Abstract
The anti-HCV activity of a novel monoclonal antibody (mAb; AR4A) and Epigallocatechin-gallate (EGCG) were studied in-vitro using an HCV cell culture system and in-vivo using a humanized liver mouse model capable of supporting HCV replication. Alone, both exhibit reliable cross-genotype HCV inhibition in-vitro, and combination therapy completely prevented HCV infection. In-vivo AR4A mAb (alone and combined with EGCG) robustly protects against the establishment of HCV genotype 1a infection. EGCG alone fails to reliably protect against HCV challenge.
Conclusion
AR4A mAb represents a safe and efficacious broadly neutralising antibody against HCV applicable to strategies to safely prevent HCV re-infection following liver transplantation, and lends further support to the concept of HCV vaccine development. The poor bioavailability of EGCG limits HCV anti-viral activity in-vivo.
Keywords: Liver transplantation, HCV prevention, anti-HCV monoclonal antibody, Epigallocatechin-gallate
The management of chronic Hepatitis C virus (HCV) infection continues to rapidly evolve. Novel direct acting anti-virals (DAAs) capable of achieving universally high cure rates carry much promise (1). Equitable access to these agents represents a global challenge with a large proportion of those chronically infected worldwide likely to have very limited access for the considerable future (2, 3).
DAAs will also feature prominently in the global resolve to eradicate HCV infection. HCV prevention, however, will remain fundamental to realising this aspiration (3, 4). Primary prevention via vaccination has been undermined by the lack of a convenient animal model and the challenge presented by the diversity amongst HCV genotypes and quasispecies (4-6). Likewise capitalising on the unique opportunity afforded by liver transplant to prevent HCV re-infection has been unsuccessful to date (7–9).
HCV associated liver disease (cirrhosis or hepatocellular carcinoma) is long established as the leading indication for liver transplantation worldwide (10). In those undergoing this life saving intervention, HCV re-infection is presently universal and drives inferior post-transplant outcomes (10–12). A window exists peri-transplantation to optimise post transplant outcomes by preventing allograft HCV infection. A precedent exists within liver transplantation whereby Hepatitis B virus re-infection can be prevented post-transplant (13, 14). To date replicating this in relation to HCV has proven elusive (15).
Strategies to employ existing therapies (Pegylated-Interferon/Ribavirin with or without first generation protease inhibitors) in a pre-emptive role pre-transplant or early post transplant have been unsuccessful. The intolerability and contraindications of these agents in highly complex and medically unstable patients severely limits the applicability of such approaches (7, 8). A randomised trial using hepatitis C immune globulin concluded that this was a safe and tolerable agent in liver transplant recipients but no beneficial effect on the rate of HCV recurrence was observed (9).
Very recently, limited data in select populations employing sofosbuvir and ribavirin pre-transplantation have proven the feasibility of this approach (16). Generalising this approach to complex, and often compromised patients pre and post-liver transplant is however the subject of ongoing studies.
Many monoclonal (mAb) and polyclonal antibodies targeting linear or conformational epitopes within the HCV surface glycoprotein (E1-E2) have been described that effect HCV neutralization in-vitro (17–19). E1/E2 interacts with a number of cell surface molecules to mediate HCV entry, e.g. C-type lectins, CD81, Scavenger receptor-B1 (20). The in-vivo performance of HCV neutralizing antibody has been more variable (21). Law et al characterised a number of antigenic regions on E2 and identified numerous human mAbs with cross neutralizing activity (22). This group has identified further antigenic regions of E1/E2, and generated a human mAb (AR4A) targeting a discontinuous epitope, outside the CD81 binding site on HCV E1/E2. This epitope is highly conserved across genotypes, and AR4A demonstrates potent, cross neutralizing activity in-vitro (23, 24).
EGCG is the most abundant catechin present in green tea extract. Green tea extracts have a long history of safe human consumption and have many purported benefits (25, 26). Green tea extracts are safe, widely available, and inexpensive (27). Two groups independently published data reporting an in-vitro anti-viral effect of EGCG against HCV (28, 29). This was primarily attributed to prevention of initial attachment of the viral particle to hepatocytes. EGCG, however, also inhibited the direct cell-cell mode of HCV transmission (28). In-vivo evidence of the anti-HCV effect of EGCG is lacking.
Using the HCV cell culture system and the SCID/uPA humanised liver mouse model we examined the anti-HCV effect of AR4A and EGCG alone and in combination. We hypothesised that combining safe, tolerable and effective HCV therapies acting at different sites of HCV cell entry could reliably protect against HCV challenge in-vivo.
Materials and Methods
Cells and viruses
Huh7.5 cells (kindly provided by Dr. Charles Rice) were maintained in complete Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum (FBS), 0.1mM non-essential amino acids (NEAA), 100U/mL penicillin and streptomycin 100 mg/mL. Cells were incubated at 37°C in conditions of 5% CO2 (30).
Plasmids encoding chimeric HCV genomes (provided by Dr. Charles Rice) representing genotypes 1–6 were used to generate cell culture derived HCV (HCVcc) as previously described (31).
Compounds
EGCG (Cat. number: E4143, 95% purity, Sigma-Aldrich, St Louis, MO) was dissolved in double distilled H2O and stored at −20°C. Recombinant human interferon alpha 2a (IFN-α2a, positive control) was obtained from PBL interferon source, (Piscataway, NJ, cat. number: 11100-1) and stored as instructed. Human anti-E1/E2 mAb AR4A has previously been characterised in detail (23). Murine IgG obtained from BD biosciences (Franklin Lakes, NJ) was used as an isotype control antibody for in-vitro assays. Mouse monoclonal anti-human CD81 antibody from BD biosciences (Franklin Lakes, NJ, Cat number 555675) represented a positive antibody control. An isotype human anti-HIV-1 IgG was administered to the control group of mice during in-vivo studies. All antibodies were stored at 4°C.
HCV inhibition assays
96 well plates were coated with poly-lysine (Trevigen, Gaithersberg, MD, Cat number: 3438-100-01) prior to plating with 104 Huh 7.5 cells in 100 μL of growth media and incubated overnight. Serially diluted concentrations of EGCG were added as appropriate pre, simultaneous with, or after inoculation with HCVcc at a multiplicity of infection (MOI) of 0.01. For antibody neutralization assays the relevant antibody was serially diluted to the required concentrations and pre-incubated with HCVcc for one hour prior to addition to Huh 7.5 cells. IFN-α2a 100 IU/mL and anti-human CD81 antibody (2.5μg/mL) served as positive controls. Murine IgG (10μg/mL) served as an isotype antibody control. After ten hours of incubation with HCVcc the cells were washed to remove unbound virus and fresh media or the relevant concentration of the investigational agents was added. HCV infectivity was determined at 48 hours using NS5a immune-histostaining (mouse monoclonal NS5a antibody (9E10), Dr. Charles Rice).
