also predicted that early experience would result in morphological changes in basal dendrites of hippocampal CA1 neurons, which are central to spatial processes important for navigation in the eight-arm radial water maze.
2. Methods
2.1 Subjects
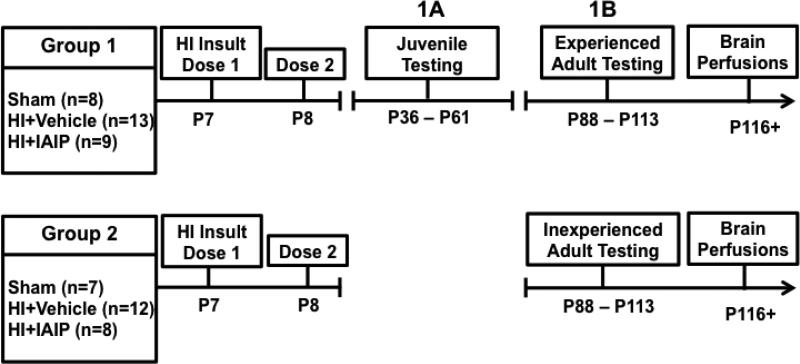
Subjects were 57 male Wistar rats born from time-mated dams shipped on embryonic day five of pregnancy and (Charles River Laboratories, Wilmington, MA) housed in the Fogarty Life Sciences Vivarium at Rhode Island College. On post-natal day 1 (P1), pups were separated into litters of eight males and two females to control for sex ratio and litter size. This study was limited to the assessment of male subjects due to prior research showing that rodent males exhibit more prominent deficits following neonatal HI brain injury as compared to females [45,46], findings that parallel observations in humans [12,47-53]. Subsets of subjects were randomly assigned to receive either hypoxic-ischemic (HI) insult or Sham surgical procedure on P7. Subjects were weaned on P21, right or left ear marked, and pair housed using a 12:12 light/dark cycle with food and water available ad libitum. Prior to weaning, approximately half of the subjects from each litter were randomly assigned into two groups for behavioral testing on the eight-arm-radial water maze. To minimize between-litter effects, subjects from all litters were represented in each treatment and experience group. Group 1 (G1; Sham n = 8, HI + Vehicle n = 13, HI + IAIPs n = 9) received behavioral testing as juveniles (P36 - P61; G1A) and also as adults (P113 - 138; G1B). Group 2 (G2; Sham n = 7, HI + Vehicle n = 12, HI + IAIPs n = 8) was tested only as adults (P113 - 138) to assess maturational effects (Figure 1). All procedures were conducted in compliance with the National Institutes of Health guidelines for care and use of laboratory animals and all protocols were approved by the Rhode Island College Institutional Animal Care and Use Committee (IACUC).
Figure 1.
Timeline showing age of HI injury, treatment timing, juvenile and adult testing for group one and adult testing only for group two on the 8-arm-radial water maze and age of sacrifice.
2.2 Surgical procedure
Surgery was performed in the Fogarty Life Science Vivarium at Rhode Island College (Providence, RI) using aseptic techniques. On postnatal day 7 (P7), subjects were randomly assigned to treatment (HI + Vehicle, HI + IAIPs, or Sham) and experience groups (Group 1: juvenile and adult testing or Group 2: adult testing only). All treatment conditions were balanced across litters. A modified version of the Rice-Vannucci method was followed to induce the hypoxic-ischemic injury [54-56]. In summary, each animal was stabilized on a surgical surface and anesthetized using 3-4% isoflurane administered through a nose cone. Total absence of leg withdrawal and tail-pinch reflexes were verified prior to advancing with 1-2% isoflurane for maintenance. A midline ventral incision was made in the neck. The right common carotid artery (RCCA) was located and completely cauterized. Sham subjects followed the same procedure except for an absence of RCCA cauterization. The neck incision was stitched using vicryl sutures, and each pup was labeled with a footpad ink injection (~10 μL). Body temperature was maintained throughout the procedure at 37° C, using an isothermal heating pad (Braintree Scientific, Braintree, MA), as reduced temperature has been shown to provide a degree of neuroprotection in humans and animal models [22,57-59].
Post-surgery, all pups returned to their mothers for 2-3 hours of feeding and recovery prior to hypoxia (8% oxygen). Preceding hypoxia, all pups received an intraperitoneal (IP) injection of 30 mg/kg of either Inter-alpha Inhibitor proteins (IAIPs; ProThera Biologics, Providence, RI) or an equivalent volume of saline (vehicle). IAIPs were extracted and purified from human plasma using the methods previously described [20]. HI subjects were placed in a hypoxia chamber with 8% humidified oxygen (balanced with nitrogen) for 120-minutes. Sham subjects were exposed to room air for 120-minutes. All subjects were kept on an isothermal heating pad to prevent hypothermia during the 120-minute period. Following hypoxia or room air exposure, all pups were returned to their dam until administration of the second 30 mg/kg treatment dose of IAIPs or saline 24 hours post-surgery. The pups remained with their mother until weaning on P21.
2.3 Behavioral testing
2.3.1 Apparatus
An eight-arm-radial water maze was used for behavioral testing (as previously described by [60-66]. Maze dimensions and testing conditions have been described extensively elsewhere [66]. The escape platform locations remained fixed relative to extra-maze spatial cues for the duration of the study. Extra-maze cues included two researchers, a white board, and two walls with high contrast (one white with a black circle painted on it and one white with a black triangle). The start arm did not contain an escape platform and no more than two adjacent arms contained a platform, ensuring adequate spacing. Water temperature was maintained at 22°C.
2.3.2 Water radial-arm maze testing procedure
Behavioral testing for group 1 (G1) began on P36 (juvenile age) and continued until P61 (4 weeks of consecutive testing days excluding weekends). Starting on P88, both experience groups (G1 and G2) were tested on the same task in the same maze for 4 weeks of adult testing (consecutive testing days excluding weekends). Each testing day consisted of 4 trials with an inter-trial interval of 90-seconds. For each trial, a subject was placed into the start arm facing the middle of the maze. Each subject was allotted 120-seconds to locate one of the hidden platforms, while arm entries were recorded. If a subject was unable to locate an escape platform during that time, he was guided to the closest escape platform and remained there for 10-seconds before being returned to the holding cage to start the inter-trial interval. Researchers recorded an arm entry (see [66] for details) each time the shoulders of the rat broke the plane of a maze arm. During the inter-trial interval, the subject was warmed (37° C) in a holding cage using an isothermal heating pad (Braintree Scientific, Braintree, MA), while the platform used for escape was removed for the remainder of the subject's testing sessions that day. The stepwise reduction in platforms across the four trials each day allowed for the assessment of working memory load, given the increased probability for working memory errors (fewer correct options) as trials progressed. The first day of testing for all subjects was considered an acquisition day and was not included in the analysis. Day 14 was also eliminated after a computer malfunction resulted in the loss of data. Thus, a total of 18 days were used for analysis.
2.3.3 Quantification of errors
As previously described, orthogonal measures of working and reference memory were used to quantify errors [62,64,66-71]. Three error types were recorded for each daily session. Reference memory (RM) errors reflected the number of first entries into arms that never contained an escape platform. Working memory incorrect (WMI) errors were recorded as repeat entries into reference memory arms, which were arms that never contained an escape platform. Working memory correct (WMC) errors represented the number of first and repeat entries into any arm that previously contained an escape platform (removed during preceding trials; [62,64,66-71]). This win-shift strategy of avoiding arms previously used for escape (WMC errors) represented the most challenging aspect of the task, as rats have a natural tendency to return to locations previously used for escape on water-based maze testing [72]. This is counter to the tendency to avoid previously explored arms in the land-based version of this task [72].
2.3.4 Data analysis
We planned a priori to investigate main effects using repeated measures ANOVAs between each experience group (G1A Juvenile, G1B Experienced Adult, and G2 Inexperienced Adult). The eighteen days used for analysis were combined into 3 blocks (6 days per block): block 1 (days 2-7), block 2 (days 8-13), block 3 (days 15-20), based on previous observations of progressive stages of improvement across multiple days of testing in this paradigm [64,66,69]. For each error type, repeated measures ANOVAs were performed with experience as the between subjects variable and the number of errors on each trial as the dependent variable. Planned comparisons using oneway ANOVAs were performed to analyze effects of treatment on total errors within experience groups collapsed across block. Further, analyses of treatment across trial within experience groups were also planned, as treatment-induced differences on later sessions of water-radial-arm maze testing have been observed previously, with the most pronounced effects occurring on trial 4, (highest working memory load; [60, 63-66,69,71]). When appropriate, Tukey's HSD post hoc analyses were used to identify treatment effects within each trial for each experience group. Alpha level was set at 0.05. When Mauchly's test of sphericity was violated, degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity.
2.4 Histological Analysis
2.4.1 Golgi-Cox staining
Following the completion of behavioral testing, rats were deeply anesthetized with sodium pentobarbital (Sleepaway, 100 mg/kg) and transcardially perfused with 0.9% saline. Brains were removed and placed in amber vials containing Golgi-Cox solution (1% potassium dichromate, 1% mercuric chloride, 0.8% potassium chromate dissolved in water), refreshed after two-days, and remained in the dark for an additional 12 days. Following impregnation, the Golgi-Cox solution was replaced with 30% sucrose. Once the brains equilibrated (as shown by sinking to the bottom of the vial), they were blocked at approximate brain coordinates: Bregma 2.76 mm and Bregma -4.80 mm [73]. The brains were processed into 200 μm coronal sections using a vibrating microtome (Leica VT1000S, Leica Microsystems, Bannockburn, IL), and mounted on gelatin-coated slides. The sections were pressed onto the gelatin coated slides using bibulous paper. Staining followed the methods described by [74]. After staining, slides were coverslipped using DPX mounting medium.
