Abstract
Clock genes, such as period, which maintain an organism’s circadian rhythm, can have profound effects on metabolic activity, including ethanol metabolism. In turn, ethanol exposure has been shown in Drosophila and mammals to cause disruptions of the circadian rhythm. Previous studies from our labs have shown that larval ethanol exposure disrupted the free-running period and period expression of Drosophila. In addition, a recent study has shown that arrhythmic flies show no tolerance to ethanol exposure. As such, Drosophila period mutants, which have either a shorter than wild-type free-running period (perS) or a longer one (perL), may also exhibit altered responses to ethanol due to their intrinsic circadian differences. In this study, we tested the initial sensitivity and tolerance of ethanol exposure on Canton-S, perS, and perL, and then measured their Alcohol Dehydrogenase (ADH) and body ethanol levels. We showed that perL flies had slower sedation rate, longer recovery from ethanol sedation, and generated higher tolerance for sedation upon repeated ethanol exposure compared to Canton-S wild-type flies. Furthermore, perL flies had lower ADH activity and had a slower ethanol clearance compared to wild-type flies. The findings of this study suggest that period mutations influence ethanol induced behavior and ethanol metabolism in Drosophila and that flies with longer circadian periods are more sensitive to ethanol exposure.
Keywords: alcoholism, circadian rhythm, Drosophila, ethanol, period
INTRODUCTION
Alcohol is one of the most commonly abused drugs in the world. Consequently, alcohol use disorders (AUD) are extremely common. Therefore, it is important to understand the biological effects of ethanol and mechanisms behind ethanol metabolism. Ethanol intoxication exhibits a biphasic effect, inducing initial hyperactivity at lower doses followed by depressed activity resulting in sedation at higher doses. Alcoholism and alcohol consumption in humans are known to affect a multitude of molecules and pathways including biological rhythms e.g. melatonin [1], temperature rhythms [2], and cycling of circadian genes [3]. Interestingly, human genetics studies have linked mutations to the per genes and increased alcohol consumption [4].
Furthermore, numerous mammal studies have shown that alcohol exposure can affect the period [5-7] and phase [7-10] of the free-running locomotor activity rhythm. Additionally, alcohol can affect transcript and protein levels of core clock genes (including period (per)) [11, 12]. Alternatively, previous studies have suggested that ethanol intake and drug addictions are strongly associated with mutations in clock genes, including per. Mice with mutated per genes, including per1 and per2, exhibit increased alcohol consumption and alcohol seeking behavior [13, 14]. In turn, animals selectively bred for high or low alcohol consumption have also shown aberrations in their circadian clock function [15-17]. Taken together, these studies demonstrate clear connections between circadian mutations and alcohol exposure by showing profound effects of alcohol on the molecular rhythms and behavioral and physiological outputs of the circadian clock.
The focus of this study was to explore sedation and tolerance to high-dose acute ethanol exposure in adult Drosophila per mutants, in order to investigate how ethanol affects animals with rhythmic, but atypical circadian periods. Drosophila can be a powerful tool to investigate the physiological and behavioral effects of alcohol intoxication for the following reasons. First, Drosophila is amenable to efficient genetic and behavioral analysis. Second, the ethanol-induced biphasic behavior in humans is mirrored in Drosophila; during initial stages of exposure, hyperactivity and increased locomotion was observed, but after greater amounts of ethanol had accumulated, the flies were sedated [18]. Third, there exists a high degree of homology between the Drosophila and mammalian clock rhythm [19]. Therefore, fruit flies have been extensively used to investigate molecular and behavioral aspects of the circadian rhythm.
Work on the effects of ethanol on Drosophila has demonstrated that, similar to mammals, ethanol can disrupt the biological clock and has the capability to modulate the expression of circadian genes [20, 21]. In addition, a recent study showed that Drosophila arrhythmic mutants (including per null (per0)) do not exhibit ethanol tolerance, indicating that a circadian clock is necessary for alcohol tolerance [22]. Although such studies have found strong correlations between circadian rhythm defects and ethanol exposure, few have directly investigated the effect of acute ethanol exposure on Drosophila with altered free-running circadian periods - period Short (perS) and period Long (perL) mutants. The perS and perL flies have a stable entrainment to a LD cycle, but they have free-running circadian periods of approximately 19.5 h and 28.5 h, respectively [23]. As alcohol consumption may have differing affects on the circadian rhythm depending upon free-running rhythm length [6, 20], there may be a connection between period length and alcohol related behaviors. Here we show that the perS and perL mutations have altered sedation rates, recovery from sedation, and ethanol tolerance and demonstrate that these effects may be the result of interplay between circadian rhythms and ethanol metabolism.
MATERIALS AND METHODS
2.1 Drosophila stocks
Drosophila fly stocks Canton-S (CS, stock number 1), and adhnull (stock number 3976), were obtained from the Bloomington Drosophila Stock Center. perS and perL mutants were generated in CS flies [23] (generously provided by Dr. Michael Rosbash, Brandeis University). To rule out effects of genetic background differences, similar behavioral effects were observed in stocks independently maintained at Colby College and Bridgewater State University. The flies were raised on standard Nutri-Fly™ Bloomington Formulation (Genesee Scientific) at 25°C under 12-hour light/12-hour dark conditions, unless otherwise stated.
