Abstract
Background
Dust mite allergens can induce allergic sensitization and exacerbate asthma symptoms. Although dust mite reduction and control strategies exist, few asthmatics employ them.
Objectives
We examined whether an in-home test kit, which quantifies dust mite allergen levels, resulted in behavioral changes in implementation and maintenance of mite reduction strategies and helped reduce allergen levels in homes of dust mite-sensitive children.
Methods
We enrolled 60 households of children aged 5-15 with parent-reported dust mite allergy into a randomized controlled trial. Intervention homes (N=30) received educational material about reducing dust mites and test kits at 1,2,5, and 8 months. Control homes (N=30) received only educational material. At baseline, 6 and 12 months, study staff visited all homes, collected dust samples from 3 locations and obtained information about parents’ mite reduction behaviors by questionnaire. Allergen concentrations (Der f 2/Der p2) in dust were assessed by immunoassays. After adjusting for visit and location, allergen concentrations in intervention and control homes were compared using mixed effects model analysis.
Results
In the intervention homes, allergen concentrations in the child's bedroom and living room floors were significantly reduced over time compared to control homes. Although not all location-specific differences in allergen concentrations were statistically significant, combining data across locations, there was a differential reduction in allergen concentrations in the intervention group versus the control group (p =0.02).
Conclusion
The use of in-home test kits along with education may beneficially influence behaviors and attitudes towards dust mite reduction strategies and help reduce residential dust mite allergen levels.
Keywords: dust mites, dust mite allergen, D. pteronyssinus, D. farinae, indoor allergens, intervention trials
INTRODUCTION
Dust mites are common triggers of asthmatic symptoms among children [1]. Studies have shown that reduction in exposure to dust mite allergens leads to improvement in asthma symptoms and airway hyperresponsiveness among dust mite -sensitive individuals with asthma [2-4]. Because allergen avoidance is a fundamental part of allergic disease management, individuals suffering from allergies or asthma are encouraged to employ environmental control measures to reduce exposure to indoor allergens [5]. While dust mite reduction strategies help reduce exposure to dust mite allergens [6-8], studies suggest that patient education alone may not be effective in changing environmental control behaviors [9, 10]. According to stage models of behavior change, such as the Precaution Adoption Process Model or PAPM [11, 12], an individual's perception of personal susceptibility to a health hazard (e.g., dust mite allergen levels in the home) promotes transition to the adoption of precautionary measures. This requires some awareness of one's degree of exposure as well as an ability to determine the effectiveness of allergen reduction strategies; however, the latter typically involves expensive, periodic, commercial services. The lack of objective and easy-to-use methods to quantify dust mite allergen levels in homes has hampered patients’ ability to confirm the efficacy of their efforts to reduce allergen levels.
We hypothesized that parents of dust mite allergic children might be motivated to implement and maintain allergen reduction strategies, subsequently reducing allergen levels, if they could easily monitor allergen levels. We conducted a randomized, controlled intervention trial to determine whether patient education in conjunction with use of commercially available in-home test kits (MITE-T-FAST™ test, Aveho Biosciences, Inc, Oak Ridge, NJ), which provide semiquantitative information on dust mite allergen levels, resulted in greater reductions in dust mite allergen levels than the use of educational materials alone. We also investigated if the use of the in-home test kits resulted in attitudinal and/or behavioral changes related to the implementation and maintenance of dust mite reduction strategies.