2.4.2 CA1 neuron reconstruction
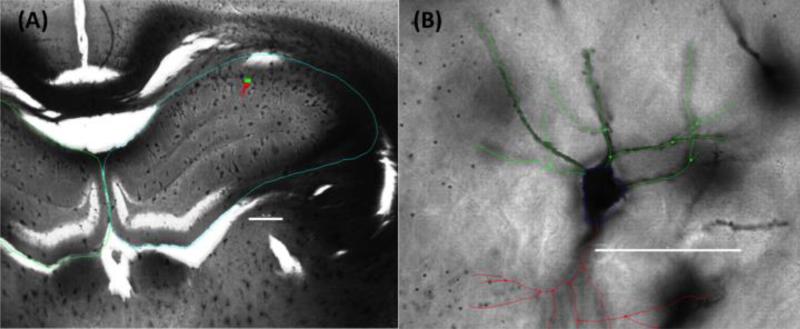
Neuronal reconstruction and tracing in three-dimensions was performed using Neurolucida 11.0 (MBF Bioscience, Burlington, VT; Figure 5) with a BX53 light microscope (Olympus) and an UPLSAPO 100x oil immersion objective of 1.4 NA. Golgi-Cox impregnated pyramidal neurons from CA1 of the left hippocampus (contralateral to injury) were identified by their characteristic triangular soma shape. The contralateral hemisphere was used for analysis given significant hippocampal atrophy and/or the absence of CA1 in the injury hemisphere of HI subjects. Neurons selected for analysis were well impregnated with no apparent truncation of the dendritic arbor, had at least three basal dendrites with two branching at least once, and a single apical dendrite that branched at least once. Inclusion criteria was similar to that described by others [18,43,75-77]. Basal pyramidal dendrites were analyzed based on previous observations of these neurons involvement in spatial processing and sensitivities to experience and other manipulations as compared to apical branches in rats [78-82].
Figure 5.
Representative photomicrographs of a Golgi-Cox stained section of dorsal (A) hippocampus (blue tracing) at low magnification, highlighting the location of a prototypical CA1 pyramidal neuron (green/red marker) and (B) a high magnification view of the same cell with basal (green) branch tracings used for analysis. Apical (red tracing) branches are included for orientation purposes. Scale bars are (A) 500μm and (B) 50μm.
2.4.3 Number of basal dendrites
The numbers of basal CA1 dendrites (dendrites projecting from the base of the neuron) were counted for each neuron. The mean number per subject was used for comparison between groups. A 3 (treatment; Sham n = 15, HI + Vehicle n = 25, HI + IAIPs n = 17) × 2 (experience; G1 n = 30, G2 n = 27) univariate ANOVA was performed to assess the average number of basal dendrites per neuron. When main effects were observed, independent samples t-tests were used to evaluate experience effects within each treatment.
2.4.4 Sholl analysis
The Sholl analysis of ring intersections is a widely used method to indirectly calculate dendritic length (in μm; [83]). Neurolucida Explorer (MBF Bioscience, Burlington, VT) was used to create concentric rings, centered at the cell body of each neuron, with 10 μm ring intervals overlaying the neuronal tracings constructed in Neurolucida 11.0 (MBF, Burlington, VT). The number of intersections of the neuronal tracing with each concentric ring in 3-dimensional space was calculated using Neurolucida explorer 11.0.
Each neuron was analyzed separately for the number of intersections. A minimum of three neurons were identified for analysis within each subject, with one subject having only two neurons that met the criteria. The mean number of dendritic intersections per subject was used as the dependent variable for comparison between treatment (Sham n = 15, HI + Vehicle n = 25, HI + IAIPs n = 17) and experience groups (G1 n = 30 & G2 n = 27). SPSS (IBM, Armonk, NY) was used to evaluate main effects using repeated measures ANOVAs, with ring radius as the repeat. Planned comparisons using one-way ANOVAs were used to identify simple effects of treatment and experience at each concentric ring. Tukey's HSD post hoc analysis was used to distinguish which of the treatment groups (HI + Vehicle, HI + IAIPs, or Sham) differed when overall treatment effects were found at each radii. When Mauchly's test of sphericity was violated, degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity.
3. Results
3.1 Behavioral Results
3.1.1 Effects of Experience Across Groups (juvenile G1A, adult experienced G1B, adult inexperienced G2)
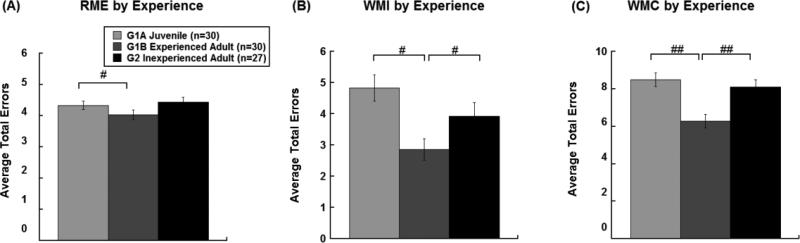
Results from three-separate 2 (age/experience) x 3 (treatment) x 3 (block) x 4 (trial) repeated measures ANOVAs for overall Reference Memory (RM) errors, revealed an effect of Age (G1A (juvenile) versus G1B (adult experienced); [F(1,27)=4.48, p=0.044; Figure 2A) but only a trend for Experience (G1B versus G2; [F(1,51)=3.27, p=0.076]; Figure 2A]) and no main effect of Maturation (G1A & G2 [F(1,51)=0.30, p=0.589]; Figure 2A). Thus, overall, subjects made fewer RM errors as experienced adults (G1B) compared to their performance as juveniles (G1A). Mature rats without early experience (G2) had comparable error rates to juvenile performance (G1A), but were not significantly different from experienced adults (G1B). Overall main effects of block across experience groups (G1A versus G2 [F(2,102)=10.484, p<0.001]; G1B versus G2 [F(2,102)=8.749, p<0.001]; G1A versus G1B [F(2,54)=12.489, p<0.001]) were indicative of fewer errors in the last third of testing as compared to earlier blocks of testing, reflecting the progression of RM learning by all subjects across the four weeks of testing. There were no overall effects of trial for RM errors (G1A versus G2 [F(2.42,123.23)=0.80,p=0.50]; G1B versus G2 [F(2.18,111.24)=1.78, p=0.17]; G1A versus G1B [F(1.84,49.55)=0.094, p=0.90]).
Figure 2.
Average number (mean±SEM) of reference memory errors (A), working memory incorrect errors (B), and working memory correct errors (C), across age (G1B, G1) and experience (G1A) conditions. Overall main effects of early experience were observed for each error type regardless of treatment, with the greatest effects seen for WMI and WMC performance (#p<0.05).
In parallel, results from 2 (age/experience) x 3 (treatment) x 3 (block) x 4 (trial) repeated measures ANOVAs for overall Working Memory Incorrect (WMI) errors, for each planned comparison revealed effects of Age (G1A versus G1B; [F(1,27)=35.97, p<0.001]; Figure 2B) and Experience (G1B versus G2; [F(1,51)=4.44, p=0.040; Figure 2B]), but no overall effect of maturation (G1A & G2 [F(1,51)=2.22, p=0.142]; Figure 2B). Thus, experienced adult rats (G1B) made fewer WMI errors overall as compared to their juvenile performance (G1A) and compared to inexperienced adults (G2). Importantly, typical maturation (G2 with no early experience) did not significantly influence overall WMI error performance, as evidenced by comparable error rates between adult inexperienced subjects and juvenile subjects. Overall main effects of block (G1A versus G2 [F(2,102)=21.741, p<0.001]; G1B versus G2 [F(2,102)=21.110, p<0.002]; G1A versus G1B [F(2,54)= 12.908, p<0.001]) with fewer errors in the last third of testing as compared to earlier blocks of testing were indicative of the progression of learning to avoid repeat entries into reference memory arms (WMI) for all groups. In contrast to RM performance, trial effects were observed, with the number of WMI errors increasing across the four trials for all groups (G1A versus G2 [F(1.61, 81.87)=195.31, p<0.0001]; G1B versus G2 [F(1.701,86.73)=141.73, p<0.0001]; G1A versus G1B [F(3.46,46.75)=3.85, p<0.0001]). Planned comparisons showed Trial x Treatment interactions with HI injured groups making more WMI errors as compared to shams as working memory load increased (Trail x Treatment interactions, G1A versus G2 [F(3.21, 81.87)=3.61, p=0.015]; G1B versus G2 [F(3.401,86.73)=3.92, p=0.008]; G1A versus G1B [F(3.46,46.75)=3.85, p=0.012]).
In addition, results from 2 (age/experience) x 3 (treatment) x 3 (block) x 3 (trial) repeated measures ANOVAs for overall WMC errors, for each planned comparison revealed main effects of Age (G1A versus G1B [F(1,27)=31.00, p<0.001; Figure 2C]) and Experience (G1B versus G2; [F(1,51)=12.25, p=0.001; Figure 2C]), but did not show an effect of Maturation (G1A versus G2; [F(1,51)=0.53, p=0.472]; Figure 2C). Specifically, experienced adults (G1B) made fewer WMC errors overall as compared to their juvenile performance (G1A) and compared to inexperienced adults (G2). These results parallel effects seen for WMI errors, suggesting that experience alone appears to improve later performance over and above an influence of typical maturation. A main effect of Block (G1A versus G2 [F(2,102)=37.520, p<0.001]; G1B versus G2 [F(2,102)=22.290, p<0.001]; G1A versus G1B [F(2,54)=15.822, p<0.001]), with fewer errors in the last third of testing as compared to earlier blocks of testing was indicative of the progression of learning of all subjects across four weeks of testing. Planned comparisons across adult groups (G1B & G2) showed Trial x Treatment interactions with HI injured groups making more WMC errors as compared to shams, as working memory load increased (G1B versus G2 [F(2.74,69.85)=3.77, p=0.017]. Further, planned comparisons across naïve groups (G1A & G2) showed Block x Trial x Treatment interactions with HI injured subjects making more WMC errors as compared to noninjured controls (G1A versus G2 [F(5.47,139.54)=3.32, P=0.006] as testing progessed and working memory load increased.