2.2 Ethanol Sedation, recovery, and tolerance assays
To determine the effect of period mutations on Drosophila behavioral responses to ethanol, a sedation assay was carried out according to previous assays outlined by Maples and Rothenfluh, with minor modifications [24]. This study used male flies due to gender-related differences in ethanol-induced behavior and their stronger tolerance to ethanol exposure [18]. CS, perS, and perL male flies of different ages (one-day, one-week, and two-week-old – typical experiments with adult flies are done on 3-7-day old flies) were collected and sorted into standard food vials of 8 flies each approximately 18 hours before sedation in order to prevent confounding effects of the CO2 sedation on ethanol-induced sedation. Flies were transferred to new empty vials at ZT4 on the day of sedation and allowed to acclimate to the new environment for 15 minutes. Sedation was carried out by dispensing 500 μL ethanol (200-proof) (Sigma-Aldrich) onto a new cotton plug, and then immediately replacing the original plug with the ethanol infused one, forming an ethanol chamber. Vials were deliberately tapped 5 times after each minute to determine the number of sedated flies. Sedation is defined as the inability of the flies to move, right themselves, or uncontrollable beating of the wings after a ten-second observational period. ST50 is defined as the time until 50% of the flies have been sedated. The flies were left in the ethanol chamber for a total of 15 minutes regardless of sedation time.
Recovery began after the flies have been removed from the ethanol chamber. The flies were returned to their original food vials with the vial laid horizontally to prevent non-sedation-related mortality. Every 30 minutes, the vials were gently rotated and observed to determine if the flies had recovered, which is indicated by the regaining of their ability to right themselves. RT50 is defined as the time required for 50% of the sedated flies to recover. Flies that have not recovered at the beginning of tolerance were excluded from the total number of flies for RT50 calculations. Tolerance after repeated exposure was tested 24 hours after the initial sedation at ZT4. The flies were once again transferred into a new empty food vial and the sedation process was repeated to determine the ST50 for tolerance measurement. For each genotype, tolerance was calculated by determining the percent change in sedation times on consecutive days (%T - to normalize tolerance between genotypes).
Statistical analyses on independent biological replicates (n=3-5) for sedation, recovery, and tolerance were performed in STATA using ANOVA; post hoc Scheffe pair-wise comparison test.
2.3 Alcohol dehydrogenase enzyme activity assay
Alcohol dehydrogenase (ADH) enzyme activity was measured for both fluctuations throughout the circadian cycle and age-dependent differences according to a previously described protocol [25, 26]. Twenty-four-hour ADH cycling differences between CS, perS and perL were measured at time points ZT0, ZT4, ZT8, ZT12, ZT16, ZT20, and ZT24, with adhnull as the control. One to three-day old flies were kept at 25°C under 12-hour light/12-hour dark standard conditions until the appropriate time point. To determine age-dependent differences, 1-day, 1-week, and 2-week-old flies of each genotype were collected at ZT4.
Five flies were homogenized from each genotype at each time point and age in 150 μL of 20 mM phosphate buffer. 100 mM potassium phosphate buffer was made with 19.2: 90.8, monobasic potassium phosphate (100 mM): dibasic potassium phosphate (100 mM) (Sigma-Aldrich). The homogenate was centrifuged at 12500 rpm in 4°C for 5 minutes, and then the supernatant collected and stored at −20°C until all extracts had been collected. ADH assay solution, 940 μL, was prepared for each sample, composed of 500 μL potassium phosphate buffer (100 mM), 40μL NAD+ (50 mM), 100 μL absolute ethanol (200-proof), and 300 μL dH2O. Directly before absorbance measurements were made, 60 μL of the sample extract was added to the assay solution to initiate the reaction. The absorbance over time of each sample produced by colorimetric changes was measured at room temperature and a wavelength of 340 nm for 10 minutes (Beckman Coulter DU530 UV/Vis Spectrophotometer). Measurements were made every 2 minutes for a total of 6 optical density readings. The data points were plotted and fitted with a regression line to determine the change in optical density (ΔOD).
In order to account for differences in protein concentration of each sample, we normalized samples for protein content using a bicinchoninic acid assay (BCA assay), also known as a Smith assay. The assay was carried out with a Pierce™ BCA Protein Assay Kit. For each sample, 200 μL of assay solution was prepared, consisting of 196 μL reagent A and 4 μL reagent B. 25 μL of sample extract was added to the assay solution and allowed to react in an incubator at 37°C for 30 minutes. After the incubation, 2 μL of the reacted sample was measured on a Thermo Scientific Nanodrop (ND-1000) spectrophotometer for the number milligrams of protein in the sample at room temperature (approximately 25°C) and a wavelength of 562 nm. The ADH activities were plotted as the normalized absorbance units (NAU - ΔOD/mg protein).
Statistical analyses on independent biological replicates (n=4-6) for the ADH activity at ZT4 were performed in STATA using ANOVA; post hoc Scheffe pair-wise comparison test. To calculate the ADH activity during lights-on and lights-off phases of the 24-hour cycle (n=3), data from ZT4 and ZT8 (lights-on) were averaged, and data from ZT16 and ZT20 (lights-off) were averaged. Lights-on and lights-off data analyses were performed in STATA using T-tests.
2.4 Ethanol pharmacokinetics assay
The ethanol accumulation assay was performed to determine the amount of ethanol accumulation that occurs after ethanol exposure treatment. CS, perS, and perL flies were exposed to ethanol vapor (200-proof) (Sigma-Aldrich) for 15 minutes, and then allowed to recover for an hour on standard Nutri-Fly™ Bloomington Formulation at room temperature (approximately 25°C). Methods to create the ethanol chamber can be found in the “Ethanol sedation, recovery, and tolerance” section.