Cell viability assay
Cell Proliferation Kit I assay (Roche, Basel, Switzerland Cat Number: 11465007001) was performed as recommended by the manufacturer.
Animal Care
Homozygous albumin/urokinase plasminogen activator severe combined immunodeficient mice (SCID/uPA) mice were kept virus- and antigen-free and housed in the provincial laboratory vivarium at the University of Alberta (32, 33). The University of Alberta Health Sciences Animal Welfare Committee approved experimental protocols, and animals were cared for in accordance with the 1993 guidelines of the Canadian Council on Animal Care. Mice were anaesthetized for transfer of human hepatocytes via intrasplenic injection. Full ethical approval for the use of human tissue was obtained from the Human Research Ethics Board of the University of Alberta Faculty of Medicine. Informed consent was obtained from all donors.
Human alpha-1-Antitrypsin levels (hAAT)
The extent and stability of human liver chimerism can be assessed by serial measurements of hAAT. Serum hAAT levels were analysed as previously described (34). Animals with low serum hAAT levels were used in tolerability/toxicology studies; high hAAT mice were used in the HCV challenge studies.
Dosing and administration of agents
Anti E1/E2 monoclonal antibody (AR4A)
An initial dose of 200mg/kg was administered via intraperitoneal (IP) injection 24 hours prior to HCV inoculation. Prior studies have shown that this dose yields mAb serum values in excess of in-vitro 90% neutralization titers (22). A further four mAb doses of 50mg/kg were administered IP at intervals of 5 days throughout the experiment. Mice in the control group received equivalent doses of an isotype antibody; human anti-HIV-1 IgG.
EGCG
Considering the available information on in-vivo EGCG pharmacokinetics, toxicity, and efficacy, a dosing schedule of 100mg/kg twice daily by gavage(IG) was employed (35). This dose was higher than that which had demonstrated efficacy in previous mouse studies but lower than that with which toxicity had been observed with repeated dosing (36). Further this dosing schedule was within that tolerated by SCID/uPA mice with reference to volume and frequency of gavage. EGCG dosing began 48 hours prior to HCV inoculation and continued for 14 days.
Experimental Protocol
Screening of serum hAAT levels was undertaken six weeks following transplantation of human hepatocytes. Mice with serum hAAT levels (>500 μg/mL) reflecting high engraftment of human hepatocytes were selected to go forward into the HCV challenge studies. In total 11 mice were assigned to each of 4 study groups:
Group 1: Control mice receiving isotype antibody IP and water IG
Group 2: Mice receiving EGCG IG only
Group 3: Mice receiving AR4A mAb IP only
Group 4: Mice receiving both AR4A IP and EGCG IG
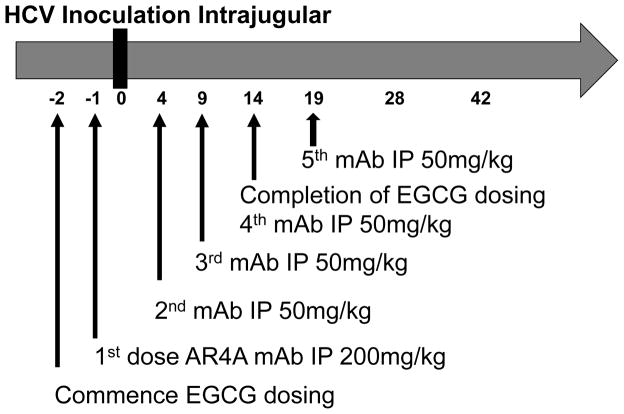
The study outline and protocol employed is illustrated in figure 1.
Figure 1.
Schematic outline of SCID/uPA mouse HCV challenge experiments. Animals were pretreated with EGCG and/or AR4A prior to challenge with HCV. Treatment continued as indicated. mAb: monoclonal antibody, IP: intraperitoneal, * four doses of mAb at 50mg/kg.
HCV inoculation was administered by intrajugular injection. The inoculum used (50 μL, 1.5 × 107 IU/mL) was patient derived HCV genotype 1a serum. Blood sampling was conducted weekly by drawing 100 μL via tail bleeds for measurement of HCV titers and serum hAAT levels.
EGCG Tolerability/Toxicology: EGCG 200mg/kg/day was administered to uninfected low hAAT level animals. Control animals received an equivalent volume of water. The health status of the animals was monitored by their general condition and weight change. At the end of the 14 day dosing period the animals were euthanized. Cardiac puncture was performed to obtain serum for measurement of EGCG levels and liver tissue was snap frozen and stored at −80°C for later measurement of EGCG.
HCV RNA quantification
Viral RNA was extracted from aliquots of mouse serum using a guanidine extraction method (Buffer AVL. Qiagen, Valencia, CA, Cat number: 19073) as per the manufacturer’s instructions and quantitated as described previously (34). The lower limit of quantification of this assay is 300 IU/mL.
EGCG quantification
Plasma and liver tissue levels of EGCG were analyzed using high performance liquid chromatography (HPLC) methodology as previously published (35).
Statistical analysis
Statistical analyses were performed using Stata (Version 12.0, StataCorp LP, College Station, TX) and GraphPad prism (version 6.0, La Jolla, CA) software. Continuous variables were compared between groups using a Mann Whitney U test. A P value less than 0.05 was considered significant. The drug concentration at which HCV infection was inhibited by 50% (IC50) was calculated using the following equations:
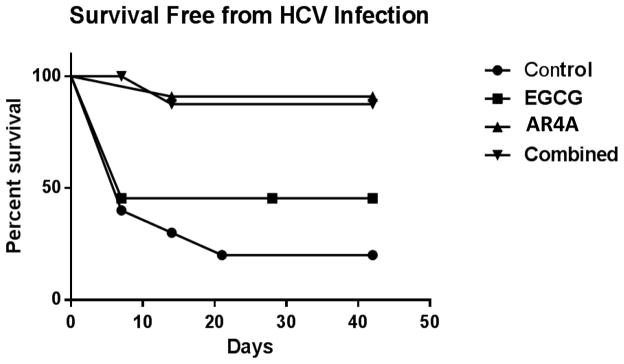
In the HCV challenge experiments, animals with HCV RNA detectable above the threshold (1000 IU/mL) by PCR at day 7 or thereafter were considered ‘infected’. Animals not reaching this threshold were ‘censored’ and considered free from HCV infection. A Kaplan-Meier survival curve (‘Survival free from infection’) was thus generated. Statistical significance between the groups was calculated using a two-tailed log rank test.