METHODS
Enrollment, Randomization and Study Procedures
Participants were recruited from central North Carolina via advertisements and recruitment campaigns from October 2006 to February 2008. To ascertain eligibility, the study coordinators scheduled a screening visit to collect dust for measuring dust mite allergen levels in the bedroom of the child with dust mite allergies. The inclusion criteria required that participants expected to remain in the same home for ≥ 12 months, have a dust mite allergic/sensitive child (aged 5-15 years) living in the household (allergy self-reported by parent or guardian), the child had to sleep in his/her own bed ≥ 3 nights out of the week, the child's bedroom floor was ≥ 50% carpet, the parents could not be using protective mattress covers at enrollment, and at least one of the child's bedroom surfaces (floor or bed) had dust mite allergen levels ≥ 2 μg/g. Homes were enrolled if at least one of the bedroom samples contained a concentration of (Der f 2/Der p2) ≥ 2 μg per gram of vacuumed dust (ELISA assessment) and ≥ 2 μg/g on the corresponding MITE-T-FAST™ test. The rapid immunoassay kit (MITE-T-FAST™) used lateral flow technology and gold labelled monoclonal antibody for allergen detection to provide information on dust mite levels (no allergen detected, < 2.0 μg/g, 2.0 μg/g, 2.0-10.0 μg/g, >10.0 μg/g). Participants were randomly assigned to control (n=30) or intervention (n=30) groups using a permuted block randomization design.
The intervention group (guardians or parents) received educational brochures on dust mites and dust mite reduction strategies and an in-home test kit at set intervals (1, 2, 5, and 8 months after the baseline visit), while the control group received either educational brochures, “thank you” notes, or reminder cards at the same intervals (Figure S1). Parents in the intervention arm were instructed to use the test kit (MITE-T-FAST™ test, Aveho Biosciences, Inc., Oak Ridge, NJ) on the child's bed and bedroom floor at 1, 2, 5, and 8 months from baseline, and return the result forms in the mail. At baseline, 6 months, and 12 months, study staff visited all homes and collected dust samples from 3 locations, completed an observation form and obtained information about parents’ mite reduction behaviors by questionnaire (e.g., washing sheets weekly in hot water, encasing mattress and pillows in allergen impermeable covers, vacuuming with HEPA filters and removing stuffed animals). The questionnaire utilized a standardized approach leveraging Likert scales to assess the PAPM stages [11, 12], and was reviewed by a panel of experts in asthma, health behaviors, clinical immunology, epidemiology, survey methods, and biostatistics. In addition, the questionnaire was cognitively tested to select language and structure of questions most effective in capturing the needed information in face-to-face interview format. Study procedures are shown in Figure S1 (Online Repository). The National Institute of Environmental Health Sciences Institutional Review Board and Copernicus Group Independent Review Board approved this study, and written informed consent was obtained from all participants. The trial was registered at http://ClinicalTrials.gov (registration identifier NCT00339690).
Dust Collection and Analysis
Baseline data on dust mite allergen levels for the child's bed and bedroom floor were collected during the eligibility screening visit. Living room floor was sampled during the baseline visit. During the follow-up visits (6 and 12 months), dust samples were collected from these 3 locations. All samples were vacuumed using a Eureka Mighty-Mite 7.0-ampere vacuum cleaner (Eureka Company, Bloomington, IL) with a dust collector (Indoor Biotechnologies, Charlottesville, VA) placed on the distal end of the vacuum's extension wand. Each sample was collected within a 1-square-yard template on the sampling surface by vacuuming for 4 minutes.
In the laboratory, vacuumed dust samples were sieved through 425μm mesh, weighed, and aliquoted. Dust samples were extracted at 50 mg/ml with PBS-T/1.0% BSA for one hour on a rocker platform at room temperature, clarified by centrifugation, aliquoted, and stored at −20°C until analysis. MARIA™-5-plex analysis (Indoor Biotechnologies, Inc., Charlottesville, VA) was performed to determine the allergen levels of Group 2-mites, using standard published techniques [13]. The study results were originally measured using ELISA, the gold standard at enrollment; however, MARIA™ technology subsequently became available, hence all samples were tested by both ELISA and MARIA™. Based upon a comparison study conducted by Filep et al, we determined that 860 ng/g for Group 2 allergens by MARIA™ corresponds closely to ELISA-based 2.0 μg/g [14]. Concentrations (MARIA™ data) are presented here in ng/g units for dust mite allergen.