Colorimetric determination of ethanol concentration was carried out using manufacturer protocol described in the EnzyChromTM Ethanol Assay Kit. Four flies from each appropriate genotype were homogenized in 40 μL of 100 mM potassium phosphate buffer and centrifuged at 12500 rpm and 4°C for 5 minutes. The supernatants were collected and stored at −20°C. The assay solution was prepared by mixing 80 μL Assay Buffer, 1 μL Enzyme A, 1 μL Enzyme B, 2.5 μL NAD, and 14 μL MTT. 90 μL of the assay solution and 10 μL of sample extract were added and mixed in a 96-well plate. The mixtures were allowed to react at room temperature (approximately 25°C) for 30 minutes undisturbed, and then 100 μL of stop solution was added and mixed into each well. The absorbance was measured at room temperature at 570 nm (BioTek ELx808 spectrophotometer). The relative ethanol concentration values were expressed as absorbance units (AU).
The measurement of the protein concentration of each sample was carried out with a Pierce™ BCA Protein Assay Kit, with slight modifications compared to the BCA assay performed for the ADH normalization, due to the Drosophila sample size variation between the ADH assay and the ethanol accumulation assay. For each sample, 200μL of assay solution was prepared, consisting of 196μL reagent A and 4μL reagent B. 10μL of sample extract and assay solution were then distributed into a 96-well plate and allowed to react in an incubator at 37°C for 30 minutes. After the incubation, the reacted samples were measured for the number of milligrams of protein in the sample at room temperature (approximately 25°C) and a wavelength of 562 nm (BioTek ELx808 spectrophotometer). The ethanol concentration values were plotted as the normalized absorbance units (NAU), which refers to ΔOD/mg protein.
Statistical analyses on independent biological replicates (n=3) for the ADH activity at ZT4 were performed in STATA using ANOVA; post hoc Scheffe pair-wise comparison test.
2.5 Ethanol Viability Assay
An ethanol viability assay was performed (n=4-5), where CS, perS, and perL flies were placed on 10% ethanol-supplemented food and the percentage of flies surviving after five days was calculated, as previously described [21].
RESULTS
3.1 Ethanol-induced behavior in period mutants
To determine the effect of period mutations on ethanol-induced behavior, we performed sedation, recovery, and tolerance assays at ZT4 on 1-day-old, 1-week-old, and 2-week-old CS, perS, and perL flies.
3.1.1 Sedation
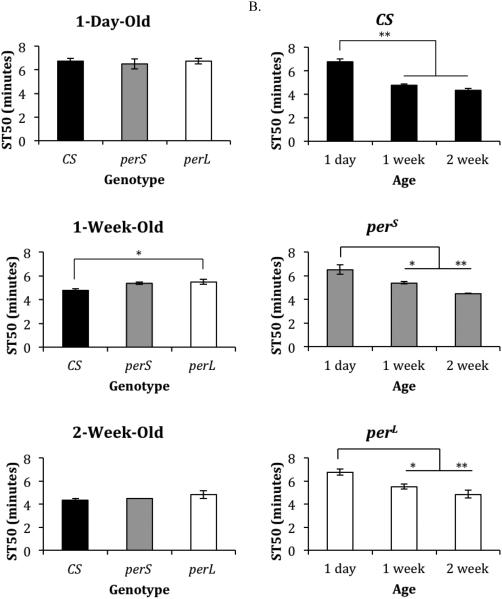
The ST50 of 1-week-old perL flies (5.5 minutes) was significantly higher than the ST50 of 1-week-old CS flies (4.75 minutes; p=0.03) (Figure 1A). All other comparisons of genotypes of the same age showed similar sedation time (ST50), which was between 6.5 minutes and 6.75 minutes for 1-day-old flies and between 4.33 minutes and 4.83 minutes for 2-week-old flies (Figure 1A). Additionally, all genotypes showed a significant age-dependent decrease in ST50 in 1-week-old and 2-week-old flies compared to 1-day-old flies (p≤0.046) (Figure 1B).
Figure 1. Ethanol-induced sedation in period mutants.
(A) Comparison of ST50 (duration of exposure to vapors generated by100% ethanol to sedate 50% of the flies) in different genotypes (wild type (CS), perS and perL) at the same age (1-day-old, 1-week-old, and 2-week-old). (B) Comparison of ST50 in each genotype with age. Columns represent mean ST50 +/− standard error of 3-4 biological replicates. For each ST50 eight male flies were used. *p<0.05 **p<0.01; ANOVA, post hoc Scheffe.
3.1.2 Recovery
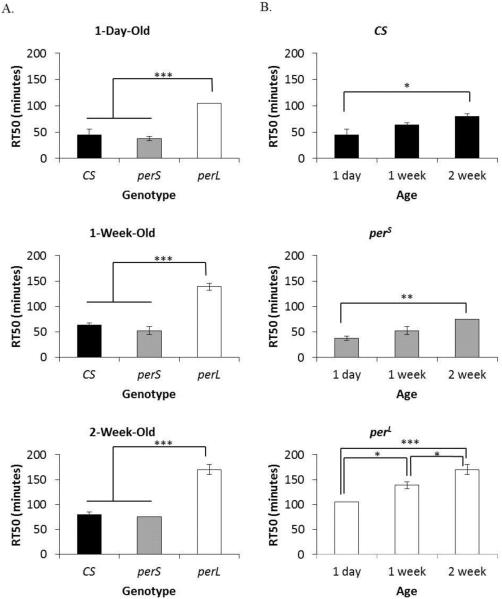
The recovery time (RT50) of CS flies and perS flies was not significantly different within the same age groups. The RT50 of CS flies and perS flies was 45 minutes and 37.5 minutes in 1-day-old, 63.75 minutes and 52.5 minutes in 1-week-old, and 80 minutes and 75 minutes in 2-week-old flies, respectively (Figure 2A). In contrast, perL flies showed a significantly longer RT50 compared to both CS flies and perS flies of the same age (p=0.0001 for all pairwise comparisons). The RT50 of perL flies was 105 minutes, 138.75 minutes, and 170 minutes in 1-day-old, 1-week-old, and 2-week-old flies, respectively. All genotypes showed a trend of age-dependent increase in RT50 (Figure 2B). The RT50 of CS flies and perS flies showed statistically significant increase between 1-day-old and 2-week-old flies (p=0.03 and p=0.005), while perL flies showed significant increase between 1-day-old and 1-week-old flies (p=0.015), 1-day-old and 2-week-old flies (p=0.0001), and 1-week-old and 2-week-old flies (p=0.032).