Finally, latency to reach each platform across trials was recorded to account for potential differences in the rate of trial completion across experience and treatment conditions. Importantly, there were no treatment effects (Sham, HI+Vehicle, HI+IAIPs) on the latency for trial completion within any of the experience groups (G1A, G1B, G2) despite differences in error (RM, WMI, WMC) rates across treatment. However, there were overall effects of Age (G1A versus G1B; [F(1,27)=21.63; p<0.001]) and Maturation (G1A versus G2; [F(1,55)=4.21, p=0.045]) as both adult groups reached the escape platforms in less total time per day as compared to juveniles. The authors take the lack of treatment based latancy effects to reflect comparable swimming performance across the treatment conditions.
3.1.2 Effects of Treatment (HI + Vehicle, HI + IAIPs, Sham) Across Experience (juvenile G1A, adult experienced G1B, adult inexperienced G2)
Within group planned comparisons for Reference Memory (RM) errors showed no overall effects of Treatment within Juvenile (G1A; Sham n = 8, HI + Vehicle n = 13, HI + IAIPs n = 9; [F(2,27)=1.80; p=0.184]), Experienced Adult (G1B; Sham n = 8, HI + Vehicle n = 13, HI + IAIPs n = 9; [F(2,27)=2.28, p=0.122]), or Inexperienced Adult groups (G2; Sham n = 7, HI + Vehicle n = 12, HI + IAIPs n = 8; [F(2,24)=1.85, p=0.180]), likely reflecting the ease with which subjects identified and avoided reference memory arms across days of testing.
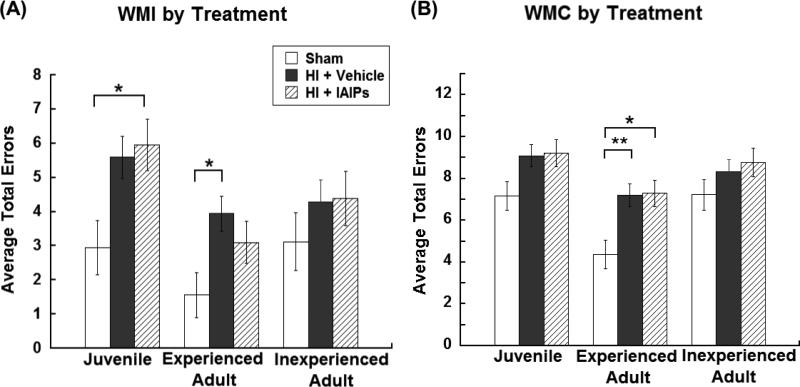
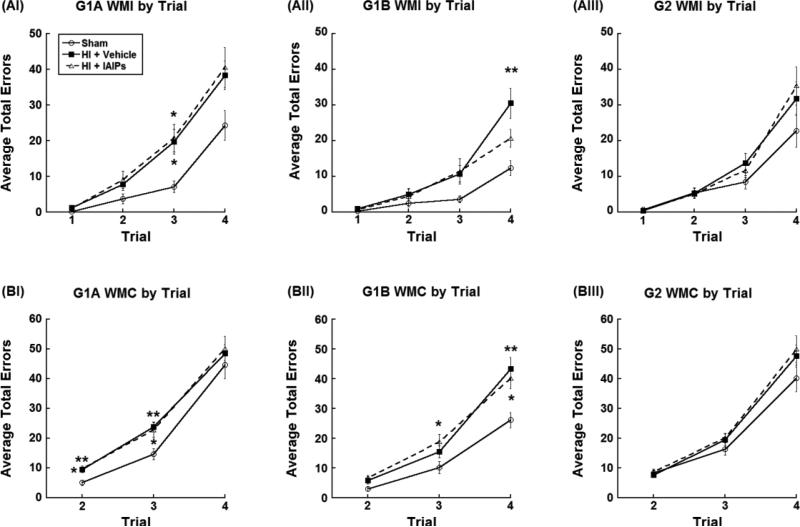
In contrast, within group planned comparisons for WMI errors revealed an overall Treatment effect within G1A Juveniles [F(2,27)=6.54, p=0.005; Figure 3A] and G1B Experienced Adults [F(2,27)=4.46, p=0.021; Figure 3B] with HI injured subjects making more WMI errors as compared to sham subjects. However, no treatment effect was observed across Inexperienced Adults (G2) [F(2,24)=1.02, p=0.377; Figure 3A], likely reflecting the moderate difficulty of this component of the task. Tukey's post hoc analysis, within G1A, showed that IAIPs treated juvenile subjects made significantly more WMI errors overall as compared to uninjured juvenile controls (p=0.048; Figure 3A). Although untreated juvenile HI subjects were not significantly different in WMI error performance, there was a trend toward more overall errors as compared to uninjured controls (p=0.068; Figure 3A). Tukey's post hoc analysis, within G1B (Adult experienced), revealed that untreated HI subjects were significantly impaired (more WMI errors) as compared to Sham subjects (p=0.008; Figure 3A), whereas IAIPs treated HI subjects were no longer impaired in adulthood as compared to shams (Figure 3A).
Figure 3.
Average number of total (mean±SEM) working memory incorrect errors (A) and working memory correct errors (B) by treatment (Juvenile/Experienced Adult: Sham n = 8, HI + Vehicle n = 13, HI + IAIPs n = 9, Inexperienced Adult: Sham n = 7, HI + Vehicle n = 13, HI + IAIPs n = 8). Effects of Treatment within experience groups are denoted as compared to Sham subjects (*(p<0.05) and **(p<0.01)).
Within group planned comparisons for WMC errors, revealed an overall Treatment effect within G1B Experienced Adults [F(2,27)=6.54, p=0.005; Figure 3B], but no overall treatment effects within G1A Juveniles [F(2,27)=3.04, p=0.065; Figure 3B] nor G2 Inexperienced Adults [F(2,26)=388.69, p=0.287]. Tukey's post hoc analysis for G1B showed significant impairments (more WMC errors) by HI subjects compared to uninjured Shams (HI + Vehicle p=0.008, HI + IAIPs p=0.011; Figure 3B]. Treatment effects for WMC errors seen within the experienced adult group likely reflected the increased demand of WMC component of the task as compared to the other error types, were only intact sham animals with early experience showed improved performance (fewer WMC errors) as compared to injured subjects. These results further highlight the stepwise level of difficulty seen across the three error types.
3.1.3 Treatment Effects (HI + Vehicle, HI + IAIPs, Sham) by Trial Across Experience (juvenile G1A, adult experienced G1B, adult inexperienced G2)
For planned comparisons within trial, when trial by treatment interactions were present, both untreated HI and IAIPs treated HI subjects made significantly more WMI errors on trial 3 (HI + Vehicle p=0.035; HI + IAIP p=0.034; Figure 4-AI) compared to shams, as juveniles (G1A). However, as experienced adults (G1B), when working memory load was at its highest (trial 4), only the untreated HI subjects showed significant impairments (more WMI errors; p=0.004; Figure 4-AII) while the IAIPs treated HI subjects showed no difference in WMI errors as compared to Shams (p=0.305; Figure 4-AII).
Figure 4.
Average total (mean±SEM) WMI errors per trial (A) and WMC errors per trial (B) reflecting planned comparisons within Juvenile (G1A, I; Sham n = 8, HI + Vehicle n = 13, HI + IAIPs n = 9), Experienced Adult (G1B, II; Sham n = 8, HI + Vehicle n = 13, HI + IAIPs n = 9) and Inexperienced Adult groups (G2, III; Sham n = 7, HI + Vehicle n = 12, HI + IAIPs n = 8). Effects of Treatment within experience groups are denoted *(p<0.05) and **(p<0.01) as compared to Sham subjects. Untreated experienced HI subjects made more WMI errors as compared to shams, when working memory load was highest (trial 4) (AII). In contrast, both experienced HI groups showed persistent WMC deficits, which were shifted to trials 3 and 4 as compared to shams and their juvenile deficit profile (BII).
As juveniles (G1A), both untreated and IAIPs treated HI subjects made significantly more WMC errors on trials 2 (HI + Vehicle p=0.01; HI + IAIPs p=0.009; Figure 4-BI) and 3 (HI + Vehicle p=0.009; HI + IAIPs p=0.036; Figure 4-BI) as compared to sham subjects. As experienced adults (G1B), only IAIPs treated HI subjects made significantly more WMC errors on trial 3 (p=0.037; Figure 3-BII) as compared to shams. However, as experienced adults (G1B), when working memory load was at its highest (trial 4), both untreated HI subjects (p=0.006) and IAIPs treated HI subjects (p=0.042) made significantly more errors as compared to Shams.
3.2 Histological Assessment
3.2.1 Number of basal hippocampal CA1 pyramidal dendrites
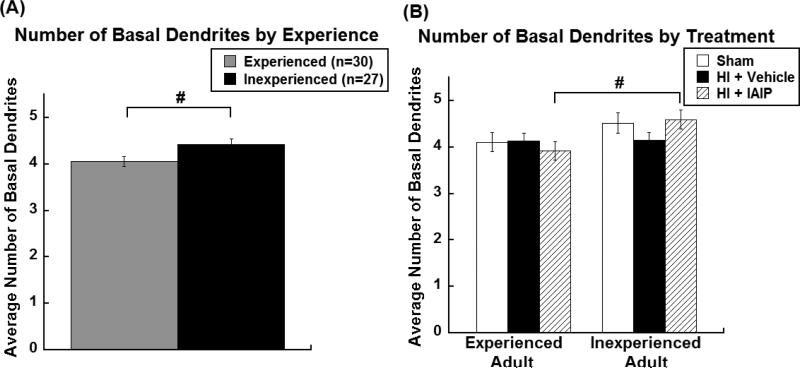
A 2 (experience; G1 n = 30 & G2 n = 27) x 3 (treatment; Sham n = 15, HI + Vehicle n = 25, HI + IAIPs n = 17) univariate ANOVA was computed to assess the number of CA1 pyramidal basal dendrites per neuron. Experienced subjects had significantly fewer basal dendrites per neuron as compared to inexperienced subjects [F(1,51)=5.45, p=0.024; Figure 6A]. Planned comparisons for effects of Experience within each Treatment (Sham n = 15, HI + Vehicle n = 25, HI + IAIPs n = 17) were evaluated using independent samples t-tests given the observed overall experience effects. Significantly fewer basal dendrites were found on Experienced HI + IAIPs subjects’ neurons compared to the Inexperienced HI + IAIPs subjects’ neurons [t(15)=−2.40, p=0.030; Figure 6B]. A similar trend was observed in the number of basal dendrites of Experienced Shams versus Inexperienced Shams [t(13)=−1.29, p=0.221; Figure 6B]. In contrast, untreated HI subjects did not show a difference in the number of basal dendrites as a result of experience [t(23)= −0.08, p=0.936; Figure 6B].