Statistical Analyses
To ascertain comparability of the intervention and control group populations, baseline characteristics and questionnaire data were compared between study arms using Fisher's exact test. The MARIA™ based dust mite allergen concentrations (Der f 2 /Der p2) were described using geometric means (GM) and distributional characteristics within each group, by location and time point. Repeated measures mixed effects models were used to compare groups with respect to reductions in mite concentrations from baseline to 6 and 12 months, for each sampling location and overall. Specifically, in this model the groups were compared with respect to the average change from baseline over the time points, regardless of whether the change was observed at 6 or 12 months or both. Descriptive and correlational analyses were conducted to examine relationships between test kit use, motivation to engage in allergen reduction behaviors, and actually engaging in such behaviors, per the PAPM model. Modeling and inference were performed using only non-missing, log-transformed data; missing data were also imputed using mixed models to ensure factorial balance in GM estimation. Of the 495 allergen measurements across 55 households in three locations, 51 (10.3%) were missing and required imputation; these imputations were equally distributed across intervention and control homes. All statistical analyses were performed using SAS for Windows (SAS Version 9.2, Cary, NC).
RESULTS
Fifty-seven of the 60 homes completed 6 months of follow-up, and 55 homes completed the full 12-month follow-up. Baseline characteristics were similar among the study groups (Table 1). Approximately half of the study participants in each study arm reported physician-diagnosed asthma. Although there were no statistically significant differences in participant characteristics, a higher percentage of participants were white and reported lower educational level in control homes versus intervention homes. Approximately half of the study participants in each study arm reported physician-diagnosed asthma.
Table 1.
Characteristics of the study participants.
| Characteristic | Treatment Group | |
|---|---|---|
| Test Kit | Control | |
| Race (p=0.34)a | N (%) | N (%) |
| White | 15 (57.7%) | 21 (72.4%) |
| Black | 8 (30.8%) | 4 (13.8%) |
| Other | 3 (11.5%) | 4 (13.8%) |
| Education (p=0.21)a | ||
| Graduate degree | 11 (42.3%) | 6 (20.7%) |
| Bachelor's degree | 8 (30.8%) | 10 (34.5%) |
| Less than bachelor's | 7 (26.9%) | 13 (44.8%) |
| Yearly household income (p=0.99)ab | ||
| $75,000+ | 7 (30.4%) | 8 (32.0%) |
| $50,000-$75,000 | 8 (34.8%) | 8 (32.0%) |
| <$50,000 | 8 (34.8%) | 9 (36.0%) |
| Type of dwelling (p=0.39)a | ||
| Detached house | 24 (92.3%) | 25 (86.2%) |
| All others | 2 (7.7%) | 4 (13.8%) |
| Child has asthma (p=0.75)a | ||
| Yes | 12 (46.2%) | 15 (51.7%) |
| No | 14 (53.8%) | 14 (48.3%) |
p-Value for association based on Fisher's Exact Test.
Seven study participants responded they ‘did not know’ or ‘refused’ to indicate their yearly income.
Total N=55 households completing the study.
Dust mite Allergen (Der f 2/Der p 2) Concentrations
Table 2 shows the geometric means (GM) and distributional characteristics for Group 2 allergen levels in each group by location and time point of study visit. In the intervention homes, dust mite allergen levels in bedroom floors were reduced throughout the follow-up. In the control homes, allergen concentrations increased slightly from baseline to 6 months, but declined thereafter. Comparing intervention homes to control homes, the differential reduction in allergen levels in bedroom floors from baseline to 6 and 12 months was statistically significant (p=0.03). For living room floors, allergen levels were reduced in both intervention and control homes over time. The differential reduction in living room floor allergen levels between intervention and control homes over the study period was marginally but not clearly statistically significant (p=0.10). In the child's bed, allergen levels declined from baseline to 6 months among intervention homes but then increased at 12 months to nearly their baseline level, in contrast to control homes in which the pattern more closely resembled that of the bedroom floor. Although the location-specific showed inconsistencies in allergen concentrations, there was a significant differential reduction in dust mite allergen concentration from baseline to 6 and 12 months, on average, between the intervention versus the control group (p=0.02).
Table 2.