Figure 2. Recovery from ethanol-induced sedation in period mutants.
(A) Comparison of RT50 (duration for 50% flies to recover after exposure to 100% ethanol for 15 minutes) in different genotypes (wild type (CS), perS and perL) at the same age (1-day-old, 1-week-old, and 2-week-old). (B) Comparison of RT50 in each genotype with age. Columns represent mean RT50 +/− standard error of 3-4 biological replicates. For each RT50 eight male flies were used. *p<0.05 **p<0.01 ***p<0.001; ANOVA, post hoc Scheffe.
3.1.3 Tolerance
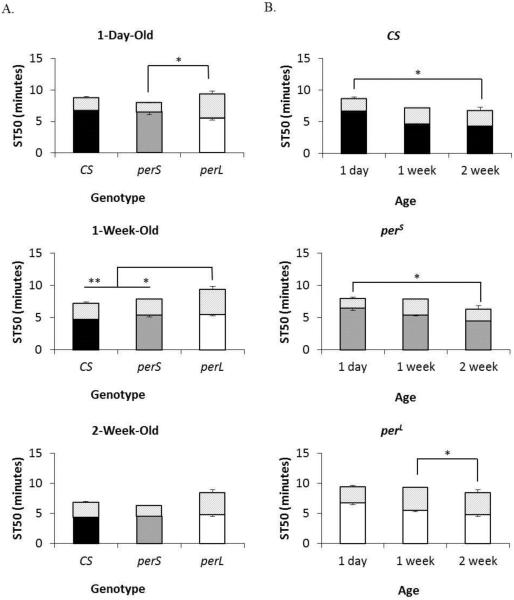
The tolerance to sedation after repeated exposure to ethanol (%T) of CS flies and perS flies was not significantly different within the same age groups. The %T of CS flies and perS flies was 25.8% and 23.5% in 1-day-old, 34.5% and 46.6% in 1-week-old, and 58.8% and 40.7% in 2-week-old flies, respectively (Figure 3A). Among the genotypes, perL flies generated the highest tolerance. The %T of 1-day-old perL flies (41.0%) was significantly higher than %T in 1-day-old perS flies (p=0.038). Furthermore, the %T in 1-week-old (69.8%) and 2-week-old (77.4%) perL flies was significantly higher than %T in 1-week-old CS flies and perS flies (p=0.001 (1wk) and 0.02 (2wk) and p=0.014 (1wk) and .009 (2wk) respectively). All genotypes showed a trend of age-dependent increase in %T (Figure 3B). The %T in CS flies and perS flies showed statistically significant increase between 1-day-old and 2-week-old flies (p=0.02 and p=0.03), while perL flies showed significant increase between 1-week-old and 2-week-old flies (p=0.05).
Figure 3. Tolerance to ethanol-induced sedation after repeated exposure in period mutants.
(A) Comparison of %T (percent change in ST50 upon ethanol exposure on consecutive days) in different genotypes (wild type (CS), perS and perL) at the same age (1-day-old, 1-week-old, and 2-week-old). (B) Comparison of %T in each genotype with age. Columns represent mean ST50 +/− standard error of second trial of 3-4 biological replicates. Shaded portion of each column depicts the difference in ST50 between trials. For each %T eight male flies were used. *p<0.05 **p<0.01; ANOVA, post hoc Scheffe.
3.2 Circadian rhythmicity of ADH activity
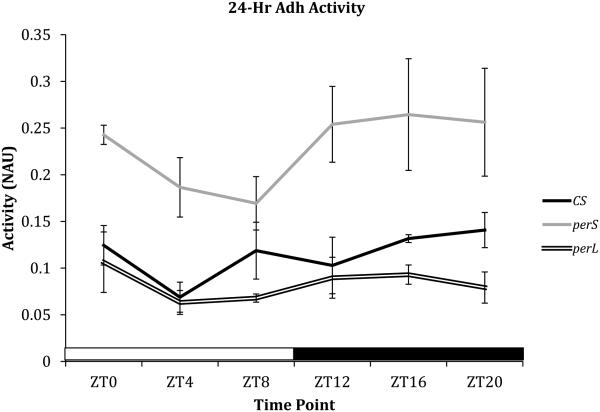
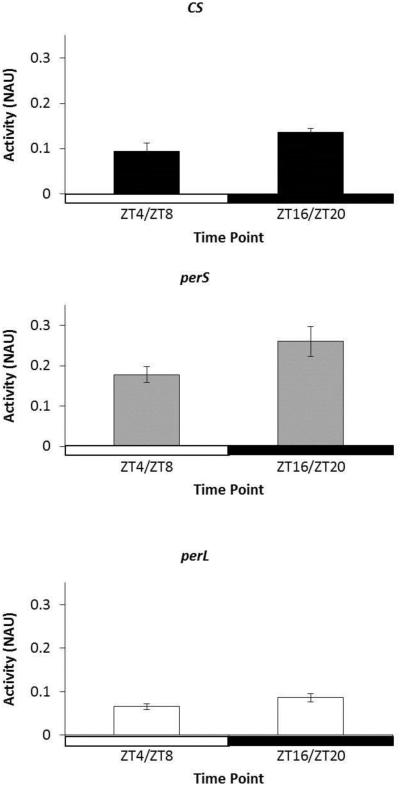
To determine if ADH activity follows a circadian pattern and the effect of period mutations on ethanol metabolism, ADH assays were performed on CS, perS, and perL flies every four hours at ZT0, ZT4, ZT8, ZT12, ZT16, and ZT20. All genotypes appeared to show an oscillating trend in ADH activity under 12-hour light/12-hour dark standard conditions (Figure 4). ADH activity appeared to decrease after ZT0 and increase after ZT12. ADH activity during lights-on (ZT4 and ZT8) and lights-off (ZT16 and ZT20) time points was averaged (Figure 5). For all genotypes, ADH activity increased in light condition compared to dark condition, although the results were not statistically significant (Figure 5). Among genotypes, perS flies showed the highest ADH activity throughout the 24-hour cycle, whereas perL flies were the lowest ADH activity at all time points (Figure 4).