Results
AR4A demonstrates effective HCV neutralizing activity in-vitro
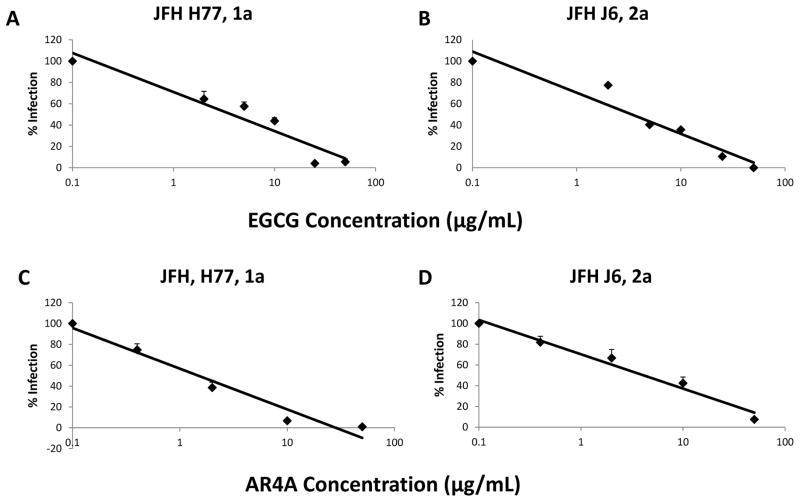
The in-vitro neutralizing capability of AR4A against HCVcc expressing surface glycoproteins of genotypes (gt) 1–6 was examined. Focusing on gt1a (genotype utilized for mouse challenge experiments) and 2a (parent JFH genotype), AR4A exhibited superior neutralizing activity against HCV genotype 1a when compared with genotype 2a; consistent with prior reports (23, 37). IC50 estimates of 1.28 μg/mL and 4.37 μg/mL were obtained for genotypes 1a and 2a, respectively (Figure 2 a,b).
Figure 2.
Anti E1/E2 mAb AR4A (A,B) and EGCG (C,D) dose dependently inhibits infection with JFH-1 1a(A,C) and 2a(B,D) constructs in-vitro. Huh 7.5 cells were treated with EGCG simultaneous with addition of JFH-1 (HCVcc MOI 0.01). AR4A was pre-incubated with JFH-1 for 1 hour prior to addition to Huh 7.5 cells. Ten hours after JFH-1 addition, cells were washed and residual infection in Huh 7.5 cells was detected by NS5A staining at 48 hours. Mean % residual HCV infection is shown. Error bars indicate sem.
Epigallocatechin-gallate (EGCG) inhibits HCV infection in-vitro
A dose dependent decline in HCV infection was observed when Huh 7.5 cells had been simultaneously treated with various concentrations of EGCG (0–100 μg/mL) (Figure 2 c,d). The EGCG IC50 was similar for HCVcc gt 1a and 2a infections (6.6 ug/ml vs. 5.6 ug/ml respectively). At concentrations greater than 25 μg/ml, EGCG consistently achieved almost complete inhibition of HCVcc infection. This inhibitory effect of EGCG was independently confirmed using a firefly luciferase reporter virus, and EGCG did not negatively impact cellular viability. Time of addition assays confirmed that optimal HCV inhibition was critically dependent upon the presence of EGCG at the time of infection (not shown).
Additive reduction in HCV infection combining AR4A and EGCG
AR4A and EGCG primarily act early in the HCV life cycle to inhibit HCV cellular attachment and entry. Using the HCVcc system, we studied the anti-HCV activity of both agents in combination.
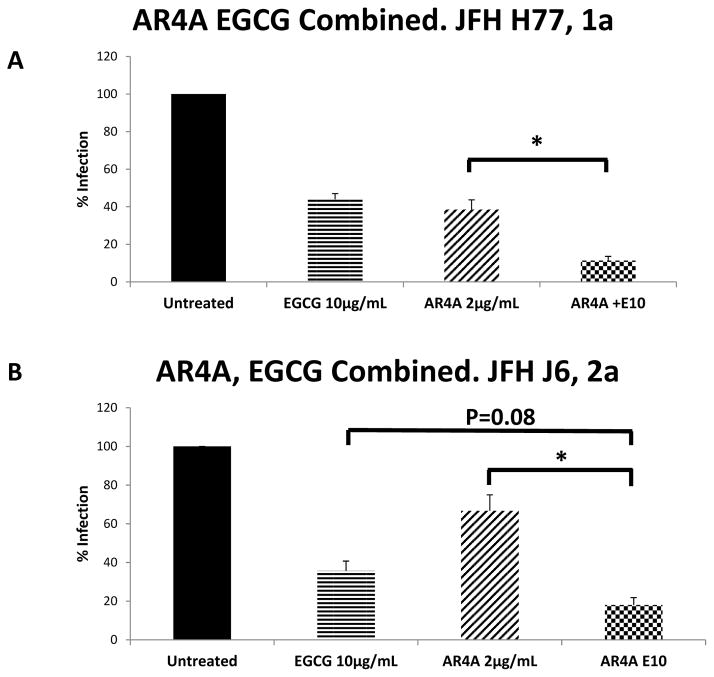
Using gt1a HCVcc, AR4A (2 μg/mL) combined with EGCG (10 μg/mL) demonstrated significantly increased inhibition compared to either agent alone; 88.7% inhibition compared to 61% for AR4A alone (P=0.01), 56% for EGCG alone (Figure 3a). Additive efficacy was again demonstrated with HCV gt 2a(Figure 3b).
Figure 3.
AR4A mAb and EGCG additively inhibit infection with JFH-1 1a(A) and 2a(B) constructs in-vitro. Huh 7.5 cells were treated with EGCG simultaneous with addition of JFH-1 (HCVcc MOI 0.01). AR4A was pre-incubated with JFH-1 for 1 hour prior to addition to Huh 7.5 cells. Ten hours after JFH-1 addition, cells were washed and residual infection in Huh 7.5 cells was detected by NS5A staining at 48 hours. Mean % residual HCV infection is shown. Error bars indicate sem. * P=0.01.