Figure 6.
The number of basal dendrites per neuron (mean±SEM) by experience groups (A) and by treatment within experience groups (B; Experienced Adult: Sham n = 8, HI + Vehicle n = 13, HI + IAIPs n =9, Inexperienced Adult: Sham n = 7, HI + Vehicle n = 12, HI + IAIPs n = 8). #p<0.05 indicates effects of Experience.
3.2.2 Hippocampal CA1 Sholl analysis: Ring intersections (dendritic length)
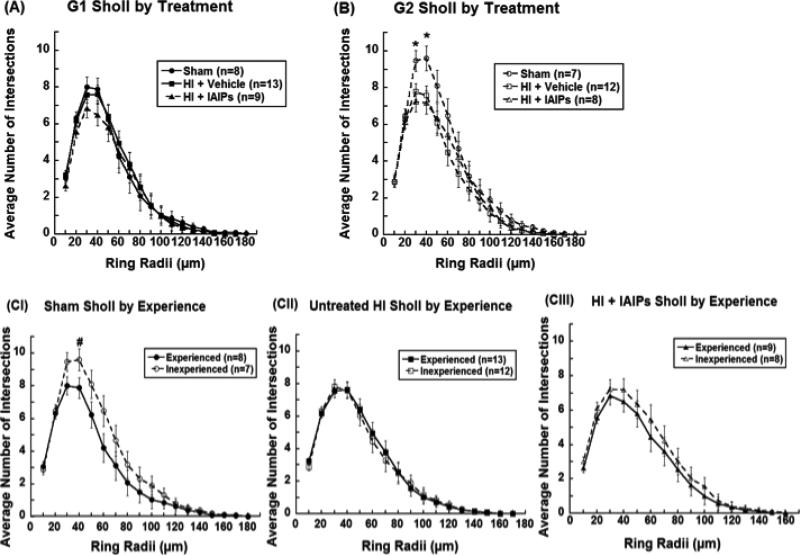
A 20 (ring; 10 μm) x 2 (experience; G1 n = 30 & G2 n = 27) x 3 (treatment; Sham n = 15, HI + Vehicle n = 25, HI + IAIPs n = 17) repeated measure ANOVA revealed an overall effect of Ring [F(3.60,183.39)=57.48 p<0.001], indicating that the number of intersections changed as the rings moved away from the cell body. Mauchly's test indicated that the assumption of sphericity was violated, χ2 (299)=1989.87, p<0.001, therefore degrees of freedom were corrected using Greenhouse-Geisser estimates of spherity (ε= 0.04). When main effects of radii were observed planned comparisons were made to identify potential differences at each intersection. Within Experienced Adults (G1), one-way ANOVAs for each concentric ring did not show a significant effect of Treatment (Sham n = 8, HI + Vehicle n = 13, HI + IAIPs n = 9) on dendritic length (Figure 7A). In the Inexperienced Adults (G2), one-way ANOVAs for each concentric ring revealed significant Treatment (Sham n = 7, HI + Vehicle n = 12, HI + IAIPs n = 8) effects at the 30 μm ring [F(2,24)= 5.29, p=0.013] and 40 μm [F(2,24)= 4.93, p=0.016] (Figure 7B). Tukey's post hoc analysis showed significantly fewer intersections in the injured groups (HI) as compared to Shams at both ring radii (HI + Vehicle p=0.046 and p=0.037 respectively; and HI + IAIPs p=0.012 and p=0.020 respectively; Figure 7B). One-way ANOVAs showed an overall effect of Experience in Sham subjects at 40 μm ring [F(1,13)=6.67, p=0.021; Figure 7-CI], indicating that the basal dendrites of Experienced Sham subjects had fewer intersections (shorter length) with the 40 μm ring as compared to the Inexperienced Shams. Basilar dendrites from the Experienced HI + IAIPs subjects did not show a significant reduction in intersections at 40 μm. However, there was an observable shift towards shorter dendritic length in Experienced HI + IAIPs subjects as compared to Inexperienced HI + IAIPs animals (Figure 7-CIII). Unlike IAIPs treated HI subjects, untreated HI subjects did not show any directional shift in dendritic length due to experience (Figure 7-CII).
Figure 7.
The average number of intersections (mean±SEM) with each concentric ring formed at 10μm intervals by Treatment within G1 Experienced adults (A) and G2 Inexperienced adults (B). Experience effects by each Treatment group (C): Sham (I), HI + Vehicle (II), and HI + IAIPs (III). Effects of Treatment (*p<0.05) as compared to both HI groups (untreated and IAIPs) and Experience effects (#p<0.05) are represented.
4. Discussion
The objectives of this study were to determine the relative effects of treatment with IAIPs and early-life experience on spatial reference and working memory and subsequent hippocampal CA1 basal pyramidal dendritic morphology following neonatal hypoxic-ischemic brain injury in rats. Currently, therapeutic hypothermia is the only clinically available treatment strategy used to attenuate tissue loss resulting from hypoxic-ischemic encephalopathy in full-term infants and treatment is not available for premature infants other than supportive care [21]. Recently, Inter-alpha Inhibitor Proteins (IAIPs) have been identified as broad-spectrum immunomodulators with cytoprotective properties that have been used to significantly increase survival following systemic infection and improve recovery after brain injury in rodent models [20,84-90]. Given the central role of inflammation in neonatal brain injury, the development of therapeutics able to target these core processes offer great advantages to improve long-term neurobehavioral outcomes [91]. We have previously shown that plasma derived IAIPs reduce acute neocortical neuronal cell death and improve brain weight outcome 72 hours after HI injury in the neonatal rat [20]. We have also shown age, task, and treatment dependent improvements in behavioral outcome for both spatial and non-spatial learning after systemic administration of IAIPs in neonatal HI injured rats [20]. In parallel, there has been increased use of early behavioral intervention programs for preterm and at risk infants in clinical settings [92,93]. However, inconsistencies in behavioral intervention approaches, duration of treatment, and results, have made it difficult to make comparisons across studies. This reinforces the need to investigate the most effective approaches to treat cognitive and behavioral deficits resulting from neonatal brain injury [93]. Despite these challenges, behavioral intervention will no doubt increase in parallel with the development of new pharmacological approaches to treat HI injury. Given the potential advantages of both experimental drug therapy and early life behavioral training, combining these approaches to treat neonatal brain injury will likely yield the most favorable benefits for functional recovery as suggested by the findings of the present study.
In general, block effects seen across the three error types, indicating more errors in the first third of testing (Days 2-7) as compared to later blocks of testing (Days 8-13 and 15-20), highlighted the potential for all groups to learn the paradigm. Our results are consistent with the findings of Smith and colleagues [19], who showed that neonatal HI injured rats tested on a modified eight-arm water maze, with incrementally increasing difficulty, were capable of learning. Specifically, improvement of HI injured animals, across several weeks of testing, was similar to the rate of learning shown by sham subjects. These findings suggested that stepwise training might facilitate improvements in long-term performance [19].
Further, the current study shows that early life eight-arm water maze experience improves long-term working and reference memory regardless of brain injury or treatment type. This is supported by the significantly fewer errors made by experienced subjects, across all three error types, as compared to inexperienced subjects. These results support the hypothesis that task specific early experience improves adult learning performance, which is consistent with human clinical findings that early intervention programs can be effective at improving behavioral and sensory processing [38,93-95]. Interestingly, inexperienced adults (G2, untrained normal maturation group), regardless of treatment, did not show significantly better performance on any of the error types as compared to immature juvenile subjects (naïve juveniles (G1A)). This lack of a maturational effect supports the notion that experience effects, shown on reference memory and working memory components of the adult task, reflect the neurobehavioral benefits of early experience rather than the influence of developmental maturation.
These results parallel our previous findings that HI injured subjects treated with IAIPs were impaired as juveniles, on the spatial Morris water maze, but deficits were no longer observed in treated animals tested as adults. However, their untreated HI injured counterparts showed persistent deficits as adults compared to control subjects [20]. Importantly, long-term follow up studies of preterm infants and children with risk factors for learning impairments have shown age dependent changes in learning impairment severity in several cognitive and sensory processing domains, including working memory [96-98]. Both the effects of maturation and experience dependent factors are likely to modify such changes, which are difficult to isolate in studies of human development. Therefore, animal models continue to provide insight into the relative influences of maturation and experience on sensory, behavioral and anatomical development. To our knowledge the current study is the first to employ complementary longitudinal and cross-sectional approaches to evaluate the effects of early life working memory experience on later performance while simultaneously assessing the relative influence of maturation in both typical and pathological conditions.
Centrally, we proposed that combining early experience and an immunomodulatory intervention with IAIPs would improve cognitive performance as task difficulty increased. When treatment groups were analyzed within each experience condition (Juvenile (G1A), Experienced Adult (G1B), Inexperienced Adult (G2)) the progressive difficulty for processing each error type was highlighted and the benefits of combining IAIPs and early training were revealed. For RM errors, there were no effects of HI injury or treatment for any of the groups across experience conditions despite an overall effect of experience. This was likely a result of the relative ease of learning the static non-escape reference arm locations across the four weeks of testing. However, for WMI errors, untreated experienced HI injured subjects made significantly more errors as compared to sham subjects. Whereas, experienced IAIPs treated HI subjects did not significantly differ from shams in the number of WMI errors committed across all days of testing. These findings contrast with the more demanding WMC error type, for which experienced sham subjects made significantly fewer errors as compared to both IAIPs treated and untreated experienced HI animals.