Figure 4. Circadian rhythm of alcohol dehydrogenase (ADH) activity in period mutants.
Comparison of ADH activity in 3-day-old wild type (CS), perS and perL flies at six time points in a 24-hour duration (ZT0, ZT4, ZT8, ZT12, ZT16, and ZT20). Each point on the line graph represents mean ADH activity +/− standard error of 3 biological replicates. ADH activity is represented as normalized absorbance units (NAU). NAU was calculated by normalizing the change in absorbance (ΔOD) due to accumulation of ADH-catalyzed NADH with the total protein concentration in each sample (BCA). X-axis is shaded clear and dark to depict light and dark conditions respectively. For each ADH activity assay five male flies were used.
Figure 5. Day vs. Night alcohol dehydrogenase (ADH) activity in period mutants.
Comparison of ADH activity in 3-day-old wild type (CS), perS and perL flies during middle of light phase (ZT4 and ZT8) and middle of dark phase (ZT16, and ZT20). Each column represents mean ADH activity +/− standard error of at two time points in 3 biological replicates. ADH activity is represented as normalized absorbance units (NAU). NAU was calculated by normalizing the change in absorbance (ΔOD) due to accumulation of ADH-catalyzed NADH with the total protein concentration in each sample (BCA). X-axis is shaded clear and dark to depict light and dark conditions respectively. For each ADH activity assay five male flies were used.
3.3 Alcohol dehydrogenase (ADH) activity in period mutants
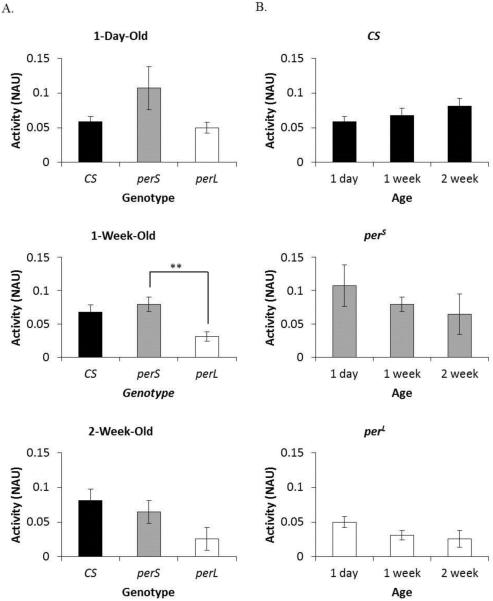
To further analyze the effect of period mutations on ethanol metabolism, ADH activity was measured at the same time points as ethanol-induced behavior assays i.e. at ZT4 in 1-day-old, 1-week-old, and 2-week-old CS, perS, and perL flies. Adhnull flies were used as control. ADH activity was not significantly different between genotypes at all ages except between 1-week-old perS flies and perL flies (p=0.01) (Figure 6A). Nevertheless, certain genotype-related trends in ADH activity were observed. perL flies showed lower ADH activity compared to CS flies and perS flies in all time points (Figure 6A). Age-dependent trends in ADH activity were also observed. CS flies appear to show an increase in ADH activity with age, while perS flies and perL flies appear to show a decline in ADH activity with age (Figure 6B).
Figure 6. Alcohol dehydrogenase (ADH) activity in period mutants.
(A) Comparison of ADH activity in different genotypes (wild type (CS), perS and perL) at the same age (1-day-old, 1-week-old, and 2-week-old). (B) Comparison of ADH activity in each genotype with age. Columns represent mean ADH activity +/− standard error of 3-4 biological replicates. ADH activity is represented as normalized absorbance units (NAU). NAU was calculated by normalizing the change in absorbance (ΔOD) due to accumulation of ADH-catalyzed NADH with the total protein concentration in each sample (BCA). For each ADH activity assay five male flies were used. **p<0.01; ANOVA, post hoc Scheffe.