High dose AR4A with low dose EGCG completely inhibits HCV infection in-vitro
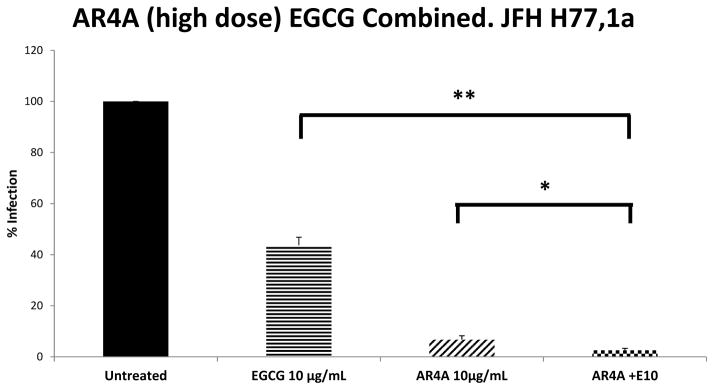
To replicate the clinical scenario whereby therapeutic antibody products are administered at high concentrations, and knowing that the in-vivo bioavailability of EGCG may prove a limiting factor we next titrated AR4a and EGCG to identify conditions capable of completely inhibiting HCV infection. The mAb AR4A, at a concentration of 10 μg/mL, consistently achieved approximately 95% neutralization of genotype 1a HCV. Even at the highest dose of AR4A used (50 μg/mL), residual infection could be detected. Despite 95% neutralization with AR4A (10 μg/mL) alone, the addition of EGCG at 10 μg/mL resulted in a significant further inhibition of HCV (P<0.001 for AR4a alone vs. combination), with complete inhibition of HCV infection attained in a number of experimental repeats (Figure 4). The activity of AR4A (10 μg/mL) and EGCG (10 μg/mL) in combination was further assessed against JFH-1 chimeric constructs expressing the structural proteins of genotypes 2–6. High titer AR4A strongly neutralized genotypes 4a, 5a and 6a; a finding in keeping with prior reports (23).
Figure 4.
High dose AR4A mAb and low dose EGCG combined robustly inhibit infection with JFH-1 genotype 1a in-vitro. Huh 7.5 cells were treated with EGCG simultaneous with addition of JFH-1 (HCVcc MOI 0.01). AR4A 10μg/mL was preincubated with JFH-1 (HCVcc MOI 0.01) and added to Huh 7.5 cells simultaneous with or without EGCG 10μg/mL. Ten hours after JFH-1 addition, cells were washed and residual infection in Huh 7.5 cells was detected by NS5A staining at 48 hours. Mean % residual HCV infection is shown. Error bars indicate sem. Data from 5 independent experiments conducted in triplicate. * P=0.02; ** P<0.001.
Animal HCV Challenge Experiments
All therapeutic regimens were well tolerated by the animals. Serum hAAT levels in mice were evenly distributed through the four groups, and remained broadly stable throughout the experiment. Two mice did not recover following the intrajugular HCV inoculation procedure, one animal in each of the control and combination groups. Additionally two mice from the EGCG alone, and the combination groups became morbid following the day seven blood draw, and one animal in the AR4A alone arm was lost after day 14.
AR4A containing treatment arms robustly protected against HCV infection in SCID/uPA mice; EGCG alone has no apparent protective effect
HCV infection was established in eight of ten control animals, with five progressing to high level replication over 42 days of follow-up.
AR4A demonstrated clear efficacy. Only 2/11 mice receiving AR4A alone and 1/10 mice in the combination arm had HCV RNA detected above the threshold of 1000IU/mL throughout study follow-up. ‘Survival free from HCV infection’ was significantly increased in AR4A treated groups. Given the small number of events in these study groups no difference was demonstrable between the groups receiving AR4A alone or both AR4A and EGCG.
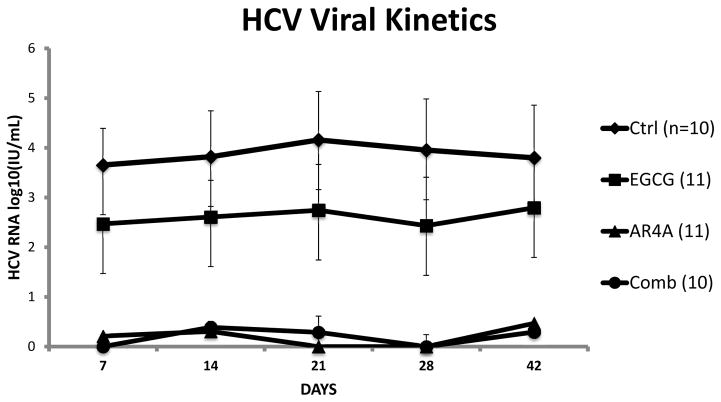
EGCG monotherapy failed to reliably protect from HCV infection and observed outcomes were no different from the control group. The viral kinetics and a Kaplan-Meier curve of ‘Survival Free from HCV Infection’ are illustrated in figure 5 and 6 respectively.
Figure 5.
HCV viral kinetics in mice according to intervention group. Mean log10 HCV RNA values are shown. Error bars indicate sem. Animal treatment groups: Control mice were treated with isotype human IgG; Animals treated with EGCG alone (200mg/kg/day for 14 days); Animals treated with AR4A alone (5 intraperitoneal injections); Animals treated with both AR4A and EGCG.
Figure 6.
Kaplan-meier ‘Survival Free From HCV Infection’. Mice failing to achieve the pre-determined threshold for active HCV replication (HCV RNA > 1000IU/mL at or after day 7) were censored. Animal treatment groups: Control mice were treated with isotype human IgG; Animals treated with EGCG alone (200mg/kg/day for 14 days); Animals treated with AR4A alone (5 intraperitoneal injections); Animals treated with both AR4A and EGCG.
EGCG levels in plasma and liver tissue of mice following 14 consecutive days of administration
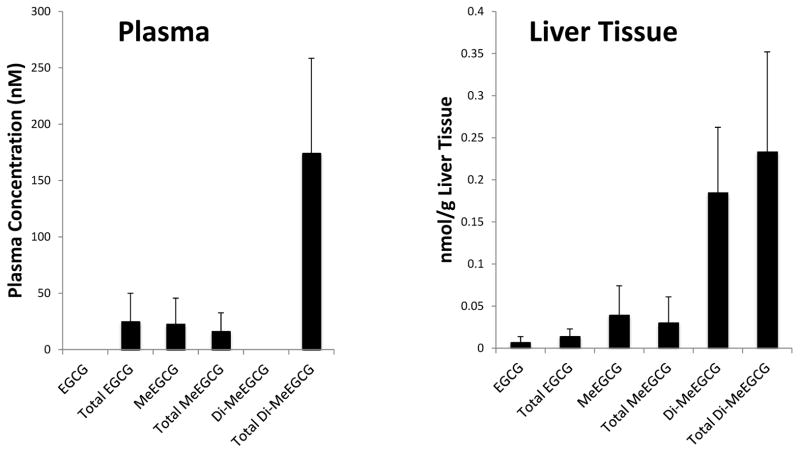
In order to assess if repeated high doses of EGCG 200mg/kg/day were capable of achieving sufficient levels of EGCG (and its metabolites) in plasma and liver we collected samples after 14 consecutive days of dosing. For this analysis samples were obtained four hours following the final dose. Figure 7 illustrates the levels of detection of EGCG and its metabolites. The parent compound itself or its metabolites were detectable in all treated animals, however importantly the levels in both plasma and liver tissue were low (in the nanomolar range) when measured four hours post administration.
Figure 7.