In addition to the three levels of cognitive demand indicated by the three error types (RM, WMI, WMC), task demand increased on subsequent trials (trials 1-4), within a given testing session, as platforms were removed and working memory load increased [61,64,66,69,71]. Previous studies have shown that HI deficits become more pronounced on tasks with higher working memory load [10,19,66]. On the moderately challenging WMI error component of the current study, planned comparisons showed that untreated experienced HI subjects (G1B/HI + Saline) made significantly more errors than sham animals at peak working memory load (trial 4). In contrast, IAIPs treated HI injured subjects with early working memory experience (G1B/HI + IAIPs) did not significantly differ from shams on the WMI error component of the task as demand increased (trials 2-4). This finding further supports the hypothesis that combining behavioral and immunomodulatory (IAIPs) treatment can yield better outcomes as compared to a single intervention, even on relatively high demand cognitive assessments (e.g., WMI).
For the most challenging error type (WMC), experienced HI injured rats (both untreated and IAIPs treated) showed robust deficits on trial 4 (highest working memory demand) as compared to sham subjects. However, during naïve (juvenile (G1A) and inexperienced adult (G2)) testing, uninjured subjects had difficulty avoiding arms previously used for escape (WMC errors), which further highlights the varying levels of difficulty embedded within the current paradigm. Future studies will seek to increase the dose of IAIPs and/or the number of testing days in order to define the limits of working memory correct performance after experimental treatment in HI injured animals. However, our current findings emphasize the importance of testing subjects on cognitive and behavioral tasks with varying levels of difficulty when evaluating experimental interventions. More broadly, the combined benefits of IAIPs administration and early working memory training after neonatal brain injury, have direct clinical implications, as preterm infants show cognitive deficits when memory demand is highest as compared to age and IQ matched term infants [10, 99,100]. Our findings may be used to inform clinical practice as new pharmacological and behavioral interventions become available.
In the current study, juvenile testing (P36+) was performed during peak periods of synaptogenesis and synaptic pruning that are important for proper formation of cognitive-dependent circuitry [101]. Experience dependent pruning has been suggested to produce more efficient cortical circuitry, which may drive higher order adult cognition [101]. Here we showed that experienced subjects had fewer basal dendrites on CA1 hippocampal neurons, in the left hemisphere (contralateral to RCCA cauterization) as compared to inexperienced subjects, regardless of treatment type. Further, experienced sham subjects showed a significant reduction in CA1 basal pyramidal dendrite length and a trend towards fewer basal dendrites, which may have contributed to the robust improvements seen in behavioral performance across ages of testing. Similarly, results showed that HI injured subjects treated with IAIPs and early working memory experience, had fewer basal CA1 dendrites and a trend towards decreased length as compared to their untrained IAIPs treated counterparts. These results are complemented by findings from other groups using alternative forms of behavioral intervention, which showed changes in dendritic morphology in frontal and parietal cortices after training. Specifically, T-maze training led to a decrease in basal dendritic length of neurons located in the medial prefrontal cortex as compared to untrained rats [42]. In addition, pyramidal neurons from parietal cortices of rats that received spatial training following experimentally induced brain lesions showed reduced branch order, number of branches, and dendritic length as compared to motor training and untrained experimentally lesioned controls [44]. Furthermore, the complexity of an enrichment task, rather than the duration of the task, has been shown to result in more robust changes in dendritic morphology during normal development and after traumatic brain injury in rats [42,102,103]. Given the complex nature of the present task and the extended testing period, both factors likely played a role in facilitating the observed anatomical alterations. Importantly, a follow up study in preterm humans at ages 7-12 years, revealed that visuospatial regions linked to working memory had increased activation in preterm subjects as compared to their aged match controls [104]. Similarly, preterm subjects tested as adolescents (9-16 years old) showed increased activation of a larger neuronal network compared to age-matched control term subjects [105]. Although it is not clear whether this change in activation/metabolism was due to increased demand on an impaired system or recruiting of alternative networks, both human and animal studies suggest that more branches and/or greater activation does not necessarily reflect better behavioral performance. Evidence of basal pyramidal CA1 neuronal pruning in sham subjects and IAIPs treated HI injured animals may reflect a greater degree of synaptic refinement, which could have improved processing efficiencies reflected in better working memory performance. Future studies will explore the relationship between training duration and task difficulty across divergent brain regions in order to identify which variables have the greatest impact on anatomical outcome. None-the-less, results of the current study support the notion that a reduction in dendritic length and number of pyramidal basal dendrites may be indicative of a more efficient circuitry following early task-specific experience and subtle changes in these morphological patterns may be further spared with the addition of IAIPs in neonatal HI injured subjects [77-81].
Interestingly, P7 HI injury has consistently been linked to decreased hippocampal volumes and associated behavioral deficits on spatially mediated learning and memory tasks [12,14,16,17,19,20,54]. In preterm human infants, reductions of hippocampal volumes have been associated with working memory deficits [106]. However, gross histological measurements of the hippocampus do not provide insight into changes in individual cell morphologies. In contrast, because Golgi-Cox staining labels a subset of neurons, the absolute number of these cells in the brain tissue cannot be determined even though the technique does permit detailed analysis of cell structure. Thus, our results do not undermine the findings of tissue loss and correlated neurobehavioral deficits as a result of HI injury shown in our previous experiments and those of other groups [20,45,55]. Instead, the results of this study are limited to commenting on the morphology of individual neurons, which may suggest that dendritic pruning leads to increased efficiency of CA1 neurons following early experience [77-81].
Conclusions
In summary, changes in neurobehavioral performance on the eight-arm-radial water maze and changes in CA1 cell morphology support the notion that performance improvements can be achieved by combining immunomodulatory IAIPs and early task-specific training in rats with neonatal HI brain injury. These findings have translational implications for a proactive, combined therapeutic intervention (IAIPs in conjunction with early task specific training) to attenuate brain injury and provide a long-term cognitive advantage for spatial working memory in rats, which could be extended to the human population. The current study also extends previous findings, which showed that treatment with IAIPs improved relativity basic spatial and non-spatial reference learning after neonatal brain injury [20], to more demanding working memory performance, at least when treatment was paired with early task specific experience.
Highlights.
Early life working memory (WM) experience improves adult memory performance.
IAIPs and early experience improve moderately demanding working memory performance in neonatal HI injured rats.
Early WM experience led to a significant reduction in hippocampal CA1 basal dendrite length in control animals.
IAIPs and early WM experience resulted in fewer basal hippocampal CA1 dendrites in HI injured rats.
Acknowledgements
The authors would like to acknowledge and thank Keyshla and Zahra Melendez, Stephanie Chauvin, and Travis Dumais for their assistance with behavioral testing and histological processing. Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under grant 1R15HD077544 and the National Institute of General Medical Sciences of the National Institutes of Health under award number NIH/NINDS: 1 R43 NS084575-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huang BY, Castillo M. Hypoxic-ischemic brain injury: Imaging findings from birth to adulthood. Radiographics. 2008;28(2):417–39. doi: 10.1148/rg.282075066. doi:10.1148/rg.282075066. [DOI] [PubMed] [Google Scholar]
- 2.Perlman JM. Intervention strategies for neonatal hypoxic-ischemic cerebral injury. Clin Ther. 2006;28(9):1353–65. doi: 10.1016/j.clinthera.2006.09.005. doi: http://dx.doi.org/10.1016/j.clinthera.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Volpe JJ. Perinatal brain injury: From pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev. 2001;7(1):56–64. doi: 10.1002/1098-2779(200102)7:1<56::AID-MRDD1008>3.0.CO;2-A. doi:10.1002/1098-2779(200102)7:1<56::AID-MRDD1008>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 4.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110–24. doi: 10.1016/S1474-4422(08)70294-1. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conklin HM, Salorio CF, Slomine BS. Working memory performance following pediatric traumatic brain injury. Brain Inj. 2008;22:847–57. doi: 10.1080/02699050802403565. doi:10.1080/02699050802403565. [DOI] [PubMed] [Google Scholar]