Geometric means and distributional characteristics for dust mite allergen (Der f 2/ Der p 2) concentrations (MARIA™ data) in each treatment group, by location and time point.
| Location and Time Point | N | Geometric Mean, ng/g (95% CI) | p-Valuea | ||
|---|---|---|---|---|---|
| Test Kit | Control | Test Kit | Control | ||
| Child's bedroom floor | |||||
| Baseline | 26 | 29 | 747 (359, 1556) | 646 (357, 1168) | 0.03 |
| 6 months | 26 | 29 | 344 (166, 715) | 701 (398, 1235) | |
| 12 months | 26 | 29 | 316 (154, 652) | 383 (156, 940) | |
| Living room floor | |||||
| Baseline | 26 | 29 | 321 (129, 801) | 351 (147, 838) | 0.10 |
| 6 months | 26 | 29 | 131 (52, 327) | 340 (137, 844) | |
| 12 months | 26 | 29 | 201 (85, 480) | 265 (121, 585) | |
| Child's bed | |||||
| Baseline | 26 | 29 | 566 (290, 1106) | 617 (299, 1273) | 0.95 |
| 6 months | 26 | 29 | 259 (120, 560) | 683 (248, 1885) | |
| 12 months | 26 | 29 | 560 (193, 1626) | 237 (74, 760) | |
p - Value for F-test of average (6- and 12-month) differential reduction in log-transformed Der f 2/ Der p 2 concentrations from baseline in the test kit group versus control, based on repeated measured model. Overall p =0.02 for model combining all data across locations.
Test Kit Findings
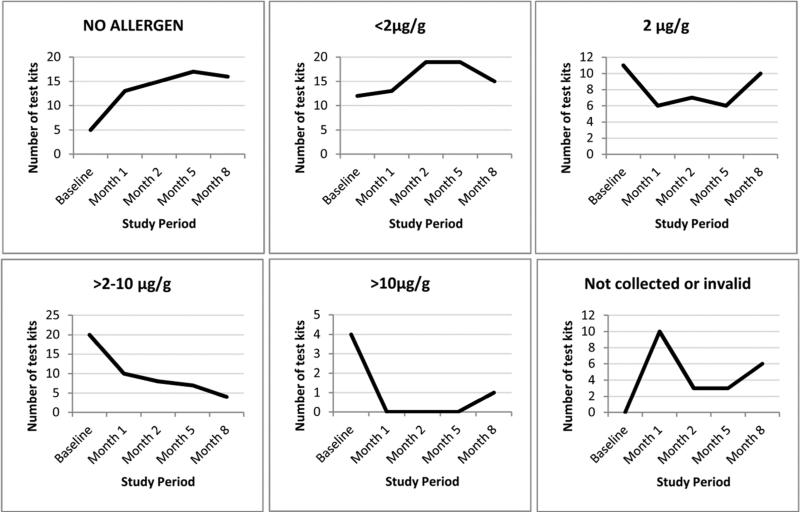
We examined results from the test kits returned by study participants in order to better understand their potential for motivation and to provide supplemental information on allergen concentrations in addition to the MARIA™ test data (technician-collected dust samples). Households in the intervention arm experienced a consistent reduction in dust mite allergen levels over the course of the study (Figure 1). The number of test kit results (child's bed and bedroom floor combined) indicating low levels of allergen (< 2μg/g or no allergen) increased from 17 tests at baseline to 31 at 8 months. Correspondingly, the number of test kits indicating elevated levels of allergen (2-10 μg/g or > 10μg/g) decreased from 24 tests at baseline to 5 tests at 8 months. Reductions in dust mite allergen levels on the bedroom floor, as measured by the test kit, from baseline to months 1, 2, 5 and 8 were statistically significant (p <0.01). More than 62% of the intervention homes had lower test kit-based allergen levels, in the child's bed and bedroom floor combined, at 8 months compared with baseline (Table 3).
Figure 1.