Detection of EGCG, Monomethylated EGCG (MeEGCG) and Dimethylated EGCG (Di-MeEGCG) in plasma and liver tissue of treated mice. SCID/uPA Mice underwent daily intragastric instillation of EGCG 200mg/kg/day for 14 days. 4 hours following the final dose the animals were euthanized. Serum was stored, and liver tissue snap frozen for later assays of levels of EGCG and its metabolites. The mean value of 3 treated mice are presented, bars indicate sem. Total: includes estimates of parent compound, in addition to the glucuronide and sulfated metabolites.
Discussion
HCV related liver disease is the leading indication for liver transplantation. Re-infection continues to drive inferior outcomes (12). Numerous unsuccessful attempts to address this discrepancy have been made. Recently, novel approaches provide some cause for optimism (16, 38), potentially shifting the paradigm whereby the treatment of HCV after liver transplantation is restricted to a delayed phase post transplantation when reinfection and histological disease has already been established (15, 39, 40).
There is a need for safe, effective and tolerable therapies that can avail of the window of opportunity provided by liver transplantation to prevent HCV re-infection. Complex drug-drug interactions require due consideration as does treatment durability in the face of a dynamic virus with high replicative capacity in the setting of impaired host humoral and cell mediated immunity. Effective inhibition of HCV entry into ‘naïve’ allograft hepatocytes represents a primary objective of preventative strategies.
In this work we investigated agents acting primarily to inhibit HCV cell entry. Consistent with prior reports AR4A and EGCG both reliably demonstrate cross genotype inhibition of HCV in-vitro. Using these agents in combination was additive and the value of EGCG maintained significance even at high AR4A titers.
SCID/uPA mice receiving AR4A mAb by intraperitoneal injection were significantly less likely to develop established infection following HCV challenge with a genotype 1a inoculum. This is the first data reporting the anti-HCV efficacy of AR4A monotherapy in an animal model capable of sustaining HCV replication. AR4A clearly provides robust protection against the initial establishment of infection, demonstrating durable activity, which compares favourably with prior studies employing immunotherapy (21, 22).
Despite clear in-vitro inhibition, administration of EGCG alone, whilst well tolerated by SCID/uPA mice, did not offer protection against HCV challenge in-vivo. This is the first report concerning the in-vivo anti-HCV activity of EGCG. This lack of efficacy was likely driven by the low bioavailability of orally-administered EGCG (41, 42). Previous studies have shown that peak plasma and liver concentrations of unconjugated EGCG in mice were 40 nmol/L and 3.5 nmol/g, respectively following treatment with intragastric EGCG (75 mg/kg). EGCG undergoes extensive Phase II metabolism resulting in the formation of glucuronide conjugates and methylated metabolites. The levels of these metabolites have been shown in animal models to exceed the levels of the parent compound (41, 43). Studies have shown that EGCG is rapidly metabolized by catechol-O-methyltransferase (COMT) resulting in the formation of MeEGCG and diMeEGCG (44). These methylated metabolites have been shown to have reduced biological activity in a number of systems compared to the unmetabolized parent compound (45, 46).
To date, no studies have examined the effects of methylation on the antiviral activity of EGCG. A recent study of influenza virus reported that methylated (−)-epigallocatechin has reduced inhibitory potency (IC50 = 33.4 μg/mL) compared to unmetabolized (−)-epigallocatechin (IC50 = 13.5 μg/mL) in vitro (47). Given the extensive Phase II biotransformation of EGCG in vivo, further studies on the anti-viral activity of the major metabolites are warranted.
Calland et al have recently proposed a novel mechanism whereby EGCG and related natural compounds disrupt initial HCV attachment in vitro. Through an interaction with surface glycoproteins such compounds alter the shape of HCV viral particles inhibiting efficient cellular attachment (48). With a proposed mechanism of action reliant on direct disruption of the initial stages of HCV cellular attachment and direct cell-cell transmission, failing to maintain adequate local concentrations will clearly limit the efficacy of EGCG in-vivo.
Prior clinical studies administering passive immunotherapy to HCV patients undergoing liver transplantation have been disappointing. Polyclonal immunoglobulin failed to prevent HCV re-infection (9). Similarly an anti-E2 mAb (HCV-AbxTL68), whilst effecting some decline in titers, did not prevent HCV recurrence in patients undergoing liver transplantation (49). Another group administered an anti-E2 mAb (MBL-HCV1) to six HCV transplant recipients. All eventually experienced re-infection with viral species harboring mutations in the target epitope (50).
AR4A consistently demonstrated a robust ability to protect against HCV challenge with a patient derived genotype 1a inoculum, and its in-vitro characteristics compare very favorably with those of other anti-HCV mAbs (23, 37, 51). The increased efficacy of AR4A mAb can be proposed by it uniquely targeting an epitope region abridging both E1 and E2, and containing a residue which is highly conserved across HCV species.
The SCID/uPA humanized liver mouse model is an extremely useful tool for conducting in-vivo HCV studies (32, 33, 37). These mice lack an adaptive immune response, thus caution is required when translating findings to the clinical situation, considering that pre-existing host antibody bound to HCV viral particles can competitively inhibit effective neutralization by anti-HCV mAbs (52, 53). Likewise it is possible that the native viral particle in the experimental systems used is physically different to that which circulates in association with human lipoproteins. An overestimation of efficacy could contribute to the comparatively poor activity observed in patient studies to date (9, 49, 50).
Another limitation is the use of a patient derived genotype 1a inoculum only. Genotype 1 is the predominant HCV genotype in Europe and North America, however the immediate generalizability to individuals with HCV of diverse genotypes awaiting liver transplant is somewhat limited. However clear in-vitro cross genotype activity of AR4A was demonstrated. Further, the patient inoculum provided a heterologous HCV species challenge against which AR4A demonstrated impressive preventative capacity.
Despite these limitations this study has yielded a number of important findings. We have demonstrated the proficiency of the anti-E1/E2 mAb AR4A to prevent the establishment of replicating HCV infection in-vivo. On the other hand, EGCG, though an effective in-vitro inhibitor, demonstrated a lack of definitive efficacy to protect SCID/uPA mice against HCV infection.
The results identify next generation anti-E1/E2 monoclonal antibodies as a potential therapeutic advance, which can constitute a primary component of future preventative approaches. The cross neutralizing ability of AR4A targeting a highly conserved epitope also provides further proof of principle that effective pre-formed antibody can contribute to protection against HCV challenge. A candidate vaccine has been shown to elicit such antibodies in vaccinated human volunteers, providing encouragement for demonstration of future preventive efficacy (54).
To optimize the efficacy of this mAb in liver transplantation, additional agents to protect against virologic breakthrough and resistant mutants will be a prerequisite. Future clinical studies of anti-HCV mAbs incorporating novel pan-genotypic DAAs in complex transplant candidates with advanced HCV-related liver disease are required.