- 6.Edgin JO, Inder TE, Anderson PJ, Hood KM, Clark CA, Woodward LJ. Executive functioning in preschool children born very preterm: Relationship with early white matter pathology. J Int Neuropsychol Soc. 2008;14(1):90–101. doi: 10.1017/S1355617708080053. doi: http://dx.doi.org/10.1017/S1355617708080053. [DOI] [PubMed] [Google Scholar]
- 7.Espy KA, Stalets MM, McDiarmid MM, Senn TE, Cwik MF, Hamby A. Executive functions in preschool children born preterm: application of cognitive neuroscience paradigms. Child Neuropsychol. 2002;8(2):83–92. doi: 10.1076/chin.8.2.83.8723. doi:10.1076/chin.8.2.83.8723. [DOI] [PubMed] [Google Scholar]
- 8.Gadian DG, Aicardi J, Watkins KE, Porter DA, Mishkin M, Vargha-Khadem F. Developmental amnesia associated with early hypoxic-ischaemic injury. Brain. 2000;123:499–507. doi: 10.1093/brain/123.3.499. doi: http://dx.doi.org/10.1093/brain/123.3.499. [DOI] [PubMed] [Google Scholar]
- 9.Hagberg H, Ichord R, Palmer C, Yager JY, Vannucci SJ. Animal models of developmental brain injury: relevance to human disease. A summary of the panel discussion from the Third Hershey Conference on Developmental Cerebral Blood Flow and Metabolism. Dev Neurosci. 2002;24:364–6. doi: 10.1159/000069040. doi:10.1159/000069040. [DOI] [PubMed] [Google Scholar]
- 10.Luciana M, Lindeke L, Georgieff M, Mills M, Nelson CA. Neurobehavioral evidence for working-memory deficits in school-aged children with histories of prematurity. Dev Med Child Neurol. 1999;41:521–33. doi: 10.1017/s0012162299001140. doi:10.1111/j.1469-8749.1999.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 11.Luu TM, Ment L, Allan W, Schneider K, Vohr BR. Executive and memory function in adolescents born very preterm. Pediatrics. 2011;127(3):e639–46. doi: 10.1542/peds.2010-1421. doi:10.1542/peds.2010-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith AL, Alexander M, Rosenkrantz TS, Sadek ML, Fitch RH. Sex differences in behavioral outcome following neonatal hypoxia ischemia: Insights from a clinical meta-analysis and a rodent model of induced hypoxic ischemic brain injury. Exp Neurol. 2014;254:54–67. doi: 10.1016/j.expneurol.2014.01.003. doi:10.1016/j.expneurol.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Woodward LJ, Edgin JO, Thompson D, Inder TE. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain. 2005;128:2578–87. doi: 10.1093/brain/awh618. doi: http://dx.doi.org/10.1093/brain/awh618. [DOI] [PubMed] [Google Scholar]
- 14.Alexander M, Garbus H, Smith AL, Rosenkrantz TS, Fitch RH. Behavioral and histological outcomes following neonatal HI injury in a preterm (P3) and term (P7) rodent model. Behav Brain Res. 2014;259:85–96. doi: 10.1016/j.bbr.2013.10.038. doi:10.1016/j.bbr.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golan H, Huleihel M. The effect of prenatal hypoxia on brain development: short- and long-term consequences demonstrated in rodent models. Dev Sci. 2006;9(4):338–49. doi: 10.1111/j.1467-7687.2006.00498.x. doi:10.1111/j.1467-7687.2006.00498.x. [DOI] [PubMed] [Google Scholar]
- 16.Pereira LO, Arteni NS, Petersen RC, da Rocha AP, Achaval M, Netto CA. Effects of daily environmental enrichment on memory deficits and brain injury following neonatal hypoxia-ischemia in the rat. Neurobiol Learn Mem. 2007;87(1):101–8. doi: 10.1016/j.nlm.2006.07.003. doi:10.1016/j.nlm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Pereira LO, Strapasson ACP, Nabinger PM, Achaval M, Netto CA. Early enriched housing results in partial recovery of memory deficits in female, but not in male, rats after neonatal hypoxia-ischemia. Brain Res. 2008;1218:257–68. doi: 10.1016/j.brainres.2008.04.010. doi: 10.1016/j.brainres.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Rojas JJ, Deniz BF, Miguel PM, Diaz R, Hermel EES, Achaval M, Netto CA, Pereira LO. Effects of daily environmental enrichment on behavior and dendritic spine density in hippocampus following neonatal hypoxia-ischemia in the rat. Exp Neurol. 2013;241:25–33. doi: 10.1016/j.expneurol.2012.11.026. doi: 10.1016/j.expneurol.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Smith AL, Hill CA, Alexander M, Szalkowski CE, Chrobak JJ, Rosenkrantz TS, Fitch RH. Spatial working memory deficits in male rats following neonatal hypoxic ischemic brain injury can be attenuated by task modifications. Brain Sci. 2014;4:240–72. doi: 10.3390/brainsci4020240. doi: 10.3390/brainsci4020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Threlkeld SW, Gaudet CM, La Rue ME, Dugas E, Hill CA, Lim YP, Stonestreet BS. Effects of inter-alpha inhibitor proteins on neonatal brain injury: Age, task and treatment dependent neurobehavioral outcomes. Exp Neurol. 2014;261(1):424–33. doi: 10.1016/j.expneurol.2014.07.012. doi: 10.1016/j.expneurol.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah PS, Ohlsson A, Perlman M. Hypothermia to treat neonatal hypoxic ischemic encephalopathy: systematic review. Arch Pediatr Adolesc Med. 2007;161(10):951–8. doi: 10.1001/archpedi.161.10.951. doi:10.1001/archpedi.161.10.951. [DOI] [PubMed] [Google Scholar]
- 22.Laptook AR, McDonald SA, Shankaran S, Stephens BE, Vohr BR, Guillet R, Higgins RD, Das A. Elevated temperature and 6- to 7-year outcome of neonatal encephalopathy. Ann Neurol. 2013;73:520–8. doi: 10.1002/ana.23843. doi:10.1002/ana.23843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encepthalopathy: mulicentre randomised trial. Lancet. 2005;354(9460):663–70. doi: 10.1016/S0140-6736(05)17946-X. doi: http://dx.doi.org/10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 24.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotton CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. doi:10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 25.Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, Gustafson KE, Leach TM, Green C, Bara R, Huitema CMP, Ehrenkranz RA, Tyson JE, Das A, Hammond J, Peralta-Carcelen M, Evans PW, Heyne RJ, Wilson-Costello DE, Vaucher YE, Bauer CR, Dusick AM, Adams-Chapman I, Goldstein RF, Guillet R, Papile LA, Higgins RD. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366(22):2085–92. doi: 10.1056/NEJMoa1112066. doi:10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bost F, Diarra-Mehrpour M, Martin JP. Inter-alpha-trypsin inhibitor proteoglycan family—a group of proteins binding and stabilizing the extracellular matrix. Eur J Biochem. 1998;252(3):339–46. doi: 10.1046/j.1432-1327.1998.2520339.x. doi: 10.1046/j.1432-1327.1998.2520339.x. [DOI] [PubMed] [Google Scholar]
- 27.Businaro R, Leali FM, De Renzis G, Pompili E, Pagliari G, Menghi G, Fumagalli L. Inter-alpha-trypsin inhibitor-related immunoreactivity in human tissues and body fluids. Cell Mol Biol. 1992;38(4):463–71. [PubMed] [Google Scholar]
- 28.Fries E, Blom AM. Bikunin—not just a plasma proteinase inhibitor. Int. J. Biochem Cell Biol. 2000;32(2):125–37. doi: 10.1016/s1357-2725(99)00125-9. doi:10.1016/S1357-2725(99)00125-9. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi H. Endogenous anti-inflammatory substances, inter-alpha-inhibitor and bikunin. Biol Chem. 2006;387(12):1545–9. doi: 10.1515/BC.2006.192. doi: 10.1515/BC.2006.192. [DOI] [PubMed] [Google Scholar]
- 30.Lim YP. ProThera Biologics, Inc.: a novel immunomodulator and biomarker for life-threatening diseases. RI Med J. 2013;96(2):16–8. [PubMed] [Google Scholar]
- 31.Salier JP, Rouet P, Raguenez G, Daveau M. The inter-alpha-inhibitor family: from structure to regulation. Biochem J. 1996;315(1):1–9. doi: 10.1042/bj3150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhuo L, Kimata K. Structure and function of inter-alpha-trypsin inhibitor heavy chains. Connect Tissue Res. 2008;49(5):311–20. doi: 10.1080/03008200802325458. doi: 10.1080/03008200802325458. [DOI] [PubMed] [Google Scholar]
- 33.Garantziotis S, Hollingsworth JW, Ghanayem RB, Timberlake S, Zhuo L, Kimata K, Schwartz DA. Inter-alpha-trypsin inhibitor attenuates complement activation and complement-induced lung injury. J Immunol. 2007;179(6):4187–92. doi: 10.4049/jimmunol.179.6.4187. doi: 10.4049/jimmunol.179.6.4187. [DOI] [PubMed] [Google Scholar]
- 34.Opal SM, Artenstein AW, Cristofaro PA, Jhung JW, Palardy JE, Parejo NA, Lim YP. Inter-alpha inhibitor proteins are endogenous furin inhibitors and provide protection against experimental anthrax intoxication. Infect Immun. 2005;73(8):5101–5. doi: 10.1128/IAI.73.8.5101-5105.2005. doi: 10.1128/IAI.73.8.5101-5105.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Threlkeld SW, Hill CA, Rosen GD, Fitch RH. Early acoustic discrimination experience ameliorates auditory processing deficits in male rats with cortical developmental disruptions. Int J Dev Neurosci. 2009;27(4):321–8. doi: 10.1016/j.ijdevneu.2009.03.007. doi: 10.1016/j.ijdevneu.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tallal P. Improving language and literacy is a matter of time. Nat Rev Neurosci. 2004;5(9):721–8. doi: 10.1038/nrn1499. doi:10.1038/nrn1499. [DOI] [PubMed] [Google Scholar]
- 37.Tallal P, Miller S, Bedi G, Byma G, Wang X, Nagarjan S, Screiner C, Jenkins W, Marzenich M. Language Comprehension in language-learning impaired children improved with acoustically modified speech. Science. 1996;271:81–4. doi: 10.1126/science.271.5245.81. doi:10.1126/science.271.5245.81. [DOI] [PubMed] [Google Scholar]