3.4 Ethanol pharmacokinetics in period mutants
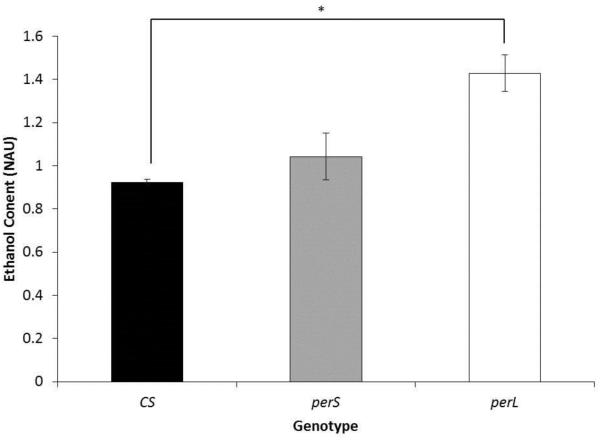
To determine if higher RT50 and low ADH activity in perL flies correlates with ethanol clearance, ethanol content was measured in 1-week-old CS, perS, and perL flies 1 hour after exposure to ethanol for 15 minutes (ethanol exposure at ZT4 followed by ethanol content assay at ZT5). perL flies showed significantly higher ethanol content compared to CS flies (CS: 0.92 NAU and perL 1.43 NAU; p-value=0.04) (Figure 7).
Figure 7. Ethanol pharmacokinetics in period mutants.
Comparison of ethanol content in 1-week-old wild type (CS), perS and perL flies. Flies were exposed to vapors generated by100% ethanol for 15 minutes at ZT4 followed by ethanol content assay at ZT5. Each column represents mean normalized ethanol content +/− standard error in 3 biological replicates. Ethanol content is represented as normalized absorbance units (NAU). NAU was calculated by normalizing the absorbance (OD) with the total protein concentration in each sample (BCA). For each ethanol content assay four male flies were used. *p<0.05; ANOVA, post hoc Scheffe.
3.5 Ethanol-induced mortality in period mutants
After five days on 10% ethanol-supplemented food, average mortality rates for each genotype were 29.3 ± 3.9, 10.0 ± 5.0, and 50.6 ± 8.8 percent for CS, perS, and perL flies respectively. After five days on control food, no mortality was observed in any genotypes. perL flies showed significantly increased mortality on the ethanol food compared to perS flies (p=0.003) and marginally more compared to CS flies (p=.080). No differences were present between CS flies and perS flies in terms of viability rates (p=0.14).
DISCUSSION
In this study, we demonstrated a strong link between mutations in the circadian rhythm gene, period, and altered behavioral and biochemical responses to acute ethanol exposure. All genotypes showed similar sedation times at each age tested except for 1-week-old perL flies, which exhibited a longer sedation time than either wild-type or perS flies. These results with rhythmic per mutants mirrors what was recently found in arrhythmic circadian mutants (including per0), which also showed no differences in sedation time to alcohol after one exposure [22]. Still, the role of the circadian clock in ethanol sedation cannot be ruled out entirely because there seems to be circadian variation in ethanol sensitivity in both wild-type flies [27] and mice [28]. The current and previous study [22] conducted these assays during the subjective day (ZT 4-9), so it is currently unknown whether circadian mutants show the same day vs. night variation in sensitivity. It is worth noting that per2Bdrm1 mutant mice (which may be arrhythmic) lack circadian variation in ethanol sensitivity, while per1Bdrm1 mice (which are rhythmic) do not [28], indicating a functional circadian clock is necessary for temporal variation in ethanol sensitivity. It may be likely that arrhythmic fly mutants, as well as wild-type flies made arrhythmic through constant light exposure, also might lack the circadian variation of sensitivity [27]. Additionally, all genotypes show a similar pattern to age effects of ethanol exposure as both one-week and two-week old flies exhibited increased sensitivity to ethanol exposure compared to one-day old flies, which has been previously well characterized (initially by Pearl et al., 1929 [29]). The lack of significant differences in sedation times may also be because sedation during acute exposure is governed by the amount of ethanol vapors absorbed [30]. Adhnull flies, which are unable to process ethanol, still maintained comparable (slightly less) sedation times to wild-type, suggesting that sedation does not depend on ADH enzymatic activity (data not shown and [31]).
While all flies showed similar patterns of sedation to ethanol exposure, perL flies did show longer recovery times and increased mortality compared to CS flies and perS flies, indicating that perL flies may be more sensitive to ethanol because of slower ethanol metabolism. perL flies exhibited increased levels of alcohol after exposure compared to CS flies and perS flies, indicating slower ethanol metabolism. Additionally, correlations have been found between internal ethanol levels and sedation and recovery time [32]. Accordingly, Adhnull flies have significantly longer recovery times after sedation with many not recovering resulting in high ethanol exposure-induced mortality (data not shown), indicating a role in ethanol exposure recovery but not sedation by ADH. Therefore, lower ADH enzymatic activity accompanied by higher amounts of residual ethanol during recovery would be associated with longer recovery times. While perL flies’ slow recovery time and differences between the alcohol levels of perS flies and perL flies can be explained by differences in ADH levels, it does not explain why CS flies had significantly faster recovery compared to perL flies. This result suggests that perL flies might be deficient in other enzymes that regulate ethanol metabolism and tolerance, and further studies will be needed to determine what else will be implicated in alcohol tolerance.
To the best of our knowledge, circadian oscillations of ADH enzymatic activity have not been previously characterized in Drosophila. Our study has found that ADH enzymatic activity follow a circadian pattern; ADH activity was observed to be moderately higher during the lights-off phases compared to lights-on. The similarity in day ADH activity fluctuation patterns between CS, perS, and perL flies suggest that the PER-mediated regulation of ADH enzymatic activity is an alternative function of PER, which is separate from the CLK/CYC and TIM/PER circadian negative feedback loop oscillator. Additionally, the ADH daily cycle in perL flies is severely blunted compared to perS flies (which was extremely robust) and CS flies. Flattened-out or blunted rhythms are indicators of impaired physiological function, which can lead to poor health and disease progression. For example, in humans, blunted melatonin rhythms are known to exacerbate alcoholism, insomnia and depression [33, 34], Parkinson’s Disease [35], and diabetes [36].