Distribution of test kit based dust mite allergen concentrations (μg/g) by time point in the intervention group (bedroom bed and floor combined, N=52). The figure shows the number of test kits in each allergen category, and the number of kits that were not collected or were invalid over the study period. p-Value <0.01 for nonparametric sign test for the decline in dust mite levels from baseline to months 1, 2, 5 and 8 for floor. p-Value <0.01 for the decline in dust levels from baseline to months 2 and 5 in bed.
Table 3.
Number of households in the intervention group (N=26) having lower, equal, and higher test kit-based allergen levels at 8 months as compared to baseline, by location.
| Dust mite level | Child's bed | Child's bedroom floor |
|---|---|---|
| Lower at 8 months | 16 | 18 |
| Equal | 5 | 6 |
| Higher at 8 months | 5 | 2 |
Test Kit Effect on Motivation to Engage in Reduction Behaviors
We assessed the extent to which participants were surprised by their home test kit results in relation to their motivation to engage in future reduction strategies. Of the participants using the test kit, 68% reported initial “surprise” or “somewhat surprise” with test kit results indicating their personal dust mite exposure was higher than anticipated. Furthermore, test kit participants “surprised” or “somewhat surprised” by their initial results tended to strongly agree that the test kit results motivated them to adopt reduction behaviors than those who were not “surprised” (76% vs. 37%, p =0.03 by Fisher's exact test).
The degree of motivation to maintain reduction behaviors over time was slightly related to the number of engaged reduction strategies at 12 months (Spearman rank correlation 0.35, p =0.08). Of the participants who reported “strongly agree” or “somewhat agree” that test kits results motivated them to maintain reduction strategies, 58% were engaging in two or more reduction behaviors at the end of the study. In contrast, none of the participants who reported “strongly disagree” or “somewhat disagree” were engaging in two or more reduction behaviors by the study end. Fifty five percent of the participants using the test kit (i.e., participants in the intervention group) and 32% of the control group participants were using allergen proof mattresses. Similarly, 63% of the participants using the test kit were using allergen proof pillow covers at 12 months compared to 33% of control group participants.
DISCUSSION
None of the previous dust mite allergen reduction studies have examined whether patient education in conjunction with the use of commercially available in-home test kits, which provide quantitative information on residential allergen levels, influences compliance with recommended dust mite reduction strategies. This is the first study to demonstrate that patient education along with objective evidence, which confirms the efficacy of patients’ efforts to reduce dust mite allergen levels in the home, may result in greater reductions in allergen levels than the use of educational materials alone. However, the results are mixed and further study would be beneficial.
In the intervention homes, dust mite allergen concentrations in the child's bedroom and living room floors were reduced over time compared to control homes (Table 2). In contrast, this pattern was not observed at the 12 month time point for concentrations in the child's bed. However, the finding may not necessarily be surprising because the use of impermeable mattress and pillow covers was never highly prevalent in the study. Studies have shown that the use of impermeable covers for mattresses and pillows can result in significant reductions in dust mite concentrations in beds [2]. Speculatively, participants in the intervention group might have also become complacent in washing sheets weekly in hot water after noticing reduction in allergen levels. Whereas control homes, while unaware of their dust mite allergen levels but continuing to receive periodic education materials and administered questionnaires, may have been more encouraged to implement avoidance strategies. Overall, control homes did ultimately experience mite reductions at 12 months, perhaps due to a Hawthorne effect, a common phenomenon when participants alter their behavior in response to being part of a study [15].
In addition to reductions seen in technician-collected dust samples, the test kit themselves were informative. From baseline to 8 months the number of test kits with undetectable allergen increased by 320% and the number of test kits with mite levels >10 μg/g decreased by 75% among the intervention group. The majority of households at 8 months had lowered test-kit based allergen levels (child's bed and bedroom floor combined) compared with baseline. These findings further support that approximately 90% of intervention participants reported that the test kit motivated them to implement and maintain allergen reduction behaviors at both 6 and 12 months.