- 38.Temple E, Deutsch G, Poldrack R, Miller S, Tallal P, Merzenich M, Gabrieli J. Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. P Natl Acad Sci USA. 2003;100(5):2860–5. doi: 10.1073/pnas.0030098100. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merzenich M, Jenkins W, Johnston P, Schreiner C, Miller S, Tallal P. Temporal processing deficit of language-learning impaired children ameliorated by training. Science. 1996;271:77–80. doi: 10.1126/science.271.5245.77. doi:10.1126/science.271.5245.77. [DOI] [PubMed] [Google Scholar]
- 40.Kraus N, Slater J, Thompson EC, Hornickel J, Strait DL, Nicol T, White-Schwoch T. Music enrichment programs improve the neural encoding of speech in at-risk children. J Neurosci. 2014;34(36):11913–8. doi: 10.1523/JNEUROSCI.1881-14.2014. doi:10.1523/JNEUROSCI.1881-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaab N, Gabrieli JDE, Deutsch GK, Tallal P, Temple E. Neural correlates of rapid auditory processing are disrupted in children with developmental dyslexia and ameliorated with training: an fMRI study. Restor Neurol Neurosci. 2007;25(3-4):295–310. [PubMed] [Google Scholar]
- 42.Comeau WL, McDonald RJ, Kolb BE. Learning-induced alterations in prefrontal cortical dendritic morphology. Behav Brain Res. 2010;214:91–101. doi: 10.1016/j.bbr.2010.04.033. doi:10.1016/j.bbr.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 43.Sutherland R, Gibb R, Kolb B. The hippocampus makes a significant contribution to experience-dependent neocortical plasticity. Behav Brain Res. 2010;214:121–4. doi: 10.1016/j.bbr.2010.05.051. doi:10.1016/j.bbr.2010.05.051. [DOI] [PubMed] [Google Scholar]
- 44.Hoh TE, Kolb B, Eppel A, Vanderwolf CH, Cain DP. Role of the neocortex in the water maze task in the rat: a detailed behavioral and Golgi-Cox analysis. Behav Brain Res. 2003;138:81–94. doi: 10.1016/s0166-4328(02)00237-1. doi:10.1016/S0166-4328(02)00237-1. [DOI] [PubMed] [Google Scholar]
- 45.Hill CA, Threlkeld SW, Fitch RH. Early testosterone modulated sex differences in behavioral outcome following neonatal hypoxia ischemia in rats. Int J Dev Neurosci. 2011;29(4):381–8. doi: 10.1016/j.ijdevneu.2011.03.005. doi:10.1016/j.ijdevneu.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peiffer AM, Rosen GD, Fitch RH. Sex differences in rapid auditory processing deficits in microgyric rats. Brain Res. Dev. Brain Res. 2004;148:53–7. doi: 10.1016/j.devbrainres.2003.09.020. doi:10.1016/j.devbrainres.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Aylward GP. Cognitive and neuropsychological outcomes: more than IQ scores. Ment Retard Dev Disabil Res Rev. 2002;8(4):234–40. doi: 10.1002/mrdd.10043. doi: 10.1002/mrdd.10043. [DOI] [PubMed] [Google Scholar]
- 48.Aylward GP. Neurodevelopmental outcomes of infants born prematurely. J Dev Behav Pediatr. 2005;26(6):427–40. doi: 10.1097/00004703-200512000-00008. doi: http://dx.doi.org/10.1097/00004703-200512000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Begega A, Mendez Lopez M, de Iscar MJ, Cuesta-Izquierdo M, Solis G, Fernandez- Colomer B, Arias JL. Assessment of the global intelligence and selective cognitive capacities in preterm 8-year-old children. Psicothema. 2010;22(4):648–53. [PubMed] [Google Scholar]
- 50.Donders J, Hoffman NM. Gender differences in learning and memory after pediatric traumatic brain injury. Neuropsychology. 2002;16(4):491–9. doi: 10.1037//0894-4105.16.4.491. doi: http://dx.doi.org/10.1037/0894-4105.16.4.491. [DOI] [PubMed] [Google Scholar]
- 51.Lauterbach MD, Raz S, Sander CJ. Neonatal hypoxic risk in preterm birth infants: the influence of sex and severity of respiratory distress on cognitive recovery. Neuropsychology. 2001;15(3):411–20. doi: http://dx.doi.org/10.1037/0894-4105.15.3.411. [PubMed] [Google Scholar]
- 52.Peacock JL, Marston L, Marlow N, Calvert SA, Greenough A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr Res. 2012;71(3):305–10. doi: 10.1038/pr.2011.50. doi: 10.1038/pr.2011.50. [DOI] [PubMed] [Google Scholar]
- 53.Raz S, Lauterbach MD, Hopkins TL, Glogowski BK, Porter CL, Riggs WW, Sander CJ. A female advantage in cognitive recovery from early cerebral insult. Dev Psychol. 1995;31(6):958–66. doi: http://dx.doi.org/10.1037/0012-1649.31.6.958. [Google Scholar]
- 54.McClure MM, Threlkeld SW, Fitch RH. Auditory processing and learning/memory following erythropoietin administration in neonatal hypoxicischemic injury. Brain Res. 2007;1132(1):203–9. doi: 10.1016/j.brainres.2006.11.006. doi:10.1016/j.brainres.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 55.McClure MM, Threlkeld SW, Rosen GD, Fitch RH. Auditory processing and learning deficits in rats with P1 versus P7 neonatal hypoxic-ischemic injury. Behav Brain Res. 2006;172:114–21. doi: 10.1016/j.bbr.2006.05.003. doi:10.1016/j.ijdevneu.2004.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rice III JE, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9(2):131–41. doi: 10.1002/ana.410090206. doi:10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 57.Mishima K, Ikeda T, Aoo N, Takai N, Takahashi S, Egashira N, Ikenoue T, Iwasaki K, Fujiwara M. Hypoxia-ischemic insult in neonatal rats induced slowly progressive brain damage related to memory impairment. Neurosci Lett. 2005;376(3):194–9. doi: 10.1016/j.neulet.2004.11.055. doi:10.1016/j.neulet.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 58.Mishima K, Ikeda T, Yoshikawa T, Aoo N, Egashira N, Xia YX, Ilenoue T, Iwasaki K, Fujiwara M. Effects of hypothermia and hyperthermia on attentional and spatial learning deficits following neonatal hypoxia-ischemic insult in rats. Behav Brain Res. 2004;151(1-2):209–17. doi: 10.1016/j.bbr.2003.08.018. doi:10.1016/j.bbr.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 59.Marks K, Shany E, Shelef I, Golan A, Zmora E. Hypothermia: A neuroprotective therapy for hypoxic ischemic encephalopathy. Isr Med Assoc J. 2010;12(8):494–500. [PubMed] [Google Scholar]
- 60.Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrino. 1999;24(2):161–73. doi: 10.1016/s0306-4530(98)00068-7. doi:10.1016/S0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- 61.Bimonte HA, Denenberg VH. Sex differences in vicarious trial-and-error behavior during radial arm maze learning. Physiol Behav. 2000;68:495–9. doi: 10.1016/s0031-9384(99)00201-2. doi:10.1016/S0031-9384(99)00201-2. [DOI] [PubMed] [Google Scholar]
- 62.Bimonte HA, Grandolm AC, Seo H, Isacson O. Spatial memory testing decreases hippocampal amyloid precursor protein in young, but not aged, female rats. Neurosci Lett. 2002;328(1):50–4. doi: 10.1016/s0304-3940(02)00442-1. doi:10.1016/S0304-3940(02)00442-1. [DOI] [PubMed] [Google Scholar]
- 63.Bimonte HA, Nelson M, Granholm AC. Age-related deficits as working memory load increases: relationships with growth factors. Neurobiol Aging. 2003;24:37–48. doi: 10.1016/s0197-4580(02)00015-5. doi:10.1016/S0197-4580(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 64.Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH. In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Phys Behav. 2000;70:311–7. doi: 10.1016/s0031-9384(00)00259-6. doi:10.1016/S0031-9384(00)00259-6. [DOI] [PubMed] [Google Scholar]
- 65.Bimonte-Nelson H, Singleton R, Hunter C, Price K, Moore A, Granholm A-CE. Ovarian hormones and cognition in the aged female rat: I. Long-term, but not short-term, ovariectomy enhances spatial performance. Behav Neurosci. 2003;117(6):1395–406. doi: 10.1037/0735-7044.117.6.1395. doi: http://dx.doi.org/10.1037/0735-7044.117.6.1395. [DOI] [PubMed] [Google Scholar]
- 66.Penley SC, Gaudet CM, Threlkeld SW. Use of an eight-arm radial water maze to assess working memory following neonatal brain injury. J Vis Exp. 2013;82:e50940. doi: 10.3791/50940. doi:10.3791/50940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Braden B, Garcia A, Mennenga S, Prokai L, Villa S, Acosta J, Lefort N, Simard AR, Bimonte-Nelson HA. Cognitive-impairing effects of medroxyprogesterone acetate in the rat: independent and interactive effects across time. Psychopharmacology (Berl) 2011;218(2):405–18. doi: 10.1007/s00213-011-2322-4. doi:10.1007/s00213-011-2322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Braden B, Talboom J, Crain I, Simard A, Lukas R, Prokai L, Scheldrup MR, Bowman BL, Bimonte-Nelson HA. Medroxyprogesterone acetate impairs memory and alters the GABAergic system in aged surgically menopausal rats. Neurobiol Learn Mem. 2010;93(3):444–53. doi: 10.1016/j.nlm.2010.01.002. doi:10.1016/j.nlm.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hyde LA, Hoplight BJ, Denenberg VH. Water version of the radial-arm maze: Learning in three inbred strains of mice. Brain Res. 1998;785:236–44. doi: 10.1016/s0006-8993(97)01417-0. doi:10.1016/S0006-8993(97)01417-0. [DOI] [PubMed] [Google Scholar]
- 70.Jarrard LE, Okaichi H, Steward O, Goldschmidt RB. On the role of hippocampal connections in the performance of place and cue tasks: comparisons with damage to hippocampus. Behav Neurosci. 1984;98:946–54. doi: 10.1037//0735-7044.98.6.946. doi: http://dx.doi.org/10.1037/0735-7044.98.6.946. [DOI] [PubMed] [Google Scholar]
- 71.Mennenga SE, Gerson JE, Koebele SV, Kingston ML, Tsang CWS, Engler-Chiurazzi EB, Baxter LC, Bimonte-Nelson HA. Understanding the cognitive impact of the contraceptive estrogen Ethinyl Estradiol: Tonic and cyclic administration impairs memory, and performance correlates with basal forebrain cholinergic system integrity. Psychoneuroendocrino. 2015;54:1–13. doi: 10.1016/j.psyneuen.2015.01.002. doi:10.1016/j.psyneuen.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whishaw IQ, Pasztor TJ. Rats alternate on dry-and but not swimming-pool (Morris Task) place task: Implications for spatial processing. Behav Neurosci. 2000;114(2):442–6. doi: 10.1037//0735-7044.114.2.442. doi: http://dx.doi.org/10.1037/0735-7044.114.2.442. [DOI] [PubMed] [Google Scholar]