In the current study, perS flies had significantly more ADH, a more robust rhythm of ADH, and lower mortality on ethanol-containing food than perL flies. These results may indicate that individuals with longer circadian periods might be more sensitive to ethanol exposure compared to shorter period individuals. Ethanol (in most, but not all cases) tends to lead to period shortening in a wide variety of species including Drosophila [21] including perL [20], mice [7], rats [5], and humans [37]. Additionally, per2Bdrm1 mutants, which initially exhibit shorter circadian periods in DD (before becoming arrhythmic), also are less sensitive to ethanol exposure and in fact have increased preference for it [8, 13, 14]. In addition, alcohol consumption also shortens REM cycles and decreases sleep time [38]. Furthermore, humans with a mutation PER2, which leads to increased alcohol drinking similar to what is seen in per2Bdrm1 mutant mice, exhibit less sleep due to earlier awaking times [39]. These studies all indicate there are possible connections among alcohol consumption, period mutations, and shortened circadian/sleep rhythms.
With this study, we add to a body of knowledge that strongly links mutations in the circadian rhythm gene, period, and altered behavioral and biochemical responses to acute ethanol exposure. Taken together, we conclude that recovery from acute ethanol exposure is largely dependent on ADH enzymatic activity and that perL flies exhibit poorer ethanol sensitivity and recovery from exposure than flies with normal rhythms due to blunted ADH activity.
Highlights.
Drosophila show faster ethanol-induced sedation and slower recovery with age.
perL flies have longer recovery time from ethanol sedation compared to wild-type.
perL flies have higher residual ethanol post-sedation compared to wild-type.
Alcohol dehydrogenase activity appears to fluctuate in a circadian pattern and influence ethanol response.
ACKNOWLEDGEMENTS
This work was supported by an investigator grant of Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM0103423 to STA, Natural Science Division Grant, Colby College (STA) and ATP, CARS, and Daniel Smith Awards, Bridgewater State University to JAS. We would like to acknowledge Joshua Kavaler and Ahmad lab members for suggestions on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Danel T, et al. Inversion of melatonin circadian rhythm in chronic alcoholic patients during withdrawal: preliminary study on seven patients. Alcohol Alcohol. 2009;44(1):42–5. doi: 10.1093/alcalc/agn091. [DOI] [PubMed] [Google Scholar]
- 2.Danel T, Libersa C, Touitou Y. The effect of alcohol consumption on the circadian control of human core body temperature is time dependent. Am J Physiol Regul Integr Comp Physiol. 2001;281(1):R52–5. doi: 10.1152/ajpregu.2001.281.1.R52. [DOI] [PubMed] [Google Scholar]
- 3.Huang MC, et al. Reduced expression of circadian clock genes in male alcoholic patients. Alcohol Clin Exp Res. 2010;34(11):1899–904. doi: 10.1111/j.1530-0277.2010.01278.x. [DOI] [PubMed] [Google Scholar]
- 4.Partonen T. Clock genes in human alcohol abuse and comorbid conditions. Alcohol. 2015;49(4):359–65. doi: 10.1016/j.alcohol.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Dwyer SM, Rosenwasser AM. Neonatal clomipramine treatment, alcohol intake and circadian rhythms in rats. Psychopharmacology (Berl) 1998;138(2):176–83. doi: 10.1007/s002130050660. [DOI] [PubMed] [Google Scholar]
- 6.Rosenwasser AM, Fecteau ME, Logan RW. Effects of ethanol intake and ethanol withdrawal on free-running circadian activity rhythms in rats. Physiol Behav. 2005;84(4):537–42. doi: 10.1016/j.physbeh.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Seggio JA, et al. Chronic ethanol intake alters circadian phase shifting and free-running period in mice. J Biol Rhythms. 2009;24(4):304–12. doi: 10.1177/0748730409338449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brager AJ, et al. Chronic ethanol disrupts circadian photic entrainment and daily locomotor activity in the mouse. Alcohol Clin Exp Res. 2010;34(7):1266–73. doi: 10.1111/j.1530-0277.2010.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruby CL, et al. Chronic ethanol attenuates circadian photic phase resetting and alters nocturnal activity patterns in the hamster. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R729–37. doi: 10.1152/ajpregu.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seggio JA, Logan RW, Rosenwasser AM. Chronic ethanol intake modulates photic and non-photic circadian phase responses in the Syrian hamster. Pharmacol Biochem Behav. 2007;87(3):297–305. doi: 10.1016/j.pbb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen CP, et al. Chronic ethanol consumption impairs the circadian rhythm of pro-opiomelanocortin and period genes mRNA expression in the hypothalamus of the male rat. J Neurochem. 2004;88(6):1547–54. doi: 10.1046/j.1471-4159.2003.02300.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen CP, et al. Prenatal ethanol exposure alters the expression of period genes governing the circadian function of beta-endorphin neurons in the hypothalamus. J Neurochem. 2006;97(4):1026–33. doi: 10.1111/j.1471-4159.2006.03839.x. [DOI] [PubMed] [Google Scholar]