The Precaution Adoption Process Model [16, 17], like other health behavior theories, considers personal susceptibility to risks as a significant factor in motivating behavior [18, 19]. We hypothesized that parents of dust mite allergic children might be more likely to move from a stage of “preparing to act” to “taking action”, if they had information on their child's risk level (dust mite allergen levels). Although both the intervention and control groups were seemingly motivated to engage in dust mite reduction behaviors, the findings suggested that the personal susceptibility information provided by the test kit was more likely to result in reduced dust mite allergen levels reported at 12 months than the information provided in the educational materials alone. Although not explicitly measured in this study, educational material may have bridged the gap between intending to act (based on test kit feedback) and carrying out intent (adopting mite reduction behaviors) [20]. Detailed information on dust mite allergen levels may have motivated participants to reduce barriers and successfully implement reduction strategies.
This study has several limitations. The findings may not be generalizable to other populations. The small study size due to the stringent eligibility criteria also limited our ability to evaluate the effect of test kit usage on asthma morbidity and other allergic outcomes. On the other hand, several studies have demonstrated the clinical importance of dust mite allergens on asthma and allergies; reductions in exposure can lead to decreased bronchial hyperresponsiveness, decreased morbidity and decreased need for medications [1-4]. Because we did not ascertain dust mite sensitization by clinical measures we were not able to assess the severity of dust allergy among participants. Hence, some children may have been misclassified due to the self-report measures. Some parents may have also been less motivated to reduce allergen levels if their child showed little/no change in symptoms. Although the precision of allergen concentrations measured by test kit results were contingent on test kit participant's ability to read color intensity charts which corresponded to allergen levels, serious differential misclassification of exposure is unlikely. Due to differences in sampling times, test kits results and MARIA™ data were not compatible. However, these measures were designed to assess different study components. While the primary assessment of exposure was based on results from MARIA™, an objective measurement, the test kits provided semiquantitative information on dust mite allergen concentrations and helped to determine if the use of test kits resulted in behavioral changes in implementation and maintenance of dust mite reduction strategies. Lastly, the selected test kit did not quantify most commonly measured mite allergens, group 1 mite, allergens; however, both group 1 and 2 allergens are major allergens and are considered clinically important because >80% of mite-allergic individuals have IgE antibodies to these proteins [21, 22]. Despite the limitations, this is the first study to show that patient education along with objective evidence can result in greater reductions in residential dust mite allergen levels than educational materials alone.
CONCLUSION
In conclusion, this study demonstrated a possible association between the use of in-home test kits along with educational materials and a reduction in dust mite allergen levels in homes of children with reported dust mite allergies. While the results were mixed, there is at least some evidence to suggest the efficacy of patients’ efforts to reduce allergen levels in the home, which may beneficially influence allergic and asthmatic patients’ and their families’ behaviors and attitudes towards environmental control measures. Additional studies with larger sample sizes and health outcome measures are needed to further investigate strategies to motivate allergic people with asthma to employ environmental control measures that reduce asthma morbidity.