- 73.Paxinos G, Watson C. The Rat Brain In Stereotaxic Coordinates. Compact 6th ed Elsevier; London: 2009. [Google Scholar]
- 74.Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J Neurosci Methods. 1998;79:1–4. doi: 10.1016/s0165-0270(97)00163-5. doi:10.1016/S0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- 75.Kolb B, Gibb R, Gorny G. Experience-dependent changes in dendritic arbor and spine density in neocortex vary qualitatively with age and sex. Neurobiol Learn Mem. 2003;79(1):1–10. doi: 10.1016/s1074-7427(02)00021-7. doi:10.1016/S1074-7427(02)00021-7. [DOI] [PubMed] [Google Scholar]
- 76.Maiti P, Muthuraju S, Ilavazhagan G, Singh S. Hypobaric hypoxia induces dendritic plasticity in cortical and hippocampal pyramidal neurons in rat brain. Behav Brain Res. 2008;189:233–43. doi: 10.1016/j.bbr.2008.01.007. doi:10.1016/j.bbr.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 77.Zhao YD, Ou S, Cheng SY, Xiao Z, He WJ, Zhang JH, Ruan HZ. Dendritic development of hippocampal CA1 pyramidal cells in neonatal hypoxia-ischemia injury model. J Neurosci Res. 2013;91(9):1165–73. doi: 10.1002/jnr.23247. doi: 10.1002/jnr.23247. [DOI] [PubMed] [Google Scholar]
- 78.Globus A, Rosenzweig MR, Bennett EL, Diamond MC. Effects of differential experience on dendritic spine counts in rat cerebral cortex. Journal of Comparative and Physiological Psychology. 1973;82:175–81. doi: 10.1037/h0033910. doi: http://dx.doi.org/10.1037/h0033910. [DOI] [PubMed] [Google Scholar]
- 79.Gordon U, Polsky A, Schiller J. Plasticity compartments in basal dendrites of neocortex. J Neurosci. 2006;26(49):12717–12726. doi: 10.1523/JNEUROSCI.3502-06.2006. doi:10.1523/JNEUROSCI.3502-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Greenough WT. Enduring brain effects of differential experience and training. In: Rosenzweig MR, Bennett EL, editors. Neural mechanisms of learning and memory. MIT Press; Cambridge, MA: 1976. pp. 255–278. [Google Scholar]
- 81.Mychasiuk R, Gibb R, Kolb B. Prenatal stress alters dendritic morphology and synaptic connectivity in the prefrontal cortex and hippocampus of developing offspring. Synapse. 2012;66:308–14. doi: 10.1002/syn.21512. doi: 10.1002/syn.21512. [DOI] [PubMed] [Google Scholar]
- 82.Wiener SI. Spatial, behavioral and sensory correlates of hippocampal CA1 complex spike cell activity: Implications for information processing functions. Progress in Neurobio. 1996;49(4):335–61. doi: 10.1016/0301-0082(96)00019-6. doi:10.1016/0301-0082(96)00019-6. [DOI] [PubMed] [Google Scholar]
- 83.Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the rat. J Anat. 1953;87(Pt 4):387–406. [PMC free article] [PubMed] [Google Scholar]
- 84.Baek YW, Brokat S, Padbury JF, Pinar H, Hixson DC, Lim YP. Inter-alpha inhibitor proteins in infants and decreased levels in neonatal sepsis. J Pediatr. 2003;143(1):11–5. doi: 10.1016/S0022-3476(03)00190-2. doi:10.1016/S0022-3476(03)00190-2. [DOI] [PubMed] [Google Scholar]
- 85.Chaaban H, Shin M, Sirya E, Lim YP, Caplan M, Padbury JF. Inter-alpha inhibitor proteins level in neonates predicts necrotizing enterocolitis. J Pediatr. 2010;157(5):757–61. doi: 10.1016/j.jpeds.2010.04.075. doi:10.1016/j.jpeds.2010.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chaaban H, Singh K, Huang J, Siryaporin E, Lim YP, Padbury JF. The role of inter-alpha inhibitor protein in the diagnosis of neonatal sepsis. J Pediatr. 2009;154(4):620–2. doi: 10.1016/j.jpeds.2008.10.008. doi:10.1016/j.jpeds.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 87.Koga Y, Fujita M, Tsuruta R, Koda Y, Nakahara T, Yagi T, Aoki T, Kobayashi C, Izumi T, Kasaoka S, Yuasa M, Maekawa T. Urinary trypsin inhibitor suppresses excessive superoxide anion radical generation in blood, oxidative stress, early inflammation, and endothelial injury in forebrain ischemia/reperfusion rats. Neurol Res. 2010;32:925–32. doi: 10.1179/016164110X12645013515133. doi: http://dx.doi.org/10.1179/016164110X12645013515133. [DOI] [PubMed] [Google Scholar]
- 88.Singh K, Zhang LX, Bendelja K, Heath R, Murphy S, Sharma S, Padbury JF, Lim YP. Inter-alpha inhibitor protein administration improves survival from neonatal sepsis in mice. Pediatr Res. 2010;68(3):242–7. doi: 10.1203/PDR.0b013e3181e9fdf0. doi:10.1203/PDR.0b013e3181e9fdf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang X, Xue Q, Yan F, Li L, Liu J, Li S, Hu S. Ulinastatin as a neuroprotective and anti-inflammatory agent in infant piglets model undergoing surgery on hypothermic low-flow cardiopulmonary bypass. Paediatr Anaesth. 2013;23:209–16. doi: 10.1111/pan.12073. doi: 10.1111/pan.12073. [DOI] [PubMed] [Google Scholar]
- 90.Yano T, Anraku S, Nakayama R, Ushijima K. Neuroprotective effect of urinary trypsin inhibitor against focal cerebral ischemia-reperfusion injury in rats. Anesthesiology. 2003;98(2):465–73. doi: 10.1097/00000542-200302000-00028. doi: 10.1097/00000542-200302000-00028. [DOI] [PubMed] [Google Scholar]
- 91.Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351(19):1985–95. doi: 10.1056/NEJMra041996. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 92.Ramachandran S, Dutta S. Early developmental care interventions of preterm very low birth weight infants. Indian Pediatr. 2013;50(8):765–70. doi: 10.1007/s13312-013-0221-y. doi: 10.1007/s13312-013-0221-y. [DOI] [PubMed] [Google Scholar]
- 93.Spittle A, Orton J, Anderson P, Boyd R, Doyle LW. Early developmental intervention programmes post-hospital discharge to prevent motor and cognitive impairments preterm infants. Cochrane Database Syst Rev. 2012;12:CD005495. doi: 10.1002/14651858.CD005495.pub3. doi: 10.1002/14651858.CD005495.pub3. [DOI] [PubMed] [Google Scholar]
- 94.Buschkuehl M, Hernandez-Garcia L, Jaeggi SM, Bernard JA, Jonides J. Neural effects of short-term training on working memory. Cogn Affect Behav Neurosci. 2014;14(1):147–60. doi: 10.3758/s13415-013-0244-9. doi: 10.3758/s13415-013-0244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Buschkuehl M, Jaeggi SM, Jonides J. Neuronal effects following working memory training. Dev Cogn Neurosci. 2012;2:S167–79. doi: 10.1016/j.dcn.2011.10.001. doi:10.1016/j.dcn.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Vries LS, Jongmans MJ. Long-term outcome after neonatal hypoxic- ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2010;95:F220–4. doi: 10.1136/adc.2008.148205. doi:10.1136/adc.2008.148205. [DOI] [PubMed] [Google Scholar]
- 97.Hautus MJ, Setchell GJ, Waldie KE, Kirk IJ. Age-related improvement in auditory temporal resolution in reading-impaired children. Dyslexia. 2003;9(1):37–45. doi: 10.1002/dys.234. doi:10.1002/dys.234. [DOI] [PubMed] [Google Scholar]
- 98.Tideman E. Longitudinal follow-up of children born preterm: Cognitive development at age 19. Early Hum Dev. 2000;58(2):81–90. doi: 10.1016/s0378-3782(00)00055-4. doi:10.1016/S0378-3782(00)00055-4. [DOI] [PubMed] [Google Scholar]
- 99.Curtis WJ, Lindeke LL, Georgieff MK, Nelson CA. Neurobehavioural functioning in neonatal intensive care unit graduates in late childhood and early adolescence. Brain. 2002;125:1646–59. doi: 10.1093/brain/awf159. doi: http://dx.doi.org/10.1093/brain/awf159. [DOI] [PubMed] [Google Scholar]
- 100.Vicari S, Caravale B, Carlesimo GA, Casadei AM, Allemand F. Spatial working memory deficits in children at ages 3-4 who were low birth weight, preterm infants. Neuropsychology. 2004;18(4):673–8. doi: 10.1037/0894-4105.18.4.673. doi: http://dx.doi.org/10.1037/0894-4105.18.4.673. [DOI] [PubMed] [Google Scholar]
- 101.Semple BD, Blomgran K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks and vulnerability to injury across species. Prog Neurobiol 106. 2013;107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. doi:10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Faherty CJ, Kerley D, Smeyne RJ. A Golgi-Cox morphological analysis of neuronal changes induced by environmental enrichment. Dev Brain Res. 2003;141(1-2):55–61. doi: 10.1016/s0165-3806(02)00642-9. doi:10.1016/S0165-3806(02)00642-9. [DOI] [PubMed] [Google Scholar]
- 103.Hoffman AN, Malena RR, Westergom BP, Luthra P, Cheng JP, Aslam HA, Zafonte RD, Kline AE. Environmental enrichment-mediated functional improvement after experimental traumatic brain injury is contingent on task-specific neurobehavioral experience. Neurosci Lett. 2008;431(3):226–30. doi: 10.1016/j.neulet.2007.11.042. doi:10.1016/j.neulet.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murner-Lavanchy I, Ritter BC, Spencer-Smith MM, Perrig WJ, Schroth G, Steinlin M, Everts R. Visuospatial working memory in very preterm and term born children- Impact of age and performance. Dev Cogn Neurosci. 2014;9:106–16. doi: 10.1016/j.dcn.2014.02.004. doi:10.1016/j.dcn.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barde LH, Yeatman JD, Lee ES, Glover G, Feldman HM. Differences in neural activation between preterm and full term born adolescents on sentence comprehension task: implications for educational accommodations. Dev Cogn Neurosci. 2012;2(1):S114–28. doi: 10.1016/j.dcn.2011.10.002. doi:10.1016/j.dcn.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Beauchamp MH, Thompson DK, Howard K, Doyle LW, Egan GF, Inder TE, Anderson PJ. Preterm infant hippocampal volumes correlate with later working memory deficits. Brain. 2008;131:2986–94. doi: 10.1093/brain/awn227. doi: http://dx.doi.org/10.1093/brain/awn227. [DOI] [PubMed] [Google Scholar]