The application of EGCG, though eminently more available worldwide than DAAs, is limited by poor bioavailability. Further characterization of the interaction between the HCV viral particle and EGCG carries the potential to identify new agents targeting HCV cell entry (48). Moreover, capitalizing upon the unique circumstances of liver transplantation, novel applications of non-toxic compounds such as EGCG or derivatives could provide an opportunity to prime liver allografts ex-vivo against HCV reinfection following implantation.
Employing therapies pre or immediately at transplantation stands to deliver timely, effective, and tolerable HCV prophylactic combinations. This provides considerable optimism that in the future routine prevention of HCV re-infection after liver transplantation is in fact attainable. In achieving this goal, outcomes for patients with HCV undergoing liver transplant will be brought back in line with that of their non-HCV counterparts, whilst ensuring optimal use of a highly valuable resource – human livers.
Supplementary Material
Figure S1: In vitro effects of EGCG on cell activity and viability. Cell Proliferation Kit I assay (Roche, Basel, Switzerland Cat Number: 11465007001) was used. Ninety-six well plates were prepared and seeded with Huh7.5 cells in an identical manner to that for inhibition assays. Following incubation overnight, serial dilutions of EGCG were added to cells. After 24hrs incubation, 10μL of MTT labelling reagent was added for 4 hours. Then 100 μL solubilisation solution was added and the plates underwent further overnight incubation. Spectrophotometrical absorbance was measured using an ELISA reader. The mean % change in cellular metabolic activity (compared to control wells) is shown, error bars indicate sem.
Figure S2: Serum human alpha-1-antitrypsin levels in animal groups. During HCV challenge experiments serial serum hAAT levels were determined to assess viability of engrafted human hepatocytes. Mean serum hAAT levels of animal treatment groups are shown. Error bars indicate sem. Animal treatment groups: Control mice were treated with isotype human IgG; Animals treated with EGCG alone (200mg/kg/day for 14 days); Animals treated with AR4A alone (5 intraperitoneal injections); Animals treated with both AR4A and EGCG.
Figure S3: HCV viral kinetics in individual mice across treatment groups. Weekly PCR for HCV RNA levels was undertaken. Log10 HCV RNA values are shown. Animal treatment groups: Control mice were treated with isotype human IgG; Animals treated with EGCG alone (200mg/kg/day for 14 days); Animals treated with AR4A alone (5 intraperitoneal injections); Animals treated with both AR4A and EGCG.
Acknowledgments
Financial Support
MH was funded by the Canada Excellence in Research Chair program. A.E. is supported by a Swiss National Fund (PBBSP3-130963) and a Lichtenstein Foundation grant. M.L. and D.R.B. were supported in part by grants R01AI079031 (to M.L.) and R01AI071084 (to D.R.B) from the National Institute for Allergy and Infectious Disease. D.L.J.T. is supported by a CIHR grant.
We thank Chelcey Dibben and Brandie Greaves for provision of expert animal care. Additional thanks to Erick Giang of The Scripps Research Institute for antibody production.
Abbreviations
- DAA
direct acting antivirals
- Di-Me EGCG
Dimethylated Epigallocatechin gallate
- DMEM
Dulbecco’s Modified Eagle’s Medium
- EGCG
Epigallocatechin gallate
- FBS
Fetal bovine serum
- h-AAT
human alpha-1-antitrypsin
- HCV
Hepatitis C virus
- HCVcc
cell culture derived Hepatitis C Virus
- HPLC
High performance liquid chromatography
- IC50
50% inhibitory concentration
- IFN
Interferon
- IG
Intragastric
- IP
Intraperitoneal
- mAb
Monoclonal antibody
- Me-EGCG
Methylated epigallocatechin gallate
- MOI
multiplicity of infection
- NEAA
Non-essential amino acids
- PCR
Polymerase Chain reaction
- SCID/uPA
Severe combined immunodeficiency/Albumin/urokinase plasminogen activator
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. Jama. 2014;312(6):631–40. doi: 10.1001/jama.2014.7085. [DOI] [PubMed] [Google Scholar]
- 2.Feeney ER, Chung RT. Antiviral treatment of hepatitis C. BMJ (Clinical research ed) 2014;348:g3308. doi: 10.1136/bmj.g3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ansaldi F, Orsi A, Sticchi L, Bruzzone B, Icardi G. Hepatitis C virus in the new era: perspectives in epidemiology, prevention, diagnostics and predictors of response to therapy. World journal of gastroenterology : WJG. 2014;20(29):9633–52. doi: 10.3748/wjg.v20.i29.9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strickland GT, El-Kamary SS, Klenerman P, Nicosia A. Hepatitis C vaccine: supply and demand. The Lancet Infectious diseases. 2008;8(6):379–86. doi: 10.1016/S1473-3099(08)70126-9. [DOI] [PubMed] [Google Scholar]
- 5.Honegger JR, Zhou Y, Walker CM. Will there be a vaccine to prevent HCV infection? Seminars in liver disease. 2014;34(1):79–88. doi: 10.1055/s-0034-1371081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Law JL, Chen C, Wong J, Hockman D, Santer DM, Frey SE, et al. A hepatitis C virus (HCV) vaccine comprising envelope glycoproteins gpE1/gpE2 derived from a single isolate elicits broad cross-genotype neutralizing antibodies in humans. PloS one. 2013;8(3):e59776. doi: 10.1371/journal.pone.0059776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everson GT, Terrault NA, Lok AS, Rodrigo DR, Brown RS, Jr, Saab S, et al. A randomized controlled trial of pretransplant antiviral therapy to prevent recurrence of hepatitis C after liver transplantation. Hepatology. 2012 doi: 10.1002/hep.25976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terrault NA. Hepatitis C therapy before and after liver transplantation. Liver Transpl. 2008;14 (Suppl 2):S58–66. doi: 10.1002/lt.21624. [DOI] [PubMed] [Google Scholar]
- 9.Davis GL, Nelson DR, Terrault N, Pruett TL, Schiano TD, Fletcher CV, et al. A randomized, open-label study to evaluate the safety and pharmacokinetics of human hepatitis C immune globulin (Civacir) in liver transplant recipients. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2005;11(8):941–9. doi: 10.1002/lt.20405. [DOI] [PubMed] [Google Scholar]
- 10.Brown RS. Hepatitis C and liver transplantation. Nature. 2005;436(7053):973–8. doi: 10.1038/nature04083. [DOI] [PubMed] [Google Scholar]
- 11.Gane EJ. The natural history of recurrent hepatitis C and what influences this. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2008;14 (Suppl 2):S36–44. doi: 10.1002/lt.21646. [DOI] [PubMed] [Google Scholar]