- 13.Gamsby JJ, et al. The circadian Per1 and Per2 genes influence alcohol intake, reinforcement, and blood alcohol levels. Behav Brain Res. 2013;249:15–21. doi: 10.1016/j.bbr.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spanagel R, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005;11(1):35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- 15.Hofstetter JR, Grahame NJ, Mayeda AR. Circadian activity rhythms in high-alcohol-preferring and low-alcohol-preferring mice. Alcohol. 2003;30(1):81–5. doi: 10.1016/s0741-8329(03)00095-8. [DOI] [PubMed] [Google Scholar]
- 16.Rosenwasser AM, et al. Circadian activity rhythms in selectively bred ethanol-preferring and nonpreferring rats. Alcohol. 2005;36(2):69–81. doi: 10.1016/j.alcohol.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 17.McCulley WD, 3rd, et al. Selective breeding for ethanol-related traits alters circadian phenotype. Alcohol. 2013;47(3):187–94. doi: 10.1016/j.alcohol.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devineni AV, Heberlein U. Acute ethanol responses in Drosophila are sexually dimorphic. Proc Natl Acad Sci U S A. 2012;109(51):21087–92. doi: 10.1073/pnas.1218850110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helfrich-Forster C. Development of pigment-dispersing hormone-immunoreactive neurons in the nervous system of Drosophila melanogaster. J Comp Neurol. 1997;380(3):335–54. doi: 10.1002/(sici)1096-9861(19970414)380:3<335::aid-cne4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Ahmad ST, et al. Larval ethanol exposure alters free-running circadian rhythm and per Locus transcription in adult D. melanogaster period mutants. Behav Brain Res. 2013;241:50–5. doi: 10.1016/j.bbr.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seggio JA, Possidente B, Ahmad ST. Larval ethanol exposure alters adult circadian free-running locomotor activity rhythm in Drosophila melanogaster. Chronobiol Int. 2012;29(1):75–81. doi: 10.3109/07420528.2011.635236. [DOI] [PubMed] [Google Scholar]
- 22.Pohl JB, et al. Circadian genes differentially affect tolerance to ethanol in Drosophila. Alcohol Clin Exp Res. 2013;37(11):1862–71. doi: 10.1111/acer.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971;68(9):2112–6. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maples T, Rothenfluh A. A simple way to measure ethanol sensitivity in flies. J Vis Exp. 2011;(48) doi: 10.3791/2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merçot H. Phenotypic expression of ADH regulatory genes inDrosophila melanogaster: a comparative study between a paleartic and a tropical population. Genetica. 1994;94(1):37–41. doi: 10.1007/BF01429218. [DOI] [PubMed] [Google Scholar]
- 26.Walker JRL. Spectrophotometric determination of enzyme activity: Alcohol dehydrogenase (ADH) Biochemical Education. 1992;20(1):42–43. [Google Scholar]
- 27.van der Linde K, Lyons LC. Circadian modulation of acute alcohol sensitivity but not acute tolerance in Drosophila. Chronobiol Int. 2011;28(5):397–406. doi: 10.3109/07420528.2011.577921. [DOI] [PubMed] [Google Scholar]
- 28.Perreau-Lenz S, et al. Circadian regulation of central ethanol sensitivity by the mPer2 gene. Addict Biol. 2009;14(3):253–9. doi: 10.1111/j.1369-1600.2009.00165.x. [DOI] [PubMed] [Google Scholar]
- 29.Pearl R, White FB, Miner JR. Age Changes in Alcohol Tolerance in Drosophila Melanogaster. Proc Natl Acad Sci U S A. 1929;15(5):425–9. doi: 10.1073/pnas.15.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heberlein U, et al. Molecular Genetic Analysis of Ethanol Intoxication in Drosophila melanogaster. Integrative and Comparative Biology. 2004;44(4):269–274. doi: 10.1093/icb/44.4.269. [DOI] [PubMed] [Google Scholar]
- 31.Singh CM, Heberlein U. Genetic control of acute ethanol-induced behaviors in Drosophila. Alcohol Clin Exp Res. 2000;24(8):1127–36. [PubMed] [Google Scholar]
- 32.Devineni AV, et al. The genetic relationships between ethanol preference, acute ethanol sensitivity, and ethanol tolerance in Drosophila melanogaster. Fly (Austin) 2011;5(3):191–9. doi: 10.4161/fly.5.3.16987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quera Salva MA, et al. Circadian rhythms, melatonin and depression. Curr Pharm Des. 2011;17(15):1459–70. doi: 10.2174/138161211796197188. [DOI] [PubMed] [Google Scholar]
- 34.Hasler BP, Clark DB. Circadian misalignment, reward-related brain function, and adolescent alcohol involvement. Alcohol Clin Exp Res. 2013;37(4):558–65. doi: 10.1111/acer.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Videnovic A, et al. 'The clocks that time us'--circadian rhythms in neurodegenerative disorders. Nat Rev Neurol. 2014;10(12):683–93. doi: 10.1038/nrneurol.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris CJ, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci U S A. 2015;112(17):E2225–34. doi: 10.1073/pnas.1418955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reinberg A, et al. Habitual moderate alcohol consumption desynchronizes circadian physiologic rhythms and affects reaction-time performance. Chronobiol Int. 2010;27(9-10):1930–42. doi: 10.3109/07420528.2010.515763. [DOI] [PubMed] [Google Scholar]
- 38.Brower KJ. Alcohol's effects on sleep in alcoholics. Alcohol Res Health. 2001;25(2):110–25. [PMC free article] [PubMed] [Google Scholar]
- 39.Comasco E, et al. Alcohol consumption among pregnant women in a Swedish sample and its effects on the newborn outcomes. Alcohol Clin Exp Res. 2012;36(10):1779–86. doi: 10.1111/j.1530-0277.2012.01783.x. [DOI] [PubMed] [Google Scholar]