Supplementary Material
Abbreviations and Acronyms
- ELISA
Enzyme-Linked Immunosorbent Assay
- HEPA
High Efficiency Particulate Air
- IgE
Immunoglobulin E
- MARIA™
Multiplex Array for Indoor Allergens
- PAPM
Precaution Adoption Process Model
Footnotes
Declaration of interests: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- 1.Portnoy J, Miller JD, Williams PB, Chew GL, Miller JD, Zaitoun F, et al. Environmental assessment and exposure control of dust mites: a practice parameter. Ann Allergy Asthma Immunol. 2013;111:465–507. doi: 10.1016/j.anai.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halken S, Host A, Niklassen U, Hansen LG, Nielsen F, Pederson S, et al. Effect of mattress and pillow encasings on children with asthma and house dust mite allergy. J Allergy Clin Immunol. 2003;111:169–76. doi: 10.1067/mai.2003.5. [DOI] [PubMed] [Google Scholar]
- 3.El-Ghitany EM, Abd El-Salam MM. Environmental intervention for house dust mite control in childhood bronchial asthma. Environ Health Prev Med. 2012;17:377–84. doi: 10.1007/s12199-011-0263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Committee on the Assessment of Asthma and Indoor Air . Clearing the air: asthma and indoor exposures. National Academy Press; Washington (DC): 2000. Division of Health Promotion and Disease Prevention, Institute of Medicine. pp. 143–144. [Google Scholar]
- 5.Myers TR. Guidelines for asthma management: a review and comparison of 5 current guidelines. Respiratory Care. 2008;53:751–67. discussion 67-9. [PubMed] [Google Scholar]
- 6.Calderón MA, Linneberg A, Kleine-Tebbe J, De Blay F, Hernandez Fernandez de Rojas D, Virchow, et al. Respiratory allergy caused by house dust mites: What do we really know? J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.10.012. Epub pii:S0091-6749(14)01482-1. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Caldas E. Dust mite allergens: mitigation and control. Current Allergy and Asthma Reports. 2002;2:424–31. doi: 10.1007/s11882-002-0077-z. [DOI] [PubMed] [Google Scholar]
- 8.Eggleston PA. Improving indoor environments: Reducing allergen exposures. J Allergy Clin Immunol. 2005;116:122–6. doi: 10.1016/j.jaci.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Busse PJ, Wang JJ, Halm EA. Allergen sensitization evaluation and allergen avoidance education in an inner-city adult cohort with persistent asthma. J Allergy Clin Immunol. 2005;116:146–52. doi: 10.1016/j.jaci.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 10.Wu F, Takaro TK. Childhood asthma and environmental interventions. Environmental Health Perspectives. 2007;115:971–5. doi: 10.1289/ehp.8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott JO, Seals BF, Jacobson MP. Use of the Precaution Adoption Process Model to examine predictors of osteoprotective behavior in epilepsy. Seizure. 2007;16:424–37. doi: 10.1016/j.seizure.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Weinstein ND, Lyon JE, Sandman PM, Cuite CL. Experimental evidence for stages of health behavior change: the precaution adoption process model applied to home radon testing. Health Psychol. 1998;17:445–53. doi: 10.1037//0278-6133.17.5.445. [DOI] [PubMed] [Google Scholar]
- 13.Earle CD, King EM, Tsay A, Pittman K, Saric B, Vailes L. High-throughout fluorescent multiplex array for indoor allergen exposure assessment. J Clin Immunol. 2007;11:428–33. doi: 10.1016/j.jaci.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Filep S, Tsay A, Vailes L, Gadermaier G, Ferreira F, Matsui E, et al. A multi allergen standard for the calibration of immunoassays: CREATE principles applied to eight purified allergens. Allergy. 2012;67:235–41. doi: 10.1111/j.1398-9995.2011.02750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P. The Hawthorne Effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7:30. doi: 10.1186/1471-2288-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinstein ND. The precaution adoption process. Health Psychol. 1988;7:355–86. doi: 10.1037//0278-6133.7.4.355. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein ND, Sandman PM. A model of the precaution adoption process: evidence from home radon testing. Health Psychol. 1992;11:170–80. doi: 10.1037//0278-6133.11.3.170. [DOI] [PubMed] [Google Scholar]
- 18.Conner M, Norman P. Predicting Health Behavior. Open University Press; Philadelphia: 1995. [Google Scholar]
- 19.Glanz K, Rimer B, Lewis FM. Health Behavior and Health Education: Theory, Research and Practice. Josey-Bass; San Francisco: 2002. [Google Scholar]
- 20.Gollwitzer PM. Implementation intentions: Strong effects of simple plans. American Psychologist. 1999;54:493–503. [Google Scholar]
- 21.Bessot JC, Pauli G. Mite allergens: an overview. Eur Ann Allergy Clin Immunol. 2011;43:141–56. [PubMed] [Google Scholar]
- 22.Chruszcz M, Pomés A, Glesner J, Vailes LD, Osinski T, Porebski PJ, et al. Molecular determinants for antibody binding on group 1 house dust mite allergens. J Biol Chem. 2012;287:7388–98. doi: 10.1074/jbc.M111.311159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.