- 12.Shiffman ML, Saab S, Feng S, Abecassis MI, Tzakis AG, Goodrich NP, et al. Liver and intestine transplantation in the United States, 1995–2004. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(5 Pt 2):1170–87. doi: 10.1111/j.1600-6143.2006.01273.x. [DOI] [PubMed] [Google Scholar]
- 13.Dumortier J, Chevallier P, Scoazec JY, Berger F, Boillot O. Combined lamivudine and hepatitis B immunoglobulin for the prevention of hepatitis B recurrence after liver transplantation: long-term results. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2003;3(8):999–1002. doi: 10.1034/j.1600-6143.2003.00191.x. [DOI] [PubMed] [Google Scholar]
- 14.Markowitz JS, Martin P, Conrad AJ, Markmann JF, Seu P, Yersiz H, et al. Prophylaxis against hepatitis B recurrence following liver transplantation using combination lamivudine and hepatitis B immune globulin. Hepatology (Baltimore, Md) 1998;28(2):585–9. doi: 10.1002/hep.510280241. [DOI] [PubMed] [Google Scholar]
- 15.Berenguer M, Palau A, Aguilera V, Rayon JM, Juan FS, Prieto M. Clinical benefits of antiviral therapy in patients with recurrent hepatitis C following liver transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(3):679–87. doi: 10.1111/j.1600-6143.2007.02126.x. [DOI] [PubMed] [Google Scholar]
- 16.Curry MP, Forns X, Chung RT, Terrault NA, Brown R, Jr, Fenkel JM, et al. Sofosbuvir and Ribavirin Prevent Recurrence of HCV Infection after Liver Transplantation: An Open-Label Study. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Owsianka A, Tarr AW, Juttla VS, Lavillette D, Bartosch B, Cosset FL, et al. Monoclonal antibody AP33 defines a broadly neutralizing epitope on the hepatitis C virus E2 envelope glycoprotein. Journal of virology. 2005;79(17):11095–104. doi: 10.1128/JVI.79.17.11095-11104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broering TJ, Garrity KA, Boatright NK, Sloan SE, Sandor F, Thomas WD, Jr, et al. Identification and characterization of broadly neutralizing human monoclonal antibodies directed against the E2 envelope glycoprotein of hepatitis C virus. Journal of virology. 2009;83(23):12473–82. doi: 10.1128/JVI.01138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson DX, Voisset C, Tarr AW, Aung M, Ball JK, Dubuisson J, et al. Human combinatorial libraries yield rare antibodies that broadly neutralize hepatitis C virus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(41):16269–74. doi: 10.1073/pnas.0705522104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartosch B, Dubuisson J. Recent advances in hepatitis C virus cell entry. Viruses. 2010;2(3):692–709. doi: 10.3390/v2030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meuleman P, Bukh J, Verhoye L, Farhoudi A, Vanwolleghem T, Wang RY, et al. In vivo evaluation of the cross-genotype neutralizing activity of polyclonal antibodies against hepatitis C virus. Hepatology (Baltimore, Md) 2011;53(3):755–62. doi: 10.1002/hep.24171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Law M, Maruyama T, Lewis J, Giang E, Tarr AW, Stamataki Z, et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nature medicine. 2008;14(1):25–7. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- 23.Giang E, Dorner M, Prentoe JC, Dreux M, Evans MJ, Bukh J, et al. Human broadly neutralizing antibodies to the envelope glycoprotein complex of hepatitis C virus. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(16):6205–10. doi: 10.1073/pnas.1114927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlsen TH, Pedersen J, Prentoe JC, Giang E, Keck ZY, Mikkelsen LS, et al. Breadth of neutralization and synergy of clinically relevant human monoclonal antibodies against HCV genotypes 1a, 1b, 2a, 2b, 2c, and 3a. Hepatology (Baltimore, Md) 2014;60(5):1551–62. doi: 10.1002/hep.27298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan N, Mukhtar H. Tea polyphenols for health promotion. Life sciences. 2007;81(7):519–33. doi: 10.1016/j.lfs.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boehm K, Borrelli F, Ernst E, Habacher G, Hung SK, Milazzo S, et al. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane database of systematic reviews (Online) 2009;(3):CD005004. doi: 10.1002/14651858.CD005004.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ullmann U, Haller J, Decourt JD, Girault J, Spitzer V, Weber P. Plasma-kinetic characteristics of purified and isolated green tea catechin epigallocatechin gallate (EGCG) after 10 days repeated dosing in healthy volunteers. International journal for vitamin and nutrition research Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung Journal international de vitaminologie et de nutrition. 2004;74(4):269–78. doi: 10.1024/0300-9831.74.4.269. [DOI] [PubMed] [Google Scholar]
- 28.Ciesek S, von Hahn T, Colpitts CC, Schang LM, Friesland M, Steinmann J, et al. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology (Baltimore, Md) 2011;54(6):1947–55. doi: 10.1002/hep.24610. [DOI] [PubMed] [Google Scholar]
- 29.Calland N, Albecka A, Belouzard S, Wychowski C, Duverlie G, Descamps V, et al. (−)-Epigallocatechin-3-gallate is a new inhibitor of hepatitis C virus entry. Hepatology (Baltimore, Md) 2012;55(3):720–9. doi: 10.1002/hep.24803. [DOI] [PubMed] [Google Scholar]
- 30.Lindenbach BD, Evans MJ, Syder AJ, Wolk B, Tellinghuisen TL, Liu CC, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005;309(5734):623–6. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 31.Gottwein JM, Scheel TK, Jensen TB, Lademann JB, Prentoe JC, Knudsen ML, et al. Development and characterization of hepatitis C virus genotype 1–7 cell culture systems: role of CD81 and scavenger receptor class B type I and effect of antiviral drugs. Hepatology (Baltimore, Md) 2009;49(2):364–77. doi: 10.1002/hep.22673. [DOI] [PubMed] [Google Scholar]
- 32.Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, et al. Hepatitis C virus replication in mice with chimeric human livers. Nature medicine. 2001;7(8):927–33. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- 33.Kneteman NM, Weiner AJ, O’Connell J, Collett M, Gao T, Aukerman L, et al. Anti-HCV therapies in chimeric scid-Alb/uPA mice parallel outcomes in human clinical application. Hepatology (Baltimore, Md) 2006;43(6):1346–53. doi: 10.1002/hep.21209. [DOI] [PubMed] [Google Scholar]
- 34.Steenbergen RH, Joyce MA, Lund G, Lewis J, Chen R, Barsby N, et al. Lipoprotein profiles in SCID/uPA mice transplanted with human hepatocytes become human-like and correlate with HCV infection success. American journal of physiology Gastrointestinal and liver physiology. 2010;299(4):G844–54. doi: 10.1152/ajpgi.00200.2010. [DOI] [PubMed] [Google Scholar]
- 35.Lambert JD, Lee MJ, Diamond L, Ju J, Hong J, Bose M, et al. Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues. Drug metabolism and disposition: the biological fate of chemicals. 2006;34(1):8–11. doi: 10.1124/dmd.104.003434. [DOI] [PubMed] [Google Scholar]
- 36.Lambert JD, Kennett MJ, Sang S, Reuhl KR, Ju J, Yang CS. Hepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in mice. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2010;48(1):409–16. doi: 10.1016/j.fct.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwards VC, Tarr AW, Urbanowicz RA, Ball JK. The role of neutralizing antibodies in hepatitis C virus infection. The Journal of general virology. 2012;93(Pt 1):1–19. doi: 10.1099/vir.0.035956-0. [DOI] [PubMed] [Google Scholar]
- 38.Charlton M, Gane E, Manns MP, Brown RS, Jr, Curry MP, Kwo PY, et al. Sofosbuvir and Ribavirin for Treatment of Compensated Recurrent Hepatitis C Virus Infection After Liver Transplantation. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Chalasani N, Manzarbeitia C, Ferenci P, Vogel W, Fontana RJ, Voigt M, et al. Peginterferon alfa-2a for hepatitis C after liver transplantation: two randomized, controlled trials. Hepatology (Baltimore, Md) 2005;41(2):289–98. doi: 10.1002/hep.20560. [DOI] [PubMed] [Google Scholar]
- 40.Terrault NA, Berenguer M. Treating hepatitis C infection in liver transplant recipients. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2006;12(8):1192–204. doi: 10.1002/lt.20865. [DOI] [PubMed] [Google Scholar]
- 41.Lambert JD, Lee MJ, Lu H, Meng X, Hong JJ, Seril DN, et al. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J Nutr. 2003;133(12):4172–7. doi: 10.1093/jn/133.12.4172. [DOI] [PubMed] [Google Scholar]
- 42.Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9(9):3312–9. [PubMed] [Google Scholar]
- 43.Meng X, Sang S, Zhu N, Lu H, Sheng S, Lee MJ, et al. Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice, and rats. Chemical research in toxicology. 2002;15(8):1042–50. doi: 10.1021/tx010184a. [DOI] [PubMed] [Google Scholar]
- 44.Chen D, Wang CY, Lambert JD, Ai N, Welsh WJ, Yang CS. Inhibition of human liver catechol-O-methyltransferase by tea catechins and their metabolites: structure-activity relationship and molecular-modeling studies. Biochemical pharmacology. 2005;69(10):1523–31. doi: 10.1016/j.bcp.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 45.Landis-Piwowar KR, Wan SB, Wiegand RA, Kuhn DJ, Chan TH, Dou QP. Methylation suppresses the proteasome-inhibitory function of green tea polyphenols. Journal of cellular physiology. 2007;213(1):252–60. doi: 10.1002/jcp.21124. [DOI] [PubMed] [Google Scholar]
- 46.Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, et al. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer research. 2003;63(22):7563–70. [PubMed] [Google Scholar]
- 47.Yang ZF, Bai LP, Huang WB, Li XZ, Zhao SS, Zhong NS, et al. Comparison of in vitro antiviral activity of tea polyphenols against influenza A and B viruses and structure-activity relationship analysis. Fitoterapia. 2014;93:47–53. doi: 10.1016/j.fitote.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 48.Calland N, Sahuc ME, Belouzard S, Pene V, Bonnafous P, Mesalam AA, et al. Polyphenols Inhibit Hepatitis C Virus Entry by a New Mechanism of Action. Journal of virology. 2015 doi: 10.1128/JVI.01473-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schiano TD, Charlton M, Younossi Z, Galun E, Pruett T, Tur-Kaspa R, et al. Monoclonal antibody HCV-AbXTL68 in patients undergoing liver transplantation for HCV: results of a phase 2 randomized study. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2006;12(9):1381–9. doi: 10.1002/lt.20876. [DOI] [PubMed] [Google Scholar]
- 50.Chung RT, Gordon FD, Curry MP, Schiano TD, Emre S, Corey K, et al. Human Monoclonal Antibody MBL-HCV1 Delays HCV Viral Rebound Following Liver Transplantation: A Randomized Controlled Study. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(4):1047–54. doi: 10.1111/ajt.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Keck ZY, Foung SK. Neutralizing antibody response to hepatitis C virus. Viruses. 2011;3(11):2127–45. doi: 10.3390/v3112127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morin TJ, Broering TJ, Leav BA, Blair BM, Rowley KJ, Boucher EN, et al. Human monoclonal antibody HCV1 effectively prevents and treats HCV infection in chimpanzees. PLoS pathogens. 2012;8(8):e1002895. doi: 10.1371/journal.ppat.1002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang P, Zhong L, Struble EB, Watanabe H, Kachko A, Mihalik K, et al. Depletion of interfering antibodies in chronic hepatitis C patients and vaccinated chimpanzees reveals broad cross-genotype neutralizing activity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(18):7537–41. doi: 10.1073/pnas.0902749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong JA, Bhat R, Hockman D, Logan M, Chen C, Levin A, et al. A recombinant HCV envelope glycoprotein vaccine elicits antibodies targeting multiple epitopes on the envelope glycoproteins associated with broad cross-neutralization. Journal of virology. 2014 doi: 10.1128/JVI.01911-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: In vitro effects of EGCG on cell activity and viability. Cell Proliferation Kit I assay (Roche, Basel, Switzerland Cat Number: 11465007001) was used. Ninety-six well plates were prepared and seeded with Huh7.5 cells in an identical manner to that for inhibition assays. Following incubation overnight, serial dilutions of EGCG were added to cells. After 24hrs incubation, 10μL of MTT labelling reagent was added for 4 hours. Then 100 μL solubilisation solution was added and the plates underwent further overnight incubation. Spectrophotometrical absorbance was measured using an ELISA reader. The mean % change in cellular metabolic activity (compared to control wells) is shown, error bars indicate sem.
Figure S2: Serum human alpha-1-antitrypsin levels in animal groups. During HCV challenge experiments serial serum hAAT levels were determined to assess viability of engrafted human hepatocytes. Mean serum hAAT levels of animal treatment groups are shown. Error bars indicate sem. Animal treatment groups: Control mice were treated with isotype human IgG; Animals treated with EGCG alone (200mg/kg/day for 14 days); Animals treated with AR4A alone (5 intraperitoneal injections); Animals treated with both AR4A and EGCG.
Figure S3: HCV viral kinetics in individual mice across treatment groups. Weekly PCR for HCV RNA levels was undertaken. Log10 HCV RNA values are shown. Animal treatment groups: Control mice were treated with isotype human IgG; Animals treated with EGCG alone (200mg/kg/day for 14 days); Animals treated with AR4A alone (5 intraperitoneal injections); Animals treated with both AR4A and EGCG.